Molecular Mechanisms of Laparoscopic Ovarian Drilling and Its Therapeutic Effects in Polycystic Ovary Syndrome
Abstract
:1. Introduction
2. The Pathophysiology of Polycystic Ovary Syndrome (PCOS)
3. The Molecular Mechanisms of IR in Polycystic Ovary Syndrome (PCOS)
4. Laparoscopic Ovarian Drilling in Polycystic Ovary Syndrome
4.1. A Brief Review of the Operative Procedure of Laparoscopic Ovarian Drilling
4.2. How Many Punctures Are Needed during the Laparoscopic Ovarian Drilling Procedure?
4.3. Possible Molecular Mechanisms of Laparoscopic Ovarian Drilling
5. The Effects of Laparoscopic Ovarian Drilling
5.1. Ovulation and Pregnancy Rates after Laparoscopic Ovarian Drilling
5.2. Metabolic Effects of Laparoscopic Ovarian Drilling
5.3. Predictors of Success after Laparoscopic Ovarian Drilling
5.4. Long-Term Effects of Laparoscopic Ovarian Drilling
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AMH | anti-Müllerian hormone |
| AI | aromatase inhibitors |
| ART | assistance reproductive technique |
| BW | body weight |
| CAPN10 | Calpain-10 |
| CC | clomiphene citrate |
| CCL2 | C-C Motif Chemokine Ligand 2 |
| CD163 | cluster of differentiation 163 |
| DENND | differentially expressed in normal and neoplastic development |
| DHEA-S | dehydroepiandrosterone sulphate |
| FSH | follicle-stimulating hormone |
| GABA | gamma amino butyric acid |
| GnRH | gonadotropin releasing hormone agonist |
| GCKR | glucokinase regulatory protein |
| GCSF | granulocyte colony-stimulating factor |
| GLUT-4 | glucose transporter type 4 |
| IL | interleukin |
| ICAM1 | intercellular adhesion molecule 1 |
| iNOS | oxidase and inducible nitric oxide synthase |
| IR | insulin resistance |
| IRS | insulin receptor substrate |
| IVF | in vitro fertilization |
| ICSI-ET | intracytoplasmic sperm injection and embryo transfer |
| LH | luteinizing hormone |
| LOD | laparoscopic ovarian drilling |
| MAPK | mitogen-activated protein kinase |
| microRNAs | small noncoding micro ribonucleic acid |
| MIF | macrophage inhibitory factor |
| MTHFR | methylenetetrahydrofolate reductase |
| MMP | matrix metalloproteinase family |
| NAD | nicotinamide adenine dinucleotide |
| NAMPT | nicotinamide phosphoribosyltransferase |
| Nd:YAG | neodymium-doped yttrium aluminium garnet |
| NEGR1 | neuronal growth regulator 1 |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| OHSS | ovarian hyperstimulation syndrome |
| OMLOD | office microlaparoscopic ovarian drilling |
| PBEF1 | pre-B-cell colony-enhancing factor 1 |
| PCOS | polycystic ovary syndrome |
| PI-3K | phosphatidylinositol (PI)3-kinase |
| RAB5B | RAS-related protein 5b |
| RARRES2 | retinoic acid receptor responder protein 2 |
| ROS | reactive oxygen species |
| TGF-β1 | transforming growth factor beta 1 |
| TNF-α | tumor necrosis factor alpha |
| TLR2 | Toll-like receptor 2 |
| VEGF | vascular epithelial growth factor |
References
- Sanchez-Garrido, M.A.; Tena-Sempere, M. Metabolic dysfunction in polycystic ovary syndrome: Pathogenic role of androgen excess and potential therapeutic strategies. Mol. Metab. 2020, 35, 100937. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, W.; Bordewijk, E.M.; Legro, R.S.; Zhang, H.; Wu, X.; Gao, J.; Morin-Papunen, L.; Homburg, R.; König, T.E.; et al. Reproductive Medicine Network+; International Ovulation Induction IPDMA Collaboration. First-line ovulation induction for polycystic ovary syndrome: An individual participant data meta-analysis. Hum. Reprod. Update 2019, 25, 717–732. [Google Scholar] [CrossRef] [PubMed]
- Rajska, A.; Buszewska-Forajta, M.; Rachoń, D.; Markuszewski, M.J. Metabolomic insight into polycystic ovary syndrome—An overview. Int. J. Mol. Sci. 2020, 21, 4853. [Google Scholar] [CrossRef] [PubMed]
- Cadagan, D.; Khan, R.; Amer, S. Thecal cell sensitivity to luteinizing hormone and insulin in polycystic ovarian syndrome. Reprod. Biol. 2016, 16, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowska, A.; Osowski, A.; Jóźwik, M.; Górecki, R.; Rynkiewicz, A.; Wojtkiewicz, J. Inositols’ importance in the improvement of the endocrine-metabolic profile in PCOS. Int. J. Mol. Sci. 2019, 20, 5787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EAl Wattar, B.H.; Teede, H.; Garad, R.; Franks, S.; Balen, A.; Bhide, P.; Piltonen, T.; Romualdi, D.; Laven, J.; Thondan, M.; et al. Harmonising research outcomes for polycystic ovary syndrome: An international multi-stakeholder core outcome set. Hum. Reprod. 2020, 35, 404–412. [Google Scholar] [CrossRef]
- Forslund, M.; Landin-Wilhelmsen, K.; Trimpou, P.; Schmidt, J.; Brännström, M.; Dahlgren, E. Type 2 diabetes mellitus in women with polycystic ovary syndrome during a 24-year period: Importance of obesity and abdominal fat distribution. Hum. Reprod. Open 2020, 2020, hoz042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliver-Williams, C.; Vassard, D.; Pinborg, A.; Schmidt, L. Risk of cardiovascular disease for women with polycystic ovary syndrome: Results from a national Danish registry cohort study. Eur. J. Prev. Cardiol. 2020. [Google Scholar] [CrossRef]
- Simsir, C.; Pekcan, M.K.; Aksoy, R.T.; Ecemis, T.; Coskun, B.; Kilic, S.H.; Tokmak, A. The ratio of anterior anogenital distance to posterior anogenital distance: A novel-biomarker for polycystic ovary syndrome. J. Chin. Med. Assoc. 2019, 82, 782–786. [Google Scholar] [CrossRef]
- Teede, H.J.; Misso, M.L.; Costello, M.F.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R.J.; International PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil. Steril. 2018, 110, 364–379. [Google Scholar] [CrossRef] [Green Version]
- Aydın, G.A.; Özsoy, H.G.T.; Ankaralı, H.; Özgen, G.; Neşelioğlu, S. The association of dynamic thiol-disulfide homeostasis and inflammatory markers in patients with polycystic ovary syndrome. Taiwan J. Obstet. Gynecol. 2020, 59, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Costello, M.F.; Misso, M.L.; Balen, A.; Boyle, J.; Devoto, L.; Garad, R.M.; Hart, R.; Johnson, L.; Jordan, C.; Legro, R.S.; et al. International PCOS Network. Evidence summaries and recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome: Assessment and treatment of infertility. Hum. Reprod. Open 2019, 2019, hoy021. [Google Scholar] [CrossRef]
- Abdalla, M.A.; Deshmukh, H.; Atkin, S.; Sathyapalan, T. A review of therapeutic options for managing the metabolic aspects of polycystic ovary syndrome. Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018820938305. [Google Scholar] [CrossRef]
- Aversa, A.; La Vignera, S.; Rago, R.; Gambineri, A.; Nappi, R.E.; Calogero, A.E.; Ferlin, A. Fundamental concepts and novel aspects of polycystic ovarian syndrome: Expert consensus resolutions. Front. Endocrinol. 2020, 11, 516. [Google Scholar] [CrossRef] [PubMed]
- Baghdadi, L.R.; Abu Hashim, H.; Amer, S.A.; Palomba, S.; Falbo, A.; Al-Ojaimi, E.; Ott, J.; Zhu, W.; Fernandez, H.; Nasr, A.; et al. Impact of obesity on reproductive outcomes after ovarian ablative therapy in PCOS: A collaborative meta-analysis. Reprod. Biomed. Online 2012, 25, 227–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shang, Y.; Zhou, H.; Hu, M.; Feng, H. Effect of diet on insulin resistance in polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2020, 105, 425. [Google Scholar] [CrossRef] [PubMed]
- Legro, R.S.; Barnhart, H.X.; Schlaff, W.D.; Carr, B.R.; Diamond, M.P.; Carson, S.A.; Steinkampf, M.P.; Coutifaris, C.; McGovern, P.G.; Cataldo, N.A.; et al. Cooperative multicenter reproductive medicine network. Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. N. Engl. J. Med. 2007, 356, 551–566. [Google Scholar] [CrossRef] [Green Version]
- Utz, A.L.; Schaefer, P.W.; Snuderl, M. Case records of the Massachusetts General Hospital. Case 20-2010. A 32-year-old woman with oligomenorrhea and infertility. N. Engl. J. Med. 2010, 363, 178–186. [Google Scholar] [CrossRef]
- Berger, J.J.; Bates, G.W., Jr. Optimal management of subfertility in polycystic ovary syndrome. Int. J. Women’s Health 2014, 6, 613–621. [Google Scholar]
- Weiss, N.S.; Kostova, E.; Nahuis, M.; Mol, B.W.J.; van der Veen, F.; van Wely, M. Gonadotrophins for ovulation induction in women with polycystic ovary syndrome. Cochrane Database Syst. Rev. 2019. [Google Scholar] [CrossRef]
- Gadalla, M.A.; Norman, R.J.; Tay, C.T.; Hiam, D.S.; Melder, A.; Pundir, J.; Thangaratinam, S.; Teede, H.J.; Mol, B.W.J.; Moran, L.J. Medical and surgical treatment of reproductive outcomes in polycystic ovary syndrome: An overview of systematic reviews. Int. J. Fertil. Steril. 2020, 13, 257–270. [Google Scholar]
- Ozelci, R.; Dilbaz, S.; Dilbaz, B.; Cırık, D.A.; Yılmaz, S.; Tekin, O.M. Gonadotropin releasing hormone antagonist use in controlled ovarian stimulation and intrauterine insemination cycles in women with polycystic ovary syndrome. Taiwan. J. Obstet. Gynecol. 2019, 58, 234–238. [Google Scholar] [CrossRef]
- Shi, S.; Hong, T.; Jiang, F.; Zhuang, Y.; Chen, L.; Huang, X. Letrozole and human menopausal gonadotropin for ovulation induction in clomiphene resistance polycystic ovary syndrome patients: A randomized controlled study. Medicine 2020, 99, e18383. [Google Scholar] [CrossRef]
- Huang, J.; Ding, Y.; Li, Z. The regulation of the follicular synchronization and sensitivity of rats with PCOS by AMH during prolonged pituitary downregulation. Gene 2019, 721, 144106. [Google Scholar] [CrossRef]
- Palomba, S.; Orio, F., Jr.; Falbo, A.; Russo, T.; Caterina, G.; Manguso, F.; Tolino, A.; Colao, A.; Zullo, F. Metformin administration and laparoscopic ovarian drilling improve ovarian response to clomiphene citrate (CC) in oligo-anovulatory CC-resistant women with polycystic ovary syndrome. Clin. Endocrinol. 2005, 63, 631–635. [Google Scholar] [CrossRef]
- Farquhar, C.M.; Williamson, K.; Gudex, G.; Johnson, N.P.; Garland, J.; Sadler, L. A randomized controlled trial of laparoscopic ovarian diathermy versus gonadotropin therapy for women with clomiphene citrate-resistant polycystic ovary syndrome. Fertil. Steril. 2002, 78, 404–411. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, L.; Kong, L.; Wu, T.; Xu, L.; Pan, X.; Liu, G.J. Ultrasound-guided transvaginal ovarian needle drilling for clomiphene-resistant polycystic ovarian syndrome in subfertile women. Cochrane Database Syst. Rev. 2019, 7, CD008583. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, K.; Luo, X.; Yang, M.; Shen, X.; Xu, L. Variation of laparoscopic ovarian drilling for clomiphene citrate-resistant patients with polycystic ovary syndrome and infertility: A meta-analysis. J. Minim. Invasive Gynecol. 2020, 27, 1048–1058. [Google Scholar] [CrossRef]
- Facchinetti, F.; Unfer, V.; Dewailly, D.; Kamenov, Z.A.; Diamanti-Kandarakis, E.; Laganà, A.S.; Nestler, J.E.; Soulage, C.O.; Group of ‘Inositol in PCOS and Reproduction’. Inositols in polycystic ovary syndrome: An overview on the advances. Trends Endocrinol. Metab. 2020, 31, 435–447. [Google Scholar] [CrossRef]
- Sharpe, A.; Morley, L.C.; Tang, T.; Norman, R.J.; Balen, A.H. Metformin for ovulation induction (excluding gonadotrophins) in women with polycystic ovary syndrome. Cochrane Database Syst. Rev. 2019, 12, CD013505. [Google Scholar] [CrossRef]
- Abu Hashim, H.; Foda, O.; Ghayaty, E. Combined metformin-clomiphene in clomiphene-resistant polycystic ovary syndrome: A systematic review and meta-analysis of randomized controlled trials. Acta Obstet. Gynecol. Scand. 2015, 94, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.; Gemmell, A.; Colleran, R.; Zanuri, N.B.; O’Brien, H.; Poobalan, A. Does metformin combined with clomiphene citrate improve fertility related outcomes in clomiphene resistant women with PCOS: A systematic review. Middle East Fertil. Soc. J. 2014, 19, 81–88. [Google Scholar] [CrossRef] [Green Version]
- Rouzi, A.A.; Ardawi, M.S. A randomized controlled trial of the efficacy of rosiglitazone and clomiphene citrate versus metformin and clomiphene citrate in women with clomiphene citrate-resistant polycystic ovary syndrome. Fertil. Steril. 2006, 85, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Kocak, M.; Caliskan, M.D.; Simsir, C.; Haleral, A. Metformin therapy improves ovulatory rates, cervical scores, and pregnancy rates in clomiphene citrate-resistant women with polycystic ovary syndrome. Fertil. Steril. 2002, 77, 101–106. [Google Scholar] [CrossRef]
- Ng, E.H.; Wat, N.M.; Ho, P.C. Effects of Metformin on ovulation rate, hormonal and metabolic profiles on women with clomiphene-resistant polycystic ovaries: A randomized, double-blinded placebo-controlled trial. Hum. Reprod. 2001, 16, 1625–1631. [Google Scholar] [CrossRef] [Green Version]
- Vandermolen, D.T.; Ratts, V.S.; Evans, W.S.; Stovall, D.W.; Kauma, S.W.; Nestler, J.E. Metformin increases the ovulation rate and pregnancy rate from clomiphene citrate in patients with polycystic ovary syndrome who are resistant to Clomiphene Citrate alone. Fertil. Steril. 2001, 75, 310–315. [Google Scholar] [CrossRef]
- Sturrock, N.D.C.; Lannon, B.; Fay, T.N. Metformin does not enhance ovulation induction in Clomiphene resistant polycystic ovary syndrome in clinical practice. Br. J. Clin. Pharmacol. 2002, 53, 469–473. [Google Scholar] [CrossRef] [Green Version]
- Abu Hashim, H.; Foda, O.; El Rakhawy, M. Unilateral or bilateral laparoscopic ovarian drilling in polycystic ovary syndrome: A meta-analysis of randomized trials. Arch. Gynecol. Obstet. 2018, 297, 859–870. [Google Scholar] [CrossRef]
- Bordewijk, E.M.; Ng, K.Y.B.; Rakic, L.; Mol, B.W.J.; Brown, J.; Crawford, T.J.; van Wely, M. Laparoscopic ovarian drilling for ovulation induction in women with anovulatory polycystic ovary syndrome. Cochrane Database Syst. Rev. 2020, 2, CD001122. [Google Scholar] [CrossRef]
- Morley, L.C.; Tang, T.; Yasmin, E.; Norman, R.J.; Balen, A.H. Insulin-sensitising drugs (metformin, rosiglitazone, pioglitazone, D-chiro-inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility. Cochrane Database Syst. Rev. 2017, 11, CD003053. [Google Scholar] [CrossRef]
- Showell, M.G.; Mackenzie-Proctor, R.; Jordan, V.; Hodgson, R.; Farquhar, C. Inositol for subfertile women with polycystic ovary syndrome. Cochrane Database Syst. Rev. 2018, 12, CD012378. [Google Scholar] [CrossRef] [PubMed]
- Franik, S.; Eltrop, S.M.; Kremer, J.A.; Kiesel, L.; Farquhar, C. Aromatase inhibitors (letrozole) for subfertile women with polycystic ovary syndrome. Cochrane Database Syst. Rev. 2018, 5, CD010287. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Tu, M.; Huang, Y.; Liu, Y.; Zhang, D. Association of metformin with pregnancy outcomes in women with polycystic ovarian syndrome undergoing in vitro fertilization: A systematic review and meta-analysis. JAMA Netw. Open 2020, 3, e2011995. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Zhou, M.; Cheng, M.; Zhang, D.; Wu, X.; Si, C.; Xia, L.; Xu, H.; Li, J.; Chang, H.M.; et al. Transvaginal ovarian drilling followed by controlled ovarian stimulation from the next day improves ovarian response for the poor responders with polycystic ovary syndrome during IVF treatment: A pilot study. Reprod. Biol. Endocrinol. 2020, 18, 7. [Google Scholar] [CrossRef] [PubMed]
- González-Ortega, C.; Piña-Aguilar, R.E.; Cancino-Villarreal, P.; Pérez-Peña, E.; Gutiérrez-Gutiérrez, A.M. Natural-cycle in vitro fertilization (IVF) combined with in vitro maturation in infertile patients with polycystic ovarian syndrome (PCOS) requiring IVF. Taiwan J. Obstet. Gynecol. 2019, 58, 192–195. [Google Scholar] [CrossRef]
- Skalecki, S.; Robson, S.J. Trends in metabolic surgery in reproductive-age women in Australia. Aust. N. Z. J. Obstet. Gynaecol. 2020, 60, 622–624. [Google Scholar] [CrossRef]
- Gjonnaess, H. Polycystic ovarian syndrome treated by ovarian electrocautery through the laparoscope. Fertil. Steril. 1984, 41, 20–25. [Google Scholar] [CrossRef]
- Stein, I.F.; Leventhal, M.L. Amenorrhea associated with bilateral polycystic ovaries. Am. J. Obstet. Gynecol. 1935, 29, 181–191. [Google Scholar] [CrossRef]
- Azziz, R.; Adashi, E.Y. Stein and Leventhal: 80 years on. Am. J. Obstet. Gynecol. 2016, 214, 247.e1–247.e11. [Google Scholar] [CrossRef]
- Sunj, M.; Canic, T.; Baldani, D.P.; Tandara, M.; Jeroncic, A.; Palada, I. Does unilateral laparoscopic diathermy adjusted to ovarian volume increase the chances of ovulation in women with polycystic ovary syndrome? Hum. Reprod. 2013, 28, 2417–2424. [Google Scholar] [CrossRef] [Green Version]
- Mitra, S.; Nayak, P.K.; Agrawal, S. Laparoscopic ovarian drilling: An alternative but not the ultimate in the management of polycystic ovary syndrome. J. Nat. Sci. Biol. Med. 2015, 6, 40–48. [Google Scholar]
- Hafizi, L.; Amirian, M.; Davoudi, Y.; Jaafari, M.; Ghasemi, G.H. Comparison of laparoscopic ovarian drilling success between two standard and dose-adjusted methods in polycystic ovary syndrome: A randomized clinical trial. Int. J. Fertil. Steril. 2020, 13, 282–288. [Google Scholar] [PubMed]
- Rezk, M.; Sayyed, T.; Saleh, S. Impact of unilateral versus bilateral laparoscopic ovarian drilling on ovarian reserve and pregnancy rate: A randomized clinical trial. Gynecol. Endocrinol. 2016, 32, 399–402. [Google Scholar] [CrossRef]
- Sunj, M.; Kasum, M.; Canic, T.; Karelovic, D.; Tandara, M.; Tandara, L.; Palada, I. Assessment of ovarian reserve after unilateral diathermy with thermal doses adjusted to ovarian volume. Gynecol. Endocrinol. 2014, 30, 785–788. [Google Scholar] [CrossRef]
- Sunj, M.; Canic, T.; Jeroncic, A.; Karelovic, D.; Tandara, M.; Juric, S.; Palada, I. Anti-Müllerian hormone, testosterone and free androgen index following the dose-adjusted unilateral diathermy in women with polycystic ovary syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 179, 163–169. [Google Scholar] [CrossRef]
- Amer, S.A.; Li, T.C.; Ledger, W.L. The value of measuring anti-Mullerian hormone in women with anovulatory polycystic ovary syndrome undergoing laparoscopic ovarian diathermy. Hum. Reprod. 2009, 24, 2760–2766. [Google Scholar] [CrossRef] [Green Version]
- Elmashad, A.I. Impact of laparoscopic ovarian drilling on anti-Müllerian hormone levels and ovarian stromal blood flow using three-dimensional power Doppler in women with anovulatory polycystic ovary syndrome. Fertil. Steril. 2011, 95, 2342–2346.e1. [Google Scholar] [CrossRef] [PubMed]
- Kaya, H.; Sezik, M.; Ozkaya, O. Evaluation of a new surgical approach for the treatment of clomiphene citrate-resistant infertility in polycystic ovary syndrome: Laparoscopic ovarian multi-needle intervention. J. Minim. Invasive Gynecol. 2005, 12, 355–358. [Google Scholar] [CrossRef] [PubMed]
- Palomba, S.; Falbo, A.; Battista, L.; Russo, T.; Venturella, R.; Tolino, A.; Orio, F.; Zullo, F. Laparoscopic ovarian diathermy vs clomiphene citrate plus metformin as second-line strategy for infertile anovulatory patients with polycystic ovary syndrome: A randomized controlled trial. Am. J. Obstet. Gynecol. 2010, 202, 577.e1–577.e8. [Google Scholar] [CrossRef]
- Yu, Y.; Fang, L.; Zhang, R.; He, J.; Xiong, Y.; Guo, X.; Du, Q.; Huang, Y.; Sun, Y. Comparative effectiveness of 9 ovulation-induction therapies in patients with clomiphene citrate-resistant polycystic ovary syndrome: A network meta-analysis. Sci. Rep. 2017, 7, 3812. [Google Scholar] [CrossRef]
- Seow, K.M.; Juan, C.C.; Hwang, J.L.; Ho, L.T. Laparoscopic surgery in polycystic ovary syndrome: Reproductive and metabolic effects. Semin. Reprod. Med. 2008, 26, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Amer, S.A.; Li, T.C.; Metwally, M.; Emarh, M.; Ledger, W.L. Randomized controlled trial comparing laparoscopic ovarian diathermy with clomiphene citrate as a first-line method of ovulation induction in women with polycystic ovary syndrome. Hum. Reprod. 2009, 24, 219–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sao, C.H.; Chan-Tiopianco, M.; Chung, K.C.; Chen, Y.J.; Horng, H.C.; Lee, W.L.; Wang, P.H. Pain after laparoscopic surgery: Focus on shoulder-tip pain after gynecological laparoscopic surgery. J. Chin. Med. Assoc. 2019, 82, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.C.; Chang, W.H.; Lin, I.C.; Wang, P.H. Compartment syndrome: A rare but uregent complication after total laparoscopic hysterectomy. Taiwan J. Obstet. Gynecol. 2019, 58, 725–726. [Google Scholar] [CrossRef]
- Hager, M.; Wenzl, R.; Riesenhuber, S.; Marschalek, J.; Kuessel, L.; Mayrhofer, D.; Ristl, R.; Kurz, C.; Ott, J. The prevalence of incidental endometriosis in women undergoing laparoscopic ovarian drilling for clomiphene-resistant polycystic ovary syndrome: A retrospective cohort study and meta-analysis. J. Clin. Med. 2019, 8, 1210. [Google Scholar] [CrossRef] [Green Version]
- Debras, E.; Fernandez, H.; Neveu, M.E.; Deffieux, X.; Capmas, P. Ovarian drilling in polycystic ovary syndrome: Long term pregnancy rate. Eur. J. Obstet. Gynecol. Reprod. Biol. X 2019, 4, 100093. [Google Scholar] [CrossRef]
- Lepine, S.; Jo, J.; Metwally, M.; Cheong, Y.C. Ovarian surgery for symptom relief in women with polycystic ovary syndrome. Cochrane Database Syst. Rev. 2017, 11, CD009526. [Google Scholar] [CrossRef]
- Kinnear, H.M.; Tomaszewski, C.E.; Chang, F.L.; Moravek, M.B.; Xu, M.; Padmanabhan, V.; Shikanov, A. The ovarian stroma as a new frontier. Reproduction 2020, 160, R25–R39. [Google Scholar] [CrossRef]
- McCartney, C.R.; Marshall, J.C. Clinical practice. Polycystic ovary syndrome. N. Engl. J. Med. 2016, 375, 54–64. [Google Scholar] [CrossRef] [Green Version]
- Azziz, R. Polycystic ovary syndrome. Obstet. Gynecol. 2018, 132, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Patel, S. Polycystic ovary syndrome (PCOS), an inflammatory, systemic, lifestyle endocrinopathy. J. Steroid Biochem. Mol. Biol. 2018, 182, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Walters, K.A.; Rodriguez Paris, V.; Aflatounian, A.; Handelsman, D.J. Androgens and ovarian function: Translation from basic discovery research to clinical impact. J. Endocrinol. 2019, 242, R23–R50. [Google Scholar] [CrossRef] [Green Version]
- Rosenfield, R.L. Current concepts of polycystic ovary syndrome pathogenesis. Curr. Opin. Pediatr. 2020, 32, 698–706. [Google Scholar] [CrossRef]
- Poli, G.; Fabi, C.; Bellet, M.M.; Costantini, C.; Nunziangeli, L.; Romani, L.; Brancorsini, S. Epigenetic mechanisms of inflammasome regulation. Int. J. Mol. Sci. 2020, 21, 5758. [Google Scholar] [CrossRef] [PubMed]
- Curry, A.M.; Fernàndez, R.D.; Pagani, T.D.; Abeyawardhane, D.L.; Trahan, M.L.; Lucas, H.R. Mapping of photochemically-derived dityrosine across Fe-bound n-acetylated α-synuclein. Life 2020, 10, 124. [Google Scholar] [CrossRef]
- Mammadova-Bach, E.; Jaeken, J.; Gudermann, T.; Braun, A. Platelets and defective N-glycosylation. Int. J. Mol. Sci. 2020, 21, 5630. [Google Scholar] [CrossRef]
- Lee, W.L.; Wang, P.H. Aberrant sialylation in ovarian cancers. J. Chin. Med. Assoc. 2020, 83, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, E.A.; Kauffman, A.S. The role of the brain in the pathogenesis and physiology of polycystic ovary syndrome (PCOS). Med. Sci. 2019, 7, 84. [Google Scholar] [CrossRef] [Green Version]
- Zeng, X.; Xie, Y.J.; Liu, Y.T.; Long, S.L.; Mo, Z.C. Polycystic ovarian syndrome: Correlation between hyperandrogenism, insulin resistance and obesity. Clin. Chim. Acta 2020, 502, 214–221. [Google Scholar] [CrossRef]
- Rostamtabar, M.; Esmaeilzadeh, S.; Tourani, M.; Rahmani, A.; Baee, M.; Shirafkan, F.; Saleki, K.; Mirzababayi, S.S.; Ebrahimpour, S.; Nouri, H.R. Pathophysiological roles of chronic low-grade inflammation mediators in polycystic ovary syndrome. J. Cell. Physiol. 2020. [Google Scholar] [CrossRef]
- Sun, H.D.; Horng, H.C.; Liu, C.H.; Hsiao, S.M.; Chen, Y.J.; Chang, W.H.; Wang, P.H. Comparison of single-port and three-port laparoscopic salpingectomy in the management of tubal pregnancy. J. Chin. Med. Assoc. 2018, 81, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Song, Y.J.; Na, Y.J.; Kim, H.G. Two-port myomectomy using bag-contained manual morcellation: A comparison with three-port myomectomy using power morcellation. Taiwan J. Obstet. Gynecol. 2019, 58, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.L.; Hsu, T.F.; Jiang, L.Y.; Chao, H.T.; Wang, P.H.; Chen, Y.J. Transvaginal natural orifice transluminal endoscopic surgery for female-to-male transgender men. J. Minim. Invasive Gynecol. 2019, 26, 135–142. [Google Scholar] [CrossRef]
- Amer, S.A.; Li, T.C.; Cooke, I.D. A prospective dose-finding study of the amount of thermal energy required for laparoscopic ovarian diathermy. Hum. Reprod. 2003, 18, 1693–1698. [Google Scholar] [CrossRef] [Green Version]
- Balen, A.H.; Morley, L.C.; Misso, M.; Franks, S.; Legro, R.S.; Wijeyaratne, C.N.; Stener-Victorin, E.; Fauser, B.C.; Norman, R.J.; Teede, H. The management of anovulatory infertility in women with polycystic ovary syndrome: An analysis of the evidence to support the development of global WHO guidance. Hum. Reprod. Update 2016, 22, 687–708. [Google Scholar] [CrossRef]
- Tsai, H.W.; Wang, P.H.; Yen, M.S.; Chao, K.C.; Hsu, T.F.; Chen, Y.J. Prevention of postlaparoscopic shoulder and upper abdominal pain: A randomized controlled trial. Obstet. Gynecol. 2013, 121, 526–531. [Google Scholar] [CrossRef]
- Tsai, H.W.; Chen, Y.J.; Ho, C.M.; Hseu, S.S.; Chao, K.C.; Tsai, S.K.; Wang, P.H. Maneuvers to decrease laparoscopy-induced shoulder and upper abdominal pain: A randomized controlled study. Arch. Surg. 2011, 146, 1360–1366. [Google Scholar] [CrossRef]
- Salah, I.M. Office microlaparoscopic ovarian drilling (OMLOD) versus conventional laparoscopic ovarian drilling (LOD) for women with polycystic ovary syndrome. Arch. Gynecol. Obstet. 2013, 287, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Lebbi, I.; Ben Temime, R.; Fadhlaoui, A.; Feki, A. Ovarian drilling in PCOS: Is it really useful? Front. Surg. 2015, 2, 30. [Google Scholar] [CrossRef] [Green Version]
- Hatirnaz, S.; Tan, S.L.; Hatirnaz, E.; Celik, O.; Kanat-Pektas, M.; Dahan, M.H. Vaginal ultrasound-guided ovarian needle puncture compared to laparoscopic ovarian drilling in women with polycystic ovary syndrome. Arch. Gynecol. Obstet. 2019, 299, 1475–1480. [Google Scholar] [CrossRef]
- Ott, J.; Mayerhofer, K.; Aust, S.; Nouri, K.; Huber, J.C.; Kurz, C. A modified technique of laparoscopic ovarian drilling for polycystic ovary syndrome using the monopolar hook electrode. Acta Obstet. Gynecol. Scand. 2011, 90, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Costello, M.F.; Garad, R.M.; Hart, R.; Homer, H.; Johnson, L.; Jordan, C.; Mocanu, E.; Qiao, J.; Rombauts, L.; Teede, H.J.; et al. A review of second- and third-line infertility treatments and supporting evidence in women with polycystic ovary syndrome. Med. Sci. 2019, 7, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amer, S.A.; Shamy, T.T.E.; James, C.; Yosef, A.H.; Mohamed, A.A. The impact of laparoscopic ovarian drilling on AMH and ovarian reserve: A meta-analysis. Reproduction 2017, 154, R13–R21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seow, K.M.; Lee, W.L.; Wang, P.H. A chellange in the management of women with polycystic ovary syndrome. Taiwan J. Obstet. Gynecol. 2016, 55, 157–158. [Google Scholar] [CrossRef] [Green Version]
- Seow, K.M.; Juan, C.C.; Ho, L.T.; Hsu, Y.P.; Lin, Y.H.; Huang, L.W.; Hwang, J.L. Adipocyte resistin mRNA levels are down-regulated by laparoscopic ovarian electrocautery in both obese and lean women with polycystic ovary syndrome. Hum. Reprod. 2007, 22, 1100–1106. [Google Scholar] [CrossRef] [Green Version]
- Flyckt, R.L.; Goldberg, J.M. Laparoscopic ovarian drilling for clomiphene-resistant polycystic ovary syndrome. Semin. Reprod. Med. 2011, 29, 138–146. [Google Scholar] [CrossRef]
- Kamal, N.; Sanad, Z.; Elkelani, O.; Rezk, M.; Shawky, M.; Sharaf, A.E. Changes in ovarian reserve and ovarian blood flow in patients with polycystic ovary syndrome following laparoscopic ovarian drilling. Gynecol. Endocrinol. 2018, 34, 789–792. [Google Scholar] [CrossRef]
- Wu, R.; Fujii, S.; Ryan, N.K.; Van der Hoek, K.H.; Jasper, M.J.; Sini, I.; Robertson, S.A.; Robker, R.L.; Norman, R.J. Ovarian leukocyte distribution and cytokine/chemokine mRNA expression in follicular fluid cells in women with polycystic ovary syndrome. Hum. Reprod. 2007, 22, 527–535. [Google Scholar] [CrossRef] [Green Version]
- Kahyaoglu, I.; Yılmaz, N.; Timur, H.; Inal, H.A.; Erkaya, S. Granulocyte colony-stimulating factor: A relation between serum and follicular fluid levels and in-vitro fertilization outcome in patients with polycystic ovary syndrome. Cytokine 2015, 74, 113–116. [Google Scholar] [CrossRef]
- Inal, Z.O.; Inal, H.A.; Erdem, S. The effect of serum and follicular fluid secreted frizzle-related protein-5 on in vitro fertilization outcomes in patients with polycystic ovary syndrome. Mol. Biol. Rep. 2018, 45, 2037–2044. [Google Scholar] [CrossRef]
- Hu, C.; Pang, B.; Ma, Z.; Yi, H. Immunophenotypic profiles in polycystic ovary syndrome. Mediat. Inflamm. 2020, 2020, 5894768. [Google Scholar] [CrossRef] [Green Version]
- Connolly, F.; Rae, M.T.; Butler, M.; Klibanov, A.L.; Sboros, V.; McNeilly, A.S.; Duncan, W.C. The local effects of ovarian diathermy in an ovine model of polycystic ovary syndrome. PLoS ONE 2014, 9, e111280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, X.; Yun, C.; Sun, L.; Xia, J.; Wu, Q.; Wang, Y.; Wang, L.; Zhang, Y.; Liang, X.; Wang, L.; et al. Gut microbiota-bile acid-interleukin-22 axis orchestrates polycystic ovary syndrome. Nat. Med. 2019, 25, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Ferraretti, A.P.; Gianaroli, L.; Magli, M.C.; Iammarrone, E.; Feliciani, E.; Fortini, D. Transvaginal ovarian drilling: A new surgical treatment for improving the clinical outcome of assisted reproductive technologies in patients with polycystic ovary syndrome. Fertil. Steril. 2001, 76, 812–816. [Google Scholar] [CrossRef]
- Tulandi, T.; Saleh, A.; Morris, D.; Jacobs, H.S.; Payne, N.N.; Tan, S.L. Effects of laparoscopic ovarian drilling on serum vascular endothelial growth factor and on insulin responses to the oral glucose tolerance test in women with polycystic ovary syndrome. Fertil. Steril. 2000, 74, 585–588. [Google Scholar] [CrossRef]
- El Behery, M.M.; Diab, A.E.; Mowafy, H.; Ebrahiem, M.A.; Shehata, A.E. Effect of laparoscopic ovarian drilling on vascular endothelial growth factor and ovarian stromal blood flow using 3-dimensional power Doppler. Int. J. Gynaecol. Obstet. 2011, 112, 119–121. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.F.; Abd el-Aal, D.E.; Darwish, A.M.; Meki, A.R. Evaluation of the impact of laparoscopic ovarian drilling on Doppler indices of ovarian stromal blood flow, serum vascular endothelial growth factor, and insulin-like growth factor-1 in women with polycystic ovary syndrome. Fertil. Steril. 2003, 79, 938–941. [Google Scholar] [CrossRef]
- Giampaolino, P.; Morra, I.; De Rosa, N.; Cagnacci, A.; Pellicano, M.; Di Carlo, C.; Nappi, C.; Bifulco, G. Impact of transvaginal hydrolaparoscopy ovarian drilling on ovarian stromal blood flow and ovarian volume in clomiphene citrate-resistant PCOS patients: A case-control study. Gynecol. Endocrinol. 2017, 33, 690–693. [Google Scholar] [CrossRef] [Green Version]
- Seyam, E.; Hefzy, E. Tumor necrosis factor alpha versus LH and androstendione as a reliable predictor of spontaneous ovulation after laparoscopic ovarian drilling for women with clomiphene citrate resistance polycystic ovarian disease. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 222, 126–133. [Google Scholar] [CrossRef]
- Abuelghar, W.M.; Bayoumy, H.A.; Ellaithy, M.I.; Khalil, M.S. Women with clomiphene citrate resistant polycystic ovarian disease: Predictors of spontaneous ovulation after laparoscopic ovarian drilling. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 175, 178–185. [Google Scholar] [CrossRef]
- Saleh, A.M.; Khalil, H.S. Review of nonsurgical and surgical treatment and the role of insulin-sensitizing agents in the management of infertile women with polycystic ovary syndrome. Acta Obstet. Gynecol. Scand. 2004, 83, 614–621. [Google Scholar] [CrossRef]
- Amer, S.A.; Gopalan, V.; Li, T.C.; Ledger, W.L.; Cooke, I.D. Long term follow-up of patients with polycystic ovarian syndrome after laparoscopic ovarian drilling: Clinical outcome. Hum. Reprod. 2002, 17, 2035–2042. [Google Scholar] [CrossRef] [Green Version]
- Giampaolino, P.; Morra, I.; Tommaselli, G.A.; Di Carlo, C.; Nappi, C.; Bifulco, G. Post-operative ovarian adhesion formation after ovarian drilling: A randomized study comparing conventional laparoscopy and transvaginal hydrolaparoscopy. Arch. Gynecol. Obstet. 2016, 294, 791–796. [Google Scholar] [CrossRef]
- Giampaolino, P.; Morra, I.; Della Corte, L.; Sparice, S.; Di Carlo, C.; Nappi, C.; Bifulco, G. Serum anti-Mullerian hormone levels after ovarian drilling for the second-line treatment of polycystic ovary syndrome: A pilot-randomized study comparing laparoscopy and transvaginal hydrolaparoscopy. Gynecol. Endocrinol. 2017, 33, 26–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cleemann, L.; Lauszus, F.F.; Trolle, B. Laparoscopic ovarian drilling as first line of treatment in infertile women with polycystic ovary syndrome. Gynecol. Endocrinol. 2004, 18, 138–143. [Google Scholar] [CrossRef]
- Yu, Q.; Hu, S.; Wang, Y.; Cheng, G.; Xia, W.; Zhu, C. Letrozole versus laparoscopic ovarian drilling in clomiphene citrate-resistant women with polycystic ovary syndrome: A systematic review and meta-analysis of randomized controlled trials. Reprod. Biol. Endocrinol. 2019, 17, 17. [Google Scholar] [CrossRef]
- Kaur, M.; Pranesh, G.; Mittal, M.; Gahlan, A.; Deepika, K.; Sashikala, T.; Rao, K.A. Outcome of laparoscopic ovarian drilling in patients of clomiphene resistant polycystic ovary syndrome in a tertiary care center. Int. J. Infertil. Fetal. Med. 2013, 4, 39–44. [Google Scholar]
- Nasr, A.A.M.; El-Naser, A.; Ali, A.E.; Abdelsattar, M.; Mgeed, A.; Abolfotouh, M. A modified technique of laparoscopic ovarian drilling for polycystic ovary syndrome using harmonic scalpel. J. Diabetes Metab. 2012, 1, S6. [Google Scholar]
- Farquhar, C.; Brown, J.; Marjoribanks, J. Laparoscopic drilling by diathermy or laser for ovulation induction in anovulatory polycystic ovary syndrome. Cochrane Database Syst. Rev. 2012, 6, CD001122. [Google Scholar] [CrossRef]
- Abu Hashim, H.; El-Lakany, N.; Sherief, L. Combined metformin and clomiphene citrate versus laparoscopic ovarian diathermy for ovulation induction in clomiphene-resistant women with polycystic ovary syndrome: A randomized controlled trial. J. Obstet. Gynaecol. Res. 2011, 37, 169–177. [Google Scholar] [CrossRef]
- Abdellah, M.S. Reproductive outcome after letrozole versus laparoscopic ovarian drilling for clomiphene-resistant polycystic ovary syndrome. Int. J. Gynaecol. Obstet. 2011, 113, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Roy, K.K.; Baruah, J.; Sharma, A.; Sharma, J.B.; Kumar, S.; Kachava, G.; Karmakar, D. A prospective randomized trial comparing the clinical and endocrinological outcome with rosiglitazone versus laparoscopic ovarian drilling in patients with polycystic ovarian disease resistant to ovulation induction with clomiphene citrate. Arch. Gynecol. Obstet. 2010, 281, 939–944. [Google Scholar] [CrossRef]
- Seckin, B.; Tokmak, A.; Yumusak, O.H. The role of anti-Mullerian homrone in predeiction of pregnancy in young and older women with unexplained infertility undergoing intrauterine insemination. J. Chin. Med. Assoc. 2019, 82, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhang, Q.; Li, Y. Effect comparsion of samplingectomy versus proximal tubal occlusion on ovarian reserve: A meta-analysis. Medicine 2020, 99, e20601. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Batarfi, A.A.; Bajouh, O.S.; Bakhashab, S. Serum anti-Müllerian hormone in the diagnosis of polycystic ovary syndrome in association with clinical symptoms. Diagnostics 2019, 9, 136. [Google Scholar] [CrossRef]
- Saleh, A.; Morris, D.; Tan, S.L.; Tulandi, T. Effects of laparoscopic ovarian drilling on adrenal steroids in polycystic ovary syndrome patients with and without hyperinsulinemia. Fertil. Steril. 2001, 75, 501–504. [Google Scholar] [CrossRef]
- Dunaif, A.; Xia, J.; Book, C.B.; Schenker, E.; Tang, Z. Excessive insulin receptor serine phosphorylation in cultured fibroblasts and in skeletal muscle. A potential mechanism for insulin resistance in the polycystic ovary syndrome. J. Clin. Investig. 1995, 96, 801–810. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Fu, Z.; Chen, X.; Li, X.; Tang, Z.; Zhou, Y.; Geng, Q. Transvaginal ultrasound-guided ovarian interstitial laser treatment in anovulatory women with polycystic ovary syndrome: A randomized clinical trial on the effect of laser dose used on the outcome. Fertil. Steril. 2010, 94, 268–275. [Google Scholar] [CrossRef]
- Stegmann, B.J.; Craig, H.R.; Bay, R.C.; Coonrod, D.V.; Brady, M.J.; Garbaciak, J.A., Jr. Characteristics predictive of response to ovarian diathermy in women with polycystic ovarian syndrome. Am. J. Obstet. Gynecol. 2003, 188, 1171–1173. [Google Scholar] [CrossRef]
- Naether, O.G.; Baukloh, V.; Fischer, R.; Kowalczyk, T. Long-term follow-up in 206 infertility patients with polycystic ovarian syndrome after laparoscopic electrocautery of the ovarian surface. Hum. Reprod. 1994, 9, 2342–2349. [Google Scholar] [CrossRef]
- Amer, S.A.; Li, T.C.; Cooke, I.D. Repeated laparoscopic ovarian diathermy is effective in women with anovulatory infertility due to polycystic ovary syndrome. Fertil. Steril. 2003, 79, 1211–1215. [Google Scholar] [CrossRef]
- Johansson, J.; Redman, L.; Veldhuis, P.P.; Sazonova, A.; Labrie, F.; Holm, G.; Johannsson, G.; Stener-Victorin, E. Acupuncture for ovulation induction in polycystic ovary syndrome: A randomized controlled trial. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E934–E943. [Google Scholar] [CrossRef] [Green Version]
- Johansson, J.; Stener-Victorin, E. Polycystic ovary syndrome: Effect and mechanisms of acupuncture for ovulation induction. Evid. Based Complement. Altern. Med. 2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambert, E.A.; Teede, H.; Sari, C.I.; Jona, E.; Shorakae, S.; Woodington, K.; Hemmes, R.; Eikelis, N.; Straznicky, N.E.; De Courten, B.; et al. Sympathetic activation and endothelial dysfunction in polycystic ovary syndrome are not explained by either obesity or insulin resistance. Clin. Endocrinol. 2015, 83, 812–819. [Google Scholar] [CrossRef]
- Barra, R.; Cruz, G.; Mayerhofer, A.; Paredes, A.; Lara, H.E. Maternal sympathetic stress impairs follicular development and puberty of the offspring. Reproduction 2014, 148, 137–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Chen, D.; Liu, N. Effectiveness of acupuncture in polycystic ovary syndrome: A systematic review and meta-analysis of randomized controlled trials. Medicine 2020, 99, e20441. [Google Scholar] [CrossRef]
- Chen, C.H.; Wang, P.H.; Hsieh, M.T.; Tzeng, C.R.; Wu, Y.H.; Lee, C.S.; Chu, Y.H.; Chang, H.Y. Sexual orientations of women with polycystic ovary syndrome: Clinical observation in Taiwan. Taiwan J. Obstet. Gynecol. 2014, 53, 542–546. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.H.; Sun, K.C.; Wang, P.H. Does the observation of lower ratio of anterior anogenital distance and posterior anogenital distance appear in adolescent and remain constant in women diagnosed with polycystic ovary syndrome? J. Chin. Med. Assoc. 2020, 83, 211–212. [Google Scholar] [CrossRef]
- Chan, I.S.; Lee, W.L.; Wang, P.H. Does the ratio of anterior anogenital distance to posterior anogenital distance fit the novel biomarker for women with polycystic ovary syndrome? J. Chin. Med. Assoc. 2019, 82, 887–888. [Google Scholar] [CrossRef]
- Su, W.H.; Lee, F.K.; Wang, P.H. Recurrent pregnancy loss and thrombophilia in women with PCOS. J. Chin. Med. Assoc. 2013, 76, 243–244. [Google Scholar] [CrossRef] [Green Version]
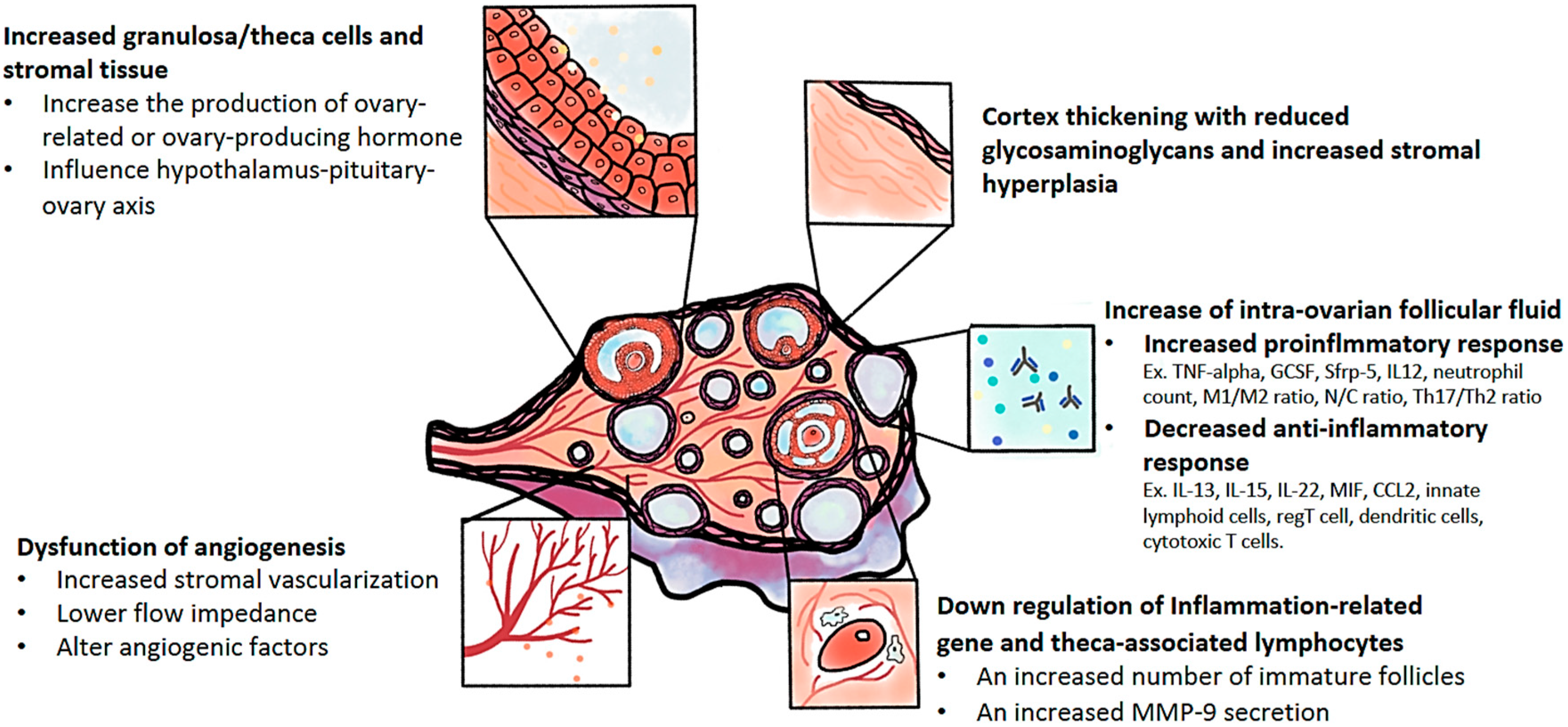
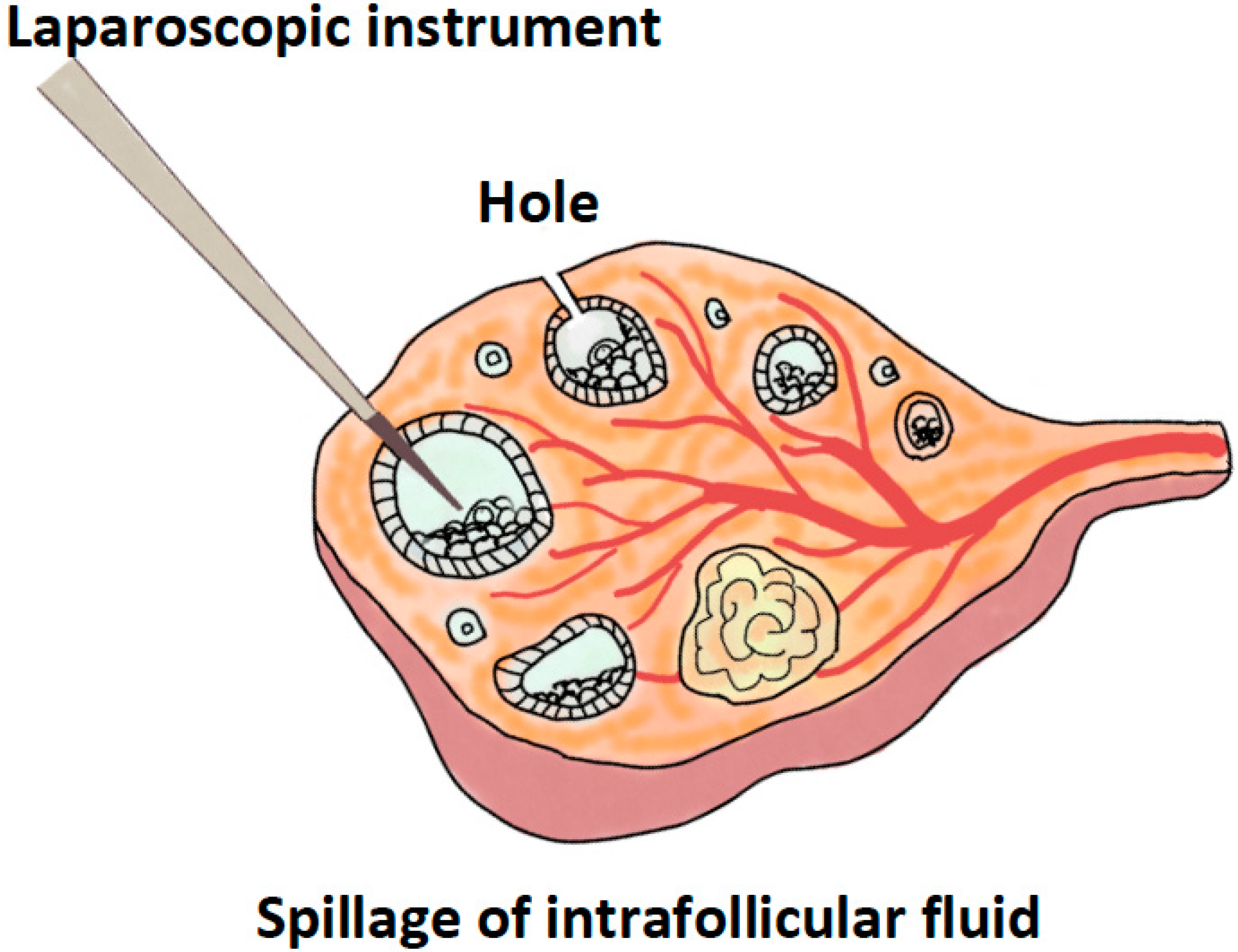
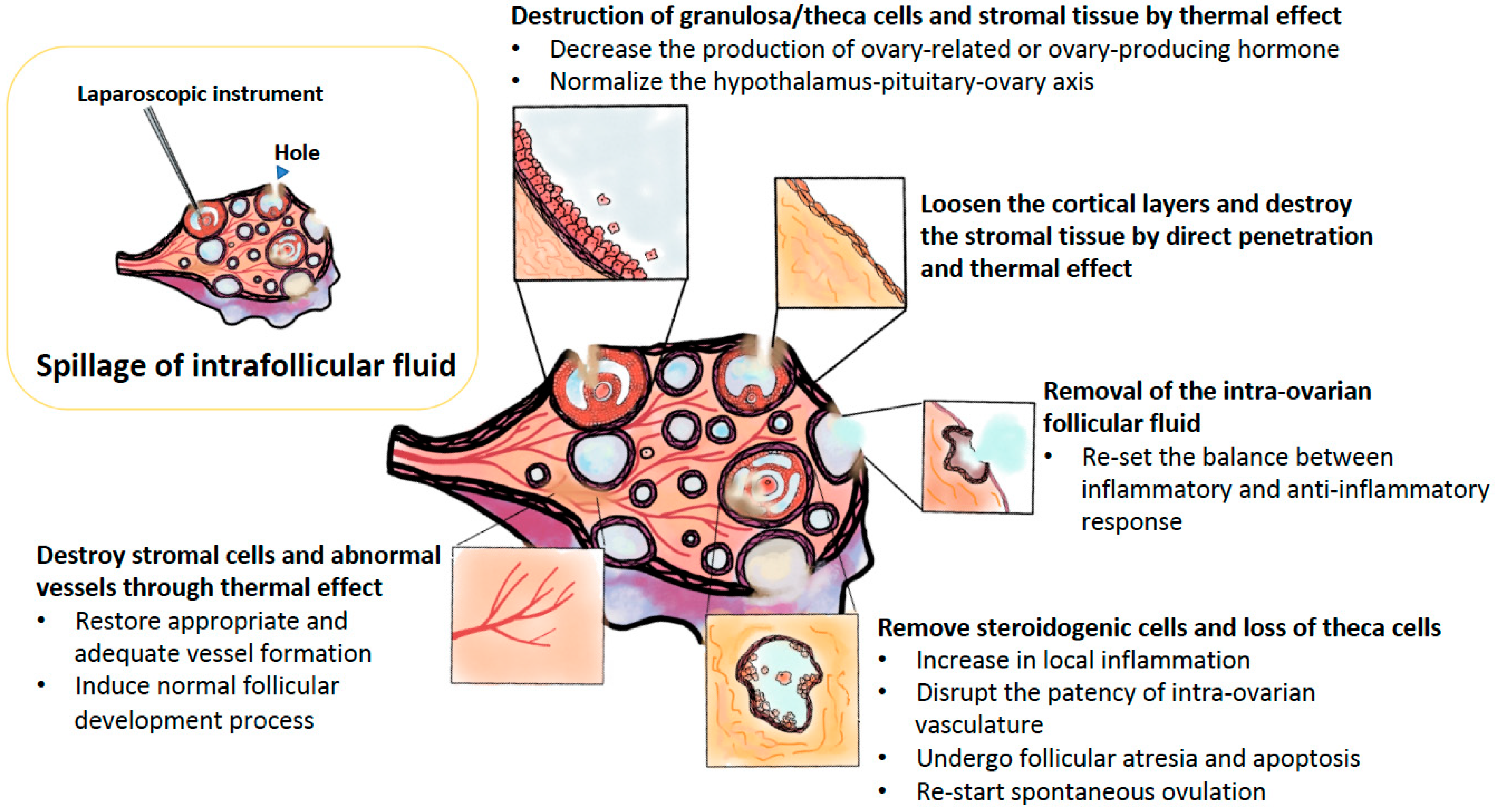
| Author (Years) [Ref] | Article | Comparison | Outcomes |
|---|---|---|---|
| Bordewijk (2020) [39] | Review | LOD with or without medical ovulation induction vs. medical ovulation induction alone | Live birth: Slightly ameliorated by LOD (OR 0.71, 95% CI 0.54–0.92) |
| Yu (2019) [116] | Review | Letrozole vs. LOD | No difference in ovulation rate (RR1.12; 95% CI 0.93–1.34), and live birth rate (RR 1.27; 95% CI 0.96–1.68) |
| Debras (2019) [66] | Multicenter study | LOD alone, long term effect | Mean follow-up period was 28.4 months (25.3–31.5). At least 47.4% women got pregnancy after a drilling. |
| Abu Hashim (2018) [38] | Review | BLOD vs. ULOD | No significant differences in ovulation (OR 0.73; 95% CI 0.47–1.11) and live birth (OR 0.77; 95% CI 0.28–2.10). |
| Franik (2018) [42] | Review | AI+/− adjuvants vs. LOD | Live birth: OR 1.38, 95% CI 0.95–2.02 |
| Abu Hashim (2015) [31] | Review | CC+M vs. LOD | Live birth: OR 2.27, 95% CI 1.22–4.17 |
| Kaur (2013) [117] | Observational study | LOD alone | Clinical pregnancy rate: 47.3%; live birth rate: 40.5% |
| Nasr (2012) [118] | RCT | Electrocautery vs. harmonic scalpel | Similar ovulation rate (89% vs. 92.9%) and pregnancy rate (50% vs. 57%). |
| Farquhar (2012) [119] | Review | LOD vs. medical treatments | Live birth: 34% vs. 38%. No significant difference. |
| Abu Hashim (2011) [120] | RCT | CC+M vs. LOD | Similar ovulation rate (67% vs. 68.4%) and pregnancy rate (15.4% vs. 17%). |
| Abdullah (2011) [121] | RCT | Letrozole vs. LOD | Ovulation rate: Significantly higher in the letrozole than LOD (59.0% vs. 47.5%). Similar live birth rate. |
| Roy (2010) [122] | RCT | Rosiglitazone + CC vs. LOD + CC | Similar ovulation (80.8 vs. 81.5%) and pregnancy rate (50 vs. 42.8%). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seow, K.-M.; Chang, Y.-W.; Chen, K.-H.; Juan, C.-C.; Huang, C.-Y.; Lin, L.-T.; Tsui, K.-H.; Chen, Y.-J.; Lee, W.-L.; Wang, P.-H. Molecular Mechanisms of Laparoscopic Ovarian Drilling and Its Therapeutic Effects in Polycystic Ovary Syndrome. Int. J. Mol. Sci. 2020, 21, 8147. https://doi.org/10.3390/ijms21218147
Seow K-M, Chang Y-W, Chen K-H, Juan C-C, Huang C-Y, Lin L-T, Tsui K-H, Chen Y-J, Lee W-L, Wang P-H. Molecular Mechanisms of Laparoscopic Ovarian Drilling and Its Therapeutic Effects in Polycystic Ovary Syndrome. International Journal of Molecular Sciences. 2020; 21(21):8147. https://doi.org/10.3390/ijms21218147
Chicago/Turabian StyleSeow, Kok-Min, Yi-Wen Chang, Kuo-Hu Chen, Chi-Chang Juan, Chen-Yu Huang, Li-Te Lin, Kuan-Hao Tsui, Yi-Jen Chen, Wen-Ling Lee, and Peng-Hui Wang. 2020. "Molecular Mechanisms of Laparoscopic Ovarian Drilling and Its Therapeutic Effects in Polycystic Ovary Syndrome" International Journal of Molecular Sciences 21, no. 21: 8147. https://doi.org/10.3390/ijms21218147







