Recent Advances in Non-Fullerene Acceptors of the IDIC/ITIC Families for Bulk-Heterojunction Organic Solar Cells
Abstract
:1. Introduction
2. Discussion
2.1. Modification of Electron-Deficient End-Capping Groups
2.2. Structural Modification of the IDIC/ITIC Core
2.3. Industrial Considerations and Scalability of NFAs
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lin, Y.; Wang, J.; Zhang, Z.-G.; Bai, H.; Li, Y.; Zhu, D.; Zhan, X. An Electron Acceptor Challenging Fullerenes for Efficient Polymer Solar Cells. Adv. Mater. 2015, 27, 1170–1174. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-X.; Chueh, C.-C.; Yip, H.-L.; Ding, F.-Z.; Li, Y.-X.; Li, C.-Z.; Li, X.; Chen, W.-C.; Jen, A.K.-Y. Improved Charge Transport and Absorption Coefficient in Indacenodithieno[3,2-b]thiophene-based Ladder-Type Polymer Leading to Highly Efficient Polymer Solar Cells. Adv. Mater. 2012, 24, 6356–6361. [Google Scholar] [CrossRef] [PubMed]
- Intemann, J.J.; Yao, K.; Li, Y.-X.; Yip, H.-L.; Xu, Y.-X.; Liang, P.-W.; Chueh, C.-C.; Ding, F.-Z.; Yang, X.; Li, X.; et al. Highly Efficient Inverted Organic Solar Cells Through Material and Interfacial Engineering of Indacenodithieno[3,2-b]thiophene-Based Polymers and Devices. Adv. Funct. Mater. 2014, 24, 1465–1473. [Google Scholar] [CrossRef]
- Li, Y.; Yao, K.; Yip, H.-L.; Ding, F.-Z.; Xu, Y.-X.; Li, X.; Chen, Y.; Jen, A.K.-Y. Eleven-Membered Fused-Ring Low Band-Gap Polymer with Enhanced Charge Carrier Mobility and Photovoltaic Performance. Adv. Funct. Mater. 2014, 24, 3631–3638. [Google Scholar] [CrossRef]
- Wang, G.; Melkonyan, S.F.; Facchetti, A.; Marks, T.J. All-Polymer Solar Cells: Recent Progress, Challenges, and Prospects. Angew. Chem. Int. Ed. 2019, 58, 4129–4142. [Google Scholar] [CrossRef]
- Chen, L.X. Organic Solar Cells: Recent Progress and Challenges. ACS Energy Lett. 2019, 4, 2537–2539. [Google Scholar] [CrossRef] [Green Version]
- Yin, H.; Yan, C.; Hu, H.; Ho, J.K.; Zhan, X.; So, G.L.S.K. Recent progress of all-polymer solar cells–From chemical structure and device physics to photovoltaic performance. Mater. Sci. Eng. R Rep. 2020, 140, 1005422. [Google Scholar] [CrossRef]
- Karunakaran, S.K.; Arumugam, G.M.; Yang, W.; Ge, S.; Khan, S.N.; Li, X.; Yang, G. Recent progress in inkjet-printed solar cells. J. Mater. Chem. A 2019, 7, 13873–13902. [Google Scholar] [CrossRef]
- Qin, J.; Lan, L.; Chen, S.; Huang, F.; Shi, H.; Chen, W.; Xia, H.; Sun, K.; Yang, C. Recent Progress in Flexible and Stretchable Organic Solar Cells. Adv. Funct. Mater. 2020, 30, 2002529. [Google Scholar] [CrossRef]
- Ryu, H.S.; Park, S.Y.; Lee, H.T.; Kim, J.Y.; Woo, H.Y. Recent progress in indoor organic photovoltaics. Nanoscale 2020, 12, 5792–5804. [Google Scholar] [CrossRef]
- Nitti, A.; Signorile, M.; Boiocchi, M.; Bianchi, G.; Po, R.; Pasini, D. Conjugated Thiophene-Fused Isatin Dyes through Intramolecular Direct Arylation. J. Org. Chem. 2016, 81, 11035–11042. [Google Scholar] [CrossRef]
- Nitti, A.; Bianchi, G.; Po, R.; Swager, T.M.; Pasini, D. Domino Direct Arylation and Cross-Aldol for Rapid Construction of Extended Polycyclic π-Scaffolds. J. Am. Chem. Soc. 2017, 139, 8788–8791. [Google Scholar] [CrossRef] [PubMed]
- Chochos, C.L.; Katsouras, A.; Gasparini, N.; Koulogiannis, C.; Ameri, T.; Brabec, C.J.; Avgeropoulos, A. Rational Design of High-Performance Wide-Bandgap (≈2 eV) Polymer Semiconductors as Electron Donors in Organic Photovoltaics Exhibiting High Open Circuit Voltages (≈1 V). Macromol. Rapid Commun. 2017, 38, 1600614. [Google Scholar] [CrossRef]
- Gasparini, N.; Katsouras, A.; Prodromidis, M.I.; Avgeropoulos, A.; Baran, D.; Salvador, M.; Fladischer, S.; Spiecker, E.; Chochos, C.L.; Ameri, T.; et al. Photophysics of Molecular-Weight-Induced Losses in Indacenodithienothiophene-Based Solar Cells. Adv. Funct. Mater. 2015, 25, 4898–4907. [Google Scholar] [CrossRef]
- Cai, Y.; Zhang, X.; Xue, X.; Wei, D.; Huo, L.; Sun, Y. High-Performance Wide-Bandgap Copolymers Based on Indacenodithiophene and Indacenodithieno[3,2-b]thiophene Units. J. Mater. Chem. C 2017, 5, 7777–7783. [Google Scholar] [CrossRef]
- Sun, H.; Liu, T.; Yu, J.; Lau, T.-K.; Zhang, G.; Zhang, Y.; Su, M.; Tang, Y.; Ma, R.; Liu, B.; et al. A monothiophene unit incorporating both fluoro and ester substitution enabling high-performance donor polymers for non-fullerene solar cells with 16.4% efficiency. Energy Environ. Sci. 2019, 12, 3328–3337. [Google Scholar] [CrossRef]
- Vandewal, K.; Tvingstedt, K.; Gadisa, A.; Inganäs, O.; Manca, J.V. Relating the open-circuit voltage to interface molecular properties of donor: Acceptor bulk heterojunction solar cells. Phys. Rev. B 2010, 81, 125204. [Google Scholar] [CrossRef] [Green Version]
- Hou, J.; Inganäs, O.; Friend, R.H.; Gao, F. Organic solar cells based on non-fullerene acceptors. Nat. Mater. 2018, 17, 119–128. [Google Scholar] [CrossRef]
- Eastham, N.D.; Logsdon, J.L.; Manley, E.F.; Aldrich, T.J.; Leonardi, M.J.; Wang, G.; Powers-Riggs, N.E.; Young, R.M.; Chen, L.X.; Wasielewski, M.R.; et al. Hole-Transfer Dependence on Blend Morphology and Energy Level Alignment in Polymer: ITIC Photovoltaic Materials. Adv. Mater. 2018, 30, 1704263. [Google Scholar] [CrossRef] [PubMed]
- Srivani, D.; Agarwal, A.; Bhosale, S.V.; Puvad, A.L.; Xiang, W.; Evans, R.A.; Gupta, A.; Bhosale, S.V. Naphthalene diimide-based non-fullerene acceptors flanked by open-ended and aromatizable acceptor functionalities. Chem. Commun. 2017, 53, 11157–11160. [Google Scholar] [CrossRef]
- Sung, M.J.; Huang, M.; Moon, S.H.; Lee, T.H.; Park, S.Y.; Kim, J.Y.; Kwon, S.K.; Choi, H.; Kim, Y.H. Naphthalene diimide-based small molecule acceptors for fullerene-free organic solar cells. Sol. Energy 2017, 150, 90–95. [Google Scholar] [CrossRef]
- Jina, R.; Wang, F.; Guana, R.; Zhenga, X.; Zhang, T. Design of perylene-diimides-based small-molecules semiconductors for organic solar cells. Mol. Phys. 2017, 115, 1591–1597. [Google Scholar] [CrossRef]
- Jo, J.W.; Jung, J.W.; Wang, H.-W.; Kim, P.; Russell, T.P.; Jo, W.H. Fluorination of Polythiophene Derivatives for High Performance Organic Photovoltaics. Chem. Mater. 2015, 27, 4865–4870. [Google Scholar] [CrossRef]
- Jung, W.J.; Jo, W.H. Low-Bandgap Small Molecules as Non-Fullerene Electron Acceptors Composed of Benzothiadiazole and Diketopyrrolopyrrole for All Organic Solar Cells. Chem. Mater. 2015, 27, 6038–6043. [Google Scholar] [CrossRef]
- Zhao, F.; Dai, S.; Wu, Y.; Zhang, Q.; Wang, J.; Jiang, L.; Ling, Q.; Wei, Z.; Ma, W.; You, W.; et al. Single-Junction Binary-Blend Nonfullerene Polymer Solar Cells with 12.1% Efficiency. Adv. Mater. 2017, 29, 1700144. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Yao, H.; Gao, B.; Qin, Y.; Zhang, S.; Yang, B.; He, C.; Xu, B.; Hou, J. Fine-Tuned Photoactive and Interconnection Layers for Achieving over 13% Efficiency in a Fullerene-Free Tandem Organic Solar Cell. J. Am. Chem. Soc. 2017, 139, 7302–7309. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Liu, T.; Gao, W.; Zhong, C.; Huo, L.; Luo, Z.; Wu, K.; Xiong, W.; Liu, F.; Sun, Y.; et al. A Novel Thiophene-Fused Ending Group Enabling an Excellent Small Molecule Acceptor for High-Performance Fullerene-Free Polymer Solar Cells with 11.8% Efficiency. Solar RRL 2017, 1, 1700044. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wang, W.; Wang, X.; Wu, Y.; Zhang, Q.; Yan, C.; Ma, W.; You, W.; Zhan, X. Enhancing Performance of Nonfullerene Acceptors via Side-Chain Conjugation Strategy. Adv. Mater. 2017, 29, 1702125. [Google Scholar] [CrossRef]
- Zhao, W.; Li, S.; Yao, H.; Zhang, S.; Zhang, Y.; Yang, B.; Hou, J. Molecular Optimization Enables over 13% Efficiency in Organic Solar Cells. J. Am. Chem. Soc. 2017, 139, 7148–7151. [Google Scholar] [CrossRef]
- Lin, Y.; He, Q.; Zhao, F.; Huo, L.; Mai, J.; Lu, X.; Su, C.-J.; Li, T.; Wang, J.; Zhu, J.; et al. A Facile Planar Fused-Ring Electron Acceptor for As-Cast Polymer Solar Cells with 8.71% Efficiency. J. Am. Chem. Soc. 2016, 138, 2973–2976. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Zhang, Y.; Zhou, L.; Zhang, G.; Yip, H.-L.; Lau, T.-K.; Lu, X.; Zhu, C.; Peng, H.; Johnson, P.A.; et al. Single-Junction Organic Solar Cell with over 15% Efficiency Using Fused-Ring Acceptor with Electron-Deficient Core. Joule 2019, 3, 1140–1151. [Google Scholar] [CrossRef]
- Fei, Z.; Eisner, F.D.; Jiao, X.; Azzouzi, M.; Röhr, J.A.; Han, Y.; Shahid, M.; Chesman, A.S.R.; Easton, C.D.; McNeill, C.R.; et al. An Alkylated Indacenodithieno[3,2-b]thiophene-Based Nonfullerene Acceptor with High Crystallinity Exhibiting Single Junction Solar Cell Efficiencies Greater than 13% with Low Voltage Losses. Adv. Mater. 2018, 30, 1705209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Guang, S.; Yu, J.; Wang, H.; Cao, J.; Du, F.; Wang, X.; Tang, W. Over 15.5% efficiency organic solar cells with triple side chain engineered ITIC. Sci. Bull. 2020, 65, 1533–1536. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, S.; Ren, J.; Gao, M.; Bi, P.; Ye, L.; Hou, J. Molecular design of a non-fullerene acceptor enables a P3HT-based organic solar cell with 9.46% efficiency. Energy Environ. Sci. 2020, 13, 2864–2869. [Google Scholar] [CrossRef]
- Asakawa, M.; Brown, C.L.; Pasini, D.; Stoddart, J.F.; Wyatt, P.G. Enantioselective Recognition of Amino Acids by Axially-Chiral π-Electron Deficient Receptors. J. Org. Chem. 1996, 61, 7234–7235. [Google Scholar] [CrossRef]
- Park, S.H.; Park, S.; Lee, S.; Kim, J.; Ahn, H.; Kim, B.J.; Chae, B.; Son, H.J. Developement of highly efficient large area organic photovoltaic module: Effects of nonfullerene acceptor. Nano Energy 2020, 77, 105147. [Google Scholar] [CrossRef]
- Meredith, P.; Li, W.; Armin, A. Nonfullerene Acceptors: A Renaissance in Organic Photovoltaics? Adv. Energy Mater. 2020, 10, 2001788. [Google Scholar] [CrossRef]
- Fox, D.; Metrangolo, P.; Pasini, D.; Pilati, T.; Resnati, G.; Terraneo, G. Site Selective Supramolecular Synthesis of Halogen Bonded Cocrystals Incorporating the Photoactive Azo Group. CrystEngComm 2008, 10, 1132–1136. [Google Scholar] [CrossRef]
- Lee, J.; Ko, S.-J.; Seifrid, M.; Lee, H.; Luginbuhl, B.R.; Karki, A.; Ford, M.; Rosenthal, K.; Cho, K.; Nguyen, T.-O.; et al. Bandgap Narrowing in Non-Fullerene Acceptors: Single Atom Substitution Leads to High Optoelectronic Response Beyond 1000 nm. Adv. Energy Mater. 2018, 8, 1801212. [Google Scholar] [CrossRef]
- Yao, H.; Ye, L.; Hou, J.; Jang, B.; Han, G.; Cui, Y.; Su, G.M.; Wang, C.; Gao, B.; Yu, R.; et al. Achieving Highly Efficient Nonfullerene Organic Solar Cells with Improved Intermolecular Interaction and Open-Circuit Voltage. Adv. Mater. 2017, 29, 1700254. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.W.; Lee, Y.J.; Lee, Y.W.; Koh, C.W.; Lee, Y.; Kim, M.J.; Liao, K.; Cho, J.H.; Kim, B.J.; Woo, H.Y. Impact of Terminal End-Group of Acceptor−Donor−Acceptor-type Small Molecules on Molecular Packing and Photovoltaic Properties. ACS Appl. Mater. Interfaces 2018, 10, 39952–39961. [Google Scholar] [CrossRef] [PubMed]
- Elumalai, N.K.; Uddin, A. Open circuit voltage of organic solar cells: An in-depth review. Energy Environ. Sci. 2016, 9, 391–410. [Google Scholar] [CrossRef]
- Tang, A.; Xiao, B.; Wang, Y.; Gao, F.; Tajima, K.; Bin, H.; Zhang, Z.-G.; Li, Y.; Wei, Z.; Zhou, E. Simultaneously Achieved High Open-Circuit Voltage and Efficient Charge Generation by Fine-Tuning Charge-Transfer Driving Force in Nonfullerene Polymer Solar Cells. Adv. Funct. Mater. 2018, 28, 1704507. [Google Scholar] [CrossRef] [Green Version]
- Tang, L.-M.; Xiaob, J.; Baia, W.-Y.; Lia, Q.-Y.; Wanga, H.-C.; Miaoc, M.-S.; Yipb, H.-L.; Xua, Y.-X. End-chain effects of non-fullerene acceptors on polymer solar cells. Org. Electron 2019, 64, 1–6. [Google Scholar] [CrossRef]
- Kolhe, N.B.; West, S.M.; Tran, D.K.; Ding, X.; Kuzuhara, D.; Yoshimoto, N.; Koganezawa, T.; Jenekhe, S.A. Designing High Performance Nonfullerene Electron Acceptors with Rylene Imides for Efficient Organic Photovoltaics. Chem. Mater. 2020, 32, 195–204. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, Q.; Zhou, J.; Lai, H.; Liu, T.; Li, D.; Chen, W.; Xie, Z.; He, F. Multiple Fused Ring-Based Near-Infrared Nonfullerene Acceptors with an Interpenetrated Charge-Transfer Network. Chem. Mater. 2019, 31, 1664–1671. [Google Scholar] [CrossRef]
- Li, X.; Li, C.; Ye, L.; Weng, K.; Fu, H.; Ryu, H.S.; Wei, D.; Sun, X.; Woo, H.Y.; Sun, Y. Asymmetric A–D–p–A-type nonfullerene small molecule acceptors for efficient organic solar cells. J. Mater. Chem. A 2019, 7, 19348–19354. [Google Scholar] [CrossRef]
- Zhang, H.; Yao, H.; Hou, J.; Zhu, J.; Zhang, J.; Li, W.; Yu, R.; Gao, B.; Zhang, S.; Hou, J. Over 14% Efficiency in Organic Solar Cells Enabled by Chlorinated Nonfullerene Small-Molecule Acceptors. Adv. Mater. 2018, 30, 1800613. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhou, Y.; Zhang, J.; Song, J.; Bo, Z. Tuning the dipole moments of nonfullerene acceptors with an asymmetric terminal strategy for highly efficient organic solar cells. J. Mater. Chem. A 2019, 7, 8889–8896. [Google Scholar] [CrossRef]
- Luo, Z.; Bin, H.; Liu, T.; Zhang, Z.-G.; Yang, Y.; Zhong, C.; Qiu, B.; Li, G.; Gao, W.; Xie, D.; et al. Fine-Tuning of Molecular Packing and Energy Level through Methyl Substitution Enabling Excellent Small Molecule Acceptors for Nonfullerene Polymer Solar Cells with Efficiency up to 12.54%. Adv. Mater. 2018, 30, 1706124. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, T.; Wang, Y.; Zhang, G.; Sun, R.; Chen, Z.; Zhong, C.; Wu, J.; Chen, Y.; Zhang, M.; et al. Reduced Energy Loss Enabled by a Chlorinated Thiophene-Fused Ending-Group Small Molecular Acceptor for Efficient Nonfullerene Organic Solar Cells with 13.6% Efficiency. Adv. Energy Mater. 2019, 9, 1900041. [Google Scholar] [CrossRef]
- Firdaus, Y.; He, Q.; Lin, Y.; Nugroho, F.A.A.; Le Corre, V.M.; Yengel, E.; Balawi, A.H.; Seitkhan, A.; Laquai, F.; Langhammer, C.; et al. Novel wide-bandgap non-fullerene acceptors for efficient tandem organic solar cells. J. Mater. Chem. A 2020, 8, 1164–1175. [Google Scholar] [CrossRef]
- Chang, S.-L.; Hung, K.-E.; Cao, F.-Y.; Huang, K.-H.; Hsu, C.-S.; Liao, C.-Y.; Lee, C.-H.; Cheng, Y.-J. Isomerically Pure Benzothiophene-Incorporated Acceptor: Achieving Improved Voc and Jsc of Nonfullerene Organic Solar Cells via End Group Manipulation. ACS Appl. Mater. Interfaces 2019, 11, 33179–33187. [Google Scholar] [CrossRef]
- Dai, S.; Li, T.; Wang, W.; Xiao, Y.; Lau, T.K.; Li, Z.; Liu, K.; Lu, X.; Zhan, X. Enhancing the Performance of Polymer Solar Cells via Core Engineering of NIR-Absorbing Electron Acceptors. Adv. Mater. 2018, 30, 1706571. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Dai, S.; Ke, Z.; Yang, L.; Wang, J.; Yan, C.; Ma, W.; Zhan, X. Fused Tris(thienothiophene)-Based Electron Acceptor with Strong Near-Infrared Absorption for High-Performance As-Cast Solar Cells. Adv. Mater. 2018, 30, 1705969. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-W.; Peng, K.-L.; Li, Y.-W.; Su, Y.-J.; Ma, K.-J.; Hong, L.; Chang, C.-C.; Hou, J.; Hsu, C.-S. A chlorinated nonacyclic carbazole-based acceptor affords over 15% efficiency in organic solar cells. J. Mater. Chem. A 2020, 8, 1131–1137. [Google Scholar] [CrossRef]
- Huang, C.; Liao, X.; Gao, K.; Zuo, L.; Lin, F.; Shi, X.; Li, C.-Z.; Liu, H.; Li, X.; Liu, F.; et al. Highly Efficient Organic Solar Cells Based on S,N-Heteroacene NonFullerene Acceptors. Chem. Mater. 2018, 30, 5429–5434. [Google Scholar] [CrossRef]
- Zhu, J.; Ke, Z.; Zhang, Q.; Wang, J.; Dai, S.; Wu, Y.; Xu, Y.; Lin, Y.; Ma, W.; You, W.; et al. Naphthodithiophene-Based Nonfullerene Acceptor for High-Performance Organic Photovoltaics: Effect of Extended Conjugation. Adv. Mater. 2018, 30, 1704713. [Google Scholar] [CrossRef]
- Yao, C.; Liu, B.; Zhu, Y.; Hong, L.; Miao, J.; Hou, J.; He, F.; Meng, H. Highly fluorescent anthracene derivative as a non-fullerene acceptor in OSCs with small non-radiative energy loss of 0.22 eV and high PCEs of over 13%. J. Mater. Chem. A 2019, 7, 10212–10216. [Google Scholar] [CrossRef]
- Feng, H.; Yi, Y.-Q.-Q.; Ke, X.; Yan, J.; Zhang, Y.; Wan, X.; Li, C.; Zheng, N.; Xie, Z.; Chen, Y. New Anthracene-Fused Nonfullerene Acceptors for High-Efficiency Organic Solar Cells: Energy Level Modulations Enabling Match of Donor and Acceptor. Adv. Energy Mater. 2019, 9, 1803541. [Google Scholar] [CrossRef]
- Liu, G.; Jia, J.; Zhang, K.; Jia, X.; Yin, Q.; Zhong, W.; Li, L.; Huang, F.; Cao, Y. 15% Efficiency Tandem Organic Solar Cell Based on a Novel Highly Efficient Wide-Bandgap Nonfullerene Acceptor with Low Energy Loss. Adv. Energy Mater. 2019, 9, 1803657. [Google Scholar] [CrossRef]
- Sun, J.; Ma, X.; Zhang, Z.; Yu, J.; Zhou, J.; Yin, X.; Yang, L.; Geng, R.; Zhu, R.; Zhang, F.; et al. Side chain engineering on Dithieno[3,2-b:2′,3′-d]pyrrol Fused Nonfullerene Acceptors Enabling Over 13% Efficiency for Organic Solar Cells. Mater. Chem. Front. 2019, 3, 702–708. [Google Scholar]
- Wan, S.-S.; Xu, X.; Jiang, Z.; Yuan, J.; Mahmood, A.; Yuan, G.-Z.; Liu, K.-K.; Ma, W.; Peng, Q.; Wang, J.-L. A bromine and chlorine concurrently functionalized end group for benzo[1,2-b:4,5-b′]diselenophene-based non-fluorinated acceptors: A new hybrid strategy to balance the crystallinity and miscibility of blend films for enabling highly efficient polymer solar cells. J. Mater. Chem. A 2020, 8, 4856–4867. [Google Scholar]
- Lin, F.; Zuo, L.; Gao, K.; Zhang, M.; Jo, S.B.; Liu, F.; Jen, A.K.-Y. Regio-Specific Selenium Substitution in Non-Fullerene Acceptors for Efficient Organic Solar Cells. Chem. Mater. 2019, 31, 6770–6778. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, M.; Liu, T.; Ming, R.; An, Q.; Wu, K.; Xie, D.; Luo, Z.; Zhong, C.; Liu, F.; et al. Asymmetrical Ladder-Type Donor-Induced Polar Small Molecule Acceptor to Promote Fill Factors Approaching 77% for High-Performance Nonfullerene Polymer Solar Cells. Adv. Mater. 2018, 30, 1800052. [Google Scholar] [CrossRef]
- Yang, L.; Song, X.; Yu, J.; Wang, H.; Zhang, Z.; Geng, R.; Cao, J.; Baran, D.; Tang, W. Tuning of the conformation of asymmetric nonfullerene acceptors for efficient organic solar cells. J. Mater. Chem. A 2019, 7, 22279–22286. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Li, M.; Zhou, X.; Jia, Q.-Q.; Feng, S.; Jiang, P.; Xu, X.; Ma, W.; Li, H.-B.; Bo, Z. Nonfullerene Acceptors with Enhanced Solubility and Ordered Packing for HighEfficiency Polymer Solar Cells. ACS Energy Lett. 2018, 3, 1832–1839. [Google Scholar] [CrossRef]
- Lee, J.; Song, S.; Huang, J.; Du, Z.; Lee, H.; Zhu, Z.; Ko, S.-J.; Nguyen, T.-Q.; Kim, J.Y.; Cho, K.; et al. Bandgap Tailored Nonfullerene Acceptors for Low-Energy-Loss Near-Infrared Organic Photovoltaics. ACS Mater. Lett. 2020, 2, 395–402. [Google Scholar] [CrossRef]
- Lee, J.; Ko, S.-J.; Seifrid, M.; Lee, H.; McDowell, C.; Luginbuhl, B.R.; Karki, A.; Cho, K.; Nguyen, T.-Q.; Bazan, G.C. Design of Nonfullerene Acceptors with Near-Infrared Light Absorption Capabilities. Adv. Energy Mater. 2018, 8, 1801209. [Google Scholar] [CrossRef]
- Chen, J.; Li, G.; Zhu, Q.; Guo, X.; Fan, Q.; Ma, W.; Zhang, M. Highly efficient near-infrared and semitransparent polymer solar cells based on an ultra-narrow bandgap nonfullerene acceptor. J. Mater. Chem. A 2019, 7, 3745–3751. [Google Scholar] [CrossRef]
- Min, J.; Luponosov, Y.N.; Cui, C.; Kan, B.; Chen, H.; Wan, X.; Chen, Y.; Ponomarenko, S.A.; Li, Y.; Brabec, C.J. Evaluation of Electron Donor Materials for Solution-Processed Organic Solar Cells via a Novel Figure of Merit. Adv. Energy Mater. 2017, 7, 1700465. [Google Scholar] [CrossRef]
- Machui, F.; Hösel, M.; Li, N.; Spyropoulos, G.D.; Ameri, T.; Sondergaard, R.R.; Jorgensen, M.; Scheel, A.; Gaiser, D.; Kreul, K.; et al. Cost analysis of roll-to-roll fabricated ITO free single and tandem organic solar modules based on data from manufacture. Energy Environ. Sci. 2014, 7, 2792–2802. [Google Scholar] [CrossRef] [Green Version]
- Po, R.; Bianchi, G.; Carbonera, C.; Pellegrino, A. “All That Glisters Is Not Gold”: An Analysis of the Synthetic Complexity of Efficient Polymer Donors for Polymer Solar Cells. Macromolecules 2015, 48, 453–461. [Google Scholar] [CrossRef]
- Guo, J.; Wu, Y.; Sun, R.; Wang, W.; Guo, J.; Wu, Q.; Tang, X.; Sun, C.; Luo, Z.; Chang, K.; et al. Suppressing photo-oxidation of non-fullerene acceptors and their blends in organic solar cells by exploring material design and employing friendly stabilizers. J. Mater. Chem. A 2019, 7, 25088–25101. [Google Scholar]
- Strohm, S.; Machui, F.; Langner, S.; Kubis, P.; Gasperini, N.; Salvador, M.; McCulloch, I.; Egelhaaf, H.-J.; Brabec, C.J. P3HT: Non-fullerene acceptor based large area, semi-transparent PV modules with power conversion efficiencies of 5%, processed by industrially scalable methods. Energy Environ. Sci. 2018, 11, 2225–2234. [Google Scholar] [CrossRef]
- Yin, A.; Zhang, D.; Cheung, S.H.; So, S.K.; Fu, Z.; Ying, L.; Huang, F.; Zhou, H.; Zhang, Y. On the understanding of energetic disorder, charge recombination and voltage losses in all-polymer solar cells. J. Mater. Chem. C 2018, 6, 7855–7863. [Google Scholar] [CrossRef]
- Cui, Y.; Yao, H.; Zhang, J.; Zhang, T.; Wang, Y.; Hong, L.; Xian, K.; Xu, B.; Zhang, S.; Peng, J.; et al. Over 16% efficiency organic photovoltaic cells enabled by a chlorinated acceptor with increased open-circuit voltages. Nat. Comm. 2019, 10, 2515. [Google Scholar] [CrossRef] [PubMed]
- Whang, Z.; Liu, X.; Jiang, H.; Zhou, X.; Zhang, L.; Pan, F.; Qiao, X.; Ma, D.; Ma, W.; Ding, L.; et al. Organic Solar Cells Based on High Hole Mobility Conjugated Polymer and Nonfullerene Acceptor with Comparable Bandgaps and Suitable Energy Level Offsets Showing Significant Suppression of Jsc–Voc Trade-Off. Solar RRL 2019, 3, 1900079. [Google Scholar] [CrossRef]
- Li, S.; Ye, L.; Zhao, W.; Liu, X.; Zhu, J.; Ade, H.; Hou, J. Design of a New Small-Molecule Electron Acceptor Enables Efficient Polymer Solar Cells with High Fill Factor. Adv. Mater. 2017, 29, 1704051. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, H.; Li, B.; Yuan, J.; Xu, X.; Wu, J.; Zhou, K.; Guo, X.; Zhang, M.; Ma, W. A blade-coated highly efficient thick active layer for non-fullerene organic solar cells. J. Mater. Chem. A 2019, 7, 22265–22273. [Google Scholar] [CrossRef]
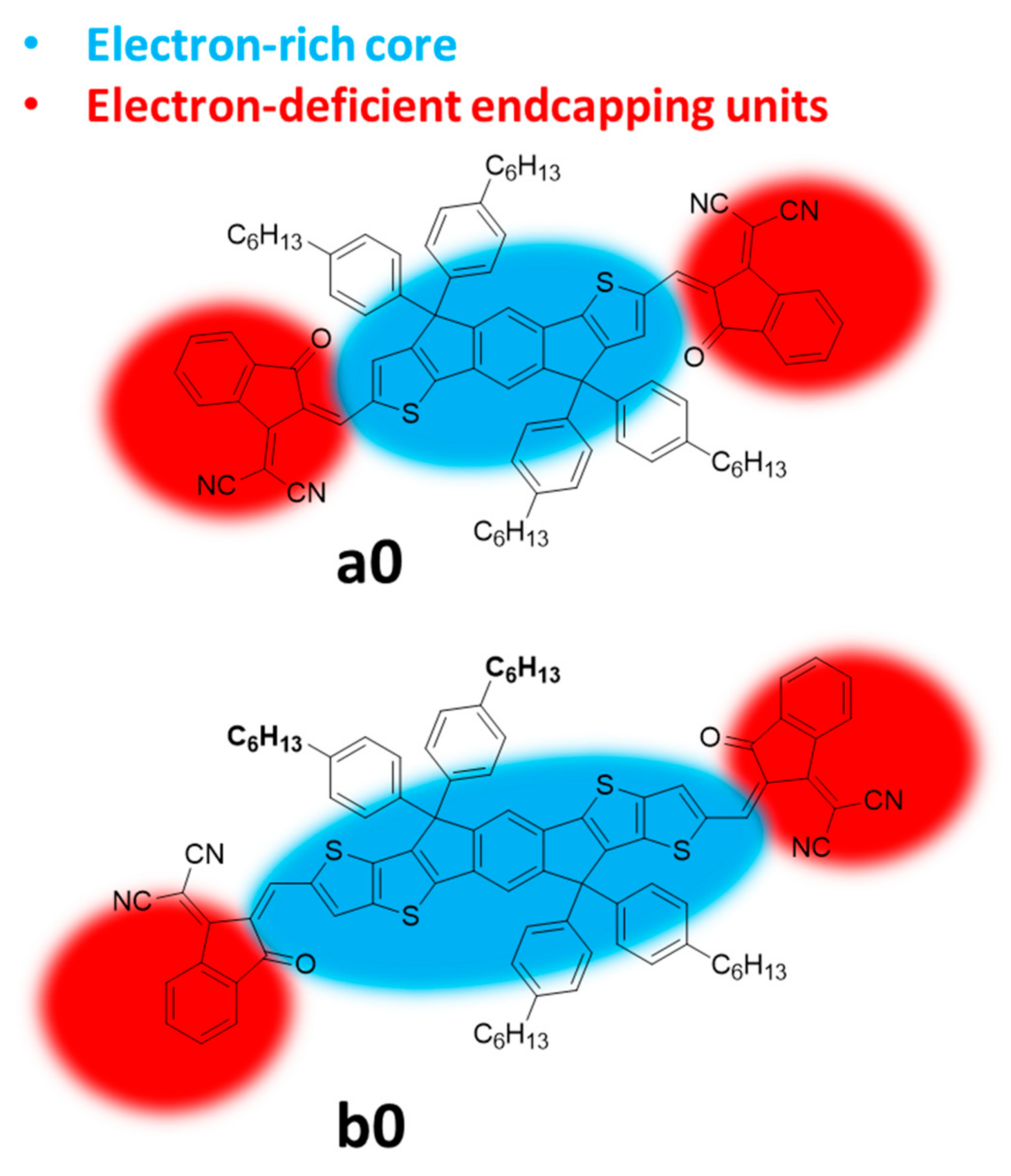
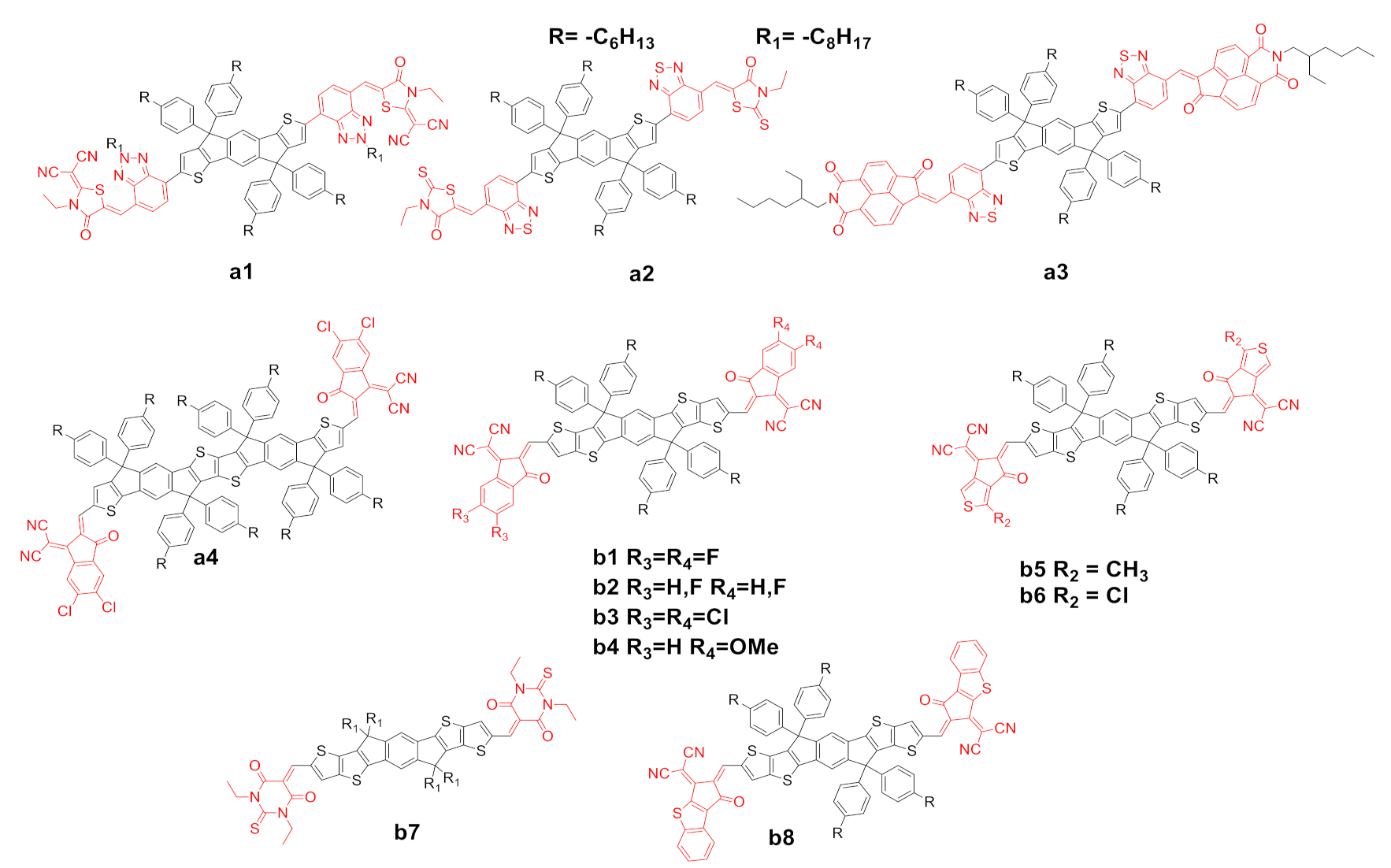
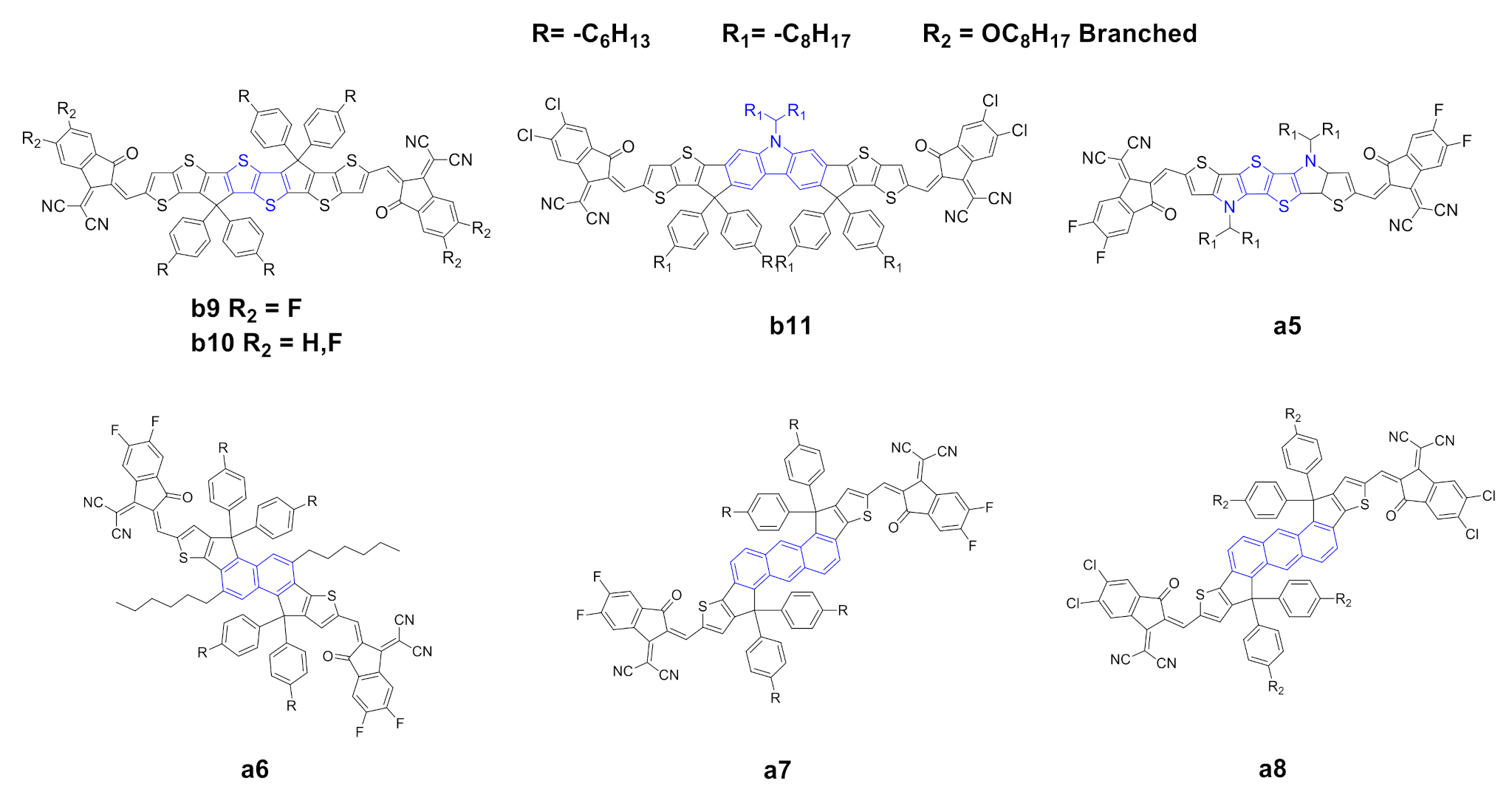
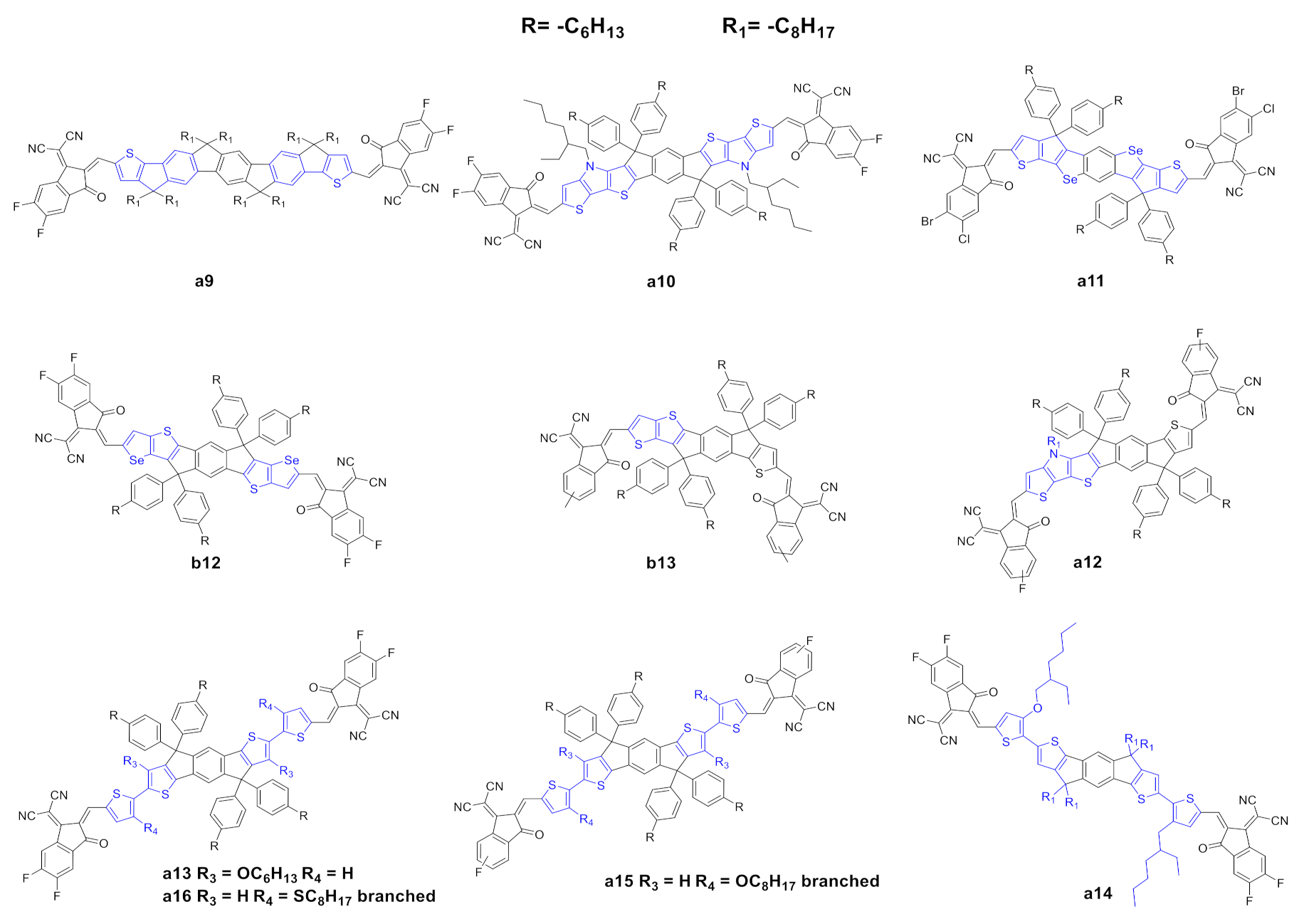
| Entry | Compound | Td (°C) a | (nm) b | (nm) c | (eV) d | ε (λmax) (M−1·L−1) e | HOMO (eV) f | LUMO (eV) f | μSCLC (cm2·V−1·s−1) g | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | a0 | 664 | 716 | 1.62 | 2.4 × 105 | −5.69 | −3.91 | 1.1 × 10−3 | [30] | |
| 2 | a1 | 396 | 613 | 636 | 1.76 | 1.14 × 105 | −5.49 | −3.57 | [43] | |
| 3 | a2 | 662 | 688 | 1.68 | −5.31 | −3.70 | [44] | |||
| 4 | a3 | 354 | 628 | 660 | 1.63 | 8.8 × 104 | −5.38 | −3.68 | 1.44 × 10−4 | [45] |
| 5 | a4 | 362 | 715 | 754 | 1.43 | 2.1 × 105 | −5.35 | −3.91 | 2.36 × 10−3 | [46] |
| 6 | b0 | 345 | 664 | 702 | 1.59 | 1.3 × 105 | −5.48 | −3.83 | 3.0 × 10−4 | [1] |
| 7 | b1 | 717 | - | 1.16 × 105 | −5.66 | −4.14 | 5.05 ×10−4 | [30] | ||
| 8 | b2 | 677 | 720 | 1.57 | −5.67 | −4.02 | 3.26 × 10−4 | [47] | ||
| 9 | b3 | 746 | 1.48 | 1.4 × 105 | −5.75 | −4.09 | [48] | |||
| 10 | b4 | 671 | 695 | 1.63 | 1.9 × 105 | −5.61 | −3.92 | [49] | ||
| 11 | b5 | 674 | 718 | 1.58 | 2.1 × 105 | −5.57 | −3.92 | 8.4 × 10−4 | [50] | |
| 12 | b6 | 709 | 746 | 1.58 | 2.3 × 105 | −5.58 | −4.01 | 7.8 × 10−4 | [51] | |
| 13 | b7 | 621 | 654 | 1.75 | 2.6 × 105 | −5.81 | −3.97 | 1.8 × 10−4 | [52] | |
| 14 | b8 | 433 | 664 | 691 | 1.59 | 1.5 × 105 | −5.49 | −3.90 | [53] |
| Entry | Device | Voc (V) i | Jsc (mA·cm−2) j | FF (%) k | PCE (%) l | Ref. |
|---|---|---|---|---|---|---|
| 1 | PDBT-T1:a0 | 0.89 | 14.61 | 63.0 | 8.19 | [30] |
| 2 | J61: a1 | 1.15 | 10.84 | 66.17 | 8.25 | [43] |
| 3 | PTB7-Th: a2 | 1.04 | 13.57 | 62.64 | 8.82 | [44] |
| 4 | PBDB-T: a3 | 1.04 | 17.34 | 60.0 | 10.08 | [45] |
| 5 | PBDB-T: a4 | 0.85 | 18.9 | 66.6 | 10.7 | [46] |
| 6 | PTB7-TH: b0 | 0.81 | 14.21 | 59.1 | 6.80 | [1] |
| 7 | PBDB-T-SF: b1 | 0.88 | 20.88 | 71.3 | 13.1 | [29] |
| 8 | PBT1-C: b2 | 0.90 | 16.50 | 70.8 | 10.54 | [47] |
| 9 | PBDB-T-2F: b3 | 0.79 | 22.67 | 75.2 | 13.45 | [48] |
| 10 | PBDB-T: b4 | 0.93 | 18.11 | 71.52 | 12.07 | [49] |
| 11 | J71: b5 | 0.92 | 18.41 | 74.2 | 12.5 | [50] |
| 12 | PM6: b6 | 0.91 | 20.1 | 74.1 | 13.6 | [51] |
| 13 | PBDB-T: b7 | 0.98 | 15.80 | 69.0 | 10.08 | [52] |
| 14 | PBDB-T: b8 | 0.94 | 19.90 | 64.51 | 12.07 | [53] |
| Entry | Compound | Td (°C) a | (nm) b | (nm) c | (eV) d | ε (max) (M−1·L−1) e | HOMO (eV) | LUMO (eV) | μSCLC (cm2·V−1·s−1) f | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | a0 | 664 | 716 | 1.62 | 2.4 × 105 | −5.69 | −3.91 | 1.1 × 10−3 | [30] | |
| 2 | a5 | 1.32 | −5.52 | −4.11 | 3.94 × 10−4 | [57] | ||||
| 3 | a6 | 384 | 696 | 730 | 1.55 | 1.8 × 105 | −5.41 | −3.78 | 1 × 10−3 | [58] |
| 4 | a7 | 718 | 778 | 1.68 | −5.69 | −4.01 | [59] | |||
| 5 | a8 | 691 | 709 | 1.60 | 2.2 × 105 | −5.71 | −3.89 | [60] | ||
| 6 | b0 | 345 | 664 | 702 | 1.59 | 1.3 × 105 | −5.48 | −3.83 | 3.0 × 10−4 | [1] |
| 7 | b9 | 331 | 788 | 862 | 1.27 | 2.3 × 105 | −5.43 | −4.00 | 1.5 × 10−3 | [54] |
| 8 | b10 | 328 | 782 | 836 | 1.32 | 2.0 × 105 | −5.36 | −3.92 | 1.2 × 10−3 | [55] |
| 9 | b11 | 338 | 690 | 753 | 1.47 | 2.3 × 105 | −5.72 | −4.03 | [56] |
| Entry | Device | Voc (V) i | Jsc (mA·cm−2) j | FF (%) k | PCE (%) l | Ref. |
|---|---|---|---|---|---|---|
| 1 | PDBT-T1: a0 | 0.89 | 14.6 | 63.0 | 8.2 | [30] |
| 2 | PBDB-T: a5 | 0.78 | 23.2 | 73.0 | 13.2 | [57] |
| 3 | FTAZ: a6 | 0.90 | 19.7 | 69.3 | 12.3 | [58] |
| 4 | PBDB-TF: a7 | 0.93 | 19.0 | 73.9 | 13.1 | [59] |
| 5 | PBDB-TF: a8 | 0.90 | 19.5 | 75.5 | 13.3 | [60] |
| 6 | PTB7-TH: b0 | 0.81 | 14.2 | 59.1 | 6.8 | [1] |
| 7 | PTB7-Th: b9 | 0.64 | 25.1 | 67.6 | 10.9 | [54] |
| 8 | PTB7-Th: b10 | 0.74 | 24.0 | 67.1 | 12.0 | [55] |
| 9 | PM6: b11 | 0.92 | 22.6 | 74.0 | 15.4 | [56] |
| Entry | Compound | Td (°C) a | (nm) b | (nm) c | (eV) d | ε (λmax) (M−1·L−1) e | HOMO (eV) | LUMO (eV) | μSCLC (cm2·V−1·s−1) f | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | a0 | 664 | 716 | 1.62 | 2.4 × 105 | −5.69 | −3.91 | 1.1 × 10−3 | [30] | |
| 2 | a9 | 678 | 704 | 1.61 | −5.71 | −3.96 | 3.53 × 10−4 | [61] | ||
| 3 | a10 | 377 | 769 | 821 | 1.39 | 3.51 × 105 | −5.42 | −3.95 | [62] | |
| 4 | a11 | 350 | 736 | 793 | 1.39 | −5.64 | −3.95 | [63] | ||
| 5 | a12 | 350 | 739 | 775 | 1.44 | −5.51 | −3.96 | [66] | ||
| 6 | a13 | 723 | 765 | 1.44 | 9.9 × 104 | −5.54 | −3.94 | [67] | ||
| 7 | a14 | 790 | 850 | 1.28 | 1.32 × 105 | −5.44 | −4.15 | [68] | ||
| 8 | a15 | 345 | 794 | 839 | 1.31 | −5.34 | −4.06 | [69] | ||
| 9 | a16 | 334 | 751 | 831 | 1.30 | −5.54 | −4.05 | 2.65 × 10−4 | [70] | |
| 10 | b0 | 345 | 664 | 702 | 1.59 | 1.3 × 105 | −5.48 | −3.83 | 3.0 × 10−4 | [1] |
| 11 | b12 | 698 | 752 | 1.44 | 2.14 × 105 | −5.52 | −3.90 | [64] | ||
| 12 | b13 | 300 | 664 | 696 | 1.65 | 2.00 × 105 | −5.60 | −3.87 | 9.64 × 10−4 | [65] |
| Entry | Device | Voc (V) i | Jsc (mA·cm−2) j | FF (%) k | PCE (%) l | Ref. |
|---|---|---|---|---|---|---|
| 1 | PDBT-T1: a0 | 0.89 | 14.61 | 63.0 | 8.19 | [30] |
| 2 | PBDB-T2F: a9 | 0.980 | 17.60 | 76.0 | 13.1 | [61] |
| 3 | PBDB-T: a10 | 0.852 | 21.9 | 69.8 | 13.1 | [62] |
| 4 | PM7: a11 | 0.830 | 22.91 | 76.5 | 14.54 | [63] |
| 5 | PBDB-T: a12 | 0.860 | 22.4 | 72.4 | 14.0 | [66] |
| 7 | PBDB-T: a13 | 0.850 | 20.87 | 72.0 | 12.79 | [67] |
| 8 | PTB7-Th: a14 | 0.74 | 26.30 | 67.0 | 13.1 | [68] |
| 9 | PTB7-Th: a15 | 0.817 | 21.90 | 65.0 | 12.1 | [69] |
| 10 | PTB7-Th: a16 | 0.750 | 25.3 | 69.3 | 13.2 | [70] |
| 11 | PTB7-TH: b0 | 0.81 | 14.21 | 59.1 | 6.80 | [1] |
| 12 | PBDB-T-2F: b12 | 0.846 | 20.21 | 75.2 | 13.05 | [64] |
| 13 | PBDB-T: b13 | 0.910 | 16.02 | 76.8 | 11.2 | [65] |
| Entry | NFA | NSS | SC | Cost (EUR/g) |
|---|---|---|---|---|
| 1 | b1 | 12 | 72 | 58 |
| 2 | b0 | 9 | 68 | 113 |
| 3 | a2 | 15 | 97 | 105 |
| 4 | a15 | 15 | 91 | 115 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forti, G.; Nitti, A.; Osw, P.; Bianchi, G.; Po, R.; Pasini, D. Recent Advances in Non-Fullerene Acceptors of the IDIC/ITIC Families for Bulk-Heterojunction Organic Solar Cells. Int. J. Mol. Sci. 2020, 21, 8085. https://doi.org/10.3390/ijms21218085
Forti G, Nitti A, Osw P, Bianchi G, Po R, Pasini D. Recent Advances in Non-Fullerene Acceptors of the IDIC/ITIC Families for Bulk-Heterojunction Organic Solar Cells. International Journal of Molecular Sciences. 2020; 21(21):8085. https://doi.org/10.3390/ijms21218085
Chicago/Turabian StyleForti, Giacomo, Andrea Nitti, Peshawa Osw, Gabriele Bianchi, Riccardo Po, and Dario Pasini. 2020. "Recent Advances in Non-Fullerene Acceptors of the IDIC/ITIC Families for Bulk-Heterojunction Organic Solar Cells" International Journal of Molecular Sciences 21, no. 21: 8085. https://doi.org/10.3390/ijms21218085
APA StyleForti, G., Nitti, A., Osw, P., Bianchi, G., Po, R., & Pasini, D. (2020). Recent Advances in Non-Fullerene Acceptors of the IDIC/ITIC Families for Bulk-Heterojunction Organic Solar Cells. International Journal of Molecular Sciences, 21(21), 8085. https://doi.org/10.3390/ijms21218085







