Wound Healing and Cell Dynamics Including Mesenchymal and Dental Pulp Stem Cells Induced by Photobiomodulation Therapy: An Example of Socket-Preserving Effects after Tooth Extraction in Rats and a Literature Review
Abstract
:1. Introduction
2. Characteristics of CO2 and Diode Lasers
3. Clinical Application and Basic Research on CO2 Laser and Diode Laser Irradiation of Tooth Extraction Sockets
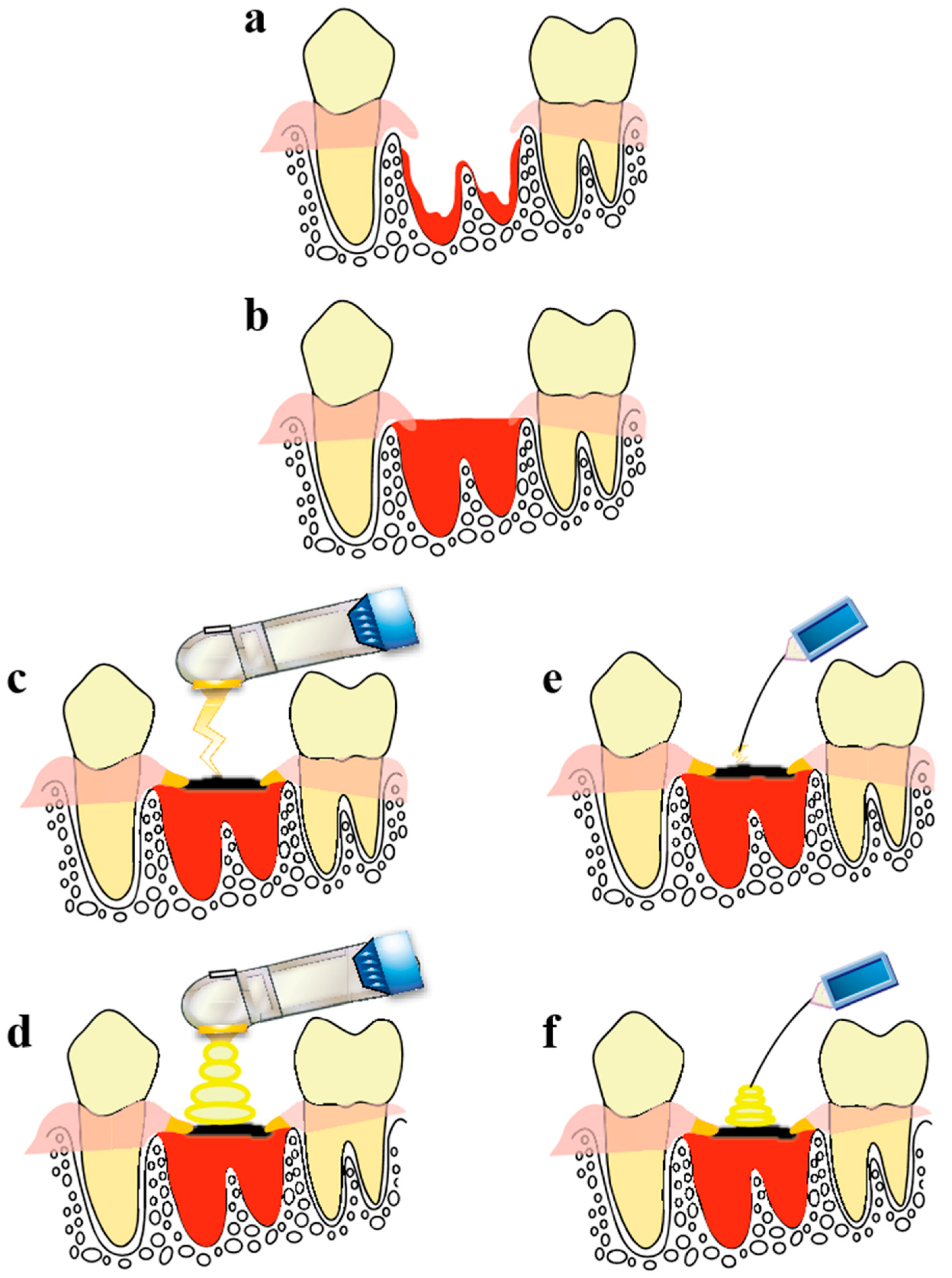
4. Observation of Wound Healing in the Tooth Extraction Socket Treated Using a CO2 Laser or Diode Laser
- (1)
- HILT: Irradiation was performed with the laser tip not in contact with the blood on the surface of the socket (1.0 W, continuous-wave mode, non-air, 30 s, 27 J).
- (2)
- PBMT: Irradiation was performed with the laser tip slightly in contact with the scab on the surface of the socket (1.0 W, Σ-mode, non-air, 15 s, 0.7 J). Σ-mode uses an ultra-short pulse width to increase peak power during irradiation, thereby enabling photobiomodulation (pulse time = 0.0008 s, pulse interval = 0.03 s, 1 cycle = 0.0308 s).
- (1)
- HILT: Irradiation was performed with the laser tip placed slightly in contact with blood on the surface of the socket (1.0 W, continuous-wave mode, 27 s, 27 J).
- (2)
- PBMT: Irradiation was performed with the laser tip placed slightly in contact with the scab on the surface of the socket (0.3 W, CP1-mode, 7 s, 0.7 J). The CP1-mode is a gated pulse mode (pulse duration, 0.0001 s; pulse interval, 0.0002 s; 1 cycle, 0.0003 s).
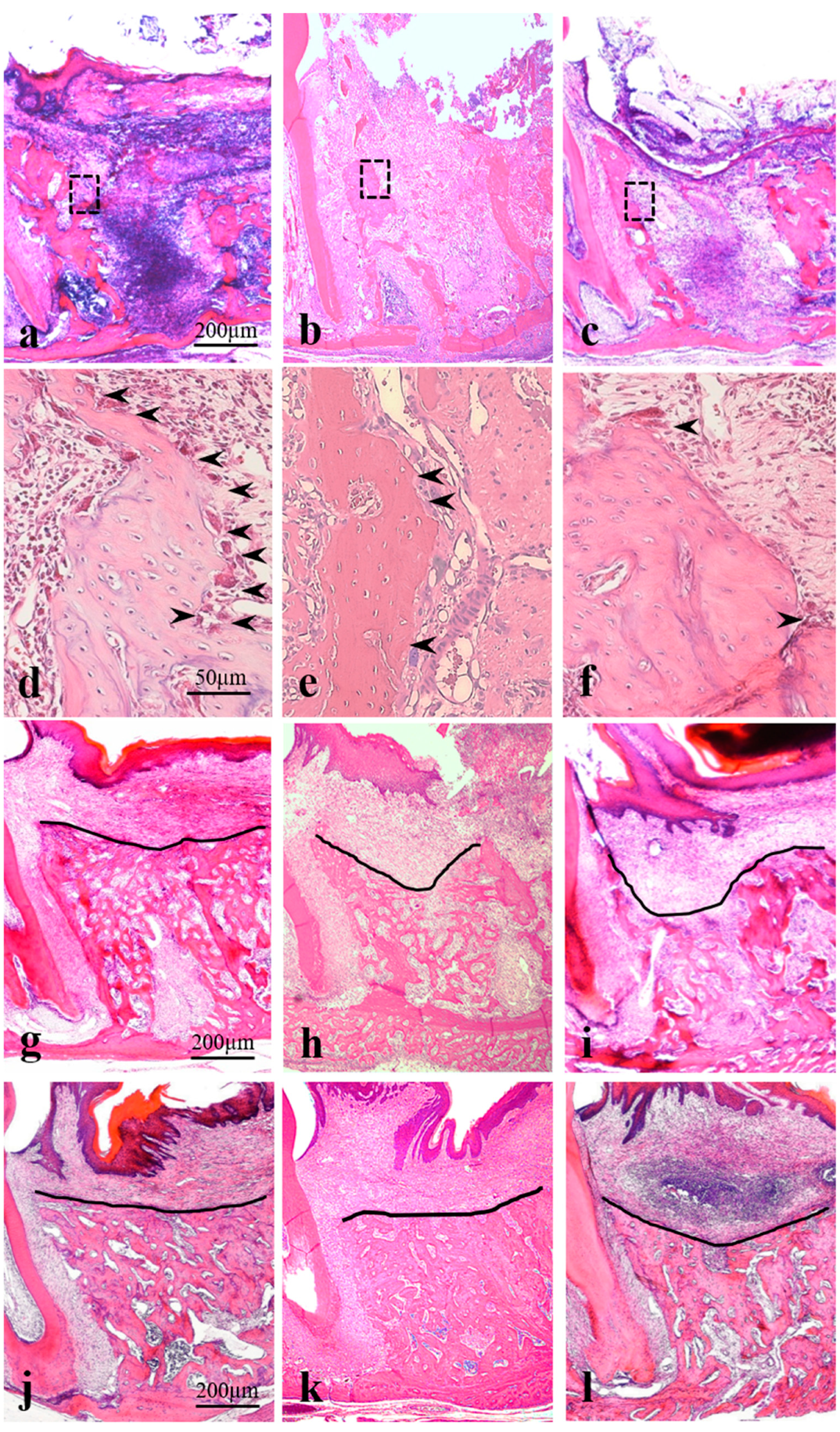
4.1. Histopathological Analysis
4.2. Results
4.3. Post-Extraction Day 3 (Corresponds to the Granulation Tissue Stage in Humans)
4.4. Post-Extraction Day 7 (Corresponds to the Temporary Bone Stage in Humans)
4.5. Post-Extraction Day 21 (Corresponds to the Healing Stage in Humans)
5. Comparison with the Current Socket Preservation Method
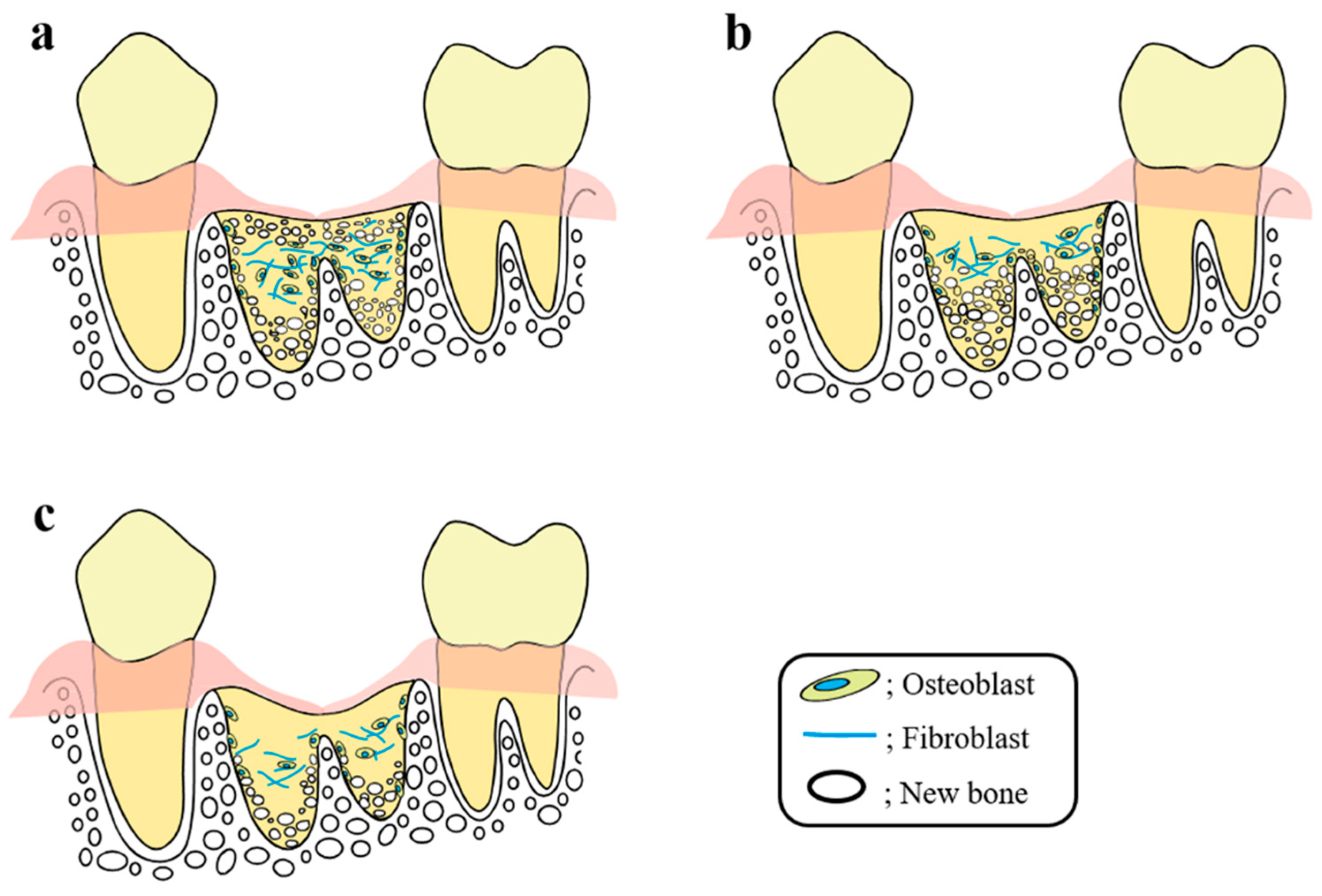
6. Mechanism of the Socket-Preserving Effect Using a CO2 Laser or Diode Laser
7. Effects of Laser Irradiation on Bone Tissues, Including Tooth Extraction Sockets, and the Underlying Cell Dynamics
8. Effect of PBMT on MSCs Transplantation in Regenerative Medicine
9. Effect of Laser Irradiation on Dental Pulp Stem Cells
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PBMT | Photobiomodulation therapy |
| HILT | High-intensity laser therapy |
| FDA | U.S. Food and Drug Administration |
| MSC | Mesenchymal stem cell |
| PDSC | Dental pulp stem cell |
References
- Sharon-Buller, A.; Sela, M. CO2-laser treatment of ulcerative lesions. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2004, 97, 332–334. [Google Scholar] [CrossRef]
- Khalil, N.M.; Noureldin, M.G. Comparison of single versus multiple low-level laser applications on bone formation in extraction socket healing in rabbits (histologic and histomorphometric study). J. Oral Maxillofac. Surg. 2019, 77, 1760–1768. [Google Scholar] [CrossRef]
- Shen, D.; Wei, J.; Chen, L.; Shen, X.; Wang, L. Besides photothermal effects, low-level CO2 laser irradiation can potentiate skin microcirculation through photobiomodulation mechanisms. Photobiomodul. Photomed. Laser Surg. 2019, 37, 151–158. [Google Scholar] [CrossRef]
- de Andrade, A.L.; Bossini, P.S.; Parizotto, N.A. Use of low level laser therapy to control neuropathic pain: A systematic review. J. Photochem. Photobiol. B 2016, 164, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Rocca, J.P.; Zhao, M.; Fornaini, C.; Tan, L.; Zhao, Z.; Merigo, E. Effect of laser irradiation on aphthae pain management: A four different wavelengths comparison. J. Photochem. Photobiol. B 2018, 189, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Tsuka, Y.; Kunimatsu, R.; Gunji, H.; Nakajima, K.; Kimura, A.; Hiraki, T.; Nakatani, A.; Tanimoto, K. Effects of Nd:YAG low-level laser irradiation on cultured human osteoblasts migration and ATP production: In vitro study. Lasers Med. Sci. 2019, 34, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Fekrazad, R.; Asefi, S.; Allahdadi, M.; Kalhori, K.A. Effect of photobiomodulation on mesenchymal stem cells. Photomed. Laser Surg. 2016, 34, 533–542. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Nicoli, F.; Xi, W.J.; Zhang, Z.; Cui, C.; Al-Mousawi, A.; Balzani, A.; Tong, Y.; Zhang, Y. The 1470 nm diode laser with an intralesional fiber device: A proposed solution for the treatment of inflamed and infected keloids. Burns Trauma 2019, 7, 5. [Google Scholar] [CrossRef] [Green Version]
- Khedr, M.M.; Mahmoud, W.H.; Sallam, F.A.; Elmelegy, N. Comparison of Nd: YAG laser and combined intense pulsed light and radiofrequency in the treatment of hypertrophic scars: A prospective clinico-histopathological study. Ann. Plast. Surg. 2020, 84, 518–524. [Google Scholar] [CrossRef]
- Ezra, N.; Arshanapalli, A.; Bednarek, R.; Akaishi, S.; Somani, A.K. The microsecond 1064 nm Nd:YAG laser as an adjunct to improving surgical scars following Mohs micrographic surgery. J. Cosmet. Laser Ther. 2016, 18, 225–229. [Google Scholar] [CrossRef]
- Ansari, F.; Sadeghi-Ghyassi, F.; Yaaghoobian, B. The clinical effectiveness and cost-effectiveness of fractional CO2 laser in acne scars and skin rejuvenation: A meta-analysis and economic evaluation. J. Cosmet. Laser Ther. 2018, 20, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Daigo, Y.; Daigo, E.; Hasegawa, A.; Fukuoka, H.; Ishikawa, M.; Takahashi, K. Utility of high intensity laser therapy combined with photobiomodulation therapy for socket preservation after tooth extraction. Photobiomodul. Photomed. Laser Surg. 2020, 38, 75–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuoka, H.; Daigo, Y.; Enoki, N.; Taniguchi, K.; Sato, H. Influence of carbon dioxide laser irradiation on the healing process of extraction sockets. Acta Odontol. Scand. 2011, 69, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Sulewski, J.G. Clearing the FDA hurdle, from initial device application through regulatory approval to the clinical operatory: An update on dental laser marketing clearances. J. Laser Dent. 2009, 17, 81–86. [Google Scholar]
- Villanueva, J.; Blanchard, S.B.; Hamada, Y. The application of a CO2 laser in implant site development: A case report. J. Dent. Maxillofac. Res. 2019, 2, 1–5. [Google Scholar]
- Araùjo, M.G.; Silva, J.C.; Mendonca, A.F.; Lindhe, J. Ridge alterations following grafting of fresh extraction sockets in man. A randomized clinical trial. Clin. Oral Implants Res. 2015, 26, 407–412. [Google Scholar] [CrossRef]
- Iasella, J.M.; Greenwell, H.; Miller, R.L.; Hill, M.; Drisko, C.; Bohra, A.A.; Scheetz, J.P. Ridge preservation with freeze-dried bone allograft and a collagen membrane compared to extraction alone for implant site development: A clinical and histologic study in humans. J. Periodontol. 2003, 74, 990–999. [Google Scholar] [CrossRef]
- Avila-Ortiz, G.; Elangovan, S.; Kramer, K.W.; Blanchette, D.; Dawson, D.V. Effect of alveolar ridge preservation after tooth extraction: A systematic review and meta-analysis. J. Dent. Res. 2014, 93, 950–958. [Google Scholar] [CrossRef]
- Chan, H.L.; Lin, G.H.; Fu, J.H.; Wang, H.L. Alterations in bone quality after socket preservation with grafting material: A systematic review. Int. J. Oral Maxillofac. Implants 2013, 28, 710–720. [Google Scholar] [CrossRef] [Green Version]
- Esposito, M.; Grusovin, M.G.; Felice, P.; Karatzopoulos, G.; Worthington, H.V.; Coulthard, P. The efficacy of horizontal and vertical bone augmentation procedures for dental implants—A Cochrane systematic review. Eur. J. Oral Implantol. 2009, 2, 167–184. [Google Scholar] [PubMed]
- Khojasteh, A.; Soheilifar, S.; Mohajerani, H.; Nowzari, H. The effectiveness of barrier membranes on bone regeneration in localized bony defects: A systematic review. Int. J. Oral Maxillofac. Implants 2013, 28, 1076–1089. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.T.; Buser, D. Esthetic outcomes following immediate and early implant placement in the anterior maxilla—A systematic review. Int. J. Oral Maxillofac. Implants 2014, 29, 186–215. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, N.; Gopalakrishna, V.; Shetty, A.; Nagraj, V.; Imran, M.; Kumar, P. Efficacy of PRF vs. PRF + biodegradable collagen plug in post-extraction preservation of Socket. J. Contemp. Dent. Pract. 2019, 20, 1323–1328. [Google Scholar] [PubMed]
- George, J.; Kuboki, Y.; Miyata, T. Differentiation of mesenchymal stem cells into osteoblasts on honeycomb collagen scaffolds. Biotechnol. Bioeng. 2006, 95, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Hisanaga, Y.; Suzuki, E.; Aoki, H.; Sato, M.; Saito, A.; Saito, A.; Azuma, T. Effect of the combined use of enamel matrix derivative and atelocollagen sponge scaffold on osteoblastic differentiation of mouse induced pluripotent stem cells in vitro. J. Periodontal Res. 2018, 53, 240–249. [Google Scholar] [CrossRef]
- Mendes, R.M.; Silva, G.A.; Lima, M.F.; Silva, G.A.; Lima, F.M.; Calliari, M.V.; Almeida, A.P.; Alves, J.B.; Ferreira, A.J. Sodium hyaluronate accelerates the healing process in tooth sockets of rats. Arch. Oral Biol. 2008, 53, 1155–1162. [Google Scholar] [CrossRef]
- Huebsch, R.F.; Hansen, L.S. A histopathologic study of extraction wounds in dogs. Oral Surg. Oral Med. Oral Pathol. 1969, 28, 187–196. [Google Scholar] [CrossRef]
- Tang, X.M.; Chai, B.P. Effect of CO2 laser irradiation on experimental fracture healing: A transmission electron microscopic study. Lasers Surg. Med. 1986, 6, 346–352. [Google Scholar] [CrossRef]
- Tsai, C.L.; Huang, L.L.; Kao, M.C. Effect of CO2 laser on healing of cultured meniscus. Lasers Surg. Med. 1997, 20, 172–178. [Google Scholar] [CrossRef]
- Korany, N.S.; Mehanni, S.S.; Hakam, H.M.; El-Maghraby, E.M. Evaluation of socket healing in irradiated rats after diode laser exposure (histological and morphometric studies). Arch. Oral Biol. 2012, 57, 884–891. [Google Scholar] [CrossRef]
- Hamad, S.A.; Naif, J.S.; Abdullah, M.A. Effect of diode laser on healing of tooth extraction socket: An experimental study in rabbits. J. Maxillofac. Oral Surg. 2016, 15, 308–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noda, M.; Aoki, A.; Mizutani, K.; Lin, T.; Komaki, M.; Shibata, S.; Izumi, Y. High-frequency pulsed low-level diode laser therapy accelerates wound healing of tooth extraction socket: An in vivo study. Lasers Surg. Med. 2016, 48, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Mergoni, G.; Vescovi, P.; Sala, R.; Merigo, E.; Passerini, P.; Maestri, R.; Corradi, D.; Govoni, P.; Nammour, S.; Bianchi, M.G. The effect of laser therapy on the expression of osteocalcin and osteopontin after tooth extraction in rats treated with zoledronate and dexamethasone. Support Care Cancer 2016, 24, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Park, J.J.; Kang, K.L. Effect of 980-nm GaAlAs diode laser irradiation on healing of extraction sockets in streptozotocin-induced diabetic rats: A pilot study. Lasers Med. Sci. 2012, 27, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Stein, E.; Koehn, J.; Sutter, W.; Wendtlandt, G.; Wanschitz, F.; Thurnher, D.; Baghestanian, M.; Turhani, D. Initial effects of low-level laser therapy on growth and differentiation of human osteoblast-like cells. Wien. Klin. Wochenschr. 2008, 120, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Saracino, S.; Mozzati, M.; Martinasso, G.; Pol, R.; Canuto, R.A.; Muzio, G. Superpulsed laser irradiation increases osteoblast activity via modulation of bone morphogenetic factors. Lasers Surg. Med. 2009, 41, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Bouvet-Gerbettaz, S.; Merigo, E.; Rocca, J.P.; Carle, G.F.; Rochet, N. Effects of low-level laser therapy on proliferation and differentiation of murine bone marrow cells into osteoblasts and osteoclasts. Lasers Surg. Med. 2009, 41, 291–297. [Google Scholar] [CrossRef]
- Shirazi, M.; Ahmad Akhoundi, M.S.; Javadi, E.; Kamali, A.; Motahhari, P.; Rashidpour, M.; Chiniforush, N. The effects of diode laser (660 nm) on the rate of tooth movements: An animal study. Lasers Med. Sci. 2015, 30, 713–718. [Google Scholar] [CrossRef]
- Hirata, S.; Kitamura, C.; Fukushima, H.; Nakamichi, I.; Abiko, Y.; Terashita, M.; Jimi, E. Low-level laser irradiation enhances BMP-induced osteoblast differentiation by stimulating the BMP/Smad signaling pathway. J. Cell. Biochem. 2010, 111, 1445–1452. [Google Scholar] [CrossRef]
- Ozawa, Y.; Shimizu, N.; Kariya, G.; Abiko, Y. Low-energy laser irradiation stimulates bone nodule formation at early stages of cell culture in rat calvarial cells. Bone 1998, 22, 347–354. [Google Scholar] [CrossRef]
- Naka, T.; Yokose, S. Application of laser-induced bone therapy by carbon dioxide laser irradiation in implant therapy. Int. J. Dent. 2012, 2012, 409496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagata, M.J.; Santinoni, C.S.; Pola, N.M.; de Campos, N.; Messora, M.R.; Bomfim, S.R.; Ervolino, E.; Fucini, S.E.; Faleiros, P.L.; Garcia, V.G.; et al. Bone marrow aspirate combined with low-level laser therapy: A new therapeutic approach to enhance bone healing. J. Photochem. Photobiol. B 2013, 121, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Bayat, M.; Jalalifirouzkouhi, A. Presenting a method to improve bone quality through stimulation of osteoporotic mesenchymal stem cells by low-level laser therapy. Photomed. Laser Surg. 2017, 35, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Fekrazad, R.; Eslaminejad, M.B.; Shayan, A.M.; Kalhori, K.A.; Abbas, F.M.; Taghiyar, L.; Sepehr Pedram, M.; Ghuchani, M.S. Effects of photobiomodulation and mesenchymal stem cells on articular cartilage defects in a rabbit model. Photomed. Laser Surg. 2016, 34, 543–549. [Google Scholar] [CrossRef]
- Yang, C.C.; Wang, J.; Chen, S.C.; Hsieh, Y.L. Synergistic effects of low-level laser and mesenchymal stem cells on functional recovery in rats with crushed sciatic nerves. J. Tissue Eng. Regen. Med. 2016, 10, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Oron, A.; Oron, U. Low-level laser therapy to the bone marrow ameliorates neurodegenerative disease progression in a mouse model of Alzheimer’s disease: A minireview. Photomed. Laser Surg. 2016, 34, 627–630. [Google Scholar] [CrossRef]
- Farfara, D.; Tuby, H.; Trudler, D.; Doron-Mandel, E.; Maltz, L.; Vassar, R.J.; Frenkel, D.; Oron, U. Low-level laser therapy ameliorates disease progression in a mouse model of Alzheimer’s disease. J. Mol. Neurosci. 2015, 55, 430–436. [Google Scholar] [CrossRef]
- Tuby, H.; Maltz, L.; Oron, U. Implantation of low-level laser irradiated mesenchymal stem cells into the infarcted rat heart is associated with reduction in infarct size and enhanced angiogenesis. Photomed. Laser Surg. 2009, 27, 227–233. [Google Scholar] [CrossRef]
- Rubio, D.; Garcia-Castro, J.; Martín, M.C.; de la Fuente, R.; Cigudosa, J.C.; Lloyd, A.C.; Bernad, A. Spontaneous human adult stem cell transformation. Cancer Res. 2005, 65, 3035–3039. [Google Scholar] [CrossRef] [Green Version]
- Farivar, S.; Malekshahabi, T.; Shiari, R. Biological effects of low level laser therapy. J. Lasers Med. Sci. 2014, 5, 58–62. [Google Scholar]
- de Freitas, L.F.; Hamblin, M.R. Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 7000417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avci, P.; Gupta, A.; Sadasivam, M.; Vecchio, D.; Pam, Z.; Pam, N.; Hamblin, M.R. Low-level laser (light) therapy (LLLT) in skin: Stimulating, healing, restoring. Semin. Cutan. Med. Surg. 2013, 32, 41–52. [Google Scholar] [PubMed]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gronthos, S.; Brahim, J.; Li, W.; Fisher, L.W.; Cherman, N.; Boyde, A.; DenBesten, P.; Robey, P.G.; Shi, S. Stem cell properties of human dental pulp stem cells. J. Dent. Res. 2002, 81, 531–535. [Google Scholar] [CrossRef]
- Hilkens, P.; Gervois, P.; Fanton, Y.; Vanormelingen, J.; Martens, W.; Struys, T.; Politis, C.; Lambrichts, I.; Bronckaers, A. Effect of isolation methodology on stem cell properties and multilineage differentiation potential of human dental pulp stem cells. Cell Tissue Res. 2013, 353, 65–78. [Google Scholar] [CrossRef]
- Pierdomenico, L.; Bonsi, L.; Calvitti, M.; Rondelli, D.; Arpinati, M.; Chirumbolo, G.; Becchetti, E.; Marchionni, C.; Alviano, F.; Fossati, V.; et al. Multipotent mesenchymal stem cells with immunosuppressive activity can be easily isolated from dental pulp. Transplantation 2005, 80, 836–842. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Walboomers, X.F.; Shi, S.; Fan, M.; Jansen, J.A. Multilineage differentiation potential of stem cells derived from human dental pulp after cryopreservation. Tissue Eng. 2006, 12, 2813–2823. [Google Scholar] [CrossRef]
- Kerkis, I.; Caplan, A.I. Stem cells in dental pulp of deciduous teeth. Tissue Eng. Part B Rev. 2012, 18, 129–138. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, Z.; Xie, Y.; Hu, J.; Wang, H.; Fan, Z.; Zhang, C.; Wang, J.; Wu, C.T.; Wang, S. Adenovirus-mediated transfer of hepatocyte growth factor gene to human dental pulp stem cells under good manufacturing practice improves their potential for periodontal regeneration in swine. Stem Cell Res. Ther. 2015, 6, 249. [Google Scholar] [CrossRef] [Green Version]
- Chamieh, F.; Collignon, A.M.; Coyac, B.R.; Lesieur, J.; Ribes, S.; Sadoine, J.; Llorens, A.; Nicoletti, A.; Letourneur, D.; Colombier, M.L.; et al. Accelerated craniofacial bone regeneration through dense collagen gel scaffolds seeded with dental pulp stem cells. Sci. Rep. 2016, 6, 38814. [Google Scholar] [CrossRef] [Green Version]
- Mata, M.; Milian, L.; Oliver, M.; Zurriaga, J.; Sancho-Tello, M.; de Llano, J.J.M.; Carda, C. In vivo articular cartilage regeneration using human dental pulp stem cells cultured in an alginate scaffold: A preliminary study. Stem Cells Int. 2017, 2017, 8309256. [Google Scholar] [CrossRef] [Green Version]
- Yanasse, R.H.; De Lábio, R.W.; Marques, L.; Fukasawa, J.T.; Segato, R.; Kinoshita, A.; Matsumoto, M.A.; Felisbino, S.L.; Solano, B.; Dos Santos, R.R.; et al. Xenotransplantation of human dental pulp stem cells in platelet-rich plasma for the treatment of full-thickness articular cartilage defects in a rabbit model. Exp. Ther. Med. 2019, 17, 4344–4356. [Google Scholar] [CrossRef] [Green Version]
- Sakai, K.; Yamamoto, A.; Matsubara, K.; Nakamura, S.; Naruse, M.; Yamagata, M.; Sakamoto, K.; Tauchi, R.; Wakao, N.; Imagama, S.; et al. Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. J. Clin. Investig. 2012, 122, 80–90. [Google Scholar] [CrossRef]
- de Eduardo, F.P.; Bueno, D.F.; de Freitas, P.M.; Marques, M.M.; Passos-Bueno, M.R.; de Eduardo, C.P.; Zatz, M. Stem cell proliferation under low intensity laser irradiation: A preliminary study. Lasers Surg. Med. 2008, 40, 433–438. [Google Scholar] [CrossRef]
- Holder, M.J.; Milward, M.R.; Palin, W.M.; Hadis, M.A.; Cooper, P.R. Effects of red light-emitting diode irradiation on dental pulp cells. J. Dent. Res. 2012, 91, 961–966. [Google Scholar] [CrossRef]
- Zaccara, I.M.; Ginani, F.; Mota-Filho, H.G.; Henriques, Á.C.; Barboza, C.A. Effect of low-level laser irradiation on proliferation and viability of human dental pulp stem cells. Lasers Med. Sci. 2015, 30, 2259–2264. [Google Scholar] [CrossRef]
- Ferreira, L.S.; Diniz, I.M.A.; Maranduba, C.M.S.; Miyagi, S.P.H.; Rodrigues, M.F.S.D.; Moura-Netto, C.; Marques, M.M. Short-term evaluation of photobiomodulation therapy on the proliferation and undifferentiated status of dental pulp stem cells. Lasers Med. Sci. 2019, 34, 659–666. [Google Scholar] [CrossRef]
- Marques, M.M.; Diniz, I.M.; de Cara, S.P.; Pedroni, A.C.; Abe, G.L.; D’Almeida-Couto, R.S.; Lima, P.L.; Tedesco, T.K.; Moreira, M.S. Photobiomodulation of dental derived mesenchymal stem cells: A systematic review. Photomed. Laser Surg. 2016, 34, 500–508. [Google Scholar] [CrossRef]
- Borzabadi-Farahani, A. Effect of low-level laser irradiation on proliferation of human dental mesenchymal stem cells; a systemic review. J. Photochem. Photobiol. B 2016, 162, 577–582. [Google Scholar] [CrossRef]
- Matsui, S.; Tsujimoto, Y.; Matsushima, K. Stimulatory effects of hydroxyl radical generation by Ga-Al-As laser irradiation on mineralization ability of human dental pulp cells. Biol. Pharm. Bull. 2007, 30, 27–31. [Google Scholar] [CrossRef] [Green Version]
- Matsui, S.; Takeuchi, H.; Tsujimoto, Y.; Matsushima, K. Effects of Smads and BMPs induced by Ga-Al-As laser irradiation on calcification ability of human dental pulp cells. J. Oral Sci. 2008, 50, 75–81. [Google Scholar] [CrossRef] [Green Version]
- Ohbayashi, E.; Matsushima, K.; Hosoya, S.; Abiko, Y.; Yamazaki, M. Stimulatory effect of laser irradiation on calcified nodule formation in human dental pulp fibroblasts. J. Endod. 1999, 25, 30–33. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daigo, Y.; Daigo, E.; Fukuoka, H.; Fukuoka, N.; Ishikawa, M.; Takahashi, K. Wound Healing and Cell Dynamics Including Mesenchymal and Dental Pulp Stem Cells Induced by Photobiomodulation Therapy: An Example of Socket-Preserving Effects after Tooth Extraction in Rats and a Literature Review. Int. J. Mol. Sci. 2020, 21, 6850. https://doi.org/10.3390/ijms21186850
Daigo Y, Daigo E, Fukuoka H, Fukuoka N, Ishikawa M, Takahashi K. Wound Healing and Cell Dynamics Including Mesenchymal and Dental Pulp Stem Cells Induced by Photobiomodulation Therapy: An Example of Socket-Preserving Effects after Tooth Extraction in Rats and a Literature Review. International Journal of Molecular Sciences. 2020; 21(18):6850. https://doi.org/10.3390/ijms21186850
Chicago/Turabian StyleDaigo, Yuki, Erina Daigo, Hiroshi Fukuoka, Nobuko Fukuoka, Masatsugu Ishikawa, and Kazuya Takahashi. 2020. "Wound Healing and Cell Dynamics Including Mesenchymal and Dental Pulp Stem Cells Induced by Photobiomodulation Therapy: An Example of Socket-Preserving Effects after Tooth Extraction in Rats and a Literature Review" International Journal of Molecular Sciences 21, no. 18: 6850. https://doi.org/10.3390/ijms21186850




