Small Ruminant Production Based on Rangelands to Optimize Animal Nutrition and Health: Building an Interdisciplinary Approach to Evaluate Nutraceutical Plants
Simple Summary
Abstract
1. Introduction
2. Unravelling the Heterogeneous Context of the Low Deciduous Forest
2.1. Botanical and Nutritional Components of the Low Deciduous Forest
2.2. Plant Secondary Compounds in the Low Deciduous Forest
2.3. The Unavoidable Occurrence of Gastrointestinal Nematodes in the Low Deciduous Forest
2.4. The Unraveled Context of the Low Deciduous Forest
3. Building an Interdisciplinary Approach to Identify Plants with Nutraceutical Potential in Heterogenous Feeding Scenarios
3.1. The “Nutraceutical” Debate
- In order to build the approach under a heterogeneous feeding scenario, we will consider the definition by Hoste et al. [35]: “a livestock feed which combines nutritional value with beneficial effects on animal health could be considered as nutraceutical”;
- The existing literature addresses several benefits of nutraceuticals [83,84,85,86,87]. In this review, we took as a basis the threat that GIN represents for small ruminants in grazing scenarios and focused on the AH properties of some LDF plant species, placing emphasis on CT, which are the most studied PSCs in relation to small ruminant nutrition and health. Nevertheless, the AH properties of other classes of PSC will be briefly discussed.
3.2. The Ethological and Botanical Approach
3.3. The Agronomical Approach
- Plant species with a null or low intake by ruminants. These plants do not classify as potential nutraceuticals, but those with high availability could be considered for their use as phytotherapeutic or herbal remedies depending on subsequent PSC content analyses;
- Plant species consumed by ruminants but with low availability in the field. These are good prospects but have the constraint of not offering enough biomass for long-term consumption. This represents the main challenge for agronomists to increase the biomass production of promising candidates by means of improved agronomic practices or identification and the selection and breeding of high yielding varieties with good bioactivity;
- Plant species consumed by ruminants and with naturally good biomass availability in the field. These plants offer the best option to be considered as potential nutraceuticals.
3.4. The Nutritional Approach
3.5. The Secondary Compounds Assessment Approach
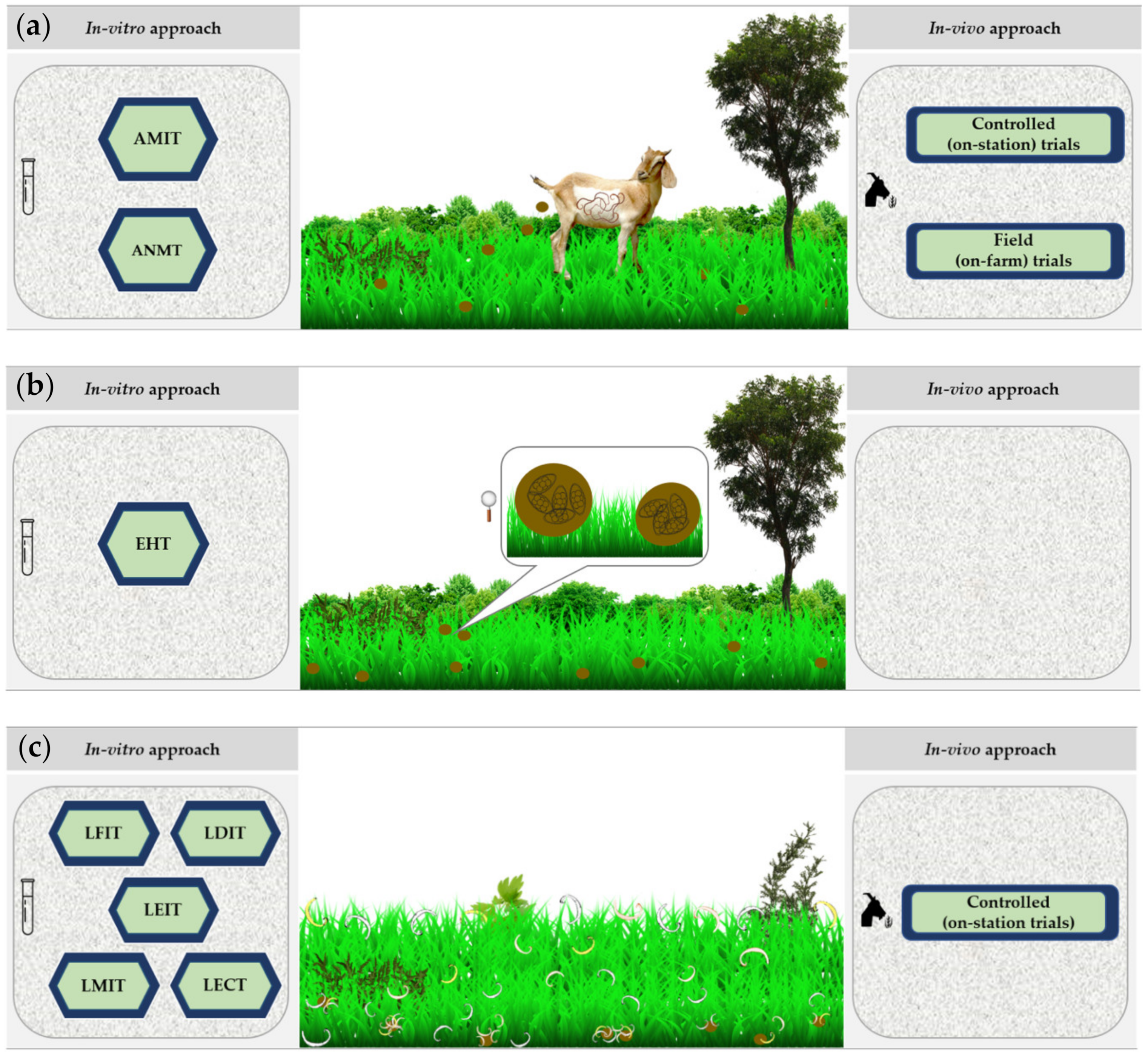
3.6. The Parasitological (Anthelmintic) Approach
3.6.1. In Vitro Screening
3.6.2. In Vivo Screening
3.7. The Animal Approach
3.8. The Selectivity Assessment Approach
4. Final Remarks and Implications
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Estell, R.E.; Havstad, K.M.; Cibilis, A.F.; Fredrickson, E.L.; Anderson, D.M.; Schrader, T.S.; James, D.K. Increasing shrub use by livestock in a world with less grass. Rangel. Ecol. Manag. 2012, 65, 553–562. [Google Scholar] [CrossRef]
- McDermott, J.J.; Staal, S.J.; Freeman, H.A.; Herrero, M.; Van de Steeg, J.A. Sustaining intensification of smallholder livestock systems in the tropics. Livest. Sci. 2010, 130, 95–109. [Google Scholar] [CrossRef]
- Flores, J.; Bautista, F. Knowledge of the Yucatec Maya in seasonal tropical forest management. Rev. Mex. Biodivers. 2012, 83, 503–512. [Google Scholar] [CrossRef]
- Accatino, F.; Sabatier, R.; De Michele, C.; Ward, D.; Wiegand, K.; Meyer, K.M. Robustness and management adaptability in tropical rangelands: A viability-based assessment under the non-equilibrium paradigm. Animal 2014, 8, 1272–1281. [Google Scholar] [CrossRef] [PubMed]
- Lund, H.G. Accounting for the world’s rangelands. Rangelands 2007, 29, 3–10. [Google Scholar] [CrossRef]
- Alonso-Díaz, M.A.; Torres-Acosta, J.F.J.; Sandoval-Castro, C.A.; Campbell, W.B. Controlling the Introduction and Augmentation of Parasites in and on Domesticated Livestock. In Sustainable Food Production Includes Human and Environmental Health; Bruce Campbell, W., López-Ortíz, S., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 191–228. [Google Scholar]
- Dumanski, J.; Desjardins, R.L.; Lal, R.; Rosegrant, M.; de Freitas, L.; Lander, J.N.; Gerber, P.; Steinfeld, H.; Verchot, L.V.; Schuman, G.E.; et al. Supporting evidence for greenhouse gas mitigation in agriculture. In Applied Agrometeorology; Stitger, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 989–996. [Google Scholar]
- Ayatunde, A.; de Leeuw, J.; Turner, M.D.; Said, M. Challenges of assessing the sustainability of (agro)-pastoral systems. Livest. Sci. 2011, 139, 30–43. [Google Scholar] [CrossRef]
- Oosting, S.J.; Udo, H.M.J.; Viets, T.C. Development of livestock production in the tropics: Farm and farmers’ perspectives. Animal. 2014, 8, 1238–1248. [Google Scholar] [CrossRef]
- Hubert, B.; Meuret, M.; Bonnemaire, J. Shepherds, sheep and forest fires: A reconception of grazinglands. In Handbook of Transdisciplinary Research; Hadorn, G.H., Hoffmann-Riem, H., Biber-Klemm, S., Grossenbacher-Mansuy, W., Joye, D., Pohl, C., Wiesmann, U., Zemp, E., Eds.; Springer Science + Business Media BV: Dordrecht, The Netherlands, 2008; pp. 103–126. [Google Scholar]
- Hubert, B.; Deverre, C.; Meuret, M. The rangelands of southern France: Two centuries of radical change. In The Art and Science of Shepherding; Meuret, M., Provenza, F.D., Eds.; Acres USA: Austin, TX, USA, 2014; pp. 27–43. [Google Scholar]
- Udo, H.M.J.; Aklilu, H.A.; Phong, L.T.; Bosma, R.H.; Budisatria, I.G.S.; Patil, B.R.; Samdup, T.; Bebe, B.O. Impact of intensification of different types of livestock production in smallholder crop-livestock systems. Livest. Sci. 2011, 139, 22–30. [Google Scholar] [CrossRef]
- Torres-Acosta, J.F.J.; Alonso-Díaz, M.; Hoste, H.; Sandoval-Castro, C.A.; Aguilar-Caballero, A.J. Positive and negative effects in goat production arising from the intake of tannin rich forage. Trop. Subtrop. Agroecosyst. 2008, 9, 83–90. [Google Scholar]
- Torres-Acosta, J.F.J.; González-Pech, P.G.; Ortíz-Ocampo, G.I.; Rodríguez-Vivas, R.I.; Tun-Garrido, J.; Ventura-Cordero, J.; Castañeda-Ramírez, G.S.; Hernández-Bolio, G.I.; Sandoval-Castro, C.A.; Ortega-Pacheco, A. Revalorizando el uso de la selva baja caducifolia para la producción de rumiantes. Trop. Subtrop. Agroecosyst. 2016, 19, 3–80. [Google Scholar]
- Ventura-Cordero, J.; Sandoval-Castro, C.A.; González-Pech, P.G.; Torres-Acosta, J.F.J. El follaje de la selva baja caducifolia como alimento nutracéutico y su potencial antihelmíntico en pequeños rumiantes. AIA 2017, 21, 55–67. [Google Scholar]
- Flores, J.; Vermont, R.; Kantún, J. Leguminosae diversity in the Yucatán peninsula and its importance for sheep and goat production. In Herbivores: The Assessment of Intake, Digestibility and the Roles of Secondary Compounds; Sandoval-Castro, C.A., Deb Hovell, F.V., Torres-Acosta, J.F.J., Ayala-Burgos, A., Eds.; Nottingham University Press: Nottingham, UK, 2006; pp. 291–299. [Google Scholar]
- Mithofer, A.; Boland, W. Plant Defense Against Herbivores: Chemical Aspects. Ann. Rev. 2012, 63, 431–450. [Google Scholar] [CrossRef] [PubMed]
- Moore, B.D.; Wiggins, N.L.; Marsh, K.J.; Dearing, M.D.; Foley, W.J. Translating physiological signals to changes in feeding behavior in mammals and the future effects of global climate change. Anim. Prod. Sci. 2015, 55, 272–283. [Google Scholar] [CrossRef]
- Janis, C. An evolutionary history of browsing and grazing ungulates. In The Ecology of Browsing and Grazing; Gordon, I.J., Prins, H.H.T., Eds.; Ecological Studies 195; Springer: Berlin/Heidelberg, Germany, 2008; pp. 21–45. [Google Scholar]
- Clauss, M.; Kaiser, T.; Hummel, J. The morphophysiological adaptations of browsing and grazing mammals. In The Ecology of Browsing and Grazing; Gordon, I.J., Prins, H.H.T., Eds.; Ecological Studies 195; Springer: Berlin/Heidelberg, Germany, 2008; pp. 47–88. [Google Scholar]
- Estell, R. Coping with shrub secondary metabolites by ruminants. Small Rum. Res. 2010, 94, 1–9. [Google Scholar] [CrossRef]
- Molento, M.B.; Fortes, F.S.; Pondelek, D.A.S.; Borges, F.A.; Chagas, A.C.S.; Torres-Acosta, J.F.J.; Geldfoh, P. Challenges of nematode control in ruminants: Focus on Latin America. Vet. Parasitol. 2011, 180, 126–132. [Google Scholar] [CrossRef]
- Miller, J.E.; Kaplan, R.M.; Pugh, D.G. Internal parasites. In Sheep and Goat Medicine, 2nd ed.; Pugh, D.G., Baird, A.N., Eds.; Saunders Elsevier: Maryland Heights, MO, USA, 2012; Volume 2, pp. 106–125. [Google Scholar]
- Charlier, J.; van der Voort, M.; Kenyon, F.; Skuce, P.; Vercruysse, J. Chasing helminths and their economic impact on farmed ruminants. Trend. Parasitol. 2014, 30, 361–367. [Google Scholar] [CrossRef]
- Makkar, H.P. Effects and fate of tannins in ruminant animals, adaptation to tannins, and strategies to overcome detrimental effects of feeding tannin-rich feeds. Small Rum. Res. 2003, 49, 241–256. [Google Scholar] [CrossRef]
- Mueller-Harvey, I. Unravelling the conundrum of tannins in animal nutrition and health. J. Sci. Food Agric. 2006, 86, 2010–2037. [Google Scholar] [CrossRef]
- Alonso-Díaz, M.A.; Torres-Acosta, J.F.J.; Sandoval-Castro, C.A.; Hoste, H. Tannins in tropical tree fodders fed to small ruminants: A friendly foe? Small Rum. Res. 2010, 89, 164–173. [Google Scholar] [CrossRef]
- Hoste, H.; Martínez-Ortíz-de-Montellano, C.; Manoralaki, F.; Brunet, S.; Ojeda-Robertos, N.; Fourquaux, I.; Torres-Acosta, J.F.J.; Sandoval-Castro, C.A. Direct and indirect effect of bioactive tannin-rich tropical and temperate legumes against nematode infections. Vet. Parasitol. 2011, 186, 18–27. [Google Scholar] [CrossRef]
- Muir, J. The multi-faceted role of condensed tannins in the goat ecosystem. Small Rumin. Res. 2011, 98, 115–120. [Google Scholar] [CrossRef]
- Piluzza, G.; Sulas, L.; Bullita, S. Tannins in forage plants and their role in animal husbandry and environmental sustainability: A review. Grass Forage Sci. 2014, 69, 32–48. [Google Scholar] [CrossRef]
- Mueller-Harvey, I.; Bee, G.; Dohme-Meier, F.; Hoste, H.; Karonen, M.; Kölliker, R.; Lüscher, A.; Niderkorn, V.; Pellikaan, W.F.; Salminen, J.P.; et al. Benefits of condensed tannins in forage legumes fed to ruminants: Importance of structure, concentration, and diet composition. Crop. Sci. 2019, 59, 861–885. [Google Scholar] [CrossRef]
- Kamaraj, C.; Rahuman, A.; Elango, G.; Bagavan, A.; Zahir, A.A. Anthelmintic activity of botanical extracts against sheep gastrointestinal nematodes, Haemonchus contortus. Parasitol. Res. 2011, 109, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Mravčáková, D.; Váradyová, Z.; Kopčáková, A.; Čobanová, K.; Grešáková, L.; Kišidayová, S.; Babják, M.; Dolinská, M.U.; Dvorožňáková, E.; Königová, A.; et al. Natural chemotherapeutic alternatives for controlling haemonchosis in sheep. BMC Vet. Res. 2019, 15, 302. [Google Scholar] [CrossRef]
- Oliveira Santos, F.; Ponce Morais Cerqueira, A.; Branco, A.; Batatinha, M.J.M.; Borges Botura, M. Anthelmintic activity of plants against gastrointestinal nematodes of goats: A review. Parasitology 2019, 146, 1233–1246. [Google Scholar] [CrossRef]
- Hoste, H.; Torres-Acosta, J.F.J.; Sandoval-Castro, C.A.; Mueller-Harvey, I.; Sotiraki, S.; Louvandini, H.; Thamsborg, S.M.; Terrill, T.H. Tannin containing legumes as a model for nutraceuticals against digestive parasites in livestock. Vet. Parasitol. 2015, 312, 5–17. [Google Scholar] [CrossRef]
- Trejo, I. Características del medio físico de la selva baja caducifolia de México. Invest. Geogr. 1999, 39, 40–52. [Google Scholar]
- Rzedowski, J. El endemismo en la flora fanerogámica mexicana: Una apreciación analítica preliminar. Acta Bot. Mex. 1991, 15, 47–64. [Google Scholar] [CrossRef]
- Leirana-Alcocer, J.L.; Hernández-Betancourt, S.; Salinas-Peba, L.; Guerrero-González, L. Cambios en la estructura y composición de la vegetación relacionados con los años de abandono de tierras agropecuarias en la selva baja caducifolia espinosa de la reserva de Dzilam, Yucatán. Polibotánica 2009, 27, 53–70. [Google Scholar]
- Trejo, I. Las selvas secas del Pacífico Mexicano. In Diversidad, Amenazas y Áreas Prioritarias Para la Conservación de Las Selvas Secas Del Pacífico de México; Ceballos, G., Martínez, L., García, A., Espinoza, E., Creel, J.B., Dirzo, R., Eds.; Fondo de cultura económica—CONABIO: Ciudad de México, Mexico, 2010; pp. 41–51. [Google Scholar]
- Ancona, J.J.; Ruenes-Morales, R.; Huchim-Herrera, J.; Montañez-Escalante, P.I.; González-Iturbe, J.A. Woody species structure, diversity and floristic affinities in seasonally dry forest in the Uxmal archeological zone. Trop. Subtrop. Agroecosyst. 2019, 22, 755–767. [Google Scholar]
- Gutiérrez, C.; Zamora, P. Especies leñosas de la selva baja caducifolia de Xmatkuil, Yucatán, México. Foresta Veracruzana 2012, 14, 9–14. [Google Scholar]
- Flores, S.; Durán, R.; Ortiz, J. Comunidades vegetales terrestres. In Biodiversidad y Desarrollo Humano en Yucatán; Durán, R., Méndez, M., Eds.; CICY; PPD-FMAM; CONABIO; SEDUMA: Mérida, Mexico, 2010; pp. 125–129. [Google Scholar]
- Ortíz-Ocampo, G.I.; Sandoval-Castro, C.A.; González-Pech, P.G.; Mancilla-Montelongo, G.; Ventura-Cordero, J.; Castañeda-Ramírez, G.S.; Tun-Garrido, J.; Torres-Acosta, J.F.J. Seasonal variation in the bromatological composition and polyphenol content of the leaves of Gymnopodium floribundum Rolfe from a tropical deciduous forest. in preparation.
- Arellano, R.J.S.; Flores, J.S.; Tun-Garrido, J. Nomenclatura, Forma de Vida, Uso, Manejo y Distribución de las Especies Vegetales de la Península de Yucatán. Fascículo No. 20. Etnoflora Yucatanense; Facultad de Medicina Veterinaria y Zootecnia: Mérida, Mexico, 2003; 320p. [Google Scholar]
- Ayala-Burgos, A.; Cetina-Góngora, R.; Capetillo-Leal, C.M.; Zapata-Campos, C.; Sandoval-Castro, C.A. Composición Química-Nutricional de Árboles Forrajeros. In Compilación de Análisis de Laboratorio de Nutrición de la Facultad de Medicina Veterinaria y Zootecnia de la Universidad Autónoma de Yucatán; Universidad Autónoma de Yucatán: Mérida, Mexico, 2006. [Google Scholar]
- González-Pech, P.; Ventura-Cordero, J.; Ortiz-Ocampo, G.; Jaimez-Rodríguez, P.; Tun, J.; Sandoval-Castro, C.; Torres-Acosta, F. Plantas Consumidas por Ovinos y Caprinos en la Selva Baja Caducifolia de Yucatán. Guía ilustrada; Colofón ediciones; Universidad Autónoma de Yucatán: Mérida, Mexico, 2017; 76p. [Google Scholar]
- Ortiz-Domínguez, G.A.; Ventura-Cordero, J.; González-Pech, P.G.; Torres-Acosta, J.F.J.; Capetillo-Leal, C.M.; Sandoval-Castro, C.A. Nutritional value and in vitro digestibility of legume pods from seven tres species present in the tropical deciduous forest. Trop. Subtrop. Agroecosyst. 2017, 20, 505–510. [Google Scholar]
- Tainton, N.M. The ecology of the main grazing lands of South Africa: The savanna biome. In Veld Management in South Africa; Tainton, N.M., Ed.; University of Natal Press: Pietermaritzburg, South Africa, 1999; pp. 23–53. [Google Scholar]
- Retama-Flores, C.; Torres-Acosta, J.F.J.; Sandoval-Castro, C.A.; Aguilar-Caballero, A.J.; Cámara-Sarmiento, R.; Canul-Ku, H.L. Maize supplementation of Pelibuey sheep in a silvopastoral system: Fodder selection, nutrient intake and resilience against gastrointestinal nematodes. Animal 2012, 6, 145–153. [Google Scholar] [CrossRef]
- Gárate-Gallardo, L.; Torres-Acosta, J.F.J.; Aguilar-Caballero, A.J.; Sandoval-Castro, C.A.; Cámara-Sarmiento, R.; Canul-Ku, H.L. Comparing different maize supplementation strategies to improve resilience and resistance against gastrointestinal nematode infections in browsing goats. Parasite 2015, 22, 19. [Google Scholar] [CrossRef]
- Pichersky, E.; Lewinsohn, E. Convergent evolution in plant specialized metabolism. Annu. Rev. Plant. Biol. 2011, 62, 49–66. [Google Scholar] [CrossRef]
- Dixon, R.A.; Strack, D. Phytochemistry meets genome analysis, and beyond. Phytochemistry 2003, 62, 815–816. [Google Scholar] [CrossRef]
- Neilson, E.H.; Goodger, J.Q.D.; Woodrow, I.E.; Møller, B.L. Plant chemical defense: At what cost? Trends Plant Sci. 2003, 18, 250–258. [Google Scholar] [CrossRef]
- Hackmann, T.J.; Spain, J.N. Invited review: Ruminant ecology and evolution: Perspectives useful to ruminant livestock research and production. J. Dairy Sci. 2010, 93, 1320–1334. [Google Scholar] [CrossRef]
- Saarinen, J. The Palaeontology of Browsing and Grazing. In The Ecology of Browsing and Grazing II, 2nd ed.; Gordon, I.J., Prins, H.H.T., Eds.; Springer: Cham, Switzerland, 2019; pp. 5–59. [Google Scholar]
- Mlambo, V.; Marume, U.; Gajana, C.S. Utility of the browser’s behavioural and physiological strategies in coping with dietary tannins: Are exogenous tannin-inactivating treatments necessary? S. Afr. J. Anim. Sci. 2015, 45, 441–451. [Google Scholar] [CrossRef]
- Villalba, J.J.; Costes-Thiré, M.; Ginane, C. Phytochemicals in animal health: Diet selection and trade-offs between costs and benefits. Proc. Nutr. Soc. USA 2017, 76, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Starkey, L.A.; Pugh, D.G. Internal Parasites of Sheep, Goats and Cervids. In Sheep, Goat and Cervid Medicine, 3rd ed.; Pugh, D.G., Baird, A.N., Edmonson, M.A., Passler, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 97–117. [Google Scholar]
- Zajac, A.M.; Garza, J. Biology, Epidemiology, and Control of Gastrointestinal Nematodes of Small Ruminants. Vet. Clin. N. Am. Food Anim. Pr. 2020, 36, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Torres-Acosta, J.F.J.; Jacobs, D.E.; Aguilar-Caballero, A.J.; Sandoval-Castro, C.A.; May-Martínez, M.; Cob-Galera, L.A. The effect of supplementary feeding on the resilience and resistance of browsing Criollo kids against natural gastrointestinal nematode infections during the rainy season in tropical México. Vet. Parasitol. 2004, 124, 217–238. [Google Scholar] [CrossRef] [PubMed]
- Torres-Acosta, J.F.J.; Hoste, H. Alternative or improved methods to limit gastro-intestinal parasitism in grazing sheep and goats. Small Rum. Res. 2008, 77, 159–173. [Google Scholar] [CrossRef]
- Mavrot, F.; Hertzberg, H.; Torgerson, P. Effect of gastro-intestinal nematode infection on sheep performance: A systematic review and meta-analysis. Parasit. Vectors. 2015, 8, 557. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, L.J.; Walkden-Brown, S.W.; Kahn, L. Ecology of the free-living stages of major trichostrongylid parasites of sheep. Vet. Parasitol. 2006, 142, 1–15. [Google Scholar] [CrossRef]
- Van Dijk, J.; de Louw, M.D.E.; Kalis, L.P.A.; Morgan, E.R. Ultraviolet light increases mortality of nematode larvae and can explain patterns of larval availability at pasture. Int. J. Parasitol. 2009, 39, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, M.; Milner, J.; Gordon, I.; Kyriazakis, I.; Jackson, F. Grazing decisions of Soay sheep (Ovis aries) on St. Kilda: A consequence of parasite distribution? Oikos 2002, 96, 235–244. [Google Scholar] [CrossRef]
- Fox, N.J.; Marion, G.; Davidson, R.S.; White, P.C.L.; Hutchings, M.R. Modelling parasite transmission in a grazing system: The importance of host behavior and immunity. PLoS ONE 2013, 8, e77996. [Google Scholar] [CrossRef]
- Hutchings, M.R.; Judge, J.; Gordon, I.J.; Athanasiadou, S.; Kyriazakis, I. Use of trade-off theory to advance the understanding of herbivore-parasite interactions. Mamm. Rev. 2006, 36, 1–16. [Google Scholar] [CrossRef]
- Vlassoff, A. Biology and population dynamics of the free living stages of gastrointestinal nematodes in sheep. In Control of Internal Parasites of Sheep; Ross, A.D., Ed.; Lincoln College: Lincoln, UK, 1982; pp. 11–20. [Google Scholar]
- Tontini, J.F.; Poli, C.H.; Bremm, C.; de Castro, J.M.; Fajardo, N.M.; Sarout, B.N.; Castilhos, Z.M. Distribution of infective gastrointestinal helminth larvae in tropical erect grass under different pasture type for lambs. Trop. Anim. Health Product. 2015, 47, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Tontini, J.F.; Poli, C.H.; Hampel, V.S.; Fajardo, N.M.; Martins, A.A.; Minho, A.P.; Muir, J.P. Dispersal and concentration of sheep gastrointestinal nematode larvae on tropical pastures. Small Rum. Res. 2019, 174, 62–68. [Google Scholar] [CrossRef]
- Jaimez-Rodríguez, P.R.; González-Pech, P.G.; Ventura-Cordero, J.; Brito, D.R.B.; Costa-Junior, L.M.; Sandoval-Castro, C.A.; Torres-Acosta, J.F.J. The worm burden of tracer kids and lambs browsing heterogeneous vegetation is influenced by strata harvested and not total dry matter intake or plant life form. Trop. Anim. Health Prod. 2019, 51, 2243–2251. [Google Scholar] [CrossRef] [PubMed]
- Stear, M.J.; Singleton, D.; Matthews, L. An evolutionary perspective on gastrointestinal nematodes of sheep. J. Helminthol. 2011, 85, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Poulin, R. Are there general laws in parasite ecology? Parasitology 2007, 134, 763–776. [Google Scholar] [CrossRef] [PubMed]
- Morrill, A.; Dargent, F.; Forbes, M.R. Explaining parasite aggregation: More than one parasite species at a time. Int. J. Parasitol. 2017, 47, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Torres-Acosta, J.F.J.; González-Pech, P.G.; Chan-Pérez, J.I.; Sandoval-Castro, C.A.; Estrada-Reyes, Z.M.; Mendoza-de-Gives, P. Experiencias en el control alternativo de nematodos gastrointestinales de pequeños rumiantes domésticos en México. In Avances en el Estudio de Helmintos Parásitos; Ortega-Pierres, M.A., Morales-Monto, J., Eds.; Editorial UNAM: Ciudad de México, Mexico, 2014; pp. 205–241. [Google Scholar]
- Torres-Acosta, J.F.J.; Hoste, H.; Sandoval-Castro, C.A.; Torres-Fajardo, R.A.; Ventura-Cordero, J.; González-Pech, P.G.; Mancilla-Montelongo, M.G.; Ojeda-Robertos, N.F.; Martínez-Ortiz-de-Montellano, C. The art of war against gastrointestinal nematodes in sheep and goat herds of the tropics. Revista Acadêmica Ciência Animal 2019, 17, 39–46. [Google Scholar]
- Coop, R.L.; Holmes, P.H. Nutrition and parasite interaction. Int. J. Parasitol. 1996, 26, 951–962. [Google Scholar] [CrossRef]
- Bishop, S.C. A consideration of resistance and tolerance for ruminant nematode infections. Front. Genet. 2012, 3, 168. [Google Scholar] [CrossRef]
- Ríos, G.; Riley, J. Preliminary studies on the utilization of the natural vegetation in the henequen zone of Yucatán for the production of goats. I. Selection and nutritive value of native plants. Trop. Anim. Health Prod. 1985, 10, 1–10. [Google Scholar]
- González-Pech, P.G.; Torres-Acosta, J.F.J.; Sandoval-Castro, C.A.; Tun-Garrido, J. Feeding behaviour of sheep and goats in a deciduous tropical forest during the dry season: The same menu consumed differently. Small Rumin. Res. 2015, 133, 128–134. [Google Scholar] [CrossRef]
- Ventura-Cordero, J.; González-Pech, P.G.; Torres-Acosta, J.F.J.; Sandoval-Castro, C.A.; Tun-Garrido, J. Sheep and goat browsing a tropical deciduous forest during the rainy season: Why does similar plant species consumption result in different nutrient intake? Anim. Prod. Sci. 2019, 59, 66–72. [Google Scholar] [CrossRef]
- Maddi, V.S.; Aragade, P.D.; Digge, V.G.; Nitalikar, M.N. Importance of Nutraceuticals in health management. Pharmacol. Rev. 2007, 1, 377–379. [Google Scholar]
- Bhattacharya, A.; Roy, D. Nutraceuticals in Livestock & Poultry; New India Publishing Agency: New Delhi, India, 2015; pp. 1–60. [Google Scholar]
- Patel, K.; Katole, S. Nutraceuticals and Ruminants nutrition—A review. Livest. Res. Int. 2018, 6, 76–85. [Google Scholar]
- Gupta, R.C.; Srivastava, A.; Lall, R. Nutraceuticals in Veterinary Medicine; Springer: Cham, Switzerland, 2019; 852p. [Google Scholar]
- Ballou, M.A.; Davis, E.M.; Kasl, B.A. Nutraceuticals. An alternative strategy for the use of antimicrobials. Vet. Clin. Food Anim. 2019, 35, 507–534. [Google Scholar] [CrossRef] [PubMed]
- Dzanis, D.A. Nutraceuticals in veterinary medicine. Aus. Vet. J. 1999, 4, 238–239. [Google Scholar] [CrossRef]
- Alamgir, A.N.M. Classification of Drugs, Nutraceuticals, Functional food, and Cosmeceuticals; Proteins, Peptides, and Enzymes as drugs. In Therapeutic Use of Medicinal Plants and their Extracts; Alamgir, A.N.M., Ed.; Springer: Cham, Switzerland, 2017; pp. 121–175. [Google Scholar]
- Boothe, D.M. Nutraceuticals in veterinary medicine. Part 1. Definitions and regulations. Compend. Contin. Educ. Vet. 1997, 19, 1248–1255. [Google Scholar]
- McCorkle, C.M. An introduction to ethnoveterinary research and development. J. Ethnobiol. 1986, 6, 129–149. [Google Scholar]
- Suroowan, S.; Mahomoodally, F. Alternative Antimicrobials: Medicinal Plants and their Influences on Animal Infectious Diseases. In Ethnoveterinary Medicine: Present and Future Concepts; McGaw, L., Abdalla, M.A., Eds.; Springer: Cham, Switzerland, 2020; pp. 23–56. [Google Scholar]
- Andlauer, W.; Fürst, P. Nutraceuticals: A piece of history, present status and outlook. Food Res. 2002, 35, 171–176. [Google Scholar] [CrossRef]
- Agreil, C.; Meuret, M. An improve method for quantifying intake rate and ingestive behavior of ruminants in diverse and variable habitats using direct observation. Small Rum. Res. 2004, 54, 99–113. [Google Scholar] [CrossRef]
- Bonnet, O.; Meuret, M.; Tischler, M.; Cezimbra, I.; Azambuja, J.; Carvalho, C. Continuous bite monitoring: A method to assess the foraging dynamics of herbivores in natural grazing conditions. Anim. Prod. Sci. 2015, 55, 339–349. [Google Scholar] [CrossRef]
- Illius, A.W.; Hodgson, J. Progress in understanding the ecology and management of grazing systems. In The Ecology and Management of Grazing Systems; Illius, A.W., Hodgson, J., Eds.; CAB International: Wallingford, UK, 1996; pp. 429–458. [Google Scholar]
- Agreil, C.; Meuret, M.; Fritz, H. Adjustment of feeding choices and intake by a ruminant foraging in varied and variable environments: New insights from continuous bite monitoring. In Feeding of Domestic Vertebrates: From Structure to Behavior; Bels, V., Ed.; CABI Publishing: Oxfordshire, UK, 2006; pp. 302–325. [Google Scholar]
- Reppert, J.N. Forage preference and grazing habits of cattle at the Eastern Colorado Range Station. J. Range Manag. 1960, 13, 58–65. [Google Scholar] [CrossRef]
- Villalba, J.J.; Provenza, F.P. Postingestive feedback from starch influences the ingestive behavior of sheep consuming wheat straw. Appl. Anim. Behav. Sci. 2000, 66, 49–63. [Google Scholar] [CrossRef]
- Damiram, D.; DelCurto, T.; Findholt, S.L.; Johnson, B.K.; Vavra, M. Comparison of bite-count and rumen evacuation techniques to estimate cattle diet quality. Rangel. Ecol. Manag. 2013, 66, 106–109. [Google Scholar] [CrossRef]
- Halls, L.K. The approximation of cattle diet through herbage sampling. J. Range Manag. 1954, 7, 269–270. [Google Scholar] [CrossRef]
- De Vries, M. Estimating forage intake and quality in grazing cattle: A reconsideration of the hand-plucking method. J. Range Manag. 1995, 48, 370–375. [Google Scholar] [CrossRef]
- Franco-Guerra, F.J.; Gómez, A.G.; Villareal, O.A.; Camacho, J.C.; Hernández, J.E.; Rodríguez, E.L.; Marcito, A. Tasa de bocados en la vegetación nativa por cabras en pastoreo trashumante en agostaderos montañosos del nudo mixteco, México. Trop. Subtrop. Agroecosyst. 2014, 17, 249–253. [Google Scholar]
- Franco-Guerra, F.J.; Sánchez, M.; Camacho, J.C.; Hernández, J.E.; Villareal, O.A.; Rodríguez, E.L.; Marcito, O. Consumo de especies arbóreas, arbustivas y sus frutos y herbáceas por cabras en pastoreo trashumante en la mixteca oaxaqueña, México. Trop. Subtrop. Agroecosyst. 2014, 17, 267–270. [Google Scholar]
- Albores-Moreno, S.; Alayón-Gamboa, J.A.; Morón-Ríos, A.; Ortiz-Colin, P.N.; Ventura-Cordero, J.; González-Pech, P.G.; Mendoza-Arroyo, G.E.; Ku-Vera, J.C.; Jiménez-Ferrer, G.; Piñeiro-Vázquez, A.T. Influence of the composition and diversity of tree fodder grazed on the selection and voluntary intake by cattle in a tropical forest. Agroforest. Syst. 2020. [Google Scholar] [CrossRef]
- Manousidis, T.; Kyriazopoulos, A.P.; Parissi, Z.M.; Abraham, E.M.; Korakis, G.; Abas, Z. Grazing behavior, forage selection and diet composition of goats in a mediterranean Woody rangeland. Small Rum. Res. 2016, 145, 142–153. [Google Scholar] [CrossRef]
- Chebli, Y.; El Otmani, S.; Chentouf, M.; Hornick, J.L.; Bindelle, J.; Cabaraux, J.F. Foraging behavior of goats browsing in Southern Mediterranean Forest Rangeland. Animals 2020, 10, 196. [Google Scholar] [CrossRef] [PubMed]
- Githiori, J.B.; Höglund, J.; Waller, P.J. Ethnoveterinary plant preparations as livestock dewormers: Practices, popular beliefs, pitfalls and prospect for the future. Anim. Health Res. Rev. 2005, 6, 91–103. [Google Scholar] [CrossRef]
- Attindéhou, S.; Houngnimassoun, M.A.; Salifou, S.; Biaout, C.F. Inventory of herbal remedies used to control small ruminant’s parasites in Southern Benin. Int. Multidiscip. Res. J. 2012, 2, 14–16. [Google Scholar]
- Vogl, C.R.; Vogl-Lukasser, B.; Walkenhorst, M. Local knowledge held by farmers in Eastern Tyrol (Austria) about the use of plants to maintain and improve animal health and welfare. J. Ethnobiol. Ethnomed. 2016, 12, 40. [Google Scholar] [CrossRef]
- Gradé, J.T.; Tabuti, J.R.S.; van Damme, P. Four footed pharmacists: Indication of self-medicating livestock in Karamoja, Uganda. Econ. Bot. 2009, 63, 29–42. [Google Scholar] [CrossRef]
- Kahiya, C.; Mukaratirwa, S.; Thamsborg, S.M. Effects of Acacia nilotica and Acacia karoo diets on Haemonchus contortus infection in goats. Vet. Parasitol. 2003, 115, 265–274. [Google Scholar] [CrossRef]
- Debela, E.; Tolera, A.; Olav, L.; Salte, R. Condensed tannins from Sebasnia sesban and Desmodium intortum as a means of Haemonchus contortus control in goats. Trop. Anim. Health Prod. 2012, 44, 1939–1944. [Google Scholar] [CrossRef] [PubMed]
- Landau, S.; Azaizeh, H.; Muklada, H.; Glasser, T.; Ungar, E.D.; Baram, H.; Abbas, N.; Markovics, A. Anthelmintic activity of Pistacia lentiscus foliage in two Middle Eastern breeds of goats differing in their propensity to consume tannin-rich browse. Vet. Parasitol. 2010, 173, 280–286. [Google Scholar] [CrossRef]
- Raju, J.; Sahoo, B.; Chandrakar, A.; Sankar, M.; Garg, A.K.; Sharma, A.K.; Pandey, A.B. Effect of feeding oak leaves (Quercus semecarpifolia vs Quercus leucotricophora) on nutrient utilization, growth performance and gastrointestinal nematodes of goats in temperate sub Himalayas. Small Rum. Res. 2015, 125, 1–9. [Google Scholar] [CrossRef]
- Paolini, V.; De La Farge, F.; Prevot, F.; Dorchies, P.H.; Hoste, H. Effects of the repeated distribution of sainfoin hay on the resistance and the resilience of goats naturally infected with gastrointestinal nematodes. Vet. Parasitol. 2005, 127, 277–283. [Google Scholar] [CrossRef]
- Moreno-Gonzalo, J.; Ferre, I.; Celaya, R.; Frutos, P.; Ferreira, L.M.M.; Hervás, G.; García, U.; Ortega-Mora, L.M.; Osoro, K. Potential use of heather to control gastrointestinal nematodes in goats. Small Rum. Res. 2012, 103, 60–68. [Google Scholar] [CrossRef]
- Moreno-Gonzalo, J.; Osoro, K.; García, U.; Frutos, P.; Celaya, R.; Ferreira, L.M.M.; Ortega-Mora, L.; Ferre, I. Anthelmintic effect of heather in goats experimentally infected with Trichostrongylus colubriformis. Parasitol. Res. 2014, 113, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Moreno, F.C.; Gordon, I.J.; Knox, M.R.; Summer, P.M.; Skerrat, L.F.; Benvenutti, M.A.; Saumell, C.A. Anthelmintic efficacy of five tropical native Australian plants against Haemonchus contortus and Trichostrongylus colubriformis in experimentally infected goats (Capra hircus). Vet. Parasitol. 2012, 187, 237–243. [Google Scholar] [CrossRef]
- Shaik, S.A.; Terrill, T.H.; Miller, J.E.; Kouakou, B.; Kannan, G.; Kaplan, R.M.; Burke, J.M.; Mosjidis, J.A. Sericea lespedeza hay as a natural deworming agent against gastrointestinal nematode infection in goats. Vet. Parasitol. 2006, 139, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Gujja, S.; Terrill, T.H.; Mosjidis, J.A.; Miller, J.E.; Mechineni, A.; Kommuru, D.S.; Shaik, S.A.; Lambert, B.D.; Cherry, N.M.; Burke, J.M. Effect of supplemental sericea lespedeza leaf meal pellets on gastrointestinal nematode infection in grazing goats. Vet. Parasitol. 2013, 191, 51–58. [Google Scholar] [CrossRef]
- Brito, D.R.B.; Costa-Junior, L.M.; García, J.L.; Torres-Acosta, J.F.J.; Louvandini, H.; Cutrim-Júnior, J.A.A.; Araújo, J.F.M.; Soares, E.D.S. Supplementation with dry Mimosa caesalpiniifolia leaves can reduce the Haemonchus contortus worm burden of goats. Vet. Parasitol. 2018, 252, 47–51. [Google Scholar] [CrossRef]
- Lima, P.M.T.; Crouzolon, P.; Sanchez, T.P.; Zabré, G.; Kabore, A.; Niderkorn, V.; Hoste, H.; Amarante, A.F.T.; Costa-Junior, L.M.; Abdalla, A.L.; et al. Effects of Acacia mearnsii supplementation on nutrition, parasitological, blood parameters and methane emissions in Santa Inês sheep infected with Trichostrongylus colubriformis and Haemonchus contortus. Exp. Parasitol. 2019, 207, 107777. [Google Scholar] [CrossRef]
- Rojas, D.; Cubides, J.; Montenegro, A.; Martínez, C.; Ríos de Álvarez, L. In vitro anthelmintic effect of four extracts obtained from Caesalpinia coriaria foliage against an Haemonchus contortus isolate. 2. Larvae exsheathment inhibition test. Poster Presentation. In Proceedings of the 27th Meeting of the World Association for the Advancement of Veterinary Parasitology (WAAVP), Madison, WI, USA, 7–11 July 2019. [Google Scholar]
- Romero, N.; Areche, C.; Cubides-Cardenas, J.; Escobar, N.; García-Beltrán, O.; Simirgiotis, M.J.; Céspedes, A. In vitro anthelmintic evaluation of Gliricidia sepium, Leucaena leucocephala, and Phitecellobium dulce: Fingerprint analysis extracts by UHPLC-Orbitrap mass spectrometry. Molecules 2020, 25, 3002. [Google Scholar] [CrossRef]
- González-Cortázar, M.; Zamilpa, A.; López-Arellano, M.E.; Aguilar-Marcelino, L.; Reyes-Guerrero, D.E.; Olazarán-Jenkins, S.; Ramírez-Vargas, G.; Olmedo-Juárez, A.; Mendoza-de-Gives, P. Lysiloma acapulcensis leaves contain anthelmintic metabolites that reduce the gastrointestinal nematode egg population in sheep faeces. Com. Clin. Path. 2017, 27, 189–197. [Google Scholar] [CrossRef]
- Higuera-Piedrahita, R.I.; López-Arellano, M.E.; López-Arellano, R.; Cuenca-Verde, C.; Cuéllar-Ordaz, J.A. Artemisia cina 30 CH como tratamiento homeopático contra el Haemonchus contortus. Rev. Mex. Cienc. Pecuarias. 2020, 11, 342–354. [Google Scholar] [CrossRef]
- Ortiz-Ocampo, G.I.; Torres-Acosta, J.F.J.; Sandoval-Castro, C.A.; Hoste, H.; Capetillo-Leal, C.M.; González-Pech, P.G.; Santos-Ricalde, R.H. In vitro and in vivo anthelmintic effect of Coffea arabica residues against an Haemonchus contortus isolate with low susceptibility to tannins. Trop. Subtrop. Agroecosyst. 2016, 19, 41–50. [Google Scholar]
- Mancilla-Montelongo, G.; Castañeda-Ramírez, G.S.; Gaudin, E.; Canul-Velázquez, M.L.; Chan-Pérez, J.I.; De La Cruz-Cortázar, A.; Mathieu, C.; Fourquaux, I.; Sandoval-Castro, C.A.; Hoste, H.; et al. In Vitro evaluation of the nutraceutical potential of Theobroma cacao pod husk and leaf extracts for ruminants. Manuscript in preparation.
- Ventura-Cordero, J.; González-Pech, P.G.; Sandoval-Castro, C.A.; Torres-Acosta, J.F.J.; Tun-Garrido, J. Feed resource selection by Criollo goats browsing a tropical deciduous forest. Anim. Prod. Sci. 2018, 58, 2314–2320. [Google Scholar] [CrossRef]
- Torres-Fajardo, R.A.; Navarro-Alberto, J.A.; Ventura-Cordero, J.; González-Pech, P.G.; Sandoval-Castro, C.A.; Chan-Pérez, J.I.; Torres-Acosta, J.F.J. Intake and selection of goats grazing heterogeneous vegetation: Effect of gastrointestinal nematodes and condensed tannins. Rangel. Ecol. Manag. 2019, 72, 946–953. [Google Scholar] [CrossRef]
- Karfs, R.A.; Abbott, B.N.; Scarth, P.F.; Wallace, J.F. Land condition monitoring information for reef catchments: A new era. Rangel. J. 2009, 31, 69–86. [Google Scholar] [CrossRef]
- Sharma, O.P.; Sharma, S.; Pattabhi, V.; Mahato, S.B.; Sharma, P.D. A review of the hepatotoxic plant Lantana camara. Crit. Rev. Toxicol. 2007, 37, 313–352. [Google Scholar] [CrossRef]
- Ávila-Cervantes, R. Caracterización Nutricional y Fitoquímica In Vitro de 10 Plantas de Bajo o Nulo Consumo de la Selva Baja Caducifolia. Master’s Thesis, Universidad Autónoma de Yucatán, Mérida, Yucatán, Mexico, 2020. [Google Scholar]
- Gusha, J.; Masocha, M.; Muchaya, M.; Ncube, S. Chemical analysis of the potential contribution of Lantana camara to the nutrition of browsing livestock. Trop. Subtrop. Agr. 2016, 19, 337–342. [Google Scholar]
- Hussein, R.A.; El-Anssary, A. Plants secondary metabolites: The key drivers of the pharmacological actions of medicinal plants. In Herbal Medicine; Intech Open: Giza, Egypt, 2019. [Google Scholar] [CrossRef]
- Barry, T.N. Condensed tannins their role in ruminant protein and carbohydrate digestion and possible effects upon the rumen ecosystem. In The Roles of Protozoa and Fungi in Ruminant Digestion; Nolan, J.V., Leng, R.A., Demeyer, D.I., Eds.; Penambul Books: Armidale, NSW, Australia, 1989; pp. 153–169. [Google Scholar]
- Alonso-Díaz, M.A.; Torres-Acosta, J.F.J.; Sandoval-Castro, C.A.; Capetillo-Leal, C.M. Polyphenolic compounds of nutraceutical trees and the variability of their biological activity measured by two methods. Trop. Subtrop. Agroecosyst. 2010, 12, 649–656. [Google Scholar]
- Hove, L.; Ndlovu, L.R.; Sibanda, S. The effects of drying temperature on chemical composition and nutritive value of some tropical fodder shrubs. Agrofor. Syst. 2003, 59, 231–241. [Google Scholar] [CrossRef]
- Ferreira, E.C.; Nogueira, A.R.; Souza, G.B.; Batista, L.A. Effect of drying method and length of storage on tannin and total phenol concentrations in Pigeon pea seeds. Food Chem. 2004, 86, 17–23. [Google Scholar] [CrossRef]
- Zeller, W.E. Activity, purification, and analysis of condensed tannins: Current state of affairs and future endeavors. Crop Sci. 2019, 59, 886–904. [Google Scholar] [CrossRef]
- Kaplan, R.M.; Vidyashankar, A.N. An inconvenient truth: Global worming and anthelmintic resistance. Vet. Parasitol. 2012, 186, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Torres-Acosta, J.F.J.; Mendoza-de-Gives, P.; Aguilar-Caballero, A.J.; Cuéllar-Ordaz, J.A. Anthelmintic resistance in sheep farms: Update of the situation in the American continent. Vet. Parasitol. 2012, 186, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Paraud, C.; Chartier, C. Facing Anthelmintic Resistance in Goats. In Sustainable Goat Production in Adverse Environments: Volume I, Welfare, Health and Breeding; Simões, J., Gutiérrez, C., Eds.; Springer: Cham, Switzerland, 2017; pp. 267–292. [Google Scholar]
- Scott, I.; Pomroy, W.E.; Kenyon, P.R.; Smith, G.; Adlington, B.; Moss, A. Lack of efficacy of monepantel against Teladorsagia circumcinta and Trichostrongylus colubriformis. Vet. Parasitol. 2013, 198, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Van de Brom, R.; Moll, L.; Kappert, C.; Vellema, P. Haemonchus contortus resistance to monepantel in sheep. Vet. Parasitol. 2015, 209, 278–280. [Google Scholar] [CrossRef] [PubMed]
- Wood, I.B.; Amaral, N.K.; Bairden, K.; Duncan, J.L.; Kassai, T.; Malone, J.B., Jr.; Pankavich, J.A.; Reinecke, R.K.; Slocombe, O.; Taylor, S.M.; et al. World Association for the Advancement of Veterinary Parasitology (W.A.A.V.P.) second edition of guidelines for evaluating the efficacy of anthelmintics in ruminants (bovine, ovine, caprine). Vet. Parasitol 1995, 58, 181–213. [Google Scholar] [CrossRef]
- Bauhaud, D.; Martínez-Ortiz-De-Montellano, C.; Chauveau, S.; Prevot, F.; Torres-Acosta, J.F.J.; Fouraste, I.; Hoste, H. Effects of four tanniniferous plant extracts on the in vitro exsheatment of third-stage larvae of parasitic nematodes. Parasitology 2006, 132, 545–554. [Google Scholar] [CrossRef]
- Jackson, F.; Hoste, H. In vitro methods for the primary screening of plant products for direct activity against ruminant gastrointestinal nematodes. In In Vitro Screening of Plant Resources for Extra Nutritional Attributes in Ruminants: Nuclear and Related Methodologies; Vercoe, P.E., Makkar, H.P.S., Schlink, A.C., Eds.; FAO/IAEA Springer Edition: Dordrecht, The Netherlands, 2010; pp. 25–45. [Google Scholar]
- Higuera-Piedrahita, R.I.; López-Arellano, M.E.; López-Arellano, R.; Cuenca-Verde, C.; Cuéllar-Ordaz, J.A. Effect evaluation of artemisins from ethanolic extract of Artemisia cina against L3 of Haemonchus contortus on a abomasal explants technique. Rev. Cien. Agri. 2016, 13, 107–116. [Google Scholar] [CrossRef]
- Quijada, J.C.; Fryganas, C.; Ropiak, H.M.; Ramsay, A.; Mueller-Harvey, I.; Hoste, H. Anthelmintic activities against Haemonchus contortus or Trichostrongylus colubriformis from small ruminants are influenced by structural features of condensed tannins. J. Agric. Food Chem. 2015, 63, 6346–6354. [Google Scholar] [CrossRef]
- Borges, D.G.L.; Borges, F.A. Plants and their medicinal potential for controlling gastrointestinal nematodes in ruminants. Nematoda 2016, 3, 1–7. [Google Scholar] [CrossRef]
- Villalba, J.J.; Provenza, F.D. Challenges in Extrapolating In vitro Findings to In Vivo Evaluation of Plant Resources. In In Vitro Screening of Plant Resources for Extra Nutritional Attributes in Ruminants: Nuclear and Related Methodologies; Vercoe, P.E., Makkar, H.P.S., Schlink, A.C., Eds.; FAO/IAEA Springer Edition: Dordrecht, The Netherlands, 2010; pp. 233–242. [Google Scholar]
- Meier, J.S.; Kreuzer, M.; Marquardt, S. Design and methodology of choice feeding experiments with ruminant livestock. Appl. Anim. Behav. Sci. 2012, 140, 105–120. [Google Scholar] [CrossRef]
- Ben Salem, H.; Nefzaoui, A.; Abdouli, H. Palatability of shrubs and fodder trees measured on sheep and dromedaries: 1. Methodological approach. Anim. Feed. Sci. Technol. 1994, 46, 143–153. [Google Scholar] [CrossRef]
- Coop, R.L.; Kyriazakis, I. Nutrition-parasite interaction. Vet. Parasitol. 1999, 84, 187–204. [Google Scholar] [CrossRef]
- Coop, R.L.; Kyriazakis, I.K. Influence of host nutrition on the development and consequences of nematode parasitism in ruminants. Trends Parasitol. 2001, 17, 325–330. [Google Scholar] [CrossRef]
- Hoste, H.; Torres-Acosta, J.F.J.; Quijada, J.; Chan-Pérez, I.; Dakheel, M.M.; Kommuru, D.S.; Mueller-Harvey, I.; Terrill, T.H. Interactions between nutrition and infections with Haemonchus contortus and related gastrointestinal nematodes in small ruminants. Adv. Parasitol. 2016, 93, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Mohrand-Fehr, P. Recent development in goat nutrition and application: A review. Small Rumin. Res. 2005, 60, 25–34. [Google Scholar] [CrossRef]
- Mancilla-Montelongo, G.M.; Castañeda-Ramírez, G.S.; Can-Celis, A.; Chan-Pérez, J.I.; Sandoval-Castro, C.A.; Torres-Acosta, J.F.J. Optimal age of Trichostrongylus colubriformis larvae (L3) for the in vitro larval exsheathment inhibition test under tropical conditions. Vet. Parasitol. 2020, 278, 109027. [Google Scholar] [CrossRef] [PubMed]
- Perevolotsky, A.; Landau, S.; Silanikove, N.; Provenza, F. Upgrading tannin-rich forages by supplementing ruminants with polyethylene glycol (PEG). In Herbivores: The Assessment of Intake, Digestibility and the Roles of Secondary Compounds; Sandoval-Castro, C.A., Deb Hovell, F.V., Torres-Acosta, J.F.J., Ayala-Burgos, A., Eds.; Nottingham University Press: Nottingham, UK, 2006; pp. 221–233. [Google Scholar]
- Bhat, T.K.; Kannan, A.; Singh, B.; Sharma, O.P. Value addition of feed and fodder by alleviating the antinutritional effects of tannins. Agric. Res. 2013, 2, 189–206. [Google Scholar] [CrossRef]
- Jackson, F.; Varady, M.; Bartley, D.J. Managing anthelmintic resistance in goats—Can we learn lessons from sheep? Small Rum. Res. 2012, 103, 3–9. [Google Scholar] [CrossRef]
- Lespine, A.; Chartier, C.; Hoste, H.; Alvinerie, M. Endectocides in goats: Pharmacology, efficacy and use conditions in the context of anthelmintics resistance. Small Rum. Res. 2012, 103, 10–17. [Google Scholar] [CrossRef]
- Rostang, A.; Devos, J.; Chartier, C.H. Review of the Eprinomectin effective doses required for dairy goats: Where do we go from here? Vet. Parasitol. 2020, 277, 1089–1092. [Google Scholar] [CrossRef]
- Codron, D.; Hofmann, R.R.; Clauss, M. Morphological and Physiological Adaptations for Browsing and Grazing. In The Ecology of Browsing and Grazing II, 2nd ed.; Gordon, I.J., Prins, H.H.T., Eds.; Springer: Cham, Switzerland, 2019; pp. 81–125. [Google Scholar]
- Gordon, I.J.; Prins, H.H.T. The Ecology of Browsing and Grazing II, 2nd ed.; Gordon, I.J., Prins, H.H.T., Eds.; Springer: Cham, Switzerland, 2019; pp. 1–4. [Google Scholar]
- Silanikove, N. The physiological basis of adaptation in goats to harsh environment. Small Rum. Res. 2000, 35, 181–193. [Google Scholar] [CrossRef]
- Hofmann, R.R. Evolutionary steps of ecophysiological adaptation and diversification of ruminants: A comparative view of their digestive system. Oecologia 1989, 78, 443–457. [Google Scholar] [CrossRef] [PubMed]
- Pfister, J.A.; Malecheck, J.C. Dietary selection by goats and sheep in deciduous woodland of northeastern Brazil. J. Range Manag 1986, 39, 24–28. [Google Scholar] [CrossRef]
- Lu, C.D. Grazing behavior and diet selection of goats. Small Rum. Res. 1988, 3, 205–216. [Google Scholar] [CrossRef]
- Decandia, M.; Yiakoulaki, M.D.; Pinna, G.; Cabiddu, A.; Molle, G. Foraging behavior and intake of goats browsing on Mediterranean shrublands. In Dairy Goats Feeding and Nutrition; Cannas, A., Pulina, G., Eds.; CAB international: Wallingford, Oxfordshire, UK, 2005; pp. 161–188. [Google Scholar]
- Dove, H. Ingestive behavior, diet selection, and feed intake. In Goat Science and Production; Solaiman, S.G., Ed.; Wiley-Blackwell: State Avenue, IA, USA, 2010; pp. 179–193. [Google Scholar]
- Goetsch, A.L.; Gipson, T.A.; Askar, A.R.; Puchala, R. Invited review: Feeding behavior of goats. J. Anim. Sci. 2010, 88, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Hoste, H.; Sotiraki, S.; Landau, S.Y.; Jackson, F.; Beveridge, I. Goat-Nematode interactions: Think differently. Trends Parasitol. 2010, 26, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Huntley, J.F.; Patterson, M.; Mackellar, A.; Jackson, F.; Stevenson, L.M.; Coop, R.L. A comparison of the mast cell and eosinophil responses of sheep and goats to gastrointestinal nematode infections. Res. Vet. Sci. 1995, 58, 5–10. [Google Scholar] [CrossRef]
- Silvestre, A.; Leignel, V.; Berrag, B.; Gasnier, N.; Humbert, J.F.; Chartier, C.; Cabaret, J. Sheep and goat nematode resistance to anthelmintics: Pro and cons breeding management factors. Vet. Res. 2002, 33, 465–480. [Google Scholar] [CrossRef]
- Amit, M.; Cohen, I.; Marcovics, A.; Muklada, H.; Glasser, T.A.; Ungar, E.D.; Landau, S.Y. Self-medication with tannin rich browse in goats infected with gastrointestinal nematodes. Vet. Parasitol. 2013, 198, 305–311. [Google Scholar] [CrossRef]
- Forbes, J.M. Voluntary Intake and Diet Selection in Farm Animals, 2nd ed.; CABI International: Wallingford, Oxfordshire, UK, 2007; pp. 41–171. [Google Scholar]
- Carvalho, P. Harry Stobbs Memorial Lecture: Can grazing behavior support innovations in grassland management? Trop. Grassl. 2013, 1, 137–155. [Google Scholar] [CrossRef]
- Johnson, D. The comparison of usage and availability measurements for evaluating resource preference. Ecology 1980, 61, 65–71. [Google Scholar] [CrossRef]
- Manly, B.F.J.; McDonald, L.L.; Thomas, D.L.; McDonald, T.L.; Erickson, W.P. Resource Selection by Animals, Statistical Design and Analysis for Field Studies, 2nd ed.; Kluwer Academic Publishers: New York, NY, USA, 2004; pp. 1–15. [Google Scholar]
- Lechowicz, M.J. The sampling characteristics of electivity indexes. Oecologia 1982, 52, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Ventura-Cordero, J.; González-Pech, P.G.; Jaimez-Rodríguez, P.R.; Ortíz-Ocampo, G.I.; Sandoval-Castro, C.A.; Torres-Acosta, J.F.J. Gastrointestinal nematode infection does not affect selection of tropical foliage by goats in a cafeteria trial. Trop. Anim. Health Prod. 2017, 49, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Ventura-Cordero, J.; González-Pech, P.G.; Jaimez-Rodríguez, P.R.; Ortíz-Ocampo, G.I.; Sandoval-Castro, C.A.; Torres-Acosta, J.F.J. Feed resource selection of Criollo goats artificially infected with Haemonchus contortus: Nutritional wisdom and prophylactic self-medication. Animal 2017, 12, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- Torres-Fajardo, R.A.; González-Pech, P.G.; Ventura-Cordero, J.; Ortíz-Ocampo, G.I.; Sandoval-Castro, C.A.; Torres-Acosta, J.F.J. Feed resource selection of Criollo goats is the result of an interaction between plant resources, condensed tannins and Haemonchus contortus infection. Appl. Anim. Behav. Sci. 2018, 208, 49–55. [Google Scholar] [CrossRef]
- Pisani, J.M.; Distel, R.A.; Bontti, E.E. Diet selection by goats on a semi-arid shrubland in central Argentina. Ecol. Austral. 2001, 10, 103–108. [Google Scholar]
- Egea, A.V.; Allegretti, L.; Paez Lama, S.; Grilli, D.; Sartor, C.; Fucili, M.; Guevara, J.C.; Passera, C. Selective behavior of Creole goats in response to the functional heterogeneity of native forage species in the central Monte desert, Argentina. Small Rum. Res. 2014, 120, 90–99. [Google Scholar] [CrossRef]
- Bernhoft, A. A brief review on bioactive compounds in plants. In Proceedings of the Bioactive compounds in plants—Benefits and risk for man and animals, Oslo, Norway, 13–14 November 2008; Bernhoft, A., Ed.; The Norwegian Academy of Science and Letters: Oslo, Norway, 2010; pp. 11–18. [Google Scholar]
- Zeineldin, M.M.; Sabek, A.A.; Barakat, R.A.; Elghandour, M.M.M.Y.; Salem, A.Z.; Jiménez, R.M. Potential contribution of plants bioactive in ruminant productive performance and their impact on gastrointestinal parasites elimination. Agroforest. Syst. 2018. [Google Scholar] [CrossRef]
- Eguale, T.; Tilahun, G.; Debella, A.; Feleke, A.; Makonnen, E. Haemonchus contortus: In Vitro and In Vivo anthelmintic activity of aqueous and hidro-alcoholic extracts of Hedera helix. Exp. Parasitol. 2007, 116, 340–345. [Google Scholar] [CrossRef]
- Marie-Magdeleine, C.; Udino, L.; Philibert, L.; Bocage, B.; Archimede, H. In vitro effects of Cassava (Manihot esculenta) leaf extracts on four development stages of Haemonchus contortus. Vet. Parasitol. 2010, 173, 85–92. [Google Scholar] [CrossRef]
- Klongsiriwet, C.; Quijada, J.; Williams, A.R.; Mueller-Harvey, I.; Williamson, E.M.; Hoste, H. Synergistic inhibition of Haemonchus contortus exsheathment by flavonoid monomers and condensed tannins. Int. J. Parasitol. 2015, 5, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Fomum, S.W.; Nsahlai, I.V. In Vitro nematicidal activity of plant species possessing alkaloids and tannins. Cogent Food Agric. 2017, 3, 1334295. [Google Scholar] [CrossRef]
- Spiegler, V.; Liebau, E.; Hensel, A. Medicinal plant extracts and plant-derived polyphenols with anthelmintic activity against intestinal nematodes. Nat. Prod. Rep. 2017, 34, 627–643. [Google Scholar] [CrossRef] [PubMed]
- Maestrini, M.; Tava, A.; Mancini, S.; Tedesco, D.; Perrucci, S. In vitro anthelmintic activity of saponins from Medicago spp. against sheep gastrointestinal nematodes. Molecules 2020, 25, 242. [Google Scholar] [CrossRef] [PubMed]
- Zarza-Albarrán, M.A.; Olmedo-Juárez, A.; Rojo-Rubio, R.; Mendoza-de-Gives, P.; González-Cortázar, M.; Tapia-Maruri, D.; Mondragón-Ancelmo, J.; García-Hernández, C.; Blé-González, E.A.; Zamilpa, A. Galloyl flavonoids from Acacia farnesiana pods possess potent anthelmintic activity against Haemonchus contortus eggs and infective larvae. J. Ethnopharmacol. 2020, 249, 112402. [Google Scholar] [CrossRef]
- Banner, R.E.; Rogosic, J.; Burritt, E.A.; Provenza, F.D. Supplemental barley and charcoal increase intake of sagebrush by lambs. J. Range Manag. 2000, 53, 415–420. [Google Scholar] [CrossRef]
- Rogosic, J.; Moe, S.R.; Skobic, D.; Knezovic, Z.; Rozic, I.; Rozic, I.; Zivkovic, M.; Pavlicevic, J. Effect of supplementation with barley and activated charcoal on intake of biochemically diverse Mediterranean shrubs. Small Rum. Res. 2009, 81, 79–84. [Google Scholar] [CrossRef]




| Concept | Definition | Comment | Reference |
|---|---|---|---|
| Feed additive | A compound generally of synthetic origin added to the feed | Dependent of VFI and long-term administration to exert their effects | [35,86,89] |
| Cosmeceutical | A cosmetic product with active ingredients | Independent of VFI. Medium to long-term administration to exert their effects | [78,88] |
| Functional food | A food containing nutrients and ingredient(s) with beneficial effects beyond adequate nutritional properties | Dependent of VFI and need long-term administration to exert their effects. The closest to the nutraceutical definition, however functional food includes fortified foods while nutraceuticals do not | [85,86] |
| Phytotherapeutic | A compound of natural origin. It can be a plant, or its extracts used instead of “conventional drugs” | Independent of VFI and need short-term administration to exert their effects | [35,85] |
| Ethnoveterinary or ethnomedicine | Practices derived from ancient knowledge, beliefs and skills to prevent and treat animal diseases | Dependent of VFI and need long-term administration to exert their effects | [90,91] |
| Definition | Year | Reference |
|---|---|---|
| Food or part of a food that provides health benefits and is used for prevention or treatment of a disease | 1989 | [82,83] |
| A nondrug substance that is produced in a purified or extracted form and administered orally to provide agents required for normal body structure and function with the intent of improving the health and wellbeing of animals | 1997 | [91] |
| Any substance that may be considered a food or part of a food which provides health benefits, including the prevention and treatment of disease | 2002 | [92] |
| A livestock feed which combines nutritional value with beneficial effects on animal health | 2015 | [35] |
| Natural compounds and/or microbes that offer potentially advantageous effects to ruminant health and productivity, including improved efficiency, milk production, and disease resistance through immune modulation or decrease disease pressure | 2019 | [86] |
| Plant Species | Family | Life Form | Availability (Kilograms of Dry Matter per Hectare) | % | Reference |
|---|---|---|---|---|---|
| Acacia collinsi | Fabaceae | Tree | 4.8 | 0.2 | [130] |
| Acacia pennatula | Fabaceae | Tree | 37.6 | 2.5 | [129] |
| 26.8 | 0.9 | [130] | |||
| Bahuinia divaricata | Fabaceae | Shrub/Tree | 2.6 | 0.1 | [130] |
| Caesalpinia gaumeri | Fabaceae | Tree | 15.3 | 1.0 | [129] |
| 10.3 | 3.9 | [129] | |||
| 183.8 | 6.2 | [130] | |||
| Caesalpinia yucatanensis | Fabaceae | Shrub/Tree | 1.7 | 0.1 | [130] |
| Chamaecrista glandulosa | Fabaceae | Herb/Shrub | 0.3 | 0.1 | [129] |
| 1.7 | 0.1 | [130] | |||
| Diphysa yucatanensis | Fabaceae | Shrub/Tree | 5.7 | 0.2 | [130] |
| Havardia albicans | Fabaceae | Tree | 118.9 | 4.0 | [130] |
| Leucaena leucocephala | Fabaceae | Shrub/Tree | 15.6 | 1.0 | [129] |
| 25.4 | 0.9 | [130] | |||
| Lysiloma latisiliquum | Fabaceae | Tree | 0.3 | <0.1 | [130] |
| Mimosa bahamensis | Fabaceae | Shrub/Tree | 4.3 | 0.3 | [129] |
| 0.9 | 0.3 | [129] | |||
| 83.5 | 2.8 | [130] | |||
| Piscidia piscipula | Fabaceae | Shrub/Tree | 0.3 | 0.1 | [129] |
| 6.7 | 0.2 | [130] | |||
| Senegalia gaumeri | Fabaceae | Tree | 16.3 | 0.5 | [130] |
| Senna villosa | Fabaceae | Shrub/Tree | 7.4 | 0.3 | [130] |
| Ipomoea crynicalix | Convolvulaceae | Vine | 119.7 | 8.0 | [129] |
| 72.1 | 27.4 | [129] | |||
| 115.3 | 3.9 | [130] | |||
| Ipomoea nil | Convolvulaceae | Vine | 1.2 | 0.1 | [129] |
| 1.8 | 0.1 | [130] | |||
| Jaquemontia penthanta | Convolvulaceae | Vine | 0.3 | <0.1 | [130] |
| Merremia aegyptia | Convolvulaceae | Vine | 5.0 | 1.9 | [129] |
| 0.3 | <0.1 | [130] | |||
| Helicteres baruensis | Malvaceae | Shrub | 2.5 | 0.9 | [129] |
| Sida acuta | Malvaceae | Herb | 1.7 | 0.1 | [130] |
| Waltheria indica | Malvaceae | Herb | 66.6 | 4.4 | [129] |
| 10.7 | 4.1 | [129] | |||
| 26.3 | 0.9 | [130] | |||
| Bidens pilosa | Asteraceae | Herb | 28.5 | 1.9 | [129] |
| Porophylum punctatum | Asteraceae | Herb | 3.4 | 0.2 | [129] |
| 3.5 | 0.1 | [130] | |||
| Viguiera dentata | Asteraceae | Herb | 5.3 | 2 | [129] |
| 3.8 | 0.1 | [130] | |||
| Gymnopodium floribundum | Polygonaceae | Shrub/Tree | 1132.8 | 75.5 | [129] |
| 129.7 | 49.3 | [129] | |||
| 1653.8 | 55.5 | [130] | |||
| Neomillspaughia emarginata | Polygonaceae | Shrub/Tree | 16.3 | 1.1 | [129] |
| 66.3 | 2.2 | [129] | |||
| Podopterus mexicanus | Polygonaceae | Shrub/Tree | 60.6 | 2 | [130] |
| Morinda royoc | Rubiaceae | Vine | 1.8 | 0.1 | [130] |
| Randia aculeata | Rubiaceae | Shrub | 2.8 | 0.2 | [129] |
| 4.3 | 0.1 | [130] | |||
| Randia obcordata | Rubiaceae | Shrub/Tree | 94.9 | 3.2 | [130] |
| Cordia alliodora | Boraginaceae | Tree | 30.7 | 1 | [130] |
| Cordia globosa boroginosa | Boraginaceae | Shrub/Tree | 35.8 | 2.4 | [129] |
| Stachytarpheta jamaicensis | Verbenaceae | Shrub | 0.4 | <0.1 | [130] |
| Lantana camara | Verbenaceae | Shrub | 3 | 0.1 | [130] |
| Acalyphaspp. | Euphorbiaceae | Herb | 9.6 | 3.6 | [129] |
| Cnidoscolus aconitifolius | Euphorbiaceae | Shrub/Tree | 3.4 | <0.1 | [130] |
| Tetramerium nervosum | Acanthaceae | Herb | 0.3 | <0.1 | [130] |
| Parmentiera millspaughiana | Bignoniaceae | Shrub/Tree | 2.4 | 0.1 | [130] |
| Bursera simaruba | Burseraceae | Tree | 2 | 0.1 | [130] |
| Dioscorea polygonoides | Dioscoraceae | Vine | 2.1 | 0.8 | [129] |
| 0.3 | <0.1 | [130] | |||
| Diospyros anisandra | Ebenaceae | Shrub/Tree | 65.6 | 2.2 | [130] |
| Mentha villosa | Lamiaceae | 15.2 | 0.5 | [130] | |
| Bunchosia swartziana | Malpighiaceae | Shrub/Tree | 15.4 | 0.5 | [130] |
| Passiflora biflora | Passifloraceae | Herb | 1.8 | 0.1 | [130] |
| Eragrostis ciliaris | Poaceae | Grass | 15.3 | 1 | [129] |
| 14.5 | 5.5 | [129] | |||
| 283.8 | 9.5 | [130] | |||
| Karwinskia humbdoltiana | Rhamnaceae | Tree | 5.8 | 0.4 | [129] |
| Cardiospermum corindum | Sapindaceae | Vine | 1.5 | 0.1 | [130] |
| Alvaradoa amorphoides | Simaroubaceae | Shrub/Tree | 23 | 0.8 | [130] |
| Solanum tridynamum | Solanaceae | Shrub | 4.8 | 0.2 | [130] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Fajardo, R.A.; González-Pech, P.G.; Sandoval-Castro, C.A.; Torres-Acosta, J.F.d.J. Small Ruminant Production Based on Rangelands to Optimize Animal Nutrition and Health: Building an Interdisciplinary Approach to Evaluate Nutraceutical Plants. Animals 2020, 10, 1799. https://doi.org/10.3390/ani10101799
Torres-Fajardo RA, González-Pech PG, Sandoval-Castro CA, Torres-Acosta JFdJ. Small Ruminant Production Based on Rangelands to Optimize Animal Nutrition and Health: Building an Interdisciplinary Approach to Evaluate Nutraceutical Plants. Animals. 2020; 10(10):1799. https://doi.org/10.3390/ani10101799
Chicago/Turabian StyleTorres-Fajardo, Rafael Arturo, Pedro Geraldo González-Pech, Carlos Alfredo Sandoval-Castro, and Juan Felipe de Jesús Torres-Acosta. 2020. "Small Ruminant Production Based on Rangelands to Optimize Animal Nutrition and Health: Building an Interdisciplinary Approach to Evaluate Nutraceutical Plants" Animals 10, no. 10: 1799. https://doi.org/10.3390/ani10101799
APA StyleTorres-Fajardo, R. A., González-Pech, P. G., Sandoval-Castro, C. A., & Torres-Acosta, J. F. d. J. (2020). Small Ruminant Production Based on Rangelands to Optimize Animal Nutrition and Health: Building an Interdisciplinary Approach to Evaluate Nutraceutical Plants. Animals, 10(10), 1799. https://doi.org/10.3390/ani10101799






