Breaking Molecular Symmetry through Biocatalytic Reactions to Gain Access to Valuable Chiral Synthons
Abstract
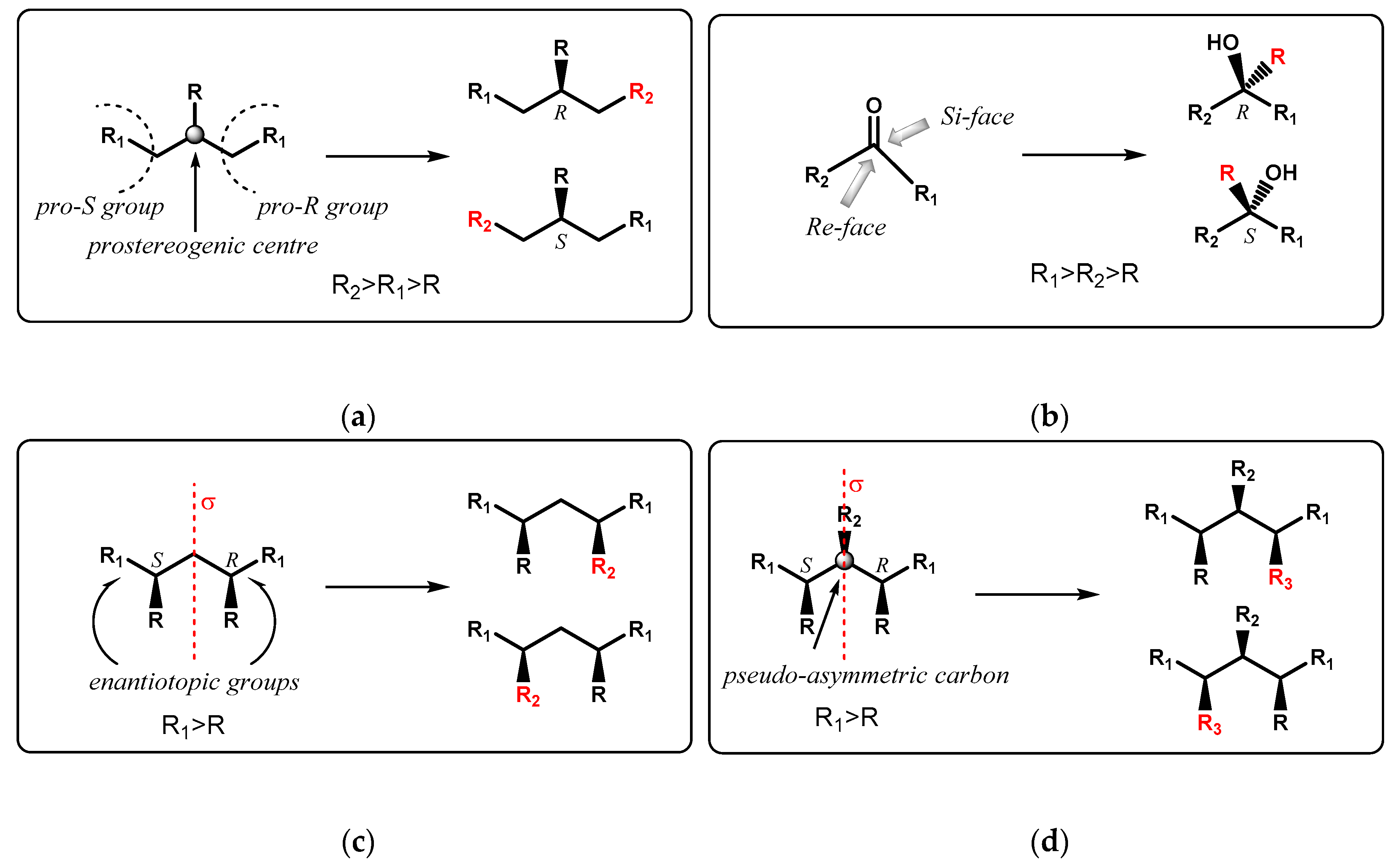
:1. Introduction
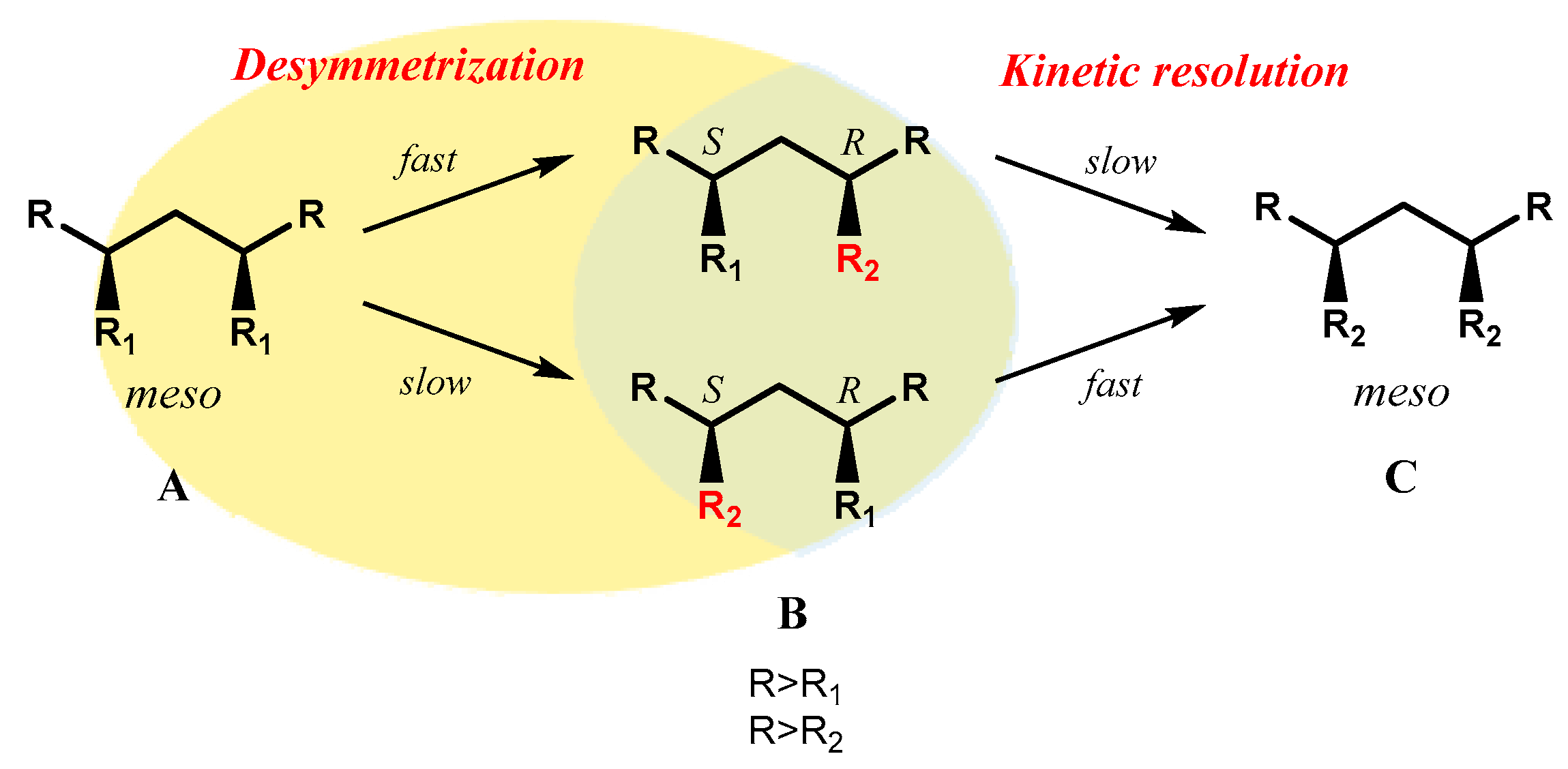
2. Desymmetrization of Meso-Compounds
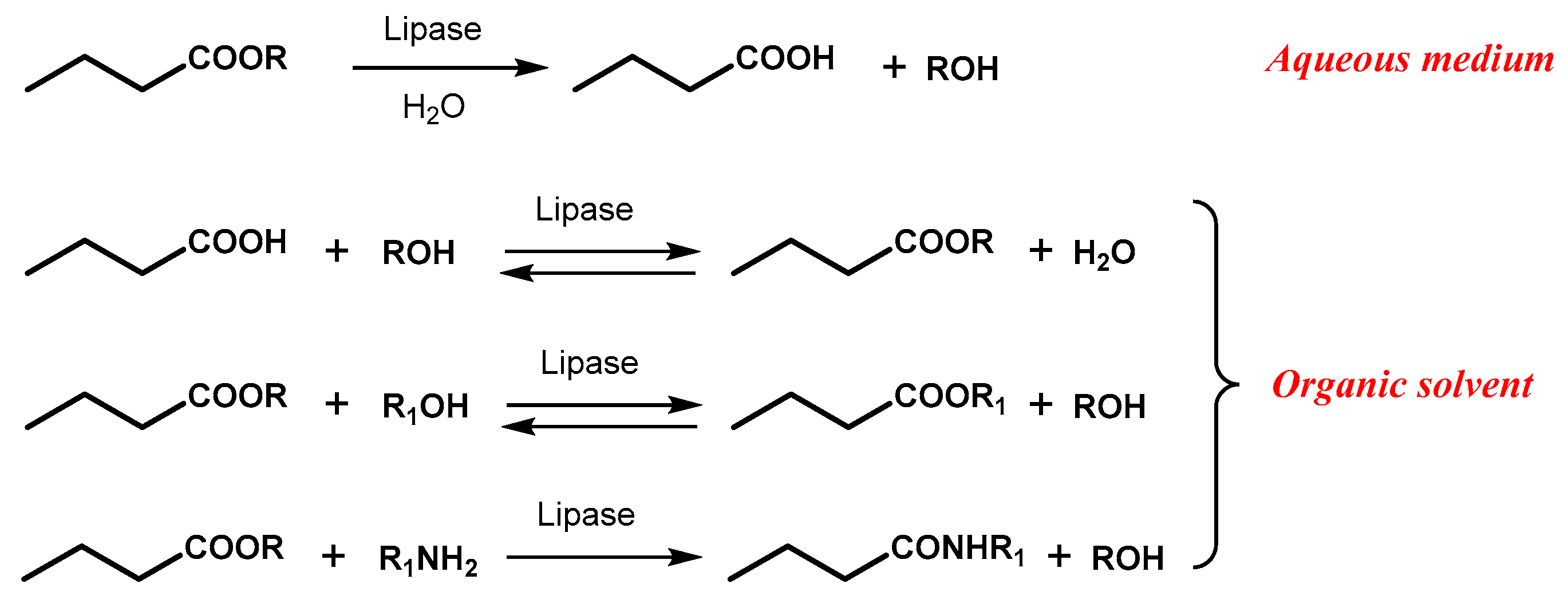
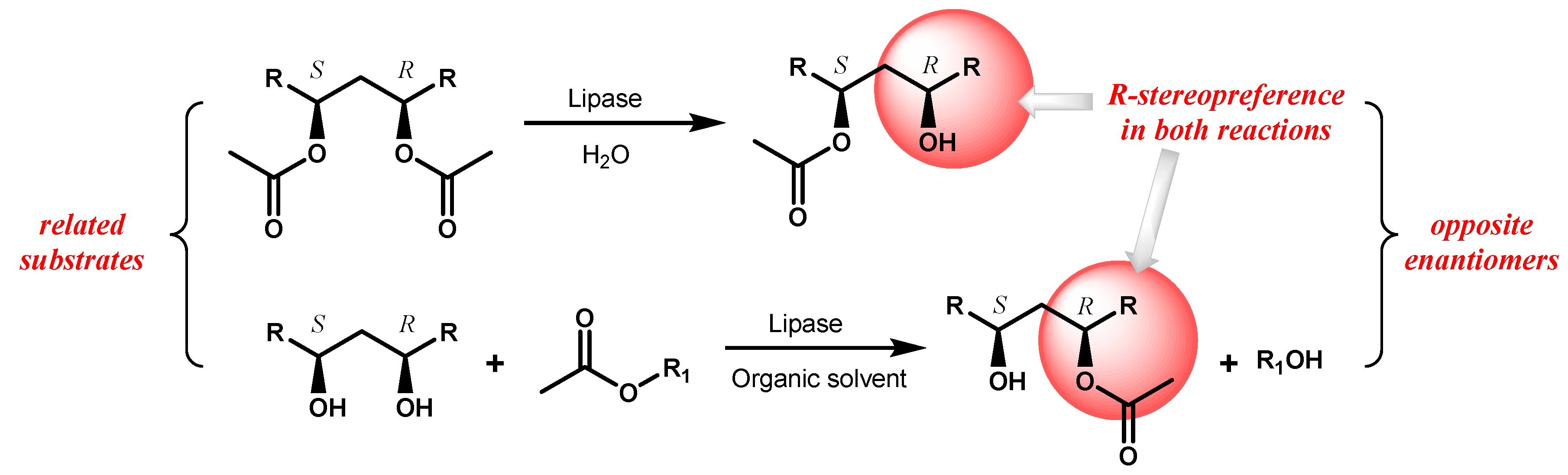
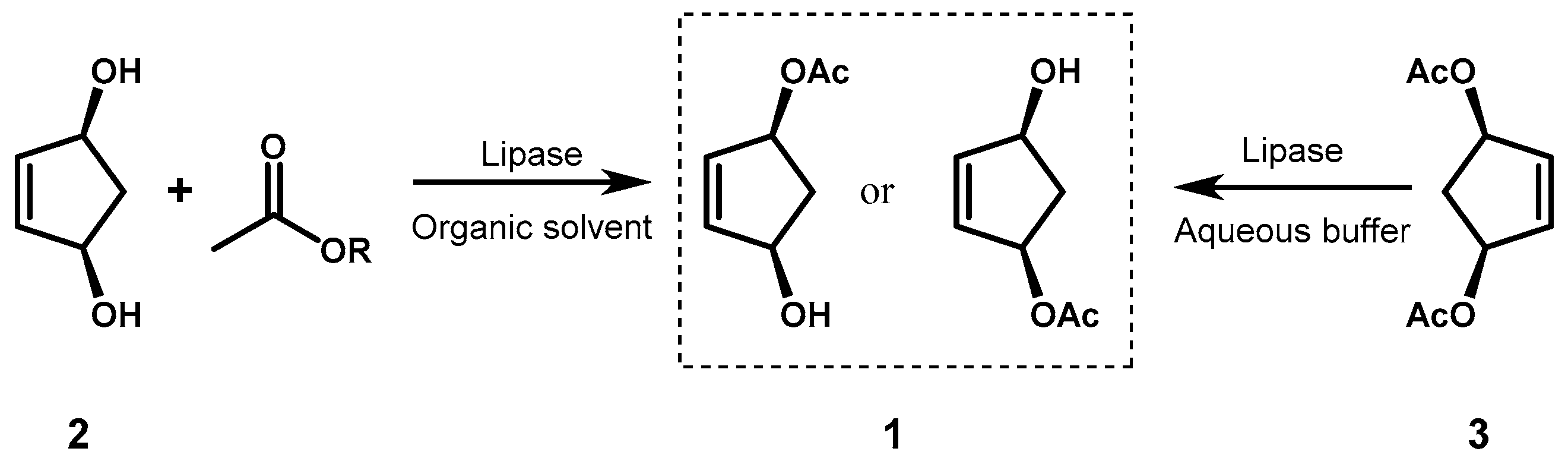
2.1. Lipase-Catalyzed Reactions
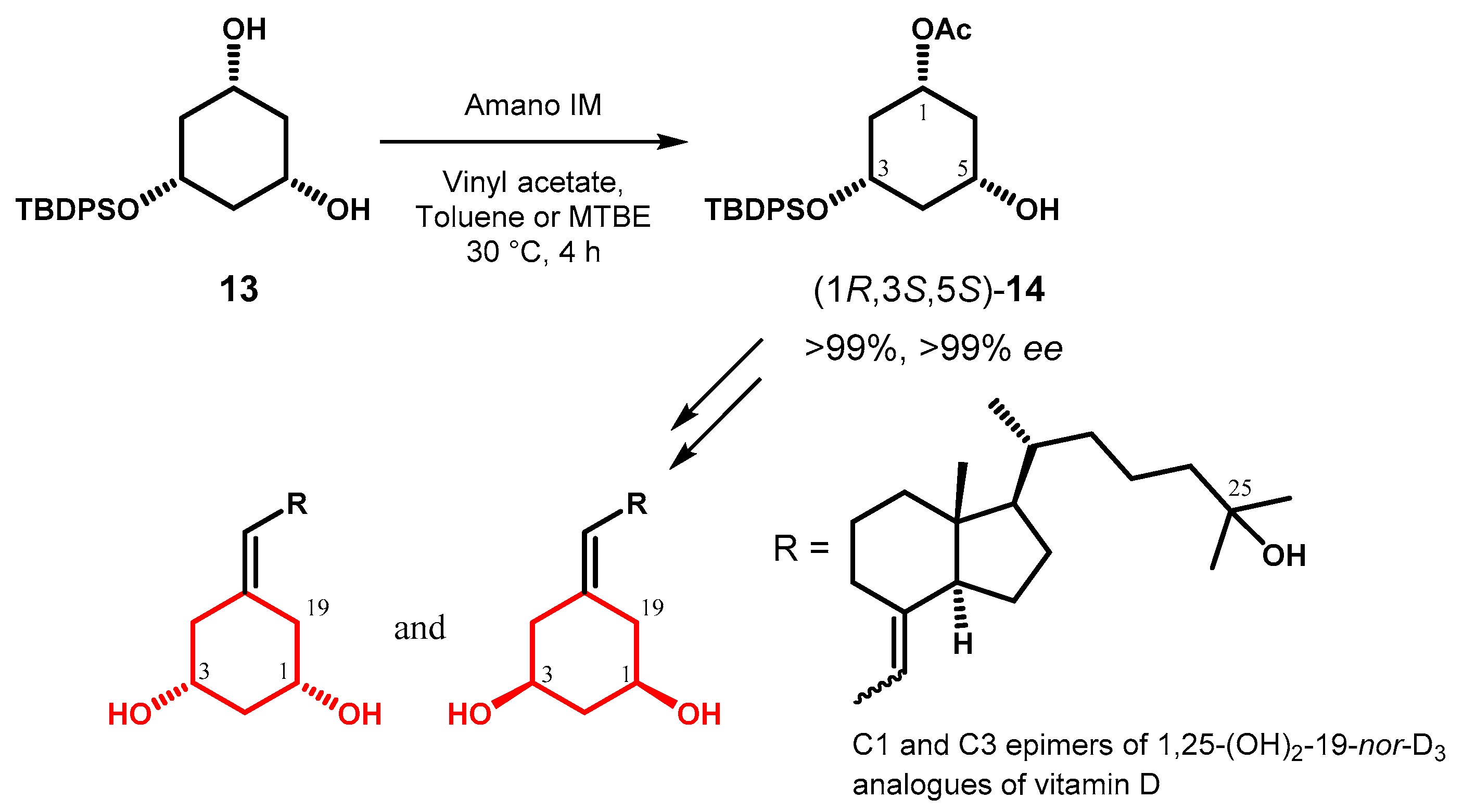
2.2. Reactions Catalyzed by other Enzymes than Lipases
3. Desymmetrization of Prochiral Compounds
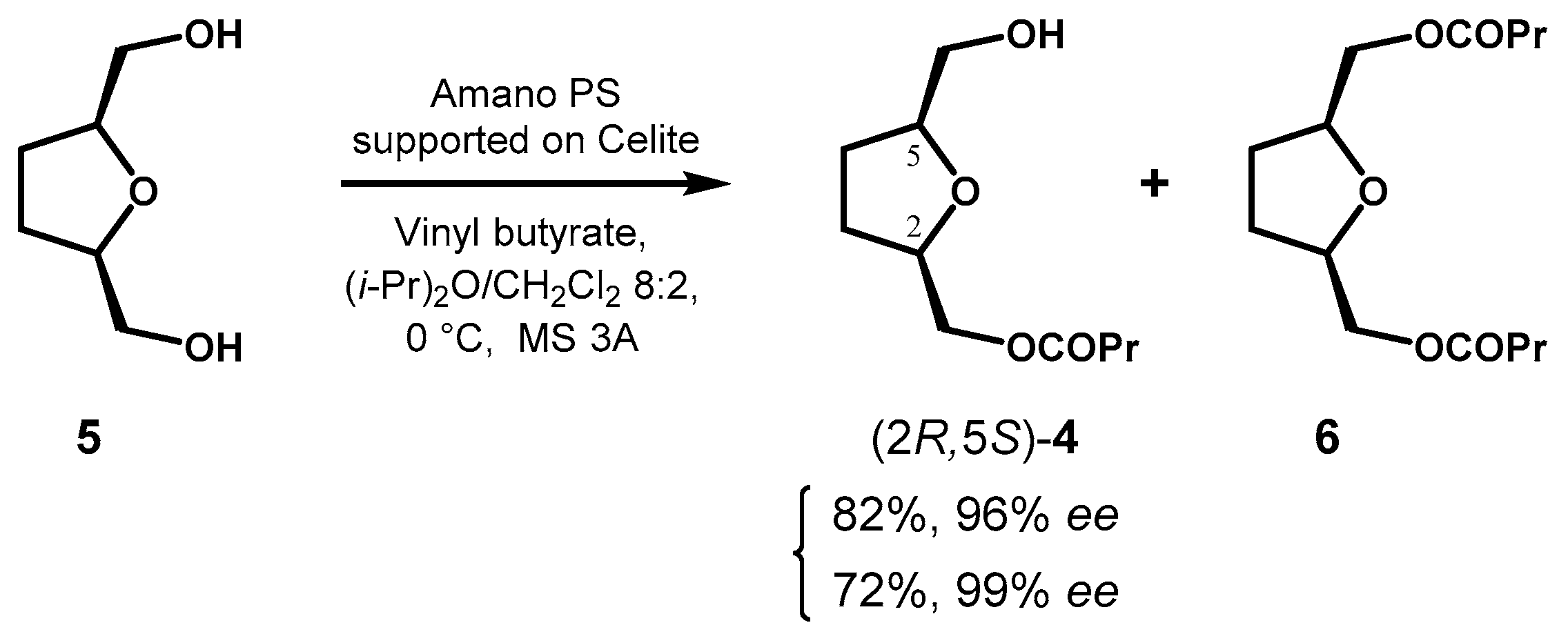
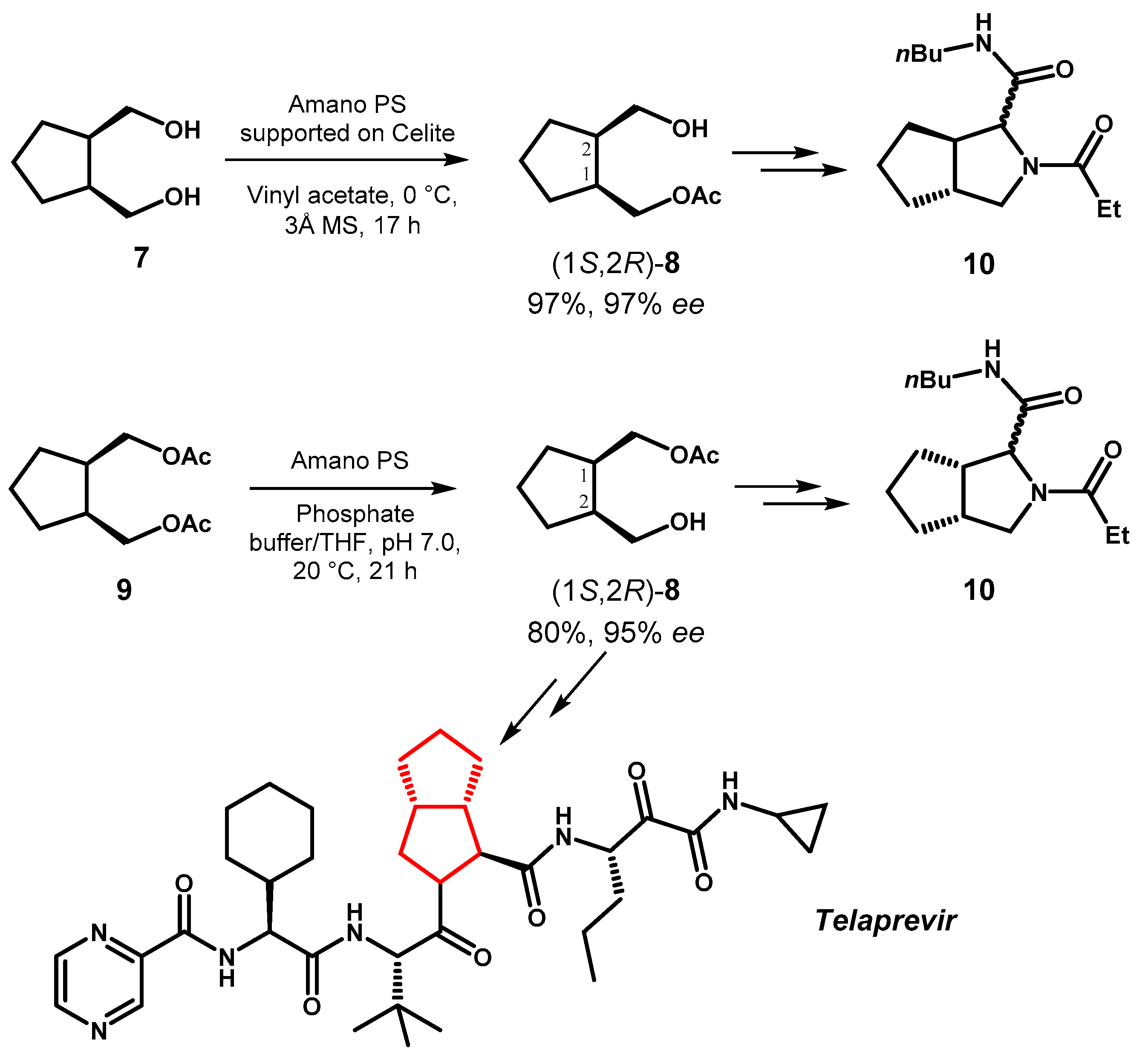
3.1. Lipase-Catalyzed Reactions
3.2. Reactions Catalyzed by Other Enzymes than Lipases
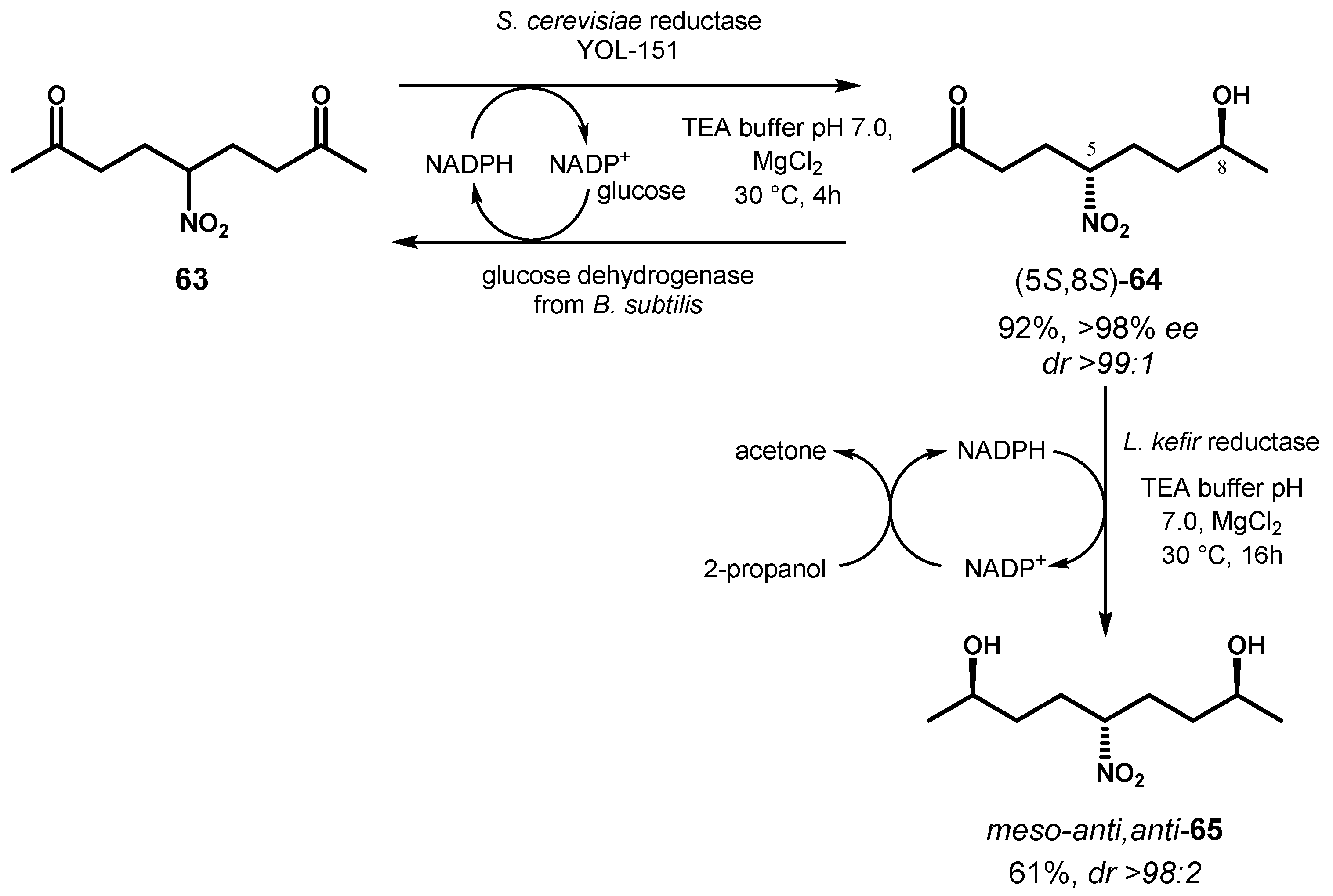
4. Biocatalyzed Desymmetrization of Diketones
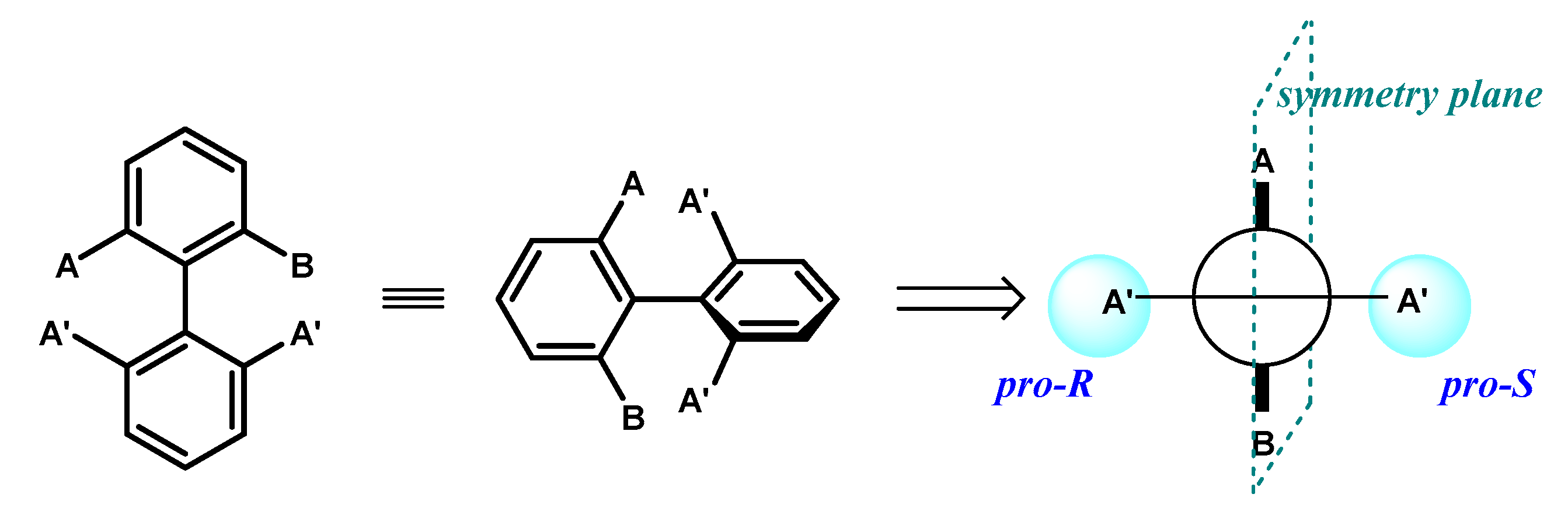
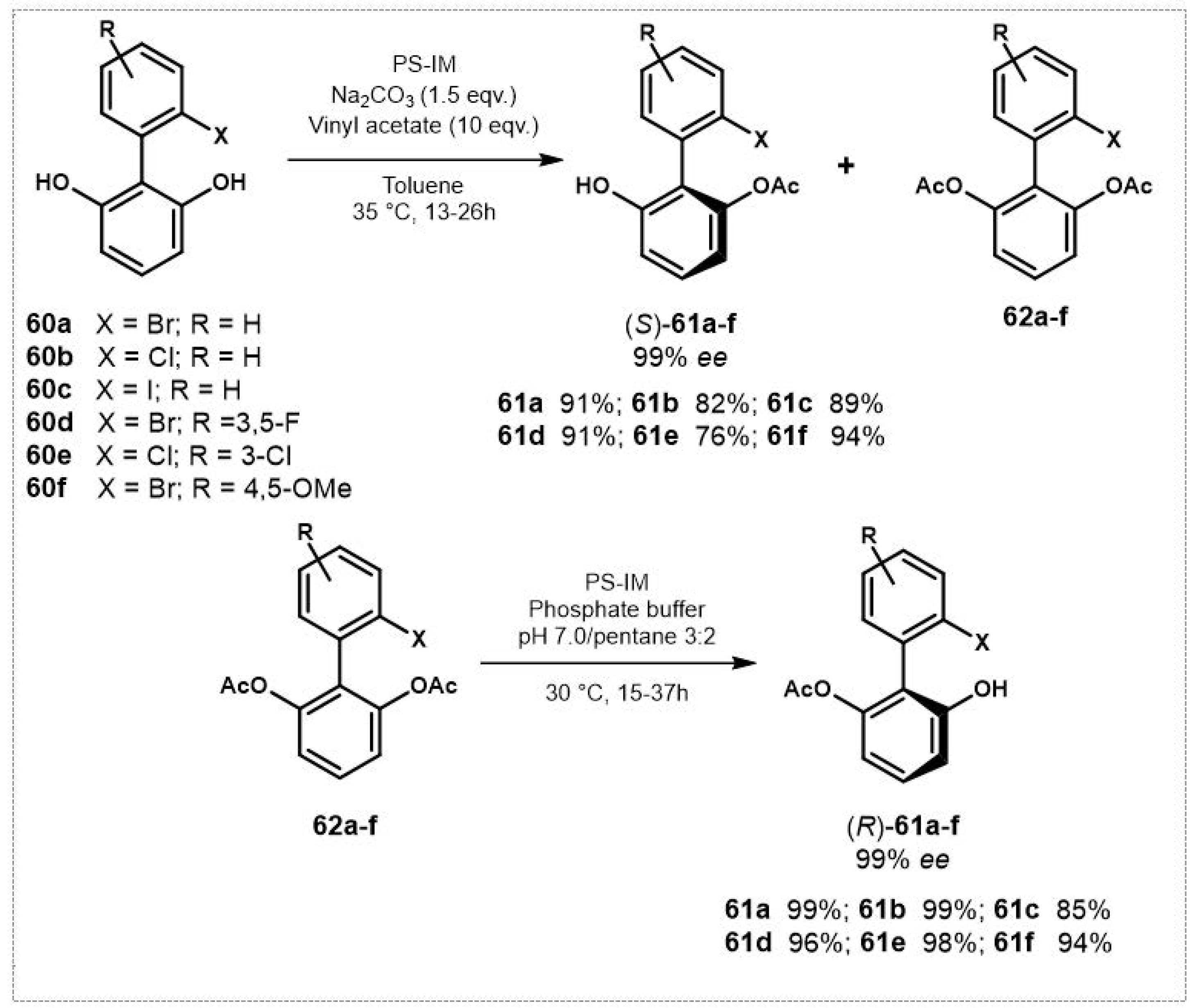
5. Desymmetrization of Challenging Substrates
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Crossley, R. The relevance of chirality to the study of biological activity. Tetrahedron 1992, 48, 8155–8178. [Google Scholar] [CrossRef]
- Brooks, W.H.; Guida, W.C.; Daniel, K.G. The significance of chirality in drug design and development. Curr. Top. Med. Chem. 2011, 11, 760–770. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration. Development of New Stereoisomeric Drugs; U.S. Food & Drug: Washington, DC, USA, 1992. Available online: https://www.fda.gov/drugs/guidancecomplianceregulatoryinformation/guidances/ucm122883.html (accessed on 7 April 2017).
- Mannschreck, A.; Kiesswetter, R.; von Angerer, E. Unequal activities of enantiomers via biological receptors: Examples of chiral drug, pesticide, and fragrance molecules. J. Chem. Educ. 2007, 84, 2012–2018. [Google Scholar] [CrossRef]
- Prusov, E.V. Stereoselective Synthesis of Drugs and Natural Products; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 1–1836. [Google Scholar] [CrossRef]
- Willis, M.C. Enantioselective desymmetrization. J. Chem. Soc. Perkin Trans. 1 1999, 1765–1784. [Google Scholar] [CrossRef]
- Merad, J.; Candy, M.; Pons, J.-M.; Bressy, C. Catalytic enantioselective desymmetrization of Meso compounds in total synthesis of natural products: Towards an economy of chiral reagents. Synth. Stuttg. 2017, 49, 1938–1954. [Google Scholar] [CrossRef] [Green Version]
- IUPAC. Basic terminology of stereochemistry. Pure Appl. Chem. 1996, 68, 2193–2222. [Google Scholar] [CrossRef]
- Vigneron, J.P.; Dhaenens, M.; Horeau, A. Nouvelle methode pour porter au maximum la purete optique d’un produit partiellement dedouble sans l’aide d’aucune substance chirale. Tetrahedron 1973, 29, 1055–1059. [Google Scholar] [CrossRef]
- Garcìa-Urdiales, E.; Alfonso, I.; Gotor, V. Enantioselective enzymatic desymmetrizations in organic synthesis. Chem. Rev. 2005, 105, 313–354. [Google Scholar] [CrossRef]
- Borissov, A.; Davies, T.Q.; Ellis, S.R.; Fleming, T.A.; Richardson, M.S.W.; Dixon, D.J. Organocatalytic enantioselective desymmetrization. Chem. Soc. Rev. 2016, 45, 5474–5540. [Google Scholar] [CrossRef]
- Suzuki, T. Recent topics in the desymmetrization of meso-diols. Tetrahedron Lett. 2017, 58, 4731–4739. [Google Scholar] [CrossRef]
- Zeng, X.-P.; Cao, Z.-Y.; Wang, Y.-H.; Zhou, F.; Zhou, J. Catalytic enantioselective desymmetrization reactions to all-carbon quaternary stereocenters. Chem. Rev. 2016, 116, 7330–7396. [Google Scholar] [CrossRef]
- Patel, R.N. Biocatalysis: Synthesis of key intermediates for development of pharmaceuticals. ACS Catal. 2011, 1056–1074. [Google Scholar] [CrossRef]
- Bode, S.E.; Wolberg, M.; Müller, M. Stereoselective synthesis of 1,3-diols. Synthesis 2006, 4, 557–588. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Brady, D. Broadening the scope of biocatalysis in sustainable organic synthesis. ChemSusChem 2019, 12, 2859–2881. [Google Scholar] [CrossRef]
- Zeymer, C.; Hilvert, D. Directed evolution of protein catalysts. Annu. Rev. Biochem. 2018, 87, 131–157. [Google Scholar] [CrossRef]
- Reetz, M.T. Lipases as practical biocatalysts. Curr. Opin. Chem. Biol. 2002, 6, 145–150. [Google Scholar] [CrossRef]
- Gotor-Fernández, V.; Brieva, R.; Gotor, V. Lipases: Useful biocatalysts for the preparation of pharmaceuticals. J. Mol. Catal. B Enzym. 2006, 40, 111–120. [Google Scholar] [CrossRef]
- Angajala, G.; Pavan, P.; Subashini, R. Lipases: An overview of its current challenges and prospectives in the revolution of biocatalysis. Biocatal. Agric. Biotechnol. 2016, 7, 257–270. [Google Scholar] [CrossRef]
- Carrea, G.; Riva, S. Medium Engineering. In Asymmetric Organic Synthesis with Enzymes; Gotor, V., Alfonso, I., García-Urdiales, E., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008; Chapter 1; pp. 3–18. [Google Scholar]
- Castillo, E.; Casas-Godoy, L.; Sandoval, G. Medium-engineering: A useful tool for modulating lipase activity and selectivity. Biocatalysis 2015, 178–188. [Google Scholar] [CrossRef]
- Quilles, J.C.J.; Brito, R.R.; Borges, J.P.; Aragonc, C.C.; Fernandez-Lorente, G.; Bocchini-Martins, D.A.; Gomes, E.; da Silvaa, R.; Boscoloa, M.; Guisan, J.M. Modulation of the activity and selectivity of the immobilized lipases by surfactants and solvents. Biochem. Eng. J. 2015, 93, 274–280. [Google Scholar] [CrossRef] [Green Version]
- Datta, S.; Christena, R.L.; Rajaram, Y.R.S. Enzyme immobilization: An overview on techniques and support materials. 3 Biotech 2013, 3, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Zoumpanioti, M.; Stamatis, H.; Xenakis, A. Microemulsion-based organogels as matrices for lipase immobilization. Biotechnol. Adv. 2010, 28, 395–406. [Google Scholar] [CrossRef]
- Rodriguesa, R.C.; Virgen-Ortízb, J.J.; dos Santosc, J.C.S.; Berenguer-Murciad, Á.; Alcantarae, A.R.; Barbosaf, O.; Ortizg, C.; Fernandez-Lafuente, R. Immobilization of lipases on hydrophobic supports: Immobilization mechanism, advantages, problems, and solutions. Biotechnol. Adv. 2019, 37, 746–770. [Google Scholar] [CrossRef] [Green Version]
- Uppenberg, J.; Oehrner, N.; Norin, M.; Hult, K.; Kleywegt, G.J.; Patkar, S.; Waagen, V.; Anthonsen, T.; Jones, T.A. Crystallographic and molecular-modeling studies of lipase B from Candida antarctica reveal a stereospecificity pocket for secondary alcohols. Biochemistry 1995, 34, 16838–16851. [Google Scholar] [CrossRef]
- Lang, D.A.; Mannesse, M.L.M.; De Haas, G.H.; Verheij, H.M.; Dijkstra, B.W. Structural basis of the chiral selectivity of Pseudomonas cepacia lipase. Eur. J. Biochem. 1998, 254, 333–340. [Google Scholar] [CrossRef] [Green Version]
- Khan, F.I.; Lan, D.; Durrani, R.; Huan, W.; Zhao, Z.; Wang, Y. The lid domain in lipases: Structural and functional determinant of enzymatic properties. Front. Bioeng. Biotechnol. 2017, 5, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Theil, F.; Schick, H.; Lapitskaya, M.A.; Pivnitsky, K.K. Enzymes in organic synthesis, 4. Investigation of the pancreatin-catalyzed acylation of cis-cyclopent-2-ene-1,4-diol with various trichloroethyl and vinyl alkanoates. Liebigs Annal. Chem. 1991, 195–200. [Google Scholar] [CrossRef]
- Ghorpade, S.R.; Kharul, R.K.; Joshi, R.R.; Kalkote, U.R.; Ravindranathan, T. Desymmetrization of meso-cyclopenten-cis-1,4-diol to 4-(R)-hydroxycyclopent-2-en-1-(S)-acetate by irreversible transesterification using Chirazyme®. Tetrahedron Asymmetry 1999, 891–899. [Google Scholar] [CrossRef]
- Sharifabad, M.E.; Hodgson, B.; Jellite, M.; Mercer, T.; Sen, T. Enzyme immobilised novel core-shell superparamagnetic nanocomposites for enantioselective formation of 4-(R)-hydroxycyclopent-2-en-1-(S)-acetate. Chem. Commun. 2014, 50, 11185–11187. [Google Scholar] [CrossRef]
- Hinze, J.; Süss, P.; Strohmaier, S.; Bornscheuer, U.T.; Wardenga, R.; von Langermann, J. Recombinant pig liver esterase-catalyzed synthesis of (1S,4R)-4-hydroxy-2-cyclopentenyl acetate combined with subsequent enantioselective crystallization. Org. Process Res. Dev. 2016, 20, 1258–1264. [Google Scholar] [CrossRef]
- Putta, S.; Reddy, A.M.; Shelu, G.R.; Reddy, B.V.S.; Kumaraguru, T. Preparation of (1R,4S)-4-hydroxycyclopent-2-en-1-yl acetate via Novozym-435® catalyzed desymmetrization of cis-3,5-diacetoxy-1-cyclopentene. Tetrahedron 2018, 74, 6673–6679. [Google Scholar] [CrossRef]
- Moni, L.; Banfi, L.; Cartagenova, D.; Cavalli, A.; Lambruschini, C.; Martino, E.; Orru, R.V.A.; Ruijter, E.; Saya, J.M.; Sgrignani, J.; et al. Zinc (II)-mediated diastereoselective Passerini reactions of biocatalytically desymmetrised renewable inputs. Org. Chem. 2020, 7, 380–398. [Google Scholar] [CrossRef]
- Estermann, H.; Prasad, K.; Shapiro, M.J.; Bolsterli, J.J.; Walkinshaw, M.D. Enzyme-catalyzed asymmetrization of bis(hydroxymethyl)-tetrahydrofurans. Tetrahedron Lett. 1990, 31, 445–448. [Google Scholar] [CrossRef]
- Naemura, K.; Fukuda, R.; Konishi, N.M.; Hirose, Y.; Tobe, Y. Enzyme-catalyzed asymmetric acylation and hydrolysis of cis-2,5-disubstituted tetrahydrofuran derivatives: Contribution to development of models for reactions catalyzed by porcine liver esterase and porcine pancreatic lipase. Tetrahedron Asymmetry 1993, 4, 911–918. [Google Scholar] [CrossRef]
- Moni, L.; Banfi, L.; Basso, A.; Carcone, L.; Rasparini, M.; Riva, R. Ugi and Passerini reactions of biocatalytically derived chiral aldehydes: Application to the synthesis of bicyclic pyrrolidines and of antiviral agent telaprevir. J. Org. Chem. 2015, 80, 3411–3428. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Sarkar, A. Enantioselective syntheses of (–)-alloyohimbane and (–)-yohimbane by an efficient enzymatic desymmetrization process. Eur. J. Org. Chem. 2016, 6001–6009. [Google Scholar] [CrossRef] [Green Version]
- Wirz, B.; Idinga, H.; Hilpert, H. Multiselective enzymatic reactions for the synthesis of protected homochiral cis- and trans-1,3,5-cyclohexanetriols. Tetrahedron Asymmetry 2000, 11, 4171–4178. [Google Scholar] [CrossRef]
- González-García, T.; Verstuyf, A.; Verlinden, L.; Fernández, S.; Ferrero, M. Enzymatic desymmetrization of 19-nor-vitamin D3 a-ring synthon precursor: Synthesis, structure elucidation, and biological activity of 1α,25-Dihydroxy-3-epi-19-nor-vitamin D3 and 1β,25-dihydroxy-19-nor-vitamin D3. Adv. Synth. Catal. 2018, 360, 2762–2772. [Google Scholar] [CrossRef]
- Ribeiro, M.F.P.; Pais, K.C.; de Jesus, B.S.M.; Fernandez-Lafuente, R.; Freire, D.M.G.; Manoel, E.A.; Simas, A.B.C. Lipase regioselective O-acetylations of a myo-inositol derivative: Efficient desymmetrization of 1,3-di-O-benzyl-myo-inositol. Eur. J. Org. Chem. 2018, 386–391. [Google Scholar] [CrossRef]
- Liang, J.; Cochran, J.E.; Dorsch, W.A.; Davies, I.; Clark, M.P. Development of a scalable synthesis of an azaindolyl-pyrimidine inhibitor of influenza virus replication. Org. Process Res. Dev. 2016, 20, 965–969. [Google Scholar] [CrossRef]
- Matsuda, T.; Yamanaka, R.; Nakamura, K. Recent progress in biocatalysis for asymmetric oxidation and reduction. Tetrahedron Asymmetry 2009, 20, 513–557. [Google Scholar] [CrossRef]
- Martínez, A.T.; Ruiz-Dueñas, F.J.; Camarero, S.; Serrano, A.; Linde, D.; Lund, H.; Vind, J.; Tovborg, M.; Herold-Majumdar, O.M.; Hofrichter, M.; et al. Oxidoreductases on their way to industrial biotransformations. Biotechnol. Adv. 2017, 35, 815–831. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.; Fernández-Fueyo, E.; Hollmann, F.; Paul, C.E.; Pesic, M.; Schmidt, S.; Wang, Y.; Younes, S.; Zhang, W. Biocatalytic oxidation reactions: A chemist’s perspective. Angew. Chem. Int. Ed. 2018, 57, 9238–9261. [Google Scholar] [CrossRef]
- Kroutil, W.; Mang, H.; Edegger, K.; Faber, K. Recent advances in the biocatalytic reduction of ketones and oxidation of sec-alcohols. Curr. Opin. Chem. Biol. 2004, 8, 120–126. [Google Scholar] [CrossRef]
- Musa, M.M.; Phillips, R.S. Recent advances in alcohol dehydrogenase-catalyzed asymmetric production of hydrophobic alcohols. Catal. Sci. Technol. 2011, 1, 1311–1323. [Google Scholar] [CrossRef]
- Nealon, C.M.; Musa, M.M.; Patel, J.M.; Phillips, R.S. Controlling substrate specificity and stereospecificity of alcohol dehydrogenases. ACS Catal. 2015, 5, 2100–2114. [Google Scholar] [CrossRef]
- Zheng, Y.G.; Yin, H.H.; Yu, D.F.; Chen, X.; Tang, X.-L.; Zhang, X.-J.; Xue, Y.-P.; Wang, Y.-J.; Liu, Z.-Q. Recent advances in biotechnological applications of alcohol dehydrogenases. Appl. Microbiol. Biotechnol. 2017, 101, 987–1001. [Google Scholar] [CrossRef]
- Jones, J.B. Horse Liver Alcohol Dehydrogenase: An Illustrative Example of the Potential of Enzymes in Organic Synthesis. In Enzyme Engineering; Chibata, I., Fukui, S., Wingard, L.B., Eds.; Springer: Boston, MA, USA, 1982; pp. 107–116. [Google Scholar] [CrossRef]
- De Smidt, O.; Du Preez, J.C.; Albertyn, J. The alcohol dehydrogenases of Saccharomyces cerevisiae: A comprehensive review. FEMS Yeast Res. 2008, 8, 967–978. [Google Scholar] [CrossRef] [Green Version]
- Van der Donk, W.A.; Zhao, H. Recent developments in pyridine nucleotide regeneration. Curr. Opin. Biotechnol. 2003, 14, 421–442. [Google Scholar] [CrossRef]
- Hollmann, F.; Arends, I.W.C.E.; Buehler, K. Biocatalytic redox reactions for organic synthesis: Nonconventional regeneration methods. ChemCatChem 2010, 2, 762–782. [Google Scholar] [CrossRef]
- Hildebrandt, P.; Musidlowska, A.; Bornscheuer, U.T.; Altenbuchner, J. Cloning, functional expression and biochemical characterization of a stereoselective alcohol dehydrogenase from Pseudomonas fluorescens DSM50106. Appl. Microbiol. Biotechnol. 2002, 59, 483–487. [Google Scholar] [CrossRef]
- Pennacchio, A.; Pucci, B.; Secundo, F.; La Cara, F.; Rossi, M.; Raia, C.A. Purification and characterization of a novel recombinant highly enantioselective short-chain NAD(H)-dependent alcohol dehydrogenase from Thermus thermophiles. Appl. Environ. Microbiol. 2008, 74, 3949–3958. [Google Scholar] [CrossRef] [Green Version]
- Goldberg, K.; Schroer, K.; Lütz, S.; Liese, A. Biocatalytic ketone reduction-A powerful tool for the production of chiral alcohols-Part II: Whole-cell reductions. Appl. Microbiol. Biotechnol. 2007, 76, 249–255. [Google Scholar] [CrossRef]
- Carballeira, J.D.; Quezada, M.A.; Hoyos, P.; Simeó, Y.; Hernaiza, M.J.; Alcantara, A.R.; Sinisterra, J.V. Microbial cells as catalysts for stereoselective red-ox reactions. Biotechnol. Adv. 2009, 27, 686–714. [Google Scholar] [CrossRef]
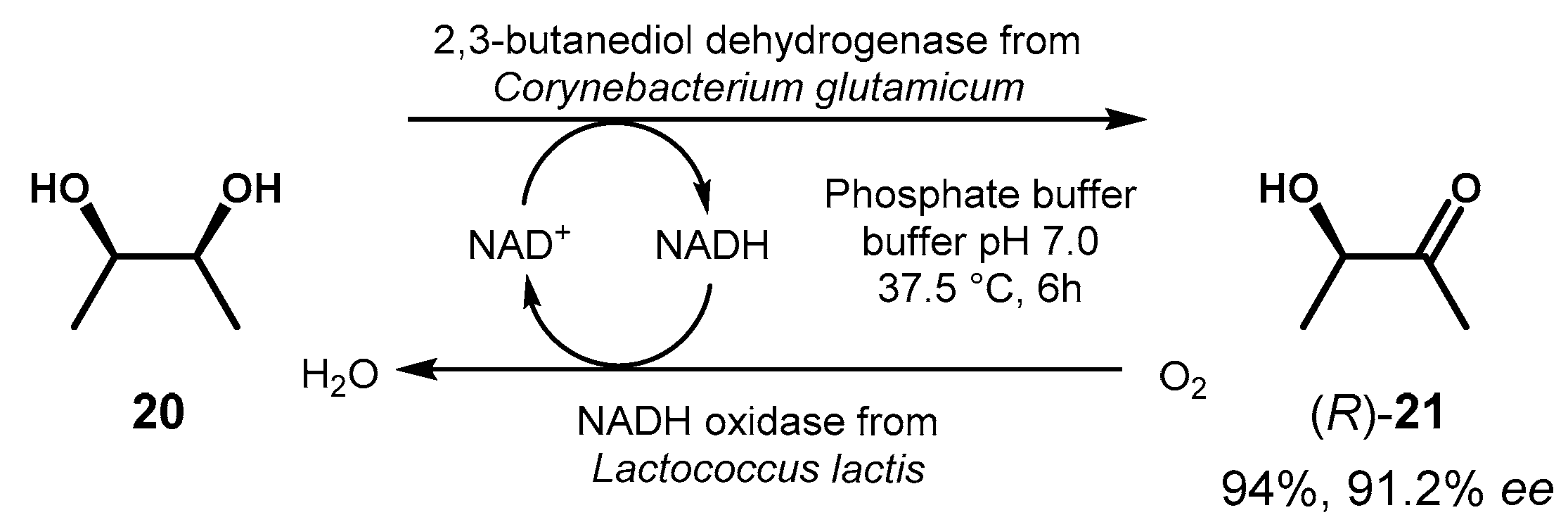
- Cui, Z.; Zhao, Y.; Mao, Y.; Shi, T.; Lu, L.; Ma, H.; Wanga, Z.; Chen, T. In vitro biosynthesis of optically pure D-(−)-acetoin from meso-2,3-butanediol using 2,3-butanediol dehydrogenase and NADH oxidase. J. Chem. Technol. Biotechnol. 2019, 94, 2547–2554. [Google Scholar] [CrossRef]
- Holec, C.; Sandkuhl, D.; Rother, D.; Kroutil, W.; Pietruszka, J. Chemoenzymatic synthesis towards the active agent travoprost. ChemCatChem 2015, 7, 3125–3130. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Horn, M.; Martinez-Rojas, E.; Görisch, H.; Tressl, R.; Garbe, L.A. Oxidation of 1,4-alkanediols into γ-lactones via γ-lactols using Rhodococcus erythropolis as biocatalyst. J. Mol. Catal. B Enzym. 2007, 49, 24–27. [Google Scholar] [CrossRef]
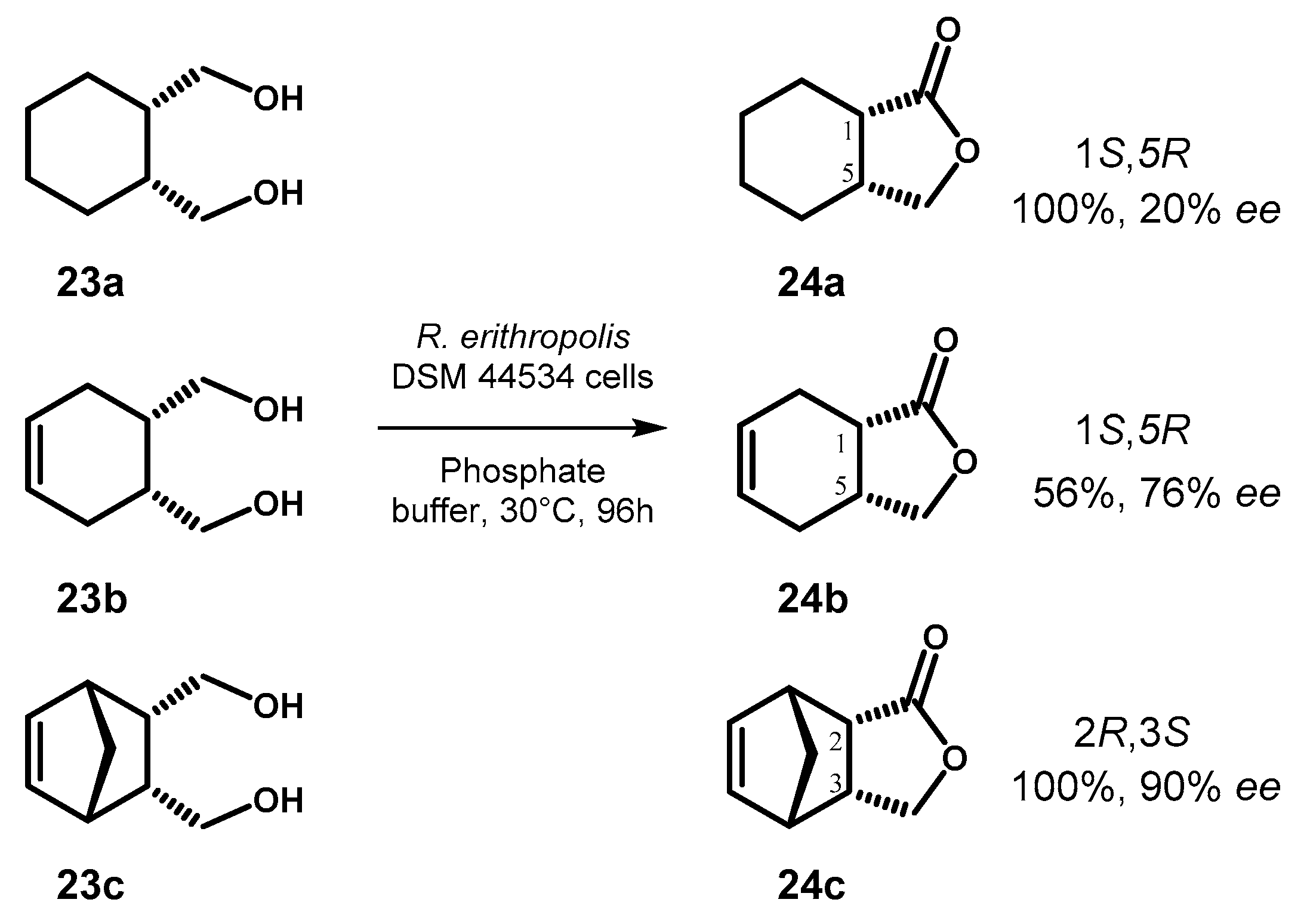
- Martinez-Rojas, E.; Olejniczak, T.; Neumann, K.; Garbe, L.-A.; Boratyński, F. Simple preparation of Rhodococcus erythropolis DSM 44534 as biocatalyst to oxidize diols into the optically active lactones. Chirality 2016, 28, 623–627. [Google Scholar] [CrossRef]
- Sugai, T.; Yamazaki, T.; Yokoyama, M.; Ohta, H. Biocatalysis in organic synthesis: The use of nitrile- and amide-hydrolyzing microorganisms. Biosci. Biotechnol. Biochem. 1997, 61, 1419–1427. [Google Scholar] [CrossRef]
- Bhalla, T.C.; Kumar, V.; Kumar, V.; Thakur, N.S. Nitrile metabolizing enzymes in biocatalysis and biotransformation. Appl. Biochem. Biotechnol. 2018, 185, 925–946. [Google Scholar] [CrossRef]
- Wang, M.-X. Enantioselective biotransformations of nitriles in organic synthesis. Acc. Chem. Res. 2015, 48, 602–611. [Google Scholar] [CrossRef] [PubMed]
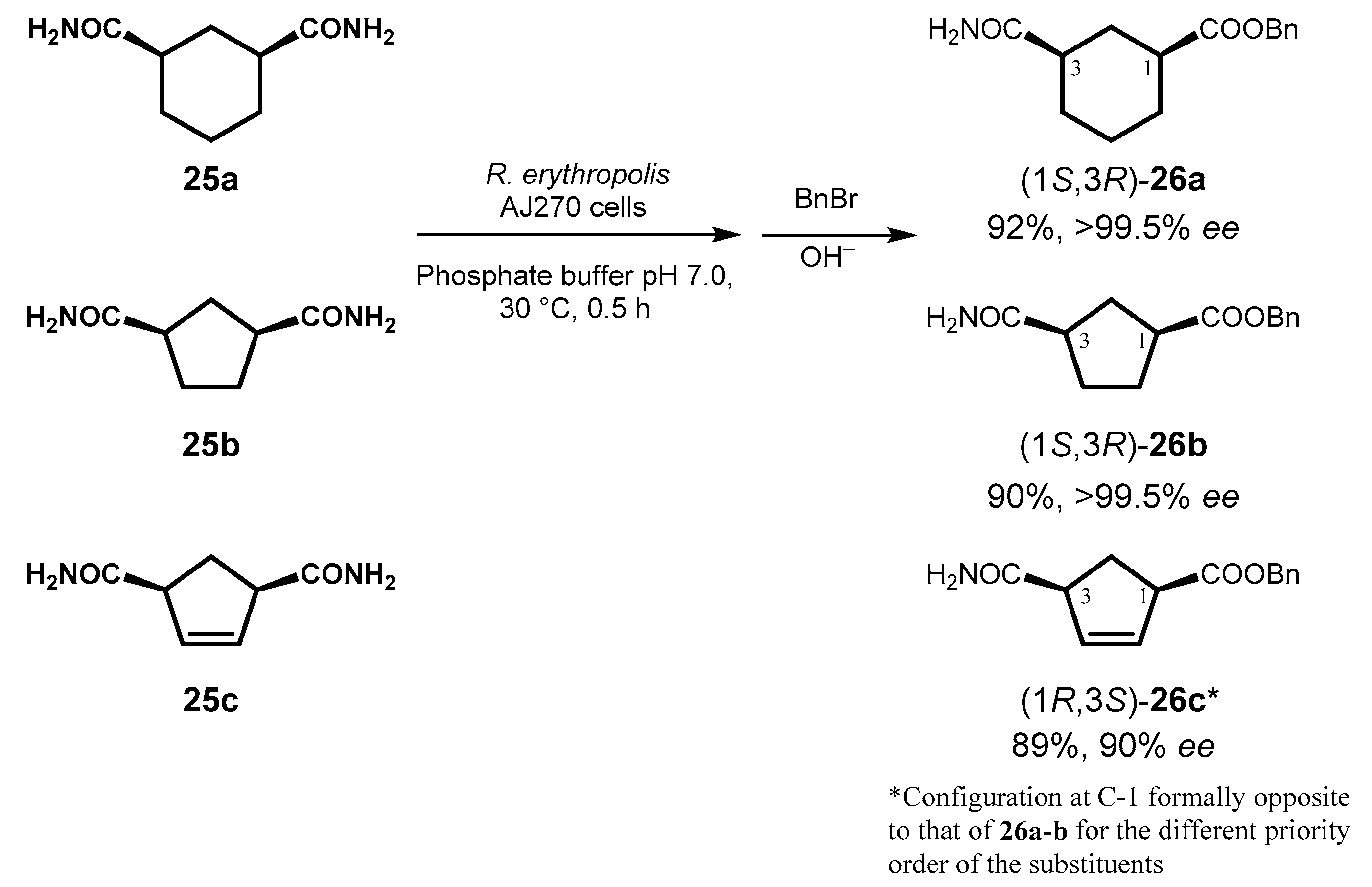
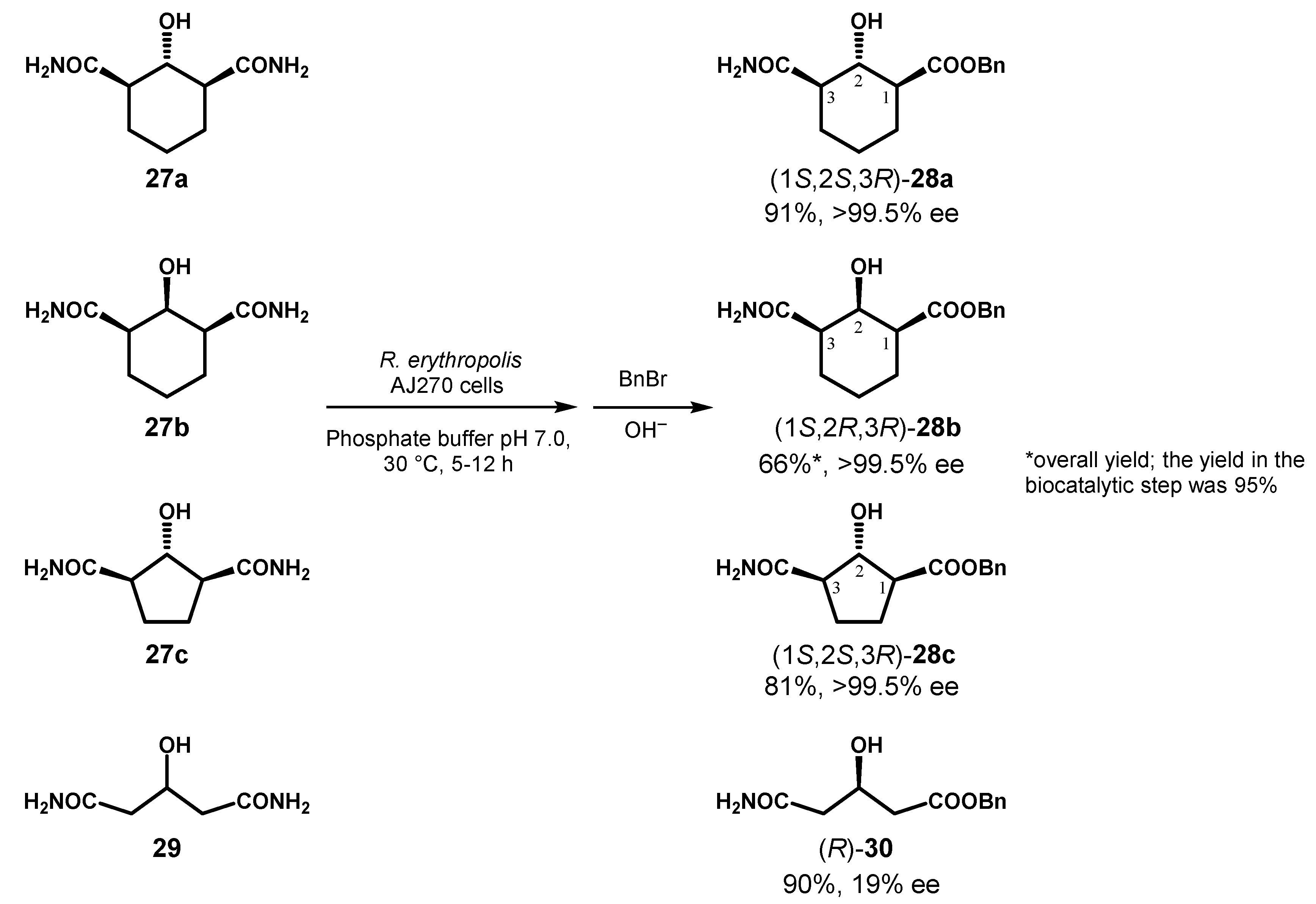
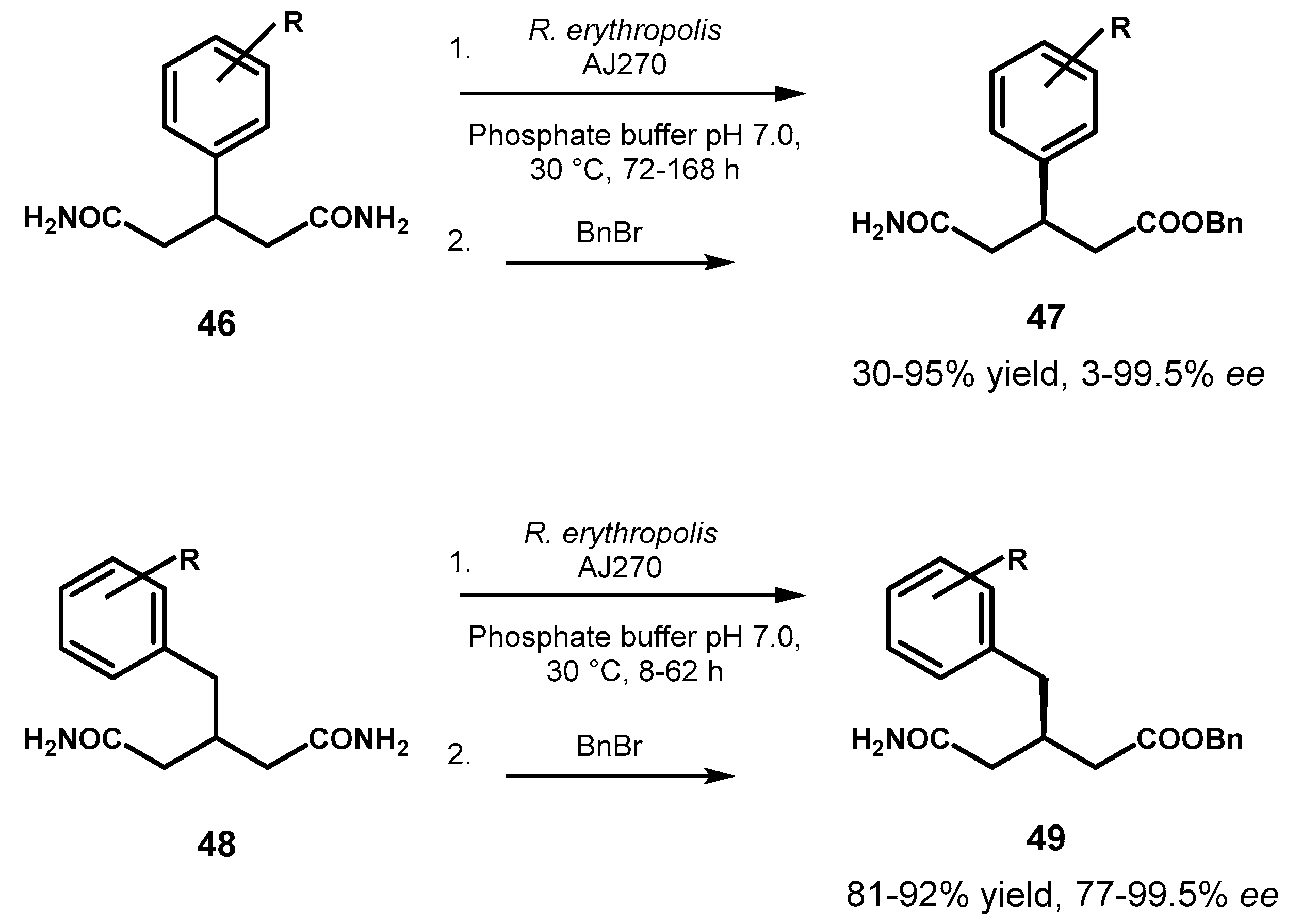
- Hu, H.-J.; Chen, P.; Ao, Y.-F.; Wang, Q.-Q.; Wang, D.-X.; Wang, M.-X. Highly efficient biocatalytic desymmetrization of meso carbocyclic 1,3-dicarboxamides: A versatile route for enantiopure 1,3-disubstituted cyclohexanes and cyclopentanes. Org. Chem. Front. 2019, 6, 808–812. [Google Scholar] [CrossRef]
- Ao, Y.-F.; Wang, D.-X.; Zhao, L.; Wang, M.-X. Synthesis of quaternary-carbon-containing and functionalized enantiopure pentanecarboxylic acids from biocatalytic desymmetrization of meso-cyclopentane-1,3-dicarboxamides. Chem. Asian J. 2015, 10, 938–947. [Google Scholar] [CrossRef] [PubMed]
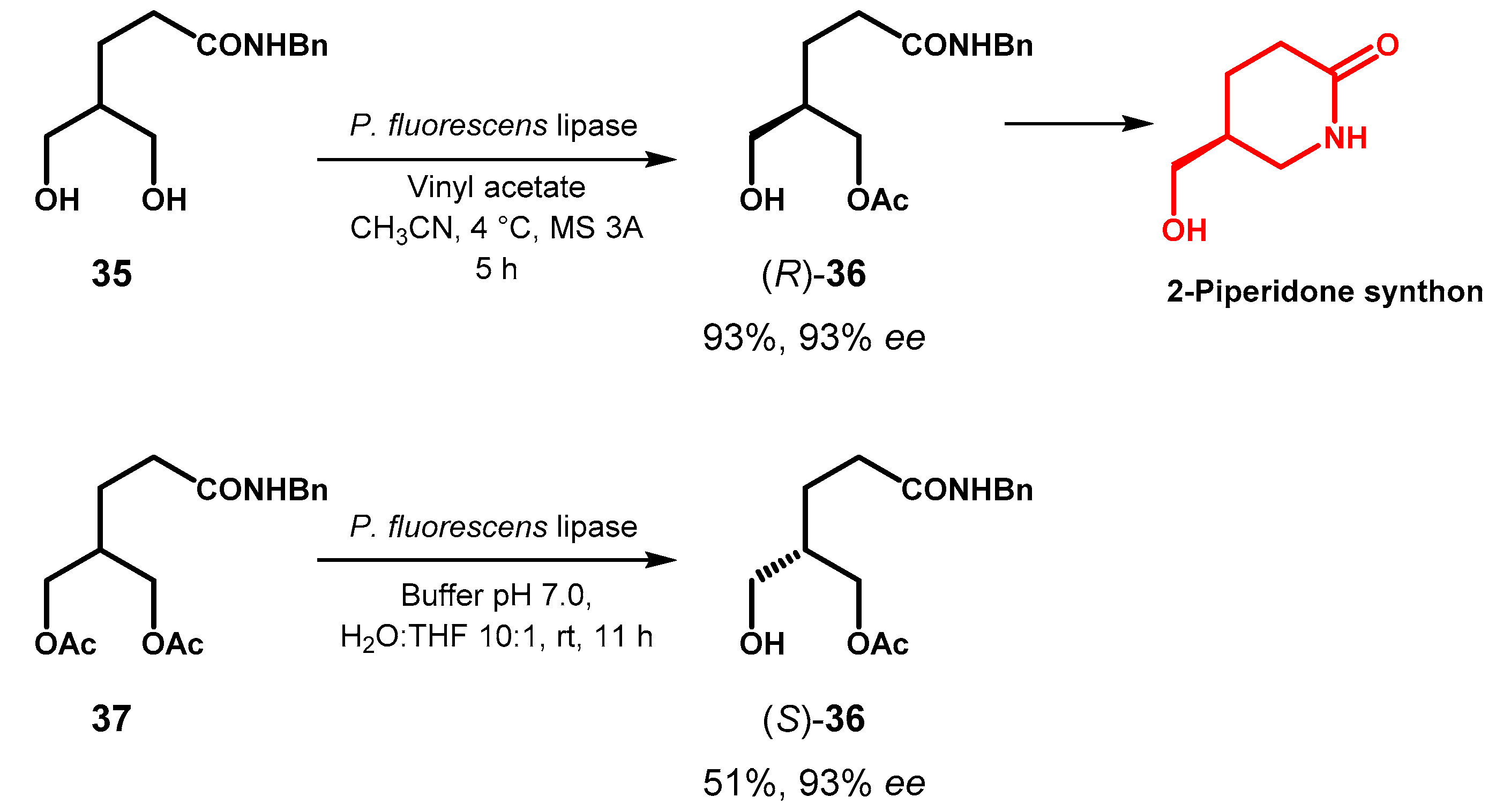
- Khong, D.T.; Pamarthy, V.S.; Gallagher, T.; Judeh, Z.M.A. Chemoenzymatic synthesis of chiral 1-benzyl-5-(hydroxymethyl)-2-piperidone enabled by lipase AK-mediated desymmetrization of prochiral 1,3-diol and its diacetate. Eur. J. Org. Chem. 2016, 3084–3089. [Google Scholar] [CrossRef] [Green Version]
- Bhuniya, R.; Nanda, S. Asymmetric total synthesis of (–)-rasfonin. Tetrahedron 2013, 69, 1153–1165. [Google Scholar] [CrossRef]
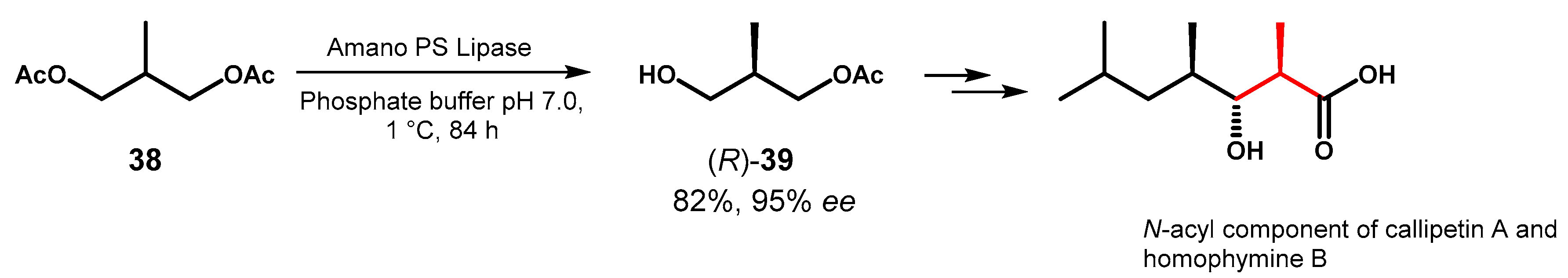
- Tokairin, Y.; Konno, H. Preparation of (2R, 3R, 4R)-3-hydroxy-2,4,6-trimethylheptanoic acid via enzymatic desymmetrization. Tetrahedron 2017, 73, 39–45. [Google Scholar] [CrossRef]
- Kołodziejskaa, R.; Kwit, M.; Studzinska, R.; Jelecki, M. Enantio- and diastereoselective acylation of prochiral hydroxyl group of pyrimidine acyclonucleosides. J. Mol. Catal. B Enzym. 2016, 133, 98–106. [Google Scholar] [CrossRef]
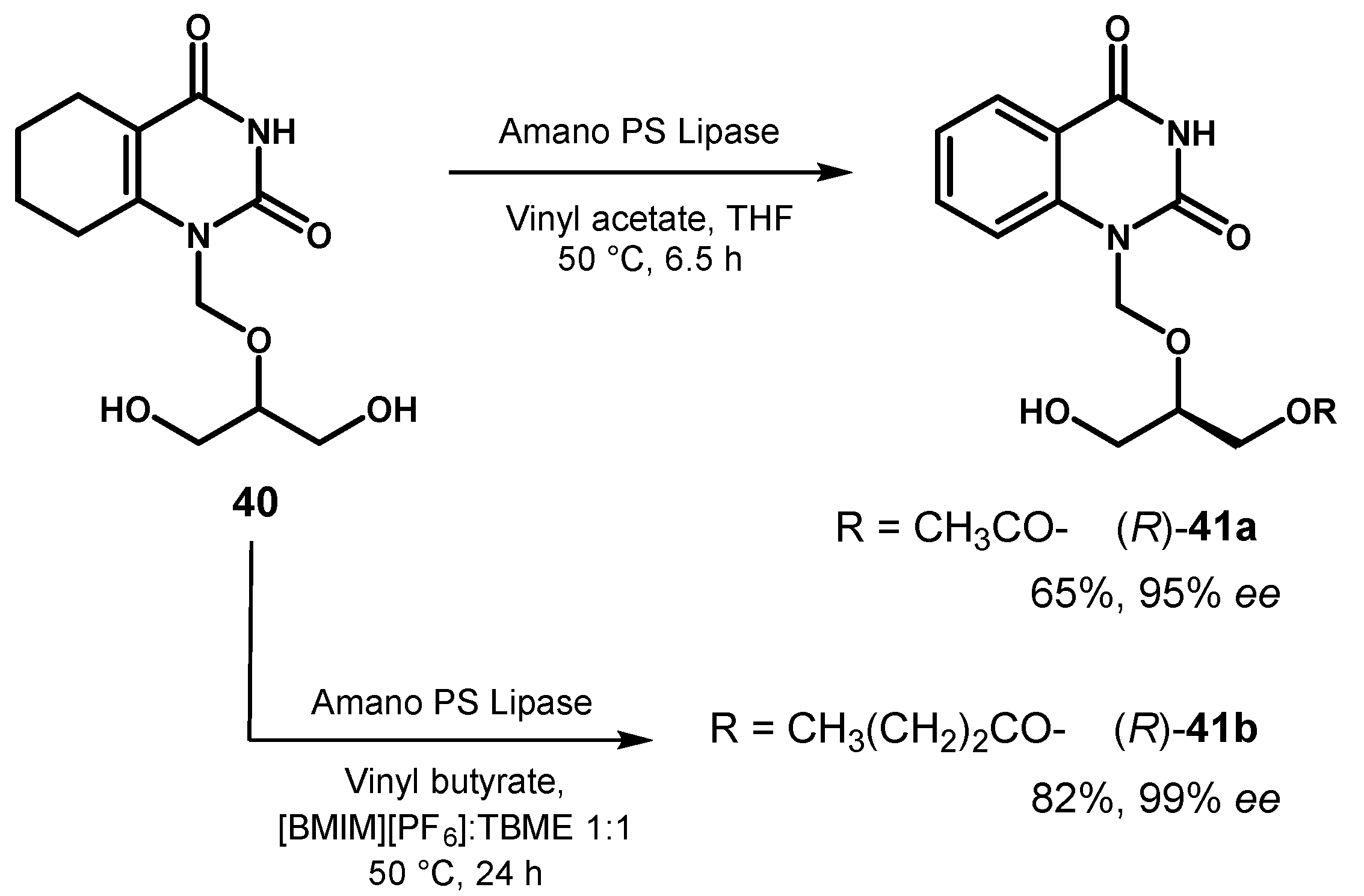
- Kołodziejska, R.; Studzińska, R.; Pawluk, H. Lipase-catalyzed enantioselective transesterification of prochiral 1-((1,3-dihydroxypropan-2-yloxy)methyl)-5,6,7,8-tetrahydroquinazoline-2,4 (1H,3H)-dione in ionic liquids. Chirality 2018, 30, 206–214. [Google Scholar] [CrossRef]
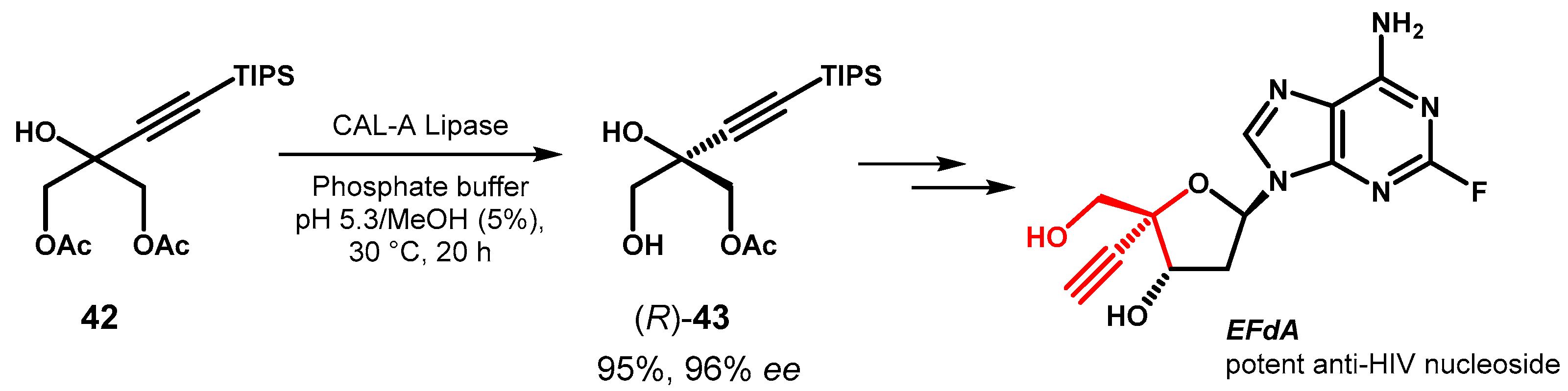
- McLaughlin, M.; Kong, J.; Belyk, K.M.; Chen, B.; Gibson, A.W.; Keen, S.P.; Lieberman, D.R.; Milczek, E.M.; Moore, J.C.; Murray, D.; et al. Enantioselective synthesis of 4′-ethynyl-2-fluoro-2′-deoxyadenosine (EFdA) via enzymatic desymmetrization. Org. Lett. 2017, 19, 926–929. [Google Scholar] [CrossRef]
- Romano, D.; Villa, R.; Molinari, F. Preparative biotransformations: Oxidation of alcohols. ChemCatChem 2012, 4, 739–749. [Google Scholar] [CrossRef]
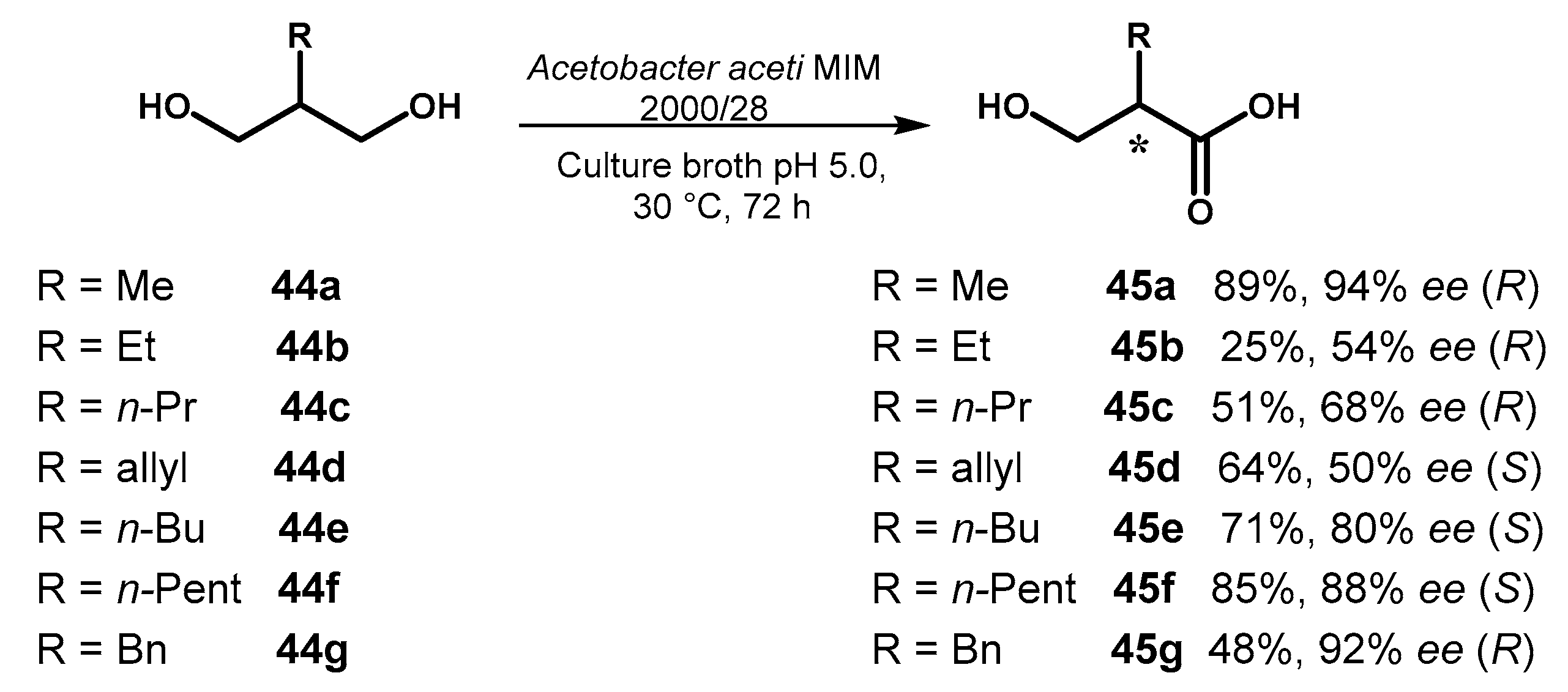
- Brenna, E.; Cannavale, F.; Crotti, M.; De Vitis, V.; Gatti, F.G.; Migliazza, G.; Molinari, F.; Parmeggiani, F.; Romano, D.; Santangelo, S. Synthesis of enantiomerically enriched 2-hydroxymethylalkanoic acids by oxidative desymmetrisation of achiral 1,3-diols mediated by Acetobacter aceti. ChemCatChem 2016, 8, 3796–3803. [Google Scholar] [CrossRef] [Green Version]
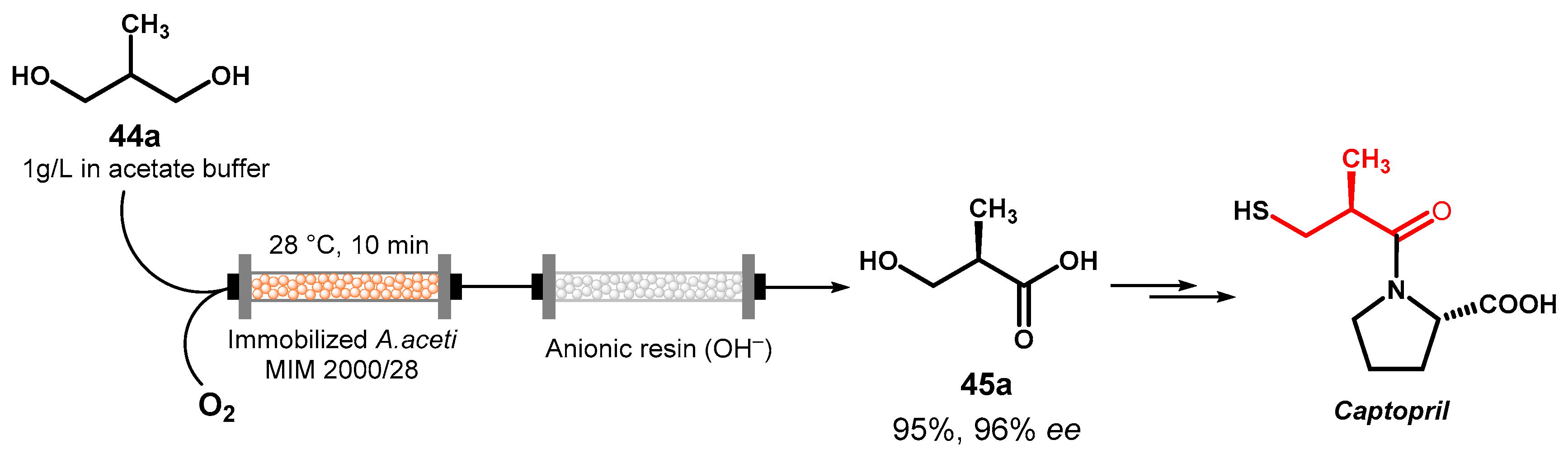
- De Vitis, V.; Dall’Oglio, F.; Pinto, A.; De Micheli, C.; Molinari, F.; Conti, P.; Romano, D.; Tamborini, L. Chemoenzymatic synthesis in flow reactors: A rapid and convenient preparation of captopril. ChemistryOpen 2017, 6, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Ao, Y.-F.; Zhang, L.-B.; Wang, Q.-Q.; Wang, D.-X.; Wang, M.-X. Biocatalytic desymmetrization of prochiral 3-aryl and 3-arylmethyl glutaramides: Different remote substituent effect on catalytic efficiency and enantioselectivity. Adv. Synth. Catal. 2018, 360, 4594–4603. [Google Scholar] [CrossRef]
- Nakamura, K.; Yamanaka, R.; Matsuda, T.; Harada, T. Recent developments in asymmetric reduction of ketones with biocatalysts. Tetrahedron Asymmetry 2003, 14, 2659–2681. [Google Scholar] [CrossRef]
- Wolfson, A.; Dlugy, C.; Tavor, D. Baker’s yeast catalyzed asymmetric reduction of prochiral ketones in different reaction mediums. Org. Commun. 2013, 6, 1–11. [Google Scholar]
- De Souza Pereira, R. The use of baker’s yeast in the generation of asymmetric centers to produce chiral drugs and other compounds. Crit. Rev. Biotechnol. 1998, 18, 25–64. [Google Scholar] [CrossRef]
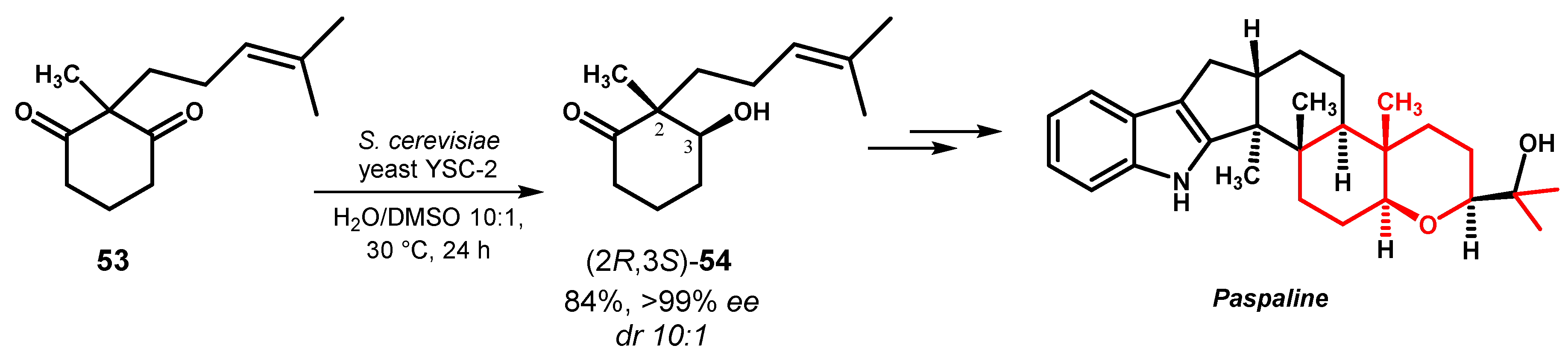
- Sharpe, R.J.; Johnson, J.S. A global and local desymmetrization approach to the synthesis of steroidal alkaloids: Stereocontrolled total synthesis of paspaline. J. Am. Chem. Soc. 2015, 137, 4968–4971. [Google Scholar] [CrossRef] [Green Version]
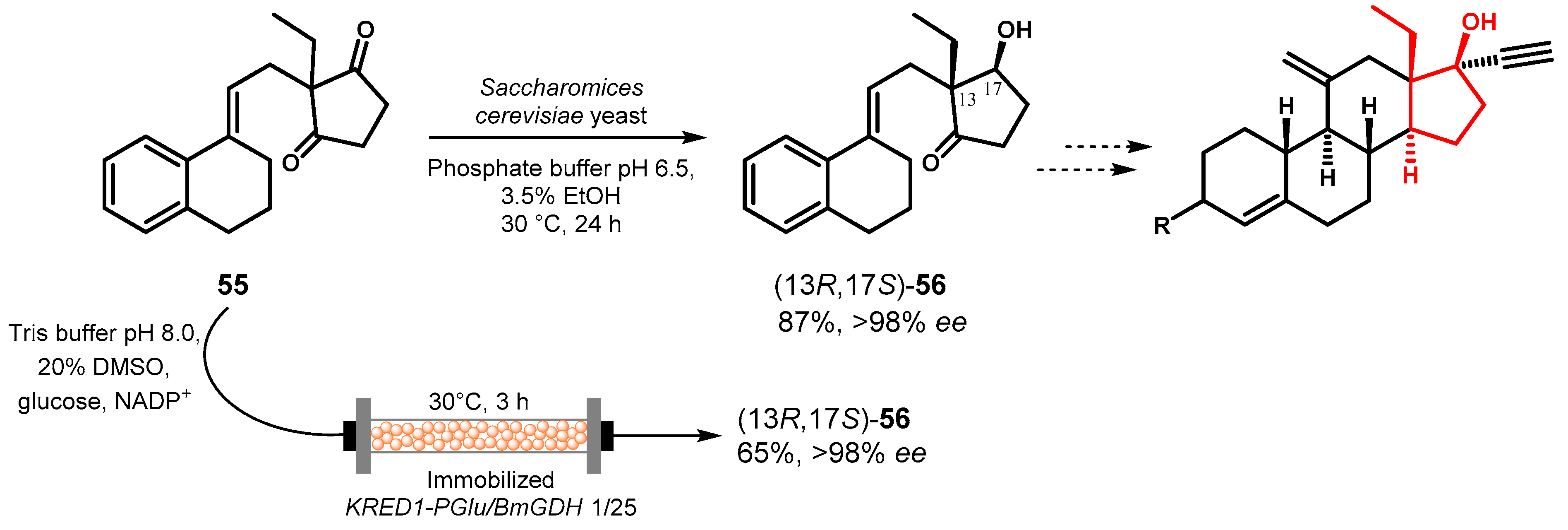
- Contente, M.L.; Molinari, F.; Serra, I.; Pinto, A.; Romano, D. Stereoselective enzymatic reduction of ethyl secodione: Preparation of a key intermediate for the total synthesis of steroids. Eur. J. Org. Chem. 2016, 1260–1263. [Google Scholar] [CrossRef]
- Dall’Oglio, F.; Contente, M.L.; Conti, P.; Molinari, F.; Monfredi, D.; Pinto, A.; Romano, D.; Ubiali, D.; Tamborini, L.; Serra, I. Flow-based stereoselective reduction of ketones using an immobilized ketoreductase/glucose dehydrogenase mixed bed system. Catal. Commun. 2017, 93, 29–32. [Google Scholar] [CrossRef]
- Chebil, L.; Humeau, C.; Falcimaigne, A.; Engasser, J.-M.; Ghoul, M. Enzymatic acylation of flavonoids. Process Biochem. 2006, 41, 2237–2251. [Google Scholar] [CrossRef]
- De Araújo, M.E.M.B.; Franco, Y.E.M.; Messias, M.C.F.; Longato, G.B.; Pamphile, J.A.; de Carvalho, P. Biocatalytic synthesis of flavonoid esters by lipases and their biological benefits. Planta Med. 2017, 7–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gascoyne, D.G.; Finkbeiker, H.L.; Chan, K.P.; Gordon, J.L.; Stewart, K.R.; Kazlauskas, R.J. Molecular Basis for Enantioselectivity of Lipase from Chromobacterium viscosum toward the Diesters of 2,3-Dihydro-3-(4′-hydroxyphenyl)-1,1,3-trimethyl-1H-inden-5-ol. J. Org. Chem. 2001, 66, 3041–3048. [Google Scholar] [CrossRef]
- Lehmann, S.V.; Breinholt, J.; Bury, P.S.; Nielsen, T.E. Enzymatic resolution to (−)-ormeloxifene intermediates from their racemates using immobilized Candida rugosa lipase. Chirality 2000, 12, 568–573. [Google Scholar] [CrossRef]
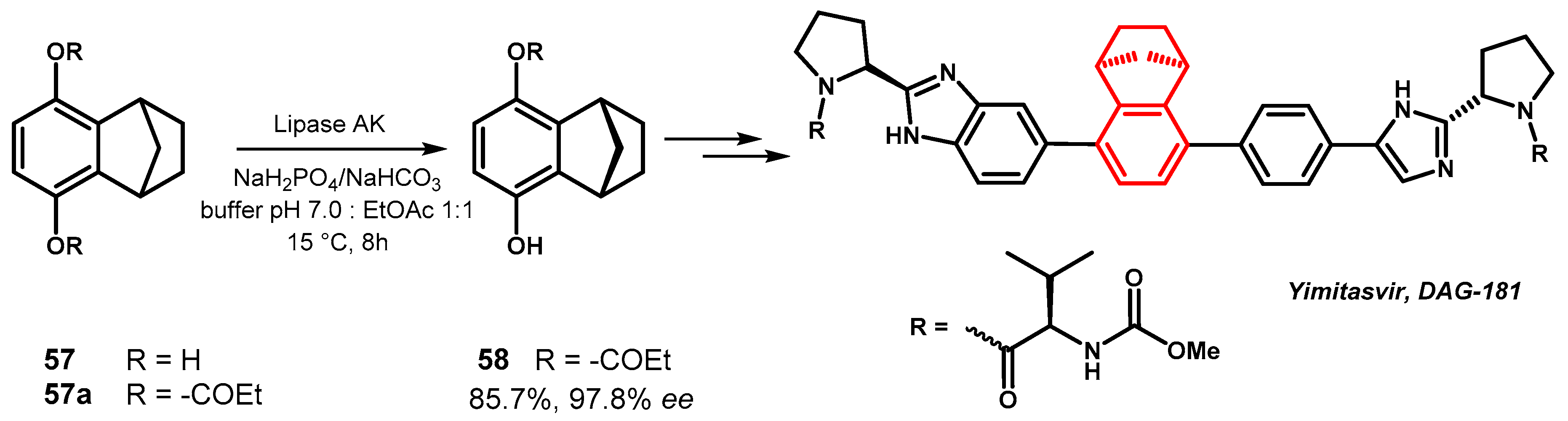
- Zhang, Y.; Zhang, J.; Xie, H.; Ren, Q.; Tan, Y.; Luo, H. Bridged Ring Compounds as Hepatitis C Virus (HCV) Inhibitors and Pharmaceutical Applications Thereof. U.S. Patent 0,193,44, 5 August 2013. [Google Scholar]
- Feng, Y.; Wang, Z.; Luo, Z.; Lai, J.; Xie, H.; Luo, Z.; Zhang, L.; Li, R.; Zhang, Y. Development of an efficient and scalable biocatalytic route to (1S,4R)-8-Hydroxy-1,2,3,4-tetrahydro-1,4-methano-naphthalen-5-yl propionate via enantioselective enzymatic desymmetrization of a prochiral diester. Org. Process Res. Dev. 2019, 23, 1243–1251. [Google Scholar] [CrossRef]
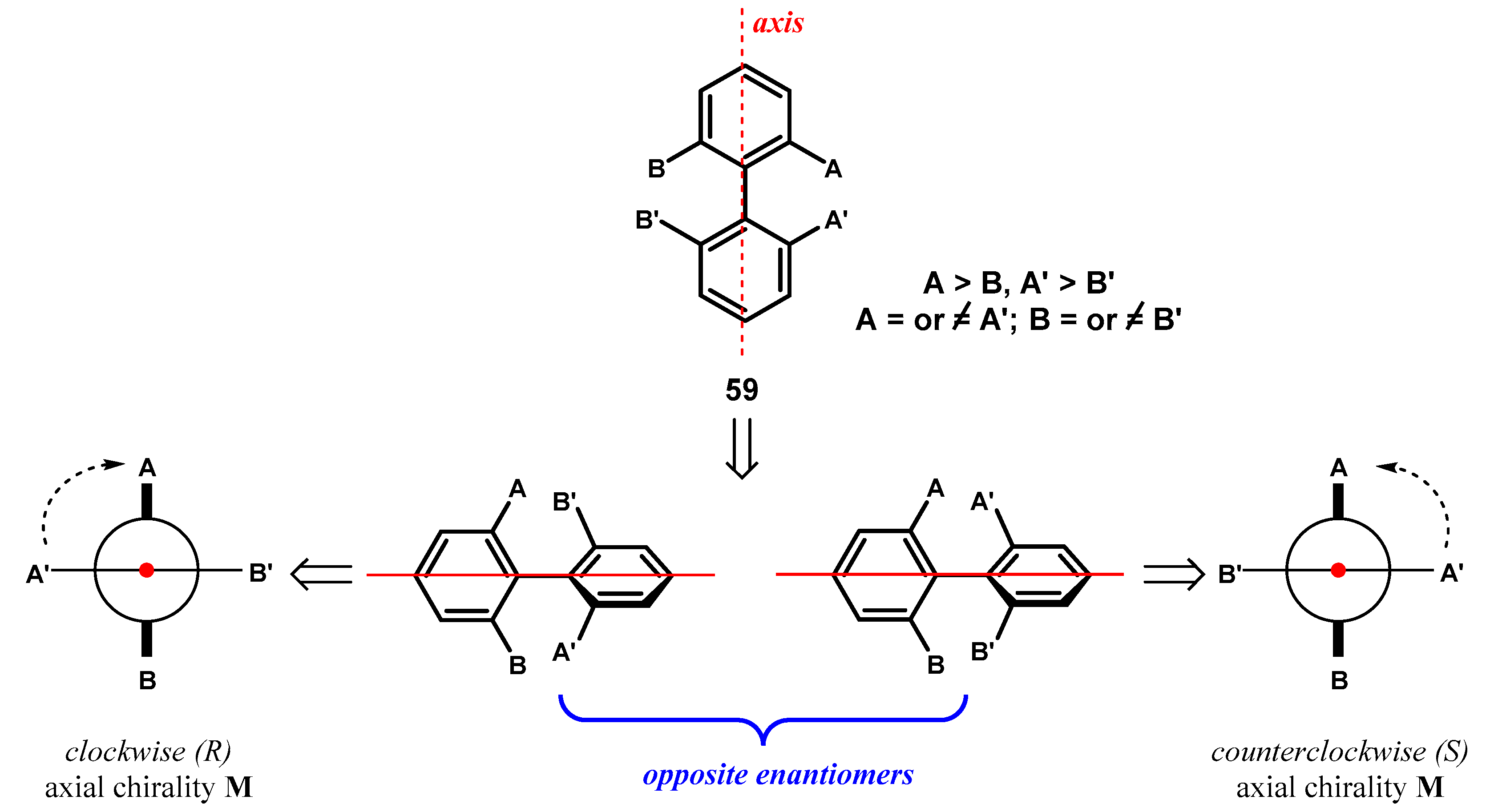
- Bringmann, G.; Günther, C.; Ochse, M.; Schupp, O.; Tasler, S. Biaryls in nature: A multi-facetted class of stereochemically, biosynthetically, and pharmacologically intriguing secondary metabolites. Fortschr. Chem. Org. Naturst. 2001, 82, 1–249. [Google Scholar] [CrossRef]
- Baudoin, O.; Guéritte, F. Natural Bridged Biaryls with Axial Chirality and Antimitotic Properties. In Studies in Natural Products Chemistry, 1st ed.; Atta-ur-Rahman, Ed.; Elsevier Science: Amsterdam, The Netherlands, 2003; Volume 29, part J; pp. 355–417. [Google Scholar] [CrossRef]
- Bringmannm, G.; Price, A.J.; Keller, M.P.A.; Gresser, M.J.; Garner, J.; Breuning, M. Atroposelective synthesis of axially chiral biaryl compounds. Angew. Chem. Int. Ed. 2005, 44, 5384–5427. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-B.; Tan, B. Construction of axially chiral compounds via asymmetric organocatalysis. Acc. Chem. Res. 2018, 51, 534–547. [Google Scholar] [CrossRef]
- Loxq, P.; Manoury, E.; Poli, R.; Deydier, E.; Labande, A. Synthesis of axially chiral biaryl compounds by asymmetric catalytic reactions with transition metals. Coord. Chem. Rev. 2016, 308, 131–190. [Google Scholar] [CrossRef]
- Ma, G.; Sibi, M.P. Catalytic kinetic resolution of biaryl compounds. Chem. Eur. J. 2015, 21, 11644–11657. [Google Scholar] [CrossRef]
- Moustafa, G.A.I.; Oki, Y.; Akai, S. Lipase-catalyzed dynamic kinetic resolution of C1-and C2-symmetric racemic axially chiral 2,2′-dihydroxy-1,1′-biaryls. Angew. Chem. Int. Ed. 2018, 57, 10278–10282. [Google Scholar] [CrossRef]
- Harada, T.; Tuyet, T.M.T.; Oku, A. Asymmetric desymmetrization of 2,2‘,6,6‘-tetrahydroxybiphenyl through annulation with enantiomerically pure bis (mesylate). Org. Lett. 2000, 2, 1319–1322. [Google Scholar] [CrossRef]
- Staniland, S.; Yuan, B.; Giménez-Agulló, N.; Marcelli, T.; Willies, S.C.; Grainger, D.M.; Turner, N.J.; Clayden, J. Enzymatic desymmetrising redox reactions for the asymmetric synthesis of biaryl atropisomers. Chem. Eur. J. 2014, 20, 13084–13088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumoto, T.; Konegawa, T.; Nakamura, T.; Suzuki, K. Facile and highly enantioselective synthesis of axially chiral biaryls by enzymatic desymmetrization. Synlett 2002, 122–124. [Google Scholar] [CrossRef]
- Kasama, K.; Aoyama, H.; Akai, S. Enantiodivergent synthesis of axially chiral biphenyls from σ-symmetric 1,1’-biphenyl-2,6-diol derivatives by single lipase-catalyzed acylative and hydrolytic desymmetrization. Eur. J. Org. Chem. 2020, 654–661. [Google Scholar] [CrossRef]
- Moustafa, G.A.I.; Kasama, K.; Higashio, K.; Akai, S. Base-promoted lipase-catalyzed kinetic resolution of atropisomeric 1,1′-biaryl-2,2′-diols. RSC Adv. 2019, 9, 1165–1175. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, A.G. Recent advances in acyclic stereocontrol. Tetrahedron 2011, 67, 9639–9667. [Google Scholar] [CrossRef]
- Chauhan, P.; Kaya, U.; Enders, D. Advances in organocatalytic 1,6-addition reactions: Enantioselective construction of remote stereogenic centers. Adv. Synth. Catal. 2017, 359, 888–912. [Google Scholar] [CrossRef]
- Alfaro Blasco, M.; Gröger, H. Enzymatic resolution of racemates with a ‘remote’ stereogenic center as an efficient tool in drug, flavor and vitamin synthesis. Bioorg. Med. Chem. 2014, 22, 5539–5546. [Google Scholar] [CrossRef]
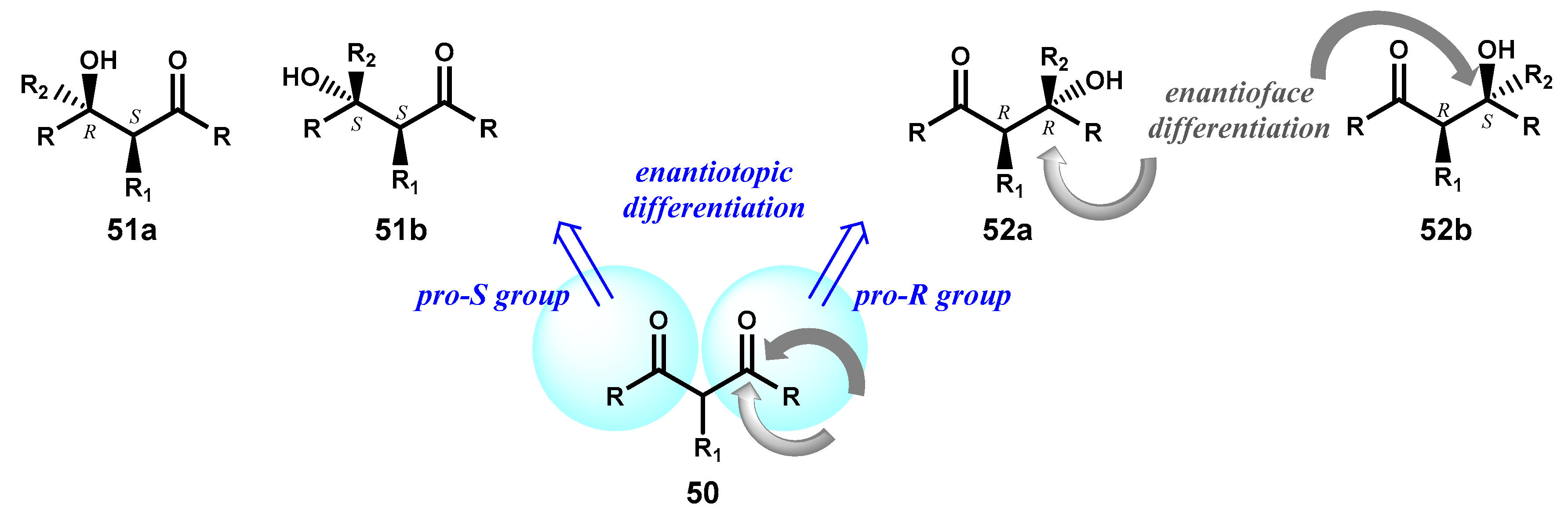
- Cunha, R.L.O.R.; Ferreira, E.A.; Oliveira, C.S.; Omori, Á.T. Biocatalysis for desymmetrization and resolution of stereocenters beyond the reactive center: How far is far enough? Biotechnol. Adv. 2015, 33, 614–623. [Google Scholar] [CrossRef]
- Skoupi, M.; Vaxelaire, C.; Strohmann, C.; Christmann, M.; Niemeyer, C.M. Enantiogroup-differentiating biocatalytic reductions of prochiral Cs-symmetrical dicarbonyl compounds to meso compounds. Chem. Eur. J. 2015, 21, 8701–8705. [Google Scholar] [CrossRef]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patti, A.; Sanfilippo, C. Breaking Molecular Symmetry through Biocatalytic Reactions to Gain Access to Valuable Chiral Synthons. Symmetry 2020, 12, 1454. https://doi.org/10.3390/sym12091454
Patti A, Sanfilippo C. Breaking Molecular Symmetry through Biocatalytic Reactions to Gain Access to Valuable Chiral Synthons. Symmetry. 2020; 12(9):1454. https://doi.org/10.3390/sym12091454
Chicago/Turabian StylePatti, Angela, and Claudia Sanfilippo. 2020. "Breaking Molecular Symmetry through Biocatalytic Reactions to Gain Access to Valuable Chiral Synthons" Symmetry 12, no. 9: 1454. https://doi.org/10.3390/sym12091454





