Functionally Relevant Differences in Plasma Fatty Acid Composition and Expression of Cytotoxic and Inhibitory NK Cell Receptors between Healthy Young and Healthy Elder Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Blood Samples
2.3. Fatty Acid Analysis in Plasma
2.4. NK Cells
2.5. Incubation of NK Cells with the Fatty Acids
2.6. Statistical Analysis
3. Results
3.1. Circulating Fatty Acids
3.2. NK Cell Number and Purification
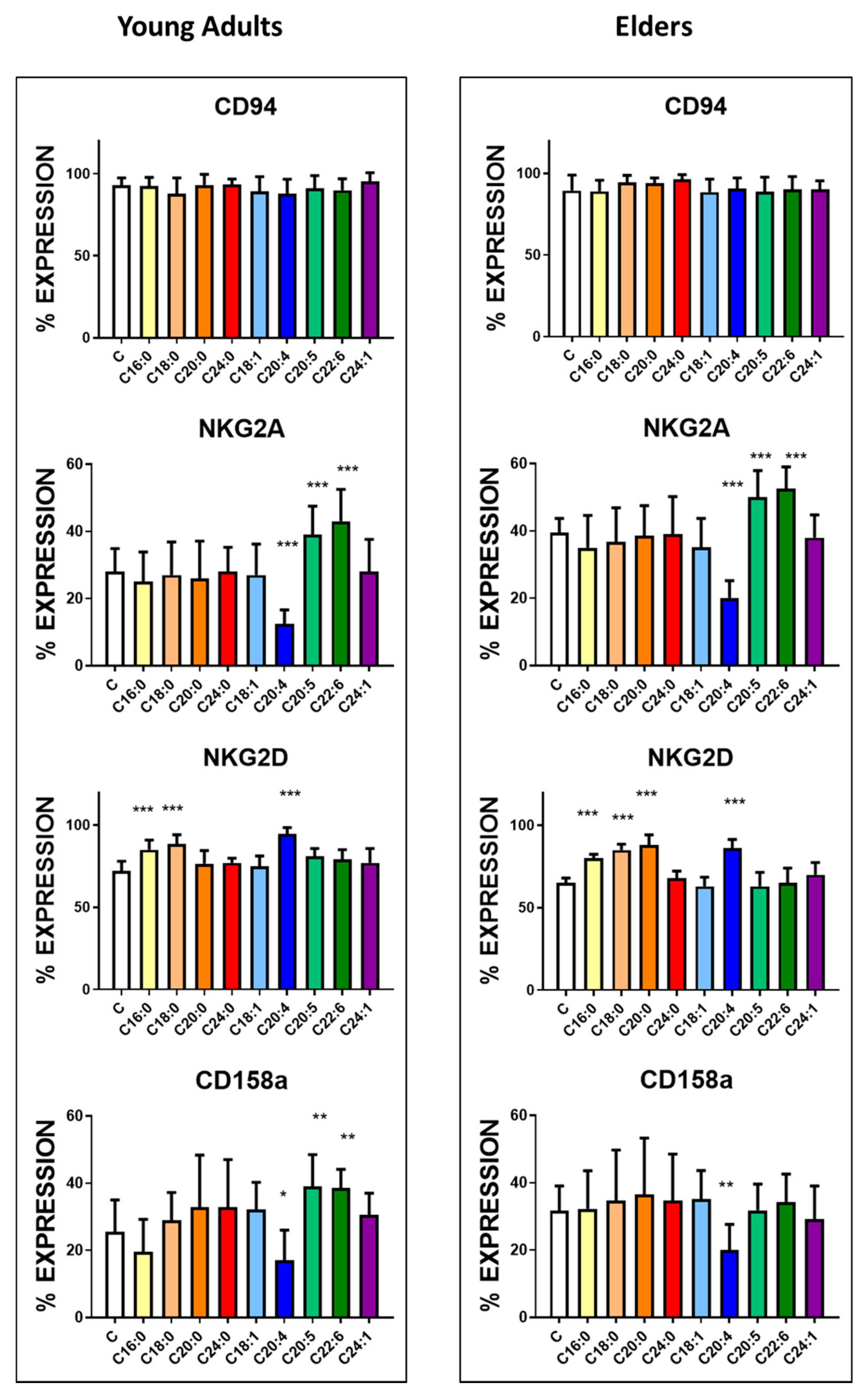
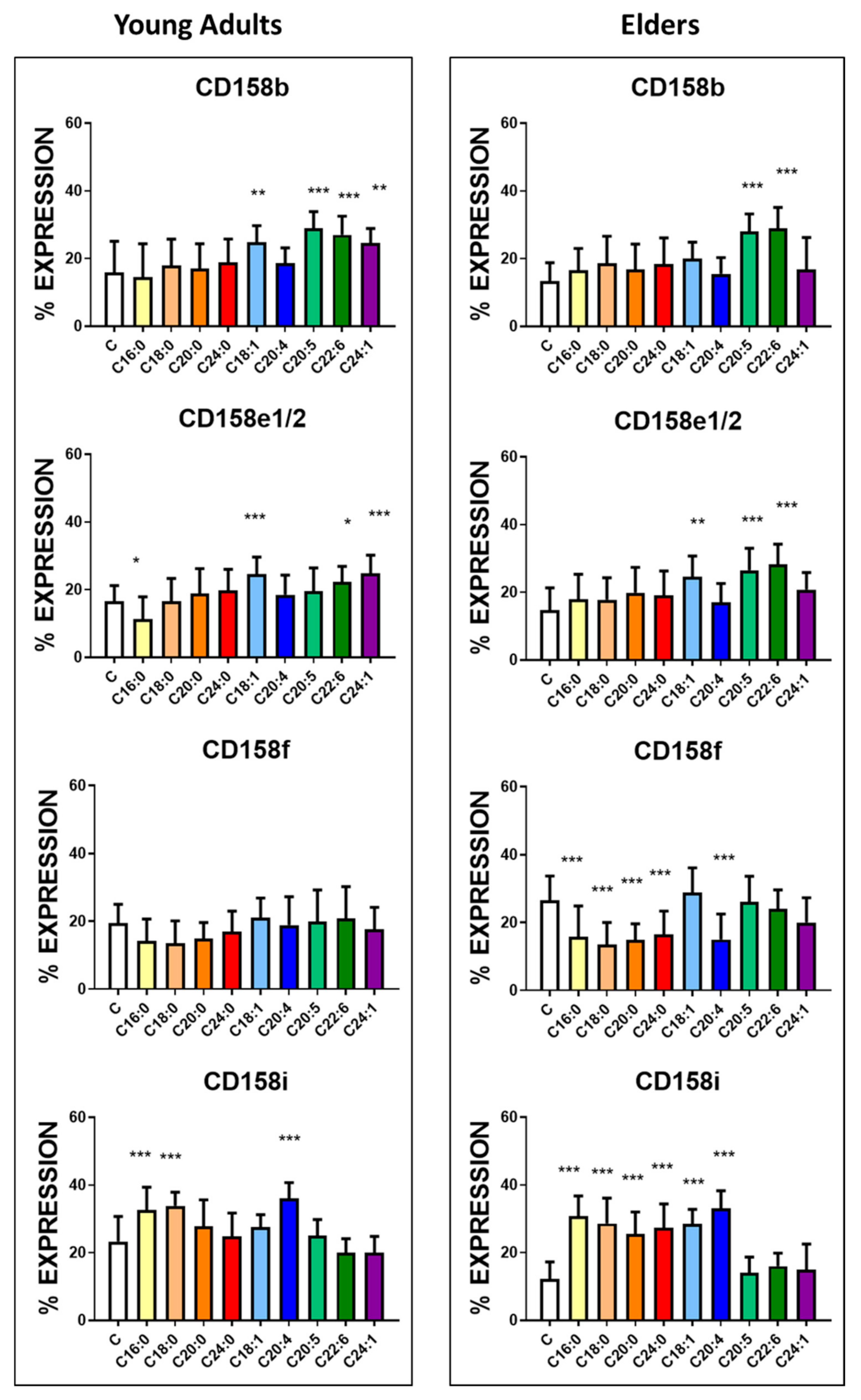
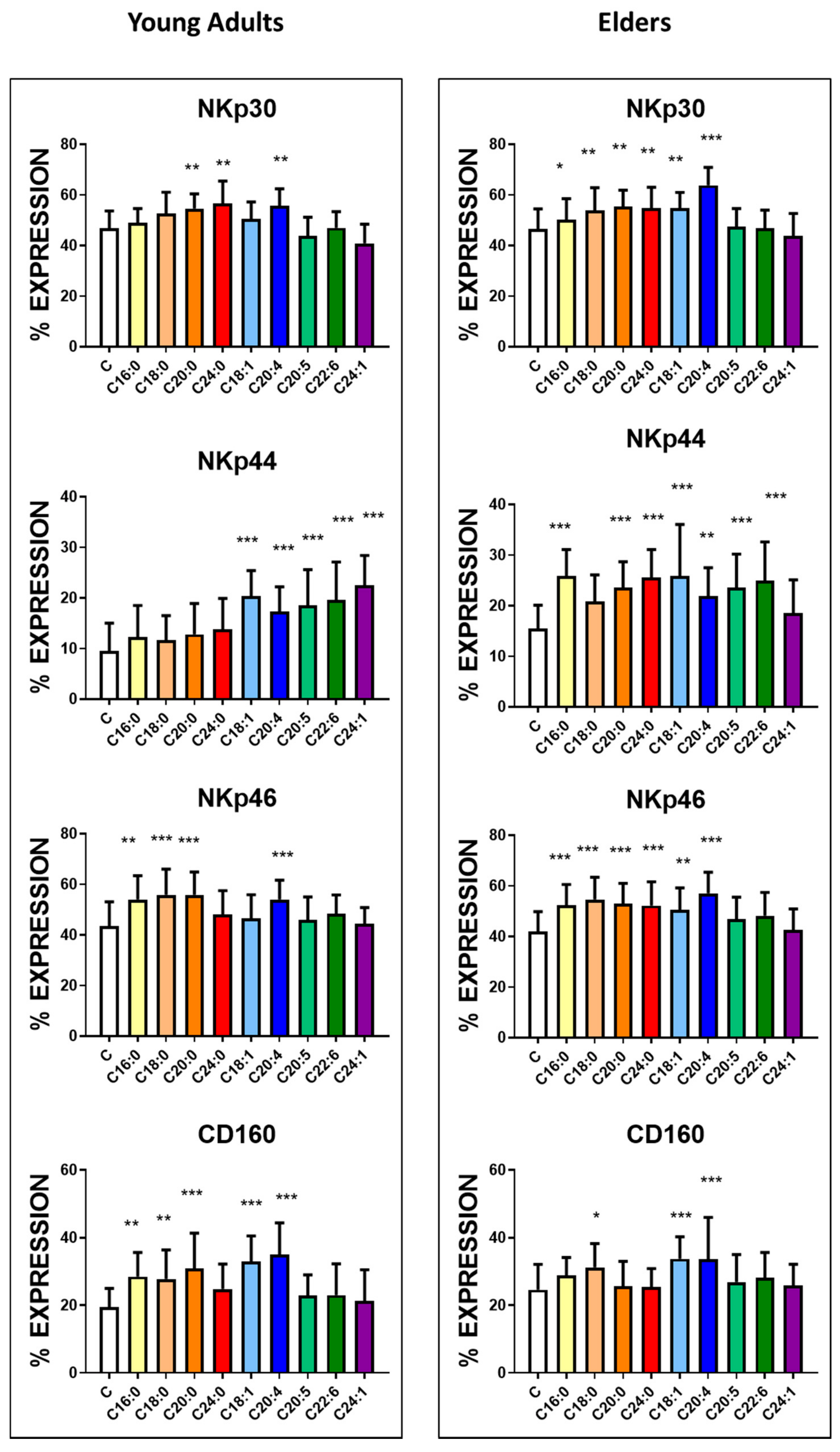
3.3. Basal Expression of Killing and KIR Receptors
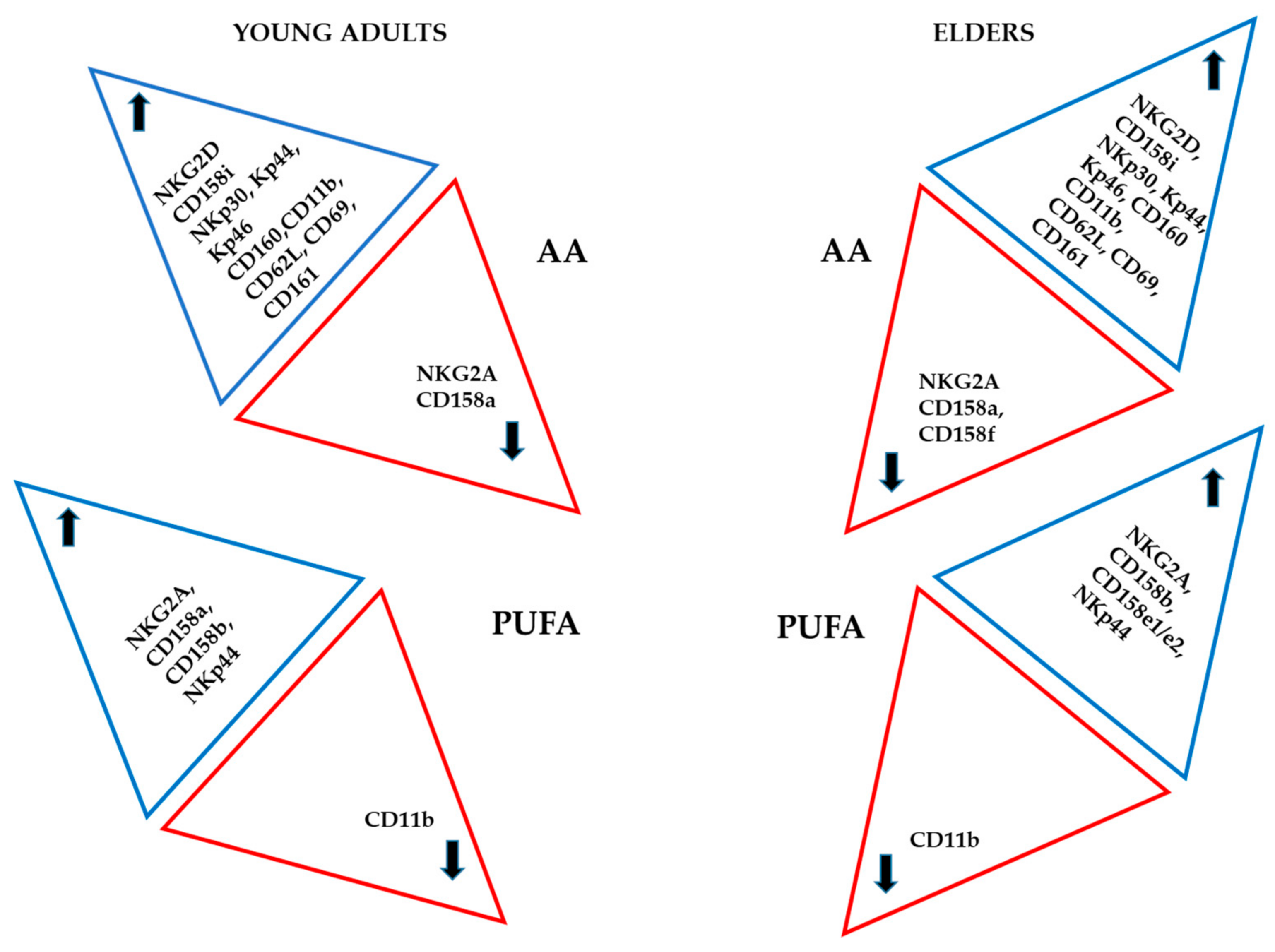
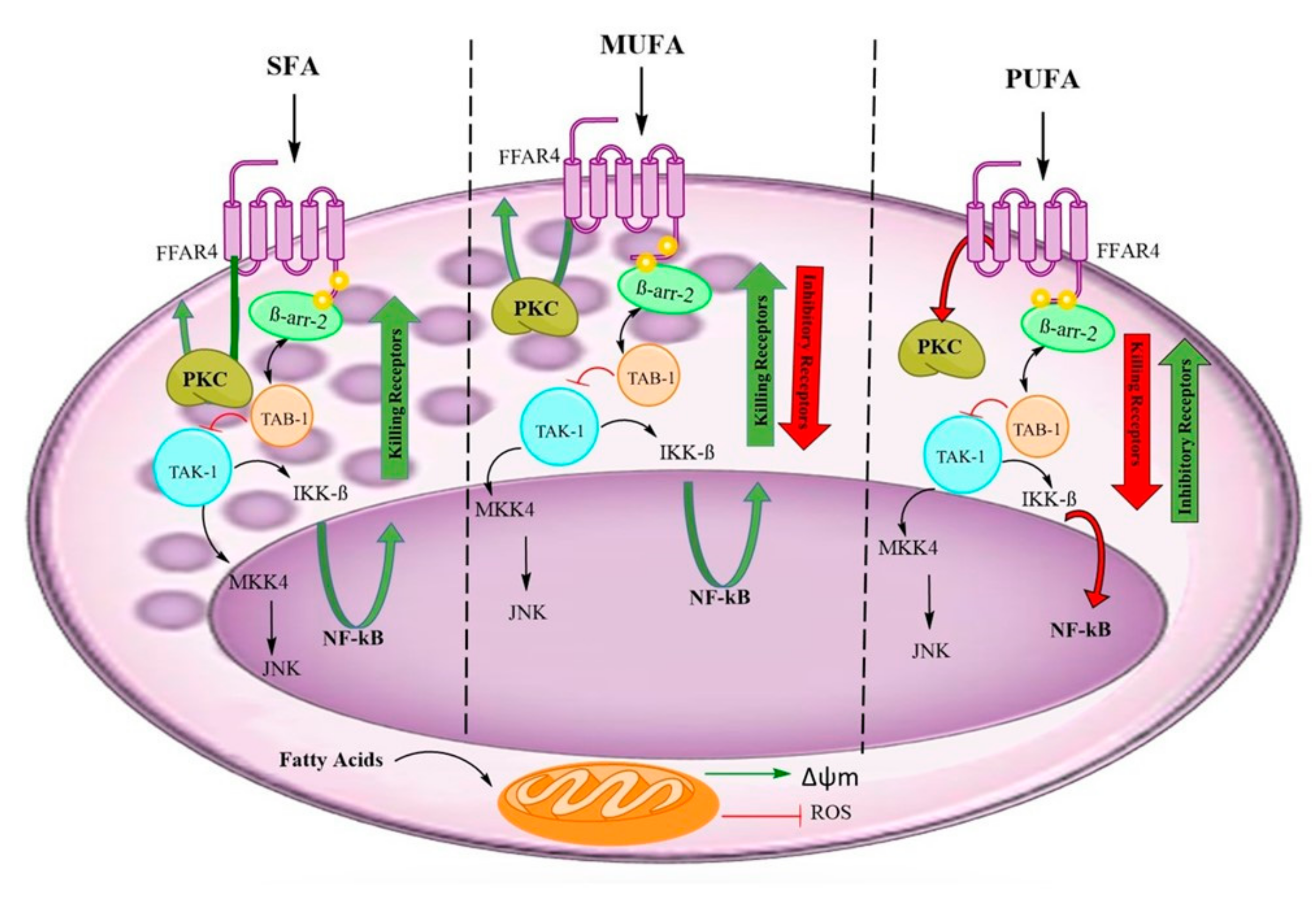
4. Discussion
5. Strengths and Limitations of the Study
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Caligiuri, M.A. Human natural killer cells. Blood 2008, 112, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Del Zotto, G.; Marcenaro, E.; Vacca, P.; Sivori, S.; Pende, D.; Della Chiesa, M.; Moretta, F.; Ingegnere, T.; Mingari, M.C.; Moretta, A.; et al. Markers and function of human NK cells in normal and pathological conditions. Cytom. B Clin. Cytom. 2017, 92, 100–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morvan, M.G.; Lanier, L.L. NK cells and cancer: You can teach innate cells new tricks. Nat. Rev. Cancer 2016, 16, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Fiatarone, M.A.; Morley, J.E.; Bloom, E.T.; Benton, D.; Solomon, G.F.; Makinodan, T. The Effect of Exercise on Natural Killer Cell Activity in Young and Old Subjects. J. Gerontol. 1989, 44, M37–M45. [Google Scholar] [CrossRef]
- Solana, R.; Campos, C.; Pera, A.; Tarazona, R. Shaping of NK cell subsets by aging. Curr. Opin. Immunol. 2014, 29, 56–61. [Google Scholar] [CrossRef]
- Mace, E.; Orange, J.S. Emerging insights into human health and NK cell biology from the study of NK cell deficiencies. Immunol. Rev. 2018, 287, 202–225. [Google Scholar] [CrossRef]
- Reed, R.G. Stress and immunological aging. Curr. Opin. Behav. Sci. 2019, 28, 38–43. [Google Scholar] [CrossRef]
- Fülöp, T.; Larbi, A.; Witkowski, J.M. Human Inflammaging. Gerontology 2019, 3, 1–10. [Google Scholar] [CrossRef]
- Caligiuri, S.P.B.; Parikh, M.; Stamenkovic, A.; Pierce, G.N.; Aukema, H.M. Dietary modulation of oxylipins in cardiovascular disease and aging. Am. J. Physiol. Circ. Physiol. 2017, 313, H903–H918. [Google Scholar] [CrossRef] [Green Version]
- Bäck, M.; Hansson, G.K. Omega-3 fatty acids, cardiovascular risk, and the resolution of inflammation. FASEB J. 2019, 33, 1536–1539. [Google Scholar] [CrossRef]
- Kyaw, T.; Peter, K.; Li, Y.; Tipping, P.; Toh, B.-H.; Bobik, A. Cytotoxic lymphocytes and atherosclerosis: Significance, mechanisms and therapeutic challenges. Br. J. Pharmacol. 2017, 174, 3956–3972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Sanctis, J.B.; Blanca, I.; Bianco, N.E. Expression of different lipoprotein receptors in natural killer cells and their effect on natural killer proliferative and cytotoxic activity. Immunology 1995, 86, 399–407. [Google Scholar] [PubMed]
- Abdelmagid, S.A.; Clarke, S.E.; Nielsen, D.E.; Badawi, A.; El-Sohemy, A.; Mutch, D.M.; Ma, D.W. Comprehensive profiling of plasma fatty acid concentrations in young healthy Canadian adults. PLoS ONE 2015, 10, e0116195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pararasa, C.; Ikwuobe, J.; Shigdar, S.; Boukouvalas, A.; Nabney, I.T.; Brown, J.E.; Devitt, A.; Bailey, C.J.; Bennett, S.J.; Griffiths, H.R. Age-associated changes in the long-chain fatty acid profile during healthy aging promote pro-inflammatory monocyte polarisation via PPARγ. Aging Cell. 2016, 15, 128–139. [Google Scholar] [CrossRef]
- Beyene, H.B.; Olshansky, G.; Smith, A.A.T.; Giles, C.; Huynh, K.; Cinel, M.; Mellett, N.A.; Cadby, G.; Hung, J.; Hui, J.; et al. High-coverage plasma lipidomics reveals novel sex-specific lipidomic fingerprints of age and BMI: Evidence from two large population cohort studies. PLoS Biol. 2020, 18, e3000870. [Google Scholar] [CrossRef]
- Purasiri, P.; McKechnie, A.; Heys, S.D.; Eremin, O. Modulation in vitro of human natural cytotoxicity, lymphocyte proliferative response to mitogens and cytokine production by essential fatty acids. Immunology 1997, 92, 166–172. [Google Scholar] [CrossRef]
- Calder, P.C.; Yaqoob, P.; Thies, F.; Wallace, F.A.; Miles, E.A. Fatty acids and lymphocyte functions. Br. J. Nutr. 2002, 87 (Suppl. 1), S31–S48. [Google Scholar] [CrossRef] [Green Version]
- Léveillé, P.; Chouinard-Watkins, R.; Windust, A.; Lawrence, P.; Cunnane, S.C.; Brenna, J.T.; Plourde, M. Metabolism of uniformly labeled 13 C-eicosapentaenoic acid and 13 C-arachidonic acid in young and old men. Am. J. Clin. Nutr. 2017, 106, 467–474. [Google Scholar] [CrossRef] [Green Version]
- Yaqoob, P. Ageing alters the impact of nutrition on immune function. Proc. Nutr. Soc. 2017, 76, 347–351. [Google Scholar] [CrossRef]
- De Sanctis, J.B.; Blanca, I.; Bianco, N.E. Regulatory effects of lipoprotein lipase on proliferative and cytotoxic activity of NK cells. J. Lipid Res. 1996, 37, 1987–2000. [Google Scholar]
- Gunturi, A.; Berg, R.E.; Forman, J. The Role of CD94/NKG2 in Innate and Adaptive Immunity. Immunol. Res. 2004, 30, 029–034. [Google Scholar] [CrossRef]
- Cruz-Muñoz, M.E.; Valenzuela-Vázquez, L.; Sánchez-Herrera, J.; Tapia, J.S. From the “missing self” hypothesis to adaptive NK cells: Insights of NK cell-mediated effector functions in immune surveillance. J. Leukoc. Biol. 2019, 105, 955–971. [Google Scholar] [CrossRef] [PubMed]
- Solana, R.; Tarazona, R.; Gayoso, I.; Lesur, O.; Dupuis, G.; Fulop, T. Innate immunosenescence: Effect of aging on cells and receptors of the innate immune system in humans. Semin. Immunol. 2012, 24, 331–341. [Google Scholar] [CrossRef]
- Le Garff-Tavernier, M.; Béziat, V.; Decocq, J.; Siguret, V.; Gandjbakhch, F.; Pautas, E.; Debré, P.; Merle-Beral, H.; Vieillard, V. Human NK cells display major phenotypic and functional changes over the lifespan. Aging Cell 2010, 9, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Oliveira, A.; Smith-Carvalho, M.; Porto, L.C.; Cardoso-Oliveira, J.; Ribeiro, A.D.S.; Falcão, R.R.; Abdelhay, E.; Bouzas, L.F.; Thuler, L.C.S.; Ornellas, M.H.; et al. Age-related changes in natural killer cell receptors from childhood through old age. Hum. Immunol. 2011, 72, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Manser, A.R.; Uhrberg, M. Age-related changes in natural killer cell repertoires: Impact on NK cell function and immune surveillance. Cancer Immunol. Immunother. 2016, 65, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Campos, C.; Pera, A.; Lopez-Fernandez, I.; Alonso, C.; Tarazona, R.; Solana, R. Proinflammatory status influences NK cells subsets in the elderly. Immunol. Lett. 2014, 162, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Keenan, C.R.; Allan, R.S. Epigenomic drivers of immune dysfunction in aging. Aging Cell 2018, 18, e12878. [Google Scholar] [CrossRef] [Green Version]
- Phan, M.-T.; Chun, S.; Kim, S.-H.; Ali, A.K.; Lee, S.-H.; Kim, S.; Kim, S.-H.; Cho, D. Natural killer cell subsets and receptor expression in peripheral blood mononuclear cells of a healthy Korean population: Reference range, influence of age and sex, and correlation between NK cell receptors and cytotoxicity. Hum. Immunol. 2017, 78, 103–112. [Google Scholar] [CrossRef]
- Mahapatra, S.; Mace, E.M.; Minard, C.G.; Forbes, L.R.; Vargas-Hernandez, A.; Duryea, T.K.; Makedonas, G.; Banerjee, P.P.; Shearer, W.T.; Orange, J.S. High-resolution phenotyping identifies NK cell subsets that distinguish healthy children from adults. PLoS ONE 2017, 12, e0181134. [Google Scholar] [CrossRef]
- Van der Geest, K.; Kroesen, B.J.; Horst, G.; Abdulahad, W.H.; Brouwer, E.; Boots, A. Impact of Aging on the Frequency, Phenotype, and Function of CD161-Expressing T Cells. Front. Immunol. 2018, 9, 752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McPhail, L.; Clayton, C.; Snyderman, R. A potential second messenger role for unsaturated fatty acids: Activation of Ca2+-dependent protein kinase. Science 1984, 224, 622–625. [Google Scholar] [CrossRef]
- Ma, J.S.; Haydar, T.F.; Radoja, S. Protein kinase C delta localises to secretory lysosomes in CD8+ CTL and directly mediates TCR signals leading to granule exocytosis-mediated cytotoxicity. J. Immunol. 2008, 181, 4716–4722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez-Curto, E.; Milligan, G. Metabolism meets immunity: The role of free fatty acid receptors in the immune system. Biochem. Pharmacol. 2016, 114, 3–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Dietetic Association; Dietitians of Canada, American College of Sports Medicine; Rodriguez, N.R.; Di Marco, N.M.; Langley, S. American College of Sports Medicine position stand. Nutrition and athletic performance. Med. Sci. Sports Exerc. 2009, 41, 709–731. [Google Scholar]
- Guilbault, C.; Wojewodka, G.; Saeed, Z.; Hajduch, M.; Matouk, E.; De Sanctis, J.B.; Radzioch, D. Cystic Fibrosis Fatty Acid Imbalance Is Linked to Ceramide Deficiency and Corrected by Fenretinide. Am. J. Respir. Cell Mol. Biol. 2009, 41, 100–106. [Google Scholar] [CrossRef]
- Franceschi, C.; Zaikin, A.; Gordleeva, S.; Ivanchenko, M.; Bonifazi, F.; Storci, G.; Bonafè, M. Inflammaging 2018: An update and a model. Semin. Immunol. 2018, 40, 1–5. [Google Scholar] [CrossRef]
- Rasmussen, L.B.; Kiens, B.; Pedersen, B.K.; Richter, E.A. Effect of diet and plasma fatty acid composition on immune status in elderly men. Am. J. Clin. Nutr. 1994, 59, 572–577. [Google Scholar] [CrossRef] [Green Version]
- Steffen, B.T.; Duprez, D.; Szklo, M. (Moyses); Guan, W.; Tsai, M. (Michael). Circulating oleic acid levels are related to greater risks of cardiovascular events and all-cause mortality: The Multi-Ethnic Study of Atherosclerosis. J. Clin. Lipidol. 2018, 12, 1404–1412. [Google Scholar] [CrossRef]
- González, S.; López, P.; Margolles, A.; Suárez, P.L.; Patterson, A.M.; Cuervo, A.; Reyes-Gavilán, C.G.D.L.; Gueimonde, M. Fatty acids intake and immune parameters in the elderly. Nutr. Hosp. 2013, 28, 474–478. [Google Scholar]
- Mizota, T.; Fujita-Kambara, C.; Matsuya, N.; Hamasaki, S.; Fukudome, T.; Goto, H.; Nakane, S.; Kondo, T.; Matsuo, H. Effect of dietary fatty acid composition on Th1/Th2 polarisation in lymphocytes. JPEN J Parenter Enteral Nutr. 2009, 33, 390–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thies, F.; Nebe-Von-Caron, G.; Powell, J.R.; Yaqoob, P.; Newsholme, E.A.; Calder, P.C. Dietary supplementation with eicosapentaenoic acid, but not with other long-chain n−3 or n−6 polyunsaturated fatty acids, decreases natural killer cell activity in healthy subjects aged >55 y. Am. J. Clin. Nutr. 2001, 73, 539–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamashita, N.; Yokoyama, A.; Hamazaki, T.; Yano, S. Inhibition of natural killer cell activity of human lymphocytes by eicosapentaenoic acid. Biochem. Biophys. Res. Commun. 1986, 138, 1058–1067. [Google Scholar] [CrossRef]
- Dalli, J.; Pistorius, K.; Walker, M.E. Novel n-3 Docosapentaneoic Acid-Derived Pro-resolving Mediators Are Vasculoprotective and Mediate the Actions of Statins in Controlling Inflammation. Retinal. Degener. Dis. 2019, 1161, 65–75. [Google Scholar] [CrossRef]
- Poznanski, S.M.; Ashkar, A.A. What Defines NK Cell Functional Fate: Phenotype or Metabolism? Front. Immunol. 2019, 10, 1414. [Google Scholar] [CrossRef] [PubMed]
- Le Bouteiller, P.; Tabiasco, J.; Polgar, B.; Kozma, N.; Giustiniani, J.; Siewiera, J.; Berrebi, A.; Aguerre-Girr, M.; Bensussan, A.; Jabrane-Ferrat, N. CD160: A unique activating NK cell receptor. Immunol. Lett. 2011, 138, 93–96. [Google Scholar] [CrossRef]
- Zuo, J.; Shan, Z.; Zhou, L.; Yu, J.; Liu, X.; Gao, Y. Increased CD160 expression on circulating natural killer cells in atherogenesis. J. Transl. Med. 2015, 13, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Fu, B.; Tian, Z.; Wei, H. Subsets of human natural killer cells and their regulatory effects. Immunology 2014, 141, 483–489. [Google Scholar] [CrossRef]
- Pinkosky, S.L.; Scott, J.W.; Desjardins, E.M.; Smith, B.K.; Day, E.A.; Ford, R.J.; Langendorf, C.G.; Ling, N.X.Y.; Nero, T.L.; Loh, K.; et al. Long-chain fatty acyl-CoA esters regulate metabolism via allosteric control of AMPK β1 isoforms. Nat. Metab. 2020, 2, 873–881. [Google Scholar] [CrossRef]
- Le Luduec, J.-B.; Boudreau, J.E.; Freiberg, J.C.; Hsu, K.C. Novel Approach to Cell Surface Discrimination Between KIR2DL1 Subtypes and KIR2DS1 Identifies Hierarchies in NK Repertoire, Education, and Tolerance. Front. Immunol. 2019, 10, 734. [Google Scholar] [CrossRef] [Green Version]
- Gumá, M.; Angulo, A.; Vilches, C.; Gómez-Lozano, N.; Malats, N.; López-Botet, M. Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood 2004, 104, 3664–3671. [Google Scholar] [CrossRef] [Green Version]
- Juárez-Vega, G.; Rangel-Ramírez, V.; Monsiváis-Urenda, A.; Niño-Moreno, P.; Garcia-Sepúlveda, C.; Noyola, D.E.; González-Amaro, R. Comparative analysis of NK cell receptor repertoire in adults and very elderly subjects with cytomegalovirus infection. Hum. Immunol. 2017, 78, 274–280. [Google Scholar] [CrossRef] [PubMed]


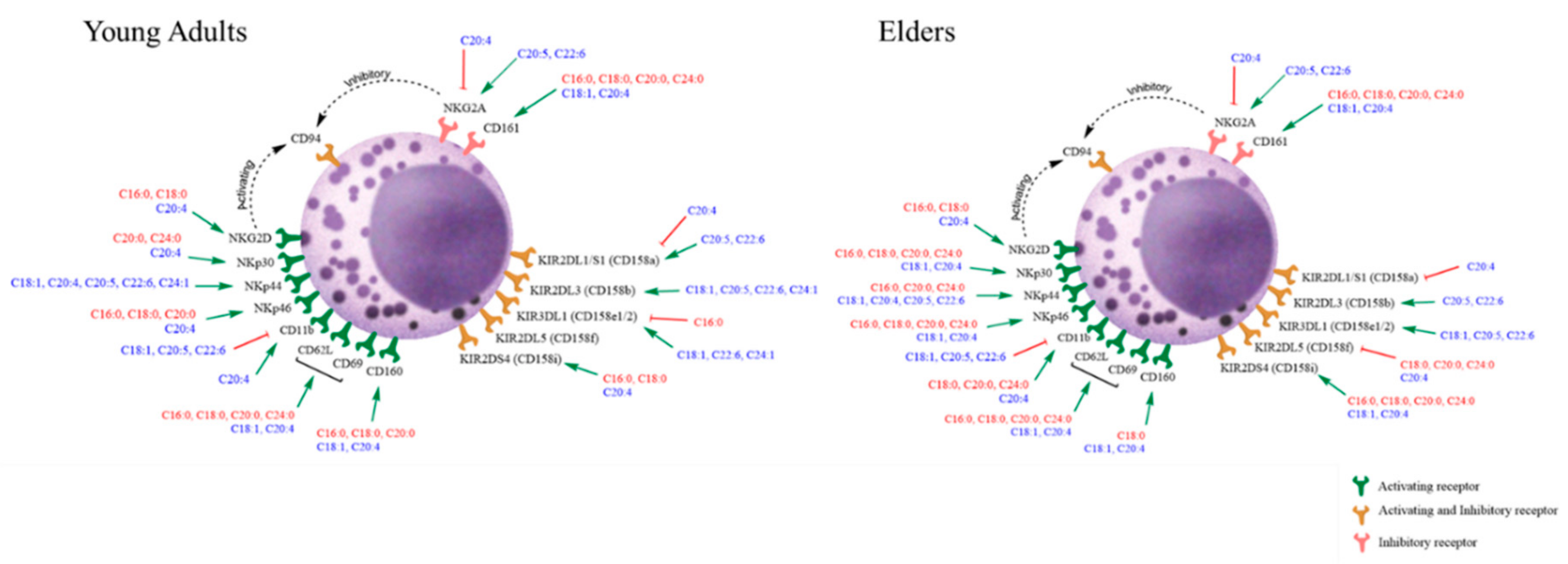
| Group of Individuals | Young Adults | Elders | p |
| N | 30 | 30 | |
| Age | 23 ± 4 | 63 ± 5 | 0.0001 |
| BMI | 22.1 ± 1.3 | 22.9 ± 2.5 | 0.95 |
| Non sterified fatty acids µmol/L | 398 ± 126 | 415 ± 125 | 0.5 |
| Total Fatty Acid (FAA) | FAA µmol/L | FAA µmol/L | p |
| Palmitic (PA) | 275.5 ± 15.6 | 250.1 ± 25.2 | 0.3 |
| Stearic (SA) | 29.2 ± 11.3 | 26.3 ± 16.1 | 0.5 |
| Oleic (OA) | 35.1 ± 14.4 | 50.4 ± 10.6 | 0.01 |
| Arachidic (ARA) | 6.1 ± 2.2 | 7.0 ± 4.6 | 0.8 |
| Arachidonic (AA) | 43.6 ± 18.2 | 68.3 ± 12.2 | 0.001 |
| Eicosapentaenoic (EPA) | 12.1 ± 5.4 | 9.4 ± 4.1 | 0.3 |
| Docosapentaenoic (DPA) | 21.8± 7.6 | 14.8± 6.1 | 0.01 |
| Docosahexaenoic (DHA) | 21.2 ± 5.1 | 10.2 ± 4.3 | 0.01 |
| Lignoceric (LA) | 5.1 ± 2.1 | 15.1 ± 7.4 | 0.001 |
| Nervonic (NA) | 11.3 ± 3.1 | 36.3 ± 4.1 | 0.0001 |
| Young Adults | Elders | Young Adults | Elders | |||
|---|---|---|---|---|---|---|
| % | % | p | MFI | MFI | p | |
| CD94 | 95.5 ± 4.6 | 89.6 ± 9.5 | <0.005 | 1250 ± 85 | 1075 ± 115 | <0.0001 |
| NKG2A | 28.6 ± 3.9 | 39.5 ± 4.2 | <0.0001 | 355 ± 55 | 501 ± 92 | <0.0001 |
| NKG2D | 76.3 ± 6.1 | 65.1 ± 3.1 | <0.0001 | 1072 ± 60 | 910 ± 55 | <0.0001 |
| NKp30 | 46.8 ± 6.9 | 43.4 ± 8.1 | NS | 1299 ± 69 | 1270 ± 81 | NS |
| NKp44 | 9.5 ± 5.5 | 15.5 ± 4.6 | <0.0001 | 430 ± 35 | 548 ± 46 | <0.0001 |
| NKp46 | 43.5 ± 9.6 | 41.9 ± 7.9 | NS | 1115 ± 56 | 1080 ± 79 | NS |
| CD160 | 19.5 ± 5.5 | 24.6 ± 7.5 | <0.005 | 495 ± 56 | 546 ± 65 | <0.005 |
| CD161 | 22.2 ± 12.2 | 25.2 ± 6.8 | NS | 1072 ± 82 | 1112 ± 68 | NS |
| KIR2DL1/S1(CD158a) | 25.5± 9.5 | 31.6 ± 7.4 | <0.01 | 475 ± 58 | 572 ± 74 | <0.0001 |
| KIR2DL3 (CD158b) | 15.8 ± 9.3 | 13.4 ± 5.4 | NS | 390 ± 33 | 376 ± 22 | NS |
| KIR3DL1 (CD158e1/2) | 16.6 ± 4.6 | 14.8 ± 6.5 | <0.05 | 550 ± 46 | 504 ± 76 | <0.01 |
| KIR2DL5 (CD158f) | 15.9 ± 5.5 | 26.5 ± 7.2 | <0.0001 | 354 ± 45 | 388 ± 32 | <0.005 |
| KIR2DS4 (CD158i) | 23.3 ± 7.4 | 12.2 ± 5.1 | <0.0001 | 650 ± 74 | 390 ± 51 | <0.0001 |
| CD62L | 30.5 ± 6.5 | 42.6 ± 7.2 | <0.001 | 708 ± 95 | 805 ± 92 | <0.0001 |
| CD69 | 4.5 ± 3.2 | 8.9 ± 3.6 | <0.0001 | 455 ± 75 | 583 ± 72 | <0.0001 |
| CD11b | 85.9 ± 8.6 | 75.9 ± 9.5 | <0.0001 | 2859 ± 96 | 2396 ± 125 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Sanctis, J.B.; Dumut, D.C.; Radzioch, D.; Hajdúch, M. Functionally Relevant Differences in Plasma Fatty Acid Composition and Expression of Cytotoxic and Inhibitory NK Cell Receptors between Healthy Young and Healthy Elder Adults. Nutrients 2020, 12, 3641. https://doi.org/10.3390/nu12123641
De Sanctis JB, Dumut DC, Radzioch D, Hajdúch M. Functionally Relevant Differences in Plasma Fatty Acid Composition and Expression of Cytotoxic and Inhibitory NK Cell Receptors between Healthy Young and Healthy Elder Adults. Nutrients. 2020; 12(12):3641. https://doi.org/10.3390/nu12123641
Chicago/Turabian StyleDe Sanctis, Juan Bautista, Daciana Catalina Dumut, Danuta Radzioch, and Marián Hajdúch. 2020. "Functionally Relevant Differences in Plasma Fatty Acid Composition and Expression of Cytotoxic and Inhibitory NK Cell Receptors between Healthy Young and Healthy Elder Adults" Nutrients 12, no. 12: 3641. https://doi.org/10.3390/nu12123641
APA StyleDe Sanctis, J. B., Dumut, D. C., Radzioch, D., & Hajdúch, M. (2020). Functionally Relevant Differences in Plasma Fatty Acid Composition and Expression of Cytotoxic and Inhibitory NK Cell Receptors between Healthy Young and Healthy Elder Adults. Nutrients, 12(12), 3641. https://doi.org/10.3390/nu12123641






