The Extent of Intracellular Accumulation of Bilirubin Determines Its Anti- or Pro-Oxidant Effect
Abstract
1. Introduction
2. Results
2.1. UCB Cytotoxicity
2.2. The Effect of UCB Exposure to Intracellular UCB Concentration
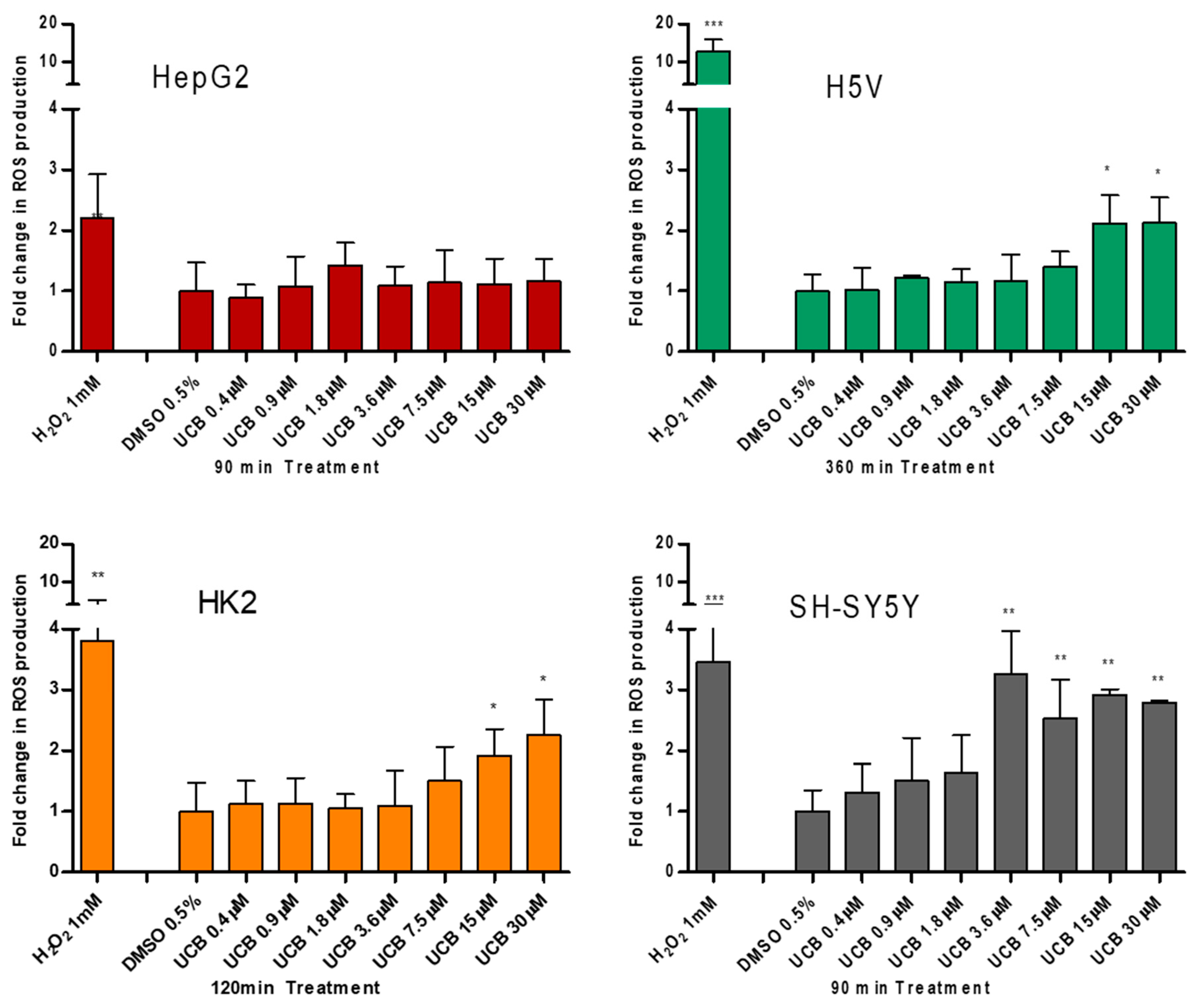
2.3. The Effect of UCB Exposure on Intracellular ROS Production
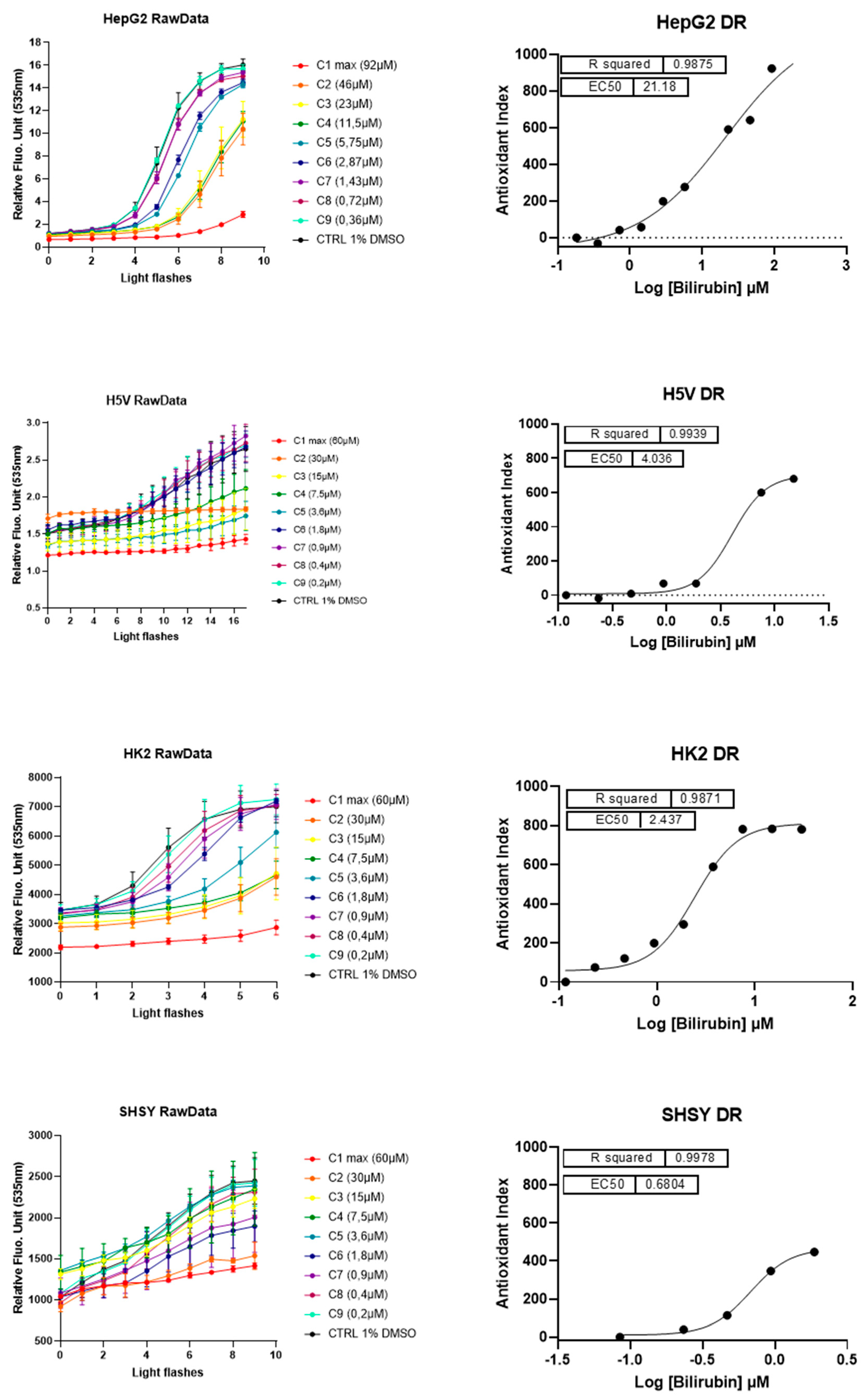
2.4. The Antioxidant Effect of UCB on Live Cells Measured by LUCS Technology AOP1
2.5. The Effect of UCB on Total GSH and SOD Activity
3. Discussion
4. Materials and Methods
4.1. Cell Cultures
4.2. Treatments
4.3. Quantification of Intracellular UCB Concentration
4.4. Cytotoxicity Test (PI) and Metabolic Activity Test (MTT)
4.5. Determination of Intracellular ROS Production
4.6. Anti Oxidant Power 1 (AOP1)
4.7. GSH Determinations
4.8. SOD Activity
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| iUCB | Intracellular unconjugated bilirubin concentrations |
| UCB | Unconjugated bilirubin |
| EC50 | Half maximal effective concentration |
| ROS | Reactive oxygen species |
| HMOX | Heme oxygenase |
| BLVR | Biliverdin reductase |
| Bf | Free bilirubin |
| CYPs | Cytochrome P-450-oxygenases |
| GSTs | Glutathione S-transferases |
| UGTs | UDP-Glucuronosyl-transferase |
| ABC | ATP Binding Cassette |
| ABCC1 | ATP Binding Cassette Subfamily C Member 1 |
| ABCC2 | ATP Binding Cassette Subfamily C Member 2 |
| ABCC3 | ATP Binding Cassette Subfamily C Member 3 |
| ABCB1 | ATP Binding Cassette Subfamily B Member 1 |
| MDR1 | Multidrug resistance protein 1 |
| MRP3 | Multidrug resistance-associated Protein 3 |
| PGP1 | P-glycoprotein 1 |
| BBB | Blood–brain barrier |
| GS | Gilbert syndrome |
| CVD | Cardiovascular diseases |
| PI | Propidium iodide |
| DMSO | Dimethyl sulfoxide |
| MTT | 3(4,5-dimethylthiazolyl-2)-2,5 diphenyl tetrazolium |
| H2DCFDA | 2′7′-dichlorodihydrofluorescein diacetate |
| DCF | 2′,7-dichlorofluorescein |
| H2O2 | Hydrogen peroxide |
| BSA | Bovine serum albumin |
| LUCs | Light-Up Cell System |
| AOP | Antioxidant powerl |
| RFU | Relative fluorescence units |
| SOD | Superoxide dismutase |
| UGT1A1 | UDP Glucuronosyltransferase family 1 member A1 |
| GSH | Reduced glutathione |
| NBT | Nitro blue tetrazolium |
| NADH | β-Nicotinamide adenine dinucleotide reduced |
| PMS | Phenazine methosulfate |
| CuZnSOD | Copper- and zinc-containing superoxide dismutase |
| SOD1 | Superoxide dismutase [Cu-Zn] |
References
- Ahlfors, C.E.; Wennberg, R.P.; Ostrow, J.D.; Tiribelli, C. Unbound (free) bilirubin: Improving the paradigm for evaluating neonatal jaundice. Clin. Chem. 2009, 55, 1288–1299. [Google Scholar] [CrossRef] [PubMed]
- Zucker, S.D.; Goessling, W.; Hoppin, A.G. Unconjugated bilirubin exhibits spontaneous diffusion through model lipid bilayers and native hepatocyte membranes. J. Biol. Chem. 1999, 274, 10852–10862. [Google Scholar] [CrossRef] [PubMed]
- Zelenka, J.; Lenícek, M.; Muchová, L.; Jirsa, M.; Kudla, M.; Balaz, P.; Zadinová, M.; Ostrow, J.D.; Wong, R.J.; Vítek, L. Highly sensitive method for quantitative determination of bilirubin in biological fluids and tissues. J. Chromatogr. B Analyt. Technol. Biomed. Life. Sci. 2008, 867, 37–42. [Google Scholar] [CrossRef]
- Kapitulnik, J.; Gonzalez, F.J. Marked endogenous activation of the CYP1A1 and CYP1A2 genes in the congenitally jaundiced Gunn rat. Mol. Pharmacol. 1993, 43, 722–725. [Google Scholar]
- De Matteis, F.; Lord, G.A.; Kee Lim, C.; Pons, N. Bilirubin degradation by uncoupled cytochrome P450. Comparison with a chemical oxidation system and characterization of the products by high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. RCM 2006, 20, 1209–1217. [Google Scholar] [CrossRef]
- Coles, B.F.; Kadlubar, F.F. Human Alpha Class Glutathione S-Transferases: Genetic Polymorphism, Expression, and Susceptibility to Disease. In Methods in Enzymology; Sies, H., Packer, L., Eds.; Gluthione Transferases and Gamma-Glutamyl Transpeptidases; Academic Press: Cambridge, MA, USA, 2005; Volume 401, pp. 9–42. [Google Scholar]
- Nakamura, A.; Nakajima, M.; Yamanaka, H.; Fujiwara, R.; Yokoi, T. Expression of UGT1A and UGT2B mRNA in human normal tissues and various cell lines. Drug Metab. Dispos. Biol. Fate Chem. 2008, 36, 1461–1464. [Google Scholar] [CrossRef]
- Schinkel, A.H. The physiological function of drug-transporting P-glycoproteins. Semin. Cancer Biol. 1997, 8, 161–170. [Google Scholar] [CrossRef]
- Falcão, A.S.; Bellarosa, C.; Fernandes, A.; Brito, M.A.; Silva, R.F.M.; Tiribelli, C.; Brites, D. Role of multidrug resistance-associated protein 1 expression in the in vitro susceptibility of rat nerve cell to unconjugated bilirubin. Neuroscience 2007, 144, 878–888. [Google Scholar] [CrossRef]
- Corich, L.; Aranda, A.; Carrassa, L.; Bellarosa, C.; Ostrow, J.D.; Tiribelli, C. The cytotoxic effect of unconjugated bilirubin in human neuroblastoma SH-SY5Y cells is modulated by the expression level of MRP1 but not MDR1. Biochem. J. 2009, 417, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Rigato, I.; Pascolo, L.; Fernetti, C.; Ostrow, J.D.; Tiribelli, C. The human multidrug-resistance-associated protein MRP1 mediates ATP-dependent transport of unconjugated bilirubin. Biochem. J. 2004, 383, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Calligaris, S.; Cekic, D.; Roca-Burgos, L.; Gerin, F.; Mazzone, G.; Ostrow, J.D.; Tiribelli, C. Multidrug resistance associated protein 1 protects against bilirubin-induced cytotoxicity. FEBS Lett. 2006, 580, 1355–1359. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, G.L.; Kool, M.; de Haas, M.; de Vree, J.M.L.; Pijnenborg, A.C.L.M.; Bosman, D.K.; Elferink, R.P.J.O.; van der Valk, P.; Borst, P.; Scheper, R.J. Tissue distribution and induction of human multidrug resistant protein 3. Lab. Investig. J. Tech. Methods Pathol. 2002, 82, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Bellarosa, C.; Bortolussi, G.; Tiribelli, C. The role of ABC transporters in protecting cells from bilirubin toxicity. Curr. Pharm. Des. 2009, 15, 2884–2892. [Google Scholar] [CrossRef]
- Watchko, J.F.; Tiribelli, C. Bilirubin-induced neurologic damage. N. Engl. J. Med. 2014, 370, 979. [Google Scholar] [CrossRef] [PubMed]
- Bosma, P.J.; Chowdhury, J.R.; Bakker, C.; Gantla, S.; de Boer, A.; Oostra, B.A.; Lindhout, D.; Tytgat, G.N.; Jansen, P.L.; Oude Elferink, R.P. The genetic basis of the reduced expression of bilirubin UDP-glucuronosyltransferase 1 in Gilbert’s syndrome. N. Engl. J. Med. 1995, 333, 1171–1175. [Google Scholar] [CrossRef]
- Vítek, L. The role of bilirubin in diabetes, metabolic syndrome, and cardiovascular diseases. Front. Pharmacol. 2012, 3, 55. [Google Scholar] [CrossRef] [PubMed]
- Baranano, D.E.; Rao, M.; Ferris, C.D.; Snyder, S.H. Biliverdin reductase: A major physiologic cytoprotectant. Proc. Natl. Acad. Sci. USA 2002, 99, 16093–16098. [Google Scholar] [CrossRef]
- Gopinathan, V.; Miller, N.J.; Milner, A.D.; Rice-Evans, C.A. Bilirubin and ascorbate antioxidant activity in neonatal plasma. FEBS Lett. 1994, 349, 197–200. [Google Scholar] [CrossRef]
- Farrera, J.A.; Jaumà, A.; Ribó, J.M.; Peiré, M.A.; Parellada, P.P.; Roques-Choua, S.; Bienvenue, E.; Seta, P. The antioxidant role of bile pigments evaluated by chemical tests. Bioorg. Med. Chem. 1994, 2, 181–185. [Google Scholar] [CrossRef]
- Doré, S.; Snyder, S.H. Neuroprotective action of bilirubin against oxidative stress in primary hippocampal cultures. Ann. N. Y. Acad. Sci. 1999, 890, 167–172. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, B.; Wang, X.; Luo, L.; Li, P.; Paty, D.W.; Cynader, M.S. Bilirubin as a potent antioxidant suppresses experimental autoimmune encephalomyelitis: Implications for the role of oxidative stress in the development of multiple sclerosis. J. Neuroimmunol. 2003, 139, 27–35. [Google Scholar] [CrossRef]
- Gazzin, S.; Strazielle, N.; Tiribelli, C.; Ghersi-Egea, J.-F. Transport and Metabolism at Blood–Brain Interfaces and in Neural Cells: Relevance to Bilirubin-Induced Encephalopathy. Front. Pharmacol. 2012, 3. [Google Scholar] [CrossRef]
- Derick, S.; Gironde, C.; Perio, P.; Reybier, K.; Nepveu, F.; Jauneau, A.; Furger, C. LUCS (Light-Up Cell System), a universal high throughput assay for homeostasis evaluation in live cells. Sci. Rep. 2017, 7, 18069. [Google Scholar] [CrossRef]
- Gironde, C.; Rigal, M.; Dufour, C.; Furger, C. AOP1, a New Live Cell Assay for the Direct and Quantitative Measure of Intracellular Antioxidant Effects. Antioxid. Basel Switz. 2020, 9, 471. [Google Scholar] [CrossRef]
- Vitek, L.; Bellarosa, C.; Tiribelli, C. Induction of Mild Hyperbilirubinemia: Hype or Real Therapeutic Opportunity? Clin. Pharmacol. Ther. 2018. [Google Scholar] [CrossRef]
- Mirabelli, P.; Coppola, L.; Salvatore, M. Cancer Cell Lines Are Useful Model Systems for Medical Research. Cancers 2019, 11, 1098. [Google Scholar] [CrossRef]
- Keppler, D. The roles of MRP2, MRP3, OATP1B1, and OATP1B3 in conjugated hyperbilirubinemia. Drug Metab. Dispos. Biol. Fate Chem. 2014, 42, 561–565. [Google Scholar] [CrossRef]
- Iusuf, D.; van de Steeg, E.; Schinkel, A.H. Hepatocyte hopping of OATP1B substrates contributes to efficient hepatic detoxification. Clin. Pharmacol. Ther. 2012, 92, 559–562. [Google Scholar] [CrossRef]
- Abu-Bakar, A.; Arthur, D.M.; Wikman, A.S.; Rahnasto, M.; Juvonen, R.O.; Vepsäläinen, J.; Raunio, H.; Ng, J.C.; Lang, M.A. Metabolism of bilirubin by human cytochrome P450 2A6. Toxicol. Appl. Pharmacol. 2012, 261, 50–58. [Google Scholar] [CrossRef]
- Brites, D. Bilirubin injury to neurons and glial cells: New players, novel targets, and newer insights. Semin. Perinatol. 2011, 35, 114–120. [Google Scholar] [CrossRef]
- Jenkinson, S.E.; Chung, G.W.; van Loon, E.; Bakar, N.S.; Dalzell, A.M.; Brown, C.D.A. The limitations of renal epithelial cell line HK-2 as a model of drug transporter expression and function in the proximal tubule. Pflugers Arch. 2012, 464, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Ngai, K.-C.; Yeung, C.-Y.; Leung, C.-S. Difference in susceptibilities of different cell lines to bilirubin damage. J. Paediatr. Child Health 2000, 36, 51–55. [Google Scholar] [CrossRef]
- Ollinger, R.; Bilban, M.; Erat, A.; Froio, A.; McDaid, J.; Tyagi, S.; Csizmadia, E.; Graça-Souza, A.V.; Liloia, A.; Soares, M.P.; et al. Bilirubin: A natural inhibitor of vascular smooth muscle cell proliferation. Circulation 2005, 112, 1030–1039. [Google Scholar] [CrossRef]
- Chan, G.K.Y.; Kleinheinz, T.L.; Peterson, D.; Moffat, J.G. A Simple High-Content Cell Cycle Assay Reveals Frequent Discrepancies between Cell Number and ATP and MTS Proliferation Assays. PLoS ONE 2013, 8, e63583. [Google Scholar] [CrossRef] [PubMed]
- Tell, G.; Gustincich, S. Redox state, oxidative stress, and molecular mechanisms of protective and toxic effects of bilirubin on cells. Curr. Pharm. Des. 2009, 15, 2908–2914. [Google Scholar] [CrossRef]
- Brito, M.A.; Lima, S.; Fernandes, A.; Falcão, A.S.; Silva, R.F.M.; Butterfield, D.A.; Brites, D. Bilirubin injury to neurons: Contribution of oxidative stress and rescue by glycoursodeoxycholic acid. Neurotoxicology 2008, 29, 259–269. [Google Scholar] [CrossRef]
- Kumar, S.; Guha, M.; Choubey, V.; Maity, P.; Srivastava, K.; Puri, S.K.; Bandyopadhyay, U. Bilirubin inhibits Plasmodium falciparum growth through the generation of reactive oxygen species. Free Radic. Biol. Med. 2008, 44, 602–613. [Google Scholar] [CrossRef]
- Oakes, G.H.; Bend, J.R. Early steps in bilirubin-mediated apoptosis in murine hepatoma (Hepa 1c1c7) cells are characterized by aryl hydrocarbon receptor-independent oxidative stress and activation of the mitochondrial pathway. J. Biochem. Mol. Toxicol. 2005, 19, 244–255. [Google Scholar] [CrossRef]
- Hopkins, P.N.; Wu, L.L.; Hunt, S.C.; James, B.C.; Vincent, G.M.; Williams, R.R. Higher Serum Bilirubin Is Associated With Decreased Risk for Early Familial Coronary Artery Disease. Arterioscler. Thromb. Vasc. Biol. 1996, 16, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.W.; Lee, E.S.; Kim, S.; Na, K.Y.; Chae, D.W.; Kim, S.; Chin, H.J. Bilirubin attenuates the renal tubular injury by inhibition of oxidative stress and apoptosis. BMC Nephrol. 2013, 14, 105. [Google Scholar] [CrossRef] [PubMed]
- Fujii, M.; Inoguchi, T.; Sasaki, S.; Maeda, Y.; Zheng, J.; Kobayashi, K.; Takayanagi, R. Bilirubin and biliverdin protect rodents against diabetic nephropathy by downregulating NAD(P)H oxidase. Kidney Int. 2010, 78, 905–919. [Google Scholar] [CrossRef] [PubMed]
- Marilena, G. New physiological importance of two classic residual products: Carbon monoxide and bilirubin. Biochem. Mol. Med. 1997, 61, 136–142. [Google Scholar] [CrossRef]
- Stocker, R.; Yamamoto, Y.; McDonagh, A.F.; Glazer, A.N.; Ames, B.N. Bilirubin is an antioxidant of possible physiological importance. Science 1987, 235, 1043–1046. [Google Scholar] [CrossRef]
- Stocker, R.; Glazer, A.N.; Ames, B.N. Antioxidant activity of albumin-bound bilirubin. Proc. Natl. Acad. Sci. USA 1987, 84, 5918–5922. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.-W.; Carey, D.; Wu, J.; Sugiyama, H. The cytoprotective effects of bilirubin and biliverdin on rat hepatocytes and human erythrocytes and the impact of albumin. Biochem. Cell Biol. 1991, 69, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Giraudi, P.J.; Bellarosa, C.; Coda-Zabetta, C.D.; Peruzzo, P.; Tiribelli, C. Functional induction of the cystine-glutamate exchanger system Xc(-) activity in SH-SY5Y cells by unconjugated bilirubin. PLoS ONE 2011, 6, e29078. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef]
- McDonagh, A.F.; Assisi, F. The ready isomerization of bilirubin IX- in aqueous solution. Biochem. J. 1972, 129, 797–800. [Google Scholar] [CrossRef]
- Jašprová, J.; Dvořák, A.; Vecka, M.; Leníček, M.; Lacina, O.; Valášková, P.; Zapadlo, M.; Plavka, R.; Klán, P.; Vítek, L. A novel accurate LC-MS/MS method for quantitative determination of Z-lumirubin. Sci. Rep. 2020, 10, 4411. [Google Scholar] [CrossRef]
- Nieminen, A.-L.; Gores, G.J.; Bond, J.M.; Imberti, R.; Herman, B.; Lemasters, J.J. A novel cytotoxicity screening assay using a multiwell fluorescence scanner. Toxicol. Appl. Pharmacol. 1992, 115, 147–155. [Google Scholar] [CrossRef]
- Dengler, W.A.; Schulte, J.; Berger, D.P.; Mertelsmann, R.; Fiebig, H.H. Development of a propidium iodide fluorescence assay for proliferation and cytotoxicity assays. Anticancer Drugs 1995, 6, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Ewing, J.F.; Janero, D.R. Microplate superoxide dismutase assay employing a nonenzymatic superoxide generator. Anal. Biochem. 1995, 232, 243–248. [Google Scholar] [CrossRef]



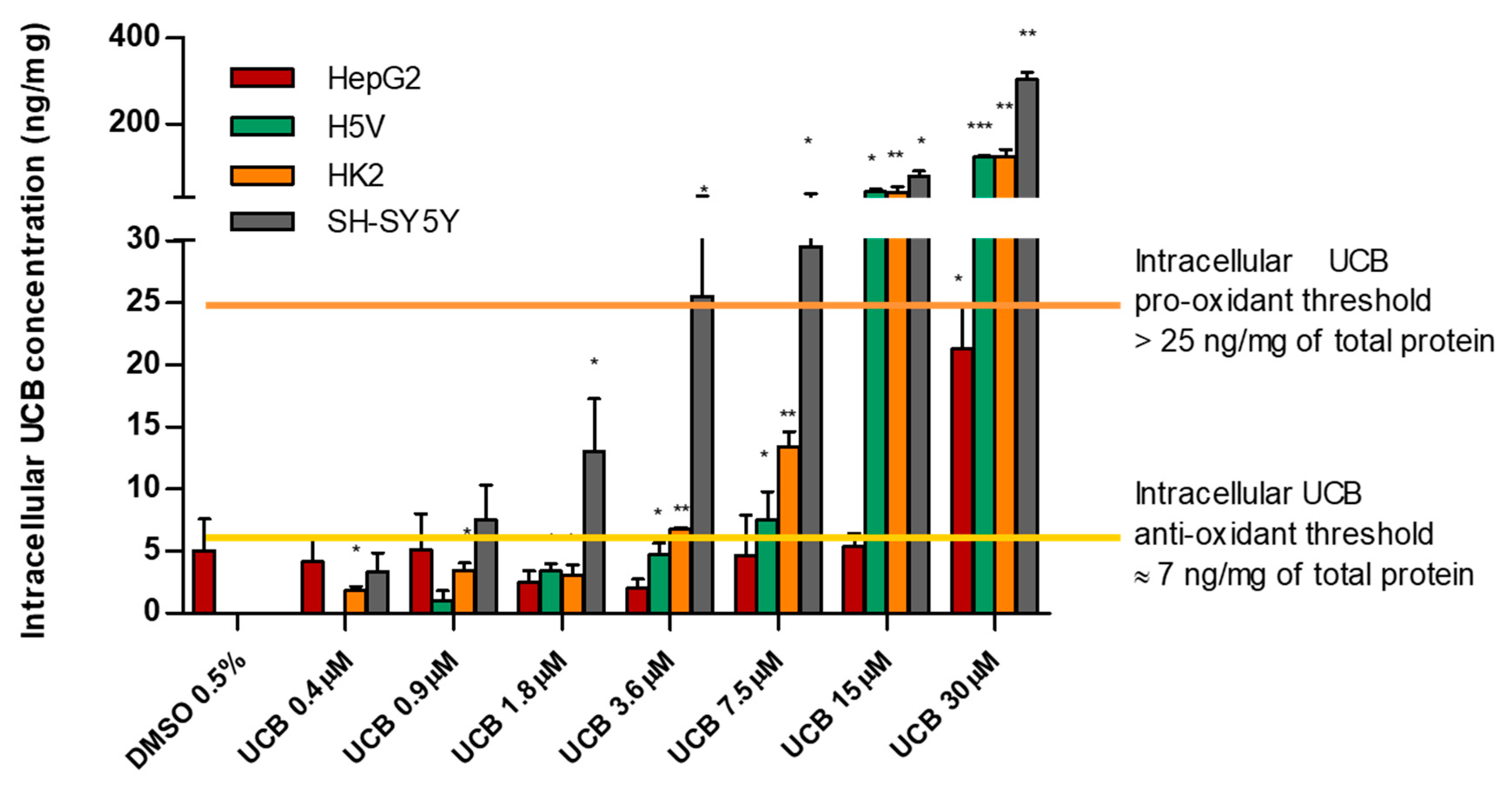
| HepG2 | H5V | HK2 | SH-SY5Y | |||||
|---|---|---|---|---|---|---|---|---|
| Treatment | Mean ± SD (ng/mg) | p-Value (vs. Ctrl) | Mean ± SD (ng/mg) | p-Value (vs. Ctrl) | Mean ± SD (ng/mg) | p-Value (vs. Ctrl) | Mean ± SD (ng/mg) | p-Value (vs. Ctrl) |
| Control | 5.0 ± 1.8 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | ||||
| UCB 0.4 µM | 4.1 ± 1.5 | 0.735 | 0.0 ± 0.0 | 1.8 ± 0.2 | 0.014 | 3.34 ± 1.06 | 0.503 | |
| UCB 0.9 µM | 5.1 ± 2.0 | 0.984 | 1 ± 1.09 | 0.546 | 3.5 ± 0.4 | 0.013 | 7.5 ± 2.0 | 0.309 |
| UCB 1.8 µM | 2.4 ± 0.7 | 0.311 | 3.4 ± 3.51 | 0.415 | 3.0 ± 0.6 | 0.037 | 13.0 ± 3.0 | 0.163 |
| UCB 3.6 µM | 2.0 ± 0.5 | 0.245 | 4.7 ± 0.6 | 0.018 | 6.7 ± 0.1 | 0.000 | 25.5 ± 5.4 | 0.043 |
| UCB 7.5 µM | 4.6 ± 2.3 | 0.900 | 7.5 ± 1.6 | 0.042 | 13.4 ± 0.9 | 0.004 | 29.5 ± 6.3 | 0.043 |
| UCB 15 µM | 5.4 ± 0.7 | 0.874 | 42.4 ± 4.5 | 0.011 | 40.1 ± 9.5 | 0.052 | 79.1 ± 8.4 | 0.011 |
| UCB 30 µM | 21.3 ± 2.4 | 0.033 | 122.3 ± 2.8 | 0.001 | 123.8 ± 11.5 | 0.009 | 303.3 ± 11.2 | 0.001 |
| Cell Lines | Antioxidant EC50 | Cytotoxic Effect | ||
|---|---|---|---|---|
| UCB Treatment | Intracellular UCB Content (ng/mg Total Protein) | UCB Treatment | Intracellular UCB Content (ng/mg Total Protein) | |
| HepG2 | 21.2 µM | between 5.4 and 21.3 ng/mg total protein | ||
| H5V | 4.04 µM | between 4.7 and 7.5 ng/mg total protein | >30 µM | >122.3 ng/mg |
| HK2 | 2.44 µM | between 3 and 6.7 ng/mg total protein | ||
| SH-SY5Y | 0.68 µM | between 3.3 and 7.5 ng/mg total protein | >3.6 µM | >25.5 ng/mg |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bianco, A.; Dvořák, A.; Capková, N.; Gironde, C.; Tiribelli, C.; Furger, C.; Vitek, L.; Bellarosa, C. The Extent of Intracellular Accumulation of Bilirubin Determines Its Anti- or Pro-Oxidant Effect. Int. J. Mol. Sci. 2020, 21, 8101. https://doi.org/10.3390/ijms21218101
Bianco A, Dvořák A, Capková N, Gironde C, Tiribelli C, Furger C, Vitek L, Bellarosa C. The Extent of Intracellular Accumulation of Bilirubin Determines Its Anti- or Pro-Oxidant Effect. International Journal of Molecular Sciences. 2020; 21(21):8101. https://doi.org/10.3390/ijms21218101
Chicago/Turabian StyleBianco, Annalisa, Aleš Dvořák, Nikola Capková, Camille Gironde, Claudio Tiribelli, Christophe Furger, Libor Vitek, and Cristina Bellarosa. 2020. "The Extent of Intracellular Accumulation of Bilirubin Determines Its Anti- or Pro-Oxidant Effect" International Journal of Molecular Sciences 21, no. 21: 8101. https://doi.org/10.3390/ijms21218101
APA StyleBianco, A., Dvořák, A., Capková, N., Gironde, C., Tiribelli, C., Furger, C., Vitek, L., & Bellarosa, C. (2020). The Extent of Intracellular Accumulation of Bilirubin Determines Its Anti- or Pro-Oxidant Effect. International Journal of Molecular Sciences, 21(21), 8101. https://doi.org/10.3390/ijms21218101







