Soft Template Electropolymerization of Polypyrrole for Improved pH-Induced Drug Delivery
Abstract
1. Introduction
2. Materials
3. Methods
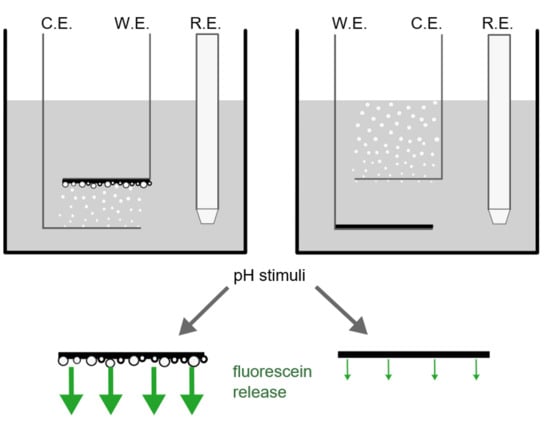
3.1. Vertical Electrode Configuration
3.2. Horizontal Electrode Configuration
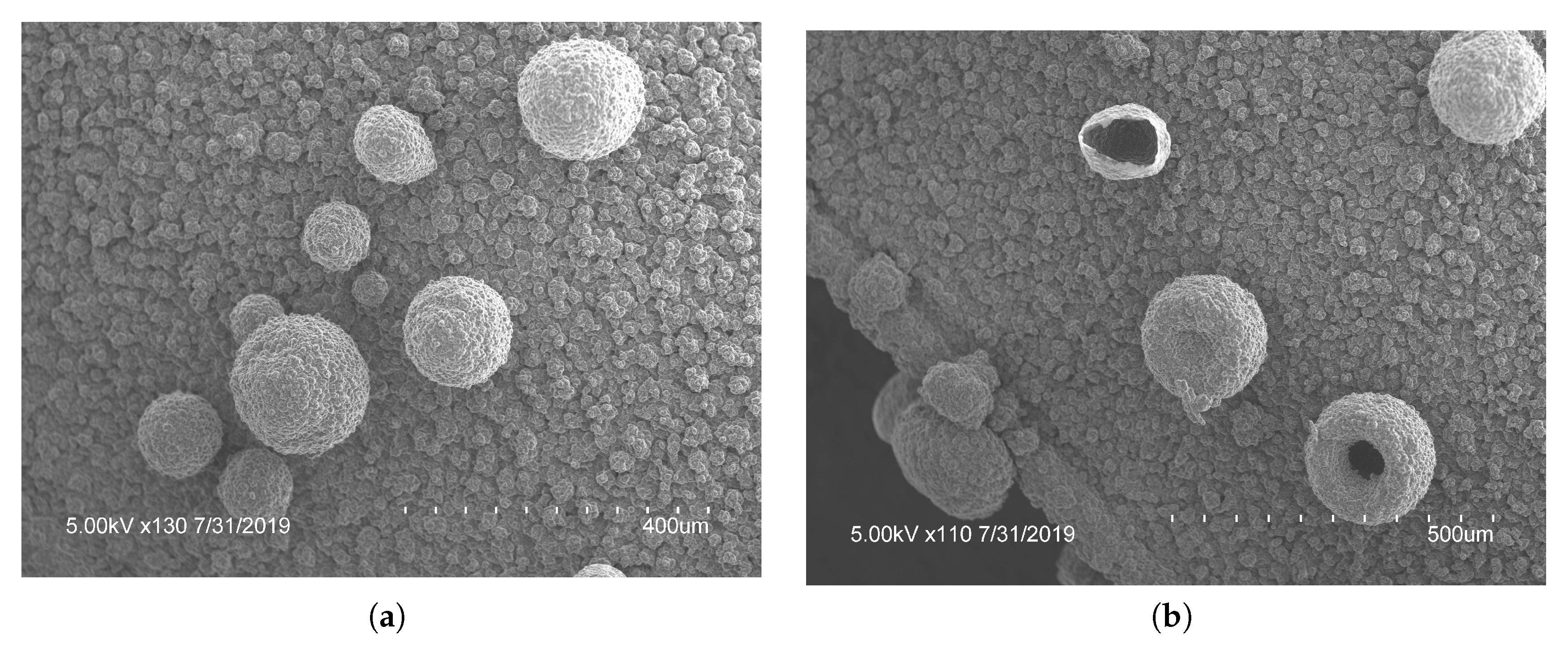
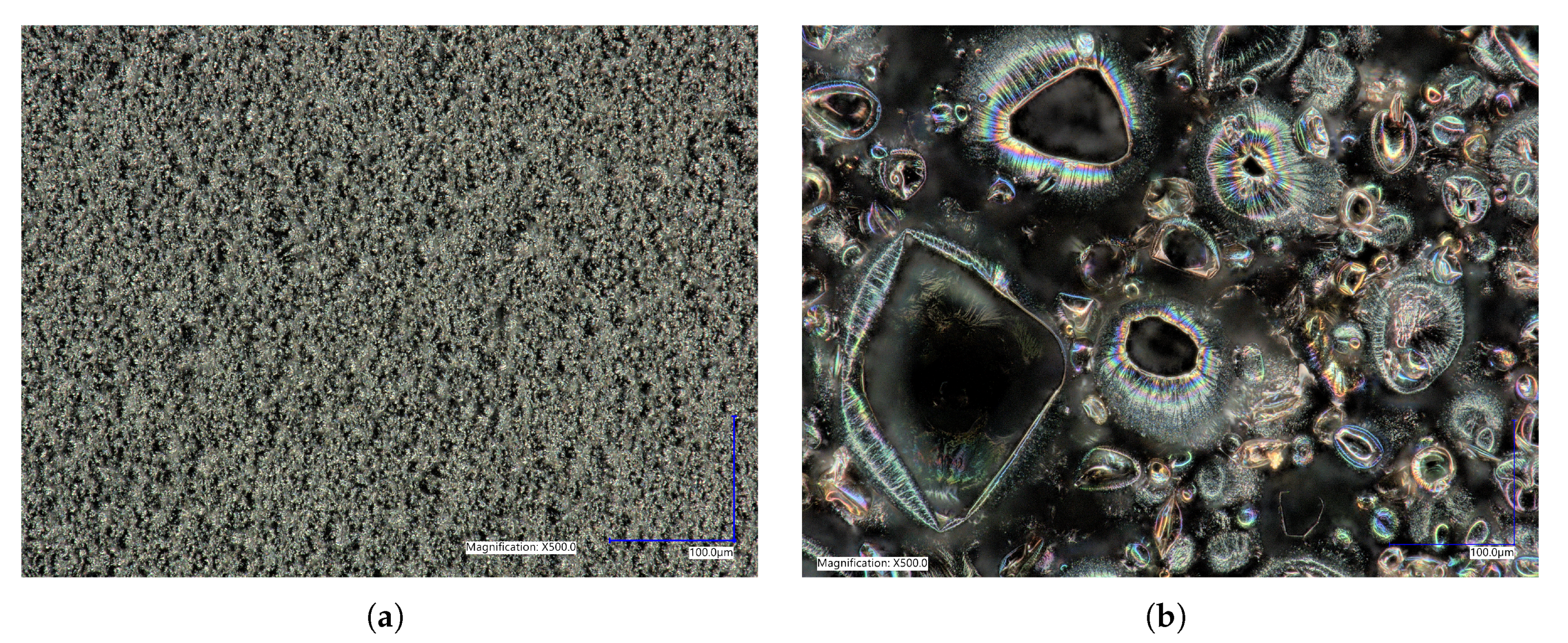
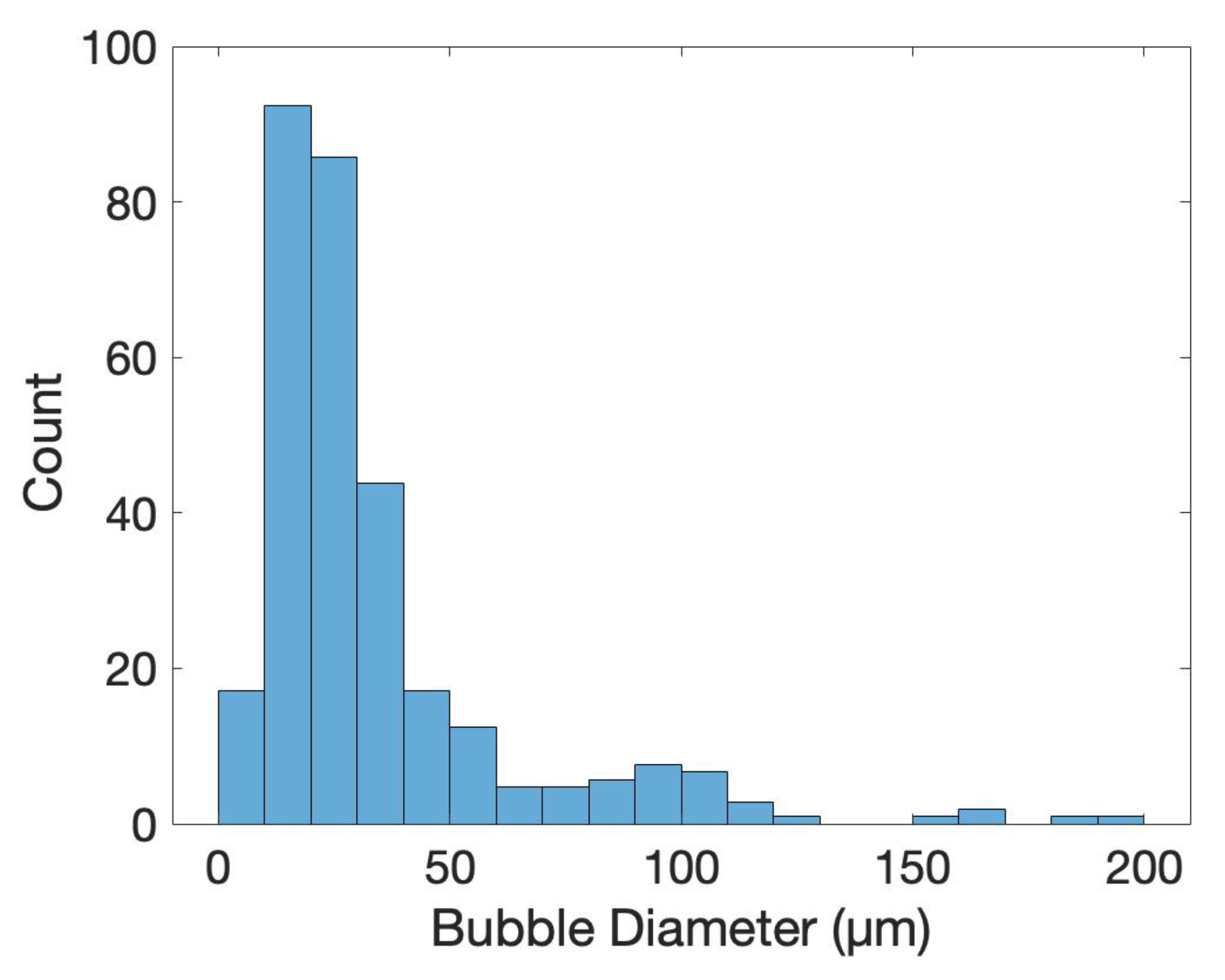
3.3. Morphology
3.4. Conductivity
3.5. pH Sensitivity and Drug Loading Capacity
4. Results and Discussion
4.1. Vertical Electrode Configuration
4.2. Horizontal Electrode Configuration
4.3. Morphology
4.4. Conductivity
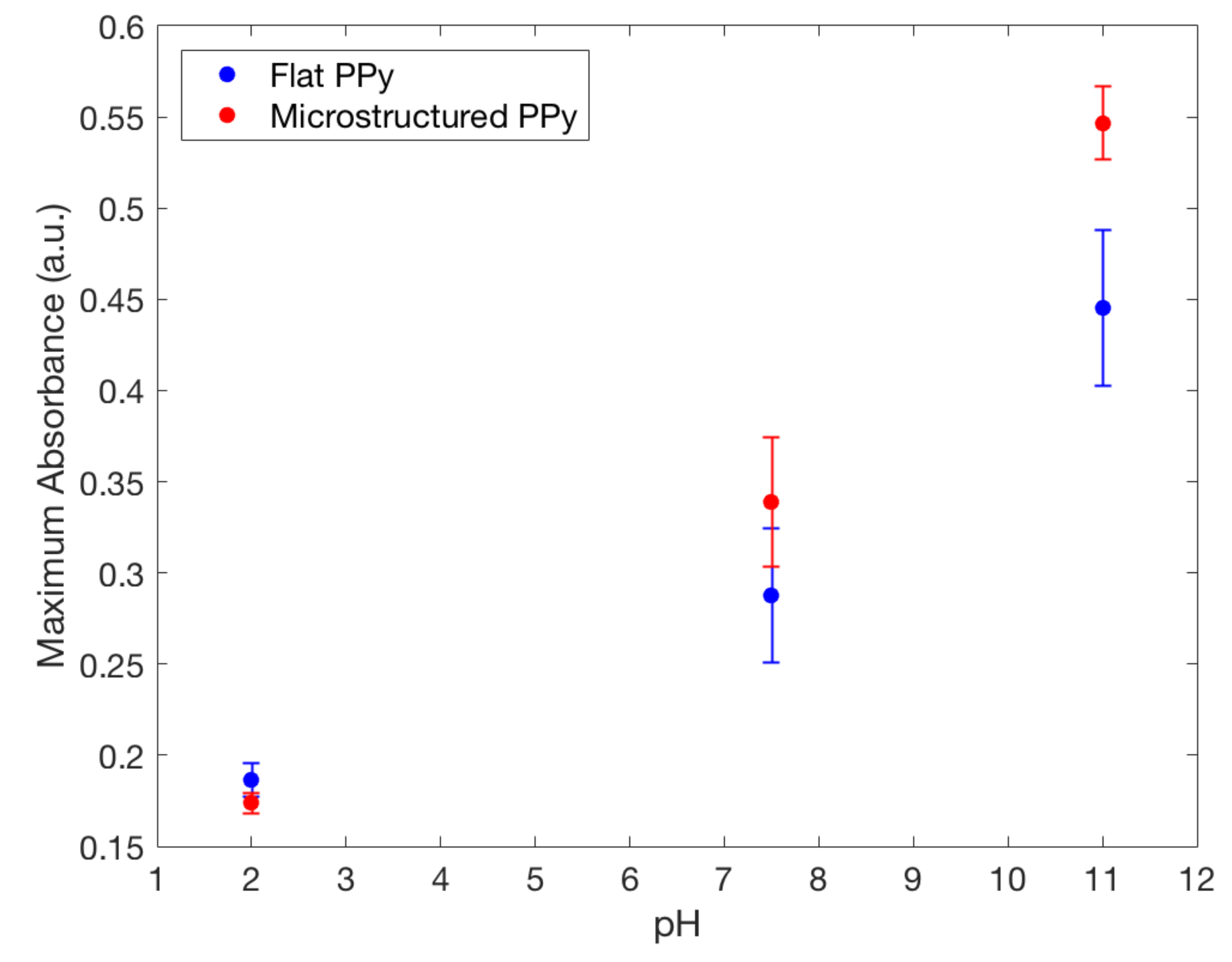
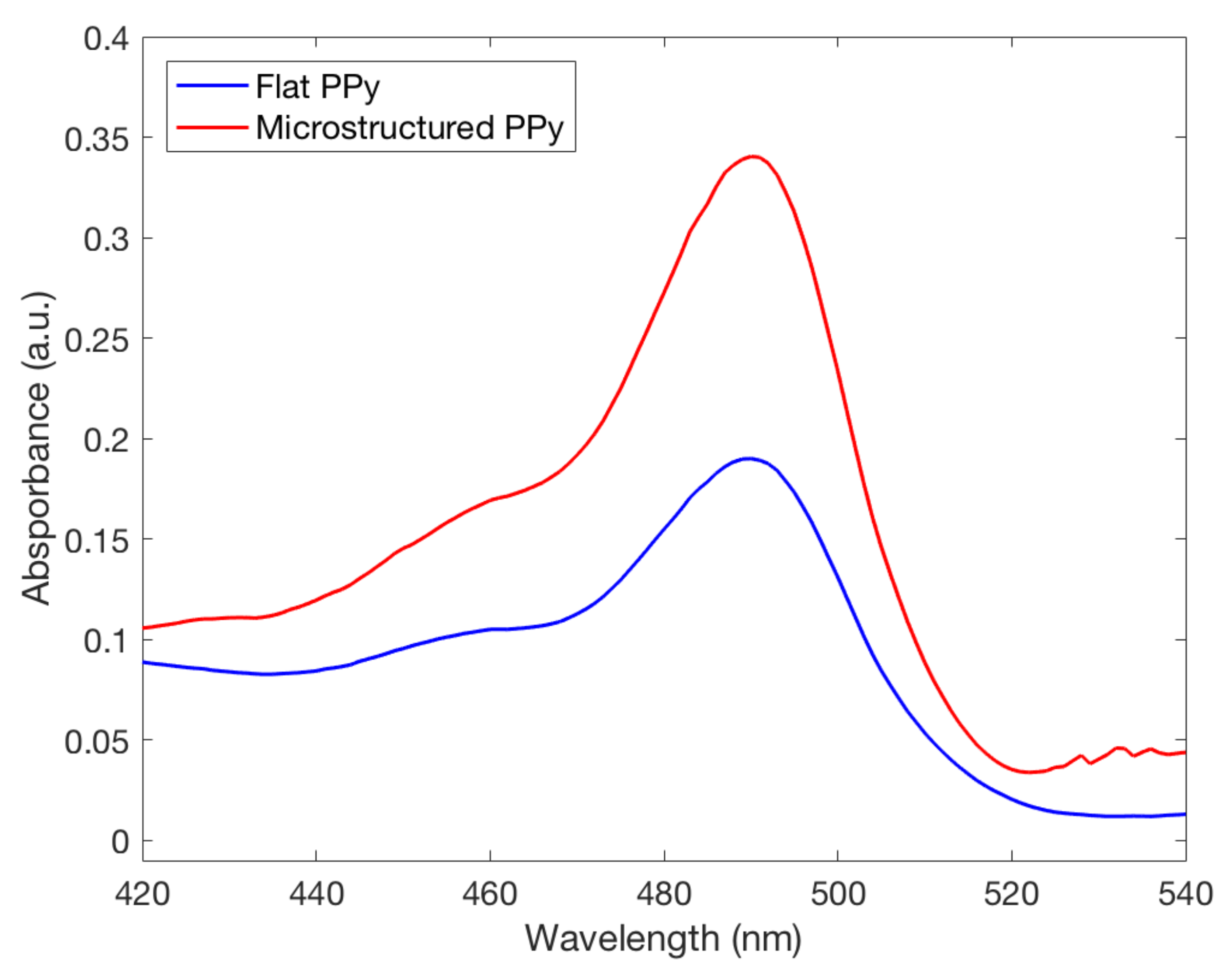
4.5. pH Sensitivity
4.6. Drug-Loading Capacity
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CE | Counter-Electrode |
| CV | Cyclic Voltammetry |
| FL | Fluorescein |
| NaCl | Sodium Chloride |
| ICP | Intrinsically conductive polymers |
| PPy | Polypyrrole |
| RE | Reference Electrode |
| SEM | Scanning Electron Microscope |
| WE | Working Electrode |
References
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair Regen. 2009, 17, 763–771. [Google Scholar] [PubMed]
- Schneider, L.A.; Korber, A.; Grabbe, S.; Dissemond, J. Influence of pH on wound-healing: A new perspective for wound-therapy? Arch. Dermatol. Res. 2007, 298, 413–420. [Google Scholar] [PubMed]
- Krukiewicz, K.; Stokfisz, A.; Zak, J.K. Two approaches to the model drug immobilization into conjugated polymer matrix. Mater. Sci. Eng. C 2015, 54, 176–181. [Google Scholar]
- Svirskis, D.; Travas-Sejdic, J.; Rodgers, A.; Garg, S. Electrochemically controlled drug delivery based on intrinsically conducting polymers. J. Control Release 2010, 146, 6–15. [Google Scholar] [PubMed]
- Sattari, S.; Tehrani, A.D.; Adeli, M. pH-Responsive Hybrid Hydrogels as Antibacterial and Drug Delivery Systems. Polymers 2018, 10, 660. [Google Scholar] [CrossRef]
- Fahlgren, A.; Bratengeier, C.; Gelmi, A.; Semeins, C.M.; Klein-Nulend, J.; Jager, E.W.H.; Bakker, A.D. Biocompatibility of Polypyrrole with Human Primary Osteoblasts and the Effect of Dopants. PLoS ONE 2015, 10, e0134023. [Google Scholar]
- Luo, X.; Cui, X.T. Sponge-like nanostructured conducting polymers for electrically controlled drug release. Electrochem. Commun. 2009, 11, 1956–1959. [Google Scholar]
- Shah, S.; Firlak, M.; Berrow, S.; Halcovitch, N.; Baldock, S.; Yousafzai, B.; Hathout, R.; Hardy, J. Electrochemically Enhanced Drug Delivery Using Polypyrrole Films. Materials 2018, 11, 1123. [Google Scholar] [CrossRef]
- Roghani-Mamaqani, H.; Yussuf, A.; Al-Saleh, M.; Al-Enezi, S.; Abraham, G. Synthesis and Characterization of Conductive Polypyrrole: The Influence of the Oxidants and Monomer on the Electrical, Thermal, and Morphological Properties. Int. J. Polym. Sci. 2018, 2018, 4191747. [Google Scholar] [CrossRef]
- Samanta, D.; Meiser, J.L.; Zare, R.N. Polypyrrole nanoparticles for tunable, pH-sensitive and sustained drug release. Nanoscale 2015, 7, 9497–9504. [Google Scholar]
- Jain, R.; Jadon, N.; Pawaiya, A. Polypyrrole based next generation electrochemical sensors and biosensors: A review. TrAC Trends Anal. Chem. 2017, 97, 363–373. [Google Scholar] [CrossRef]
- Tandon, B.; Magaz, A.; Balint, R.; Blaker, J.J.; Cartmell, S.H. Electroactive biomaterials: Vehicles for controlled delivery of therapeutic agents for drug delivery and tissue regeneration. Adv. Drug Deliv. Rev. 2018, 129, 148–168. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, R.; Lagenaur, C.F.; Cui, X.T. Electrochemically controlled release of dexamethasone from conducting polymer polypyrrole coated electrode. J. Control Release 2006, 110, 531–541. [Google Scholar] [CrossRef] [PubMed]
- MacDiarmid, A.G. “Synthetic Metals”: A Novel Role for Organic Polymers (Nobel Lecture). Angew. Chem. Int. Ed. 2001, 40, 2581–2590. [Google Scholar] [CrossRef]
- Qu, L.; Shi, G.; Chen, F.; Zhang, J. Electrochemical Growth of Polypyrrole Microcontainers. Macromolecules 2003, 36, 1063–1067. [Google Scholar] [CrossRef]
- Qu, L.; Shi, G.; Yuan, J.; Han, G.; Chen, F. Preparation of polypyrrole microstructures by direct electrochemical oxidation of pyrrole in an aqueous solution of camphorsulfonic acid. J. Electroanal. Chem. 2004, 561, 149–156. [Google Scholar] [CrossRef]
- Bajpai, V.; He, P.; Dai, L. Conducting-Polymer Microcontainers: Controlled Syntheses and Potential Applications. Adv. Funct. Mater. 2004, 14, 145–151. [Google Scholar] [CrossRef]
- Jiang, S.; Sun, Y.; Cui, X.; Huang, X.; He, Y.; Ji, S.; Shi, W.; Ge, D. Enhanced drug loading capacity of polypyrrole nanowire network for controlled drug release. Synth. Met. 2013, 163, 19–23. [Google Scholar] [CrossRef]
- Wang, W.Q.; Zhang, R.F.; Lu, L.C. The Electrochemical Polymerization of Polypyrrole Microstructures in an Aqueous Solution of Dodecylbenzenesulfonic Acid. Adv. Mater. Res. 2011, 306–307, 696–700. [Google Scholar] [CrossRef]
- Parakhonskiy, B.; Shchukin, D. Polypyrrole Microcontainers: Electrochemical Synthesis and Characterization. Langmuir 2015, 31, 9214–9218. [Google Scholar] [CrossRef]
- Xiao, Y.; Che, J.; Li, C.M.; Sun, C.Q.; Chua, Y.T.; Lee, V.S.; Luong, J.H. Preparation of nano-tentacle polypyrrole with pseudo-molecular template for ATP incorporation. J. Biomed. Mater. Res. Part A 2007, 80A, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Uppalapati, D.; Boyd, B.J.; Garg, S.; Travas-Sejdic, J.; Svirskis, D. Conducting polymers with defined micro- or nanostructures for drug delivery. Biomaterials 2016, 111, 149–162. [Google Scholar] [CrossRef]
- Pernaut, J.M.; Reynolds, J.R. Use of Conducting Electroactive Polymers for Drug Delivery and Sensing of Bioactive Molecules. A Redox Chemistry Approach. J. Phys. Chem. B 2000, 104, 4080–4090. [Google Scholar] [CrossRef]
- Wiegand, C.; Abel, M.; Ruth, P.; Elsner, P.; Hipler, U.C. pH Influence on Antibacterial Efficacy of Common Antiseptic Substances. Skin Pharmacol. Physiol. 2015, 28, 147–158. [Google Scholar] [CrossRef]
- Yang, L.; Wang, K.; Li, H.; Denstedt, J.D.; Cadieux, P.A. The Influence of Urinary pH on Antibiotic Efficacy Against Bacterial Uropathogens. Urology 2014, 84, 731.e1–731.e7. [Google Scholar] [CrossRef] [PubMed]
- Harrison, I.; Spada, F. Hydrogels for Atopic Dermatitis and Wound Management: A Superior Drug Delivery Vehicle. Pharmaceutics 2018, 10, 71. [Google Scholar] [CrossRef]
- Chougule, M.A.; Pawar, S.G.; Godse, P.R.; Mulik, R.N.; Sen, S.; Patil, V.B. Synthesis and Characterization of Polypyrrole (PPy) Thin Films. Soft Nanosci. Lett. 2011, 1, 6–10. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liubchak, I.; Lawrence, M.T.; Holness, F.B.; Price, A.D. Soft Template Electropolymerization of Polypyrrole for Improved pH-Induced Drug Delivery. Int. J. Mol. Sci. 2020, 21, 8114. https://doi.org/10.3390/ijms21218114
Liubchak I, Lawrence MT, Holness FB, Price AD. Soft Template Electropolymerization of Polypyrrole for Improved pH-Induced Drug Delivery. International Journal of Molecular Sciences. 2020; 21(21):8114. https://doi.org/10.3390/ijms21218114
Chicago/Turabian StyleLiubchak, Iryna, Matthew T. Lawrence, F. Benjamin Holness, and Aaron D. Price. 2020. "Soft Template Electropolymerization of Polypyrrole for Improved pH-Induced Drug Delivery" International Journal of Molecular Sciences 21, no. 21: 8114. https://doi.org/10.3390/ijms21218114
APA StyleLiubchak, I., Lawrence, M. T., Holness, F. B., & Price, A. D. (2020). Soft Template Electropolymerization of Polypyrrole for Improved pH-Induced Drug Delivery. International Journal of Molecular Sciences, 21(21), 8114. https://doi.org/10.3390/ijms21218114