miRNA Expression Profiles in Luminal A Breast Cancer—Implications in Biology, Prognosis, and Prediction of Response to Hormonal Treatment
Abstract
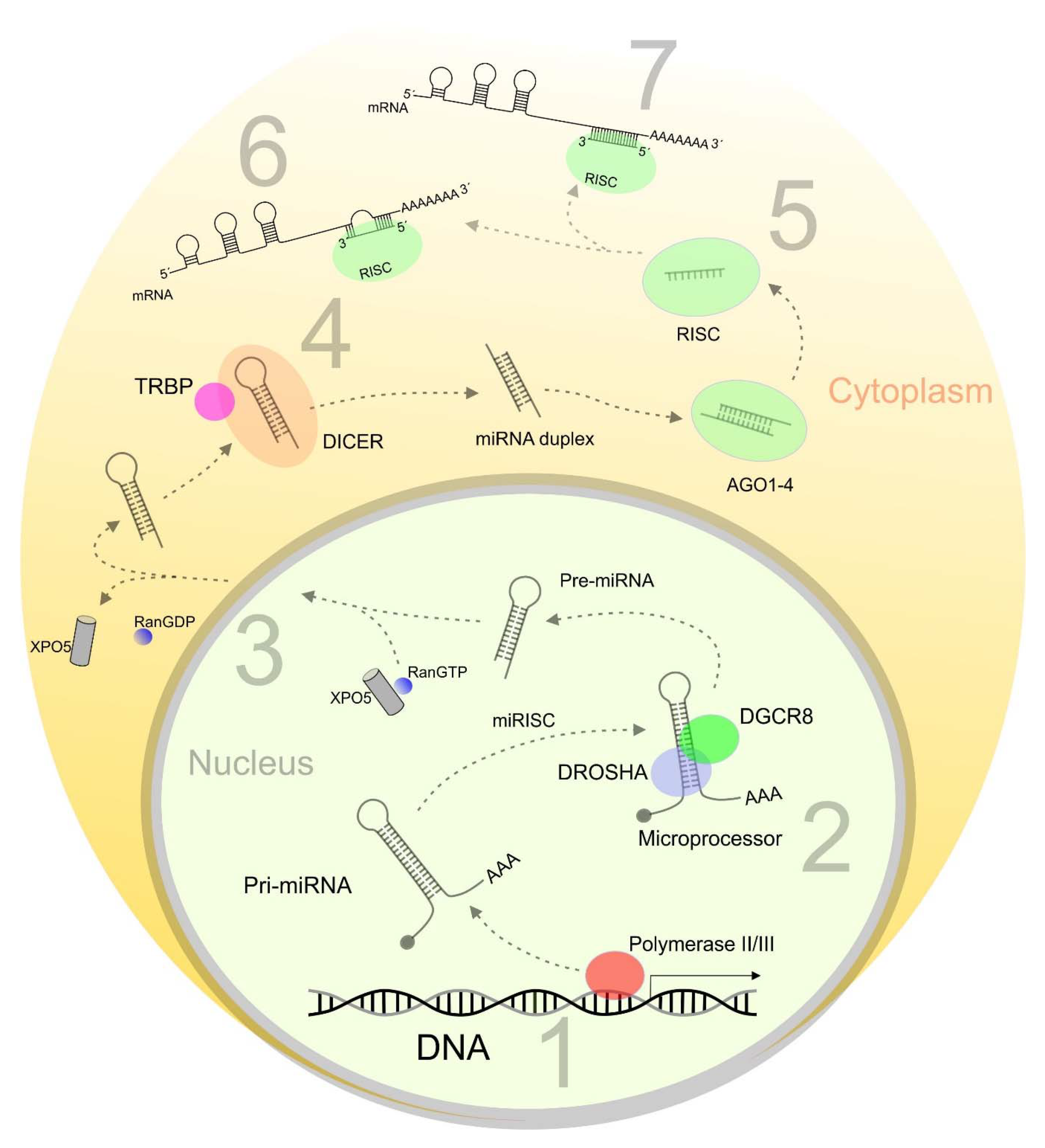
:1. Introduction
2. DifferentialMicroRNA Expression in Luminal BCSubtypes
3. Specific miRNA Expressionin Early and Metastatic Stage in LumA BC
4. miRNAs and BC Prognosis
5. miRNAs and Their Role in Endocrine Resistance
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Hammond, M.E.H.; Hayes, D.F.; Dowsett, M.; Allred, D.C.; Hagerty, K.L.; Badve, S.; Fitzgibbons, P.L.; Francis, G.; Goldstein, N.S.; Hayes, M.; et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J. Clin. Oncol. 2010, 28, 2784–2795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ethier, J.L.; Ocaña, A.; Rodríguez Lescure, A.; Ruíz, A.; Alba, E.; Calvo, L.; Ruíz-Borrego, M.; Santaballa, A.; Rodríguez, C.A.; Crespo, C.; et al. Outcomes of single versus double hormone receptor–positive breast cancer. A GEICAM/9906 sub-study. Eur. J. Cancer 2018, 94, 199–205. [Google Scholar] [CrossRef]
- Cantini, L.; Bertoli, G.; Cava, C.; Dubois, T.; Zinovyev, A.; Caselle, M.; Castiglioni, I.; Barillot, E.; Martignetti, L. Identification of microRNA clusters cooperatively acting on epithelial to mesenchymal transition in triple negative breast cancer. Nucleic Acids Res. 2019, 47, 2205–2215. [Google Scholar] [CrossRef] [Green Version]
- Peppercorn, J.; Perou, C.M.; Carey, L.A. Molecular subtypes in breast cancer evaluation and management: Divide and conquer. Cancer Invest. 2008, 26, 1–10. [Google Scholar] [CrossRef]
- Russnes, H.G.; Lingjærde, O.C.; Børresen-Dale, A.-L.; Caldas, C. Breast Cancer Molecular Stratification: From Intrinsic Subtypes to Integrative Clusters. Am. J. Pathol. 2017, 187, 2152–2162. [Google Scholar] [CrossRef]
- Curtis, C.; Shah, S.P.; Chin, S.-F.; Turashvili, G.; Rueda, O.M.; Dunning, M.J.; Speed, D.; Lynch, A.G.; Samarajiwa, S.; Yuan, Y.; et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature 2012, 486, 346–352. [Google Scholar] [CrossRef]
- ENCODE Project Consortium An integrated encyclopedia of DNA elements in the human genome. Nature 2012, 489, 57–74. [CrossRef]
- Qi, X.; Zhang, D.-H.; Wu, N.; Xiao, J.-H.; Wang, X.; Ma, W. ceRNA in cancer: Possible functions and clinical implications. J. Med. Genet. 2015, 52, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Tay, Y.; Rinn, J.; Pandolfi, P.P. The multilayered complexity of ceRNA crosstalk and competition. Nature 2014, 505, 344–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzuti, M.; Filosa, G.; Melzi, V.; Calandriello, L.; Dioni, L.; Bollati, V.; Bresolin, N.; Comi, G.P.; Barabino, S.; Nizzardo, M.; et al. MicroRNA expression analysis identifies a subset of downregulated miRNAs in ALS motor neuron progenitors. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Paul, J.; Muthuswami, R. MicroRNA: Perspectives in Health and Diseases; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Zubor, P.; Kubatka, P.; Dankova, Z.; Gondova, A.; Kajo, K.; Hatok, J.; Samec, M.; Jagelkova, M.; Krivus, S.; Holubekova, V.; et al. miRNA in a multiomic context for diagnosis, treatment monitoring and personalized management of metastatic breast cancer. Future Oncol. 2018, 14, 1847–1867. [Google Scholar] [CrossRef] [PubMed]
- Jevšinek Skok, D.; Hauptman, N.; Boštjančič, E.; Zidar, N. The integrative knowledge base for miRNA-mRNA expression in colorectal cancer. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Al-thoubaity, F.K. Molecular classification of breast cancer: A retrospective cohort study. Ann. Med. Surg. 2020, 49, 44–48. [Google Scholar] [CrossRef]
- Kudela, E.; Samec, M.; Kubatka, P.; Nachajova, M.; Laucekova, Z.; Liskova, A.; Dokus, K.; Biringer, K.; Simova, D.; Gabonova, E.; et al. Breast Cancer in Young Women: Status Quo and Advanced Disease Management by a Predictive, Preventive, and Personalized Approach. Cancers 2019, 11, 1791. [Google Scholar] [CrossRef] [Green Version]
- Fragomeni, S.M.; Sciallis, A.; Jeruss, J.S. Molecular subtypes and local-regional control of breast cancer. Surg. Oncol. Clin. N. Am. 2018, 27, 95–120. [Google Scholar] [CrossRef]
- Dai, X.; Xiang, L.; Li, T.; Bai, Z. Cancer Hallmarks, Biomarkers and Breast Cancer Molecular Subtypes. J. Cancer 2016, 7, 1281–1294. [Google Scholar] [CrossRef] [Green Version]
- Tang, P.; Tse, G.M. Immunohistochemical Surrogates for Molecular Classification of Breast Carcinoma: A 2015 Update. Arch. Pathol. Lab. Med. 2016, 140, 806–814. [Google Scholar] [CrossRef] [Green Version]
- Jin, C.; Yan, B.; Lu, Q.; Lin, Y.; Ma, L. Reciprocal regulation of Hsa-miR-1 and long noncoding RNA MALAT1 promotes triple-negative breast cancer development. Tumour Biol. 2016, 37, 7383–7394. [Google Scholar] [CrossRef] [PubMed]
- Crippa, E.; Lusa, L.; De Cecco, L.; Marchesi, E.; Calin, G.A.; Radice, P.; Manoukian, S.; Peissel, B.; Daidone, M.G.; Gariboldi, M.; et al. miR-342 regulates BRCA1 expression through modulation of ID4 in breast cancer. PLoS ONE 2014, 9, e87039. [Google Scholar] [CrossRef] [PubMed]
- Shin, V.Y.; Kwong, A. Response to: Comment on “Circulating cell-free miRNAs as biomarker for triple-negative breast cancer”. Br. J. Cancer 2016, 114, e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braicu, C.; Raduly, L.; Morar-Bolba, G.; Cojocneanu, R.; Jurj, A.; Pop, L.-A.; Pileczki, V.; Ciocan, C.; Moldovan, A.; Irimie, A.; et al. Aberrant miRNAs expressed in HER-2 negative breast cancers patient. J. Exp. Clin. Cancer Res. 2018, 37, 257. [Google Scholar] [CrossRef] [Green Version]
- Bai, X.; Han, G.; Liu, Y.; Jiang, H.; He, Q. miRNA-20a-5p promotes the growth of triple-negative breast cancer cells through targeting RUNX3. Biomed. Pharmacother. 2018, 103, 1482–1489. [Google Scholar] [CrossRef]
- Savad, S.; Mehdipour, P.; Miryounesi, M.; Shirkoohi, R.; Fereidooni, F.; Mansouri, F.; Modarressi, M.H. Expression analysis of MiR-21, MiR-205, and MiR-342 in breast cancer in Iran. Asian Pac. J. Cancer Prev. 2012, 13, 873–877. [Google Scholar] [CrossRef] [Green Version]
- Søkilde, R.; Persson, H.; Ehinger, A.; Pirona, A.C.; Fernö, M.; Hegardt, C.; Larsson, C.; Loman, N.; Malmberg, M.; Rydén, L.; et al. Refinement of breast cancer molecular classification by miRNA expression profiles. BMC Genom. 2019, 20, 503. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Zou, W.; Wang, Y.; Liao, Z.; Li, L.; Zhai, Y.; Zhang, L.; Gu, S.; Zhao, X. Plasma-based microRNA signatures in early diagnosis of breast cancer. Mol. Genet. Genomic Med. 2020, 8. [Google Scholar] [CrossRef] [Green Version]
- Haakensen, V.D.; Nygaard, V.; Greger, L.; Aure, M.R.; Fromm, B.; Bukholm, I.R.K.; Lüders, T.; Chin, S.-F.; Git, A.; Caldas, C.; et al. Subtype-specific micro-RNA expression signatures in breast cancer progression. Int. J. Cancer 2016, 139, 1117–1128. [Google Scholar] [CrossRef]
- Moi, L.; Braaten, T.; Al-Shibli, K.; Lund, E.; Busund, L.-T.R. Differential expression of the miR-17-92 cluster and miR-17 family in breast cancer according to tumor type; results from the Norwegian Women and Cancer (NOWAC) study. J. Transl. Med. 2019, 17, 334. [Google Scholar] [CrossRef] [Green Version]
- Arabkari, V.; Clancy, E.; Dwyer, R.M.; Kerin, M.J.; Kalinina, O.; Holian, E.; Newell, J.; Smith, T.J. Relative and Absolute Expression Analysis of MicroRNAs Associated with Luminal A Breast Cancer- A Comparison. Pathol. Oncol. Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Toyama, T.; Takahashi, S.; Yoshimoto, N.; Iwasa, M.; Asano, T.; Fujii, Y.; Yamashita, H. miR-1290 and its potential targets are associated with characteristics of estrogen receptor α-positive breast cancer. Endocr. Relat. Cancer 2013, 20, 91–102. [Google Scholar] [CrossRef] [Green Version]
- Van Schooneveld, E.; Wildiers, H.; Vergote, I.; Vermeulen, P.B.; Dirix, L.Y.; Van Laere, S.J. Dysregulation of microRNAs in breast cancer and their potential role as prognostic and predictive biomarkers in patient management. Breast Cancer Res. 2015, 17. [Google Scholar] [CrossRef] [Green Version]
- Iorio, M.V.; Ferracin, M.; Liu, C.-G.; Veronese, A.; Spizzo, R.; Sabbioni, S.; Magri, E.; Pedriali, M.; Fabbri, M.; Campiglio, M.; et al. MicroRNA Gene Expression Deregulation in Human Breast Cancer. Cancer Res. 2005, 65, 7065–7070. [Google Scholar] [CrossRef] [Green Version]
- Blenkiron, C.; Goldstein, L.D.; Thorne, N.P.; Spiteri, I.; Chin, S.-F.; Dunning, M.J.; Barbosa-Morais, N.L.; Teschendorff, A.E.; Green, A.R.; Ellis, I.O.; et al. MicroRNA expression profiling of human breast cancer identifies new markers of tumor subtype. Genome Biol. 2007, 8, R214. [Google Scholar] [CrossRef] [Green Version]
- Weigelt, B.; Peterse, J.L.; van ’t Veer, L.J. Breast cancer metastasis: Markers and models. Nat. Rev. Cancer 2005, 5, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Calin, G.A.; Croce, C.M. MicroRNA signatures in human cancers. Nat. Rev. Cancer 2006, 6, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Nassar, F.J.; Nasr, R.; Talhouk, R. MicroRNAs as biomarkers for early breast cancer diagnosis, prognosis and therapy prediction. Pharmacol. Ther. 2017, 172, 34–49. [Google Scholar] [CrossRef]
- Fan, T.; Mao, Y.; Sun, Q.; Liu, F.; Lin, J.; Liu, Y.; Cui, J.; Jiang, Y. Branched rolling circle amplification method for measuring serum circulating microRNA levels for early breast cancer detection. Cancer Sci. 2018, 109, 2897–2906. [Google Scholar] [CrossRef] [Green Version]
- Heneghan, H.M.; Miller, N.; Kelly, R.; Newell, J.; Kerin, M.J. Systemic miRNA-195 Differentiates Breast Cancer from Other Malignancies and Is a Potential Biomarker for Detecting Noninvasive and Early Stage Disease. Oncologist 2010, 15, 673–682. [Google Scholar] [CrossRef] [Green Version]
- McDermott, A.M.; Miller, N.; Wall, D.; Martyn, L.M.; Ball, G.; Sweeney, K.J.; Kerin, M.J. Identification and validation of oncologic miRNA biomarkers for luminal A-like breast cancer. PLoS ONE 2014, 9, e87032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kodahl, A.R.; Zeuthen, P.; Binder, H.; Knoop, A.S.; Ditzel, H.J. Alterations in Circulating miRNA Levels following Early-Stage Estrogen Receptor-Positive Breast Cancer Resection in Post-Menopausal Women. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Guo, H.; Zeng, X.; Li, H.; Guo, Y.; Wang, T.; Guo, H.; Zhu, G.; Wang, L.; Zhou, H.; Liu, K.; et al. Plasma miR-1273g-3p acts as a potential biomarker for early Breast Ductal Cancer diagnosis. An. Acad. Bras. Cienc. 2020, 92, e20181203. [Google Scholar] [CrossRef] [Green Version]
- McAnena, P.; Tanriverdi, K.; Curran, C.; Gilligan, K.; Freedman, J.E.; Brown, J.A.L.; Kerin, M.J. Circulating microRNAs miR-331 and miR-195 differentiate local luminal a from metastatic breast cancer. BMC Cancer 2019, 19, 436. [Google Scholar] [CrossRef] [Green Version]
- Wanandi, S.I.; Syahrani, R.A.; Arumsari, S.; Wideani, G.; Hardiany, N.S. Profiling of Gene Expression Associated with Stemness and Aggressiveness of ALDH1A1-Expressing Human Breast Cancer Cells. Malays. J. Med. Sci. 2019, 26, 38–52. [Google Scholar] [CrossRef]
- Holliday, D.L.; Speirs, V. Choosing the right cell line for breast cancer research. Breast Cancer Res. 2011, 13, 215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guttilla, I.K.; Adams, B.D.; White, B.A. ERα, microRNAs, and the epithelial-mesenchymal transition in breast cancer. Trends Endocrinol. Metab. 2012, 23, 73–82. [Google Scholar] [CrossRef]
- Lin, J.; Wang, L.; Gao, J.; Zhu, S. MiR-203 inhibits estrogen-induced viability, migration and invasion of estrogen receptor α-positive breast cancer cells. Exp. Ther. Med. 2017, 14, 2702–2708. [Google Scholar] [CrossRef] [Green Version]
- Rennebeck, G.; Martelli, M.; Kyprianou, N. Anoikis and Survival Connections in the Tumor Microenvironment: Is There a Role in Prostate Cancer Metastasis? Cancer Res. 2005, 65, 11230–11235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malagobadan, S.; Ho, C.S.; Nagoor, N.H. MicroRNA-6744-5p promotes anoikis in breast cancer and directly targets NAT1 enzyme. Cancer Biol. Med. 2020, 17, 101–111. [Google Scholar] [CrossRef]
- Van Zijl, F.; Krupitza, G.; Mikulits, W. Initial steps of metastasis: Cell invasion and endothelial transmigration. Mutat. Res. 2011, 728, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Yang, Y.; Wu, H. MicroRNA-765 alleviates the malignant progression of breast cancer via interacting with EZH1. Am. J. Transl. Res. 2019, 11, 4500–4507. [Google Scholar]
- Lin, C.; Gao, B.; Yan, X.; Lei, Z.; Chen, K.; Li, Y.; Zeng, Q.; Chen, Z.; Li, H. MicroRNA 628 suppresses migration and invasion of breast cancer stem cells through targeting SOS1. Onco Targets Ther. 2018, 11, 5419–5428. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.; Wang, Y.; Peng, J.; Shen, Q.; Chen, M.; Tang, W.; Li, X.; Cai, C.; Wang, B.; Cai, S.; et al. Mitochondrial calcium uniporter as a target of microRNA-340 and promoter of metastasis via enhancing the Warburg effect. Oncotarget 2017, 8, 83831–83844. [Google Scholar] [CrossRef] [Green Version]
- Rahimi, M.; Sharifi-Zarchi, A.; Zarghami, N.; Geranpayeh, L.; Ebrahimi, M.; Alizadeh, E. Down-Regulation of miR-200c and Up-Regulation of miR-30c Target both Stemness and Metastasis Genes in Breast Cancer. Cell J. 2020, 21, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Örs Kumoğlu, G.; Döşkaya, M.; Gulce Iz, S. The biomarker features of miR-145-3p determined via meta-analysis validated by qRT-PCR in metastatic cancer cell lines. Gene 2019, 710, 341–353. [Google Scholar] [CrossRef]
- Tang, C.-P.; Zhou, H.-J.; Qin, J.; Luo, Y.; Zhang, T. MicroRNA-520c-3p negatively regulates EMT by targeting IL-8 to suppress the invasion and migration of breast cancer. Oncol. Rep. 2017, 38, 3144–3152. [Google Scholar] [CrossRef] [Green Version]
- Yin, K.; Yin, W.; Wang, Y.; Zhou, L.; Liu, Y.; Yang, G.; Wang, J.; Lu, J. MiR-206 suppresses epithelial mesenchymal transition by targeting TGF-β signaling in estrogen receptor positive breast cancer cells. Oncotarget 2016, 7, 24537–24548. [Google Scholar] [CrossRef] [Green Version]
- Sun, G.; Liu, M.; Han, H. Overexpression of microRNA-190 inhibits migration, invasion, epithelial-mesenchymal transition, and angiogenesis through suppression of protein kinase B-extracellular signal-regulated kinase signaling pathway via binding to stanniocalicin 2 in breast cancer. J. Cell. Physiol. 2019, 234, 17824–17838. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, X.; Huang, Z.; Xu, L.; Zhu, W.; Liu, P. An ER-associated miRNA signature predicts prognosis in ER-positive breast cancer. J. Exp. Clin. Cancer Res. 2014, 33, 94. [Google Scholar] [CrossRef] [PubMed]
- Amorim, M.; Lobo, J.; Fontes-Sousa, M.; Estevão-Pereira, H.; Salta, S.; Lopes, P.; Coimbra, N.; Antunes, L.; Palma de Sousa, S.; Henrique, R.; et al. Predictive and Prognostic Value of Selected MicroRNAs in Luminal Breast Cancer. Front. Genet. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Sevinc, E.D.; Egeli, U.; Cecener, G.; Tezcan, G.; Tunca, B.; Gokgoz, S.; Tasdelen, I.; Tolunay, S.; Evrensel, T. Association of miR-1266 with recurrence/metastasis potential in estrogen receptor positive breast cancer patients. Asian Pac. J. Cancer Prev. 2015, 16, 291–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Xu, L.; He, L.; Wang, J.; Shi, X.; Li, Z.; Shi, S.; Hou, K.; Teng, Y.; Qu, X. MiR-891a-5p as a prognostic marker and therapeutic target for hormone receptor-positive breast cancer. J. Cancer 2020, 11, 3771–3782. [Google Scholar] [CrossRef]
- Emmadi, R.; Canestrari, E.; Arbieva, Z.H.; Mu, W.; Dai, Y.; Frasor, J.; Wiley, E. Correlative Analysis of miRNA Expression and Oncotype Dx Recurrence Score in Estrogen Receptor Positive Breast Carcinomas. PLoS ONE 2015, 10, e0145346. [Google Scholar] [CrossRef]
- Milevskiy, M.J.G.; Gujral, U.; Del Lama Marques, C.; Stone, A.; Northwood, K.; Burke, L.J.; Gee, J.M.W.; Nephew, K.; Clark, S.; Brown, M.A. MicroRNA-196a is regulated by ER and is a prognostic biomarker in ER+ breast cancer. Br. J. Cancer 2019, 120, 621–632. [Google Scholar] [CrossRef] [Green Version]
- Sahlberg, K.K.; Bottai, G.; Naume, B.; Burwinkel, B.; Calin, G.A.; Børresen-Dale, A.-L.; Santarpia, L. A Serum MicroRNA Signature Predicts Tumor Relapse and Survival in Triple-Negative Breast Cancer Patients. Clin. Cancer Res. 2015, 21, 1207–1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Zhan, Y.; Chi, J.; Guo, S.; Zhong, X.; He, A.; Zheng, J.; Gong, Y.; Li, X.; Zhou, L. Using microRNAs as Novel Predictors of Urologic Cancer Survival: An Integrated Analysis. EBioMedicine 2018, 34, 94–107. [Google Scholar] [CrossRef] [Green Version]
- Aure, M.R.; Vitelli, V.; Jernström, S.; Kumar, S.; Krohn, M.; Due, E.U.; Haukaas, T.H.; Leivonen, S.-K.; Vollan, H.K.M.; Lüders, T.; et al. Integrative clustering reveals a novel split in the luminal A subtype of breast cancer with impact on outcome. Breast Cancer Res. 2017, 19, 44. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zhao, J.; Shi, M.; Ding, Y.; Sun, H.; Yuan, F.; Zou, Z. Elevated Expression of miR-210 Predicts Poor Survival of Cancer Patients: A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e89223. [Google Scholar] [CrossRef]
- Qin, Q.; Furong, W.; Baosheng, L. Multiple functions of hypoxia-regulated miR-210 in cancer. J. Exp. Clin. Cancer Res. 2014, 33, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.; Marian, C.; Makambi, K.H.; Kosti, O.; Kallakury, B.V.S.; Loffredo, C.A.; Zheng, Y.-L. MicroRNA-9 as potential biomarker for breast cancer local recurrence and tumor estrogen receptor status. PLoS ONE 2012, 7, e39011. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, H.; Tang, D.; Wu, J.; Yao, G.; Zhang, Q. Overexpressions of MicroRNA-9 and MicroRNA-200c in Human Breast Cancers Are Associated with Lymph Node Metastasis. Cancer Biother. Radiopharm. 2013, 28, 283–288. [Google Scholar] [CrossRef] [Green Version]
- Hoppe, R.; Achinger-Kawecka, J.; Winter, S.; Fritz, P.; Lo, W.-Y.; Schroth, W.; Brauch, H. Increased expression of miR-126 and miR-10a predict prolonged relapse-free time of primary oestrogen receptor-positive breast cancer following tamoxifen treatment. Eur. J. Cancer 2013, 49, 3598–3608. [Google Scholar] [CrossRef] [PubMed]
- Győrffy, B.; Hatzis, C.; Sanft, T.; Hofstatter, E.; Aktas, B.; Pusztai, L. Multigene prognostic tests in breast cancer: Past, present, future. Breast Cancer Res. 2015, 17. [Google Scholar] [CrossRef] [Green Version]
- Aushev, V.N.; Lee, E.; Zhu, J.; Gopalakrishnan, K.; Li, Q.; Teitelbaum, S.L.; Wetmur, J.; Esposti, D.D.; Hernandez-Vargas, H.; Herceg, Z.; et al. Novel Predictors of Breast Cancer Survival Derived from miRNA Activity Analysis. Clin. Cancer Res. 2018, 24, 581–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.J.; Seo, J.W.; Chae, Y.S.; Kim, J.G.; Kang, B.W.; Kim, W.W.; Jung, J.H.; Park, H.Y.; Jeong, J.Y.; Park, J.-Y. Genetic polymorphism of miR-196a as a prognostic biomarker for early breast cancer. Anticancer Res. 2014, 34, 2943–2949. [Google Scholar] [PubMed]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG); Davies, C.; Godwin, J.; Gray, R.; Clarke, M.; Cutter, D.; Darby, S.; McGale, P.; Pan, H.C.; Taylor, C.; et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: Patient-level meta-analysis of randomised trials. Lancet 2011, 378, 771–784. [Google Scholar] [CrossRef]
- Muluhngwi, P.; Krishna, A.; Vittitow, S.L.; Napier, J.T.; Richardson, K.M.; Ellis, M.; Mott, J.L.; Klinge, C.M. Tamoxifen differentially regulates miR-29b-1 and miR-29a expression depending on endocrine-sensitivity in breast cancer cells. Cancer Lett. 2017, 388, 230–238. [Google Scholar] [CrossRef] [Green Version]
- Bergamaschi, A.; Katzenellenbogen, B.S. Tamoxifen down-regulation of miR-451 increases 14-3-3ζ and promotes breast cancer cell survival and endocrine resistance. Oncogene 2012, 31, 39–47. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, F.; Costa, A.; Senkus, E.; Aapro, M.; André, F.; Barrios, C.H.; Bergh, J.; Bhattacharyya, G.; Biganzoli, L.; Cardoso, M.J.; et al. 3rd ESO-ESMO international consensus guidelines for Advanced Breast Cancer (ABC 3). Breast 2017, 31, 244–259. [Google Scholar] [CrossRef] [Green Version]
- Sadat Alamolhodaei, N.; Behravan, J.; Mosaffa, F.; Karimi, G. MiR 221/222 as New Players in Tamoxifen Resistance. Curr. Pharm. Des. 2016, 22, 6946–6955. [Google Scholar] [CrossRef] [PubMed]
- Joshi, T.; Elias, D.; Stenvang, J.; Alves, C.L.; Teng, F.; Lyng, M.B.; Lykkesfeldt, A.E.; Brünner, N.; Wang, J.; Gupta, R.; et al. Integrative analysis of miRNA and gene expression reveals regulatory networks in tamoxifen-resistant breast cancer. OncoTarget 2016, 7, 57239–57253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nam, S.; Long, X.; Kwon, C.; Kim, S.; Nephew, K.P. An integrative analysis of cellular contexts, miRNAs and mRNAs reveals network clusters associated with antiestrogen-resistant breast cancer cells. BMC Genom. 2012, 13, 732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.-J.; Lin, J.; Yang, H.; Kong, W.; He, L.; Ma, X.; Coppola, D.; Cheng, J.Q. MicroRNA-221/222 negatively regulates estrogen receptor α and is associated with tamoxifen resistance in breast cancer. J. Biol. Chem. 2016, 291, 22859. [Google Scholar] [CrossRef] [Green Version]
- Miller, T.E.; Ghoshal, K.; Ramaswamy, B.; Roy, S.; Datta, J.; Shapiro, C.L.; Jacob, S.; Majumder, S. MicroRNA-221/222 confers tamoxifen resistance in breast cancer by targeting p27Kip1. J. Biol. Chem. 2008, 283, 29897–29903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cun, J.; Yang, Q. Bioinformatics-based interaction analysis of miR-92a-3p and key genes in tamoxifen-resistant breast cancer cells. Biomed. Pharmacother. 2018, 107, 117–128. [Google Scholar] [CrossRef]
- Li, J.; Lu, M.; Jin, J.; Lu, X.; Xu, T.; Jin, S. miR-449a Suppresses Tamoxifen Resistance in Human Breast Cancer Cells by Targeting ADAM22. CPB 2018, 50, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wu, M.; Chong, Q.-Y.; Zhang, M.; Zhang, X.; Hu, L.; Zhong, Y.; Qian, P.; Kong, X.; Tan, S.; et al. Loss of Estrogen-Regulated MIR135A1 at 3p21.1 Promotes Tamoxifen Resistance in Breast Cancer. Cancer Res. 2018, 78, 4915–4928. [Google Scholar] [CrossRef] [Green Version]
- Ljepoja, B.; García-Roman, J.; Sommer, A.-K.; Wagner, E.; Roidl, A. MiRNA-27a sensitizes breast cancer cells to treatment with Selective Estrogen Receptor Modulators. Breast 2019, 43, 31–38. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, J.; Shi, Y.; Sun, Q.; Zhang, Q.; Guan, X. The novel role of miRNAs for tamoxifen resistance in human breast cancer. Cell. Mol. Life Sci. 2015, 72, 2575–2584. [Google Scholar] [CrossRef]
- Cui, J.; Yang, Y.; Li, H.; Leng, Y.; Qian, K.; Huang, Q.; Zhang, C.; Lu, Z.; Chen, J.; Sun, T.; et al. MiR-873 regulates ERα transcriptional activity and tamoxifen resistance via targeting CDK3 in breast cancer cells. Oncogene 2015, 34, 3895–3907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, L.; Meng, X.; Li, X.; Zhang, Y.; Li, C.; Xiang, C.; Xing, Y.; Xia, Y.; Xi, T. miR-125a-3p inhibits ERα transactivation and overrides tamoxifen resistance by targeting CDK3 in estrogen receptor-positive breast cancer. FASEB J. 2018, 32, 588–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, P.; Fang, C.; Zeng, H.; Shi, Y.; Pan, Z.; An, N.; He, K.; Zhang, L.; Long, X. Differential microRNA expression profiles in tamoxifen-resistant human breast cancer cell lines induced by two methods. Oncol. Lett. 2018, 15, 3532–3539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, R.; Wang, Y.; Wang, C.-X.; Yin, M.; Liu, H.-L.; Chen, J.-P.; Han, J.-Q.; Wang, W.-B. miRNA-155 mediates TAM resistance by modulating SOCS6-STAT3 signalling pathway in breast cancer. Am. J. Transl. Res. 2015, 7, 2115–2126. [Google Scholar]
- Kim, Y.S.; Park, S.J.; Lee, Y.S.; Kong, H.K.; Park, J.H. miRNAs involved in LY6K and estrogen receptor α contribute to tamoxifen-susceptibility in breast cancer. Oncotarget 2016, 7, 42261–42273. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Luo, A.; Liu, Y.; Wang, S.; Li, Y.; Shi, W.; Liu, Z.; Qu, X. MiR-214 increases the sensitivity of breast cancer cells to tamoxifen and fulvestrant through inhibition of autophagy. Mol. Cancer 2015, 14. [Google Scholar] [CrossRef] [Green Version]
- He, M.; Jin, Q.; Chen, C.; Liu, Y.; Ye, X.; Jiang, Y.; Ji, F.; Qian, H.; Gan, D.; Yue, S.; et al. The miR-186-3p/ EREG axis orchestrates tamoxifen resistance and aerobic glycolysis in breast cancer cells. Oncogene 2019, 38, 5551–5565. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Wang, M.; Xiao, G.; Yang, G.; Wang, H.; Li, Y.; Sun, X.; Qin, S.; Du, N.; et al. A miR-26a/E2F7 feedback loop contributes to tamoxifen resistance in ER-positive breast cancer. Int. J. Oncol. 2018, 53, 1601–1612. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Zou, Z.; Nie, P.; Kou, X.; Wu, B.; Wang, S.; Song, Z.; He, J. Downregulation of microRNA-27b-3p enhances tamoxifen resistance in breast cancer by increasing NR5A2 and CREB1 expression. Cell Death Dis. 2016, 7, e2454. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, K.; Horie-Inoue, K.; Ueno, T.; Suzuki, T.; Sato, W.; Shigekawa, T.; Osaki, A.; Saeki, T.; Berezikov, E.; Mano, H.; et al. miR-378a-3p modulates tamoxifen sensitivity in breast cancer MCF-7 cells through targeting GOLT1A. Sci. Rep. 2015, 5, 13170. [Google Scholar] [CrossRef]
- Ahmad, A.; Ginnebaugh, K.R.; Yin, S.; Bollig-Fischer, A.; Reddy, K.B.; Sarkar, F.H. Functional role of miR-10b in tamoxifen resistance of ER-positive breast cancer cells through down-regulation of HDAC4. BMC Cancer 2015, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luengo-Gil, G.; García-Martínez, E.; Chaves-Benito, A.; Conesa-Zamora, P.; Navarro-Manzano, E.; González-Billalabeitia, E.; García-Garre, E.; Martínez-Carrasco, A.; Vicente, V.; Ayala de la Peña, F. Clinical and biological impact of miR-18a expression in breast cancer after neoadjuvant chemotherapy. Cell Oncol. 2019, 42, 627–644. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Roy, S.; Nuovo, G.; Ramaswamy, B.; Miller, T.; Shapiro, C.; Jacob, S.T.; Majumder, S. Anti-microRNA-222 (anti-miR-222) and -181B suppresses growth of tamoxifen-resistant xenografts in mouse by targeting TIMP3 protein and modulating mitogenic signal. J. Biol. Chem. 2018, 293, 3588. [Google Scholar] [CrossRef] [Green Version]
- Song, B.; Wang, C.; Liu, J.; Wang, X.; Lv, L.; Wei, L.; Xie, L.; Zheng, Y.; Song, X. MicroRNA-21 regulates breast cancer invasion partly by targeting tissue inhibitor of metalloproteinase 3 expression. J. Exp. Clin. Cancer Res. 2010, 29, 29. [Google Scholar] [CrossRef] [Green Version]
- Hafez, M.M.; Hassan, Z.K.; Zekri, A.R.N.; Gaber, A.A.; Al Rejaie, S.S.; Sayed-Ahmed, M.M.; Al Shabanah, O. MicroRNAs and metastasis-related gene expression in Egyptian breast cancer patients. Asian Pac. J. Cancer Prev. 2012, 13, 591–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Y.; Lai, X.; Yu, S.; Chen, S.; Ma, Y.; Zhang, Y.; Li, H.; Zhu, X.; Yao, L.; Zhang, J. Exosomal miR-221/222 enhances tamoxifen resistance in recipient ER-positive breast cancer cells. Breast Cancer Res. Treat. 2014, 147, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.C.; Conger, A.K.; Yan, T.J.; Hoang, V.T.; Miller, D.F.B.; Buechlein, A.; Rusch, D.B.; Nephew, K.P.; Collins-Burow, B.M.; Burow, M.E. MicroRNA-335-5p and -3p synergize to inhibit estrogen receptor alpha expression and promote tamoxifen resistance. FEBS Lett. 2017, 591, 382–392. [Google Scholar] [CrossRef] [Green Version]
- Ward, A.; Shukla, K.; Balwierz, A.; Soons, Z.; König, R.; Sahin, Ö.; Wiemann, S. MicroRNA-519a is a novel oncomir conferring tamoxifen resistance by targeting a network of tumour-suppressor genes in ER+ breast cancer. J. Pathol. 2014, 233, 368–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.; Cheng, L.; Hu, P.; Liu, R. MicroRNA-663b mediates TAM resistance in breast cancer by modulating TP73 expression. Mol. Med. Rep. 2018, 18, 1120–1126. [Google Scholar] [CrossRef] [Green Version]
- Shi, W.; Gerster, K.; Alajez, N.M.; Tsang, J.; Waldron, L.; Pintilie, M.; Hui, A.B.Y.; Sykes, J.; P’ng, C.; Miller, N.; et al. MicroRNA-301 Mediates Proliferation and Invasion in Human Breast Cancer. Cancer Res. 2011. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.-J.; Cheng, Y.-M.; Chen, C.-C.; Chen, Y.-C.; Shen, C.-J. MiR-148a and miR-152 reduce tamoxifen resistance in ER+ breast cancer via downregulating ALCAM. Biochem. Biophys. Res. Commun. 2017, 483, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Manavalan, T.T.; Teng, Y.; Litchfield, L.M.; Muluhngwi, P.; Al-Rayyan, N.; Klinge, C.M. Reduced Expression of miR-200 Family Members Contributes to Antiestrogen Resistance in LY2 Human Breast Cancer Cells. PLoS ONE 2013, 8, e62334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; Zhang, W.; Liu, C.; Li, G. miR-200 affects tamoxifen resistance in breast cancer cells through regulation of MYB. Sci. Rep. 2019, 9, 18844. [Google Scholar] [CrossRef]
- Hrstka, R.; Nenutil, R.; Fourtouna, A.; Maslon, M.M.; Naughton, C.; Langdon, S.; Murray, E.; Larionov, A.; Petrakova, K.; Muller, P.; et al. The pro-metastatic protein anterior gradient-2 predicts poor prognosis in tamoxifen-treated breast cancers. Oncogene 2010, 29, 4838–4847. [Google Scholar] [CrossRef] [PubMed]
- Achari, C.; Winslow, S.; Ceder, Y.; Larsson, C. Expression of miR-34c induces G2/M cell cycle arrest in breast cancer cells. BMC Cancer 2014, 14, 538. [Google Scholar] [CrossRef] [Green Version]
- Antonini, D.; Russo, M.T.; De Rosa, L.; Gorrese, M.; Del Vecchio, L.; Missero, C. Transcriptional Repression of miR-34 Family Contributes to p63-Mediated Cell Cycle Progression in Epidermal Cells. J. Investig. Dermatol. 2010, 130, 1249–1257. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Shi, Y.; Zheng, L.; Zhou, B.; Inose, H.; Wang, J.; Guo, X.E.; Grosschedl, R.; Karsenty, G. miR-34s inhibit osteoblast proliferation and differentiation in the mouse by targeting SATB2. J. Cell Biol. 2012, 197, 509–521. [Google Scholar] [CrossRef] [Green Version]
- Ward, A.; Balwierz, A.; Zhang, J.D.; Küblbeck, M.; Pawitan, Y.; Hielscher, T.; Wiemann, S.; Sahin, Ö. Re-expression of microRNA-375 reverses both tamoxifen resistance and accompanying EMT-like properties in breast cancer. Oncogene 2013, 32, 1173–1182. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Zeng, H.; Ye, P.; Shi, Y.; Guo, J.; Long, X. Differential microRNA profiles between fulvestrant-resistant and tamoxifen-resistant human breast cancer cells. Anticancer Drugs 2018, 29, 539–548. [Google Scholar] [CrossRef]
- Tangkeangsirisin, W.; Serrero, G. GP88 (Progranulin) Confers Fulvestrant (Faslodex, ICI 182,780) Resistance to Human Breast Cancer Cells. Adv. Breast Cancer Res. 2014, 3, 68–78. [Google Scholar] [CrossRef] [Green Version]
- Tsuboi, K.; Kaneko, Y.; Nagatomo, T.; Fujii, R.; Hanamura, T.; Gohno, T.; Yamaguchi, Y.; Niwa, T.; Hayashi, S.-I. Different epigenetic mechanisms of ERα implicated in the fate of fulvestrant-resistant breast cancer. J. Steroid Biochem. Mol. Biol. 2017, 167, 115–125. [Google Scholar] [CrossRef]
- Rao, X.; Di Leva, G.; Li, M.; Fang, F.; Devlin, C.; Hartman-Frey, C.; Burow, M.E.; Ivan, M.; Croce, C.M.; Nephew, K.P. MicroRNA-221/222 confers breast cancer fulvestrant resistance by regulating multiple signaling pathways. Oncogene 2011, 30, 1082–1097. [Google Scholar] [CrossRef] [Green Version]
- Fan, M.; Yan, P.S.; Hartman-Frey, C.; Chen, L.; Paik, H.; Oyer, S.L.; Salisbury, J.D.; Cheng, A.S.L.; Li, L.; Abbosh, P.H.; et al. Diverse gene expression and DNA methylation profiles correlate with differential adaptation of breast cancer cells to the antiestrogens tamoxifen and fulvestrant. Cancer Res. 2006, 66, 11954–11966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xin, F.; Li, M.; Balch, C.; Thomson, M.; Fan, M.; Liu, Y.; Hammond, S.M.; Kim, S.; Nephew, K.P. Computational analysis of microRNA profiles and their target genes suggests significant involvement in breast cancer antiestrogen resistance. Bioinformatics 2009, 25, 430–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, J.; He, K.; Zeng, H.; Shi, Y.; Ye, P.; Zhou, Q.; Pan, Z.; Long, X. Differential microRNA expression profiles determined by next-generation sequencing in three fulvestrant-resistant human breast cancer cell lines. Oncol. Lett. 2019, 17, 3765–3776. [Google Scholar] [CrossRef] [Green Version]
- Vilquin, P.; Donini, C.F.; Villedieu, M.; Grisard, E.; Corbo, L.; Bachelot, T.; Vendrell, J.A.; Cohen, P.A. MicroRNA-125b upregulation confers aromatase inhibitor resistance and is a novel marker of poor prognosis in breast cancer. Breast Cancer Res. 2015, 17. [Google Scholar] [CrossRef] [Green Version]
- Bacci, M.; Giannoni, E.; Fearns, A.; Ribas, R.; Gao, Q.; Taddei, M.L.; Pintus, G.; Dowsett, M.; Isacke, C.M.; Martin, L.-A.; et al. miR-155 Drives Metabolic Reprogramming of ER+ Breast Cancer Cells Following Long-Term Estrogen Deprivation and Predicts Clinical Response to Aromatase Inhibitors. Cancer Res. 2016, 76, 1615–1626. [Google Scholar] [CrossRef] [Green Version]
- Masri, S.; Liu, Z.; Phung, S.; Wang, E.; Yuan, Y.-C.; Chen, S. The role of microRNA-128a in regulating TGFbeta signaling in letrozole-resistant breast cancer cells. Breast Cancer Res Treat 2010, 124, 89–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoppe, R.; Fan, P.; Büttner, F.; Winter, S.; Tyagi, A.K.; Cunliffe, H.; Jordan, V.C.; Brauch, H. Profiles of miRNAs matched to biology in aromatase inhibitor resistant breast cancer. Oncotarget 2016, 7, 71235–71254. [Google Scholar] [CrossRef] [Green Version]
- Citron, F.; Segatto, I.; Vinciguerra, G.L.R.; Musco, L.; Russo, F.; Mungo, G.; D’Andrea, S.; Mattevi, M.C.; Perin, T.; Schiappacassi, M.; et al. Downregulation of miR-223 expression is an early event during mammary transformation and confers resistance to CDK4/6 inhibitors in luminal breast cancer. Cancer Res. 2019. [Google Scholar] [CrossRef] [Green Version]
| miRNA | Patients/Specimen Characteristics (Number of Patients/Specimens) | Results | Reference |
|---|---|---|---|
| miR-16, miR-21, miR-155, miR-195 | Serum from patients with BC (n = 49) and healthy controls (n = 19) | Increased level in LumA EBC | [40] |
| miR-195 | Blood from patients with BC (n = 83) (ductal type—71%, LumA epithelial subtype—63%, early stage—71%, in situ—12%) and healthy controls (n= 63) | Higher expression in EBC patients | [41] |
| miR-29a, miR-181a, miR-652 | Blood from patients with a new diagnosis of LumA-like BC (n = 54) and healthy control participants (n = 56) | Reduced expression in LumA-like BC women | [42] |
| miR-23a-3p, miR-152-3p | Blood samples from patients with BC (n = 106) (LumA, n = 23) and healthy control (n = 96) | Lower level in patients with LumA | [29] |
| miR-338-3p, miR-223, miR-148a | Blood samples before and after surgery of post-menopausal patients with ER+and HER2-early stage of BC (n = 24) | Lower level in post-operative ER+ EBC post-menopausal women | [43] |
| miR-1273g-3p | MCF-7 BC cells; patients with BC (n = 39) and healthy controls (n = 40) | Increased expression in MCF-7 cells and BC patients | [44] |
| miRNA Pattern | Study Design/Model | Result | Ref. |
|---|---|---|---|
| ↑ miR-331 ↓ miR-195 | Metastasized BC LumA patients vs. patients with local disease or healthy controls | [45] | |
| ↑ miR-203 | MCF-7 | ↓ estradiol-induced viability, migration and invasion ↓ ERα protein expression | [49] |
| ↓ miR-6744-5p | Anoikis-resistant sub-cell line (MCF-7-AR6) | [50] | |
| ↑ miR-6744-5p | MCF-7 | ↑ anoikis sensitivity ↑ E-cadherin | [50] |
| ↑ miR-765 | ↓ proliferation, migration, invasiveness | [53] | |
| ↑ miR-628 | ↑ migration, invasiveness ↑ E-cadherin ↓ vimentin, Snail | [54] | |
| ↓ miR-340 | ↑ migration, invasiveness | [55] | |
| ↓ miR-200c ↑ miR-30c | MCF-7-derived mammospheres | ↑ stem cells markers (OCT4, SOX2, c-MYC) ↑ EMT-related genes (SNAIL1, CDH2, TWIST1/2) | [56] |
| ↓ miR-145-3p | MCF-7 (metastasisinduced and cancer environment imitated) | [57] | |
| ↑ miR-520c-3p | MCF-7, T47D | ↓ migration ↑ E-cadherin ↓ vimentin ↓ fibronectin | [58] |
| ↑ miR-206 | ↓ migration, invasiveness, EMT ↓ TGF-β, NRP1, SMAD2 | [59] | |
| ↓ miR-190 | T47D | [60] |
| Upregulated miRNA | Reference | Downregulated miRNA | Reference |
|---|---|---|---|
| miR-187 | [61] | miR-203 | [49] |
| miR-210 | [61] | miR-182-5p | [62] |
| miR-224 | [61] | miR-200b-3p | [62] |
| miR-9 | [61] | miR-30b-5p | [62] |
| miR-1266 | [63] | miR-30c-5p | [62] |
| miR-128-3p | [64] | Let-7 family | [65] |
| miR-661 | [64] | miR-891a-5p | [64] |
| miR-296-3p | [64] | miR-383-5p | [64] |
| miR-196a | [66] | miR-1295a | [64] |
| Downregulated miRNA in Tamoxifen-Resistant BC | ||
| miRNA | Target Gene | Reference |
| miR-106b | YWHAG, YWHAZ | [83] |
| miR-125a-3p | CDK3 | [93] |
| miR-135a | FOXM1, ERK1/2, AKT1 | [83] |
| miR-186-3p | EREG | [98] |
| miR-26a | E2F7 | [99] |
| miR-27b-3p | NR5A2, CREB1 | [100] |
| miR-33b | FYN | [83] |
| miR-342-3p/5p | FYN | [83] |
| miR-378a-3p | GOLT1A | [101] |
| miR-449a | ADAM22 | [88] |
| miR-491-5p | YWHAG, YWHAZ | [83] |
| miR-577 | YWHAG, YWHAZ | [83] |
| miR-593 | SNAI2 | [83] |
| miR-873 | CDK3 | [92] |
| miR-942 | YWHAG, YWHAZ | [83] |
| miR-96 | YWHAG, YWHAZ | [83] |
| Upregulated miRNA in Tamoxifen-Resistant BC | ||
| miRNA | Target Gene | Reference |
| miR-10b | HDAC4 | [102] |
| miR-18a | MYBL2 | [103] |
| miR-101 | MAGI2, Akt | [91] |
| miR-155 | SOCS6-STAT3 | [95] |
| miR-181b | STAT1,MYB, BCL2, SOX9 | [83,104] |
| miR-192-5p | ERα | [96] |
| miR-196a | Hox, Fox, Cdkinhib. | [66] |
| miR-21 | TIMP3, ADAM | [105,106] |
| miR-221 | P27, ERɑ | [107] |
| miR-222 | P27, ERɑ | [107] |
| miR-335-5p/3p | [108] | |
| miR-519a | CDKN1 | [109] |
| miR-663b | TP73 | [110] |
| miR-92a-3p | [87] | |
| Downregulated miRNA in Tamoxifen Sensitive BC | ||
| miRNA | Target Gene | Reference |
| miR-301 | PTEN, Akt | [111] |
| Upregulated miRNA in Tamoxifen Sensitive BC | ||
| miRNA | Target Gene | Reference |
| miR-148a | ALCAM | [112] |
| miR-152 | ALCAM | [112] |
| miR-200b/c | ZEB1 | [113,114] |
| miR-214 | UCP2 | [97] |
| miR-261 | AGR | [115] |
| miR-27a | ZBTB10 | [90] |
| miR-320a | ARPP-19 | [93] |
| miR-34 | CCND1, CDK4/6 | [116,117,118] |
| miR-375 | MTDH, ZEB1, SNAI2 | [119] |
| miR-451 | HER, EGFR, MAPK | [80] |
| miR-575 | AGR | [115] |
| Downregulated miRNA in Fulvestrant-Resistant BC | ||
| miRNA | Target Gene | Reference |
| miR-137 | SRC3 | [126] |
| miR-143 | [126] | |
| miR-145 | [126] | |
| miR-424 | PI3K/Akt/mTOR | [126] |
| Upregulated miRNA in Fulvestrant-Resistant BC | ||
| miRNA | Target Gene | Reference |
| miR-21 | PI3K/Akt/mTOR | [126] |
| miR-221 | PCDH10 | [126] |
| miR-222 | CAMs KEGG pathway | [126] |
| Upregulated miRNA in Aromatase Inhibitor-Resistant BC | |||
|---|---|---|---|
| miRNA | Target Gene | Reference | Aromatase inhibitor |
| miR-125b | Akt/mTOR | [127] | Letrozole, anastrozole |
| miR-128a | TGFbRI | [129] | Letrozole |
| miR-155 | HK2, STAT3 | [128] | Anastrozole |
| miR-205 | Akt/mTOR | [127] | Letrozole/anastrozole |
| miR-432-5p | TGF-β | [130] | Letrozole/anastrozole |
| miR-433-3p | MAPK | [130] | Letrozole/anastrozole |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudela, E.; Samec, M.; Koklesova, L.; Liskova, A.; Kubatka, P.; Kozubik, E.; Rokos, T.; Pribulova, T.; Gabonova, E.; Smolar, M.; et al. miRNA Expression Profiles in Luminal A Breast Cancer—Implications in Biology, Prognosis, and Prediction of Response to Hormonal Treatment. Int. J. Mol. Sci. 2020, 21, 7691. https://doi.org/10.3390/ijms21207691
Kudela E, Samec M, Koklesova L, Liskova A, Kubatka P, Kozubik E, Rokos T, Pribulova T, Gabonova E, Smolar M, et al. miRNA Expression Profiles in Luminal A Breast Cancer—Implications in Biology, Prognosis, and Prediction of Response to Hormonal Treatment. International Journal of Molecular Sciences. 2020; 21(20):7691. https://doi.org/10.3390/ijms21207691
Chicago/Turabian StyleKudela, Erik, Marek Samec, Lenka Koklesova, Alena Liskova, Peter Kubatka, Erik Kozubik, Tomas Rokos, Terezia Pribulova, Eva Gabonova, Marek Smolar, and et al. 2020. "miRNA Expression Profiles in Luminal A Breast Cancer—Implications in Biology, Prognosis, and Prediction of Response to Hormonal Treatment" International Journal of Molecular Sciences 21, no. 20: 7691. https://doi.org/10.3390/ijms21207691
APA StyleKudela, E., Samec, M., Koklesova, L., Liskova, A., Kubatka, P., Kozubik, E., Rokos, T., Pribulova, T., Gabonova, E., Smolar, M., & Biringer, K. (2020). miRNA Expression Profiles in Luminal A Breast Cancer—Implications in Biology, Prognosis, and Prediction of Response to Hormonal Treatment. International Journal of Molecular Sciences, 21(20), 7691. https://doi.org/10.3390/ijms21207691






