2.1. Whole Genome Sequencing and Annotations
The whole genome shotgun sequencing (WGS) project for the
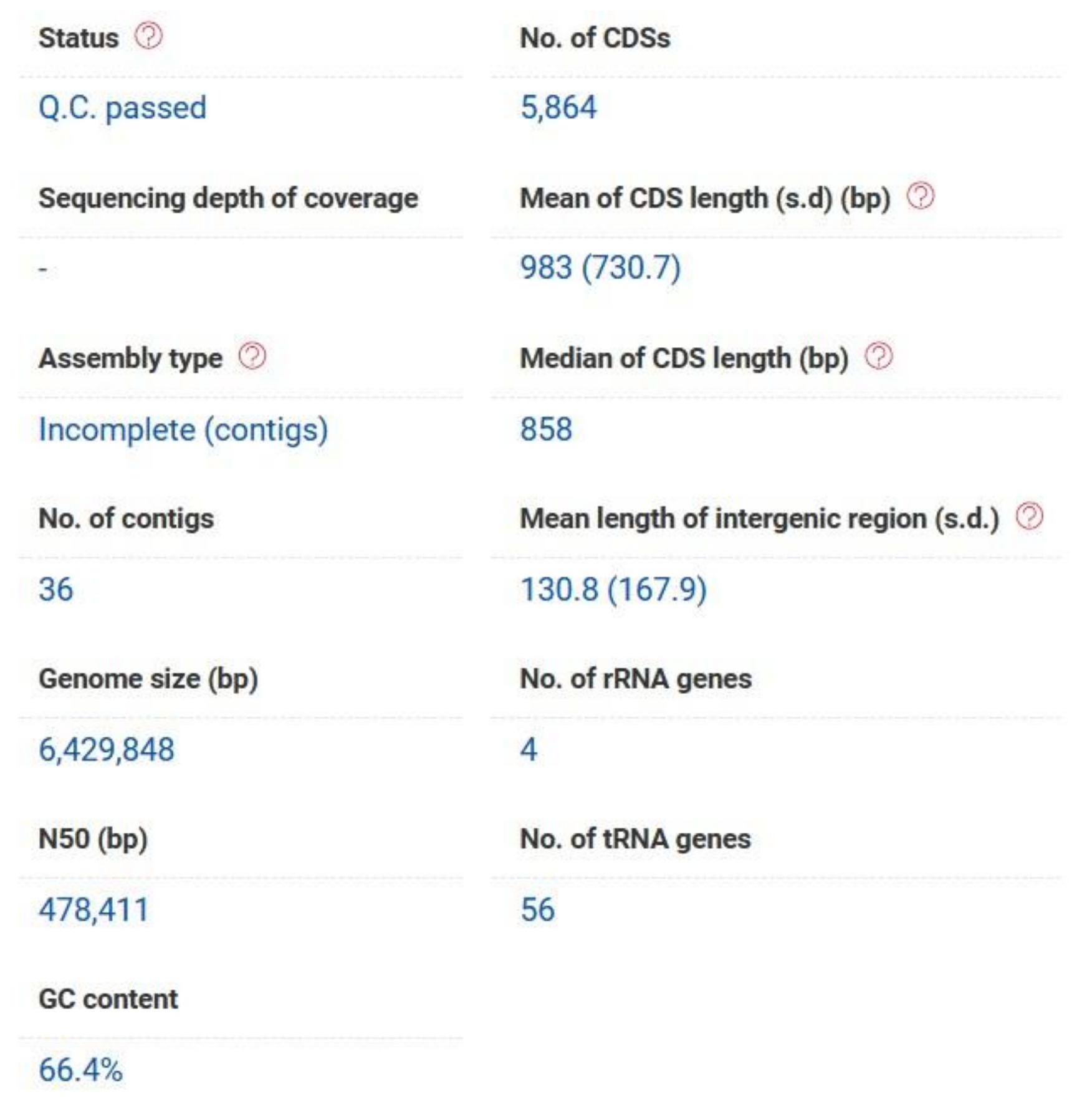
P. aeruginosa strain PAEM (6.4 Mb) isolated from a frozen ready-to-cook meat (the environment of meat (EM) processing) has been deposited at DDBJ/ENA/GenBank under the accession JACBNI010000000 (BioProject: PRJNA644471). The genome sequence has been annotated by the NCBI Prokaryotic Genome Annotation Pipeline (PGAP; Annotation Method: Best-placed reference protein set; GeneMarkS-2). A total of 5986 genes have been identified, including 5884 protein-coding sequences (CDSs) and 62 genes for RNA (3 rRNA, 55 tRNA, 4 ncRNA). The pseudogenes (40) contain frameshifted (27), incomplete (16), internal stop (6), and multiple problems (7) genes. The genome contains three Clustered Regularly Interspaced Short Palindromic Repeat (CRISPR) arrays (JACBNI010000000). In respect that the large portion of
P. aeruginosa CDSs are not functionally verified and, therefore, annotated as hypothetical proteins, they have been additionally analyzed through the RAST (Rapid Annotation Sequence Tool) and EzBioCloud servers [
24,
25]. Orthologous Average Nucleotide Identity (OrthoANIu) is the standard algorithm used to build the EzBioCloud database [
26]. The
P. aeruginosa PAEM genome (6.4 Mb) has a G+C content of 66.4%, and the assemblies contain 6185 (207 contigs with protein-encoding genes, PEG), and 5864 (36 contigs with PEG of more than 500 bp) potential CDSs with the bacterial core genes coverage 98.9% according to the RAST and EzBiocloud annotations, respectively (
Figure 1,
Table S1).
There are 33% CDSs (1994 genes including 109 hypothetical) with the functional features and 67% CDSs (4191 genes including 1531 hypothetical) non-belonging to the metabolic pathways identified by the RAST Subsystems [
24]. The prevalent number of genes belongs to such functional categories as amino acids and derivatives (550), carbohydrates (290), membrane transport (261), and protein metabolism (231). The category “Secondary metabolism” includes the genes related to plant hormones (auxin biosynthesis) and bacterial cytostatics (paerucumarin biosynthesis) (data not shown). The results from the
classifier Levofloxacin has identified three genome regions responsible for the predicted levofloxacin sensitivity in
P. aeruginosa PAEM [
24]. The predicted regions 1 and 2 were found to be identical with the genome sequences of the reference strain
P. aeruginosa PAO1 by the DNA Motif Search tool in the current Pseudomonas Genome Database (DB) [
23]. The genome examination for the presence of resistance genes by RAST found 30 CDSs for colicin E2, polymyxin, vancomycin, fosfomycin, and multidrug resistance proteins, including responsibility for heavy metals, cobalt–zinc–cadmium, chromium, copper, tellurium, and organic hydroperoxide, which are used in some industrial processes. The protectants against oxidative damage and other stress factors (85 searching results) are encoded by a multitude of stress response regulators, such as sodium hydrogen antiporters, outer membrane stress sensor proteases (proteolysis and periplasmic stress response), SOS—and NO—response regulators, uptake regulators of free iron and heme, paraquat-inducible proteins, alkyl hydroperoxide reductases, starvation, osmotic, temperature response regulators, and DNA repair proteins (44 results) [
24].
According to the EzBiocloud annotation [
25], there are 1706 genes encoding hypothetical proteins and about 158 results related to CDSs with multidrug resistance, stress response, or antibiotic biosynthesis functions (by EzBioCloud similarity) (
Tables S1–S3). These include the resistance to neomycin, paromomycin, ribostamycin, butirosin, gentamicin B, trimethoprim, methotrexate, azomycin, acriflavin, antimicrobial lipopeptide surfactants, puromycin, tetraphenylarsonium chloride, mycotoxin fusaric acid, sulfonamide bicyclomycin, chloramphenicol, carbenicillin, erythromycin, novobiocin, streptomycin, tetracycline, macrolides–lincosamides–streptogramin B (MLS), teicoplanin, antimicrobial peptides (colistin, bacitracin, polymyxin), beta-lactams (methicillin and carbapenem resistance), the herbicide dinoseb, metronidazole, carbonyl cyanide m-chlorophenyl-hydrazone (CCCP), carbonyl cyanide 4-trifluoromethoxyphenylhydrazone (FCCP), 2,4- dinitrophenol (DNP), nalidixic acid, fosfomycin, benzalkonium chloride, and fluoroquinolones (
Tables S1 and S2). Apart from the predicted resistance to most antibiotics, the strain PAEM possesses the genes responsible for resistance against broad-spectrum antiseptics and damaging environmental factors, including the resistance to ethidium bromide and quaternary ammonium compounds, organic hydroperoxides, arsenic, polyamines, acids (the acidic stomach environment, high levels of acetic acid, dichloroacetic acid), tributyltin chloride, toluene, phenol, alcohol, cumene hydroperoxide, and dichloroacetic acid (glutathione-dependent dehalogenation) (
Tables S1 and S2). About 44 multidrug-resistance and 87 CDSs for stress-response proteins were found. Numerous CDSs related to chaperones (62 CDSs); cold shock CapA, CspA, CapB (6 CDSs); heat shock (19 CDSs) and phage shock proteins, holin-like proteins, and other osmoprotectants (40 CDSs); miniconductance mechanosensitive channels (against hypoosmotic shock); sodium, potassium, lithium, rubidium, and organophosphate Pi antiporters, chloride/bicarbonate exchanger (25 CDSs); photoreceptors and light response regulators (25 CDSs), DNA damage protectors (72 CDSs) and antiviral defense factors, such as CRISPR-associated proteins (7 CDSs) (
Tables S1 and S2). The antibiotic biosynthesis genes of PAEM are similar by the genes from the reference strain PAO1 with 99.2–99.7% of identity, excluding the activator of bacteriocin biosynthesis PrtN (97.7%) (
Table S3).
Transcriptional regulators and their regulatory proteins related to quorum sensing (17 CDSs), biofilm formation (16 CDSs), and polysaccharide metabolism/catabolism (70 CDSs) have also been identified as being involved in the resistance to damaging environment factors, as well as in the virulence and pathogenicity of
P. aeruginosa PAEM, according to the EzbioCloud searching results (
Table S4). However, many of the diguanilate cyclases (16 CDSs), histidine kinases, and other two-component response regulators (102 CDSs) are also known to be involved in the processes of cell adhesion, formation, maturation, and degradation of the
P. aeruginosa biofilm (
Table S5).
2.2. Comparative Pathogenomics, Serotyping, and Multilocus Sequence Typing
Searching for virulence and pathogenicity factors by the EzBiocloud similarity resulted in 80 and six CDSs, respectively (
Table S1). However, the comparative pathogenomics with the use of database VFPB (Virulence Factors of Pathogenic Bacteria,
http://www.mgc.ac.cn/VFs/) revealed that the strain PAEM from the environment of meat processing has the similar sets of genes; most of which are of a high level of mosaic identity (average 99.2–100%) with the corresponding sequences of the reference strains
P. aeruginosa PAO1 and LESB58 (
Table S6). They include all the known
P. aeruginosa virulence factors, namely: 46 flagella genes (
flg,
fli,
fla,
flh,
mot) related to the adherence; 33 genes (
pil,
fim,
chp) of the type IV pili and twitching motility proteins biosynthesis; 11 genes of the lipopolysaccharide (LPS) O-antigen biosynthesis; 16 genes (
phz) related to antimicrobial activity (phenazines biosynthesis pathway); 24 genes (
alg,
muc) related to antyphagocytosis (alginate biosynthesis and regulation); 3 genes (
rhl) related to biosurfactants (rhamnolipid biosynthesis); 4 genes (
plc,
pld) of hemolytic and non-hemolytic phospholipases C and D; 26 genes (
pch,
fpt,
pvd) for iron uptake (pyochelin, pyoverdin, and their receptors); 4 genes (
aprA,
lasA and
B,
prpL) of proteases (alkaline phosphatase, elastase, and protease IV); 7 genes (
hdtS,
lasR and
lasl,
ahlR and
ahll,
rhll) related to quorum sensing; the sensor kinase’s, GacS, and its response regulator’s, GacA, (GacS/GacA) two-component system genes
gacS and
gacA; 20 genes related to Hcp secretion island-1 encoding type VI secretion system (H-T6SS); 35 genes of the
P. aeruginosa type III secretion system (TTSS) and 3 genes related to the
P. aeruginosa TTSS translocated effectors (
exo S,
T and
Y); exotoxin-A (
toxA); and three genes (
hcnA, B and C) for hydrogen cyanide production (
Table S6). The environmental strain PAEM mostly distinguishes from the both comparative strains PAO1 and LESB58 by the genes of QS-dependent protease IV
prpL (98.6 and 98.2%, respectively), elastase
lasA (98.8 and 99.4%), QS-related genes for acylhomoserine lactone synthase
htdS (99.2 and 99.1%), N-(butanoyl)-L-homoserine lactone QS system
rhll (98.6 and 99.5%) and
rhlR (99.6 and 99.7%), two-component system
gacS (99.7%), sulfate transporter
pcrV from TTSS (99.7 and 99.4%, respectively), (ABC)-importer for iron acquisition
pchI (98.3 and 98.4%), pyoverdine-related
pvdD (70.0 and 99.9%),
pvdJ (73.8 and 99.8%) and
pvdY (99.1 and 99.3%), rhamnosyltransferase (aclacinomycin-T 2-deoxy-L-fucose transferase, by EzBiocloud)
rhlB (99.3 and 99.5%), exotoxin
toxA (99.5 and 99.0%), exoenzymes
exoS (99.3 and 100%),
exoT (98.8 and 99.0%) and
exoY (99.4 and 99.6%), and alginate regulation
mucD (99.9 and 99.2%) and
algW (99.8 and 99.3%) (
Table S6).
The differences in such category of virulence factors as LPS O-antigens are also observed among the strains
P. aeruginosa PAO1, LESB58, and PAEM (
Table S6). No hits were found in the sequences of the strains LESB58 (serotype O6) and PAEM against the Wzx/Wzy-dependent pathway genes (
wbpA,
wbpB,
wbpE,
wbpD, and
wbpI), which are essential for B-band O-antigen biosynthesis in
P. aeruginosa serotype O5 (PAO1) and O16 [
27,
28]. The O-antigen biosynthesis gene cluster of the PAEM strain was successfully aligned to the
P. aeruginosa serotype O6 O-antigen biosynthesis cluster (GenBank ID: AF498417.1) with the identity 99.9 at 99.97% of coverage [
29].
The multilocus sequence typing (MLST) by the “mlst” software with the use of the
P. aeruginosa PubMLST database (
https://pubmlst.org/) attributed the PAEM genes (
acsA,
aroE,
guaA,
mutL,
nuoD,
ppsA, and
trpE) to the sequence type (ST) 2502, whose clonal complex is not defined [
30]. Two records belonging to the unpublished isolates from a bottled natural mineral water in Brazil (2018) and from a dog with otitis in France (2010) were found in the
P. aeruginosa PubMLST database. The phylogenetically closest STs were from two isolates of STs 131 and 957 (UK) and one isolate of ST 663 from France [
30].
The environmental
P. aeruginosa PAEM possesses the most abundant CRISPR/Cas system of the type I-F, an antiviral adaptive immune system conferring resistance to foreign genetic elements from plasmids and phages [
31]. This type was shown to correlate strongly with the susceptibility of the strain to multiple clinically relevant antibiotics for the prevalent STs 111 and 235 [
31].
2.3. Comparative Genomics and Singletons Analysis
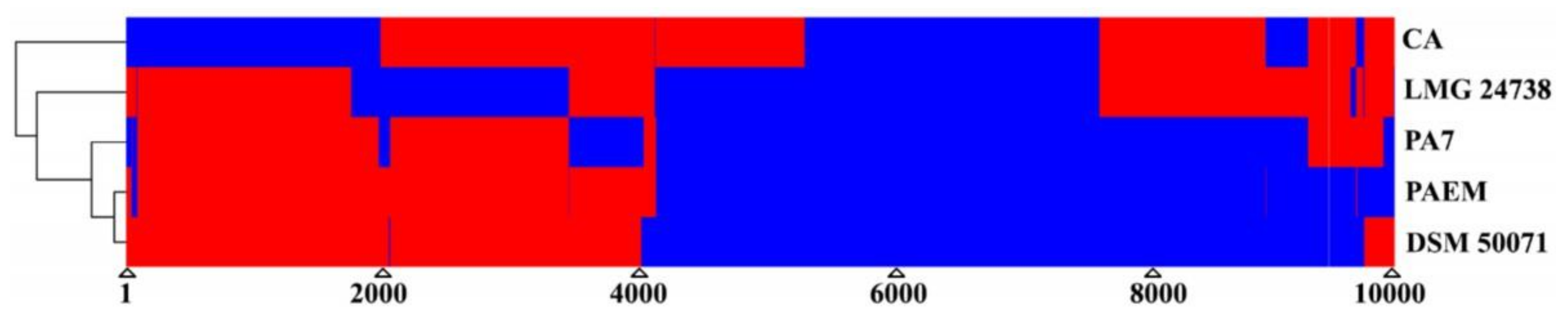
The strains
P. aeruginosa DSM 50071
T,
P. aeruginosa PA7,
Pseudomonas panipatensis LMG 24738, and
Azotobacter vinilandii CA were selected and analyzed by the EzBiocloud Comparative Genomics (CG) (
Table S7,
Figure 2 and
Figure 3). The common 2293 genes and 5075 of the
P. aeruginosa core genes were found in the sampling (
Figure 2 and
Figure 3). Although
P. aeruginosa PAEM has the expected core and pangenome, it revealed 150 and 157 singletons aligned against the orthologues and
P. aeruginosa strains, respectively (
Figure 2 and
Figure 3). In addition, the PAEM singletones have either low identity or no homologues when aligned against the reference
P. aeruginosa PAO1 or LESB58 sequences by the Basic Local Alignment Search Tool (BLAST) in the Pseudomonas Genome DB (
http://www.pseudomonas.com/). The PAEM singletons found by the EzBioCloud CG tools (
Table S7) without any similarity with the reference strains
P. aeruginosa DSM 50071
T, PA7, PAO1 and LESB58 have been additionally analyzed by the BLAST programs in the National Center for Biotechnology Information (NCBI) and Pseudomonas Genome DB (
https://www.ncbi.nlm.nih.gov/;
http://www.pseudomonas.com/).
There were no best reciprocal blast hits found for the CDSs for RNA-directed DNA polymerase PAEM_00124 (
Tables S1 and S7) with the exception of an urine-derived isolate (95.8%) of carbapenem-resistant
P. aeruginosa BH9 from Brazil (GenBank: CP029713.1) and
Sinimarinibacterium sp. NLF-5-8 (93%) isolated from the wastewater treatment plants for livestock in South Korea (GenBank: CP048030.1). However, the reference non-redundant protein sequence (RefSeq: WP_023102177.1) containing isolates of
P. aeruginosa from the human bloodstream, urogenital and eye infections, as well as of the foodborne pathogen
Salmonella enterica isolated from ground chicken (USA) (GenBank: EAU0154325.1), the clinical isolates of
Alcaligenes faecalis from wound, and several strains (
Spongiibacter tropicus,
Rheinheimera sp.,
Haliea sp.), with metagenome-assembled genomes, from the Pacific ocean were identical with PAEM_00124 by 100, 97, 95, and 87%, respectively.
The DNA polymerase IV (EC 2.7), similar to PAEM_00120 (
Tables S1 and S7), is widely distributed among the intestinal bacteria
P. putida and
P. fluorescence (NCBI Multispecies Reference Sequence (RefSeq): WP_003160626.1) [
32]. However, this record of five hypotetical proteins identical to PAEM_00120 include
P. aeruginosa BL12 from the eye infection (GenBank: ERY33665.1) and the multidrug-resistant (MDR) Japanese strain of
P. aeruginosa NCGM1179 (GenBank: GAA18645.1) from the respiratory tract. The strain NCGM1179 was highly resistant to carbapenems, aminoglycosides, and fluoroquinolones [
33].
The singleton PAEM_00125 (
Tables S1 and S7) with the predicted function of the XRE family transcriptional regulator or master regulator for biofilm formation (by EzBioCloud annotation) is identical to NCBI RefSeq WP_023102178.1 that corresponds to five records for
P. aeruginosa, including the same clinical strains described above and one crude oil-degrading isolate from the Gulf of Mexico (GeneBank: RFK48079.1).
The adenine-specific DNA-methyltransferase PAEM_00130 (
Tables S1 and S7), belonging to the type I restriction system, did not find the identical CDSs, but its protein sequence has the identity of 96–100% with the proteins from the same
P. aeruginosa isolates (RefSeq WP_079265932.1).
The probable tyrosine recombinase PAEM_00144 in
P. aeruginosa (
Tables S1 and S7) is of 100% protein identity with RefSeq WP_003160612.1, which includes the Japanese MDR clinical isolate NCGM1179. The PAEM singletons encoding for the itegrases/recombinases PAEM_00143, PAEM_00145, PAEM_04638 do not have any similarity, as well as orthologues among Pseudomonadaceae, according to the NCBI and EzBioCloud (EggNog/COG) databases (
Tables S1 and S7). In addition, the nucleotide and protein sequences of PAEM_00143, PAEM_00144 are well aligned against the sequences of
Comamonas testosteroni P19 isolated from the biphenyl as the sole carbon source from the waste water of South Korea (GenBank: LN879547).
The singleton PAEM_00412 (
Tables S1 and S7), encoding for the predicted tRNA(fMet)-specific endonuclease VapC2, a toxic component of a toxin–antitoxin (TA) system, was aligned against the multispecies RefSeq WP_003105740.1, including the putative nucleic acid binding proteins of
P. aeruginosa (GenBank: YP_006960443.1; AGO43171.1), endonuclease VapC for
Salmonella enterica (GenBank: ECA4546738.1), or hypothetical proteins for such strains as
P. aeruginosa PABE173_5365 isolated from a glass-crusher air plate (GenBank: EKA41331.1) and BL12 from the eye infection (GenBank: ERY24639.1). The antitoxin component VapB2 (PAEM_00413) of the (TA)-module has the lower number of identical sequences (
Tables S1 and S7). Among 24 genes from the 4662 genome assemblies of
P. aeruginosa aligned against PAEM_00413 (VapB2) in the specific Pseudomonas Genome DB, only 12 genes from clinical isolates were without mismatches.
The singleton PAEM_00443 and PAEM_00444 (
Tables S1 and S7), encoding for the reparative/replicative DNA helicase of subfamily UvrD/REP and hypothetical DNA repair protein RecF, respectively, found the nucleotide matches (100%) with only the
P. fluorescence strain NCTC10783 isolated from the respiratory tract (GenBank LR134300.1), but with 99.8% of the protein identity with the multispecies AAA family ATPase [Pseudomonas] (NCBI RefSeq: WP_003125302.1) that include again the clinical isolates
P. aeruginosa B12, NCMG1179, and 3575.
From 341,478 to 347,944 nucleotides (
Tables S1 and S7), there are nine PAEM-specific CDSs for hypothetical proteins related to the family RebB (Pfam: Family 11747) killing trait of the endosymbiont bacteria, methyl-accepting chemotaxis protein, RNA polymerase sigma factors, and cAMP-dependent regulator, which have orthologues in all available species from EggNOG (evolutionary genealogy of genes: Non-supervised Orthologous Groups).
The extended clusters of PAEM singletons from c(426058..428253) to c(445219..445413) in the contig 15 (
Tables S1 and S7) include 13 hypothetical proteins predicted as adenine-specific DNA-methyltransferase (type II restriction system, defense mechanism), multispecies transposases, and TonB-dependent siderophore-related receptor. The hypothetical proteins PAEM_03139 and PAEM_03140 also relate to the probable TonB-dependent receptors, which are similar to the PAO1 and LESB58 hypothetical proteins (99.0 and 99.6%). From c(420623..421225) to c(423573..424352) in the contig 21 (
Tables S1 and S7), there are four hypothetical proteins with no predicted function and orthologues, but with the identity to hypothetical proteins from the
P. aeruginosa LESlike isolates (data not shown).
The protein RelA (PAEM_03870), containing the regulatory domain of the bifunctional enzyme SpoT (Pfam PF0467), predicted as GTP diphosphokinase (absent in PAO1 and LESB58) has 100% identity with six reference sequences of the
P. aeruginosa (p)ppGpp synthetase or GTP pyrophosphokinase (RefSeq: WP_079393115.1). In eubacteria, ppGpp (guanosine 3′-diphosphate 5-′diphosphate) is a mediator of the stringent response that coordinates a variety of cellular activities in response to changes in nutritional abundance [
34].
The singleton PAEM_03874 (Pyocin-S2) (
Tables S1 and S7), belonging to the colicin/pyocin nuclease family, has 98% identity with the homing endonuclease from the structural family HNH (Pfam CL0263) of
Salmonella enterica (GenBank: ECA4547102.1), 100% with
P. aeruginosa RefSeq (WP_144126122.1, WP_148711685.1, WP_121379800.1, ERU77283.1), and 99.4% with the PAO1 strain (absent in LESB58). In addition, the HNH nuclease PAEM_04208 (
Tables S1 and S7) is identical by 100% to the PAO1 hypothetical protein PA0820 (absent in LES58). Pyocins belong to a larger group of bacteriocins, which are used by bacteria to compete for resources by killing competitors, usually of the same bacterial species [
34].
The singletons PAEM_03937 and PAEM_03938 (
Tables S1 and S7) were predicted to have the putative salinity stress responsive phosphoserine phosphatase (M:COG1213) or sugar nucleotidyltransferase (POG001052) functions, which are of 99.3–99.5 and 99.0–99.2% identity to the hypothetical proteins in PAO1 and LESB58, respectively (
Tables S1 and S7). However, the hypothetical protein PAEM_04645 (
Tables S1 and S7), which is related to a metallo-beta-lactamase domain protein according to EggNOG function, was found to have no matches within the comparative genomes suggested by EzBioCloud, as well as with PAO1 and LESB58. The CDS homologues of 100% identity were found in the hospital strains
P. aeruginosa DVT401 (GenBank: CP050335) and DVT 425 (GenBank: CP050325), and
P. fluorescens NCTC10783 (GenBank: LR134300). It has 100% amino acid identity with the multispecies metallo-beta-lactamase (MBL) fold hydrolase [Pseudomonas] (NCBI RefSeq: WP_003126469.1), including the environmental and host-associated
P. aeruginosa strains, such as ATCC 25324 from air from the glass-crusher (GenBank: EKA34652.1), BL12 from the eye infection (GenBank: ERY39277.1), and the human isolate 3575 (GenBank: EZO25326.1). Only two CDSs for the MBL fold metallo-hydrolase from the human isolate
P. aeruginosa N15-01092 (GenBank: CP012901.1) and sheep mastitis PSE305 (GenBank: HG974234) were found in the Pseudomonas Genome DB with 92.2% of identity (
http://www.pseudomonas.com/).
PAEM_04659 encoding for the 2-hydroxymuconate (4-oxalocrotonate) tautomerase family protein, related to the metabolism of aromatic compounds, particularly aromatic amines, has no matches within the DSM 500071
T (
Table S7), PAO1, and LESB58 genomes, but 100% identity with the multispecies protein [Pseudomonas] (RefSeq: WP_003159697.1), including 13 sequences from the MDR
P. aeruginosa strains BL12, BL24, BWHPSA003, BWHPSA017, CF18, X24509, Z61, 3575, BWH050, BWH055, NCMG1179,
Pseudomonas knackmussii, and
Acinetobacter baumannii, which degrade chloroaromatic compounds [
35,
36].
The gene cluster containing the putative stress-responsible OsmC-like porin, dihydrolipoyllysine-residue acetyltransferase PAEM_04661 (
Tables S1 and S7); disulfide-isomerase PAEM_04662 (putative polyketide synthase from 25 strains of
P. aeruginosa, particularly involved in the aromatic antibiotic production, such as isochromanequinones frenolicin and nanaomycins); the tetR family transcriptional regulator-like protein PAEM_04665, negative regulator of growth toxin ParE (induces the SOS response, inhibits cell division, and promotes biofilm formation) aligned against nine hypothetical proteins from the listed above strains (MULTISPECIES: [Pseudomonas] Ref Seq: WP_003125306.1; WP_015060171.1; WP_003125102.1).
From (197,990...198,724) to (203,991...204,881) bases (
Tables S1 and S7), there is a locus of seven PAEM singletons encoding for tRNA-dependent cyclodipeptide synthase proX—PAEM_05797 (MULTISPECIES: tRNA-dependent cyclodipeptide synthase [Pseudomonas] Ref Seq: WP_003158562.1) and five paralogues of hypothetical proteins with the EggNog function of 2OG-Fe(II) oxygenase superfamily, namely the multiply duplicated isopenicillin N synthase family oxygenase—PAEM_05798, PAEM_05799, PAEM_05801, PAEM_05802 (Ref Seq: WP_023132409.1), and gibberellin 3-beta-dioxygenase PAEM_05803 (
Tables S1 and S7). The identical proteins exist in many environmental strains, including the sewage
P. aeruginosa ENV-208, as well as the rhizosphere
P. aeruginosa M18 [
37]. The available orthologues were found in
Photorhabdus luminescens subsp.
laumondii TTO1, the marine gamma proteobacterium HTCC2080, and
Photorhabdus asymbiotica subsp.
asymbiotica ATCC 43949 (data not shown). The evolutionary significance of paralogues or gene duplication is to increase the likelihood of their expression and thus to strengthen the reliability of signs in the coding in which they participate. On the other hand, repeatedly duplicated genes probably serve as a “storage” of mutations that are harmful for the current environmental conditions, but giving the evolutionary advantage of any other conditions. Thus, when environmental conditions change quickly, organisms can “find” the most beneficial mutations from an accumulated set under these conditions and therefore quickly adapt to them [
37].
One more PAEM singleton locus contains the Type-I restriction system S of methylase family
hsdM (PAEM_05832), type I site-specific deoxyribonucleases
hsdS (PAEM_05832) and
hsdR (PAEM_05834), RNA-directed DNA polymerase (PAEM_05836) and hypothetical proteins PAEM_05833, PAEM_05835, and PAEM_05837, which putatively also related to the restriction-modification_system (
Tables S1 and S7). Only
hsdS found six matches in NCBI with 100% identity with the restriction endonuclease subunit S from the carbapenem- and colistin-degrading
P. aeruginosa (Ref Seq: WP_079386772.1). In Pseudomonas Genome DB, 30 hits were found against PAEM_05832 (
hsdS), including the veterinary strain VET-48 from the dog’s ear secretion (GenBank: RAED01000237).
2.4. Genomic Islands Analysis
Genomic islands (GIs) are commonly defined as clusters of genes of probable horizontal origin in bacterial or archaeal genomes. They often provide adaptive traits that enhance the fitness of a microorganism within a niche, encoding novel genes, environmentally relevant adaptations such as metal resistance, and entire metabolic pathways, such as the degradation of monoaromatic hydrocarbons. One of the important goals of GI predictions is pathogen outbreak analysis. The presence/absence of GIs, prophages, and other regions of genome plasticity supported the subdivision of
P. aeruginosa into two main groups [
38,
39]. Each group may be drawing upon distinct mobile gene pools at the largest clade grouping level (group 1 vs. group 2). This was in accordance with the core gene-based population structure of
P. aeruginosa, which contains 4009 core genes within 103
P. aeruginosa genomes [
40]. However, due to the highly adaptive divergence within the GIs regions, the
P. aeruginosa pan-genome is continuously extended. Nearly half of the new environmental and industrial isolates had new genotypes, indicating the wide geographically dispersing of the distinct clones [
40,
41].
Although
P. aeruginosa PAEM, similar to the strains DSM 50071
T, PAO1, and LESB58, is included into the most represented
P. aeruginosa phylogenetic group based on the whole-genome analysis [
39,
40], the comparable part of its genome (35 contigs) distinguishes from the DSM 50071
T and PAO1 genomes by 10 GIs, containing 122 and 151 CDSs, respectively (
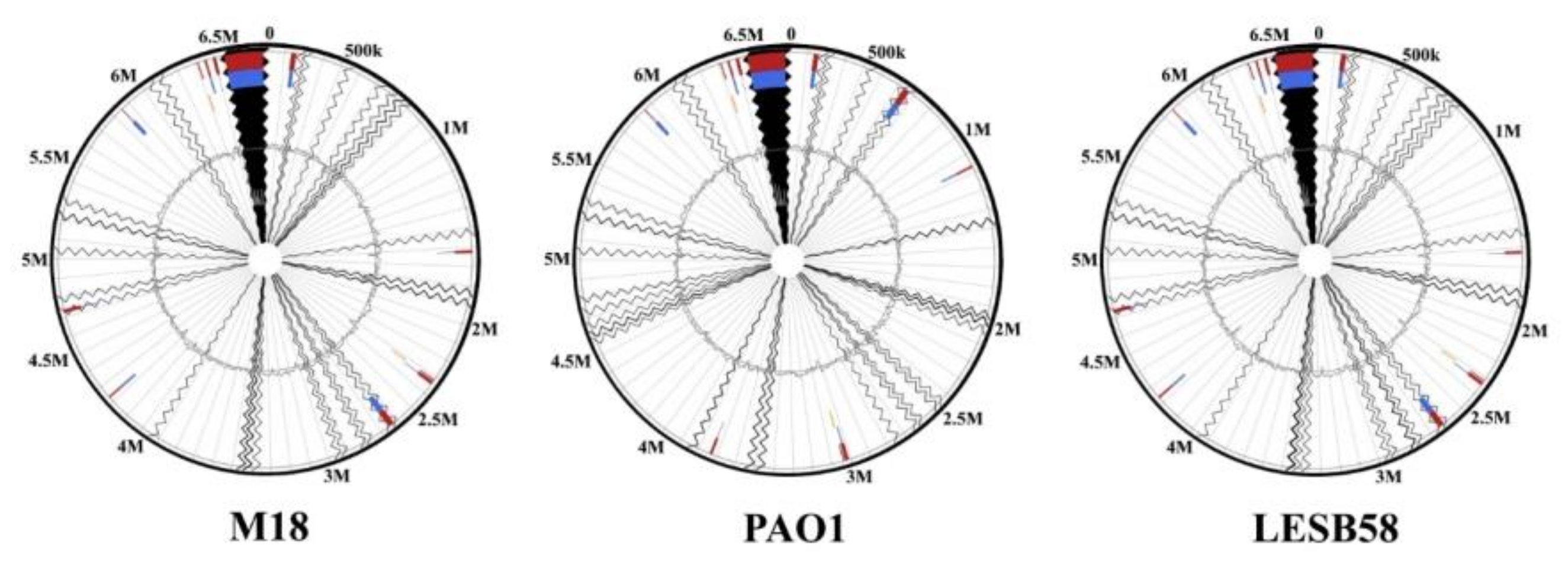
Figure 4). Meanwhile, it distinguishes from LESB58 by 11 GIs, containing 160 CDSs, with the longest GI of 34497 bp, according to the analysis by IslandViewer 4 [
38].
The
P. aeruginosa PAEM alignment against the reference genomes of the environmental rizosphere- and rice-derived strains M18 and F9676 resulted in 11 (160 CDSs) and 10 (121 CDSs) GIs, respectively (
Figure 4). The largest lengths of the PAEM GIs are 34749 and 28865 bp aligned against the M18 and F9676 genomes, respectively. The locations of PAEM GIs against M18 in comparison with LESB8 are identical (
Figure 4). Remarkably, the phylogenetic outlier strain
P. aeruginosa PA7 [
40,
41], with the lowest nucleotide sequences similarity (≤90–95%) with PAEM, revealed eight GIs (158 CDSs), with the largest length of 59,689 bp (data not shown). The CDSs of GIs found by IslandViewer 4 coincide with the singletons from the results of the EzBioCloud CG analysis (
Table S7).
As the main distinctive traits for
P. aeruginosa PAEM, the IslandViewer 4 found the GIs containing recombinational DNA repair, replication, and DNA/RNA metabolism systems, such as exodeoxyribonucleases V alpha (RecD), exonucleases SbcCD, RecA/RadA recombinases, superfamily II DNA/RNA helicases (SNF2 family), ribonucleotide reductases (aerobic and anaerobic), error-prone repair homolog of DNA polymerases III, DNA polymerase IV-like protein ImuB, ParA-like proteins; the antibiotic biosynthesis/resistance and iron transport genes encoding colicin/pyosin nuclease family proteins, pyocin S2 immunity protein, ferrichrome–iron receptor, pyoverdine sidechain non-ribosomal peptide synthetases PvdJ; the malonate pathway proteins; T6SS components VgrG proteins and virulence determinants Rhs-family proteins (type VI secretion system), Vap toxin system proteins, cold shock proteins of the CSP family; trans-2,3-dihydro-3-hydroxyanthranilate isomerase (EC 5.3.3.17), conjugative transfer proteins PilL, P, Q, S, U, M in a PFGI-1-like cluster, large exoproteins involved in heme utilization or adhesion, secreted proteins Hcp, thiol peroxidase of Tpx-type, bacteriocin/lantibiotic efflux ABC transporter/ATP-binding proteins, aliphatic amidase AmiE (EC 3.5.1.4), and hypothetical and phage-related proteins. Evidently, the restriction–modification systems are most highly variable among the prokaryotes under an evolutionary drive, since they have themselves been ranked with mobile elements [
42].
Nevertheless, many protein sequences, encoded by the GIs regions against PAO1 and LESB58, have been found in PAEM with the high similarity by BLAST in EzBioCloud (data not shown). Some of the PAEM CDSs (absent in PAO1 or/and LESB58) are mainly similar to hypothetical or phage proteins (99–100%) and the functional genes related to the traits of
P. aeruginosa strains isolated from the hexachlorocyclohexane (chlorinated organic insecticide)-contaminated soil MBT-1 (NCBI ID: CP006853), crude oil IMP66 (NCBI ID: CP028959), rice F9676 (NCBI ID: CP012066.1), tung meal LYT4 (NCBI ID: CP052759), swab from a not healing wound (NCBI ID: CP041785), and mink raising farm PA59 (NCBI ID: NZ_CP024630.1). No sequence similarity at all was found for PAEM_02237 (hypothetical protein) and PAEM_03871 (chromosome partitioning protein ParA) (
Tables S1 and S7).
Generally, the results on the PAEM strain are in agreement with the evidence about the low gene variability of
P. aeruginosa with high levels of their recombination within the species [
43,
44].
2.5. Phylogenetic Analysis
Although most of virulence-, pathogenicity-, and biofilm-associated factors of
P. aeruginosa PAEM from the frozen meat ready-to cook product showed the higher similarity with the reference genes of PAO1 and LESB58 (
Tables S3–S6), one gene, putatively possessing the cold shock protein (Csp) function, has been suggested as a marker gene for the strains adapted to the cold environment. The DNA:DNA hybridization (DDH), which was carried out by the TYGS server (
https://tygs.dsmz.de), confirmed the similarity of the PAEM strain to the reference strains with the following percentages: LESB58–96.6%; M18–95.5%; F9676–95.4%; PAO1–95.4%; VET-48–95.2%; and PSE305–94.7% [
45].
The genome of the true environmental isolate, such as the biocontrol strain M18 from the sweet melon rhizosphere, is also most similar to that of one of the severe clinical pathogens
P. aeruginosa strain LESB58 [
37]. However, the whole transcriptomic analysis of M18 indicated that 10.6% of the expressed genes are temperature-dependent, with the up-regulation at lower temperature than at 37 °C [
37].
Cold shock proteins (Csps) are mainly known to respond to the stabilization of the transcription and translation of proteins to prevent the cell membrane fluidity and enzyme activity decrease due to the temperature downshift (cold shock). Csps may contribute to tolerance against osmotic, oxidative, starvation, pH, and ethanol stress, as well as to host cell invasion for enteropathogens [
46]. The CDS for hypothetical protein PAEM_03055 in the cold-adapted
P. aeruginosa PAEM (
Table S1) was predicted as a CspA family protein of stress response and cold shock (beta-ribbon DNA-binding domain), according to the EGGNOG/KEGG/SEED function from the EzBioCloud genome feature data [
25].
From all sequenced genomes (4660) from Pseudomonas Genomes DB, 238 BLASTN hits against PAEM_03055 returned [
23]. However, the homologues of CspA PAEM_03055 (GeneBank: HZL38_16480) are mostly annotated as “Main chromosome cold shock protein CapB” or “DUF1294 domain-containing protein” with unknown function. The alignment of an amino acid sequence of PAEM_03055 (Diamond BLASTP) resulted in finding 3954 homologues (including resubmitted genome versions) among the complete assemblies of
P. aeruginosa [
23].
Phylogenetic analysis of the nucleotide sequences (codons) for the close homologues of PAEM_03055 was conducted in MEGA X [
47]. The evolutionary history (evolutionary probability, EP) was inferred using the Maximum Likelihood method and Hasegawa–Kishino–Yano model, plus a discrete Gamma distribution (+G), which due to the lowest BIC scores (Bayesian Information Criterion) were taken to describe the substitution pattern the best [
48,
49]. The bootstrap consensus tree inferred from 500 replicates [
50] is taken to represent the evolutionary history (
Figure 5). Branches corresponding to partitions reproduced in less than 50% bootstrap replicates are collapsed. The percentage of replicate trees, in which the associated taxa clustered together in the bootstrap test (100 replicates), is shown next to the branches [
50]. Initial trees for the heuristic search were obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the Maximum Composite Likelihood (MCL) approach and then selecting the topology with a superior log likelihood value. A discrete Gamma distribution was used to model evolutionary rate differences among sites (five categories (+G, parameter = 0.2394)). This analysis involved 111 nucleotide sequences. Codon positions included were 1st+2nd+3rd+Noncoding [
47]. There were in total 728 positions in the final dataset. The CspA sequence of the
P. aeruginosa strain PA7 was chosen as an outgroup because of the highest bootstrap values in the tree nodes (
Figure 5), while the strains clustered similarly independently on outgroups and methods (data not shown).
Although no possibility of inferring a common ancestor of clones for the species
P. aeruginosa, due to their capability of the fast intraspecific chromosomal recombination [
43,
44], the cold shock protein CspA has shown an evolutionary probability of the strains origin depending on the sources and geography of their isolation (
Figure 5).
The branching pattern of the tree for the strain PAEM and reference agricultural isolates, such as M18 from melon rhizosphere (100% identity) and F9676 from rice (99.85%), is identical. However, the clinical MDR isolates of
P. aeruginosa, including host-associated 012 (Switzerland, diseased humans); Y71, Y89 (South Korea, sputum of 70-year-old male); E80 (Washington, cystic fibrosis patient); X78812, H5708, W60856, (New York, Memorial Hospital, cancer patients), and PA_150577 (Hong Kong, human) are also with 100% identity of their CspA with that of the agricultural strains and cold-adapted PAEM_03055 (
Table S1; GenBank: HZL38_16480).
The strains
P. aeruginosa 8380 and BH9 from human gut and urine samples, respectively; USDA-ARS-USMARC-41639 from nasopharynx of domestic cow; and NCTC13359 from water bottles are also clustered in the same clade with PAEM, M18, and F9676 (
Figure 5). Probably, they all may be highly adapted to negative temperatures, because PAEM withstands long-term storage of at least up to −20 °C in the frozen meat mince containing soy (NCBI BioSample: SAMN15461260). The agricultural soils used for growing plants can also freeze during the winter at least in northern latitudes.
There is the same possibility that the source of the isolate PAEM could be the meat-processing environment or the farm animal itself infected by the soil-derived pseudomonad. The farm animal-associated strain PSE350 is placed in the neighbor clade of the industrial strains, such as the RW109-like strains with the large multireplicon genomes (about 7.0–7.9 million bp) [
40].
The farm or agricultural strains N17-1 and HS9 from the soil; AJD2 from the cotton rizosphere; FDAARGOS, NCTC 13359, and PRD10 (animal room) from water bottles, as well as the strains M8A1, M8A4, and Pb18 isolated from the crude oil; and FA-HZ1 and PA1RG from wastewater seem to originate by parallel evolution from the same ancestor as the strains from two clusters of the PAEM- and industrial RW109-related strains (
Figure 5). Thus, the representatives of these phylogenetic branches, inferred from the common ancestor, including human-associated strains and the reference strains PAO1 and DSM 50071
T, may be originated from the environment, particularly the agricultural soils and plants, which, in turn, may be contaminated by the industrial waste. Probably, all of them can be adapted to negative temperatures to varying degrees and other negative environmental factors for their survival.
However, the topology of the ML tree branches indicates that the
P. aeruginosa strains, with the Csp related to the cold-adapted strains, could have a probability of secondary origination from the high-temperature tolerant pathogenic strains, such as PA7, UCBPP-PA14, or LES-like strains (
Figure 5).
Notably, the isolates from the ocean, clustered with an algae-associated DN1 and L10 from a holobiotic reed, are also close to the earlier common ancestor with the LES-like isolates or the isolates mainly causing bacteremia or associated with the burn pathology, which clustered in the separate branch. The strain DK2 that suggested having the traits for early-stage adaptation to the human organism are included in the neighbor branch (
Figure 5). Thus, their ancestors putatively belonged to the strains from southerly latitude or from warm-blooded mammals. This is also indicated by the close location of the environmental strains MBT-1 (soil, India), B10W (wastewater, Honolulu), as well as the strains associated with the community-acquired diarrhea B136-33 (Taiwan) or community-acquired pneumonia PA58 (ventilator-associated, Mexico) and PASGNDM345 (sputum, Singapore) (
Figure 5).
The phylogenetic outlier strain PA7 from a non-respiratory patient clustered closely to the branch with the strain UCBPP-PA14 from a human burn patient (
Figure 5), which is reference for the strains containing the additional exotoxin-encoding gene
exoU [
41]. Remarkably, the route branch PA7 contains the strain CR1 isolated from a chilli rhizosphere in India (NCBI, BioSample: SAMN06673526) (
Figure 5). Therefore, it is not surprising that the
P. aeruginosa strains, including PAEM, have the genes responsible for the plant growth promotion (or pathogenesis) and biocontrol activity (
Tables S1 and S3) [
37,
40,
41].
2.6. Gene Expression Analysis after Cultivation of PAEM with the Marine Bacterial Alpha-Galactosidase α-PsGal
The highly biofilm-forming strain
P. aeruginosa PAEM has been found to decrease its newly biofilm formation by 60% under supplementation with the cold-active alpha-galactosidase
α-PsGal from the marine bacterium
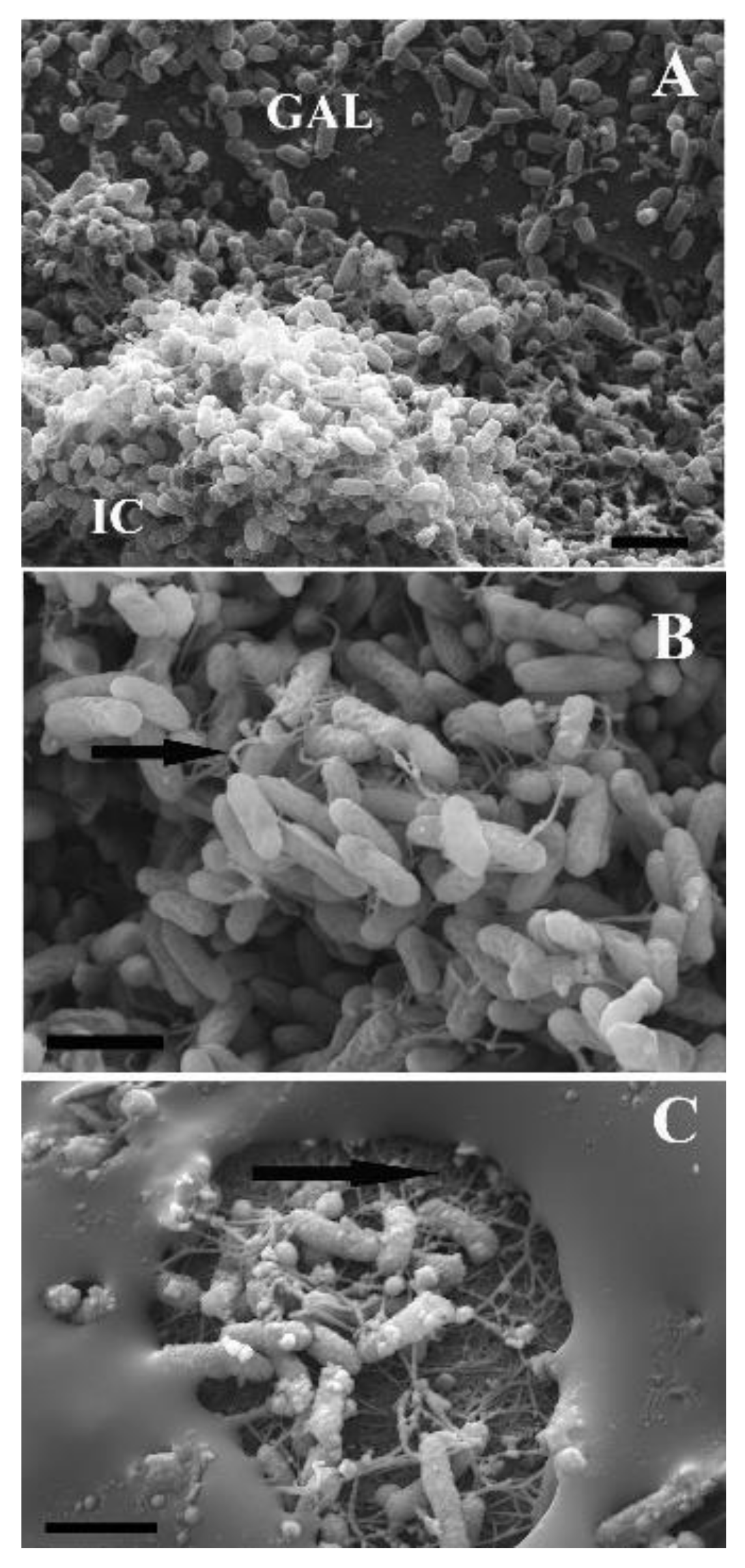
Pseudoalteromonas sp. KMM 701 into the liquid growth medium (Luria-Bertani, LB) at a room temperature without shaking (data not shown). In addition, the mature biofilm destruction was observed as gullies, channels, and deep pits between the colonies, when 15 μL of α-PsGal at a concentration of 0.08 mg/mL (with the activity 1–20 U/mg protein) was applied above the plated
P. aeruginosa and kept for 12 h at 22 °C (
Figure 6A–C). However, the results of electron microscopy, along with the biofilm-degrading effect, revealed a significant change in the morphology of the extracellular matrix after 24 h of incubation with the enzyme α-PsGal (
Figure 6). The biofilm changed its structure from being uniformly loose due to the formed voids and channels between the clusters of colonies to an opaque monolithic substance covering a significant part of the cells (
Figure 6C). At the same time, the number of the cells decreased, while the remained cells produced putatively outer membrane vesicles (
Figure 6C). It is obvious that the
P. aeruginosa PAEM biofilms under study have galactose-containing substrates for the hydrolysis reaction in the presence of α-PsGal. Probably, the appearance of a large amount of free galactose as a result of the action of the galactosidase could trigger the synthesis of new and/or transformation existing oligo- and polysaccharides in the extracellular matrix by the PAEM cells themselves or with the participation of the transglycosylating activity of α-PsGal [
4].
According to the comparative genomics, the PAEM pathogenome, including most of the biofilm-regulating factors, is the same with the reference strains PAO1 and LESB58 (
Table S6). Therefore, for evaluating the effect of the cold-active alpha-galactosidase α-PsGal on
P. aeruginosa PAEM, the levels of gene expression with the established biofilm-regulation functions were determined in the presence of the different concentrations of the enzyme in the cultivation medium (
Figure 6,
Table 1).
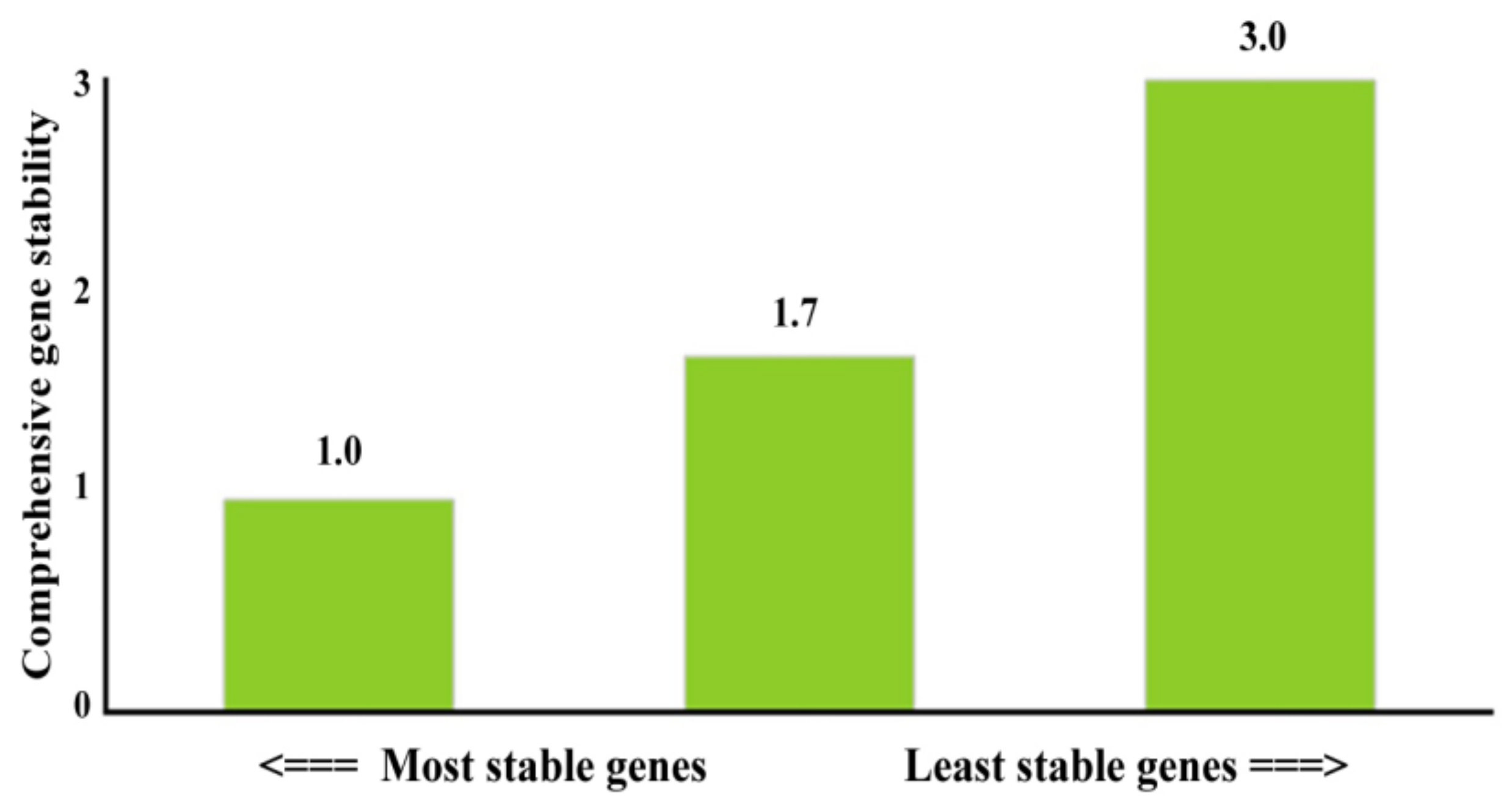
To evaluate the best reference genes for
P. aeruginosa PAEM, three candidate genes of pyrroline-5-carboxylate reductase (
proC), sigma factor RpoD (
rpoD), and chromosomal beta-lactamase (
ampC) were compared in terms of the expression stability in all experimental and control samples (
Table 1). The relative fold change in the expression was evaluated using the RefFinder [
61], which integrates different mathematic algorithms (geNorm, Normfinder, BestKeeper and the comparative deltaCt method) to compare and rank the tested candidate reference genes.
The
proC and
rpoD genes showed the most stable expression patterns in all tested experimental sets (
Figure 7); thus, this pair was used as an internal control in the relative comparison studies of the biofilm-related genes in
P. aeruginosa PAEM. Similarly, these genes formed the most stable pair among six candidate housekeeping genes earlier [
51].
The expression levels of the biofilm-related genes of
P. aeruginosa PAEM were evaluated by quantitative PCR (qPCR) (
Figure 8). These genes showed the differential expression patterns in both control and experimental conditions under 2, 10, and 20 units of the a-galactosidase α-PsGal supplemented into the growth medium (
Figure 8 and
Figure 9). Five groups of the genes were identified based on their transcriptional levels.
The first group consists of the genes
bdlA and
vsmR(rhlR), whose expression was increased by 2–3 folds upon the enzyme supplementation, and no dose-dependent effects were observed (
Figure 8 and
Figure 9). BdlA, a chemotaxis regulator, plays a pivotal role in the dispersion of biofilm, while
vsmR (
rhlR), encoding for the regulator RhlR, controls expression of the genes required for biofilm formation, including LasR-specific factors of the QS system in
P. aeruginosa [
8,
53,
59]. It has been suggested that BdlA may be a link between sensing environmental cues, c-di-GMP levels, and detachment. The
bdlA mutants were found to have increased adherent properties and intracellular levels of c-di-GMP [
59]. Meanwhile, two interconnected acyl-homoserine lactone (acyl-HSL) signal-receptor pairs, 3-oxo-dodecanoyl-HSL-LasR and butanoyl-HSL-RhlR, regulate more than 300 genes, including QS-dependent motility, virulence, and biofilm formation [
53].
The second and most abundant group consists of the genes
eddA,
eddB,
HDGYP,
rpfG/C,
lasl,
pelF, and
morA, whose expression was increased by 1.4-3.5 fold only by 10 and/or 20 units of the enzyme (
Figure 8 and
Figure 9). This group includes genes participating in QS, extracellular DNA (eDNA) degradation, production of a glucose-rich matrix exopolysaccharide Pel, and flagellar development [
53,
55,
57,
58,
60].
Many pathogens produce secreted extracellular DNases to avoid an external antimicrobial activity.
P. aeruginosa encodes an operon of two secreted enzymes, a predicted alkaline phosphatase and DNase. The DNase EddB degrades eDNA to use as a nutrient source. EddA has both alkaline phosphatase and phosphodiesterase activities and protects against antimicrobial activity [
60]. Nevertheless, the eDNA degradation may be a factor of the visible changes in the
P. aeruginosa PAEM biofilm thickness (
Figure 6).
HDGYP is a protein domain, which is encoded by the genes, signed here as
HDGYP and
rpfG/C (
Table 1,
Figure 8 and
Figure 9), is involved in the hydrolysis of the bacterial second messenger cyclic-di-GMP [
58]. Overexpression of these domain-containing proteins was found to inhibit biofilm formation [
58].
PelF, a putative glycosyltransferase, encoded by a seven-gene cluster
pel, is involved in the polysaccharide Pel biogenesis [
57].
The absence of regulator motility MorA in the
P. aeruginosa mutants led to a reduction in biofilm formation, but their motility was unaffected [
53]. In addition, the overexpression of flagellin resulted in the decreased adhesion; therefore, it has been hypothesized that flagella might be strongly regulated by MorA at certain stages of bacterial adhesion and biofilm formation [
53].
The
lasI gene was found to be down-regulated in biofilm [
55]; therefore, an increase in the level of its expression may coincide with the biofilm degradation/dispersion of
P. aeruginosa PAEM (
Figure 6).
The third group consists of the genes
pslA and
pprB, whose expression was extremely activated in a dose-dependent manner from 2 to 10 units of the enzyme, while 20 units of the enzyme slightly decreased this effect, remaining a magnitude higher compared to the control (
Figure 8 and
Figure 9). The permeability regulator encoded by
pprB serves as a receiver in a “PprAB” system, which switches bacteria from their free-living cells to multi-cellular biofilm. It was shown that
pprB activation is also associated with the increased susceptibility to antibiotics and reduced virulence [
56]
Meanwhile, PslA is the first and most important protein necessary for the synthesis of the biofilm matrix polysaccharide Psl [
8,
12,
52,
54]. The
P. aeruginosa polysaccharide Psl could be a galactose- and mannose-rich exopolysaccharide [
54]. However, the galactose-containing matrix component is still unknown, which is probably due to the strain-dependent polysaccharide structure specificity [
62].
Notably, the nanomolar concentrations of the own
P. aeruginosa glycoside hydrolases PslG and PelA, belonging to the different metabolic pathways and participating in vivo exopolysaccharide processing, also effectively degraded polysaccharide components of the biofilm matrix, preventing newly biofilms and rapidly disrupted mature biofilms in vitro [
63]. The treatments by PelA and PslG were effective against clinical and environmental
P. aeruginosa isolates and reduced biofilm biomass by 58–94% [
63].
The fourth PAEM expression pattern group consists of the genes
roeA and
sadC, whose expression was down-regulated by two units of the a-galactosidase α-PsGal, but it was activated by 10 and 20 units of the enzyme (
Figure 8 and
Figure 9). These genes also participate in the production of extracellular polysaccharides (EPS), biofilm formation, and swarming motility [
13,
14]. The digyanilate cyclases RoeA and SadC make distinct contributions to the biofilm formation, controlling polysaccharide production and flagellar motility, respectively, with no correlation between levels of c-di-GMP and the observed phenotypic traits with regard to the EPS production and swarming motility [
13].
It also can be noted that genes from the third and fourth clustered groups, such as
pprB,
pslA, and
roeA, showed the highest level of up-regulation in response to the a-galactosidase α-PsGal application. At the dose of 10 units, their expression levels were induced 6.4–10.1 fold compared to the untreated cells (
Figure 8).
The last fifth cluster group includes one gene
pelA, whose expression was not altered by α-PsGal (
Figure 8 and
Figure 9). PelA is involved in the polysaccharide Pel production and important for maintaining of cell-to-cell communication. However, loss of the Pel biosynthesis in the PAO1 strain resulted in no difference in attachment or biofilm development [
57]. Thus, the PAEM glucose-containing Pel is not absolutely affected by the presence of the galactosidase and/or free galactose (
Figure 8 and
Figure 9), and it is also not critical for the biofilm maintenance [
57].
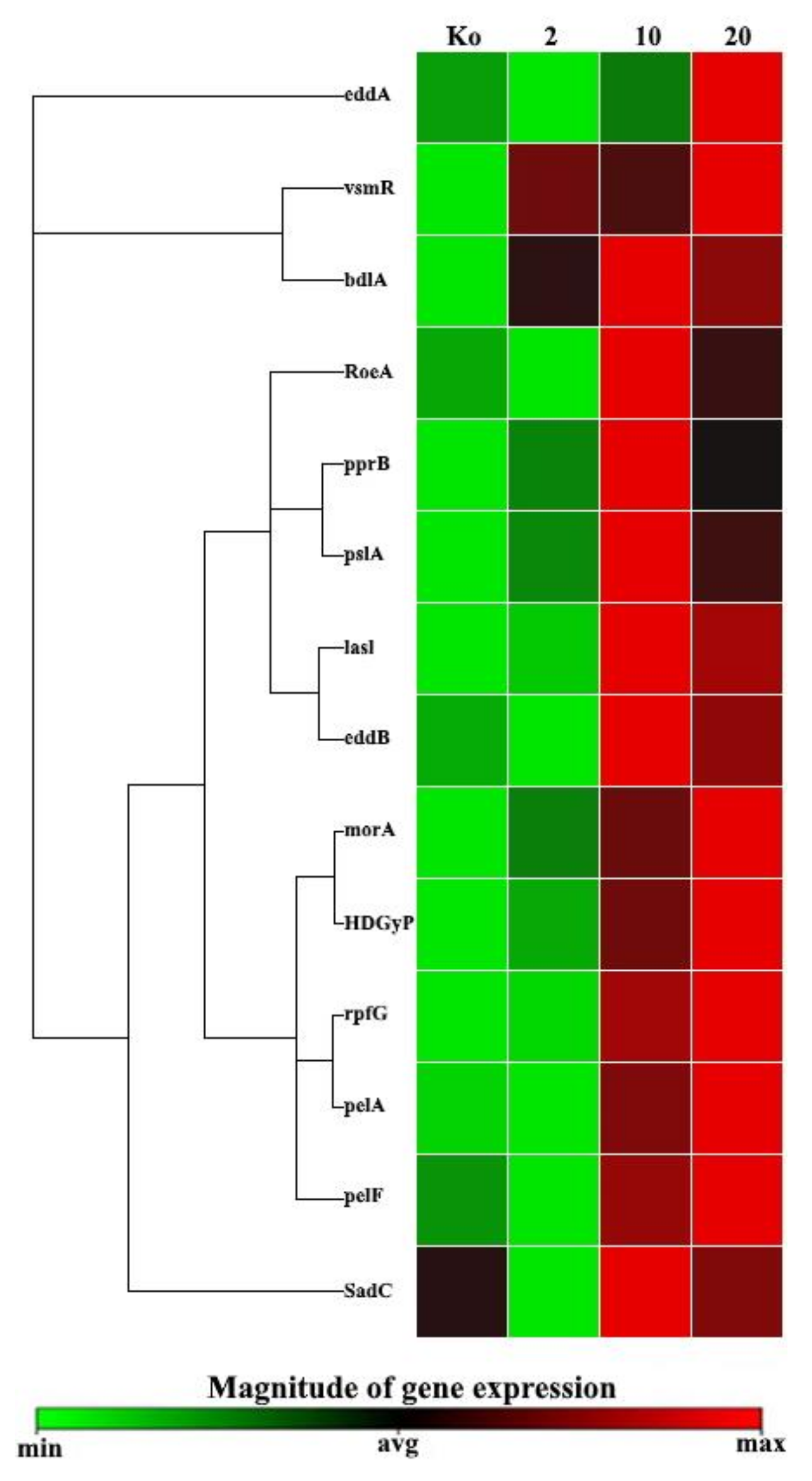
The heatmap shown in
Figure 9 partly confirmed clustering of the commonly regulated
P. aeruginosa PAEM biofilm-associated genes. However, as soon as the clustergram shows the data in a hierarchy, based on the degree of similarity of the expression for different targets and samples not considering statistical confidence, it does not fully reflect the observed biological effect.