Edible Oxya chinensis sinuosa—Derived Protein as a Potential Nutraceutical for Anticancer Immunity Improvement
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Studies
2.2. Antibodies and Reagents
2.3. Isolation of Ocs-P Extract
2.4. Treatment Conditions of Ocs-P in BMDCs
2.5. Annexin V and PI Staining
2.6. Analysis of Surface Molecules of BMDC
2.7. Detection of Intracelluar and Extracelluar Cytokines in BMDCs
2.8. Analysis of Ag Uptake Ability in BMDCs
2.9. Western Blotting Analysis
2.10. Ocs-P-treated BMDC Maturation Induced by Inhibition of the MAPK and NF-ĸB Signaling Pathways
2.11. Allogenic Mixed Lymphocyte Reaction
2.12. Cytotoxic T Lymphocyte (CTL) Function Assay
2.13. Animal Experiment for Antitumor Effect
2.14. Statistical Analysis
3. Results
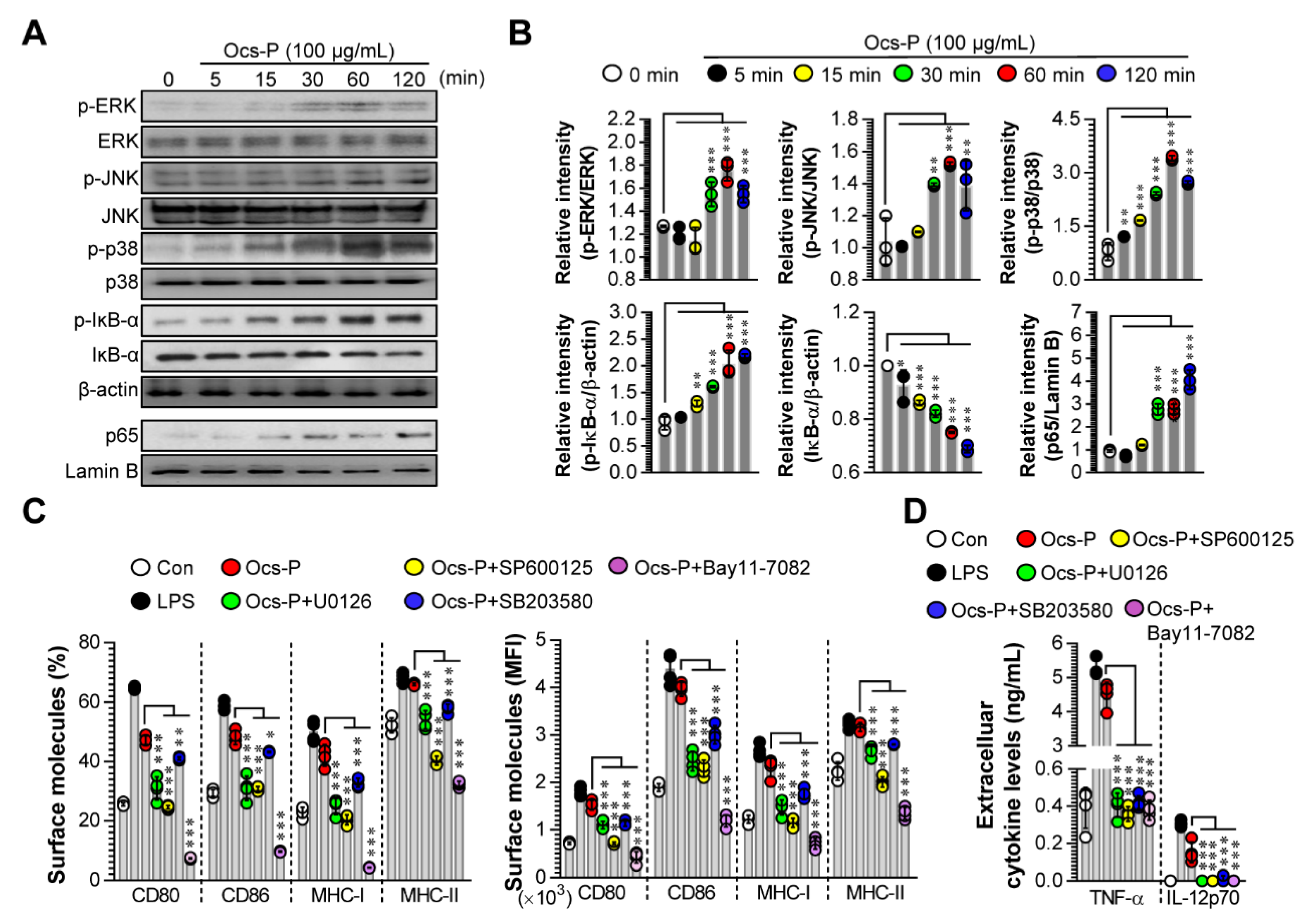
3.1. Ocs-P Promotes a Typical Maturation Profile for Potential Th1 Polarization and Cytotoxic CD8+ T Cell Responses in Primary BMDCs
3.2. Ocs-P Induces DC Maturation by Activating MAPK and NF-ĸB Signals
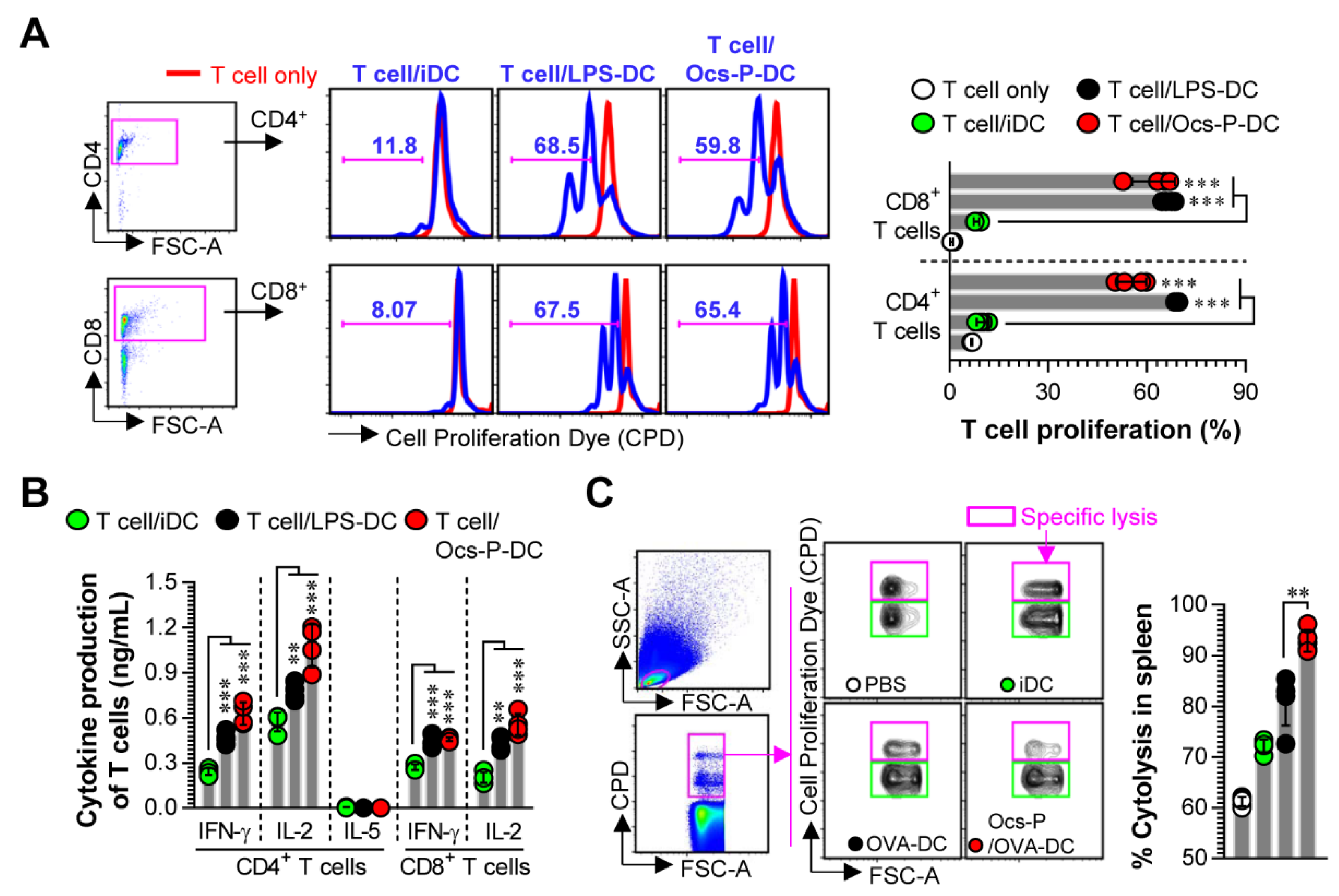
3.3. Ocs-P-stimulated DCs Induce T Cell Proliferation, Favor Th1 Polarization, and Activate Cytotoxic CD8+ T Cells
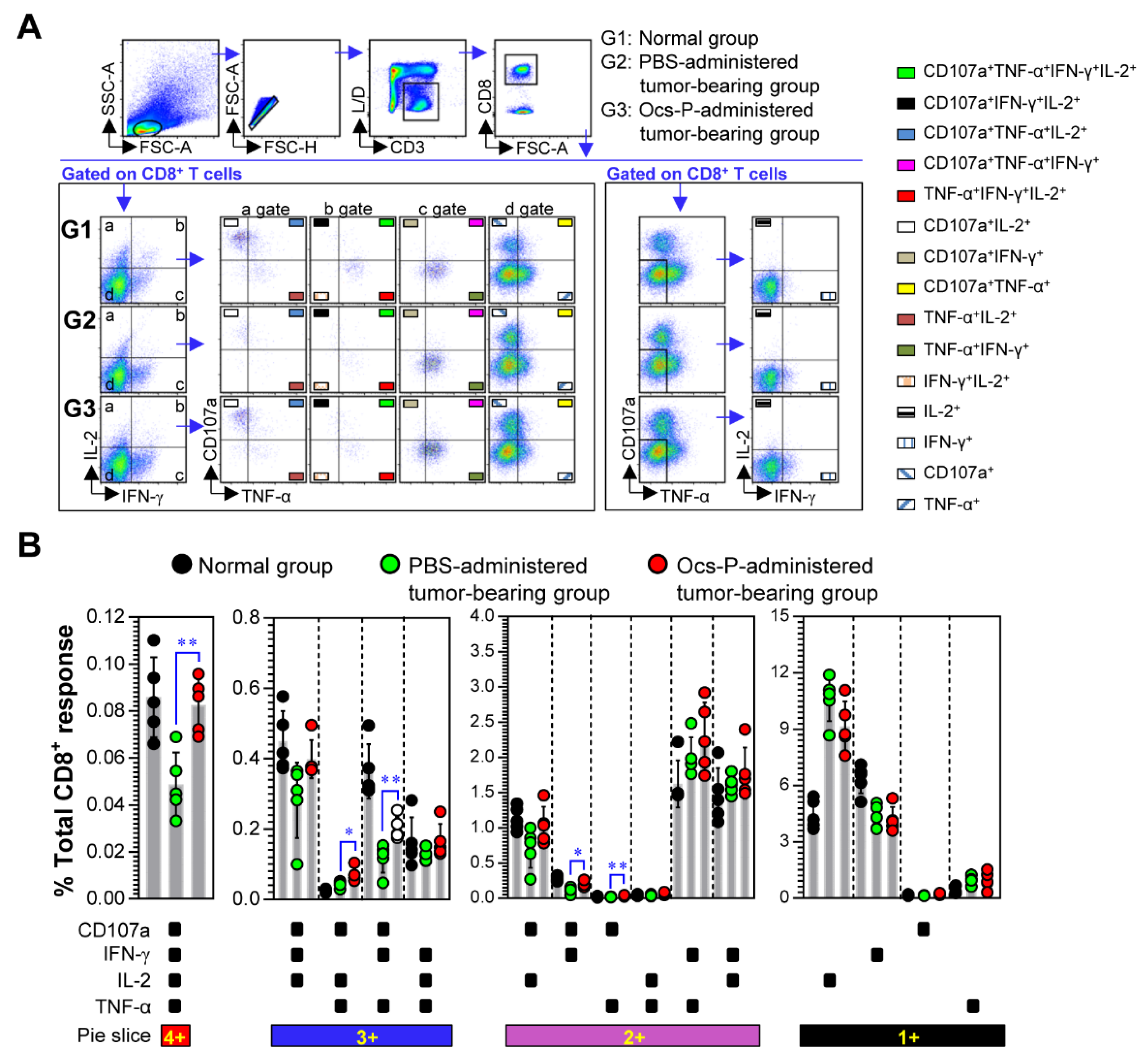
3.4. Ocs-P Inhibits Tumor Growth via Activation of Innate and Adaptive Immunity
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Huis, A.V.; Itterbeeck, J.V.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects: Future Prospects for Food and Feed Security; FAO Forestry Paper; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013. [Google Scholar]
- Cerritos, R. Insects as food: An ecological, social and economical approach. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2009, 4, 1–10. [Google Scholar] [CrossRef]
- Berenbaum, M.R.; Eisner, T. Bugs’ bugs. Science 2008, 322, 52–53. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Kim, H.; Choe, J.Y. The Role of Eco-Friendly Edible Insect Restaurants in the Field of Sustainable Tourism. Int. J. Environ. Res. Public Health 2020, 17, 4064. [Google Scholar] [CrossRef] [PubMed]
- Bukkens, S.G. The nutritional value of edible insects. Ecol. Food Nutr. 1997, 36, 287–319. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Schluter, O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef]
- Jantzen da Silva Lucas, A.; Menegon de Oliveira, L.; da Rocha, M.; Prentice, C. Edible insects: An alternative of nutritional, functional and bioactive compounds. Food Chem. 2020, 311, 126022. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; FitzGerald, R.J. Unlocking the biological potential of proteins from edible insects through enzymatic hydrolysis: A review. Innov. Food Sci. Emerg. Technol. 2017, 43, 239–252. [Google Scholar] [CrossRef]
- Kim, T.K.; Yong, H.I.; Kim, Y.B.; Kim, H.W.; Choi, Y.S. Edible Insects as a Protein Source: A Review of Public Perception, Processing Technology, and Research Trends. Food Sci. Anim. Resour. 2019, 39, 521–540. [Google Scholar] [CrossRef]
- Chatsuwan, N.; Nalinanon, S.; Puechkamut, Y.; Lamsal, B.P.; Pinsirodom, P. Characteristics, functional properties, and antioxidant activities of water-soluble proteins extracted from grasshoppers, Patanga succincta and Chondracris roseapbrunner. J. Chem. 2018, 2018. [Google Scholar] [CrossRef]
- Li, X.; Xie, H.; Chen, Y.; Lang, M.; Chen, Y.; Shi, L. Silkworm Pupa Protein Hydrolysate Induces Mitochondria-Dependent Apoptosis and S Phase Cell Cycle Arrest in Human Gastric Cancer SGC-7901 Cells. Int. J. Mol. Sci. 2018, 19, 1013. [Google Scholar] [CrossRef]
- Yu, W.; Ying, H.; Tong, F.; Zhang, C.; Quan, Y.; Zhang, Y. Protective effect of the silkworm protein 30Kc6 on human vascular endothelial cells damaged by oxidized low density lipoprotein (Ox-LDL). PLoS ONE 2013, 8, e68746. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.; Sahu, R.K.; Dey, T.; Lahkar, M.D.; Manna, P.; Kalita, J. Beneficial role of insect-derived bioactive components against inflammation and its associated complications (colitis and arthritis) and cancer. Chem. Biol. Interact. 2019, 313, 108824. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.W.; Markkandan, K.; Lee, J.H.; Subramaniyam, S.; Yoo, S.; Park, J.; Hwang, J.S. Transcriptome Profiling and In Silico Analysis of the Antimicrobial Peptides of the Grasshopper Oxya chinensis sinuosa. J. Microbiol. Biotechnol. 2016, 26, 1863–1870. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Tian, Y.; Cai, R.; Yi, T.; Jin, D.; Guo, J. Antiproliferative and Proapoptotic Effects of a Protein Component Purified from Aspongopus chinensis Dallas on Cancer Cells In Vitro and In Vivo. Evid. Based Complement Altern. Med. 2019, 2019, 8934794. [Google Scholar] [CrossRef]
- Kim, H.J.; Kang, S.J.; Kim, S.G.; Kim, J.E.; Koo, H.Y. Antioxidant activity and antimicrobial activity of the grasshopper, Oxya chinensis sinuosa. J. Sericultural Entomol. Sci. 2015, 53, 130–134. [Google Scholar] [CrossRef][Green Version]
- Lee, W.; Lee, H.; Kim, M.A.; Choi, J.; Kim, K.M.; Hwang, J.S.; Na, M.; Bae, J.S. Evaluation of novel factor Xa inhibitors from Oxya chinensis sinuosa with anti-platelet aggregation activity. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Hossain, M.K.; Wall, K.A. Use of Dendritic Cell Receptors as Targets for Enhancing Anti-Cancer Immune Responses. Cancers (Basel) 2019, 11, 418. [Google Scholar] [CrossRef]
- Kim, W.S.; Yoon, J.H.; Shin, M.K.; Shin, S.J. Infection of Dendritic Cells With Mycobacterium avium subspecies hominissuis Exhibits a Functionally Tolerogenic Phenotype in Response to Toll-Like Receptor Agonists via IL-10/Cox2/PGE2/EP2 Axis. Front. Microbiol. 2019, 10, 1795. [Google Scholar] [CrossRef]
- Kim, W.S.; Kim, K.; Byun, E.B.; Song, H.Y.; Han, J.M.; Park, W.Y.; Yuk, J.M.; Byun, E.H. RM, a novel resveratrol derivative, attenuates inflammatory responses induced by lipopolysaccharide via selectively increasing the Tollip protein in macrophages: A partial mechanism with therapeutic potential in an inflammatory setting. Int. Immunopharmacol. 2020, 78, 106072. [Google Scholar] [CrossRef]
- Burkholder, B.; Huang, R.Y.; Burgess, R.; Luo, S.; Jones, V.S.; Zhang, W.; Lv, Z.Q.; Gao, C.Y.; Wang, B.L.; Zhang, Y.M.; et al. Tumor-induced perturbations of cytokines and immune cell networks. Biochim. Biophys. Acta 2014, 1845, 182–201. [Google Scholar] [CrossRef]
- Lutz, M.B. Induction of CD4(+) Regulatory and Polarized Effector/helper T Cells by Dendritic Cells. Immune Netw. 2016, 16, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Gardner, A.; Ruffell, B. Dendritic Cells and Cancer Immunity. Trends Immunol. 2016, 37, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Croft, M. The role of TNF superfamily members in T-cell function and diseases. Nat. Rev. Immunol. 2009, 9, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Rautajoki, K.J.; Kylaniemi, M.K.; Raghav, S.K.; Rao, K.; Lahesmaa, R. An insight into molecular mechanisms of human T helper cell differentiation. Ann. Med. 2008, 40, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Perreau, M.; Mennechet, F.; Serratrice, N.; Glasgow, J.N.; Curiel, D.T.; Wodrich, H.; Kremer, E.J. Contrasting effects of human, canine, and hybrid adenovirus vectors on the phenotypical and functional maturation of human dendritic cells: Implications for clinical efficacy. J. Virol. 2007, 81, 3272–3284. [Google Scholar] [CrossRef]
- Nakahara, T.; Moroi, Y.; Uchi, H.; Furue, M. Differential role of MAPK signaling in human dendritic cell maturation and Th1/Th2 engagement. J. Dermatol. Sci. 2006, 42, 1–11. [Google Scholar] [CrossRef]
- Takeuchi, A.; Saito, T. CD4 CTL, a Cytotoxic Subset of CD4(+) T Cells, Their Differentiation and Function. Front. Immunol. 2017, 8, 194. [Google Scholar] [CrossRef]
- Chauchet, X.; Hannani, D.; Djebali, S.; Laurin, D.; Polack, B.; Marvel, J.; Buffat, L.; Toussaint, B.; Le Gouellec, A. Poly-functional and long-lasting anticancer immune response elicited by a safe attenuated Pseudomonas aeruginosa vector for antigens delivery. Mol. Ther. Oncolytics 2016, 3, 16033. [Google Scholar] [CrossRef][Green Version]
- Wylie, B.; Macri, C.; Mintern, J.D.; Waithman, J. Dendritic Cells and Cancer: From Biology to Therapeutic Intervention. Cancers (Basel) 2019, 11, 521. [Google Scholar] [CrossRef]
- Palucka, K.; Banchereau, J. Dendritic-cell-based therapeutic cancer vaccines. Immunity 2013, 39, 38–48. [Google Scholar] [CrossRef]
- Perez, C.R.; De Palma, M. Engineering dendritic cell vaccines to improve cancer immunotherapy. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Bellone, G.; Carbone, A.; Smirne, C.; Scirelli, T.; Buffolino, A.; Novarino, A.; Stacchini, A.; Bertetto, O.; Palestro, G.; Sorio, C.; et al. Cooperative induction of a tolerogenic dendritic cell phenotype by cytokines secreted by pancreatic carcinoma cells. J. Immunol. 2006, 177, 3448–3460. [Google Scholar] [CrossRef]
- Ma, Y.; Shurin, G.V.; Gutkin, D.W.; Shurin, M.R. Tumor associated regulatory dendritic cells. Semin. Cancer Biol. 2012, 22, 298–306. [Google Scholar] [CrossRef]
- Shurin, G.V.; Ma, Y.; Shurin, M.R. Immunosuppressive mechanisms of regulatory dendritic cells in cancer. Cancer Microenviron. 2013, 6, 159–167. [Google Scholar] [CrossRef]
- Bauer, C.A.; Kim, E.Y.; Marangoni, F.; Carrizosa, E.; Claudio, N.M.; Mempel, T.R. Dynamic Treg interactions with intratumoral APCs promote local CTL dysfunction. J. Clin. Investig. 2014, 124, 2425–2440. [Google Scholar] [CrossRef]
- Jia, J.; Wang, Z.; Li, X.; Wang, Z.; Wang, X. Morphological characteristics and co-stimulatory molecule (CD80, CD86, CD40) expression in tumor infiltrating dendritic cells in human endometrioid adenocarcinoma. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012, 160, 223–227. [Google Scholar] [CrossRef]
- Song, K.; Xu, L.; Zhang, W.; Cai, Y.; Jang, B.; Oh, J.; Jin, J.O. Laminarin promotes anti-cancer immunity by the maturation of dendritic cells. Oncotarget 2017, 8, 38554–38567. [Google Scholar] [CrossRef]
- Durgeau, A.; Virk, Y.; Corgnac, S.; Mami-Chouaib, F. Recent Advances in Targeting CD8 T-Cell Immunity for More Effective Cancer Immunotherapy. Front. Immunol. 2018, 9, 14. [Google Scholar] [CrossRef]
- Farhood, B.; Najafi, M.; Mortezaee, K. CD8(+) cytotoxic T lymphocytes in cancer immunotherapy: A review. J. Cell. Physiol. 2019, 234, 8509–8521. [Google Scholar] [CrossRef]
- Wimmers, F.; Aarntzen, E.H.; Duiveman-deBoer, T.; Figdor, C.G.; Jacobs, J.F.; Tel, J.; de Vries, I.J. Long-lasting multifunctional CD8(+) T cell responses in end-stage melanoma patients can be induced by dendritic cell vaccination. Oncoimmunology 2016, 5, e1067745. [Google Scholar] [CrossRef]
- Baumgaertner, P.; Jandus, C.; Rivals, J.P.; Derre, L.; Lovgren, T.; Baitsch, L.; Guillaume, P.; Luescher, I.F.; Berthod, G.; Matter, M.; et al. Vaccination-induced functional competence of circulating human tumor-specific CD8 T-cells. Int. J. Cancer 2012, 130, 2607–2617. [Google Scholar] [CrossRef]
- Imai, N.; Ikeda, H.; Tawara, I.; Shiku, H. Tumor progression inhibits the induction of multifunctionality in adoptively transferred tumor-specific CD8+ T cells. Eur. J. Immunol. 2009, 39, 241–253. [Google Scholar] [CrossRef]
- Imai, N.; Ikeda, H.; Tawara, I.; Wang, L.; Wang, L.; Nishikawa, H.; Kato, T.; Shiku, H. Glucocorticoid-induced tumor necrosis factor receptor stimulation enhances the multifunctionality of adoptively transferred tumor antigen-specific CD8+ T cells with tumor regression. Cancer Sci. 2009, 100, 1317–1325. [Google Scholar] [CrossRef]
- Yuan, J.; Gnjatic, S.; Li, H.; Powel, S.; Gallardo, H.F.; Ritter, E.; Ku, G.Y.; Jungbluth, A.A.; Segal, N.H.; Rasalan, T.S.; et al. CTLA-4 blockade enhances polyfunctional NY-ESO-1 specific T cell responses in metastatic melanoma patients with clinical benefit. Proc. Natl. Acad. Sci. USA 2008, 105, 20410–20415. [Google Scholar] [CrossRef]
- Shedlock, D.J.; Shen, H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science 2003, 300, 337–339. [Google Scholar] [CrossRef]
- Imai, N.; Tawara, I.; Yamane, M.; Muraoka, D.; Shiku, H.; Ikeda, H. CD4(+) T cells support polyfunctionality of cytotoxic CD8(+) T cells with memory potential in immunological control of tumor. Cancer Sci. 2020, 111, 1958–1968. [Google Scholar] [CrossRef]
- Juno, J.A.; van Bockel, D.; Kent, S.J.; Kelleher, A.D.; Zaunders, J.J.; Munier, C.M. Cytotoxic CD4 T Cells-Friend or Foe during Viral Infection? Front. Immunol. 2017, 8, 19. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, W.S.; Han, J.M.; Song, H.-Y.; Byun, E.-H.; Seo, H.S.; Byun, E.-B. Edible Oxya chinensis sinuosa—Derived Protein as a Potential Nutraceutical for Anticancer Immunity Improvement. Nutrients 2020, 12, 3236. https://doi.org/10.3390/nu12113236
Kim WS, Han JM, Song H-Y, Byun E-H, Seo HS, Byun E-B. Edible Oxya chinensis sinuosa—Derived Protein as a Potential Nutraceutical for Anticancer Immunity Improvement. Nutrients. 2020; 12(11):3236. https://doi.org/10.3390/nu12113236
Chicago/Turabian StyleKim, Woo Sik, Jeong Moo Han, Ha-Yeon Song, Eui-Hong Byun, Ho Seong Seo, and Eui-Baek Byun. 2020. "Edible Oxya chinensis sinuosa—Derived Protein as a Potential Nutraceutical for Anticancer Immunity Improvement" Nutrients 12, no. 11: 3236. https://doi.org/10.3390/nu12113236
APA StyleKim, W. S., Han, J. M., Song, H.-Y., Byun, E.-H., Seo, H. S., & Byun, E.-B. (2020). Edible Oxya chinensis sinuosa—Derived Protein as a Potential Nutraceutical for Anticancer Immunity Improvement. Nutrients, 12(11), 3236. https://doi.org/10.3390/nu12113236




