Noncovalent Complexes of Cyclodextrin with Small Organic Molecules: Applications and Insights into Host–Guest Interactions in the Gas Phase and Condensed Phase
Abstract
:1. Introduction
2. Applications of CD Inclusion Complexes in the Solid/Solution Phases
2.1. Pharmaceuticals
2.2. Cosmetics
2.3. Foods
2.4. Textiles
2.5. Separation Science
2.6. Essential Oils
3. Structures of CDs and Their Derivatives in the Solution- and Solid-Phases
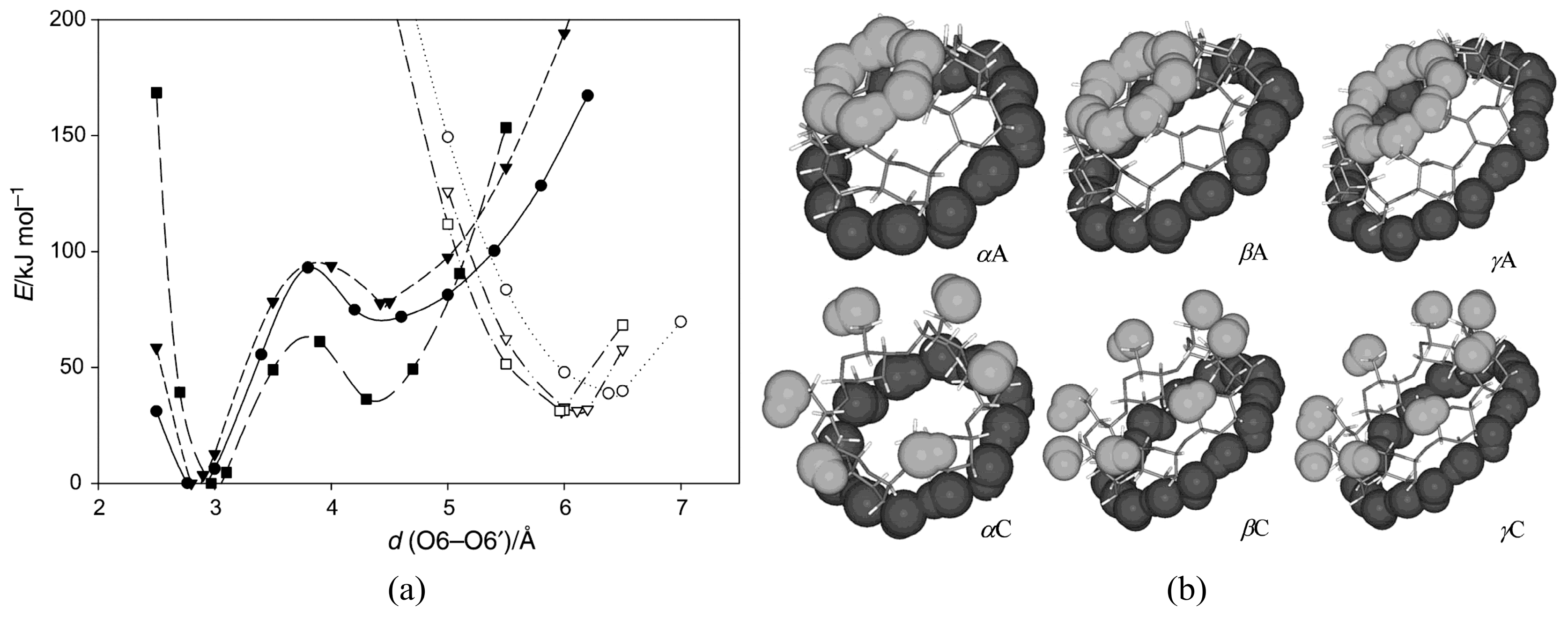
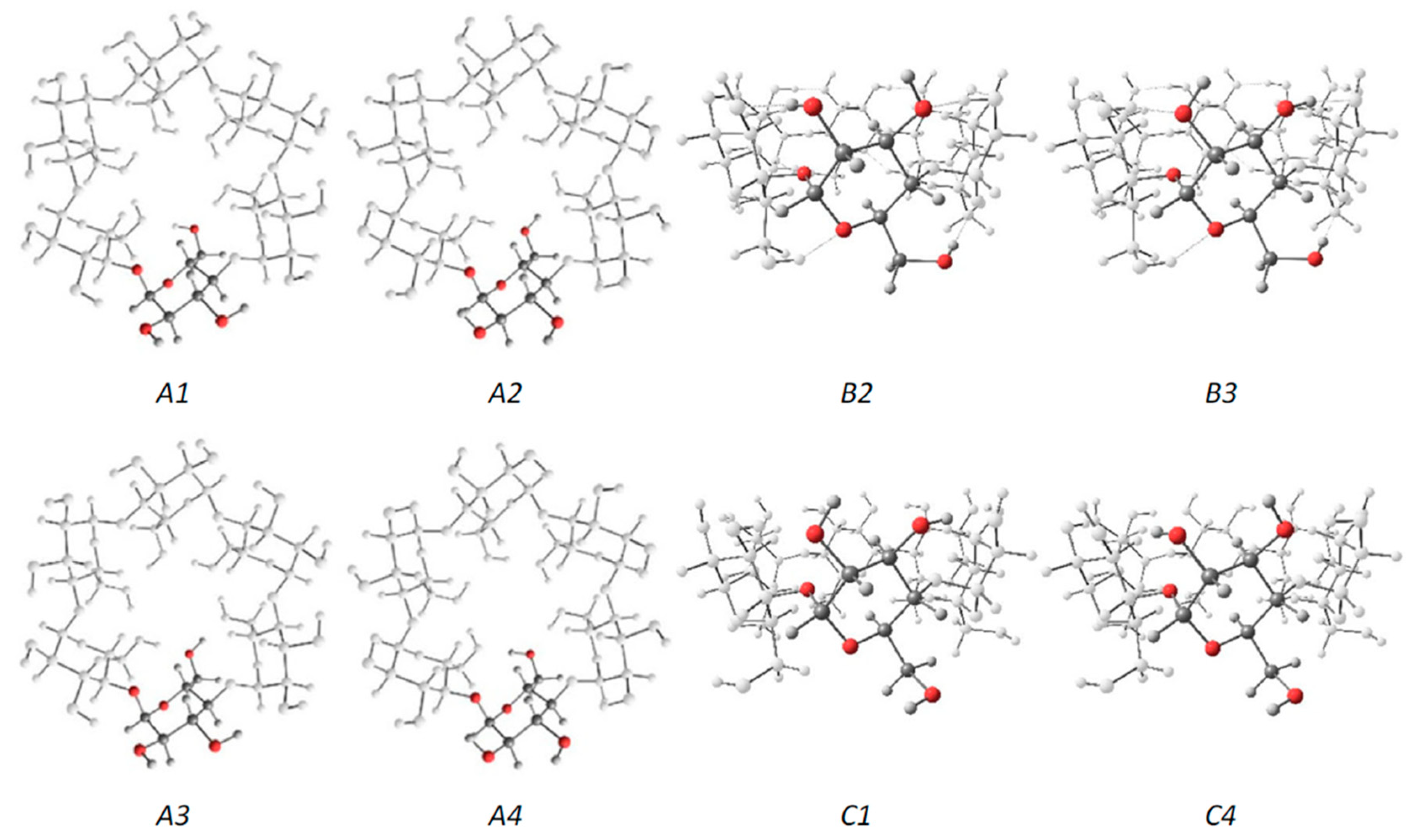
3.1. Symmetrical CDs
3.2. Permethylated β-CDs
4. Noncovalent CD Complexes in the Gas Phase
4.1. Mass Spectrometry
4.2. Ion-Mobility Spectrometry
4.2.1. Confirmation of the Formation of CD–Guest Complexes and Their Associated Conformers
4.2.2. Elucidating the Structures of CD–Guest Complexes
4.2.3. Chiral Differentiation
4.3. IRMPD Spectroscopy
5. Conclusions
Funding
Conflicts of Interest
References
- Del Valle, E.M.M. Cyclodextrins and their uses: A review. Process. Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Dodziuk, H. Molecules with Holes−Cyclodextrins. In Cyclodextrins and Their Complexes: Chemistry, Analytical Methods, Applications; Dodziuk, H., Ed.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006; pp. 1–30. ISBN 3527312803. [Google Scholar]
- Crini, G.; Fourmentinn, S.; Lichtfouse, E. Cyclodextrin Fundamentals, Reactivity and Analysis; Springer International Publishing AG: Cham, Switzerland, 2018; ISBN 9783319761589. [Google Scholar]
- Villiers, A. Sur la transformation de la fécule en dextrine par le ferment butyrique. Compt. Rendu. 1891, 112, 536. [Google Scholar]
- Armstrong, D.W.; DeMond, W.; Czech, B.P. Separation of metallocene enantiomers by liquid chromatography: Chiral recognition via cyclodextrin bonded phases. Anal. Chem. 1985, 57, 481–484. [Google Scholar] [CrossRef]
- Li, S.; Purdy, W.C. Cyclodextrins and their applications in analytical chemistry. Chem. Rev. 1992, 92, 1457–1470. [Google Scholar] [CrossRef]
- Szente, L.; Szejtli, J. Non-chromatographic analytical uses of cyclodextrins. Analyst 1998, 123, 735–741. [Google Scholar] [CrossRef]
- Moon, J.Y.; Jung, H.J.; Moon, M.H.; Chung, B.C.; Choi, M.H. Inclusion complex-based solid-phase extraction of steroidal compounds with entrapped β-cyclodextrin polymer. Steroids 2008, 73, 1090–1097. [Google Scholar] [CrossRef]
- Liu, H.; Liu, C.; Yang, X.; Zeng, S.; Xiong, Y.; Xu, W. Uniformly sized β-cyclodextrin molecularly imprinted microspheres prepared by a novel surface imprinting technique for ursolic acid. Anal. Chim. Acta 2008, 628, 87–94. [Google Scholar] [CrossRef]
- Jin, H.L.; Stalcup, A.; Armstrong, D.W. Optical enrichment of dansyl-rac-amino acids by formation of crystalline inclusion complexes with cyclodextrins. Chirality 1989, 1, 137–141. [Google Scholar] [CrossRef]
- Armstrong, D.W.; Ward, T.J.; Armstrong, R.D.; Beesley, T.E. Separation of drug stereoisomers by the formation of β-cyclodextrin inclusion complexes. Science 1986, 232, 1132–1135. [Google Scholar] [CrossRef]
- Smolková-Keulemansová, E. Cyclodextrins as stationary phases in chromatography. J. Chromatogr. A 1982, 251, 17–34. [Google Scholar] [CrossRef]
- Schneiderman, E.; Stalcup, A.M. Cyclodextrins: A versatile tool in separation science. J. Chromatogr. B 2000, 745, 83–102. [Google Scholar] [CrossRef]
- Armstrong, D.W.; Chang, S.C.; Stalcup, A.M.; Hilton, M.L.; Duncan, J.D.; Faulkner, J.R. Derivatized Cyclodextrins for Normal-Phase Liquid Chromatographic Separation of Enantiorners. Anal. Chem. 1990, 62, 1610–1615. [Google Scholar] [CrossRef]
- Bressolle, F.; Audran, M.; Pham, T.-N.; Vallon, J.-J. Cyclodextrins and enatiomeric separations of drugs by liquid chromatography and capillary electrophoresis: Basic priciples and new developments. J. Chromatogr. B 1996, 687, 303–336. [Google Scholar] [CrossRef]
- Cucci, C.; Mignani, A.G.; Dall’Asta, C.; Pela, R.; Dossena, A. A portable fluorometer for the rapid screening of M1 aflatoxin. Sens. Actuators B Chem. 2007, 126, 467–472. [Google Scholar] [CrossRef]
- Cozzini, P.; Ingletto, G.; Singh, R.; Dall’Asta, C. Mycotoxin detection plays “cops and robbers”: Cyclodextrin chemosensors as specialized police? Int. J. Mol. Sci. 2008, 9, 2474–2494. [Google Scholar] [CrossRef]
- Ortega-Caballero, F.; Rousseau, C.; Christensen, B.; Petersen, T.E.; Bols, M. Remarkable supramolecular catalysis of glycoside hydrolysis by a cyclodextrin cyanohydrin. J. Am. Chem. Soc. 2005, 127, 3238–3239. [Google Scholar] [CrossRef]
- Ortega-Caballero, F.; Bjerre, J.; Laustsen, L.S.; Bols, M. Four orders of magnitude rate increase in artificial enzyme-catalyzed aryl glycoside hydrolysis. J. Org. Chem. 2005, 70, 7217–7226. [Google Scholar] [CrossRef]
- Szente, L.; Szemán, J. Cyclodextrins in analytical chemistry: Host–guest type molecular recognition. Anal. Chem. 2013, 85, 8024–8030. [Google Scholar] [CrossRef]
- Ueno, A.; Suzuki, I.; Osa, T. Host–guest Sensory Systems for Detecting Organic Compounds by Pyrene Excimer Fluorescence. Anal. Chem. 1990, 62, 2461–2466. [Google Scholar] [CrossRef]
- Gu, L.-Q.; Braha, O.; Conlan, S.; Cheley, S.; Bayley, H. Stochastic Sensing of Organic Analytes by a Pore-Forming Protein Containing a Molecular Adapter. Nature 1999, 398, 686–690. [Google Scholar] [CrossRef]
- Wu, H.C.; Astier, Y.; Maglia, G.; Mikhailova, E.; Bayley, H. Protein nanopores with covalently attached molecular adapters. J. Am. Chem. Soc. 2007, 129, 16142–16148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kano, K. Mechanisms for chiral recognition by cyclodextrins. J. Phys. Org. Chem. 1997, 10, 286–291. [Google Scholar] [CrossRef]
- Liu, L.; Guo, Q.X. The driving forces in the inclusion complexation of cyclodextrins. J. Incl. Phenom. 2002, 42, 1–14. [Google Scholar] [CrossRef]
- Chen, Y.; Zuo, Z.; Dai, X.; Xiao, P.; Fang, X.; Wang, X.; Wang, W.; Ding, C.F. Gas-phase complexation of α-/β-cyclodextrin with amino acids studied by ion mobility-mass spectrometry and molecular dynamics simulations. Talanta 2018, 186, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Schalley, C.A. Molecular recognition and supramolecular chemistry in the gas phase. Mass Spectrom. Rev. 2001, 20, 253–309. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M.J.; Friess, D.S.; Rajagopalan, S.; Wendt, S.; Zenobi, R. Quantitative determination of noncovalent binding interactions using soft ionization mass spectrometry. Int. J. Mass Spectrom. 2002, 216, 1–27. [Google Scholar] [CrossRef]
- Przybylski, M.; Glocker, M.O. Electrospray Mass Spectrometry of Biomacromolecular Complexes with Noncovalent Interactions-New Analytical Perspectives for Supramolecular Chemistry and Molecular Recognition Processe. Angew. Chem. Int. Ed. Engl. 1996, 35, 806–826. [Google Scholar] [CrossRef]
- Ariga, K.; Kunitake, T. The Chemistry of Molecular Recognition—Host Molecules and Guest Molecules. In Supramolecular Chemistry—Fundamentals and Applications; Springer: Berlin, Germany, 2006. [Google Scholar] [CrossRef]
- Shahgaldian, P.; Pieles, U. Cyclodextrin derivatives as chiral supramolecular receptors for enantioselective sensing. Sensors 2006, 6, 593–615. [Google Scholar] [CrossRef] [Green Version]
- Fischer, E. Einfluss der Configuration auf die Wirkung der Enzyme Influence of configuration on the action of enzymes. Ber. Dtsch. Chem. Ges. 1894, 27, 2985–2993. [Google Scholar] [CrossRef] [Green Version]
- Easton, C.J.; Lincoln, S.F. Chiral discrimination by modified cyclodextrins. Chem. Soc. Rev. 1996, 25, 163–170. [Google Scholar] [CrossRef]
- Martin, J.; Díaz-Montaña, J.E.; Asuero, G.A. Cyclodextrins: Past and Present. In Cyclodextrin: A Versatile Ingredient; Arora, P., Dhingra, N., Eds.; IntechOpen: London, UK, 2018; pp. 1–43. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Wu, C.; Ma, Y.; Zhou, S.; Li, Z.; Sun, T. Effectively enhancing the enantioseparation ability of β-cyclodextrin derivatives by de novo design and molecular modeling. Analyst 2017, 142, 3699–3706. [Google Scholar] [CrossRef] [PubMed]
- Scriba, G.K.E. Chiral recognition in separation sciences. Part I: Polysaccharide and cyclodextrin selectors. TrAC Trends Anal. Chem. 2019, 120, 115639. [Google Scholar] [CrossRef]
- Lebrilla, C.B. The gas-phase chemistry of cyclodextrin inclusion complexes. Acc. Chem. Res. 2001, 34, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Chu, Y.; Wang, R.; He, X.; Ding, C. Quantifying non-covalent binding affinity using mass spectrometry: A systematic study on complexes of cyclodextrins with alkali metal cations. Rapid Commun. Mass Spectrom. 2015, 29, 927–936. [Google Scholar] [CrossRef]
- Chen, X.; Chu, Y.; Gu, L.; Zhou, M.; Ding, C.F. The non-covalent complexes of α- or γ-cyclodextrin with divalent metal cations determined by mass spectrometry. Carbohydr. Res. 2020, 492, 107987. [Google Scholar] [CrossRef]
- Wu, Y.; Shi, R.; Wu, Y.L.; Holcroft, J.M.; Liu, Z.; Frasconi, M.; Wasielewski, M.R.; Li, H.; Stoddart, J.F. Complexation of polyoxometalates with cyclodextrins. J. Am. Chem. Soc. 2015, 137, 4111–4118. [Google Scholar] [CrossRef]
- Fan, Y.X.; Lu, S.F.; Cao, J. A novel inorganic-organic hybrid complex between polyoxometalate and cyclodextrin: Synthesis, structure and catalytic activity. Int. J. Mass Spectrom. 2019, 435, 163–167. [Google Scholar] [CrossRef]
- Su, P.; Smith, J.A.; Warneke, J.; Laskin, J. Gas-phase fragmentation of host-huest complexes of cyclodextrins and polyoxometalates. J. Am. Soc. Mass Spectrom. 2019, 30, 1934–1945. [Google Scholar] [CrossRef]
- Jiang, Z.G.; Mao, W.T.; Huang, D.P.; Wang, Y.; Wang, X.J.; Zhan, C.H. A nonconventional host–guest cubic assembly based on γ-cyclodextrin and a Keggin-type polyoxometalate. Nanoscale 2020, 12, 10166–10171. [Google Scholar] [CrossRef]
- Assaf, K.I.; Ural, M.S.; Pan, F.; Georgiev, T.; Simova, S.; Rissanen, K.; Gabel, D.; Nau, W.M. Water structure recovery in chaotropic anion recognition: High-affinity binding of dodecaborate clusters to γ-cyclodextrin. Angew. Chem. Int. Ed. 2015, 54, 6852–6856. [Google Scholar] [CrossRef] [Green Version]
- Assaf, K.I.; Suckova, O.; Al Danaf, N.; Von Glasenapp, V.; Gabel, D.; Nau, W.M. Dodecaborate-Functionalized Anchor Dyes for Cyclodextrin-Based Indicator Displacement Applications. Org. Lett. 2016, 18, 932–935. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jiang, Y.; Yuan, Q.; Warneke, J.; Hu, Z.; Yang, Y.; Sun, H.; Sun, Z.; Wang, X. Bin Photoelectron spectroscopy and computational investigations of the electronic structures and noncovalent interactions of cyclodextrin- Closo -dodecaborate anion complexes χ-CD·B12X122- (χ = α, β, γ; F). Phys. Chem. Chem. Phys. 2020, 22, 7193–7200. [Google Scholar] [CrossRef] [PubMed]
- Abramov, P.A.; Ivanov, A.A.; Shestopalov, M.A.; Moussawi, M.A.; Cadot, E.; Floquet, S.; Haouas, M.; Sokolov, M.N. Supramolecular Adduct of γ-Cyclodextrin and [{Re6Q8}(H2O)6]2+ (Q=S, Se). J. Clust. Sci. 2018, 29, 9–13. [Google Scholar] [CrossRef] [Green Version]
- Ivanov, A.A.; Falaise, C.; Laouer, K.; Hache, F.; Changenet, P.; Mironov, Y.V.; Landy, D.; Molard, Y.; Cordier, S.; Shestopalov, M.A.; et al. Size-Exclusion Mechanism Driving Host–guest Interactions between Octahedral Rhenium Clusters and Cyclodextrins. Inorg. Chem. 2019, 58, 13184–13194. [Google Scholar] [CrossRef] [PubMed]
- Dantz, D.A.; Meschke, C.; Buschmann, H.J.; Schollmeyer, E. Complexation of volatile organic molecules from the gas phase with cucurbituril and β-cyclodextrin. Supramol. Chem. 1998, 9, 79–83. [Google Scholar] [CrossRef]
- Ramirez, J.; Ahn, S.; Grigorean, G.; Lebrilla, C.B. Evidence for the formation of gas-phase inclusion complexes with cyclodextrins and amino acids. J. Am. Chem. Soc. 2000, 122, 6884–6890. [Google Scholar] [CrossRef]
- Ahn, S.; Ramirez, J.; Grigorean, G.; Lebrilla, C.B. Chiral recognition in gas-phase cyclodextrin: Amino acid complexes—Is the three point interaction still valid in the gas phase? J. Am. Soc. Mass Spectrom. 2001, 12, 278–287. [Google Scholar] [CrossRef] [Green Version]
- Ahn, S.; Cong, X.; Lebrilla, C.B.; Gronert, S. Zwitterion formation in gas-phase cyclodextrin complexes. J. Am. Soc. Mass Spectrom. 2005, 16, 166–175. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Ahn, S.; Park, S.; Oh, H.B. Characterization of permethylated β-cyclodextrin-peptide noncovalently bound complexes using electron capture dissociation mass spectrometry (ECD MS). Int. J. Mass Spectrom. 2009, 279, 47–52. [Google Scholar] [CrossRef]
- Li, Z.; Couzijn, E.P.A.; Zhang, X. Intrinsic properties of α-cyclodextrin complexes with benzoate derivatives in the gas phase: An experimental and theoretical study. J. Phys. Chem. B 2012, 116, 943–950. [Google Scholar] [CrossRef]
- Ghatee, M.H.; Sedghamiz, T. Chiral recognition of propranolol enantiomers by β-cyclodextrin: Quantum chemical calculation and molecular dynamics simulation studies. Chem. Phys. 2014, 445, 5–13. [Google Scholar] [CrossRef]
- Lee, S.S.; Park, S.; Kim, J.Y.; Kim, H.R.; Lee, S.; Oh, H.B. Infrared multiple photon dissociation spectroscopy and density functional theory (DFT) studies of protonated permethylated β-cyclodextrin-water non-covalent complexes. Phys. Chem. Chem. Phys. 2014, 16, 8376–8383. [Google Scholar] [CrossRef] [PubMed]
- Ceborska, M.; Zimnicka, M.; Kowalska, A.A.; Dąbrowa, K.; Repeć, B. Structural diversity in the host–guest complexes of the antifolate pemetrexed with native cyclodextrins: Gas phase, solution and solid state studies. Beilstein J. Org. Chem. 2017, 13, 2252–2263. [Google Scholar] [CrossRef] [PubMed]
- Kfoury, M.; Landy, D.; Fourmentin, S. Characterization of cyclodextrin/volatile inclusion complexes: A review. Molecules 2018, 23, 1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
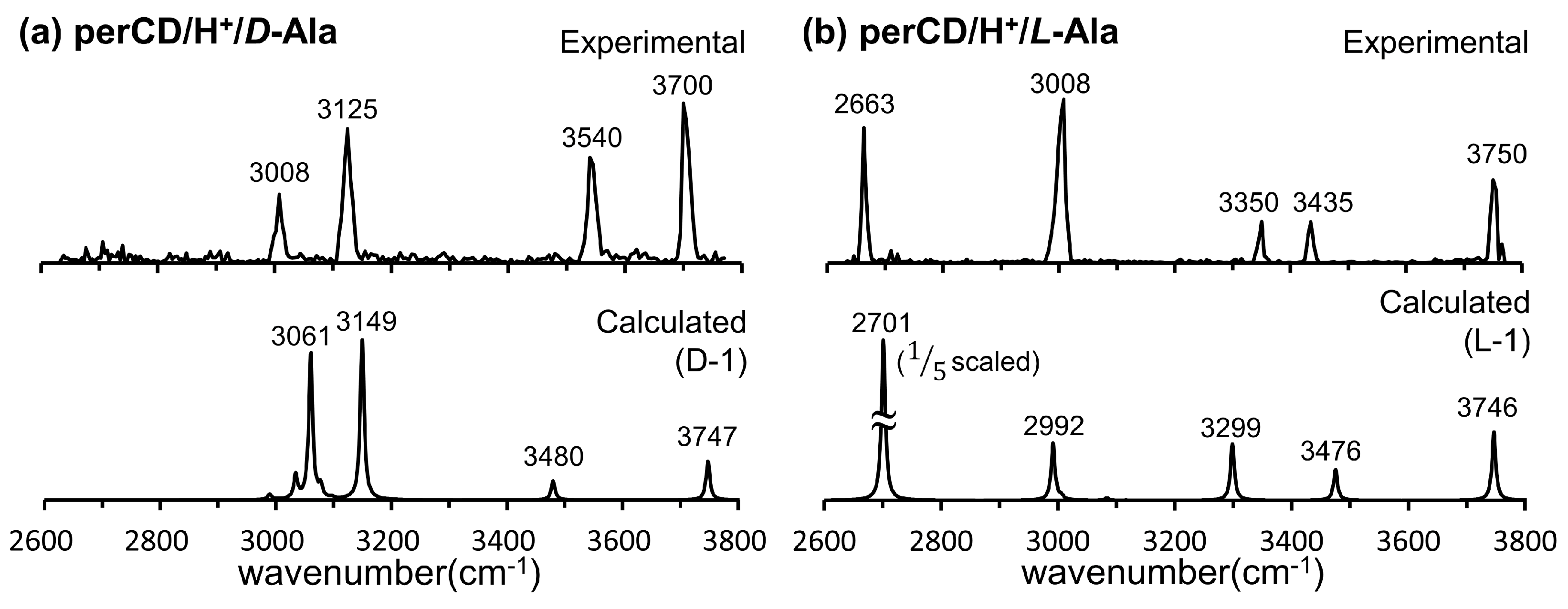
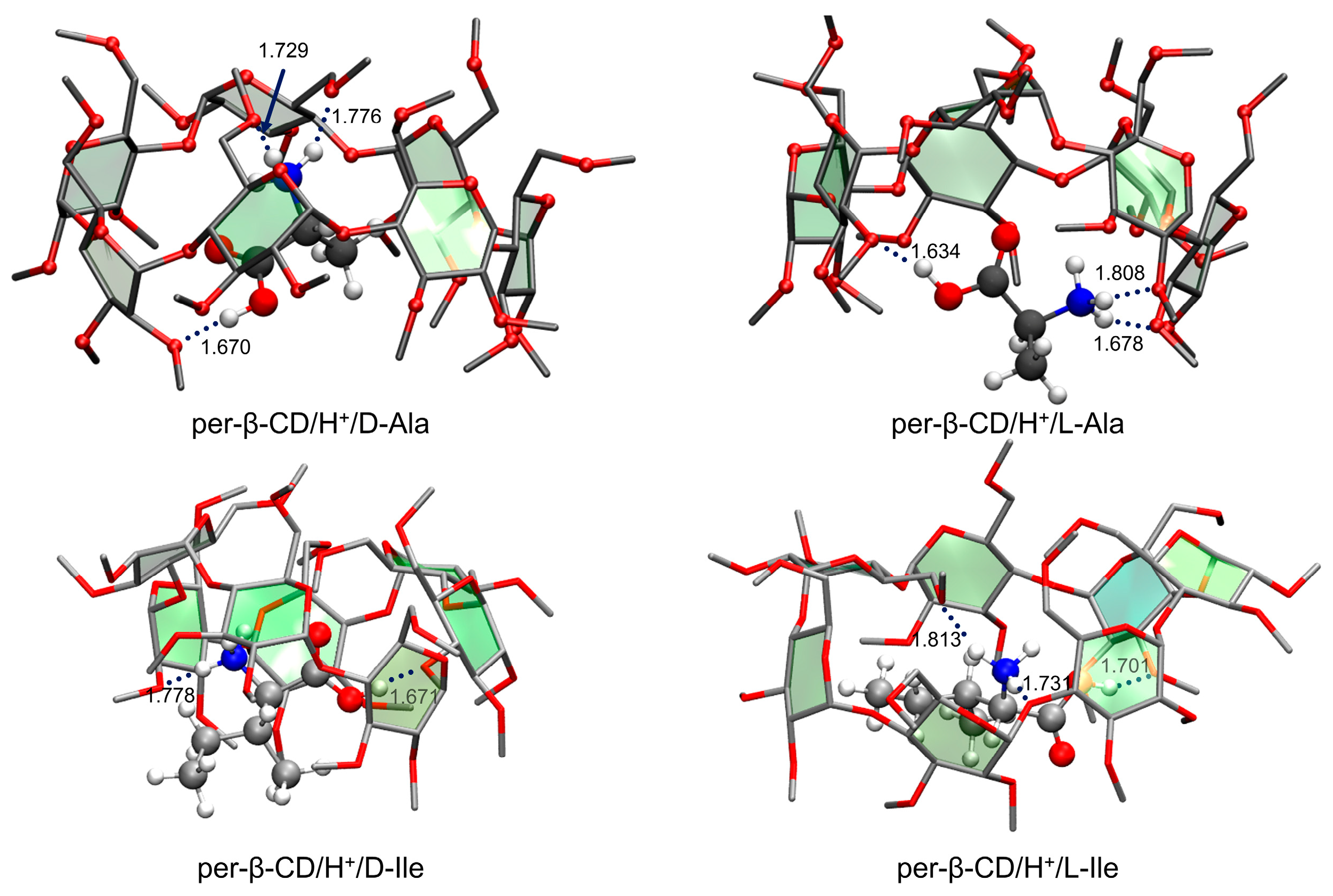
- Lee, S.S.; Park, S.; Hong, Y.; Lee, J.U.; Kim, J.H.; Yoon, D.; Kong, X.; Lee, S.; Oh, H.B. Chiral differentiation of d- and l-alanine by permethylated β-cyclodextrin: IRMPD spectroscopy and DFT methods. Phys. Chem. Chem. Phys. 2017, 19, 14729–14737. [Google Scholar] [CrossRef]
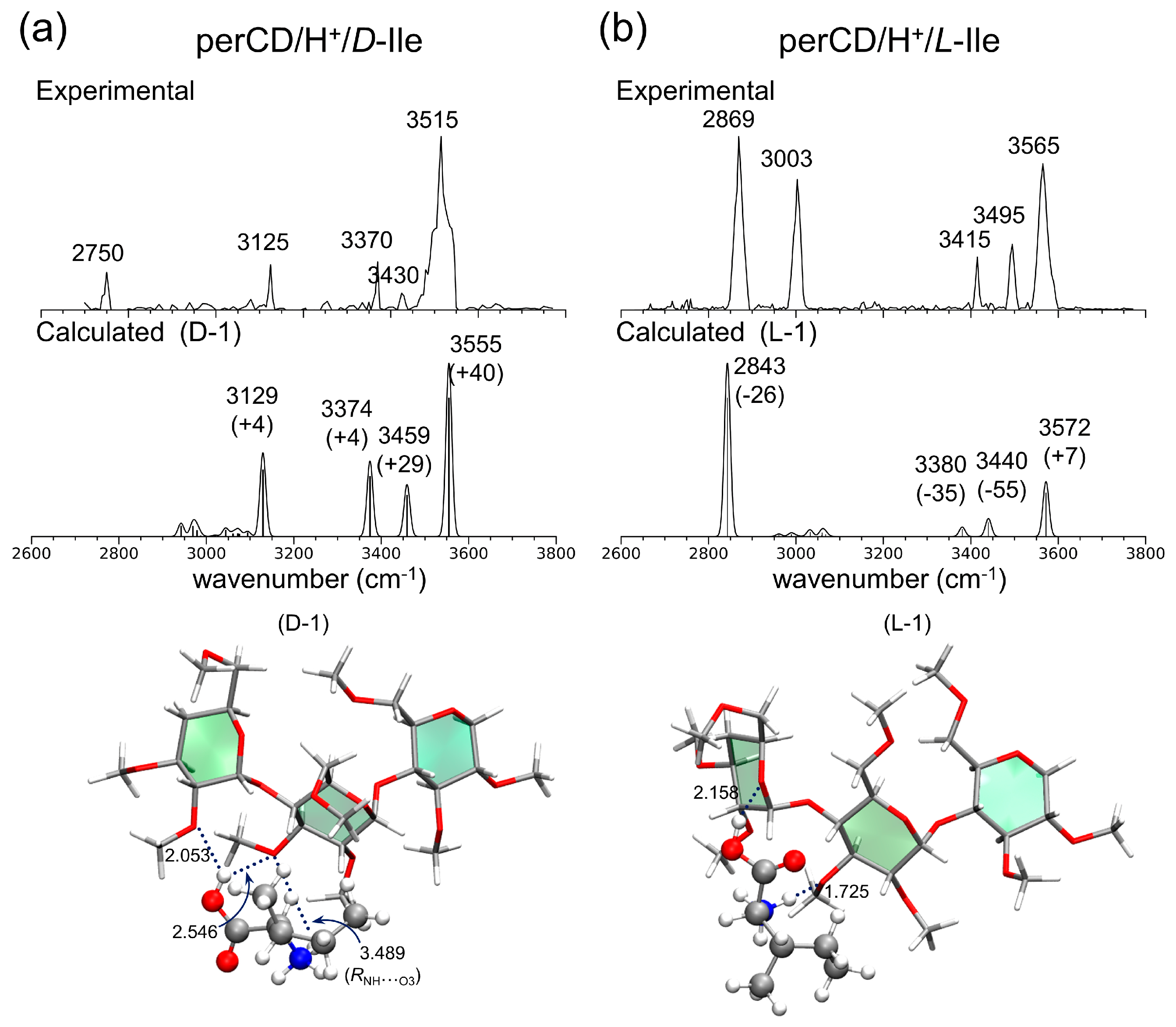
- Lee, S.S.; Lee, J.U.; Oh, J.H.; Park, S.; Hong, Y.; Min, B.K.; Lee, H.H.L.; Kim, H.I.; Kong, X.; Lee, S.; et al. Chiral differentiation of D- and L-isoleucine using permethylated β-cyclodextrin: Infrared multiple photon dissociation spectroscopy, ion-mobility mass spectrometry, and DFT calculations. Phys. Chem. Chem. Phys. 2018, 20, 30428–30436. [Google Scholar] [CrossRef]
- Duez, Q.; Knight, G.; Daly, S.; De Winter, J.; Halin, E.; MacAleese, L.; Antoine, R.; Gerbaux, P.; Dugourd, P. Action-FRET of β-cyclodextrin inclusion complexes. New J. Chem. 2017, 41, 1806–1812. [Google Scholar] [CrossRef]
- Cyclodextrin Research & Development Laboratory Ltd. Cyclolab. Available online: https://cyclolab.hu/ (accessed on 29 May 2020).
- Loftsson, T.; Brewster, M.E. Cyclodextrins as functional excipients: Methods to enhance complexation efficiency. J. Pharm. Sci. 2012, 101, 3019–3032. [Google Scholar] [CrossRef]
- Challa, R.; Ahuja, A.; Ali, J.; Khar, R.K. Cyclodextrins in drug delivery: An updated review. AAPS PharmSciTech 2005, 6, 329–357. [Google Scholar] [CrossRef]
- Loftsson, T.; Duchêne, D. Cyclodextrins and their pharmaceutical applications. Int. J. Pharm. 2007, 329, 1–11. [Google Scholar] [CrossRef]
- Booij, L.H.D.J. Cyclodextrins and the emergence of sugammadex. Anaesthesia 2009, 64, 31–37. [Google Scholar] [CrossRef]
- Yang, L.P.H.; Keam, S.J. Sugammadex. A review of its use in anesthetic practice. Drugs 2009, 69, 919–942. [Google Scholar] [CrossRef] [PubMed]
- Di Cagno, M.P. The potential of cyclodextrins as novel active pharmaceutical ingredients: A short overview. Molecules 2017, 22, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cyclo Therapeutics, Inc. Cyclo Therapeutics, Inc. Available online: https://www.cyclotherapeutics.com/cyclodextrins/trappsol-cyclo (accessed on 29 May 2020).
- Buschmann, H.-J.; Schollmeyer, E. Applications of cyclodextrins in cosmetic products: A review. J. Cosmet. Sci. 2002, 53, 185–191. [Google Scholar] [PubMed]
- Numanoǧlu, U.; Şen, T.; Tarimci, N.; Kartal, M.; Koo, O.M.Y.; Önyüksel, H. Use of cyclodextrins as a cosmetic delivery system for fragrance materials: Linalool and benzyl acetate. AAPS PharmSciTech 2007, 8. [Google Scholar] [CrossRef] [Green Version]
- Sharma, N.; Baldi, A. Exploring versatile applications of cyclodextrins: An overview. Drug Deliv. 2016, 23, 739–757. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, S. Review of the Usability of Cyclodextrin as a Cosmetic Ingredient. Asian J. Beauty Cosmetol. 2019, 17, 545–553. [Google Scholar] [CrossRef] [Green Version]
- Chiari-Andréo, B.G.; De Almeida-Cincotto, M.G.J.; Oshiro, J.A.; Taniguchi, C.Y.Y.; Chiavacci, L.A.; Isaac, V.L.B. Nanoparticles for cosmetic use and its application. In Nanoparticles in Pharmacotherapy; Grumezescu, M.A., Ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; pp. 113–146. ISBN 9780128165041. [Google Scholar]
- Mori, T.; Tsuchiya, R.; Doi, M.; Nagatani, N.; Tanaka, T. Solubilization of ultraviolet absorbers by cyclodextrin and their potential application in cosmetics. J. Incl. Phenom. Macrocycl. Chem. 2019, 93, 91–96. [Google Scholar] [CrossRef]
- Hedges, A.R.; Shieh, W.J.; Sikorski, C.T. Use of cyclodextrins for encapsulation in the use and treatment of food products. In Encapsulation and Controlled Release of Food Ingredients; Risch, S.J., Reineccius, G.A., Eds.; American Chemical Society: Washington, DC, USA, 1995; pp. 60–71. [Google Scholar]
- Szente, L.; Szejtli, J. Cyclodextrins as food ingredients. Trends Food Sci. Technol. 2004, 15, 137–142. [Google Scholar] [CrossRef]
- Cravotto, G.; Binello, A.; Baranelli, E.; Carraro, P.; Trotta, F. Cyclodextrins as food additives and in food processing. Curr. Nutr. Food Sci. 2006, 2, 343–350. [Google Scholar] [CrossRef]
- López-Nicolás, J.M.; Pérez-López, A.J.; Carbonell-Barrachina, Á.; García-Carmona, F. Use of natural and modified cyclodextrins as inhibiting agents of peach juice enzymatic browning. J. Agric. Food Chem. 2007, 55, 5312–5319. [Google Scholar] [CrossRef]
- Ha, H.J.; Ahn, J.; Min, S.G.; Kwak, H.S. Properties of cholesterol-reduced ice cream made with cross-linked β-cyclodextrin. Int. J. Dairy Technol. 2009, 62, 452–457. [Google Scholar] [CrossRef]
- Astray, G.; Gonzalez-Barreiro, C.; Mejuto, J.C.; Rial-Otero, R.; Simal-Gándara, J. A review on the use of cyclodextrins in foods. Food Hydrocoll. 2009, 23, 1631–1640. [Google Scholar] [CrossRef]
- Ananingsih, V.K.; Sharma, A.; Zhou, W. Green tea catechins during food processing and storage: A review on stability and detection. Food Res. Int. 2013, 50, 469–479. [Google Scholar] [CrossRef]
- Kfoury, M.; Hădărugă, N.G.; Hădărugă, D.I.; Fourmentin, S. Cyclodextrins as encapsulation material for flavors and aroma. In Encapsulations; Grumezescu, M.A., Ed.; Elsvier: Amsterdam, The Netherlands, 2016; pp. 127–192. ISBN 9780128043073. [Google Scholar]
- Fenyvesi, É.; Vikmon, M.; Szente, L. Cyclodextrins in food technology and human nutrition: Benefits and limitations. Crit. Rev. Food Sci. Nutr. 2016, 56, 1981–2004. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.M.; Awad, A.C.; Bennink, M.R.; Gill, J.L. Cholesterol reduction in liquid egg yolk using beta-cyclodextrin. J. Food Sci. 1995, 60, 691–694. [Google Scholar] [CrossRef]
- Awad, A.C.; Bennink, M.R.; Smith, D.M. Composition and functional properties of cholesterol reduced egg yolk. Poult. Sci. 1997, 76, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.K.; Ahn, J.; Kwak, H.S. Cholesterol removal from homogenized milk with β-cyclodextrin. J. Dairy Sci. 1999, 82, 2327–2330. [Google Scholar] [CrossRef]
- Raben, A.; Andersen, K.; Karberg, M.A.; Holst, J.J.; Astrup, A. Acetylation of or β-cyclodextrin addition to potato starch: Beneficial effect on glucose metabolism and appetite sensations. Am. J. Clin. Nutr. 1997, 66, 304–314. [Google Scholar] [CrossRef]
- Lai, C.S.; Chow, J.; Wolf, B.W. Methods of Using Gamma Cyclodextrin to Control Blood Glucose and Insulin Secretion. U.S. Patent Appl. US 2005215523, 16 June 2005. [Google Scholar]
- Comerford, K.B.; Artiss, J.D.; Jen, K.L.C.; Karakas, S.E. The beneficial effects α-cyclodextrin on blood lipids and weight loss in healthy humans. Obesity 2011, 19, 1200–1204. [Google Scholar] [CrossRef]
- Singh, N.; Sahu, O. Sustainable cyclodextrin in textile applications. In The Impact and Prospects of Green Chemistry for Textile Technology; ul-Islam, S., Butola, S.B., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; pp. 83–105. ISBN 9780081024911. [Google Scholar]
- Voncina, B.; Vivo, V. Cyclodextrins in Textile Finishing. In Eco-Friendly Textile Dyeing and Finishing; Gunay, M., Ed.; IntechOpen: London, UK, 2013; pp. 53–75. [Google Scholar] [CrossRef] [Green Version]
- Radu, C.D.; Parteni, O.; Ochiuz, L. Applications of cyclodextrins in medical textiles—Review. J. Control. Release. 2016, 224, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Voncina, B. Application of Cyclodextrins in Textile Dyeing. In Textile Dyeing; Hauser, J.P., Ed.; IntechOpen: London, UK, 2011; pp. 373–394. [Google Scholar] [CrossRef] [Green Version]
- Morin-Crini, N.; Winterton, P.; Fourmentin, S.; Wilson, L.D.; Fenyvesi, É.; Crini, G. Water-insoluble β-cyclodextrin–epichlorohydrin polymers for removal of pollutants from aqueous solutions by sorption processes using batch studies: A review of inclusion mechanisms. Prog. Polym. Sci. 2018, 78, 1–23. [Google Scholar] [CrossRef]
- Hinze, W.L. Applications of cyclodextrins in chromatographic separations and purification methods. Sep. Purif. Methods 1981, 10, 159–237. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Tanaka, M.; Nakae, M.; Funazo, K.; Shono, T. Chemically Bonded Cyclodextrin Stationary Phases for Liquid Chromatographic Separation of Aromatic Compounds. Anal. Chem. 1983, 55, 1852–1857. [Google Scholar] [CrossRef]
- Xiao, Y.; Ng, S.C.; Tan, T.T.Y.; Wang, Y. Recent development of cyclodextrin chiral stationary phases and their applications in chromatography. J. Chromatogr. A 2012, 1269, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Scriba, G.K.E. Chiral recognition in separation science—An update. J. Chromatogr. A 2016, 1467, 56–78. [Google Scholar] [CrossRef]
- Manaf, N.A.; Saad, B.; Mohamed, M.H.; Wilson, L.D.; Latiff, A.A. Cyclodextrin based polymer sorbents for micro-solid phase extraction followed by liquid chromatography tandem mass spectrometry in determination of endogenous steroids. J. Chromatogr. A 2018, 1543, 23–33. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, X.; Chen, X.; Hao, X. Synthesis of ionic liquids functionalized β-cyclodextrin-bonded chiral stationary phases and their applications in high-performance liquid chromatography. Anal. Chim. Acta 2010, 678, 208–214. [Google Scholar] [CrossRef]
- Matencio, A.; Bermejo-Gimeno, M.J.; García-Carmona, F.; López-Nicolás, J.M. Separating and Identifying the Four Stereoisomers of Methyl Jasmonate by RP-HPLC and using Cyclodextrins in a Novel Way. Phytochem. Anal. 2017, 28, 151–158. [Google Scholar] [CrossRef]
- Xu, X.; Wang, C.; Li, H.; Li, X.; Liu, B.; Singh, V.; Wang, S.; Sun, L.; Gref, R.; Zhang, J. Evaluation of drug loading capabilities of γ-cyclodextrin-metal organic frameworks by high performance liquid chromatography. J. Chromatogr. A 2017, 1488, 37–44. [Google Scholar] [CrossRef]
- Li, L.; Cheng, B.; Zhou, R.; Cao, Z.; Zeng, C.; Li, L. Preparation and evaluation of a novel N-benzyl-phenethylamino-β-cyclodextrin-bonded chiral stationary phase for HPLC. Talanta 2017, 174, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Issaraseriruk, N.; Sritana-anant, Y.; Shitangkoon, A. Substituent effects on chiral resolutions of derivatized 1-phenylalkylamines by heptakis(2,3-di-O-methyl-6-O-tert-butyldimethylsilyl)-β-cyclodextrin GC stationary phase. Chirality 2018, 30, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Fan, J.; Liu, W.; Chen, X.; Ruan, L.; Zhang, W. A new single-urea-bound 3,5-dimethylphenylcarbamoylated β-cyclodextrin chiral stationary phase and its enhanced separation performance in normal-phase liquid chromatography. Electrophoresis 2018, 39, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Greño, M.; Marina, M.L.; Castro-Puyana, M. Effect of the combined use of γ-cyclodextrin and a chiral ionic liquid on the enantiomeric separation of homocysteine by capillary electrophoresis. J. Chromatogr. A 2018, 1568, 222–228. [Google Scholar] [CrossRef]
- Donegatti, T.A.; Lobato, A.; Moreira Gonçalves, L.; Alves Pereira, E. Cyclohexane-1,3-dione as a derivatizing agent for the analysis of aldehydes by micelar electrokinetic chromatography with diode array detection. Electrophoresis 2019, 40, 2929–2935. [Google Scholar] [CrossRef]
- Sun, J.; Ma, S.; Liu, B.; Yu, J.; Guo, X. A fully derivatized 4-chlorophenylcarbamate-β-cyclodextrin bonded chiral stationary phase for enhanced enantioseparation in HPLC. Talanta 2019, 204, 817–825. [Google Scholar] [CrossRef]
- Sun, J.; Liu, B.; Cai, L.; Yu, J.; Guo, X. Chiral liquid chromatography–mass spectrometry (LC–MS/MS) method development with β-cyclodextrin (β-CD) derivatized chiral stationary phase for the enhanced separation and determination of flurbiprofen enantiomers: Application to a stereoselective pharmacokinetic study. New J. Chem. 2020, 44, 10334–10342. [Google Scholar]
- Shuang, Y.; Cao, Z.; Zhang, T.; Li, L. Enantiomeric Separation of Chiral Triazole Pesticides by a mono-6-(4-Nitrophenyl)-ureido-β-cyclodextrin-Bonded Stationary Phase Using High-Performance Liquid Chromatography. Anal. Lett. 2020, 53, 2481–2500. [Google Scholar] [CrossRef]
- Shuang, Y.; Liao, Y.; Wang, H.; Wang, Y.; Li, L. Preparation and evaluation of a triazole-bridged bis(β-cyclodextrin)–bonded chiral stationary phase for HPLC. Chirality 2020, 32, 168–184. [Google Scholar] [CrossRef]
- Cancelliere, G.; Ciogli, A.; D’Acquarica, I.; Gasparrini, F.; Kocergin, J.; Misiti, D.; Pierini, M.; Ritchie, H.; Simone, P.; Villani, C. Transition from enantioselective high performance to ultra-high performance liquid chromatography: A case study of a brush-type chiral stationary phase based on sub-5-micron to sub-2-micron silica particles. J. Chromatogr. A 2010, 1217, 990–999. [Google Scholar] [CrossRef]
- Xiao, Y.; Tan, T.T.Y.; Ng, S.C. Enantioseparation of dansyl amino acids by ultra-high pressure liquid chromatography using cationic β-cyclodextrins as chiral additives. Analyst 2011, 136, 1433–1439. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.; Pérez-Quintanilla, D.; Morante-Zarcero, S.; Sierra, I.; Marina, M.L.; Aturki, Z.; Fanali, S. Ordered mesoporous silica functionalized with β-cyclodextrin derivative for stereoisomer separation of flavanones and flavanone glycosides by nano-liquid chromatography and capillary electrochromatography. J. Chromatogr. A 2017, 1490, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Salido-Fortuna, S.; Marina, M.L.; Castro-Puyana, M. Enantiomeric determination of econazole and sulconazole by electrokinetic chromatography using hydroxypropyl-β-cyclodextrin combined with ionic liquids based on L-lysine and L-glutamic acid. J. Chromatogr. A 2020, 1621, 461085. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cao, Y.; Wu, S.; Wang, S.; Zhao, X.; Zhou, T.; Lou, Y.; Fan, G. Determination of vancomycin in human serum by Cyclodextrin-Micellar Electrokinetic Capillary Chromatography (CD-MEKC) & application for PDAP patients. Molecules 2017, 22, 538. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Guo, J.; Wang, Z.; Zhang, G.; Yu, H.; Chang, R.; Chen, A. Simultaneous separation and determination of hirsutine and hirsuteine by cyclodextrin-modified micellar electrokinetic capillary chromatography. Phytochem. Anal. 2020, 31, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Marques, H.M.C. A review on cyclodextrin encapsulation of essential oils and volatiles. Flavour Fragr. J. 2010, 25, 313–326. [Google Scholar] [CrossRef]
- Wadhwa, G.; Kumar, S.; Chhabra, L.; Mahant, S.; Rao, R. Essential oil–cyclodextrin complexes: An updated review. J. Incl. Phenom. Macrocycl. Chem. 2017, 89, 39–58. [Google Scholar] [CrossRef]
- Abril- Sánchez, C.; Matencio, A.; Navarro-Orcajada, S.; García-Carmona, F.; López-Nicolás, J.M. Evaluation of the properties of the essential oil citronellal nanoencapsulated by cyclodextrins. Chem. Phys. Lipids 2019, 219, 72–78. [Google Scholar] [CrossRef]
- Yuan, C.; Wang, Y.; Liu, Y.; Cui, B. Physicochemical characterization and antibacterial activity assessment of lavender essential oil encapsulated in hydroxypropyl-beta-cyclodextrin. Ind. Crops Prod. 2019, 130, 104–110. [Google Scholar] [CrossRef]
- Simionato, I.; Domingues, F.C.; Nerín, C.; Silva, F. Encapsulation of cinnamon oil in cyclodextrin nanosponges and their potential use for antimicrobial food packaging. Food Chem. Toxicol. 2019, 132, 110647. [Google Scholar] [CrossRef]
- Silva, F.; Caldera, F.; Trotta, F.; Nerín, C.; Domingues, F.C. Encapsulation of coriander essential oil in cyclodextrin nanosponges: A new strategy to promote its use in controlled-release active packaging. Innov. Food Sci. Emerg. Technol. 2019, 56, 102177. [Google Scholar] [CrossRef]
- Herrera, A.; Rodríguez, F.J.; Bruna, J.E.; Abarca, R.L.; Galotto, M.J.; Guarda, A.; Mascayano, C.; Sandoval-Yáñez, C.; Padula, M.; Felipe, F.R.S. Antifungal and physicochemical properties of inclusion complexes based on β-cyclodextrin and essential oil derivatives. Food Res. Int. 2019, 121, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Lim, H.; Nicholas, D.D.; Kim, Y. Bio-based Preservative using Methyl-β-cyclodextrin-Essential Oil Complexes for Wood Protection. Int. J. Biol. Macromol. 2020, 147, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Karpfen, A.; Liedl, E.; Snor, W.; Viernstein, H.; Weiss-Greiler, P.; Wolschann, P. Density functional calculations on cyclodextrins. Monatsh Chem. 2008, 139, 363–371. [Google Scholar] [CrossRef]
- Gamboa-Carballo, J.J.; Rand, V.K.; Levalois-Grützmacher, J.; Gaspard, S.; Jáuregui-Haza, Y. Structures and stabilities of naturally occurring cyclodextrins: A theoretical study of symmetrical conformers. J. Mol. Model. 2017, 23, 318. [Google Scholar] [CrossRef]
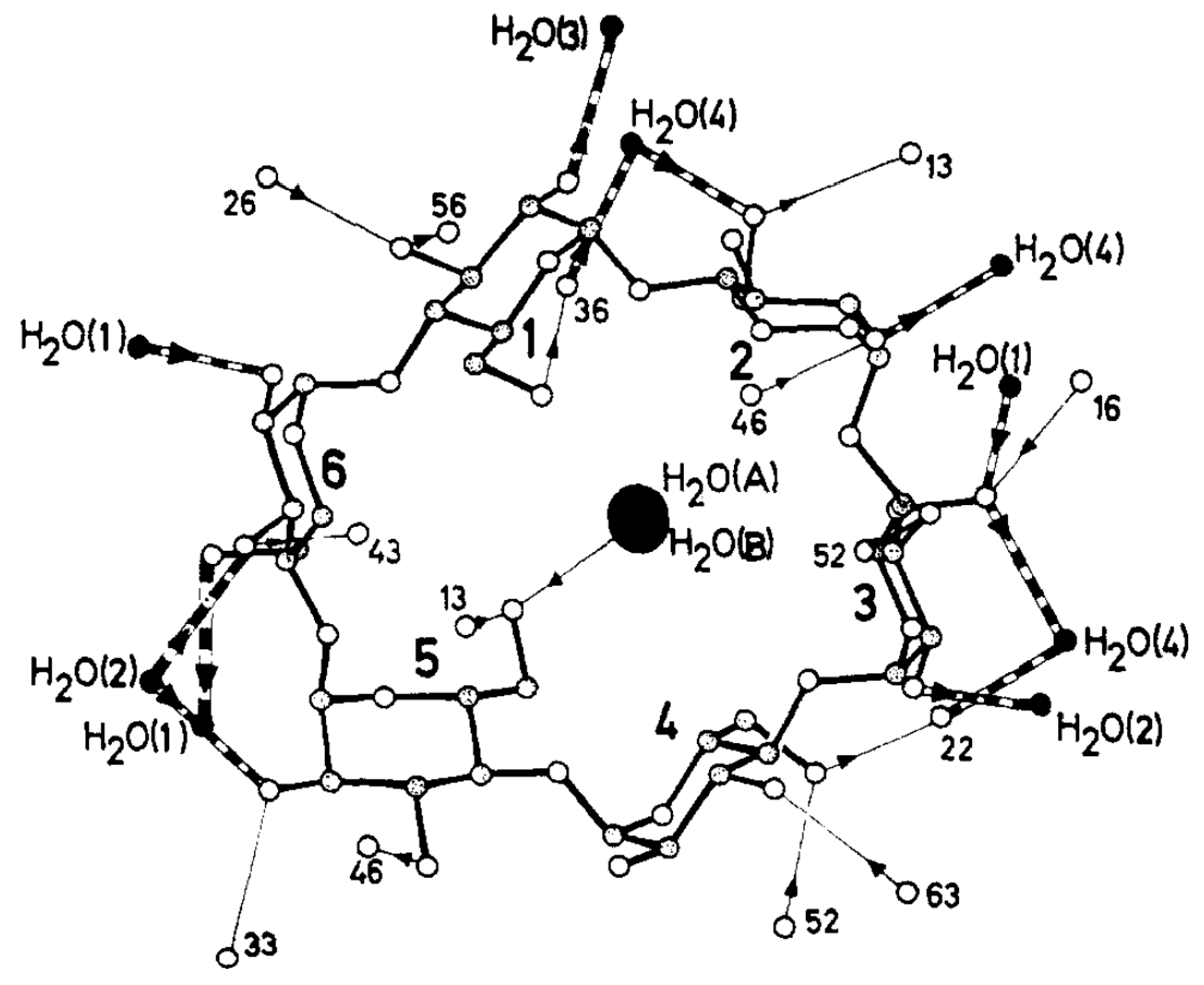
- Klar, B.; Hingerty, B.; Saenger, W. Topography of cyclodextrin inclusion complexes. XII. Hydrogen bonding in the crystal structure of α-cyclodextrin hexahydrate: The use of a multicounter detector in neutral diffraction. Acta Crystallogr. Sect. B Struct. Sci. 1980, 36, 1154–1165. [Google Scholar] [CrossRef]
- Lindner, K.; Saenger, W. Crystal and molecular structures of cyclomaltoheptaose inclusion complexes with HI and with methanol. Carbohydr. Res. 1982, 107, 7–16. [Google Scholar] [CrossRef]
- Harata, K. The structure of the cyclodextrin complex. XX. Crystal structure of uncomplexed hydrated γ-cyclodextrin. Bull. Chem. Soc. Jpn. 1987, 60, 2763–2767. [Google Scholar] [CrossRef] [Green Version]
- Manor, P.C.; Saenger, W. Topography of cyclodextrin inclusion complexes. III. Crystal and molecular structure of cyclohexaamylose hexahydrate, the water dimer inclusion complex. J. Am. Chem. Soc. 1974, 96, 3630–3639. [Google Scholar] [CrossRef]
- Steiner, T.; Koellner, G. Crystalline β-cyclodextrin hydrate at various humidities: Fast, continuous, and reversible dehydration studied by X-ray diffraction. J. Am. Chem. Soc. 1994, 116, 5122–5128. [Google Scholar] [CrossRef]
- Maclennan, J.M.; Stezowski, J.J. The crystal structure of uncomplexed-hydrated cyclooctaamylose. Biochem. Biophys. Res. Commun. 1980, 92, 926–932. [Google Scholar] [CrossRef]
- Lindner, K.; Saenger, W. Crystal and molecular structure of cyclohepta-amylose dodecahydrate. Carbohydr. Res. 1982, 99, 103–115. [Google Scholar] [CrossRef]
- Betzel, C.; Saenger, W.; Hingerty, B.E.; Brown, G.M. Circular and Flip-Flop Hydrogen Bonding in β-Cyclodextrin Undecahydrate: A Neutron Diffraction Study. J. Am. Chem. Soc. 1984, 106, 7545–7557. [Google Scholar] [CrossRef]
- Steiner, T.; Mason, S.A.; Saenger, W. Disordered Guest and Water Molecules. Three-Center and Flip-Flop O-H⋯O Hydrogen Bonds in Crystalline β-Cyclodextrin Ethanol Octahydrate at T = 295 K: A Neutron and X-ray Diffraction Study. J. Am. Chem. Soc. 1991, 113, 5676–5687. [Google Scholar] [CrossRef]
- Ganem, B.; Li, Y.T.; Henion, J.D. Detection of Noncovalent Receptor-Ligand Complexes by Mass Spectrometry. J. Am. Chem. Soc. 1991, 113, 6294–6296. [Google Scholar] [CrossRef]
- Ganem, B.; Li, Y.T.; Henion, J.D. Observation of Noncovalent Enzyme-Substrate and Enzyme-Product Complexes by Ion-Spray Mass Spectrometry. J. Am. Chem. Soc. 1991, 113, 7818–7819. [Google Scholar] [CrossRef]
- Camilleri, P.; Haskins, N.J.; New, A.P.; Saunders, M.R. Analysing the complexation of amino acids and peptides with β-cyciodextrin using electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 1993, 7, 949–952. [Google Scholar] [CrossRef]
- Cunniff, J.B.; Vouros, P. False positives and the detection of cyclodextrin inclusion complexes by electrospray mass spectrometry. J. Am. Soc. Mass Spectrom. 1995, 6, 437–447. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Kang, Y.; Zeng, S. Analysis of stereoisomers of chiral drug by mass spectrometry. Chirality 2018, 30, 609–618. [Google Scholar] [CrossRef]
- Casas-Hinestroza, J.L.; Bueno, M.; Ibáñez, E.; Cifuentes, A. Recent advances in mass spectrometry studies of non-covalent complexes of macrocycles—A review. Anal. Chim. Acta 2019, 1081, 32–50. [Google Scholar] [CrossRef]
- Haskins, N.J.; Saunders, M.R.; Camilleri, P. The Complexation and Chiral Selectivity of 2-Hydroxypropyl-β-cyclodextrin with guest molecules as studied by electrospray mass spectrometry. Rapid Commun. Mass Spectrom. 1994, 8, 423–426. [Google Scholar] [CrossRef]
- Davey, S.N.; Leigh, D.A.; Smart, J.P.; Tetler, L.W.; Truscello, A.M. Fast atom bombardment mass spectrometry as a tool for the rapid determination of enantioselective binding of methylated cyclodextrins. Carbohydr. Res. 1996, 290, 117–123. [Google Scholar] [CrossRef]
- Bakhtiar, R.; Hop, C.E.C.A. A study of the complexation between dimethyl-β cyclodextrin and steroid hormones using electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 1997, 11, 1478–1481. [Google Scholar] [CrossRef]
- Mele, A.; Malpezzi, L. Noncovalent association phenomena of 2,5-dihydroxybenzoic acid with cyclic and linear oligosaccharides. A matrix-assisted laser desorption/ionization time-of-flight mass spectrometric and X-ray crystallographic study. J. Am. Soc. Mass Spectrom. 2000, 11, 228–236. [Google Scholar] [CrossRef] [Green Version]
- Ramanathan, R.; Prokai, L. Electrospray ionization mass spectrometric study of encapsulation of amino acids by cyclodextrins. J. Am. Soc. Mass Spectrom. 1995, 6, 866–871. [Google Scholar] [CrossRef] [Green Version]
- Cescutti, P.; Garozzo, D.; Rizzo, R. Study of the inclusion complexes of aromatic molecules with cyclodextrins using ionspray mass spectrometry. Carbohydr. Res. 1996, 290, 105–115. [Google Scholar] [CrossRef]
- Druliner, J.D.; Wasserman, E. Synthesis and characterization of cyclodextrin/perfluoroalkane inclusion compounds. J. Fluor. Chem. 1995, 72, 75–78. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Naggi, A.; Guerrini, M.; Focher, B. Inclusion complexes of bropirimine with β-cyclodextrin in solution and in solid state. Int. J. Pharm. 1991, 77, 247–254. [Google Scholar] [CrossRef]
- Anguiano-Igea, S.; Otero-Espinar, F.J.; Vila-Jato, J.L.; Blanco-Méndez, J. Interaction of clofibrate with cyclodextrin in solution: Phase solubility, 1H NMR and molecular modelling studies. Eur. J. Pharm. Sci. 1997, 5, 215–221. [Google Scholar] [CrossRef]
- Castronuovo, G.; Elia, V.; Fessas, D.; Velleca, F.; Viscardi, G. Thermodynamics of the interaction of α-cyclodextrin with monocarboxylic acids in aqueous solutions: A calorimetric study at 25 °C. Carbohydr. Res. 1996, 287, 127–138. [Google Scholar] [CrossRef]
- Wilson, L.D.; Verrall, R.E. A Volumetric Study of Cyclodextrin-α,ω-Alkyl Dicarboxylate Anion Complexes in Aqueous Solutions. J. Phys. Chem. B 2000, 104, 1880–1886. [Google Scholar] [CrossRef]
- Gabelica, V.; Galic, N.; De Pauw, E. On the specificity of cyclodextrin complexes detected by electrospray mass spectrometry. J. Am. Soc. Mass Spectrom. 2002, 13, 946–953. [Google Scholar] [CrossRef] [Green Version]
- Gabelica, V.; Galic, N.; Rosu, F.; Houssier, C.; De Pauw, E. Influence of response factors on determining equilibrium association constants of non-covalent complexes by electrospray ionization mass spectrometry. J. Mass Spectrom. 2003, 38, 491–501. [Google Scholar] [CrossRef]
- Cram, D.J.; Helgeson, R.C.; Sousa, L.R.; Timoko, J.M.; Newcomb, M.; Moreau, P.; de Jong, F.; Gokel, G.W.; Hoffman, D.H.; Domeier, L.A.; et al. Chiral recognition in complexation of guests by designed host molecules. Pure Appl. Chem. 1975, 43, 327–349. [Google Scholar] [CrossRef] [Green Version]
- Davankov, V.A. The nature of chiral recognition: Is it a three-point interaction? Chirality 1997, 9, 99–102. [Google Scholar] [CrossRef]
- Lipkowitz, K.B.; Coner, R.; Peterson, M.A. Locating regions of maximum chiral discrimination: A computational study of enantioselection on a popular chiral stationary phase used in chromatography. J. Am. Chem. Soc. 1997, 119, 11269–11276. [Google Scholar] [CrossRef]
- Kitae, T.; Nakayama, T.; Kano, K. Chiral recognition of α-amino acids by charged cyclodextrins through cooperative effects of Coulomb interaction and inclusion. J. Chem. Soc. Perkin Trans. 1998, 2, 207–212. [Google Scholar] [CrossRef]
- Guo, M.; Song, F.; Liu, Z.; Liu, S. Characterization of non-covalent complexes of rutin with cyclodextrins by electrospray ionization tandem mass spectrometry. J. Mass Spectrom. 2004, 39, 594–599. [Google Scholar] [CrossRef]
- Qi, Y.; Geib, T.; Volmer, D.A. Determining the binding sites of β-cyclodextrin and peptides by electron-capture dissociation high resolution tandem mass spectrometry. J. Am. Soc. Mass Spectrom. 2015, 26, 1143–1149. [Google Scholar] [CrossRef] [Green Version]
- Lehmann, E.; Salih, B.; Gómez-López, M.; Diederich, F.; Zenobi, R. Do matrix-assisted laser desorption/ionization mass spectra reflect solution-phase formation of cyclodextrin inclusion complexes? Analyst 2000, 125, 849–854. [Google Scholar] [CrossRef]
- So, M.P.; Wan, T.S.M.; Dominic Chan, T.W. Differentiation of enantiomers using matrix-assisted laser desorption/ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2000, 14, 692–695. [Google Scholar] [CrossRef]
- Hill, H.H.; Siems, W.F.; St. Louis, R.H.; McMinn, D.G. Ion Mobility Spectrometry. Anal. Chem. 1990, 62, 1201A–1209A. [Google Scholar] [CrossRef]
- Borsdorf, H.; Eiceman, G.A. Ion mobility spectrometry: Principles and applications. Appl. Spectrosc. Rev. 2006, 41, 323–375. [Google Scholar] [CrossRef]
- Cumeras, R.; Figueras, E.; Davis, C.E.; Baumbach, J.I.; Gracia, I. Review on Ion Mobility Spectrometry. Part 1: Current Instrumentation. Analyst 2015, 140, 1376–1390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Creaser, C.S.; Griffiths, J.M.R.; Bramwell, C.J.; Noreen, S.; Hill, C.A.; Thomas, C.L.P. Ion mobility spectrometry: A review. Part 1. Structural analysis by mobility measurement. Analyst 2004, 129, 984–994. [Google Scholar] [CrossRef]
- Bohrer, B.C.; Merenbloom, S.I.; Koeniger, S.L.; Hilderbrand, A.E.; Clemmer, D.E. Biomolecule Analysis by Ion Mobility Spectrometry. Annu. Rev. Anal. Chem. 2008, 1, 293–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guharay, S.K.; Dwivedi, P.; Hill, H.H. Ion mobility spectrometry: Ion source development and applications in physical and biological sciences. IEEE Trans. Plasma Sci. 2008, 36, 1458–1470. [Google Scholar] [CrossRef]
- O’Donnell, R.M.; Sun, X.; Harrington, P.d.B. Pharmaceutical applications of ion mobility spectrometry. TrAC Trends Anal. Chem. 2008, 27, 44–53. [Google Scholar] [CrossRef]
- Armenta, S.; Alcala, M.; Blanco, M. A review of recent, unconventional applications of ion mobility spectrometry (IMS). Anal. Chim. Acta 2011, 703, 114–123. [Google Scholar] [CrossRef]
- Mäkinen, M.A.; Anttalainen, O.A.; Sillanpää, M.E.T. Ion mobility spectrometry and its applications in detection of chemical warfare agents. Anal. Chem. 2010, 82, 9594–9600. [Google Scholar] [CrossRef]
- Keller, T.; Keller, A.; Tutsch-Bauer, E.; Monticelli, F. Application of ion mobility spectrometry in cases of forensic interest. Forensic Sci. Int. 2006, 161, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Dodds, J.N.; Baker, E.S. Ion Mobility Spectrometry: Fundamental Concepts, Instrumentation, Applications, and the Road Ahead. J. Am. Soc. Mass Spectrom. 2019, 30, 2185–2195. [Google Scholar] [CrossRef]
- Mathiron, D.; Iori, R.; Pilard, S.; Rajan, T.S.; Landy, D.; Mazzon, E.; Rollin, P.; Djedaïni-Pilard, F. A combined approach of NMR and mass spectrometry techniques applied to the α-cyclodextrin/moringin complex for a novel bioactive formulation. Molecules 2018, 23, 1714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kralj, B.; Šmidovnik, A.; Kobe, J. Mass spectrometric investigations of a- and b- cyclodextrin complexes with ortho-, meta- and para- coumaric acids by negative mode electrospray ionization. Rapid Commun. Mass Spectrom. 2010, 24, 1457–1466. [Google Scholar]
- Berland, K.; Renaud, J.B.; Mayer, P.M. Utilizing ion mobility and tandem mass spectrometry to evaluate the structure and behaviour of multimeric cyclodextrin complexes. Can. J. Chem. 2014, 93, 1313–1319. [Google Scholar] [CrossRef]
- Zimnicka, M.; Troć, A.; Ceborska, M.; Jakubczak, M.; Koliński, M.; Danikiewicz, W. Structural elucidation of specific noncovalent association of folic acid with native cyclodextrins using an ion mobility mass spectrometry and theoretical approach. Anal. Chem. 2014, 86, 4249–4255. [Google Scholar] [CrossRef]
- Nagy, G.; Chouinard, C.D.; Attah, I.K.; Webb, I.K.; Garimella, S.V.B.; Ibrahim, Y.M.; Baker, E.S.; Smith, R.D. Distinguishing enantiomeric amino acids with chiral cyclodextrin adducts and structures for lossless ion manipulations. Electrophoresis 2018, 39, 3148–3155. [Google Scholar] [CrossRef]
- Bian, X.; Zhao, B.; Pang, B.; Zheng, Z.; Liu, S.; Liu, Z.; Song, F. Separation, Quantification and Structural Study of (+)-Catechin and (–)-Epicatechin by Ion Mobility Mass Spectrometry Combined with Theoretical Algorithms. Chinese J. Chem. 2019, 37, 581–587. [Google Scholar] [CrossRef]
- Polfer, N.C. Infrared multiple photon dissociation spectroscopy of trapped ions. Chem. Soc. Rev. 2011, 40, 2211–2221. [Google Scholar] [CrossRef]
- Stedwell, C.N.; Galindo, J.F.; Roitberg, A.E.; Polfer, N.C. Structures of Biomolecular Ions in the Gas Phase Probed by Infrared Light Sources. Annu. Rev. Anal. Chem. 2013, 6, 267–285. [Google Scholar] [CrossRef]
- Jašíková, L.; Roithová, J. Infrared Multiphoton Dissociation Spectroscopy with Free-Electron Lasers: On the Road from Small Molecules to Biomolecules. Chem. A Eur. J. 2018, 24, 3374–3390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oomens, J.; Sartakov, B.G.; Meijer, G.; von Helden, G. Gas-phase infrared multiple photon dissociation spectroscopy of mass-selected molecular ions. Int. J. Mass Spectrom. 2006, 254, 1–19. [Google Scholar] [CrossRef]
- Steill, J.D.; Oomens, J. Gas-phase deprotonation of p-hydroxybenzoic acid investigated by IR spectroscopy: Solution-phase structure is retained upon ESI. J. Am. Chem. Soc. 2009, 131, 13570–13571. [Google Scholar] [CrossRef] [PubMed]
- Almasian, M.; Grzetic, J.; Van Maurik, J.; Steill, J.D.; Berden, G.; Ingemann, S.; Buma, W.J.; Oomens, J. Non-equilibrium isomer distribution of the gas-phase photoactive yellow protein chromophore. J. Phys. Chem. Lett. 2012, 3, 2259–2263. [Google Scholar] [CrossRef] [PubMed]
- Scott Hopkins, W.; Marta, R.A.; Steinmetz, V.; McMahon, T.B. Mode-specific fragmentation of amino acid-containing clusters. Phys. Chem. Chem. Phys. 2015, 17, 28548–28555. [Google Scholar] [CrossRef]
- Fu, W.; Xiong, J.; Lecours, M.J.; Carr, P.J.J.; Marta, R.A.; Fillion, E.; McMahon, T.; Steinmetz, V.; Hopkins, W.S. The structures of proton-bound dimers of glycine with phenylalanine and pentafluorophenylalanine. J. Mol. Spectrosc. 2016, 330, 194–199. [Google Scholar] [CrossRef]
- Kong, X.; Lin, C.; Infusini, G.; Oh, H.B.; Jiang, H.; Breuker, K.; Wu, C.C.; Charkin, O.P.; Chang, H.C.; McLafferty, F.W. Numerous isomers of serine octamer ions characterized by infrared photodissociation spectroscopy. ChemPhysChem 2009, 10, 2603–2606. [Google Scholar] [CrossRef]
- Dunbar, R.C.; Steill, J.D.; Oomens, J. Encapsulation of metal cations by the PhePhe ligand: A cation-π ion cage. J. Am. Chem. Soc. 2011, 133, 9376–9386. [Google Scholar] [CrossRef]
- Filippi, A.; Fraschetti, C.; Piccirillo, S.; Rondino, F.; Botta, B.; D’Acquarica, I.; Calcaterra, A.; Speranza, M. Chirality effects on the IRMPD spectra of basket resorcinarene/nucleoside complexes. Chem. A Eur. J. 2012, 18, 8320–8328. [Google Scholar] [CrossRef] [Green Version]
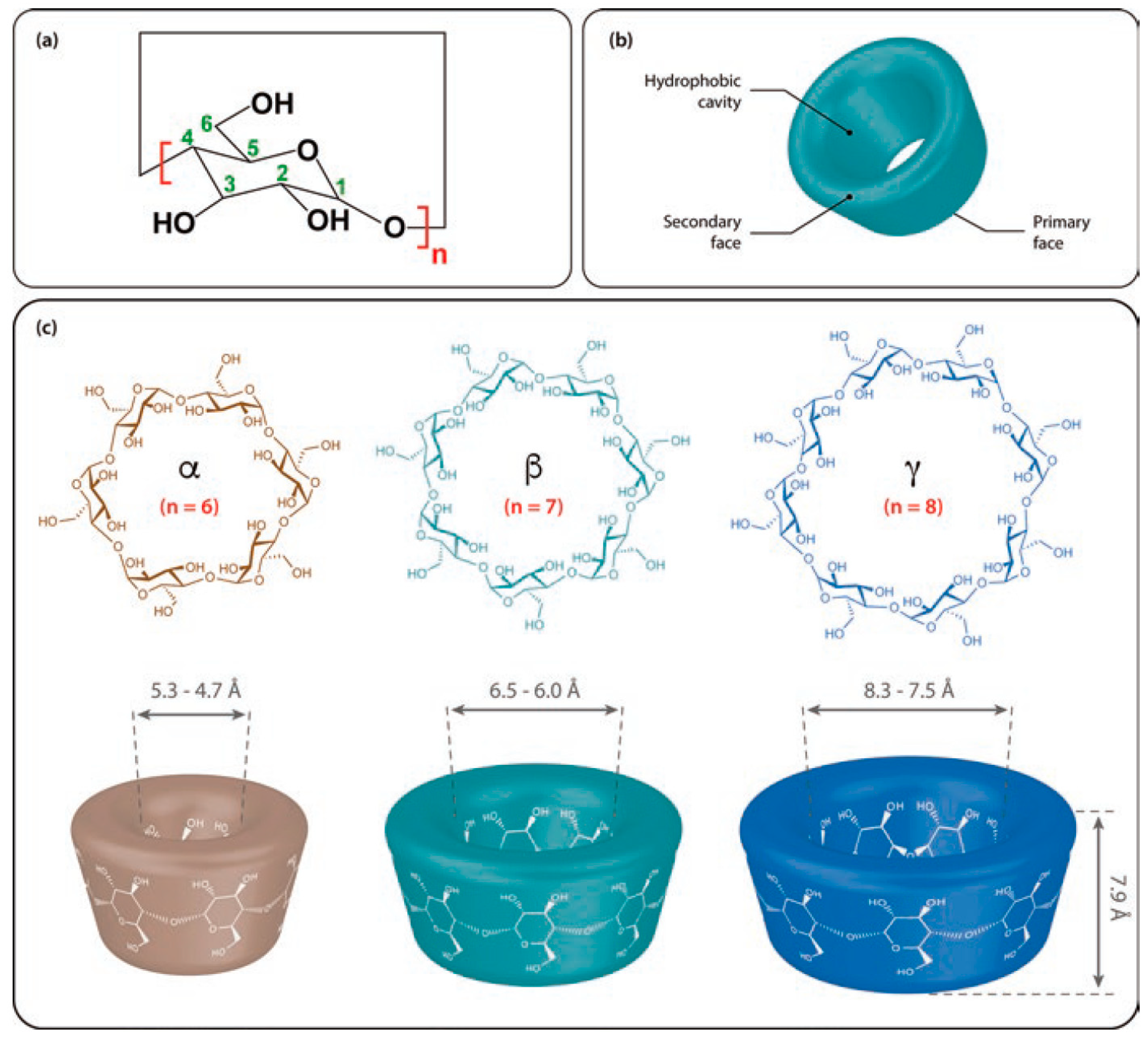
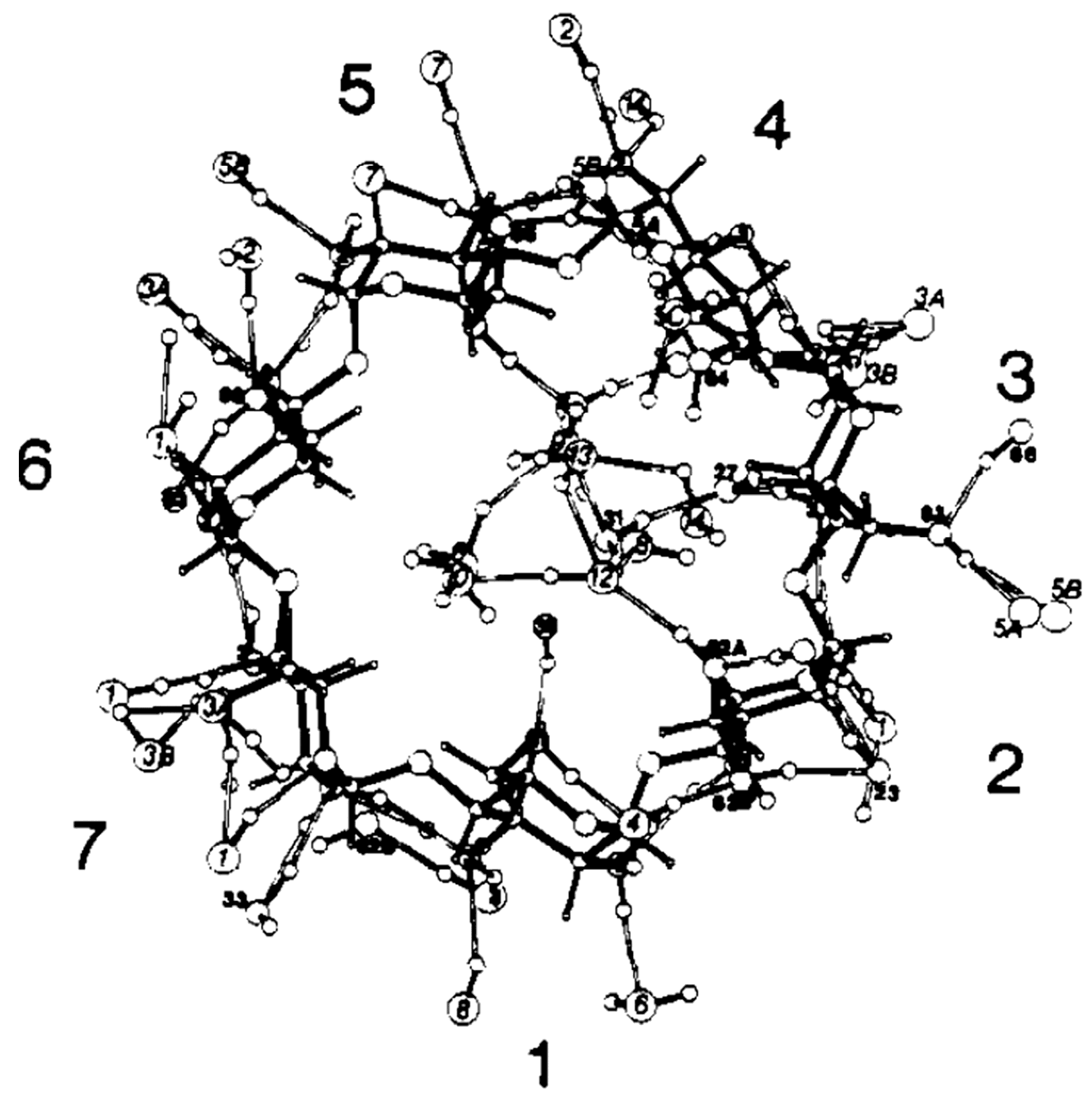
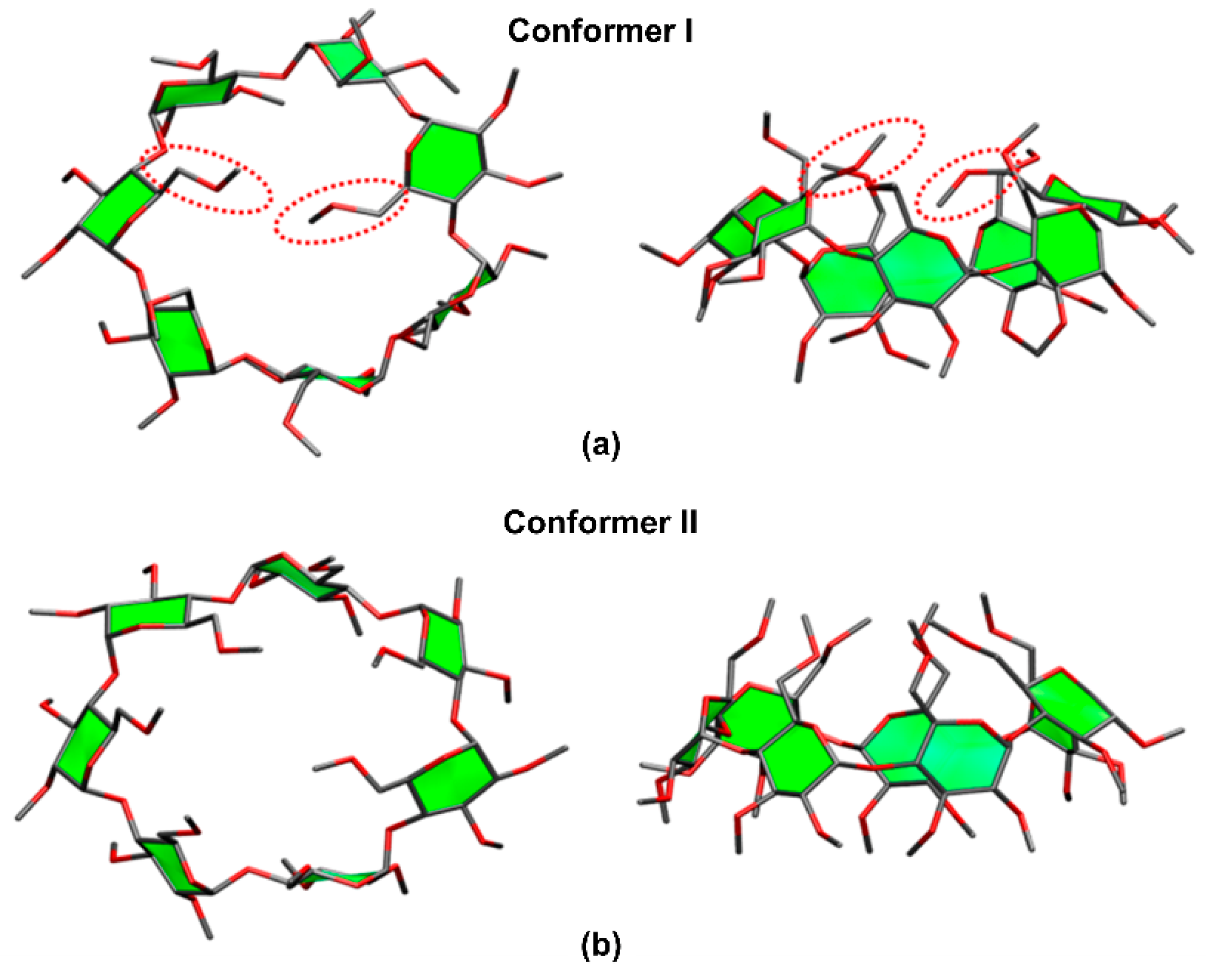
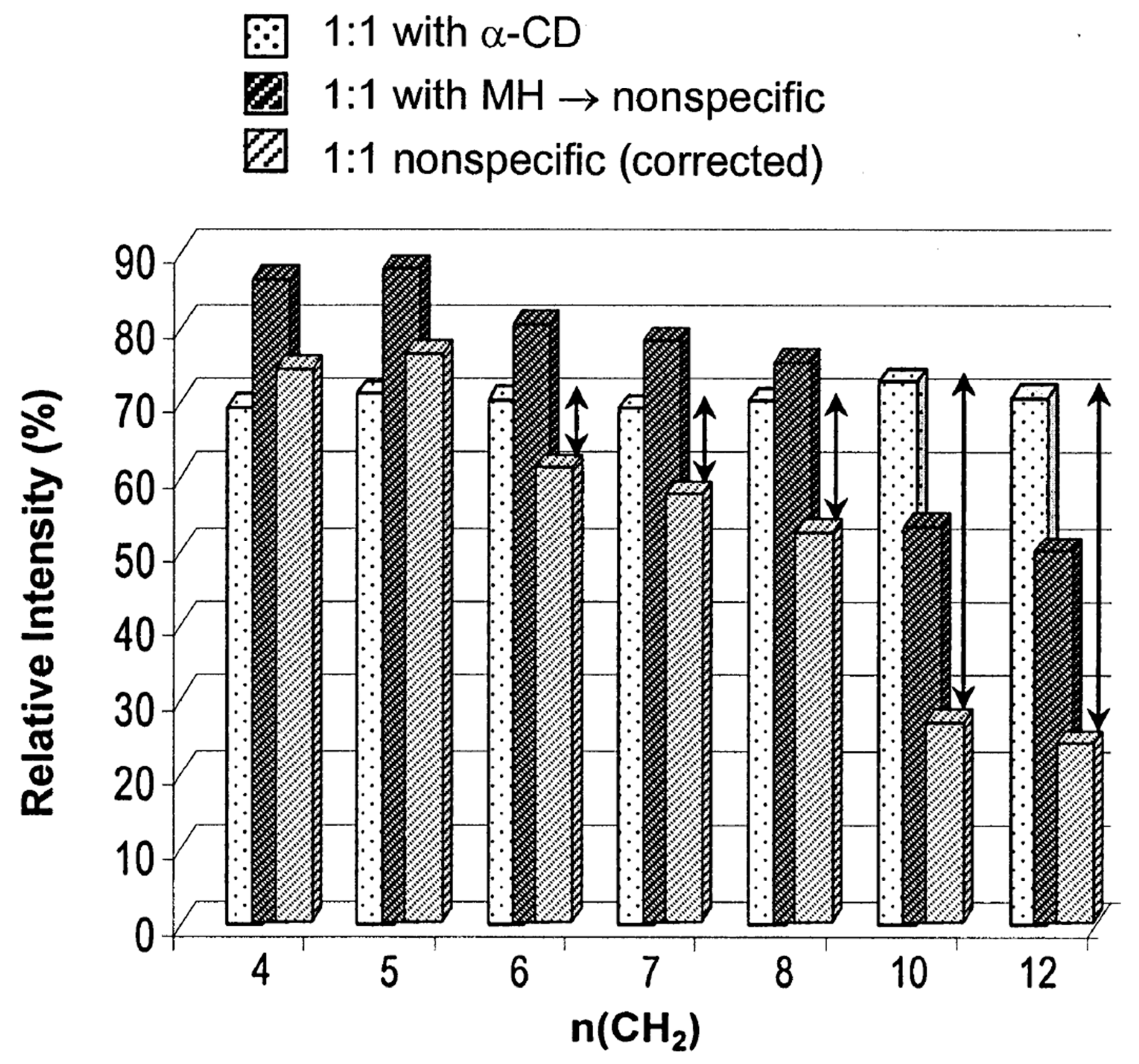
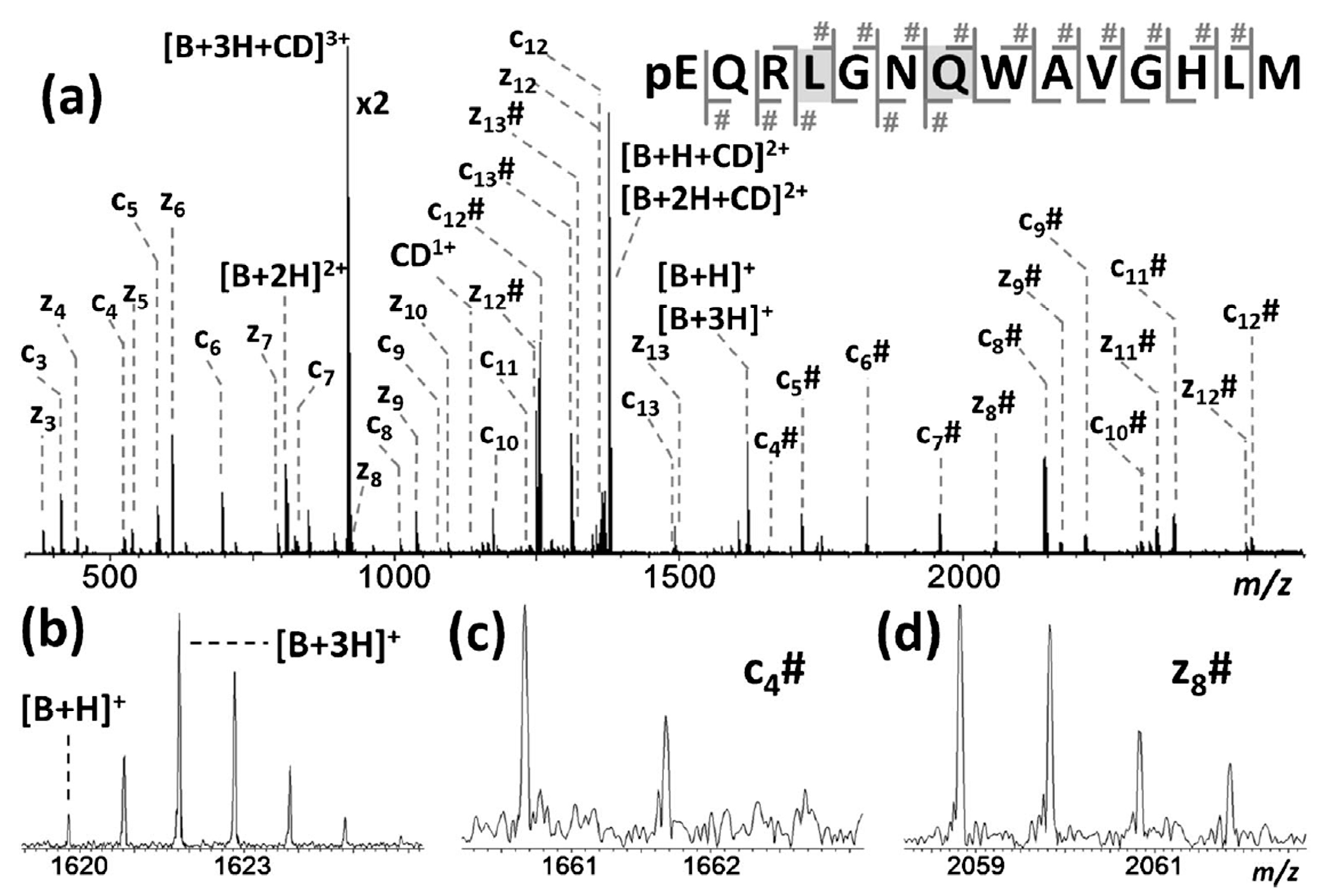
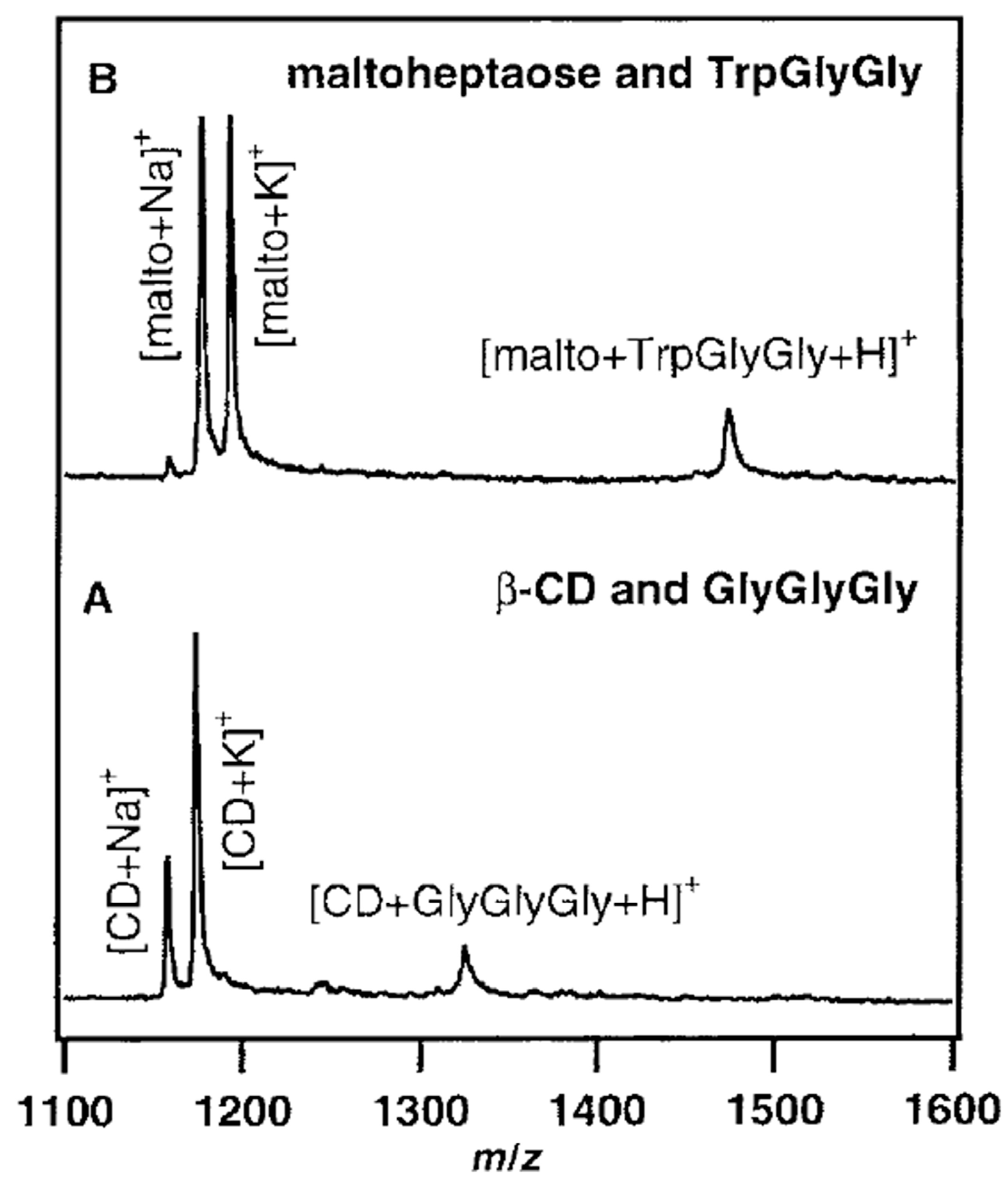
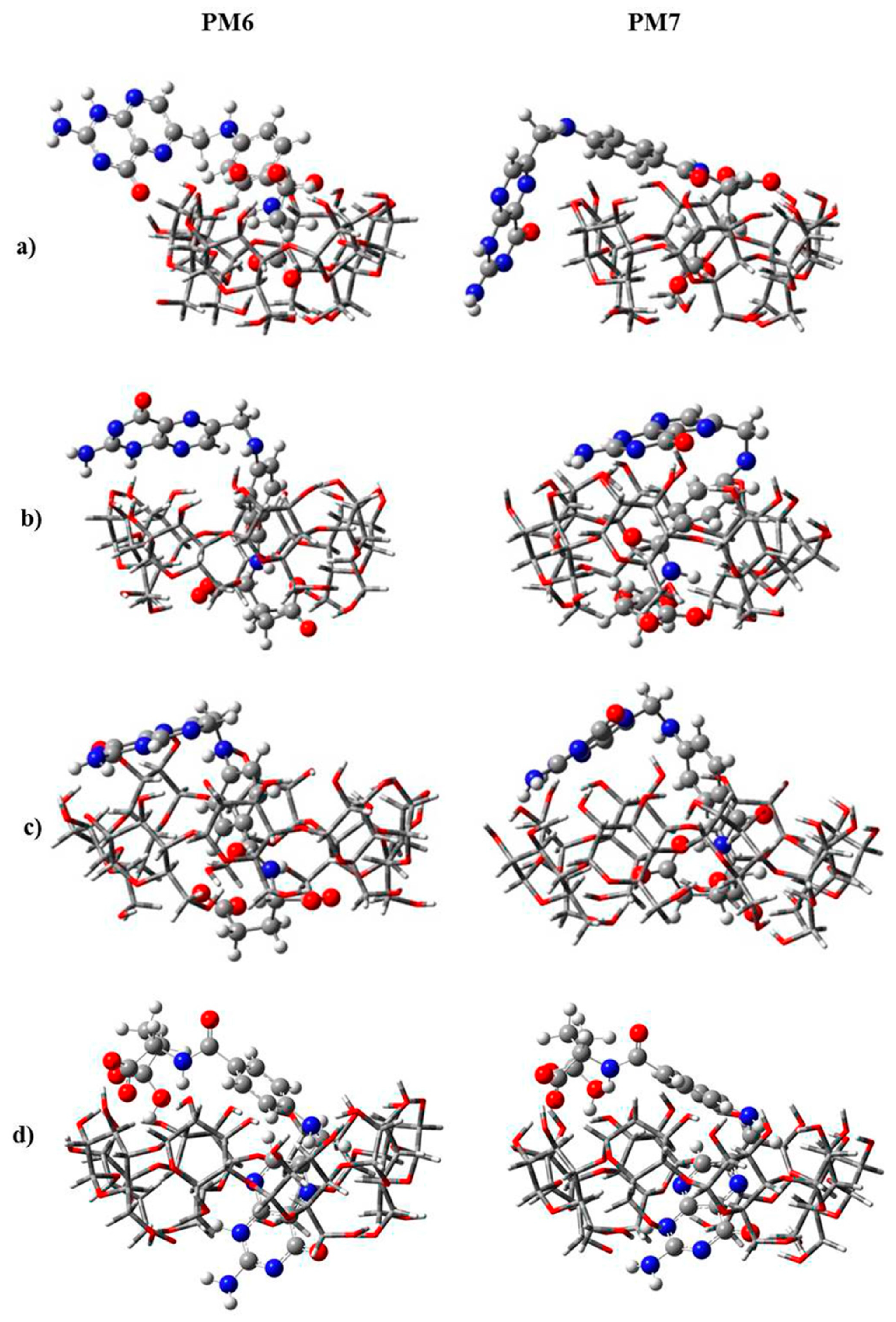
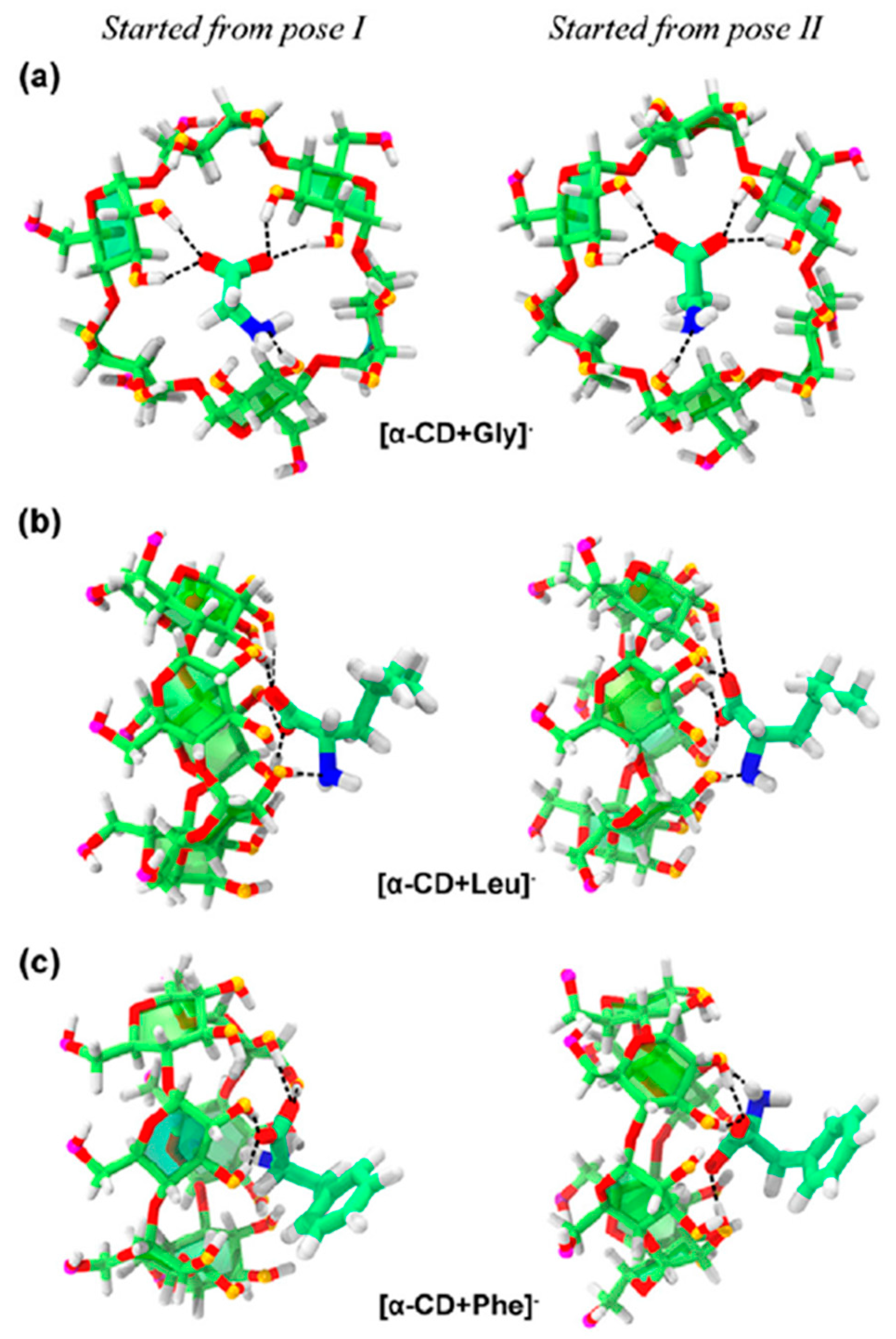
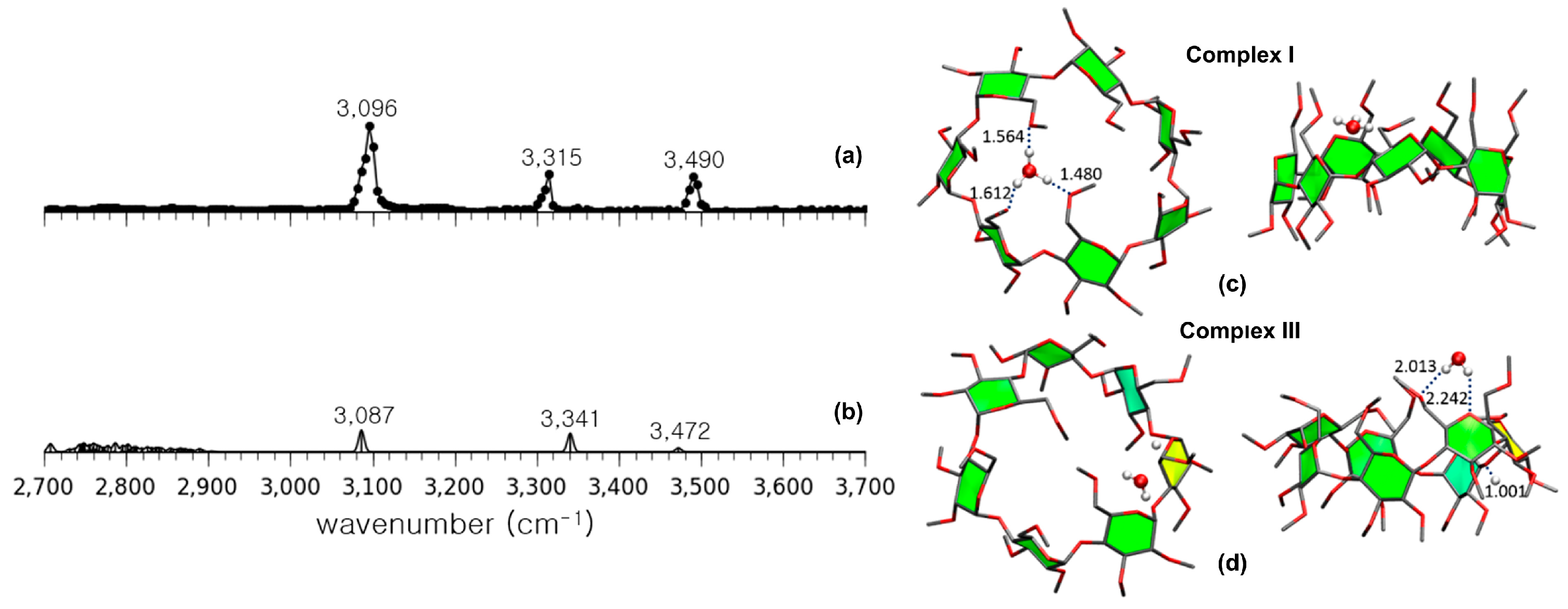
| Cyclodextrin | α | β | γ |
|---|---|---|---|
| Chemical Abstracts Service Registry Number | 100016-20-3 | 7585-39-9 | 17465-86-0 |
| Glucopyranose units | 6 | 7 | 8 |
| Formulae | C36H60O30 | C42H70O35 | C48H80O40 |
| Molecular weight (g/mol) | 972.9 | 1135.0 | 1297.1 |
| Central cavity diameter: external/internal (Å) | 5.3/4.7 | 6.5/6.0 | 8.3/7.5 |
| Height of torus (Å) | 7.9 | 7.9 | 7.9 |
| Approximate volume of cavity (Å) | 174 | 262 | 427 |
| Water solubility at 25 °C (g/L) | 145 | 18.5 | 232 |
| Number of water molecules within cavity | 6–8 | 11–12 | 13–17 |
| pKa | 12.3 | 12.2 | 12.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-u.; Lee, S.-S.; Lee, S.; Oh, H.B. Noncovalent Complexes of Cyclodextrin with Small Organic Molecules: Applications and Insights into Host–Guest Interactions in the Gas Phase and Condensed Phase. Molecules 2020, 25, 4048. https://doi.org/10.3390/molecules25184048
Lee J-u, Lee S-S, Lee S, Oh HB. Noncovalent Complexes of Cyclodextrin with Small Organic Molecules: Applications and Insights into Host–Guest Interactions in the Gas Phase and Condensed Phase. Molecules. 2020; 25(18):4048. https://doi.org/10.3390/molecules25184048
Chicago/Turabian StyleLee, Jae-ung, Sung-Sik Lee, Sungyul Lee, and Han Bin Oh. 2020. "Noncovalent Complexes of Cyclodextrin with Small Organic Molecules: Applications and Insights into Host–Guest Interactions in the Gas Phase and Condensed Phase" Molecules 25, no. 18: 4048. https://doi.org/10.3390/molecules25184048
APA StyleLee, J.-u., Lee, S.-S., Lee, S., & Oh, H. B. (2020). Noncovalent Complexes of Cyclodextrin with Small Organic Molecules: Applications and Insights into Host–Guest Interactions in the Gas Phase and Condensed Phase. Molecules, 25(18), 4048. https://doi.org/10.3390/molecules25184048








