1. Introduction
Titanium dioxide (TiO
2) inorganic semiconductors have emerged as trending materials for photocatalysis applications [
1,
2,
3]. The band gap of 3.2 eV necessitates ultraviolet (UV) light absorption to cause electron movement from the valence band to the conduction band to progress the photocatalytic reaction. However, these photoreactions only proceed within the UV portion of the solar spectrum, which leaves the broad portions of visible and near infrared (NIR) spectra undetected. Reversing the visible and NIR scattering phenomenon using TiO
2 remains an arduous task for researchers. The methods for improving spectral absorbance include doping with metals and non-metals [
4,
5,
6,
7] and coupling with other compounds to form nanocomposites [
8,
9].
Doping TiO
2 with elements such as B [
10,
11], C, N, and S [
12,
13,
14] is among the extensively studied techniques used to narrow the band gap. C and N are organic elements that possess remarkable electronic structures and lower toxicity than metallic elements. Thus, non-metallic doping is advantageous because of the smaller ionic radii that can occupy the interstitial sites of TiO
2 [
15,
16,
17]. The extra energy levels imparted by dopants in TiO
2 promote absorption of visible light photons [
18]. To date, several studies have reported alternative mechanisms for electron–hole reaction pathways to facilitate photocatalysis. Specifically, N-doped TiO
2 forms a unique band structure as a substitution at vacancy or interstitial sites in TiO
2. As a result of this, the TiO
2 band gap of 3.2 eV is narrowed to between 2.8 and 3.06 eV. The doped TiO
2 then sensitizes visible light through the low-energy sites occupied by N for photocatalytic reactions. Consequently, N-doped TiO
2 is reported to perform better in photodegradation reactions due to suppressed electron recombination [
16,
19]. However, in tri-doped CNS-TiO
2, C, N, and S elements substitute at O sites in TiO
2. In other words, the 2p orbitals of C, N, and S interact with O orbitals inside TiO
2’s conduction band. Therefore, new energy levels from the CNS doping elements lower the overall band structure of TiO
2. The great advantage of multi-element doping is that the sensitization sites increase several times as compared to singly doped element [
14,
20,
21]. However, utilizing N- or CNS-doped TiO
2 nanoparticles in aquatic and air purification methods requires a stable substrate to immobilize and prevent release of TiO
2 into the environment. There are several immobilizing substrates for practical application of TiO
2 nanoparticles, which include stainless steel [
22] and glass [
23]. However, these substrates only offer support. Their chemical composition has minimal effects in terms of improving the light absorption, which is essential for photocatalytic activity progression. Thus, there is a need to stabilize the nanoparticles in micro-sized compounds such as NaYF
4:Yb,Er phosphors, which possess light-harvesting properties in the NIR region.
Although upconversion phosphors have short photoluminescence lifetimes, if coupled with TiO
2 they promote light harvesting or photocatalysis, even under visible light photons, due to the heterojunction effect [
24,
25]. The heterojunctions that exist at interfacial peripheries of the TiO
2 catalyst and phosphor support material are associated with modified electronic structure due to defects [
24,
26]. This phenomenon distinguishes NaYF
4:Yb,Er from other compounds, since both the heterojunctions and the light upconversion effects simultaneously improve the optical and photocatalytic performance of the composites.
Coupling TiO
2 with NaYF
4:Yb,Er phosphor has been reported as a promising approach for utilizing low-energy photons in the NIR region and emitting UV-visible light [
27,
28]. Over the past decades, NaYF
4:Yb,Er has been utilized to convert NIR 980 nm photons to emitted photons at 525–550 nm [
26,
29,
30], and even at lower wavelengths such as 390–420 nm [
31]. However, only the TiO
2 coating on phosphor has been reported to cause improved photocatalytic efficiencies after a long photoreaction time above 10 h [
32]. There is a need to evaluate photocatalysis in consideration of the amount of catalyst in the reactor, the light source intensity, and the concentration of pollutant in an effort to complete the photoreaction rapidly. The effect of N doping [
33] or carbon–nitrogen–sulfur (CNS) doping [
21] has been reported as a method to promote visible light activation of TiO
2, but few studies have been reported that compare the effect of coupling N-doped or CNS-doped TiO
2 with NaYF
4:Yb,Er phosphor.
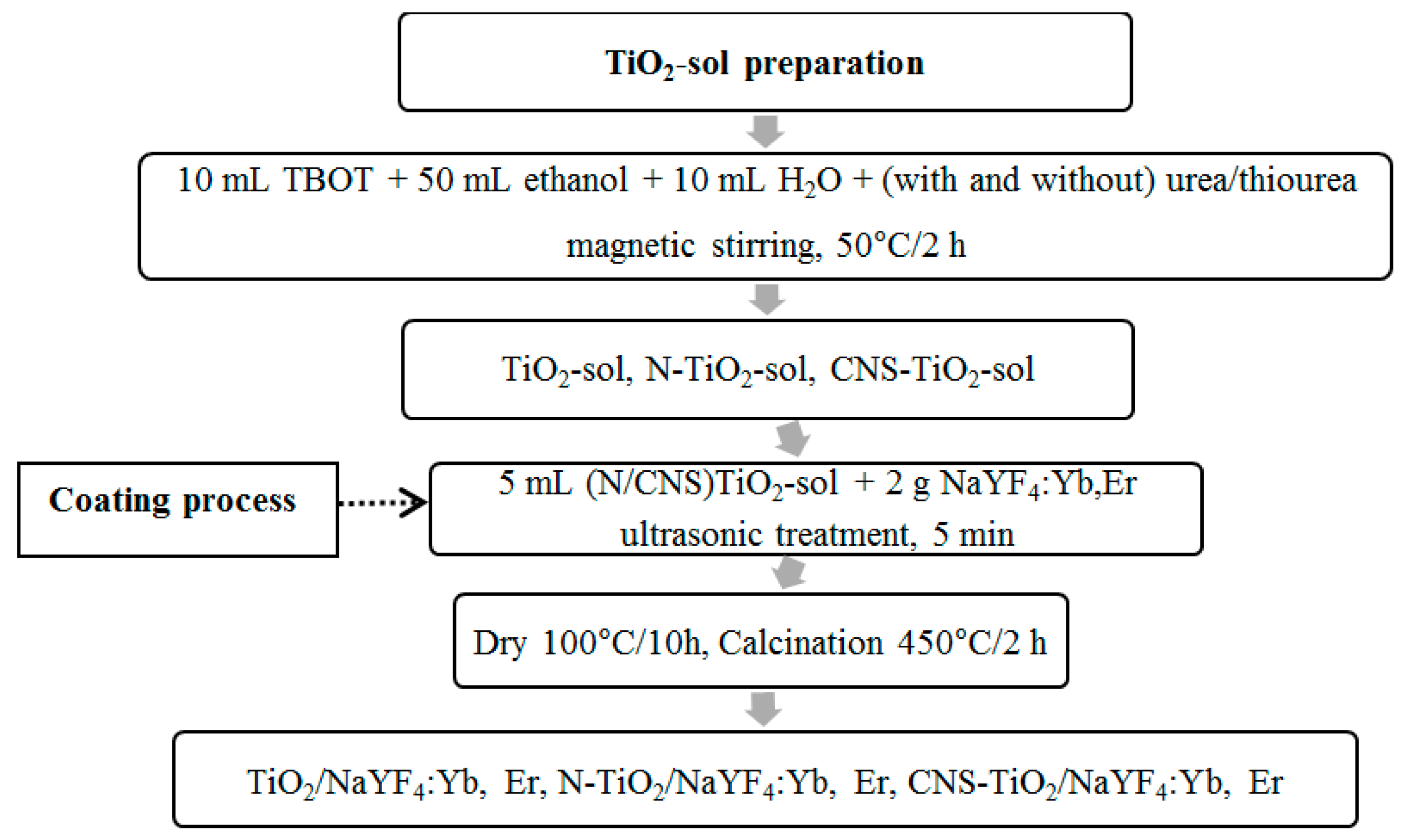
This study focuses on investigating the effects of N doping and CNS doping of TiO2 and coupling with NaYF4:Yb,Er upconversion phosphor. The stability of the organic–inorganic molecular bonding was characterized to confirm the existence of dopants in the TiO2/NaYF4:Yb,Er composites. Photocatalytic properties were evaluated with aqueous methylene blue (MB) and toluene pollutant mediums. The photocatalyst samples of N- or CNS-doped TiO2/NaYF4:Yb,Er were activated by UV, visible, and NIR light illumination of the solar spectrum.
2. Results
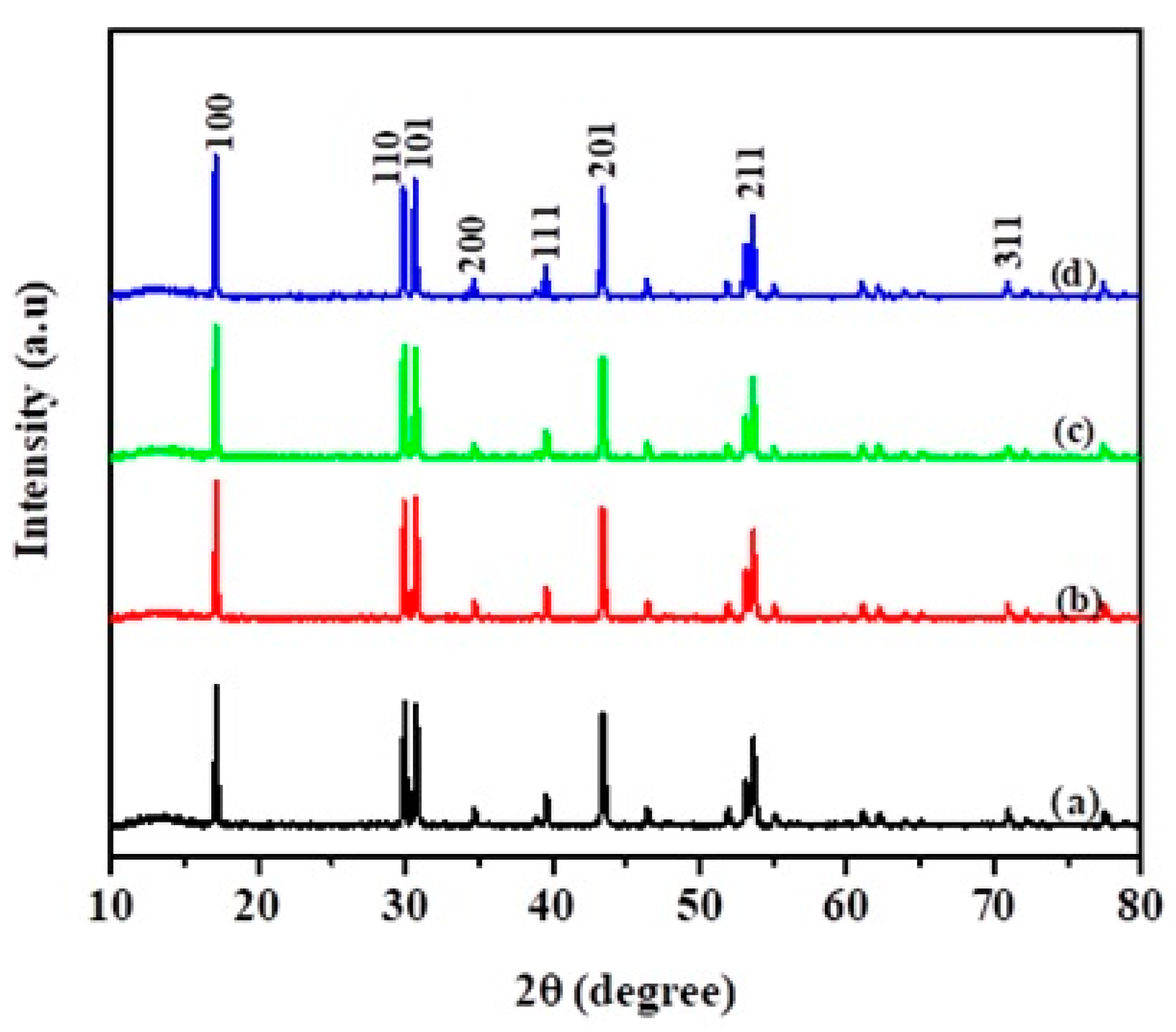
Figure 1 shows XRD spectra of NaYF
4:Yb,Er phosphor composites. The crystallinity is indexed for the phosphor in reference to hexagonal sodium, yttrium, ytterbium, erbium, and fluoride (JCPDS 00–028–1192). The NaYF
4:Yb,Er phosphor peaks were centered at (100), (110), (101), (200), (111), (201), (211), and (311). However, doping elements and TiO
2 crystal were undetectable in the composites owing to the relatively small amounts coated on the sub-micron phosphor matrix. The XRD peaks were invariant in terms of peak broadening, which normally confirms the effect of N or CNS doping in TiO
2.
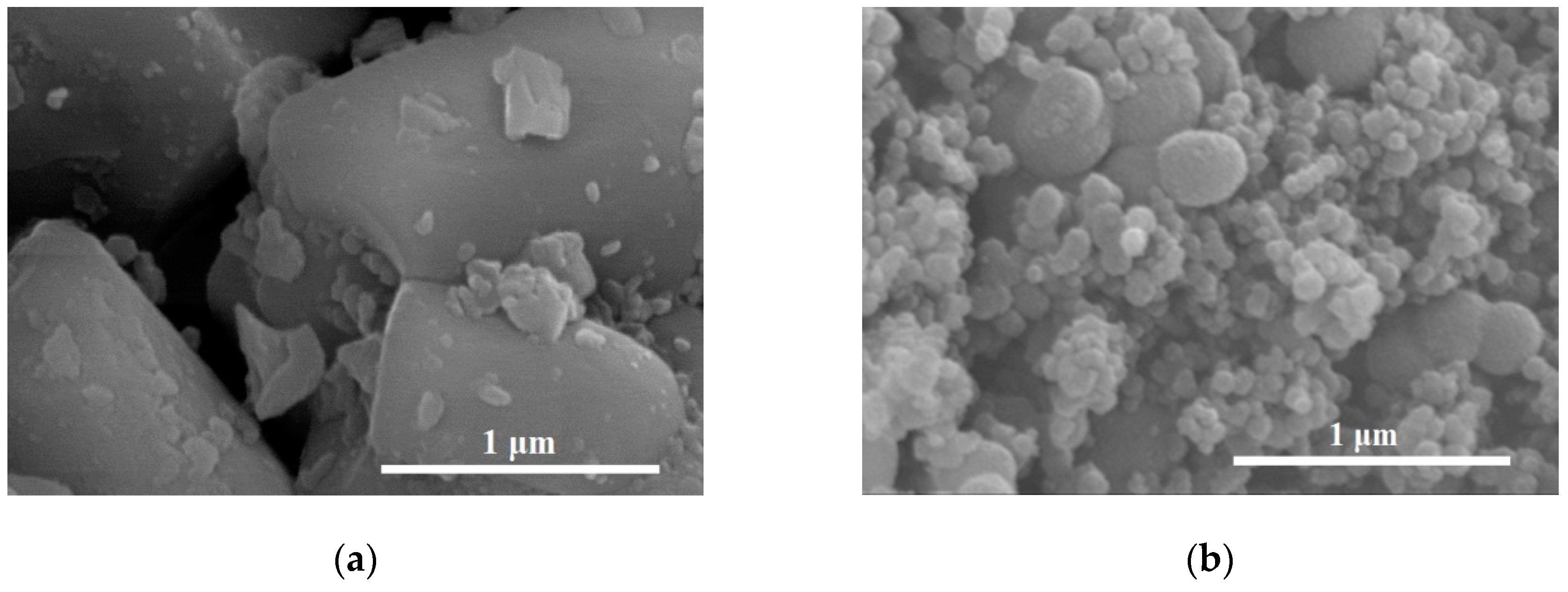
Figure 2 shows SEM images of NaYF
4:Yb,Er (
Figure 2a), TiO
2 (
Figure 2b), TiO
2/NaYF
4:Yb,Er (
Figure 2c), N-TiO
2/NaYF
4:Yb,Er (
Figure 2d), and CNS-TiO
2/NaYF
4:Yb,Er (
Figure 2e). The phosphor particles in
Figure 2a are in the ~10 micrometer range, while the undoped TiO
2 particles in
Figure 2b are agglomerates with a spherical morphology measuring 20–50 nm.
Table 1 shows the elemental compositions of F, Na, Y, Er, and Yb. When the TiO
2 sol was coated on the phosphor, its spherical morphology was maintained (
Figure 2c).
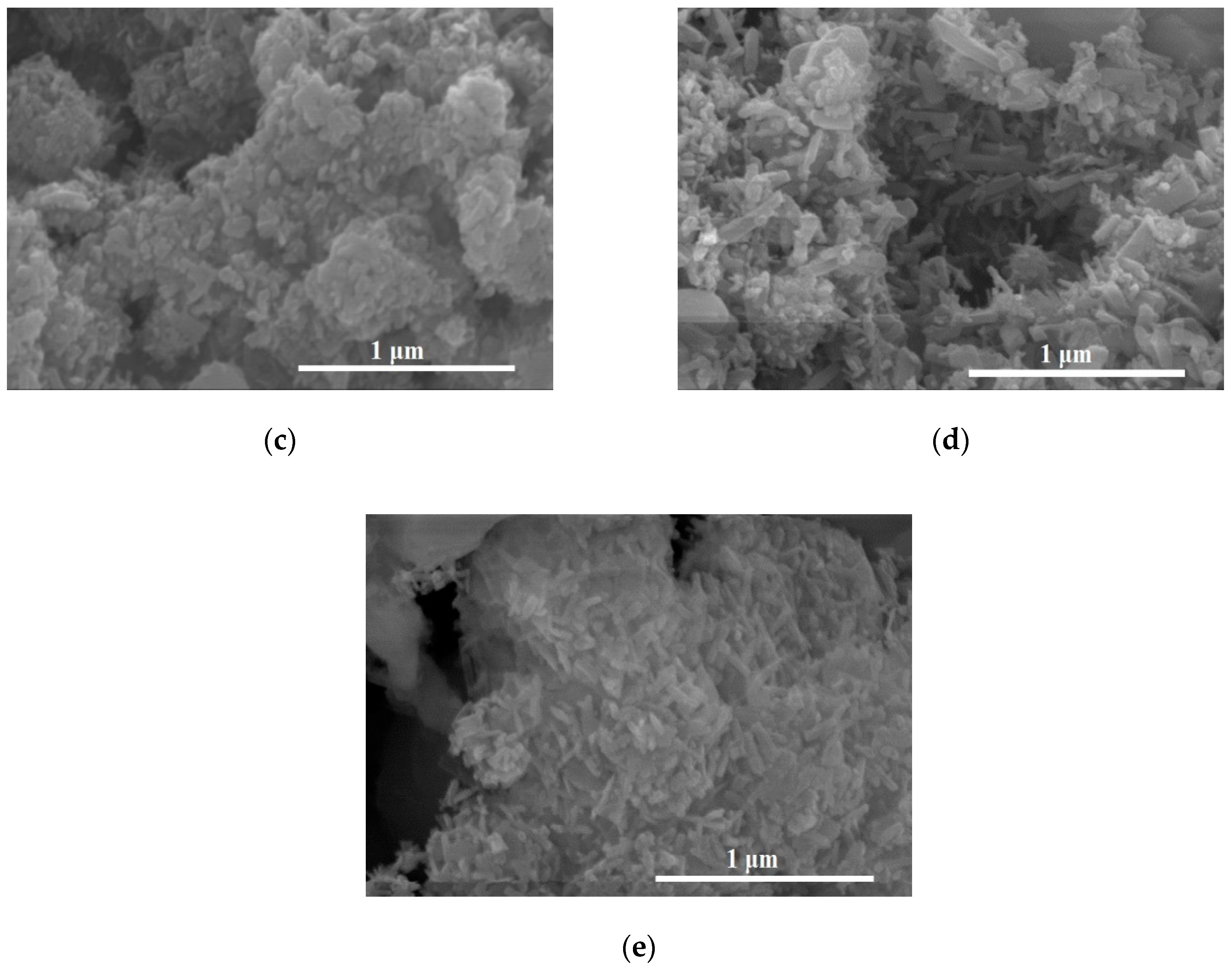
Table 2 shows the elemental compositions of O, F, Na, Ti, Y, Er, and Yb. However, with N and CNS doping in TiO
2, the TiO
2 morphology changed to clustered nanorods on the surfaces of phosphor particles (
Figure 2d,e).
Table 3 shows the elemental compositions and confirms the presence of N, O, F, Na, Ti, Y, Er, Yb, and the N-doped TiO
2 at 0.17 at.% N. The dense nanorods’ TiO
2 morphology is presumed to be because of the change in pH after addition of the urea (N) or thiourea carbon-nitrogen-sulfur (CNS) doping reagent, as described in the Materials section.
Table 4 shows the elemental compositions of C, N, O, F, Na, S, Ti, Y, Er, and Yb. The EDS spectra are shown in
Figures S1, S3, S5, and S7. The EDS elemental mapping data are shown in
Figures S2, S4, S6, and S8. Interestingly, the CNS-doped TiO
2 shows values of C 46.30 at.%, N 0.79 at.%, and 0.15 at.%. Therefore, the thiourea doping reagent is a rich source for CNS elements.
FTIR spectroscopy results for N-TiO
2/NaYF
4:Yb,Er and CNS-TiO
2/NaYF
4:Yb,Er are shown in
Figure 3a,b, respectively. The stretching vibrations at 3410 and 1624 cm
−1 are assigned to the -OH groups bonded to Ti- atoms and H
2O bending, respectively; while the stretching vibrations at 1369 and 1130 cm
−1 arise from carboxylate and S = O bonds, respectively, because of surface-adsorbed SO
42− species. The stretching vibrations at 1044 and 616 cm
−1 are because of hyponitrite and the Ti-O groups, respectively [
34].
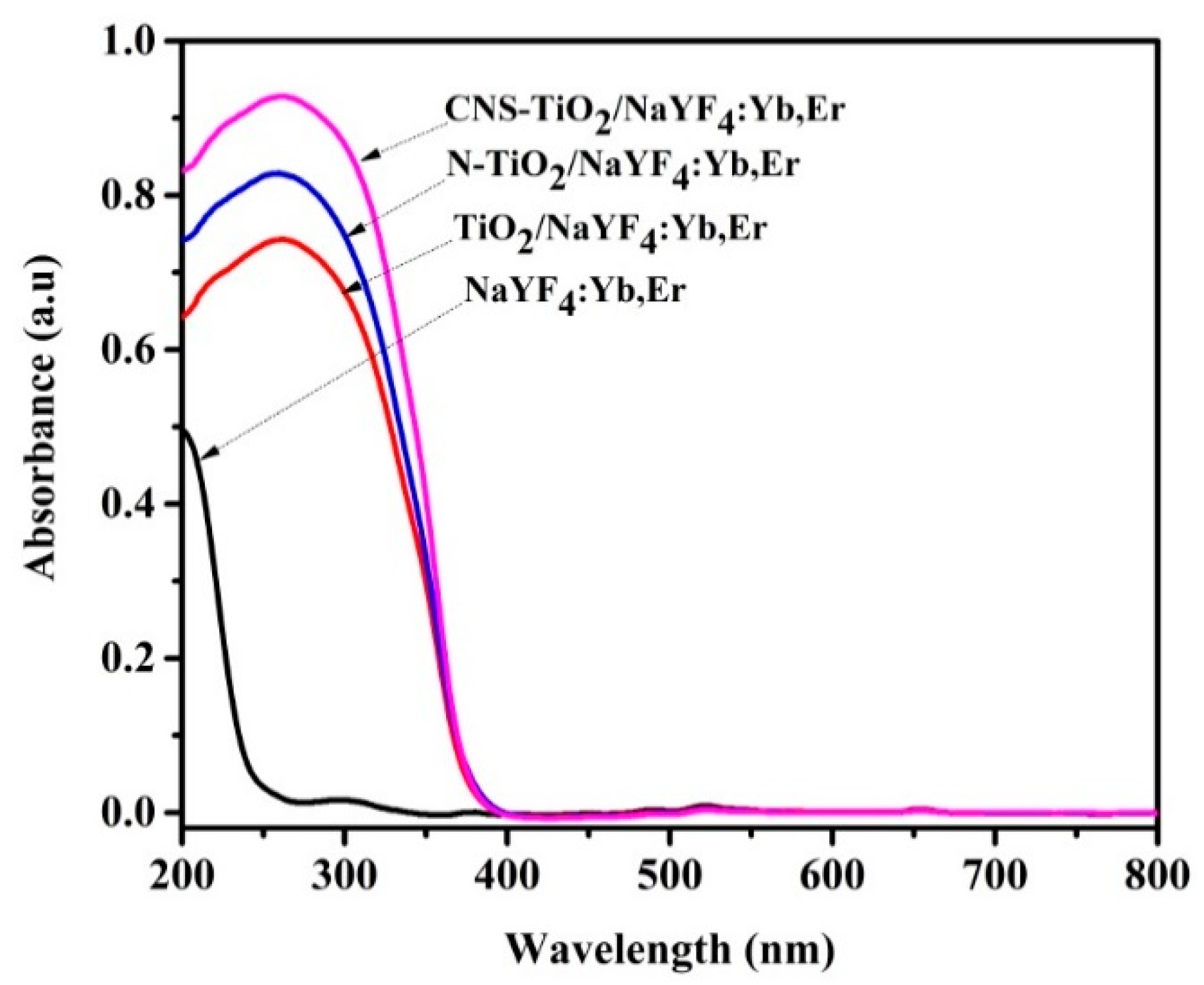
Figure 4 exhibits UV-Vis absorption spectra of NaYF
4:Yb,Er coated with undoped, N-doped, and CNS-doped TiO
2. The NaYF
4:Yb,Er phosphor absorbs UV light from 200 nm with an edge at 250 nm (due to the NaYF
4 host), and in
Figure S9 absorption peaks were centered at 524 and 654 nm due to the Er
3+ co-activator [
35]. However, coating TiO
2 on phosphor broadened the absorption spectra. Specifically, the TiO
2/NaYF
4:Yb,Er composites exhibited UV-Vis absorption between 200 and 400 nm, with peak absorption at 300 nm. The additional N-doped and CNS-doped TiO
2 phosphor composites show higher absorption intensities and a slight shift towards the visible region; the red-shift has been referenced by other researchers as being because of the lower energy levels in TiO
2 imparted by N or CNS doping elements [
20,
36].
Figure 5a shows photoluminescence (PL) spectra as obtained after 980 nm NIR irradiation of NaYF
4:Yb,Er upconversion phosphor composites. Firstly, the full emission spectra (UV-Vis–NIR emission) in
Figure 5a exhibit emissions of visible light photons at 520, 527, 540, and 548 nm. Thus, as a result of simultaneous energy transfer occurring in the excited phosphor, the energy losses resulted in lower energy photons at 652 and 658 nm. The
2H
11/2-to-
4I
15/2 transition is assigned to the emission at 527 nm, while the
4S
3/2 to
4I
15/2 is assigned to the emission at 540 nm. Additionally, the transitions emitting low-energy photons at 652 nm are from
4F
9/2 to
4I
15/2 [
25]. The overall reduction in emission peaks in the TiO
2-coated NaYF
4:Yb,Er samples was also observed in previous research [
32]. Thus, TiO
2 nanoparticles on phosphor act as barriers to emitted light.
Figure 5a was enlarged and labeled (
Figure 5b–d) to clearly observe peak variations.
Figure 5b shows UV light emission at 384 nm and visible light photons at 407 and 484 nm in the NaYF
4:Yb,Er phosphor. However, all of these peaks were suppressed in TiO
2/NaYF
4:Yb,Er and N/CNS-doped TiO
2/NaYF
4:Yb,Er composites. The effect of doping on photoluminescence was observed in the enlarged peaks in
Figure 5c,d. Specifically, at 520 and 527 nm in
Figure 5c, the TiO
2/NaYF
4:Yb,Er and CNS-doped TiO
2/NaYF
4:Yb,Er show unchanged emission intensities, while only N-doped TiO
2/NaYF
4:Yb,Er exhibits further peak suppression. However, at the peaks located at 540 and 548 nm, the CNS-doped TiO
2/NaYF
4:Yb,Er exhibits the highest photoluminescence, followed by TiO
2/NaYF
4:Yb,Er and N-doped TiO
2/NaYF
4:Yb,Er.
Figure 5d shows the enlarged PL emission spectra to clearly show peaks in the NIR region. At 652 and 658 nm, the peak intensity decreases in the order of NaYF
4:Yb,Er > TiO
2/NaYF
4:Yb,Er TiO
2 > CNS-doped TiO
2/NaYF
4:Yb,Er > N-doped TiO
2/NaYF
4:Yb,Er. The peak suppression and enhancement phenomena represent a multi-energy transfer process. However, some researchers have highlighted that the pH level in urea (the doping reagent in phosphor) tunes the PL emissions for visible and NIR emissions in Y
2O
3:Yb,Er nanophosphors [
37]. Thus, in our work we confirmed the tuning of visible and NIR emissions. In the N-doped TiO
2/NaYF
4:Yb,Er (urea additive: pH 5.79) and in CNS-doped TiO
2/NaYF
4:Yb,Er (thiourea additive: pH 5.73) as compared to undoped TiO
2/NaYF
4:Yb,Er (pH 6.39), the pH values are almost the same, however the most acidic CNS-doped phosphor exhibits the highest PL intensity (at 540 and 548 nm), followed by the TiO
2 phosphor and the doped TiO
2-coated samples. Upconversion phosphors are characterized by having short lifetimes, as exhibited in the decay curves in
Figure S10.
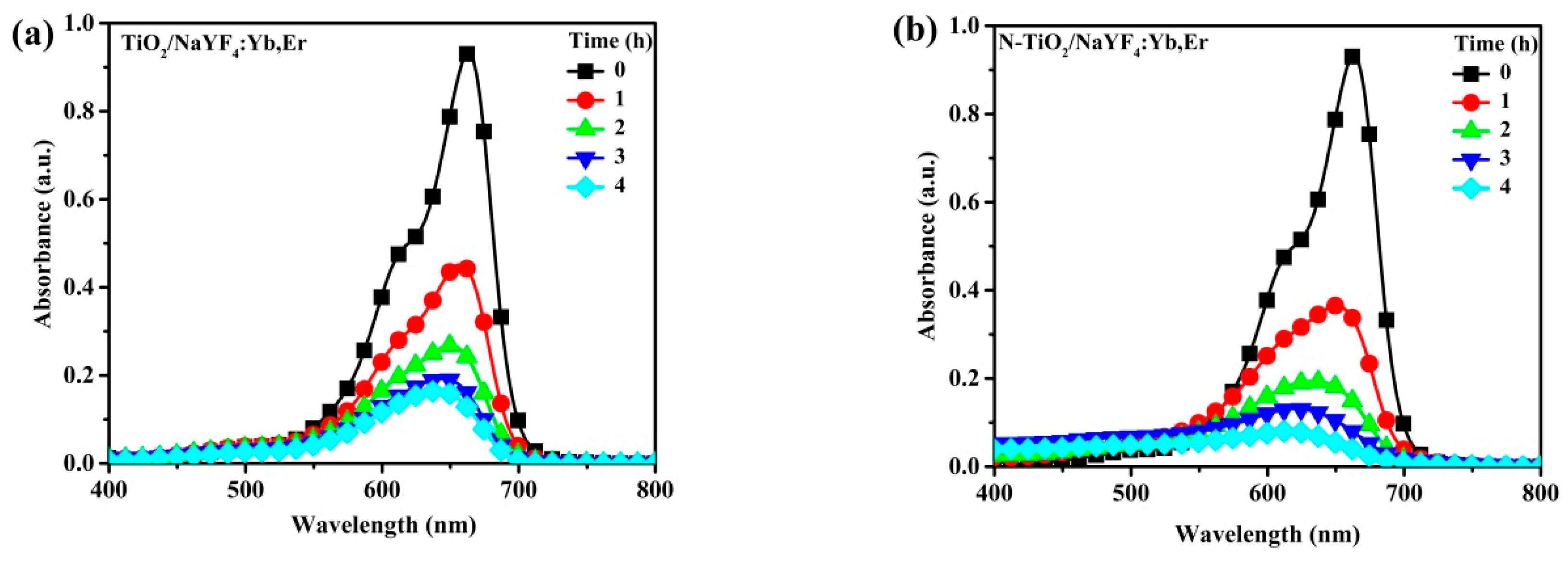
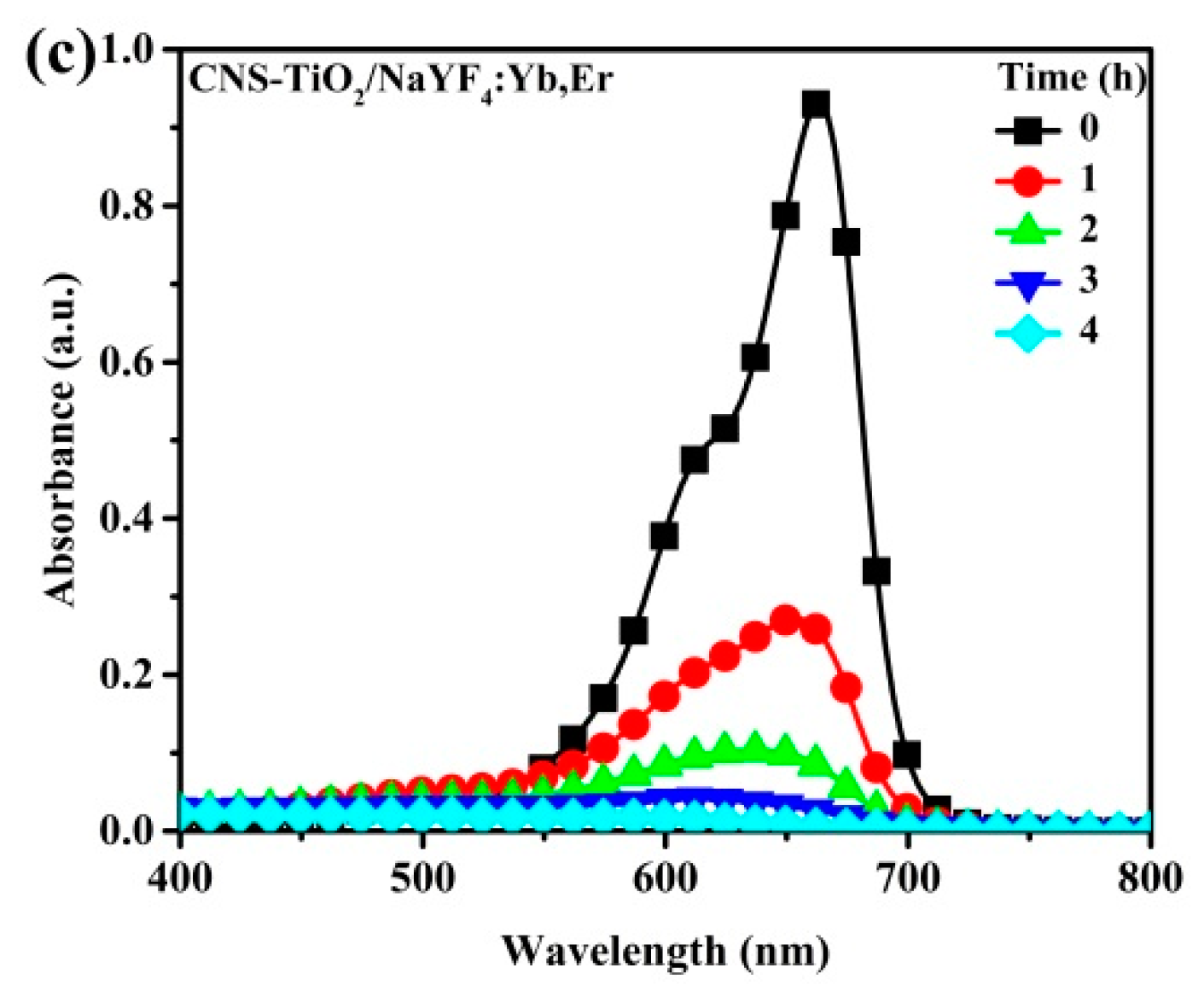
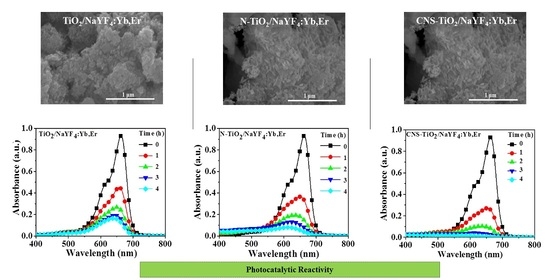
Figure 6 shows the photobleaching variation of the MB solution for NaYF
4:Yb,Er phosphor composites under UV light illumination. The maximum peak at 664 nm is the characteristic MB absorbance that is monitored to evaluate intensity variations as a measure of the degradation of the organic compound. In a 4 h reaction period, the CNS-TiO
2/NaYF
4:Yb,Er absorbance spectra with the lowest intensities at seen at 4 h. Therefore, CNS-TiO
2/NaYF
4:Yb,Er shows the highest photocatalytic reactivity among the three samples. However, the TiO
2 and N-TiO
2 upconversion phosphor composites require more time to completely degrade MB solutions (
Figure 6b,c).
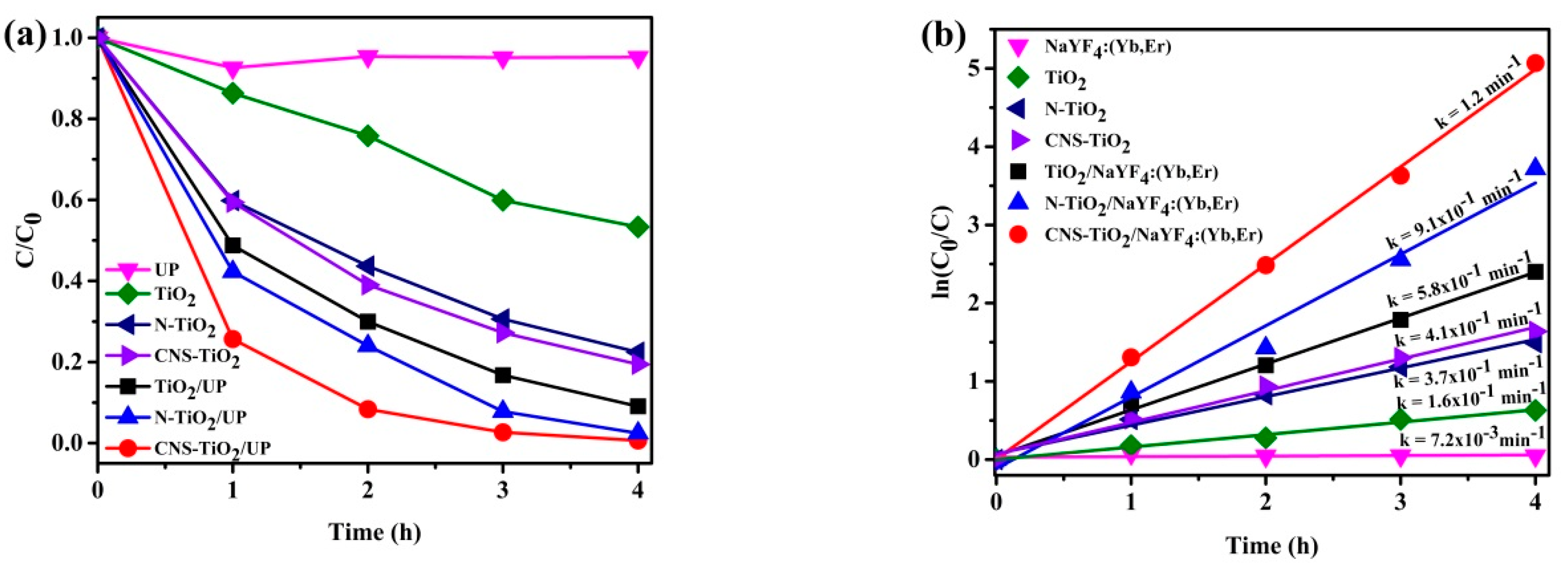
Figure 7a shows the peak absorbance variations for photodegradation of the MB solution under UV illumination. The photodegradation of the MB solution proceeded to 40% efficiency for the photoreaction mixture with TiO
2 only. However, N-doped TiO
2 and CNS-doped TiO
2 improves the efficiency to 80%. This is owing to the light absorption property related to N- and CNS-doped TiO
2. Supporting TiO
2 on the NaYF
4:Yb,Er upconversion phosphor improved the photocatalytic efficiency up to 90%. Moreover, with N-TiO
2 or CNS-TiO
2 supported on NaYF
4:Yb,Er, the photocatalytic efficiencies were enhanced to completion (100% in 4 h). Thus, N-TiO
2 or CNS-TiO
2 coupling with NaYF
4:Yb,Er phosphor improves the catalytic activity due to the improvement in light absorption by phosphor and N or CNS doping elements. In detail, the NaYF
4:Yb,Er phosphor support has light absorption properties due to the NaYF
4 host (as exhibited in
Figure 4), which coincides with the TiO
2 absorption band. Additionally, light absorption for the composite is improved with additional N and CNS doping.
Both CNS doping and N doping of TiO
2 further cause improvements in photocatalytic efficiencies. The N or CNS doping of TiO
2 incorporates new energy levels in the interstitial and substitution sites. Thus, lower energy levels lower the TiO
2 absorption band, which promotes the flow of electrons into the conduction band. As follows, the photocatalytic activity achieves completion in a 4 h cycle for phosphor coated with doped CNS or N-TiO
2.
Figure 7b illustrates the rate of kinetics for the samples in
Figure 7a. As shown in
Figure 7b, the reactions have a linear and typical relationship for first-order kinetics. The rate constants are 0.16 min
−1 for TiO
2, 0.37 min
−1 for N-TiO
2, 0.41 min
−1 for CNS-TiO
2, 0.0072 min
−1 for NaYF
4:Yb,Er, 0.58 min
−l for TiO
2/NaYF
4:Yb,Er, 0.91 min
−1 for N-TiO
2/NaYF
4:Yb,Er, and 1.2 min
−1 for CNS-TiO
2/NaYF
4:Yb,Er. This result shows that MB photodegradation with CNS-TiO
2/NaYF
4:Yb,Er is the fastest reaction by 7.5 times for TiO
2, by 3.2 times for N-TiO
2, by 2.9 times for CNS-TiO
2, by 2.1 times for TiO
2/NaYF
4:Yb,Er, and by 1.3 times for N-TiO
2/NaYF
4:Yb,Er. Therefore, CNS doping of TiO
2 and its support on NaYF
4:Yb,Er phosphor improves the photocatalytic performances.
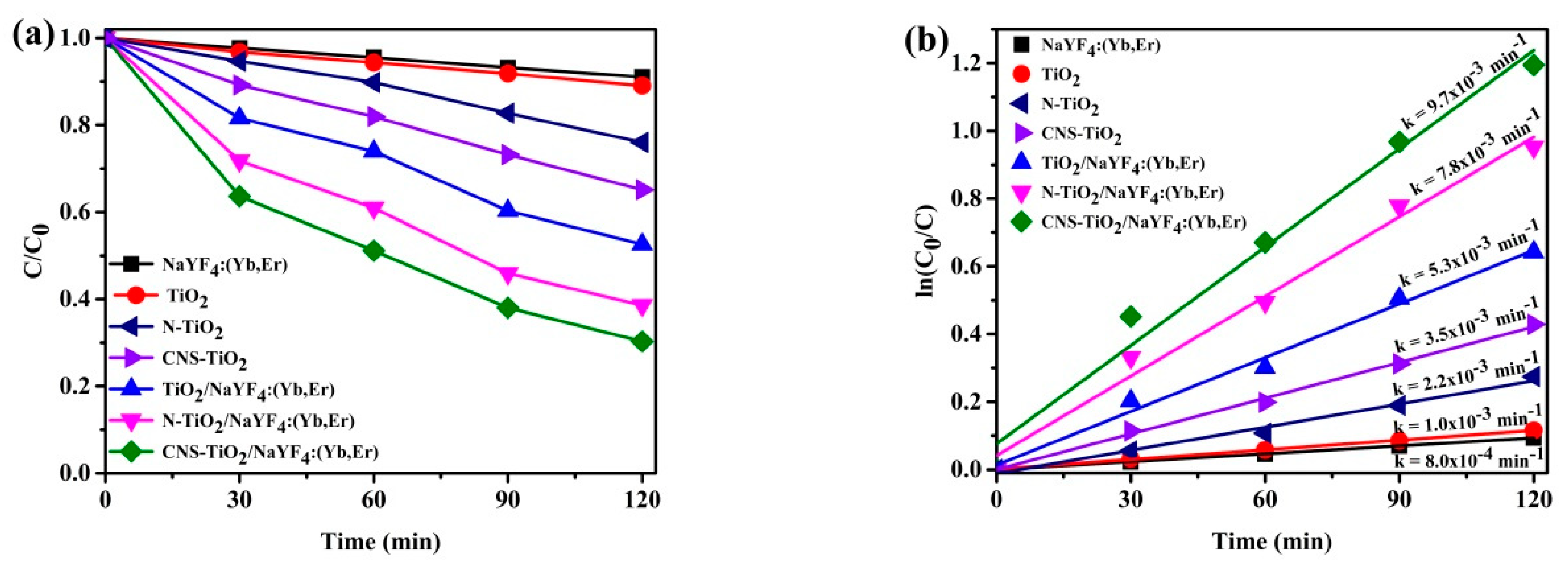
Figure 8 exhibits the MB peak absorbance against time for visible light activation. The N-doped TiO
2 and CNS-doped TiO
2 show improvements in visible light photocatalytic efficiencies as compared to TiO
2 only. Furthermore, coupling TiO
2 with phosphor and doping the TiO
2/NaYF
4:Yb,Er causes enhancements of photocatalytic efficiencies. Precisely, TiO
2/NaYF
4:Yb,Er, N-doped TiO
2/NaYF
4:Yb,Er, and CNS-doped TiO
2/NaYF
4:Yb,Er showed 50%, 60%, and 70% efficiencies after 120 min of light irradiation. The undoped TiO
2/NaYF
4:Yb,Er composite showed significant photocatalytic efficiency as compared to TiO
2, N-TiO
2, and CNS-TiO
2, mainly due to the heterojunction effect that exists between phosphor and TiO
2. The CNS-doped TiO
2/NaYF
4:Yb,Er shows the highest photocatalytic reactivity over visible light illumination. As a result, the effect of doping with N-TiO
2/NaYF
4:Yb,Er or CNS-TiO
2/NaYF
4:Yb,Er is clearly exhibited by 10% and 20% efficiency enhancements, respectively, compared with undoped-TiO
2/NaYF
4:Yb,Er. Visible light sensitization is conceptualized by the low-energy states induced by doping with N or CNS elements. Thus, tri-element doping in TiO
2 imparts more impurities in the TiO
2 band than N doping only.
Figure 8b shows the rate of kinetics for the samples in
Figure 8a. The CNS-doped TiO
2/NaYF
4:Yb,Er exhibited the swiftest reaction with a 9.7 × 10
−3 min
−1 rate constant. In comparison with other photocatalysts, the CNS-doped TiO
2/NaYF
4:Yb,Er reaction is 9.7 times greater for TiO
2 only, 4.4 times greater for N-TiO
2, 2.8 times greater for CNS-TiO
2, 1.8 times greater for TiO
2/NaYF
4:Yb,Er, and 1.2 times greater for N-TiO
2/NaYF
4:Yb,Er. Therefore, the photocatalytic efficiencies are improved by tri-doping TiO
2 and its support on the NaYF
4:Yb,Er upconversion phosphor.
Figure 9 shows the MB peak absorbance against time for NIR light activation. Undoped TiO
2–phosphor and N-doped TiO
2–phosphor composites only show ~15% efficiency with NIR illuminations, but CNS-doped TiO
2/NaYF
4:Yb,Er exhibits a ~25% improvement. Therefore, NIR irradiations in CNS-doped TiO
2/NaYF
4:Yb,Er have the highest photocatalytic activity. Under NIR, the Yb
3+ in NaYF
4:Yb,Er phosphor sensitizes the NIR photons and emits UV-Vis–NIR photons through the Er
3+ emission center, as discussed in
Figure 5. Visible light photons are sensitized through the low energy levels imparted by N- or CNS doping and electrons are injected into the TiO
2 conduction band for photocatalysis. Additionally, the doping elements promote electron–hole generation efficiencies to facilitate photocatalysis. However, the overall photocatalytic efficiencies were low due to the mono-wavelength absorption properties of Yb
3+ ions at 980 nm.
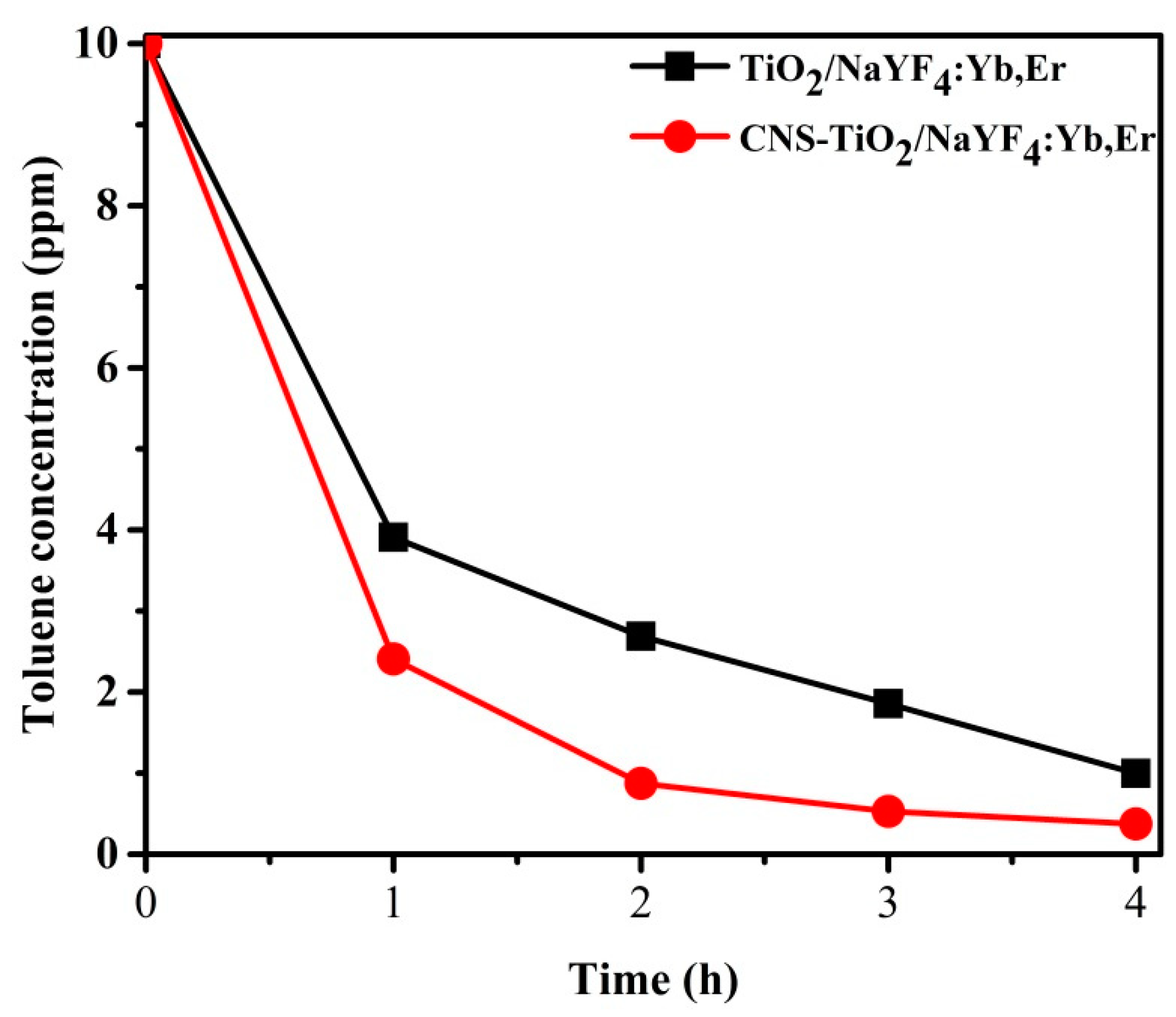
Figure 10 exhibits the concentration variations of toluene during photodegradation with TiO
2-NaYF
4:Yb,Er and CNS/TiO
2-NaYF
4:Yb,Er powders under UV light illumination. The toluene concentration in the Y-axis corresponds to the concentration of toluene remaining in the Teflon bag after 1 h sampling under UV light illumination. Through the first 1 h sampling cycle, the CNS/TiO
2-NaYF
4:Yb,Er photocatalyst has 1.5 ppm of toluene less than the undoped TiO
2-NaYF
4:Yb,Er sample. The 4 h toluene degradation is above 95% for the CNS/TiO
2-NaYF
4:Yb,Er. This result indicates that the CNS/TiO
2-NaYF
4:Yb,Er has exceptionally superior photoactivity compared with the TiO
2-NaYF
4:Yb,Er sample. Thus, CNS doping is essential for improving photocatalytic activity.
3. Discussions
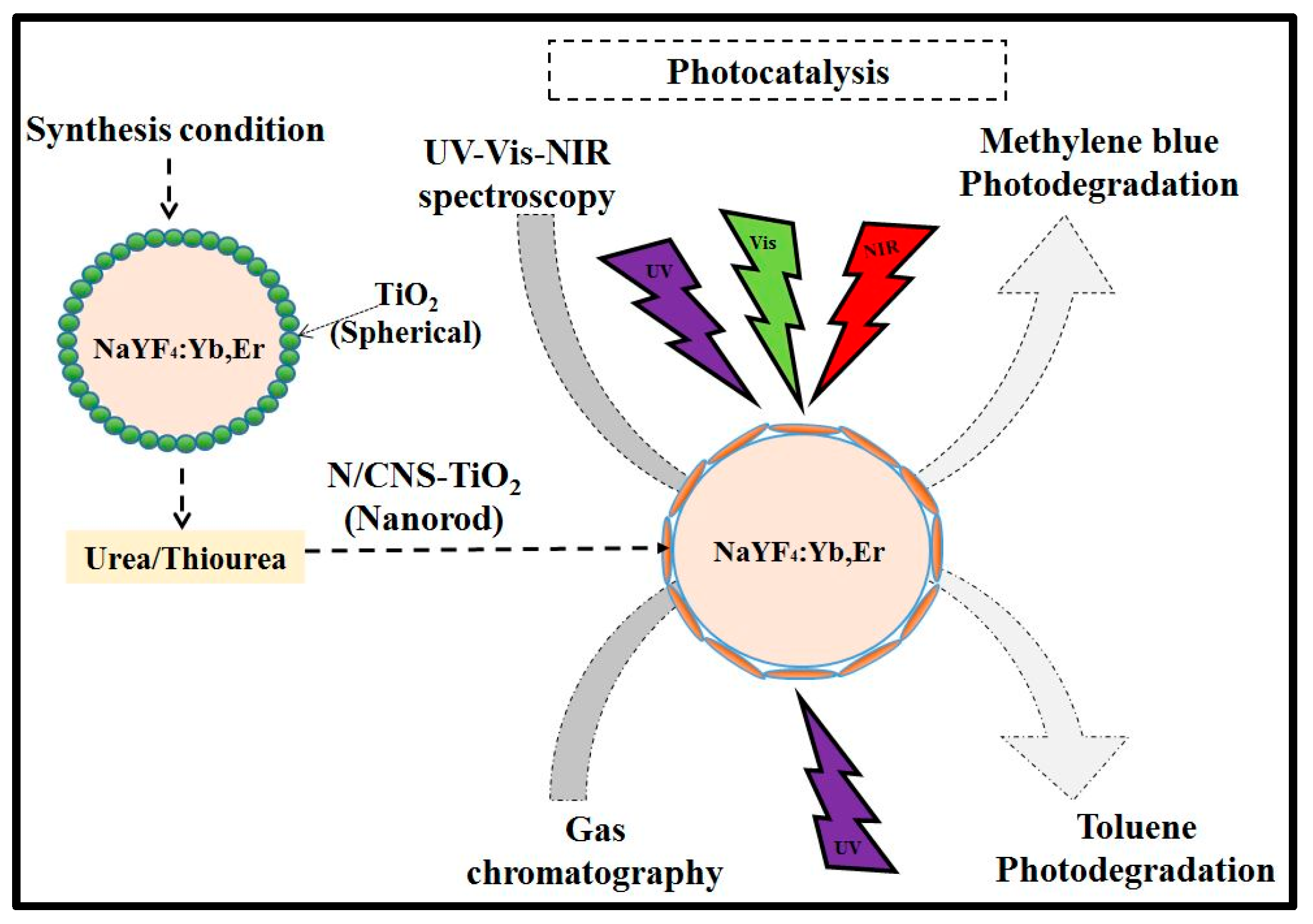
Figure 11 is a schematic diagram depicting the synthesis and photocatalytic test performances. The pH of the coating sol varied due to the interaction of the TiO
2 precursor and ethanol without pH modifiers. Thus, the TiO
2 sol without the doping reagent showed slightly alkaline conditions at pH 6.39. As a result of this slight alkalinity, the TiO
2 morphology existed as spherical particles on phosphor. However, with additional thiourea and urea as doping reagents for N and CNS in the TiO
2 sol, the pH decreased to acidic conditions. For instance, in the N-doped TiO
2 sol the pH was 5.79, while in the CNS-doped TiO
2 sol the pH was 5.73. As a result, the morphologies were changed to nanorods. Hence, the drop in pH promoted the formation of nanorods. The photoluminescence emission peaks were also observed to be tuned by urea or thiourea reagents owing to the drop in pH.
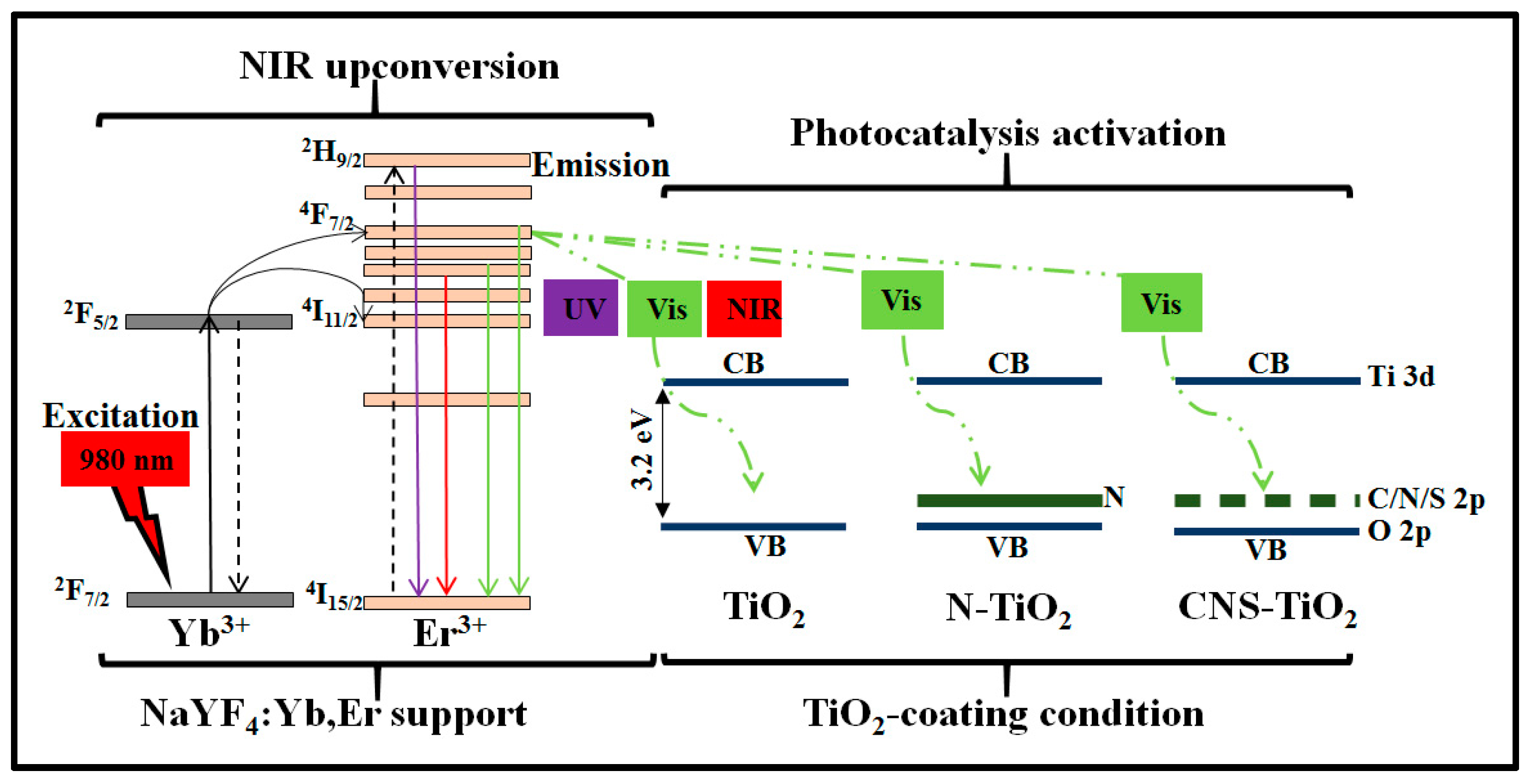
Figure 12 shows the proposed photocatalysis mechanism for the NaYF
4:Yb,Er phosphor coated with TiO
2 under different doping conditions. The three conditions for TiO
2 coating on NaYF
4:Yb,Er are illustrated in
Figure 12 as TiO
2, N-TiO
2, and CNS-TiO
2. The first condition is TiO
2 supported on NaYF
4:Yb,Er phosphor, where only the TiO
2 energy band is present. The second condition is N-doped TiO
2 supported on NaYF
4:Yb,Er, where the continuous solid line inside the TiO
2 represents N energy levels. The third condition is CNS-doped TiO
2 supported on NaYF
4:Yb,Er, where the dotted line in the TiO
2 band represents discrete energy levels imparted by C, N, or S elements. As illustrated, the upconversion phosphor as the support material contains Yb
3+ as the NIR sensitizer in the
2F
7/2 state, which transfers energy to the co-activator or Er
3+ through the
4F
7/2 and
4I
11/2 states [
35,
38,
39]. Simultaneously, the Er
3+ emits UV-Vis–NIR photons of less than 980 nm. Hence, the upconversion phosphor converts low-energy NIR photons at 980 nm to high-energy photons (
Figure 5). Peak wavelengths were observed at 384, 407, 520, 527, 540, 548, 652, 658, and 836 nm. Thus, NaYF
4:Yb,Er upconversion phosphor emits UV-Vis–NIR photons.
Photocatalytic activity proceeds under UV-Vis–NIR irradiation. The reactions are related to light sensitization centers [
40]. For instance, under UV light activation, the TiO
2-NaYF
4:Yb,Er composite absorbs light and facilitates photocatalysis. It is noteworthy that the NaYF
4 phosphor host also sensitizes UV light photons (
Figure 4), which enhances the overall light absorption by the TiO
2-NaYF
4:Yb,Er composite. Under visible light, N-TiO
2-NaYF
4:Yb,Er or CNS-TiO
2/NaYF
4:Yb,Er light is sensitized through the lower energy levels in the N or CNS, which inject electrons into the TiO
2 conduction band for photoreaction at the nanorod surface. Under NIR irradiation, the light is sensitized through the Yb
3+ and energy is transferred through Er
3+. Then, the emitted visible light is absorbed through low-energy impurities in N or CNS elements and through the low energy levels due to heterojunctions between TiO
2 and NaYF
4:Yb,Er phosphor. Consequently, after sensitization of UV-Vis–NIR light, electrons are injected into the TiO
2 conduction band, leaving holes in the valence band. At the surface of TiO
2, electrons are adsorbed by O
2 molecules while holes are adsorbed by H
2O molecules to form superoxide and hydroxyl radicals, respectively. After several intermediate reactions, superoxide or hydroxyl radicals attack and photodegrade the MB or toluene pollutants. The CNS-doped TiO
2/NaYF
4:Yb,Er outperformed the N-doped TiO
2-NaYF
4:Yb,Er and TiO
2-NaYF
4:Yb,Er in UV-Vis–NIR photocatalytic activities in methylene blue due to the existence of low-energy CNS doping elements with improved light absorption properties [
20].
This work mainly focused on adding 2.5% urea or thiourea to TiO
2 to study the effects of doping with N or CNS. Since the photoluminescence spectra exhibited interesting visible and NIR light tuning, further investigations into the effects of varying urea or thiourea (N or CNS, respectively) doping of TiO
2/NaYF
4:Yb,Er is recommended. Specifically, the photoluminescence emissions at 540 and 548 nm in CNS-doped TiO
2/NaYF
4:Yb,Er were enhanced (
Figure 5b) compared to the TiO
2-NaYF
4:Yb,Er and N-TiO
2-NaYF
4:Yb,Er. This insinuates the possibility that CNS doping improves the emission of visible light. Additionally, the photoluminescence under NIR is limited due to the narrow absorption of Yb
3+ ions to the 980 nm wavelengths [
39,
40]. It is also recommended to evaluate the effects of adding 2 co-sensitizers to improve the absorption of NIR light to achieve significant photocatalytic efficiency.