A Potential Involvement of Anandamide in the Modulation of HO/NOS Systems: Women, Menopause, and “Medical Cannabinoids”
Abstract
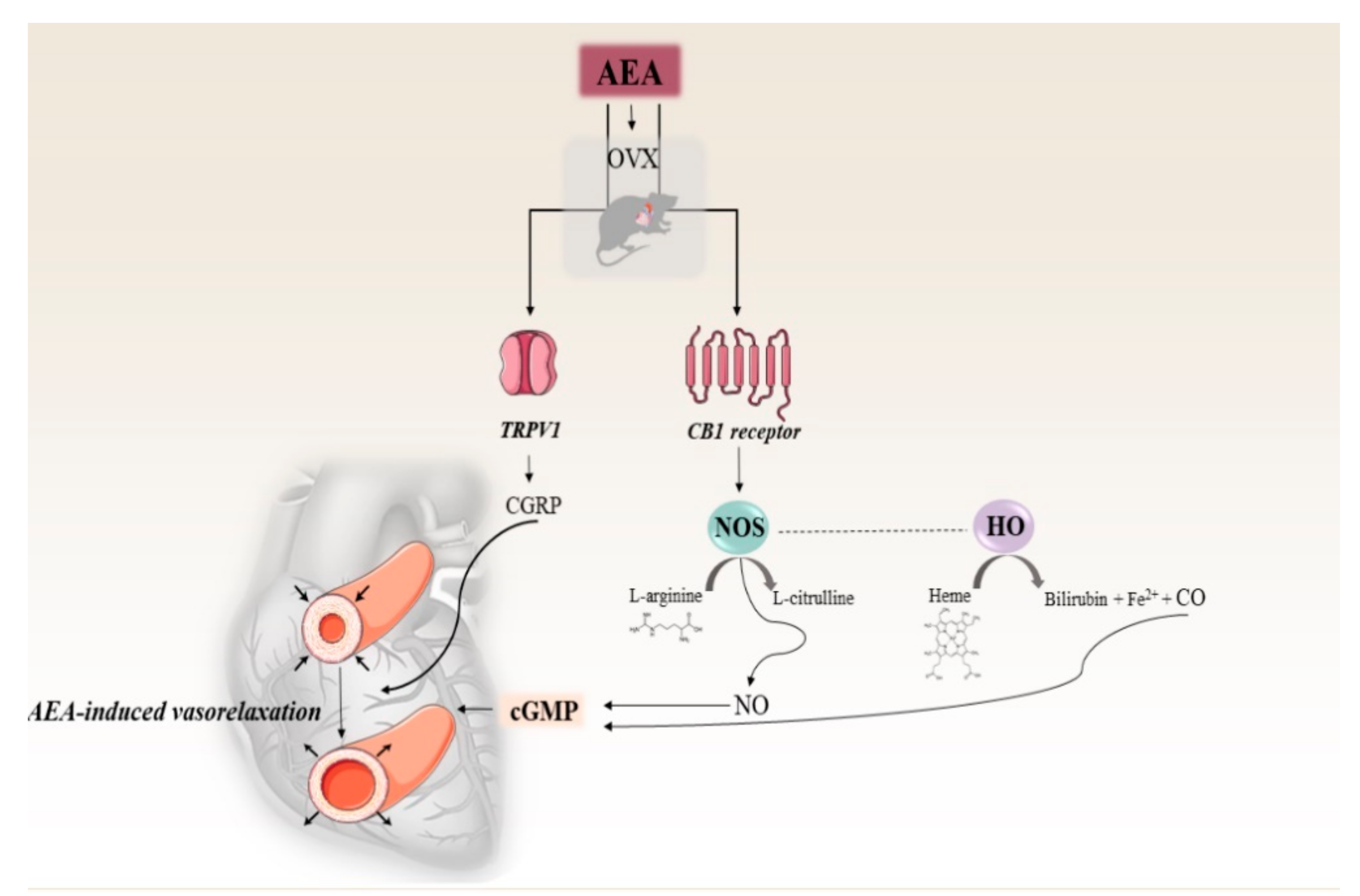
1. Introduction
2. Results
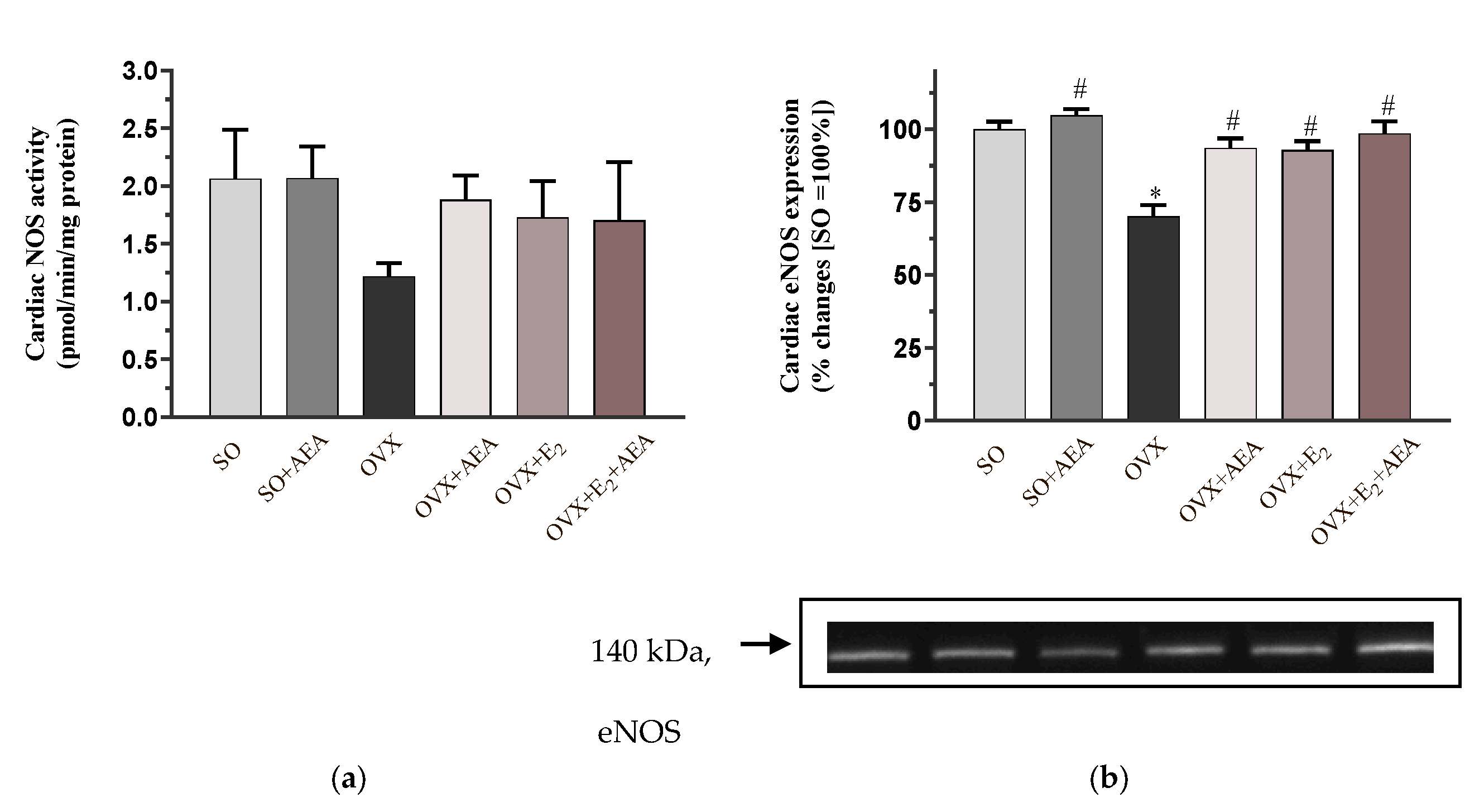
2.1. NOS Activity and eNOS Expression
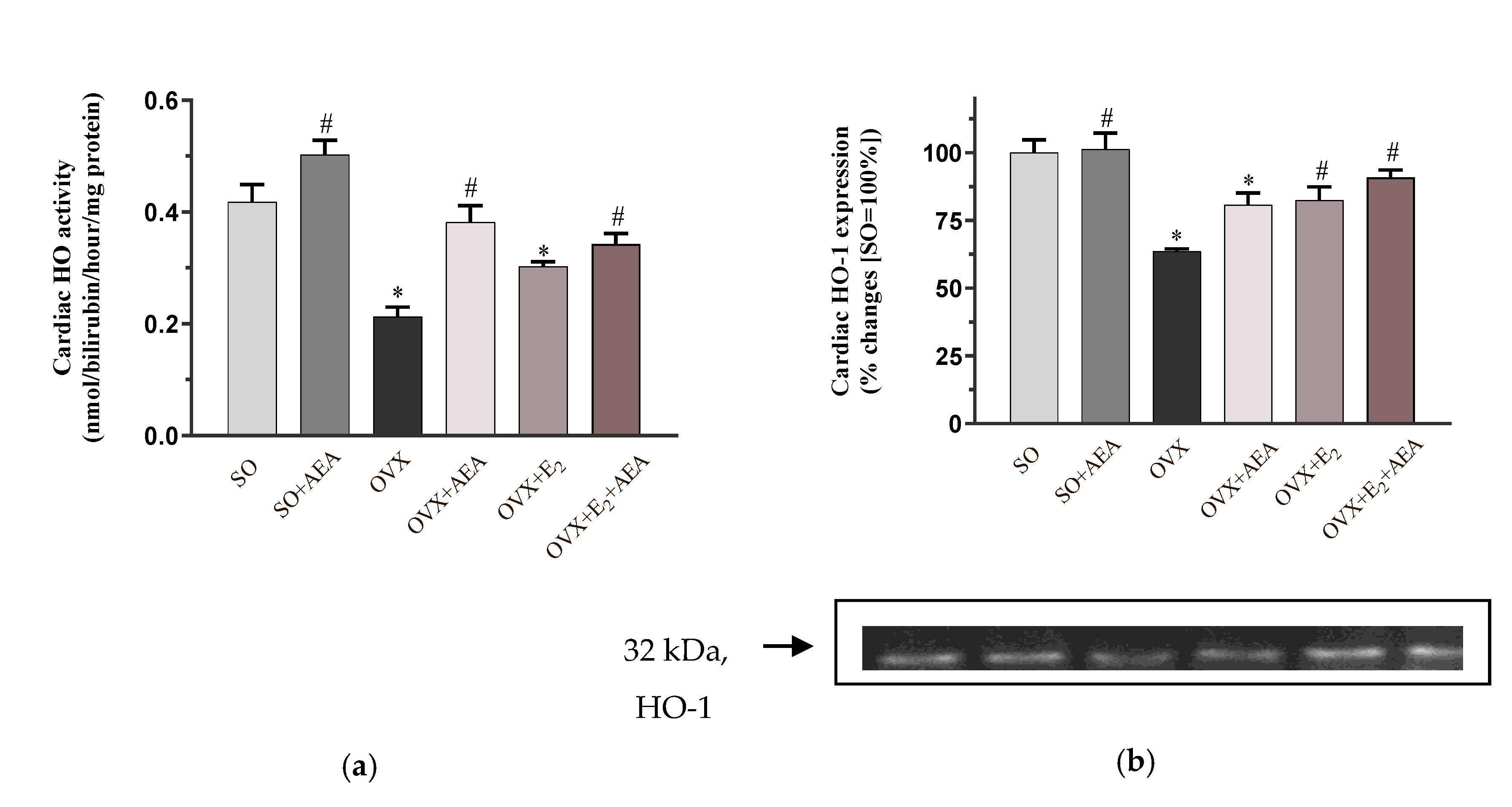
2.2. HO Activity and HO-1 Expression
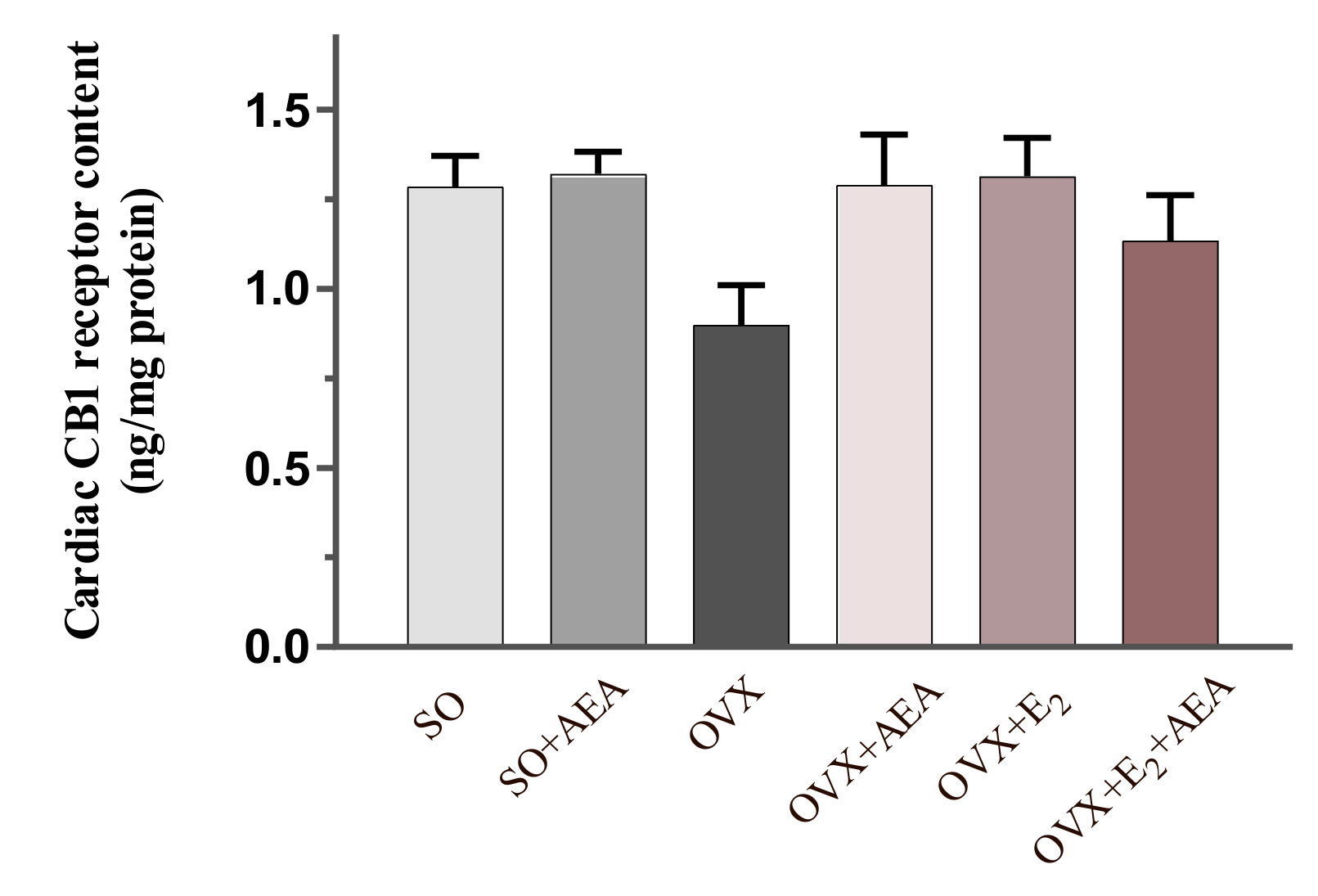
2.3. Cardiac CB1 Receptor Content
2.4. Cardiac cGMP Concentration
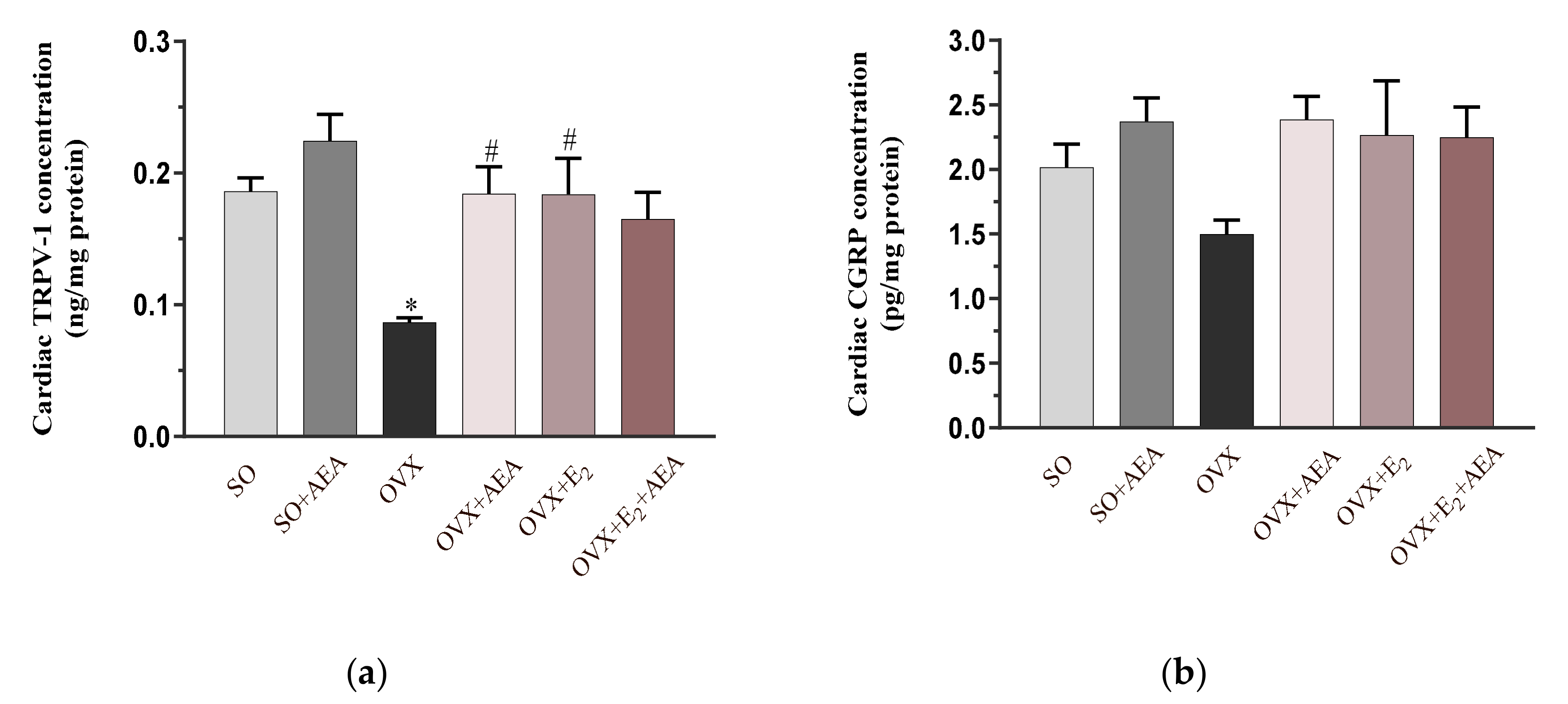
2.5. Cardiac TRPV1 and CGRP Concentrations
3. Discussion
Limitations
4. Materials and Methods
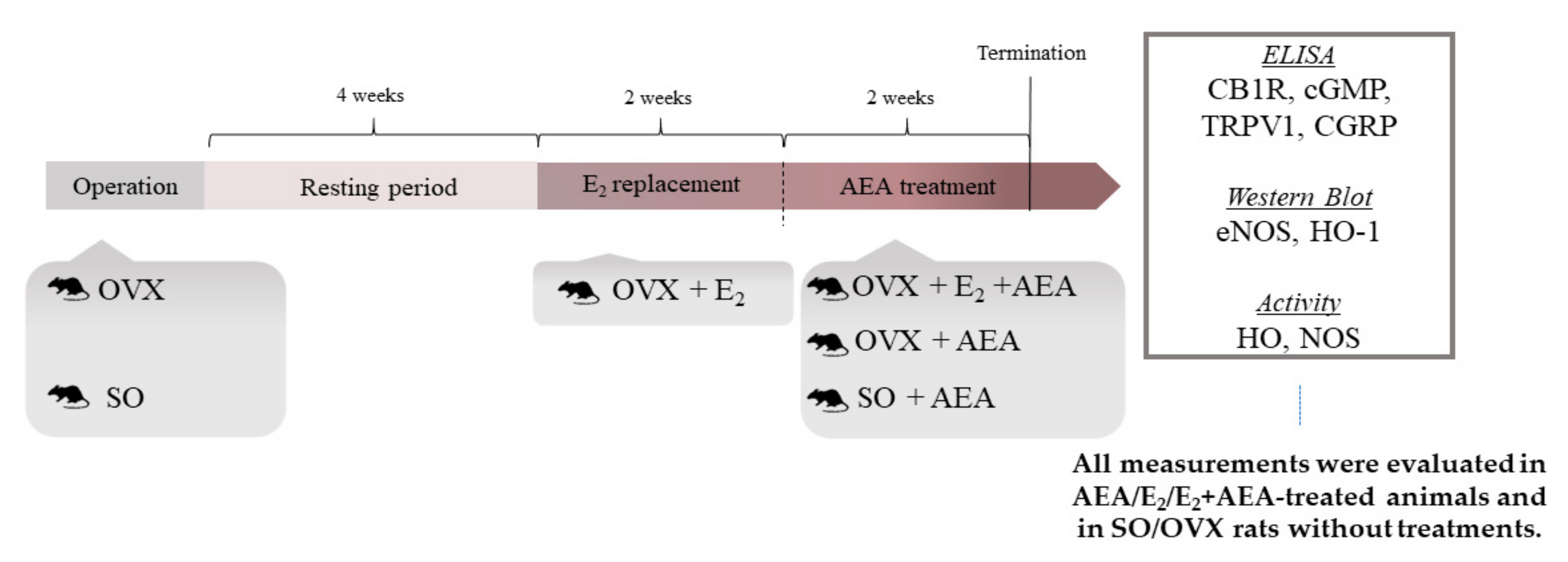
4.1. Animals
4.2. Experimental Design
4.3. Measurement of NOS Activity
4.4. Measurement of HO Activity
4.5. Measurement of eNOS and HO-1 Expression
4.6. Determination of Cardiac CB1 Receptor, cGMP, TRPV1, and CGRP Concentrations
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AEA | anandamide |
| CB1R | cannabinoid 1 receptor |
| cGMP | cyclic guanosine monophosphate |
| CGRP | calcitonin gene related peptide |
| CO | carbon monoxide |
| E2 | estrogen replacement therapy |
| eNOS | endothelial nitric oxide synthase |
| HO | heme oxygenase |
| NO | nitric oxide |
| NOS | nitric oxide synthase |
| sGC | soluble guanylate cyclase |
| SO | sham operation |
| OVX | surgical ovariectomy |
| TRPV1 | transient potential vanilloid receptor 1 |
References
- Mascarenhas-Melo, F.; Sereno, J.; Teixeira-Lemos, E.; Ribeiro, S.; Rocha-Pereira, P.; Cotterill, E.; Teixeira, F.; Reis, F. Markers of increased cardiovascular risk in postmenopausal women: Focus on oxidized-LDL and HDL subpopulations. Dis. Markers 2013, 35, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Posa, A.; Kupai, K.; Ménesi, R.; Szalai, Z.; Szabó, R.; Pintér, Z.; Pálfi, G.; Gyöngyösi, M.; Berkó, A.; Pávó, I.; et al. Sexual dimorphism of cardiovascular ischemia susceptibility is mediated by heme oxygenase. Oxid. Med. Cell. Longev. 2013, 2013, 521563. [Google Scholar] [CrossRef] [PubMed]
- Moreau, K.L.; Hildreth, K.L.; Meditz, A.L.; Deane, K.D.; Kohrt, W.M. Endothelial function is impaired across the stages of the menopause transition in healthy women. J. Clin. Endocrinol. Metab. 2012, 97, 4692–4700. [Google Scholar] [CrossRef] [PubMed]
- Kalantaridou, S.N.; Naka, K.K.; Papanikolaou, E.; Kazakos, N.; Kravariti, M.; Calis, K.A.; Paraskevaidis, E.A.; Sideris, D.A.; Tsatsoulis, A.; Chrousos, G.P.; et al. Impaired endothelial function in young women with premature ovarian failure: Normalization with hormone therapy. J. Clin. Endocrinol. Metab. 2004, 89, 3907–3913. [Google Scholar] [CrossRef]
- Posa, A.; Szabó, R.; Kupai, K.; Berkó, A.M.; Veszelka, M.; Szűcs, G.; Börzsei, D.; Gyöngyösi, M.; Pávó, I.; Deim, Z.; et al. Cardioprotective Effect of Selective Estrogen Receptor Modulator Raloxifene Are Mediated by Heme Oxygenase in Estrogen-Deficient Rat. Oxid. Med. Cell. Longev. 2017, 2017, 2176749. [Google Scholar] [CrossRef]
- Schierbeck, L.L.; Rejnmark, L.; Tofteng, C.L.; Stilgren, L.; Eiken, P.; Mosekilde, L.; Køber, L.; Jensen, J.-E.B. Effect of hormone replacement therapy on cardiovascular events in recently postmenopausal women: Randomised trial. BMJ 2012, 345, e6409. [Google Scholar] [CrossRef]
- Wu, S.; Ruan, Y.; Zhu, X.; Lai, W. Estrogen receptors and the activity of nitric oxide synthase in the artery of female rats receiving hormone replacement therapy. Horm. Res. 2000, 53, 144–147. [Google Scholar] [CrossRef]
- Chung, H.T.; Pae, H.O.; Cha, Y.N. Role of heme oxygenase-1 in vascular disease. Curr. Pharm. Des. 2008, 14, 422–428. [Google Scholar] [CrossRef]
- Pae, H.O.; Kim, N.-H.; Jeong, H.J.; Chang, K.C.; Chung, H.-T. Role of heme oxygenase in preserving vascular bioactive NO. Nitric Oxide 2010, 23, 251–257. [Google Scholar] [CrossRef]
- O’Sullivan, S.E. Endocannabinoids and the Cardiovascular System in Health and Disease. Handb. Exp. Pharmacol. 2015, 231, 393–422. [Google Scholar]
- Stanley, C.P.; Hind, W.H.; Tufarelli, C.; O’Sullivan, S.E. The endocannabinoid anandamide causes endothelium-dependent vasorelaxation in human mesenteric arteries. Pharmacol. Res. 2016, 113, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.S.; Barrett, D.A.; Randall, M.D. ‘Entourage’ effects of N-palmitoylethanolamide and N-oleoylethanolamide on vasorelaxation to anandamide occur through TRPV1 receptors. Br. J. Pharmacol. 2008, 155, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Rosano, G.M.; Silvia, M.; Andreassi, G.M.; Cristiana, V.; Cristina, V.; Marco, G.; Marco, S.; Giuseppe, M. Hormone replacement therapy and cardioprotection: A new dawn? A statement of the Study Group on Cardiovascular Disease in Women of the Italian Society of Cardiology on hormone replacement therapy in postmenopausal women. J. Cardiovasc. Med. 2009, 10, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Fuentes, H.A.; Aragones, J.; Bernhagen, J.; Boening, A.; Boisvert, W.A.; Bøtker, H.E.; Bulluck, H.; Cook, S.; di Lisa, F.; Engel, F.B.; et al. From basic mechanisms to clinical applications in heart protection, new players in cardiovascular diseases and cardiac theranostics: Meeting report from the third international symposium on “New frontiers in cardiovascular research”. Basic Res. Cardiol. 2016, 111, 69. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Juhasz, B.; Thirunavukkarasu, M.; Pant, R.; Zhan, L.; Penumathsa, S.V.; Secor, E.R., Jr.; Srivastava, S.; Raychaudhuri, U.; Menon, V.P.; Otani, H.; et al. Bromelain induces cardioprotection against ischemia-reperfusion injury through Akt/FOXO pathway in rat myocardium. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H1365–H1370. [Google Scholar] [CrossRef]
- Szabo, R.; Börzsei, D.; Karácsonyi, Z.; Gesztelyi, R.; Nemes, K.; Berkó, A.M.; Veszelka, M.; Török, S.; Kupai, K.; Varga, C.; et al. Postconditioning-like effect of exercis: New paradigm in experimental menopause. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H400–H407. [Google Scholar] [CrossRef]
- Rosano, G.M.; Vitale, C.; Marazzi, G.; Volterrani, M. Menopause and cardiovascular disease: The evidence. Climacteric 2007, 10, 19–24. [Google Scholar] [CrossRef]
- Novella, S.; Heras, M.; Hermenegildo, C.; Dantas, A.P. Effects of estrogen on vascular inflammation: A matter of timing. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2035–2042. [Google Scholar] [CrossRef]
- Posa, A.; Pavo, I.; Varga, C. Heme oxygenase contributes to estradiol and raloxifene-induced vasorelaxation in estrogen deficiency. Int. J. Cardiol. 2015, 189, 252–254. [Google Scholar] [CrossRef]
- Abraham, N.G.; Kappas, A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol. Rev. 2008, 60, 79–127. [Google Scholar] [CrossRef]
- Mihailovic-Stanojevic, N.; Miloradović, Z.; Ivanov, M.; Bugarski, B.; Jovović, Đ.; Karanović, D.; Vajić, U.; Komes, D.; Grujić-Milanović, J. Upregulation of Heme Oxygenase-1 in Response to Wild Thyme Treatment Protects against Hypertension and Oxidative Stress. Oxid. Med. Cell. Longev. 2016, 2016, 1458793. [Google Scholar] [CrossRef]
- Issan, Y.; Kornowski, R.; Aravot, D.; Shainberg, A.; Laniado-Schwartzman, M.; Sodhi, K.; Abraham, N.G.; Hochhauser, E. Heme oxygenase-1 induction improves cardiac function following myocardial ischemia by reducing oxidative stress. PLoS ONE 2014, 9, e92246. [Google Scholar] [CrossRef] [PubMed]
- Daiber, A.; Xia, N.; Steven, S.; Oelze, M.; Hanf, A.; Kröller-Schön, S.; Münzel, T.; Li, H. New Therapeutic Implications of Endothelial Nitric Oxide Synthase (eNOS) Function/Dysfunction in Cardiovascular Disease. Int. J. Mol. Sci. 2019, 20, 187. [Google Scholar] [CrossRef] [PubMed]
- Schulz, E.; Gori, T.; Munzel, T. Oxidative stress and endothelial dysfunction in hypertension. Hypertens. Res. 2011, 34, 665–673. [Google Scholar] [CrossRef]
- Ho, W.S.V.; Kelly, M.E.M. Cannabinoids in the Cardiovascular System. Adv. Pharmacol. 2017, 80, 329–366. [Google Scholar] [PubMed]
- Batkai, S.; Pacher, P.; Osei-Hyiaman, D.; Radaeva, S.; Liu, J.; Harvey-White, J.; Offertáler, L.; Mackie, K.; Rudd, A.; Bukoski, R.D.; et al. Endocannabinoids acting at cannabinoid-1 receptors regulate cardiovascular function in hypertension. Circulation 2004, 110, 1996–2002. [Google Scholar] [CrossRef]
- Pacher, P.; Mukhopadhyay, P.; Mohanraj, R.; Godlewski, G.; Bátkai, S.; Kunos, G. Modulation of the endocannabinoid system in cardiovascular disease: Therapeutic potential and limitations. Hypertension 2008, 52, 601–607. [Google Scholar] [CrossRef]
- Pacher, P.; Batkai, S.; Kunos, G. Cardiovascular pharmacology of cannabinoids. Handb. Exp. Pharmacol. 2005, 168, 599–625. [Google Scholar]
- Rajesh, M.; Bátkai, S.; Kechrid, M.; Mukhopadhyay, P.; Lee, W.; Horváth, B.; Holovac, E.; Cinar, R.; Liaudet, L.; Mackie, K.; et al. Cannabinoid 1 receptor promotes cardiac dysfunction, oxidative stress, inflammation, and fibrosis in diabetic cardiomyopathy. Diabetes 2012, 61, 716–727. [Google Scholar] [CrossRef]
- El-Remessy, A.B.; Rajesh, M.; Mukhopadhyay, P.; Horváth, B.; Patel, V.; Al-Gayyar, M.M.H.; Pillai, B.A.; Pacher, P. Cannabinoid 1 receptor activation contributes to vascular inflammation and cell death in a mouse model of diabetic retinopathy and a human retinal cell line. Diabetologia 2011, 54, 1567–1578. [Google Scholar] [CrossRef]
- Harper-Harrison, G.; Shanahan, M.M. Hormone Replacement Therapy, in StatPearls; StatPearls: Treasure Island, FL, USA, 2020. [Google Scholar]
- Underdown, N.J.; Hiley, C.R.; Ford, W.R. Anandamide reduces infarct size in rat isolated hearts subjected to ischaemia-reperfusion by a novel cannabinoid mechanism. Br. J. Pharmacol. 2005, 146, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Tuma, R.F.; Steffens, S. Targeting the endocannabinod system to limit myocardial and cerebral ischemic and reperfusion injury. Curr. Pharm. Biotechnol. 2012, 13, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Romano, M.R.; Lograno, M.D. Cannabinoid agonists induce relaxation in the bovine ophthalmic artery: Evidences for CB1 receptors, nitric oxide and potassium channels. Br. J. Pharmacol. 2006, 147, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Randhawa, P.K.; Jaggi, A.S. A Review on Potential Involvement of TRPV1 Channels in Ischemia-Reperfusion Injury. J. Cardiovasc. Pharmacol. Ther. 2018, 23, 38–45. [Google Scholar] [CrossRef]
- Peng, J.; Lu, R.; Ye, F.; Deng, H.-W.; Li, Y.-J. The heme oxygenase-1 pathway is involved in calcitonin gene-related peptide-mediated delayed cardioprotection induced by monophosphoryl lipid A in rats. Regul. Pept. 2002, 103, 1–7. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szabó, R.; Börzsei, D.; Szabó, Z.; Hoffmann, A.; Zupkó, I.; Priksz, D.; Kupai, K.; Varga, C.; Pósa, A. A Potential Involvement of Anandamide in the Modulation of HO/NOS Systems: Women, Menopause, and “Medical Cannabinoids”. Int. J. Mol. Sci. 2020, 21, 8801. https://doi.org/10.3390/ijms21228801
Szabó R, Börzsei D, Szabó Z, Hoffmann A, Zupkó I, Priksz D, Kupai K, Varga C, Pósa A. A Potential Involvement of Anandamide in the Modulation of HO/NOS Systems: Women, Menopause, and “Medical Cannabinoids”. International Journal of Molecular Sciences. 2020; 21(22):8801. https://doi.org/10.3390/ijms21228801
Chicago/Turabian StyleSzabó, Renáta, Denise Börzsei, Zsuzsanna Szabó, Alexandra Hoffmann, István Zupkó, Dániel Priksz, Krisztina Kupai, Csaba Varga, and Anikó Pósa. 2020. "A Potential Involvement of Anandamide in the Modulation of HO/NOS Systems: Women, Menopause, and “Medical Cannabinoids”" International Journal of Molecular Sciences 21, no. 22: 8801. https://doi.org/10.3390/ijms21228801
APA StyleSzabó, R., Börzsei, D., Szabó, Z., Hoffmann, A., Zupkó, I., Priksz, D., Kupai, K., Varga, C., & Pósa, A. (2020). A Potential Involvement of Anandamide in the Modulation of HO/NOS Systems: Women, Menopause, and “Medical Cannabinoids”. International Journal of Molecular Sciences, 21(22), 8801. https://doi.org/10.3390/ijms21228801






