Sex-Dependent Molecular Mechanisms of Lipotoxic Injury in Brain Microvasculature: Implications for Dementia
Abstract
1. Introduction
2. Results
2.1. Sex Differences in Weight, Lipids, Glucose and Insulin
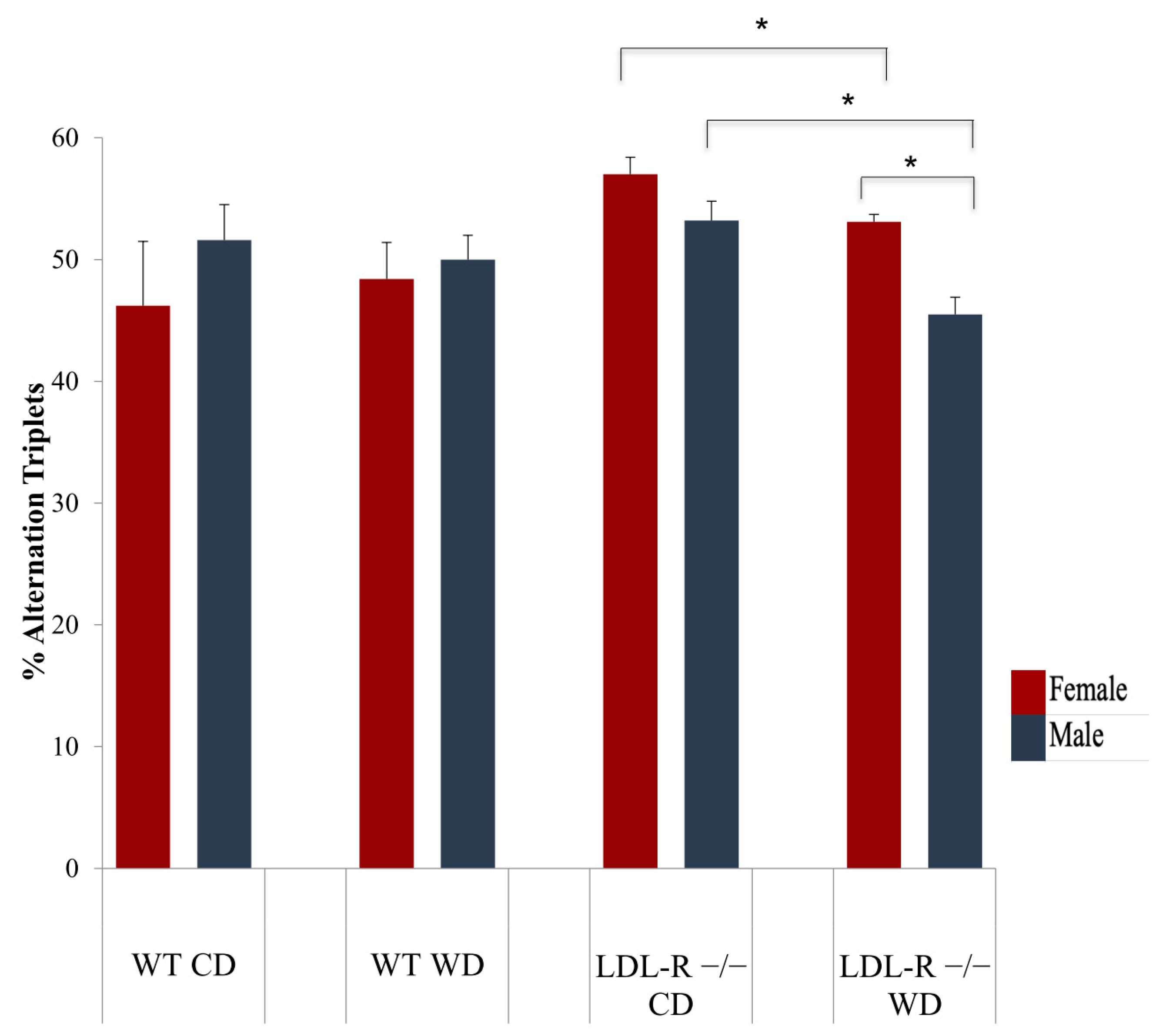
2.2. Cognitive Function
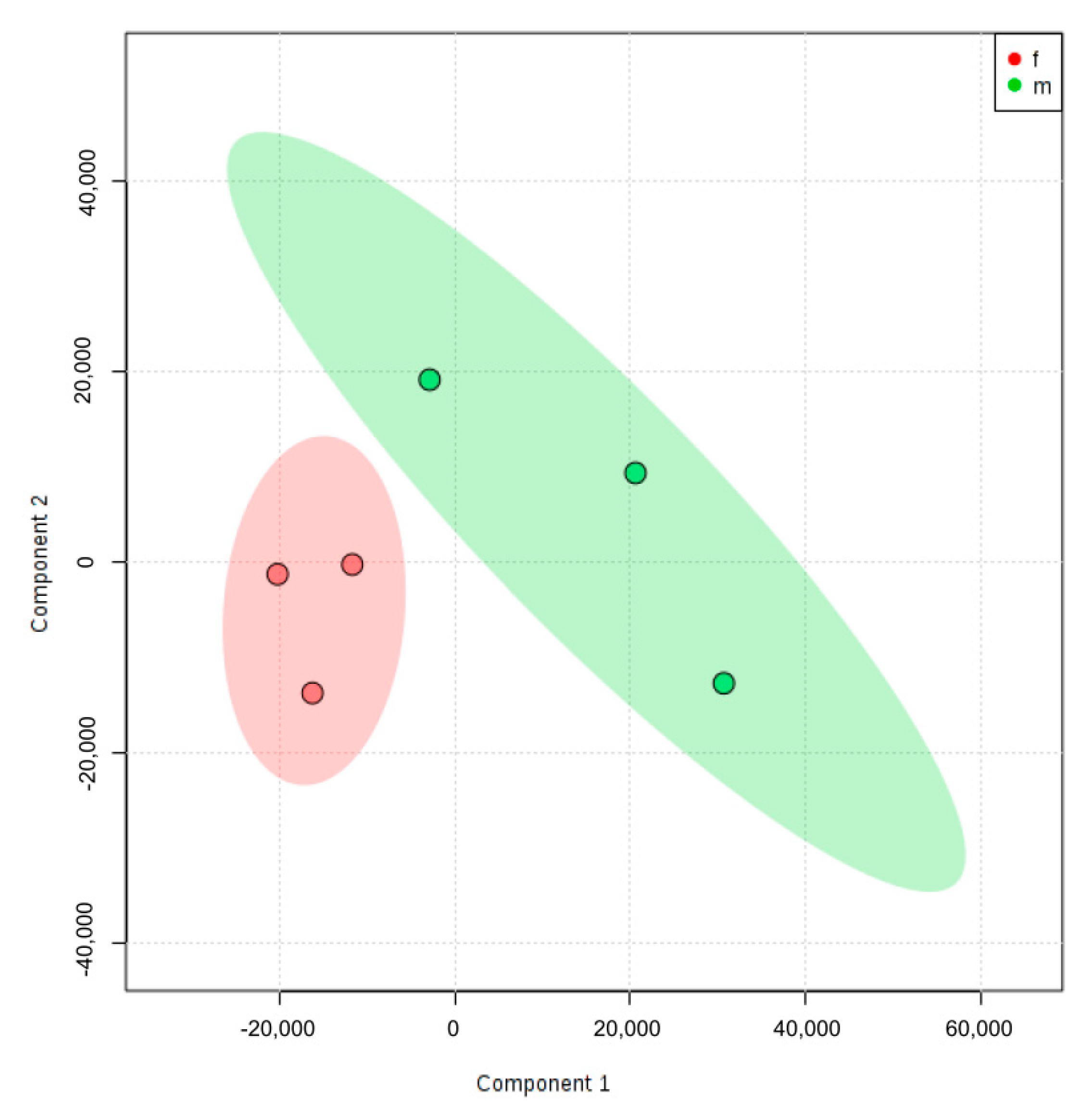
2.3. Principal Component Analysis
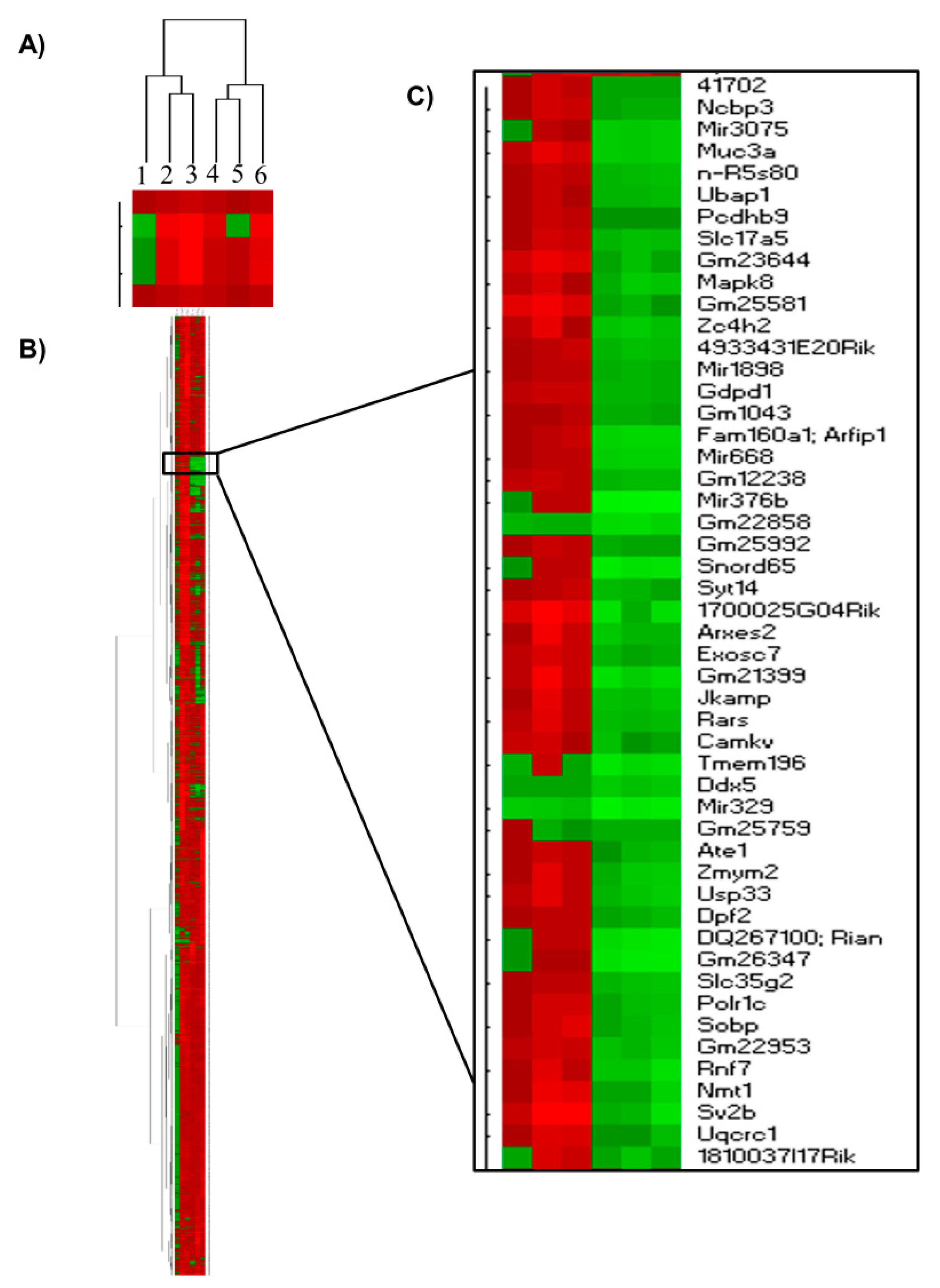
2.4. Hierarchical Clustering
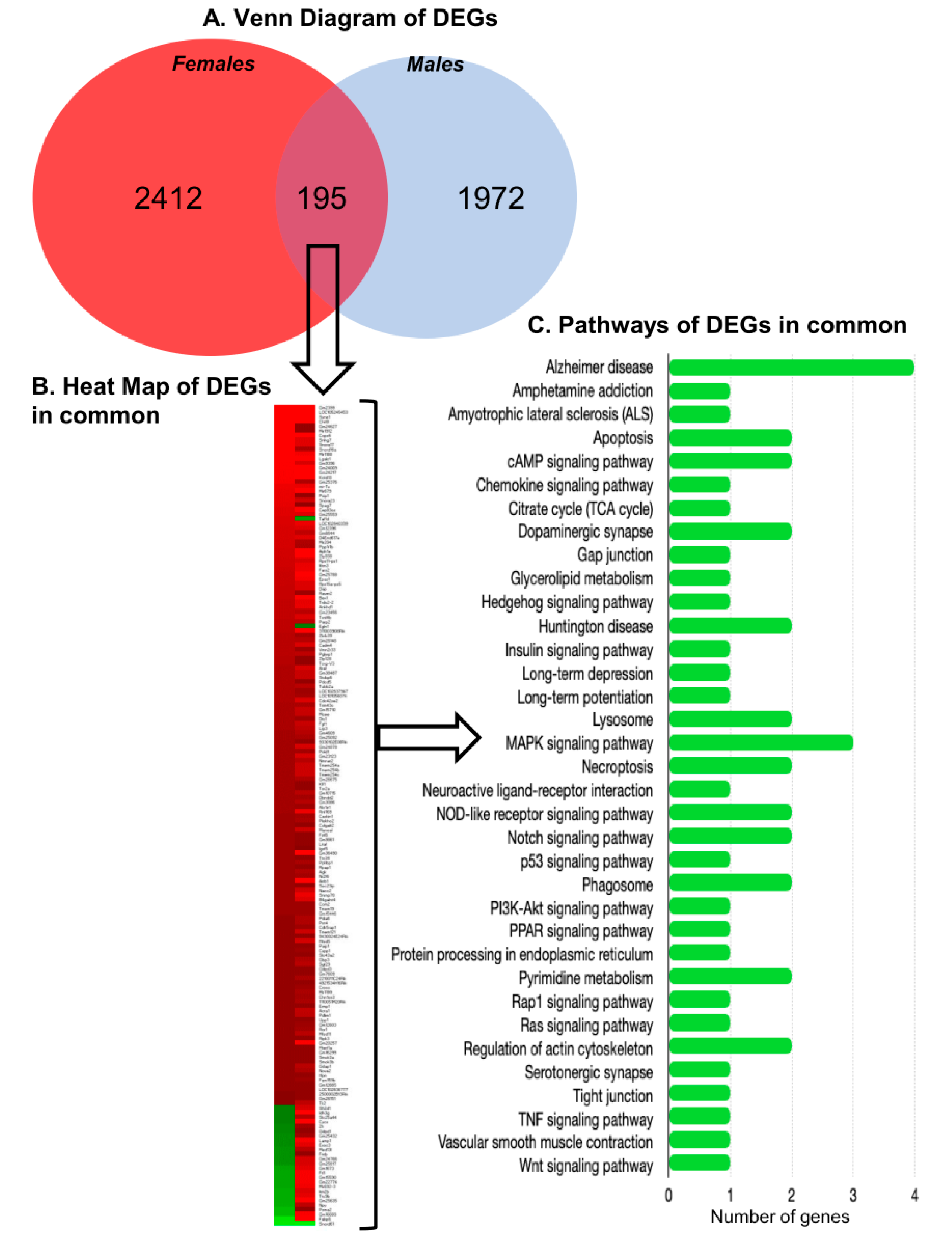
2.5. Differentially Expressed Protein Coding and Non-Coding RNAs
2.6. Differentially Expressed Genes and Their Function
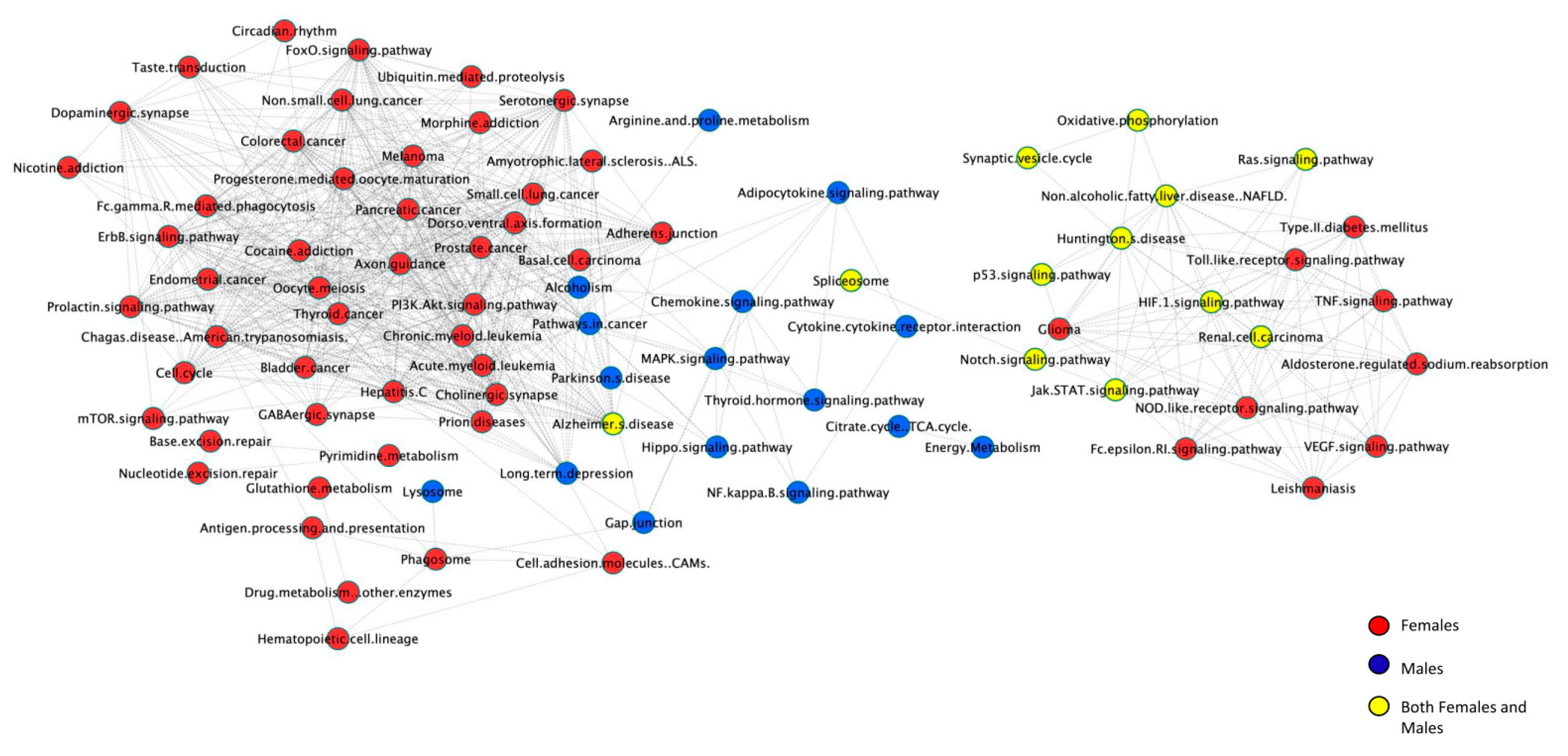
2.7. Cellular Pathways
2.8. Pathway Networks
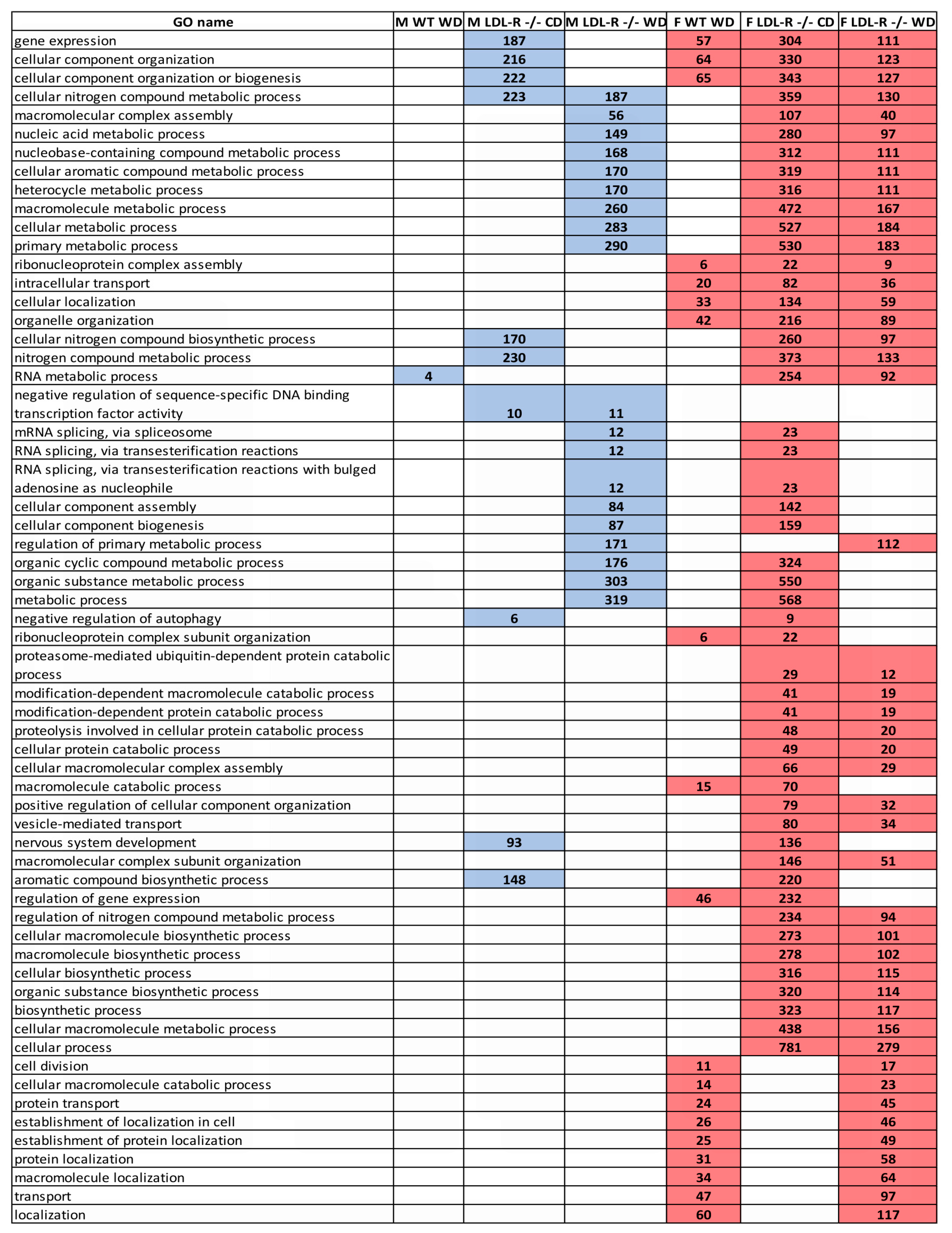
2.9. Gene Ontology
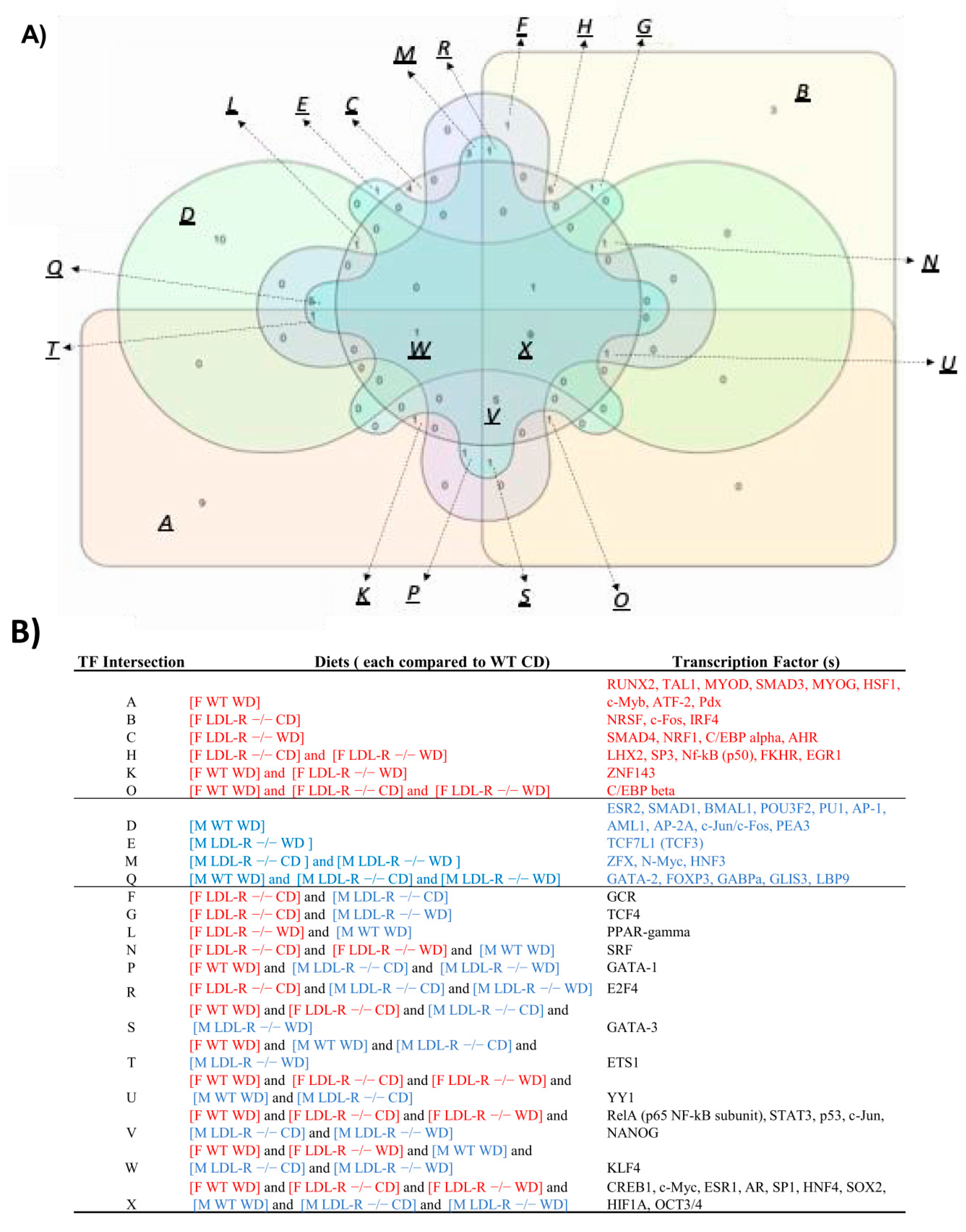
2.10. Transcription Factors
2.11. Non-Protein Coding RNAs
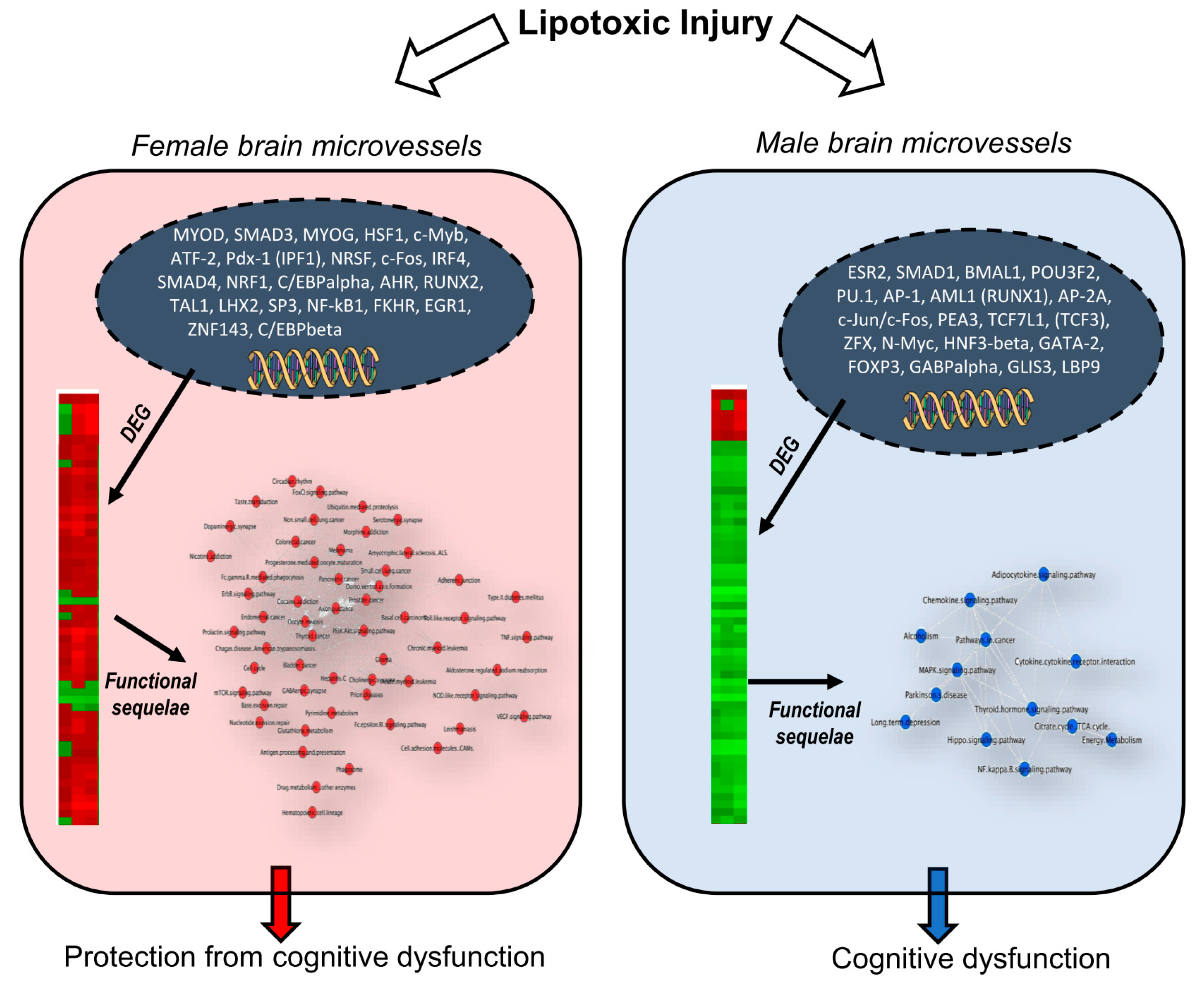
3. Discussion
3.1. Sex Differences in Hippocampal Microvascular Gene Expression and Hierarchical Clustering Following Lipotoxic Injury
3.2. Sex Differences in Differential Expression of Gene Ontology, Pathways, and Pathway Networks
3.3. Sex- Differences in Differential Regulation of Transcription Factors and their Inter- Connection to Signaling Pathways
3.4. Sex- Differences in Differential Regulation of Non-Coding RNAs
4. Methods
4.1. Experimental Animals
4.2. Cognitive Testing (Y-Maze)
4.3. Blood Metabolic and Hormone Assays
4.4. Isolation and Cryosection of Murine Brain Hippocampus
4.5. Laser Capture Microdissection (LCM) of Hippocampal Microvessels
4.6. RNA Extraction from Laser Captured Brain Microvessels
4.7. Microarray Hybridization and Transcriptome Analysis
4.8. qRT-PCR Analysis of Gene Expression in Murine Hippocampal Microvessels
4.9. Bioinformatic Analysis
4.10. Statistical Methods
5. Conclusions and Relevance
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Medeiros, A.M.; Silva, R.H. Sex differences in Alzheimer’s disease: Where do we stand? J. Alzheimers Dis. 2019, 67, 35–60. [Google Scholar] [CrossRef]
- Barnes, D.E.; Yaffe, K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011, 10, 819–828. [Google Scholar] [CrossRef]
- Morovic, S.; Budincevic, H.; Govori, V.; Demarin, V. Possibilities of dementia prevention—It is never too early to start. J. Med. Life 2019, 12, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Grammas, P. Neurovascular dysfunction, inflammation and endothelial activation: Implications for the pathogenesis of Alzheimer’s disease. J. Neuroinflamm. 2011, 8, 26. [Google Scholar] [CrossRef] [PubMed]
- Heron, M. Deaths: Leading causes for 2017. Natl. Vital Stat. Rep. 2019, 68, 6. [Google Scholar]
- Alzheimer’s Association. 2020 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2020, 16, 391–460. [Google Scholar] [CrossRef]
- Alzheimer’s Association. 2019 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2019, 15, 321–387. [Google Scholar] [CrossRef]
- Kochanek, K.D.; Murphy, S.L.; Xu, J.; Tejada-Vera, B. Deaths: Final data for 2014. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics and National Vital Statistics System. Natl. Vital Stat. Rep. 2016, 65, 1–122. [Google Scholar]
- Reitz, C.; Brayne, C.; Mayeux, R. Epidemiology of Alzheimer disease. Nat. Rev. Neurol. 2011, 7, 137–152. [Google Scholar] [CrossRef]
- Gannon, O.J.; Robison, L.S.; Custozzo, A.J.; Zuloaga, K.L. Sex differences in risk factors for vascular contributions to cognitive impairment & dementia. Neurochem. Int. 2019, 127, 38–55. [Google Scholar] [CrossRef]
- Gorelick, P.B.; Counts, S.E.; Nyenhuis, D. Vascular cognitive impairment and dementia. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2016, 1862, 860–868. [Google Scholar] [CrossRef] [PubMed]
- Podcasy, J.L.; Epperson, C.N. Considering sex and gender in Alzheimer disease and other dementias. Dialogues Clin. Neurosci. 2016, 18, 437–446. [Google Scholar] [PubMed]
- Kalaria, R.N.; Erkinjuntti, T. Small vessel disease and subcortical vascular dementia. J. Clin. Neurol. 2006, 2, 1–11. [Google Scholar] [CrossRef]
- Cohen, A.D. Distinct pathways for cognitive decline in the presence of Alzheimer’s disease pathology or cerebrovascular disease. Brain J. Neurol. 2016, 139, 2340–2341. [Google Scholar] [CrossRef]
- Aung, H.H.; Altman, R.; Nyunt, T.; Kim, J.; Nuthikattu, S.; Budamagunta, M.; Voss, J.C.; Wilson, D.; Rutledge, J.C.; Villablanca, A.C. Lipotoxic brain microvascular injury is mediated by activating transcription factor 3-dependent inflammatory and oxidative stress pathways. J. Lipid Res. 2016, 57, 955–968. [Google Scholar] [CrossRef]
- Ishibashi, S.; Brown, M.S.; Goldstein, J.L.; Gerard, R.D.; Hammer, R.E.; Herz, J. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J. Clin. Investig. 1993, 92, 883–893. [Google Scholar] [CrossRef]
- Bieghs, V.; Van Gorp, P.J.; Wouters, K.; Hendrikx, T.; Gijbels, M.J.; van Bilsen, M.; Bakker, J.; Binder, C.J.; Lutjohann, D.; Staels, B.; et al. LDL receptor knock-out mice are a physiological model particularly vulnerable to study the onset of inflammation in non-alcoholic fatty liver disease. PLoS ONE 2012, 7, e30668. [Google Scholar] [CrossRef]
- Nuthikattu, S.; Milenkovic, D.; Rutledge, J.; Villablanca, A. The western diet regulates hippocampal microvascular gene expression: An integrated genomic analyses in female mice. Sci. Rep. 2019, 9, 19058. [Google Scholar] [CrossRef]
- Nuthikattu, S.; Milenkovic, D.; Rutledge, J.C.; Villablanca, A.C. Lipotoxic Injury differentially regulates brain microvascular gene expression in male mice. Nutrients 2020, 12, 1771. [Google Scholar] [CrossRef]
- Toro, C.A.; Zhang, L.; Cao, J.; Cai, D. Sex differences in Alzheimer’s disease: Understanding the molecular impact. Brain Res. 2019, 1719, 194–207. [Google Scholar] [CrossRef]
- Ferretti, M.T.; Iulita, M.F.; Cavedo, E.; Chiesa, P.A.; Schumacher Dimech, A.; Santuccione Chadha, A.; Baracchi, F.; Girouard, H.; Misoch, S.; Giacobini, E.; et al. Sex differences in Alzheimer disease—The gateway to precision medicine. Nat. Rev. Neurol. 2018, 14, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.L.; Greaves, L.; Repta, R. Better science with sex and gender: Facilitating the use of a sex and gender-based analysis in health research. Int. J. Equity Health 2009, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Nowatzki, N.; Grant, K.R. Sex is not enough: The need for gender-based analysis in health research. Health Care Women Int. 2011, 32, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.L.; Greaves, L.; Repta, R. Better Science with Sex and Gender: A Primer for Health Research; Vancouver Women’s Health Research Network: Vancouver, BC, Canada, 2007. [Google Scholar]
- Rutkowsky, J.M.; Lee, L.L.; Puchowicz, M.; Golub, M.S.; Befroy, D.E.; Wilson, D.W.; Anderson, S.; Cline, G.; Bini, J.; Borkowski, K.; et al. Reduced cognitive function, increased blood-brain-barrier transport and inflammatory responses, and altered brain metabolites in LDLr -/-and C57BL/6 mice fed a western diet. PLoS ONE 2018, 13, e0191909. [Google Scholar] [CrossRef]
- Barron, A.M.; Rosario, E.R.; Elteriefi, R.; Pike, C.J. Sex-Specific effects of high fat diet on indices of metabolic syndrome in 3xTg-AD mice: Implications for Alzheimer’s disease. PLoS ONE 2013, 8, e78554. [Google Scholar] [CrossRef] [PubMed]
- Hwang, L.L.; Wang, C.H.; Li, T.L.; Chang, S.D.; Lin, L.C.; Chen, C.P.; Chen, C.T.; Liang, K.C.; Ho, I.K.; Yang, W.S.; et al. Sex differences in high-fat diet-induced obesity, metabolic alterations and learning, and synaptic plasticity deficits in mice. Obesity 2010, 18, 463–469. [Google Scholar] [CrossRef]
- Ingvorsen, C.; Karp, N.A.; Lelliott, C.J. The role of sex and body weight on the metabolic effects of high-fat diet in C57BL/6N mice. Nutr. Diabetes 2017, 7, e261. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, U.S.; Waldén, T.B.; Carlsson, P.O.; Jansson, L.; Phillipson, M. Female mice are protected against high-fat diet induced metabolic syndrome and increase the regulatory T cell population in adipose tissue. PLoS ONE 2012, 7, e46057. [Google Scholar] [CrossRef]
- Medrikova, D.; Jilkova, Z.M.; Bardova, K.; Janovska, P.; Rossmeisl, M.; Kopecky, J. Sex differences during the course of diet-induced obesity in mice: Adipose tissue expandability and glycemic control. Int. J. Obes. 2012, 36, 262–272. [Google Scholar] [CrossRef]
- Madhunapantula, S.V.; Mosca, P.J.; Robertson, G.P. The Akt signaling pathway: An emerging therapeutic target in malignant melanoma. Cancer Biol. Ther. 2011, 12, 1032–1049. [Google Scholar] [CrossRef]
- Mao, R.; Meng, S.; Gu, Q.; Araujo-Gutierrez, R.; Kumar, S.; Yan, Q.; Almazan, F.; Youker, K.A.; Fu, Y.; Pownall, H.J.; et al. AIBP limits angiogenesis through γ-secretase-mediated upregulation of notch signaling. Circ. Res. 2017, 120, 1727–1739. [Google Scholar] [CrossRef]
- Xiao, Z.; Wei, Z.; Deng, D.; Zheng, Z.; Zhao, Y.; Jiang, S.; Zhang, D.; Zhang, L.J.; Fan, M.; Chen, S.; et al. Downregulation of Siah1 promotes colorectal cancer cell proliferation and migration by regulating AKT and YAP ubiquitylation and proteasome degradation. Cancer Cell Int. 2020, 20, 50. [Google Scholar] [CrossRef]
- Tan, B.; Mu, R.; Chang, Y.; Wang, Y.B.; Wu, M.; Tu, H.Q.; Zhang, Y.C.; Guo, S.S.; Qin, X.H.; Li, T.; et al. RNF4 negatively regulates NF-κB signaling by down-regulating TAB2. FEBS Lett. 2015, 589, 2850–2858. [Google Scholar] [CrossRef]
- Sannino, G.; Pasqualini, L.; Ricciardelli, E.; Montilla, P.; Soverchia, L.; Ruggeri, B.; Falcinelli, S.; Renzi, A.; Ludka, C.; Kirchner, T.; et al. Acute stress enhances the expression of neuroprotection- and neurogenesis-associated genes in the hippocampus of a mouse restraint model. Oncotarget 2016, 7, 8455–8465. [Google Scholar] [CrossRef] [PubMed]
- Lovell, M.A.; Soman, S.; Bradley, M.A. Oxidatively modified nucleic acids in preclinical Alzheimer’s disease (PCAD) brain. Mech. Ageing Dev. 2011, 132, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Bossy-Wetzel, E.; Schwarzenbacher, R.; Lipton, S.A. Molecular pathways to neurodegeneration. Nat. Med. 2004, 10 (Suppl. S2–S9). [Google Scholar] [CrossRef]
- Cabrera-Reyes, E.A.; Vanoye-Carlo, A.; Rodríguez-Dorantes, M.; Vázquez-Martínez, E.R.; Rivero-Segura, N.A.; Collazo-Navarrete, O.; Cerbón, M. Transcriptomic analysis reveals new hippocampal gene networks induced by prolactin. Sci. Rep. 2019, 9, 13765. [Google Scholar] [CrossRef]
- Zhong, L.; Zhou, J.; Chen, X.; Lou, Y.; Liu, D.; Zou, X.; Yang, B.; Yin, Y.; Pan, Y. Quantitative proteomics study of the neuroprotective effects of B12 on hydrogen peroxide-induced apoptosis in SH-SY5Y cells. Sci. Rep. 2016, 6, 22635. [Google Scholar] [CrossRef] [PubMed]
- Abhinand, C.S.; Raju, R.; Soumya, S.J.; Arya, P.S.; Sudhakaran, P.R. VEGF-A/VEGFR2 signaling network in endothelial cells relevant to angiogenesis. J. Cell Commun. Signal. 2016, 10, 347–354. [Google Scholar] [CrossRef]
- Sieveking, D.P.; Lim, P.; Chow, R.W.; Dunn, L.L.; Bao, S.; McGrath, K.C.; Heather, A.K.; Handelsman, D.J.; Celermajer, D.S.; Ng, M.K. A sex-specific role for androgens in angiogenesis. J. Exp. Med. 2010, 207, 345–352. [Google Scholar] [CrossRef]
- Dobrynina, L.A.; Zabitova, M.R.; Shabalina, A.A.; Kremneva, E.I.; Akhmetzyanov, B.M.; Gadzhieva, Z.S.; Berdalin, A.B.; Kalashnikova, L.A.; Gnedovskaya, E.V.; Krotenkova, M.V. MRI types of cerebral small vessel disease and circulating markers of vascular wall damage. Diagnostics 2020, 10, 354. [Google Scholar] [CrossRef] [PubMed]
- Karar, J.; Maity, A. PI3K/AKT/mTOR Pathway in Angiogenesis. Front. Mol. Neurosci. 2011, 4, 51. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Liu, L.; Packwood, W.; Merkel, M.; Hurn, P.D.; Van Winkle, D.M. Sex differences in the mechanism of Met5-enkephalin-induced cardioprotection: Role of PI3K/Akt. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H302–H310. [Google Scholar] [CrossRef][Green Version]
- Wen, X.; Qi, D.; Sun, Y.; Huang, X.; Zhang, F.; Wu, J.; Fu, Y.; Ma, K.; Du, Y.; Dong, H.; et al. H2S attenuates cognitive deficits through Akt1/JNK3 signaling pathway in ischemic stroke. Behav. Brain Res. 2014, 269, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B. Signalling pathways of the TNF superfamily: A double-edged sword. Nat. Rev. Immunol. 2003, 3, 745–756. [Google Scholar] [CrossRef]
- Straface, E.; Gambardella, L.; Pagano, F.; Angelini, F.; Ascione, B.; Vona, R.; De Falco, E.; Cavarretta, E.; Russa, R.; Malorni, W.; et al. Sex differences of human cardiac progenitor cells in the biological response to TNF-α treatment. Stem Cells Int. 2017, 2017, 4790563. [Google Scholar] [CrossRef]
- Djouadi, F.; Weinheimer, C.J.; Saffitz, J.E.; Pitchford, C.; Bastin, J.; Gonzalez, F.J.; Kelly, D.P. A gender-related defect in lipid metabolism and glucose homeostasis in peroxisome proliferator-activated receptor alpha-deficient mice. J. Clin. Investig. 1998, 102, 1083–1091. [Google Scholar] [CrossRef]
- Wójtowicz, S.; Strosznajder, A.K.; Jeżyna, M.; Strosznajder, J.B. The novel role of PPAR alpha in the brain: Promising target in therapy of Alzheimer’s disease and other neurodegenerative disorders. Neurochem. Res. 2020, 45, 972–988. [Google Scholar] [CrossRef]
- Alaqeel, A.M.; Abou Al-Shaar, H.; Shariff, R.K.; Albakr, A. The role of RNA metabolism in neurological diseases. Balk. J. Med. Genet. 2015, 18, 5–14. [Google Scholar] [CrossRef]
- Nussbacher, J.K.; Tabet, R.; Yeo, G.W.; Lagier-Tourenne, C. Disruption of RNA metabolism in neurological diseases and emerging therapeutic interventions. Neuron 2019, 102, 294–320. [Google Scholar] [CrossRef]
- Lazrak, M.; Deleuze, V.; Noel, D.; Haouzi, D.; Chalhoub, E.; Dohet, C.; Robbins, I.; Mathieu, D. The bHLH TAL-1/SCL regulates endothelial cell migration and morphogenesis. J. Cell Sci. 2004, 117, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Hata, Y.; Duh, E.; Zhang, K.; Robinson, G.S.; Aiello, L.P. Transcription factors Sp1 and Sp3 alter vascular endothelial growth factor receptor expression through a novel recognition sequence. J. Biol. Chem. 1998, 273, 19294–19303. [Google Scholar] [CrossRef]
- Cohen-Solal, K.A.; Boregowda, R.K.; Lasfar, A. RUNX2 and the PI3K/AKT axis reciprocal activation as a driving force for tumor progression. Mol. Cancer 2015, 14, 137. [Google Scholar] [CrossRef]
- Neef, D.W.; Jaeger, A.M.; Gomez-Pastor, R.; Willmund, F.; Frydman, J.; Thiele, D.J. A direct regulatory interaction between chaperonin TRiC and stress-responsive transcription factor HSF1. Cell Rep. 2014, 9, 955–966. [Google Scholar] [CrossRef] [PubMed]
- Jochum, W.; Passegué, E.; Wagner, E.F. AP-1 in mouse development and tumorigenesis. Oncogene 2001, 20, 2401–2412. [Google Scholar] [CrossRef]
- Behrens, A.; Sibilia, M.; Wagner, E.F. Amino-Terminal phosphorylation of c-Jun regulates stress-induced apoptosis and cellular proliferation. Nat. Genet. 1999, 21, 326–329. [Google Scholar] [CrossRef]
- Herdegen, T.; Waetzig, V. AP-1 proteins in the adult brain: Facts and fiction about effectors of neuroprotection and neurodegeneration. Oncogene 2001, 20, 2424–2437. [Google Scholar] [CrossRef]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms underlying inflammation in neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [CrossRef]
- Simi, A.; Tsakiri, N.; Wang, P.; Rothwell, N.J. Interleukin-1 and inflammatory neurodegeneration. Biochem. Soc. Trans. 2007, 35, 1122–1126. [Google Scholar] [CrossRef]
- Saggu, R.; Schumacher, T.; Gerich, F.; Rakers, C.; Tai, K.; Delekate, A.; Petzold, G.C. Astroglial NF-kB contributes to white matter damage and cognitive impairment in a mouse model of vascular dementia. Acta Neuropathol. Commun. 2016, 4, 76. [Google Scholar] [CrossRef] [PubMed]
- Tai, X.; Erman, B.; Alag, A.; Mu, J.; Kimura, M.; Katz, G.; Guinter, T.; McCaughtry, T.; Etzensperger, R.; Feigenbaum, L.; et al. Foxp3 transcription factor is proapoptotic and lethal to developing regulatory T cells unless counterbalanced by cytokine survival signals. Immunity 2013, 38, 1116–1128. [Google Scholar] [CrossRef] [PubMed]
- Baruch, K.; Rosenzweig, N.; Kertser, A.; Deczkowska, A.; Sharif, A.M.; Spinrad, A.; Tsitsou-Kampeli, A.; Sarel, A.; Cahalon, L.; Schwartz, M. Breaking immune tolerance by targeting Foxp3(+) regulatory T cells mitigates Alzheimer’s disease pathology. Nat. Commun. 2015, 6, 7967. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, D.; Weyer, S.; Tolosa, E.; Gaenslen, A.; Berg, D.; Leyhe, T.; Gasser, T.; Stoltze, L. Higher frequency of regulatory T cells in the elderly and increased suppressive activity in neurodegeneration. J. Neuroimmunol. 2007, 188, 117–127. [Google Scholar] [CrossRef]
- Chen, M.J.; Ramesha, S.; Weinstock, L.D.; Gao, T.; Ping, L.; Xiao, H.; Dammer, E.B.; Duong, D.D.; Levey, A.I.; Lah, J.J.; et al. Microglial ERK signaling is a critical regulator of pro-inflammatory immune responses in Alzheimer’s disease. bioRxiv 2019, 798215. [Google Scholar] [CrossRef]
- Rustenhoven, J.; Smith, A.M.; Smyth, L.C.; Jansson, D.; Scotter, E.L.; Swanson, M.E.V.; Aalderink, M.; Coppieters, N.; Narayan, P.; Handley, R.; et al. PU.1 regulates Alzheimer’s disease-associated genes in primary human microglia. Mol. Neurodegener. 2018, 13, 44. [Google Scholar] [CrossRef]
- Zovoilis, A.; Agbemenyah, H.Y.; Agis-Balboa, R.C.; Stilling, R.M.; Edbauer, D.; Rao, P.; Farinelli, L.; Delalle, I.; Schmitt, A.; Falkai, P.; et al. microRNA-34c is a novel target to treat dementias. EMBO J. 2011, 30, 4299–4308. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Lu, H.C. microRNAs in neurodegeneration: Current findings and potential impacts. J. Alzheimers Dis. Park. 2018, 8. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, Y.; Su, L. MiR-539-5p Decreases amyloid β-protein production, hyperphosphorylation of Tau and memory impairment by regulating PI3K/Akt/GSK-3β pathways in APP/PS1 double transgenic mice. Neurotox. Res. 2020, 38, 524–535. [Google Scholar] [CrossRef]
- Logan, M.K.; Burke, M.F.; Hebert, M.D. Altered dynamics of scaRNA2 and scaRNA9 in response to stress correlates with disrupted nuclear organization. Biol. Open 2018, 7. [Google Scholar] [CrossRef]
- Cohen, E.; Avrahami, D.; Frid, K.; Canello, T.; Levy Lahad, E.; Zeligson, S.; Perlberg, S.; Chapman, J.; Cohen, O.S.; Kahana, E.; et al. Snord 3A: A molecular marker and modulator of prion disease progression. PLoS ONE 2013, 8, e54433. [Google Scholar] [CrossRef]
- Prusiner, S.B. Novel proteinaceous infectious particles cause scrapie. Science 1982, 216, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Kotraiah, V. Modulation of aldehyde dehydrogenase activity affects (±)-4-hydroxy-2E-nonenal (HNE) toxicity and HNE-protein adduct levels in PC12 cells. J. Mol. Neurosci. 2012, 47, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Sultana, R.; Butterfield, D.A. Proteomics identification of carbonylated and HNE-bound brain proteins in Alzheimer’s disease. Methods Mol. Biol. 2009, 566, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Chen, D.; Liu, Y.; Yu, J.; Qiao, L.; Lin, S.; Chen, D.; Zhong, G.; Lu, X.; Wang, Y.; et al. Long noncoding RNA HOXA-AS3 integrates NF-κB signaling to regulate endothelium inflammation. Mol. Cell. Biol. 2019, 39. [Google Scholar] [CrossRef]
- Zhong, Y.; Yu, C.; Qin, W. LncRNA SNHG14 promotes inflammatory response induced by cerebral ischemia/reperfusion injury through regulating miR-136-5p /ROCK1. Cancer Gene Ther. 2019, 26, 234–247. [Google Scholar] [CrossRef]
- Ball, H.J.; McParland, B.; Driussi, C.; Hunt, N.H. Isolating vessels from the mouse brain for gene expression analysis using laser capture microdissection. Brain Res. Brain Res. Protoc. 2002, 9, 206–213. [Google Scholar] [CrossRef]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Li, S.; Xia, J. MetaboAnalystR 3.0: Toward an optimized workflow for global metabolomics. Metabolites 2020, 10, 186. [Google Scholar] [CrossRef]
- MetaboAnalyst-Statistical, Functional and Integrative Analysis of Metabolomics Data. Available online: https://www.metaboanalyst.ca/ (accessed on 2 March 2020).
- DAVID. Available online: https://david.ncifcrf.gov/home.jsp (accessed on 2 June 2019).
- Huang da, W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Huang da, W.; Sherman, B.T.; Lempicki, R.A. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009, 37, 1–13. [Google Scholar] [CrossRef]
- GeneTrail2. Available online: https://genetrail2.bioinf.uni-sb.de (accessed on 2 June 2019).
- Stockel, D.; Kehl, T.; Trampert, P.; Schneider, L.; Backes, C.; Ludwig, N.; Gerasch, A.; Kaufmann, M.; Gessler, M.; Graf, N.; et al. Multi-Omics enrichment analysis using the GeneTrail2 web service. Bioinformatics 2016, 32, 1502–1508. [Google Scholar] [CrossRef] [PubMed]
- Metacore. Available online: https://portal.genego.com (accessed on 2 June 2019).
- Cytoscape. Available online: https://cytoscape.org/ (accessed on 2 June 2019).
- Su, G.; Morris, J.H.; Demchak, B.; Bader, G.D. Biological network exploration with Cytoscape 3. Curr. Protoc. Bioinform. 2014, 47, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Splitstackshape. Available online: https://github.com/mrdwab/splitstackshape (accessed on 2 March 2020).
- Rdata.Table. Available online: https://github.com/Rdatatable/data.table (accessed on 2 March 2020).
- Dplyr Tidyverse. Available online: http://dplyr.tidyverse.org (accessed on 2 March 2020).
- Dplyr Tidyverse Github. Available online: https://github.com/tidyverse/dplyr (accessed on 2 March 2020).
- String Tidyverse Github. Available online: https://github.com/tidyverse/stringr (accessed on 2 March 2020).
- String Tidyverse. Available online: http://stringr.tidyverse.org (accessed on 2 March 2020).
- Cytoscape Network Analyzer Application. Available online: http://apps.cytoscape.org/apps/networkanalyzer (accessed on 2 March 2020).
- PermutMatrix. Available online: http://www.atgc-montpellier.fr/permutmatrix (accessed on 2 June 2019).
- Caraux, G.; Pinloche, S. PermutMatrix: A graphical environment to arrange gene expression profiles in optimal linear order. Bioinformatics 2005, 21, 1280–1281. [Google Scholar] [CrossRef]
- VENNY 2.1. Available online: http://bioinfogp.cnb.csic.es/tools/venny/ (accessed on 2 June 2019).
- Heberle, H.; Meirelles, G.V.; da Silva, F.R.; Telles, G.P.; Minghim, R. InteractiVenn: A web-based tool for the analysis of sets through Venn diagrams. BMC Bioinform. 2015, 16, 169. [Google Scholar] [CrossRef] [PubMed]
- Metascape. Available online: http://metascape.org/ (accessed on 2 June 2019).
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Nebel, R.A.; Aggarwal, N.T.; Barnes, L.L.; Gallagher, A.; Goldstein, J.M.; Kantarci, K.; Mallampalli, M.P.; Mormino, E.C.; Scott, L.; Yu, W.H.; et al. Understanding the impact of sex and gender in Alzheimer’s disease: A call to action. Alzheimers Dement. 2018, 14, 1171–1183. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nuthikattu, S.; Milenkovic, D.; Rutledge, J.C.; Villablanca, A.C. Sex-Dependent Molecular Mechanisms of Lipotoxic Injury in Brain Microvasculature: Implications for Dementia. Int. J. Mol. Sci. 2020, 21, 8146. https://doi.org/10.3390/ijms21218146
Nuthikattu S, Milenkovic D, Rutledge JC, Villablanca AC. Sex-Dependent Molecular Mechanisms of Lipotoxic Injury in Brain Microvasculature: Implications for Dementia. International Journal of Molecular Sciences. 2020; 21(21):8146. https://doi.org/10.3390/ijms21218146
Chicago/Turabian StyleNuthikattu, Saivageethi, Dragan Milenkovic, John C. Rutledge, and Amparo C. Villablanca. 2020. "Sex-Dependent Molecular Mechanisms of Lipotoxic Injury in Brain Microvasculature: Implications for Dementia" International Journal of Molecular Sciences 21, no. 21: 8146. https://doi.org/10.3390/ijms21218146
APA StyleNuthikattu, S., Milenkovic, D., Rutledge, J. C., & Villablanca, A. C. (2020). Sex-Dependent Molecular Mechanisms of Lipotoxic Injury in Brain Microvasculature: Implications for Dementia. International Journal of Molecular Sciences, 21(21), 8146. https://doi.org/10.3390/ijms21218146





