Dihydrotestosterone (DHT) Enhances Wound Healing of Major Burn Injury by Accelerating Resolution of Inflammation in Mice
Abstract
1. Introduction
2. Results
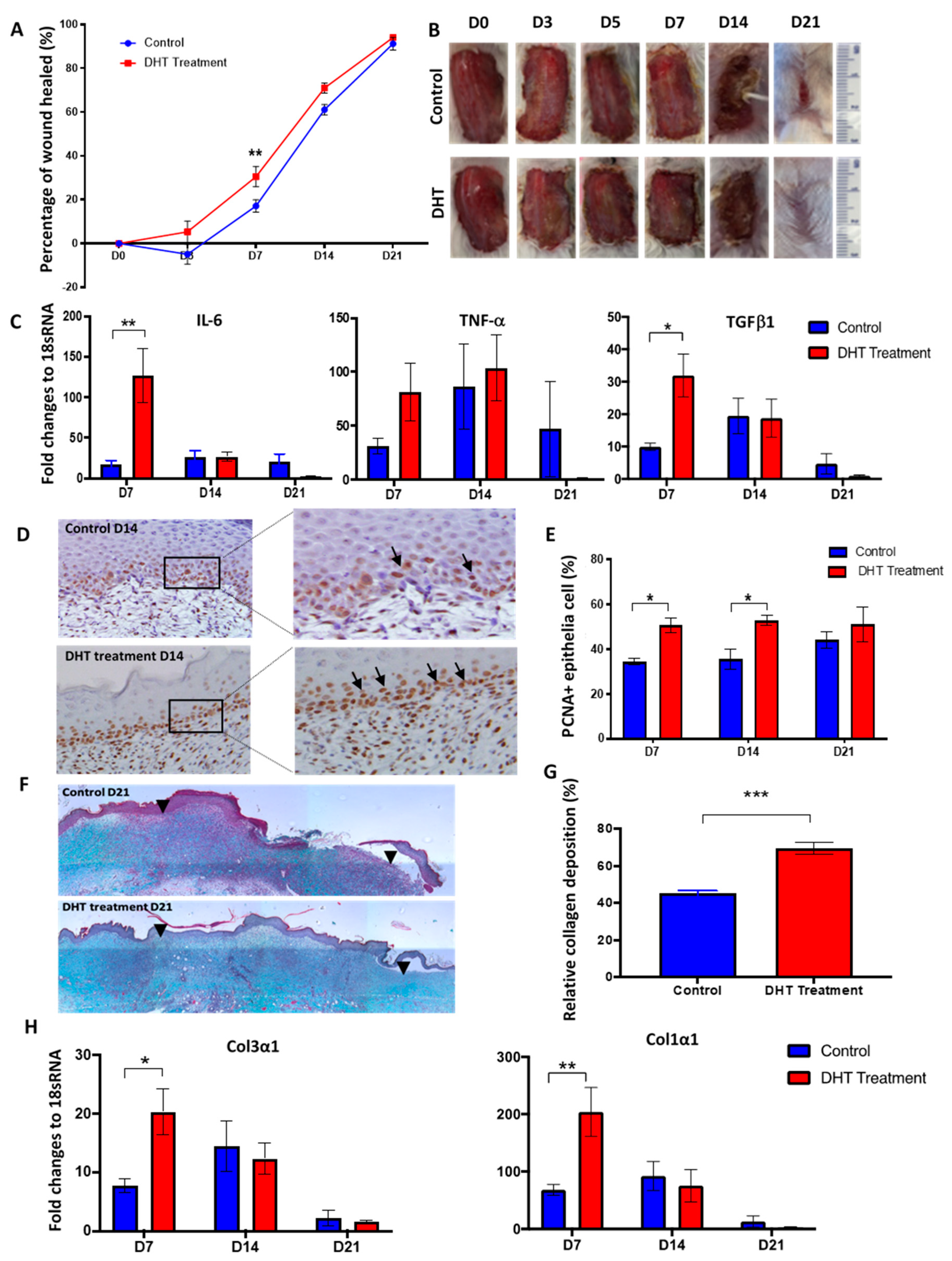
2.1. DHT Accelerates Local Wound Healing in Mice Post Major Burn Injury
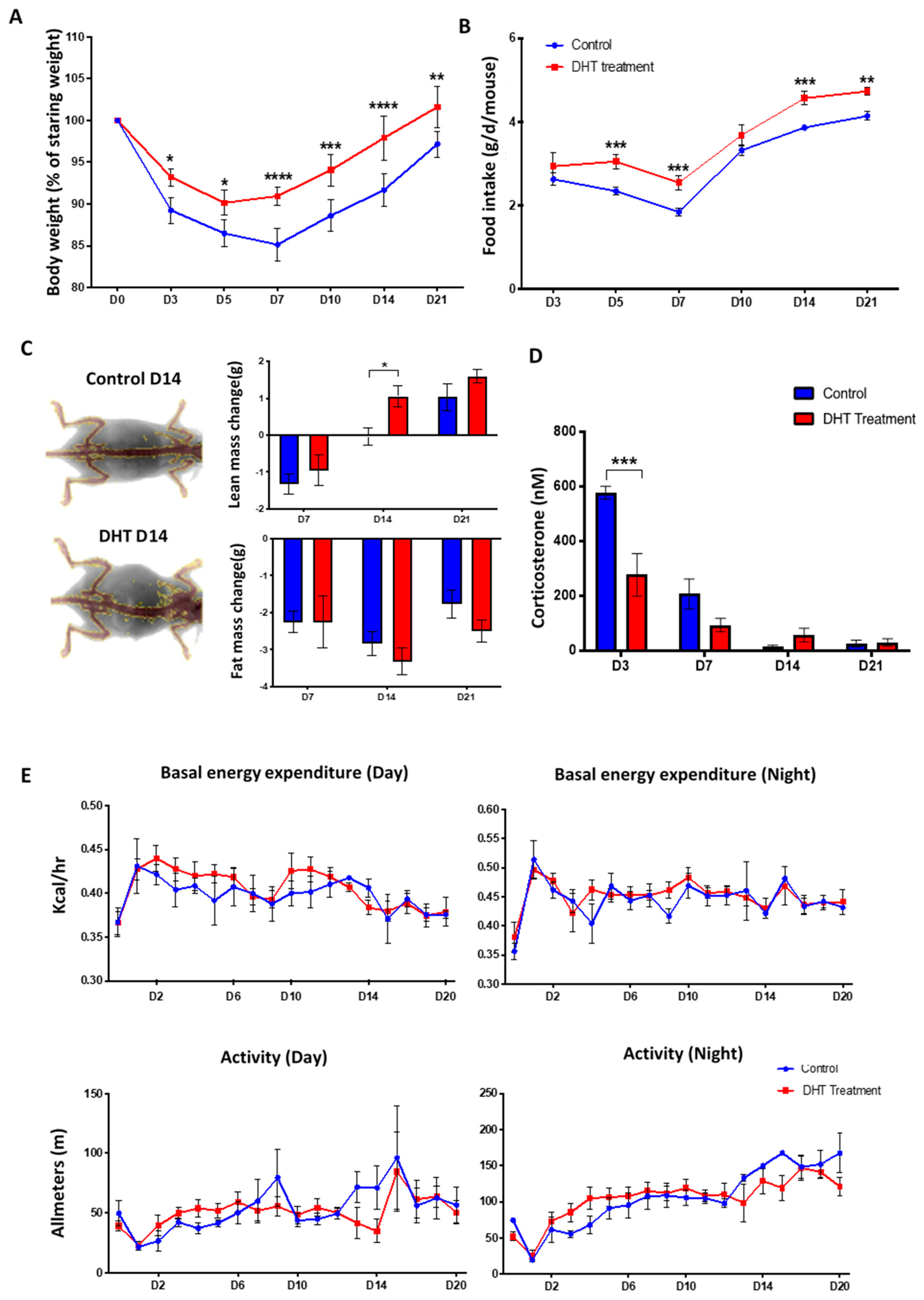
2.2. DHT Better Maintained Body Weight but Had No Effects on Metabolic Response Following Major Burn Injury
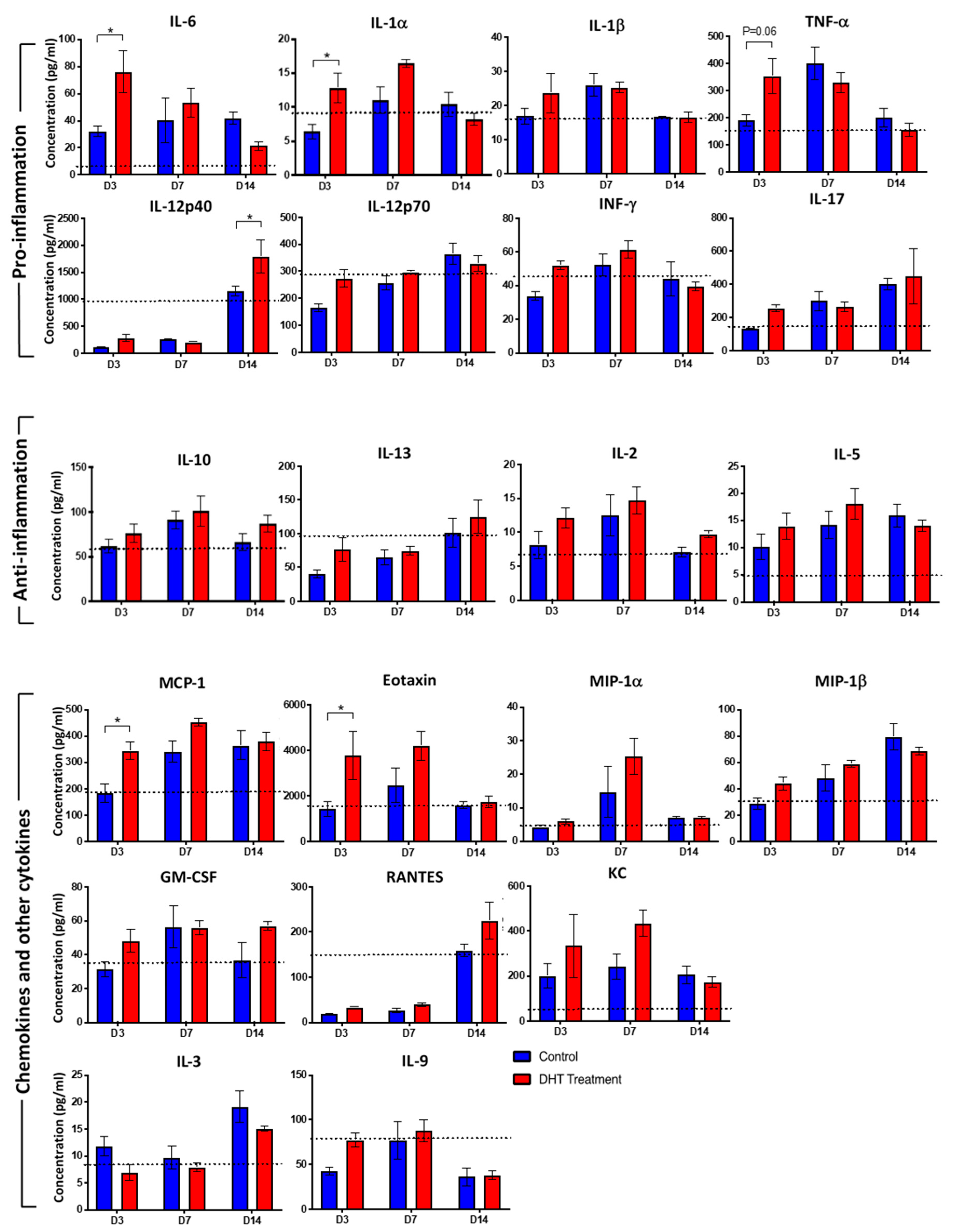
2.3. DHT Treatment Enhances Inflammatory Cytokines and Chemokines Release
2.4. DHT Treatment Preserves Normal Splenic Structure Post Major Burn Injury
2.5. Monocytes and Macrophages are Critical for Facilitating Major Burn Wound Healing
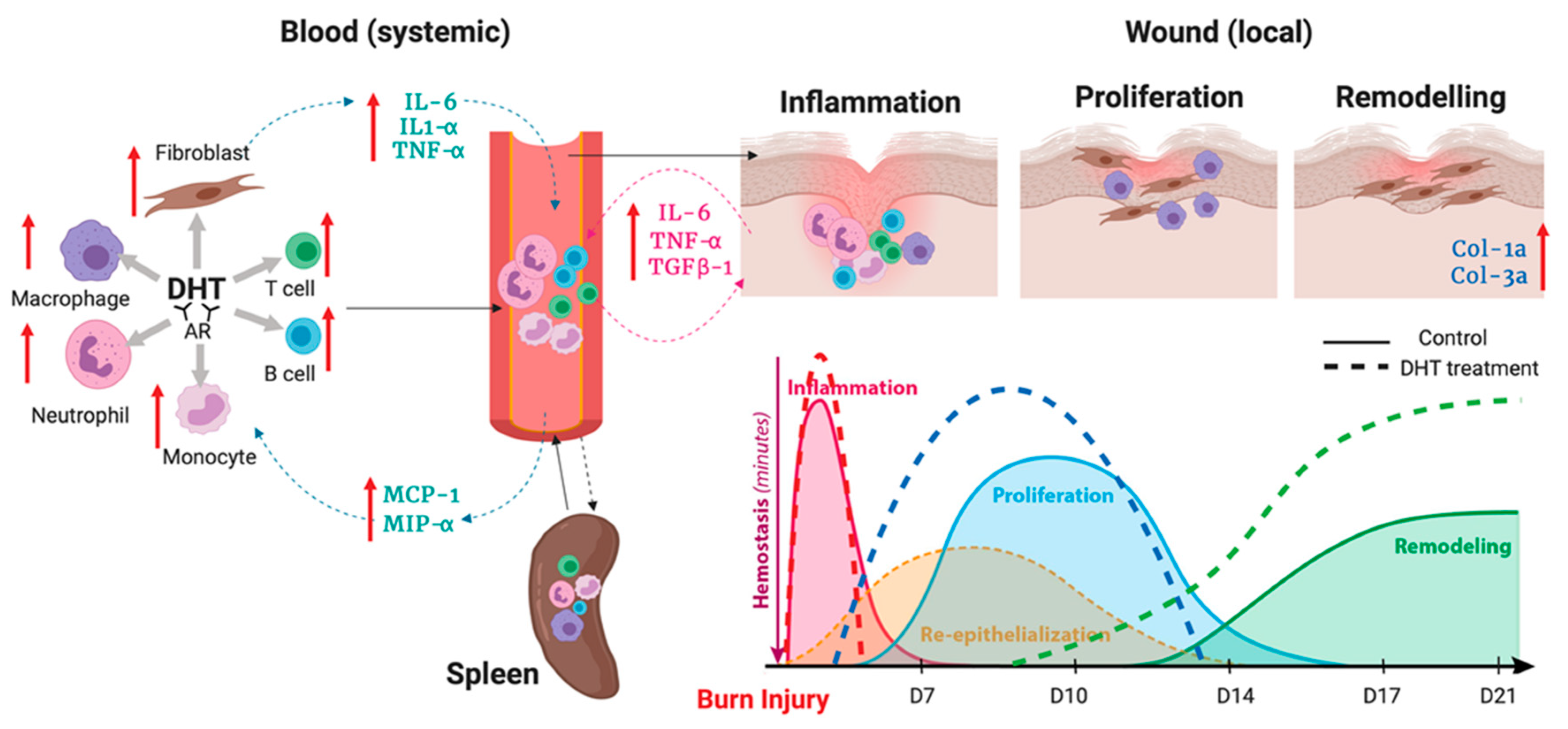
3. Discussion
4. Materials and Methods
4.1. Major Burn Injury Model and Wound Healing Experiment
4.2. Body Composition and Food Intake
4.3. Metabolism
4.4. Histology, Immunocytochemistry, and Image Analysis
4.5. Real-Time Quantitative Polymerase Chain Reaction
4.6. Obtaining Single-Cell Suspensions from Mouse Tissues
4.7. Flow Cytometry
4.8. Cytokines Expression
4.9. Corticosterone Assay
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AR | androgen receptor |
| DHT | dihydrotestosterone |
| ECM | extracellular matrix |
| TBSA | total body surface area |
| PCNA | proliferating cell nuclear antigen |
References
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Young, A.; McNaught, C.E. The physiology of wound healing. Surgery 2011, 29, 475–479. [Google Scholar] [CrossRef]
- Liao, Z.; Huan, J.; Lv, G.; Shou, Y.; Wang, Z. Multi-centre clinical study of the effect of sliver nitrate ointment on the partial thickness burn wounds. Zhonghua Shao Shang Za Zhi 2006, 22, 359–361. [Google Scholar] [PubMed]
- Jeschke, M.G. Post-burn hypermetabolism: Past, present and future. J. Burn Care Res. 2016, 37, 86–96. [Google Scholar] [CrossRef]
- Hart, D.W.; Wolf, S.E.; Mlcak, R.; Chinkes, D.L.; Ramzy, P.I.; Obeng, M.K.; Ferrando, A.A.; Wolfe, R.R.; Herndon, D.N. Persistence of muscle catabolism after severe burn. Surgery 2000, 128, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Wray, C.J.; Mammen, J.M.V.; Hasselgren, P. Catabolic response to stree and potential benefits of nutrition support. Nutr. Support Specif. Surg. Cond. 2002, 18, 971–977. [Google Scholar]
- Azzouni, F.; Godoy, A.; Li, Y.; Mohler, J. The 5 alpha-reductase isozyme family: A review of basic biology and their role in human diseases. Adv. Urol. 2012, 2012, 530121. [Google Scholar] [CrossRef]
- Handelsman, D.J. Androgen Physilogy, Pharmacology, and Abuse; Elsevier: Philadelphia, PA, USA, 2016. [Google Scholar]
- Ashcroft, G.S.; Mills, S.J. Androgen receptor-mediated inhibition of cutaneous wound healing. J. Clin. Investig. 2002, 110, 615–624. [Google Scholar] [CrossRef]
- Efron, P.A.; Moldawer, L.L. Cytokines and wound healing: The role of cytokine and anticytokine therapy in repair response. J. Burn Care Rehabil. 2004, 25, 149–160. [Google Scholar] [CrossRef]
- Gilliver, S.C.; Ashworth, J.J.; Mills, S.J.; Hardman, M.J.; Ashcroft, G.S. Androgens modulate the inflammatory response during acute wound healing. J. Cell Sci. 2006, 119, 722–732. [Google Scholar] [CrossRef]
- Gilliver, S.C.; Ruckshanthi, J.P.; Atkinson, S.J.; Ashcroft, G.S. Androgens influence expression of matrix proteins and proteolytic factors during cutaneous wound healing. Lab. Investig. 2007, 87, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.J.; Lai, K.P.; Chuang, K.H.; Chang, P.; Yu, I.C.; Lin, W.J.; Chang, C. Monocyte/macrophage androgen receptor suppresses cutaneous wound healing in mice by enhancing local TNF-alpha expression. J. Clin. Investig. 2009, 119, 3739–3751. [Google Scholar] [CrossRef]
- Demling, R.H.; DeSanti, L. Oxandrolone induced lean mass gain during recovery from severe burns is maintained after discontinuation of the anabolic steroid. Burns 2003, 29, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Hart, D.W.; Wolf, S.E.; Ramzy, P.I.; Chinkes, D.L.; Beauford, R.B.; Ferrando, A.A.; Wolfe, R.R.; Herndon, D.N. Anabolic effects of oxandrolone after severe burn. Ann. Surg. 2001, 233, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.E.; Edelman, L.S.; Kemalyan, N.; Donison, L.; Cross, J.; Underwood, M.; Spence, R.J.; Noppenberger, D.; Palmieri, T.L.; Greenhalgh, D.G.; et al. Effects of oxandrolone on outcome measures in the severely burned: A multicenter prospective randomized double-blind trial. J. Burn Care Res. 2006, 27, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Barrow, R.E.; Dasu, M.R.; Ferrando, A.A.; Spies, M.; Thomas, S.J.; Perez-Polo, J.R.; Herndon, D.N. Gene expression patterns in skeletal muscle of thermally injured children treated with oxandrolone. Ann. Surg. 2003, 237, 422–428. [Google Scholar] [CrossRef]
- Jeschke, M.G.; Finnerty, C.C.; Suman, O.E.; Kulp, G.; Mlcak, R.P.; Herndon, D.N. The effect of oxandrolone on the endocrinologic, inflammatory, and hypermetabolic responses during the acute phase postburn. Ann. Surg. 2007, 246, 351–360; discussion 360–362. [Google Scholar] [CrossRef]
- Hew, J.J.; Parungao, R.J.; Shi, H.; Tsai, K.H.; Kim, S.; Ma, D.; Malcolm, J.; Li, Z.; Maitz, P.K.; Wang, Y. Mouse models in burns research: Characterisation of the hypermetabolic respones to burn injury. Burns 2019, 46, 663–674. [Google Scholar] [CrossRef]
- Woolf, P.D. Hormonal responses to trauma. Crit. Care Med. 1992, 20, 216–226. [Google Scholar] [CrossRef]
- Ferrando, A.A.; Sheffield-Moore, M.; Wolf, S.E.; Herndon, D.N.; Wolfe, R.R. Testosterone administration in severe burns ameliorates muscle catabolism. Crit. Care Med. 2001, 29, 1936–1942. [Google Scholar] [CrossRef]
- Porter, C.; Tompkins, R.G.; Finnerty, C.C.; Sidossis, L.S.; Suman, O.E.; Herndon, D.N. The metabolic stress response to burn trauma: Current understanding and therapies. Lancet 2016, 16, 1417–1426. [Google Scholar] [CrossRef]
- Torres, S.J.; Nowson, C.A. Relationship between stress, eating behabior, and obesity. Nutrition 2007, 23, 887–894. [Google Scholar] [CrossRef]
- Yau, Y.H.C.; Potenza, M.N. Stress and eating behaviors. Minerva Endocrinol. 2013, 2013, 3. [Google Scholar]
- Hur, J.; Yang, H.; Chun, W.; Kim, J.H.; Shin, S.H.; Kang, H.J.; Kim, H.S. Inflammatory cytokines and their prognostic ability in cases of major burn injury. Ann. Lab. Med. 2015, 35, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Finnerty, C.C.; Jeschke, M.G.; Herndon, D.N.; Gamelli, R.; Gilbran, N.; Klein, M.; Silver, G.; Arnoldo, B.; Remick, D.; Tompkins, R.G. Temporal cytokine profiles in severely burned patients: A comparsion of adults and children. Mol. Med. 2008, 9–10, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Huang, W.; Ai, S.; Wang, S. Clinical significance of leukocyte infiltrative resposne in deep wound of patients with amjor burn. Burns 2006, 32, 945–950. [Google Scholar] [CrossRef]
- Tarran, S.; Langlois, N.E.; Dziewulski, P.; Sztynda, T. Using the inflammatory cell infiltrate to estimate the age of human burn wounds: A review and immunohistochemical study. Med. Sci. Law 2006, 46, 115–126. [Google Scholar] [CrossRef]
- Gilliver, S.C.; Wu, F.; Ashcroft, G.S. Regulatory roles of androgens in cutaneous wound healing. J. Thromb. Haemost. 2003, 90, 978–985. [Google Scholar] [CrossRef]
- Nahrendorf, M.; Pittet, M.J.; Swirski, F.K. Monocytes: Protagonists of infarct inflammation and repair. Circulation 2010, 121, 2437–2445. [Google Scholar] [CrossRef]
- Kratofil, R.M.; Kubes, P.; Deniset, J.F. Monocytes conversion during inflammation and injury. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 35–42. [Google Scholar] [CrossRef]
- Koh, T.J.; DiPietro, L.A. Inflammation and wound healing: The role of the macrophage. Expert Rev. Mol. Med. 2011, 13. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Huang, J.J.; Lin, M.; Xie, T.T.; You, T.H. Quercetin promotes diabetic wound heaing via switching macropjages from M1 to M2 polarization. J. Surg. Res. 2020, 245, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Wang, S.; Kwak, G.; Yang, Y.; Kwon, I.; Kim, S. Exosome-guided phenocypic switch of M1 to M2 macrophage for cutaneus wound healing. Adv. Sci. 2019, 6. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; O’Neill, C.; Handelsman, D.J. Induction of spermatogenesis by androgens in gonadotropin-deficient (hpg) mice. Endocrinology 1995, 136, 5311–5321. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, H.; Lo, T.-H.; Ma, D.; Condor, B.; Lesmana, B.; Parungao, R.J.; Tsai, K.H.-Y.; Kim, S.; Chen, H.-T.; Silveira, P.A.; et al. Dihydrotestosterone (DHT) Enhances Wound Healing of Major Burn Injury by Accelerating Resolution of Inflammation in Mice. Int. J. Mol. Sci. 2020, 21, 6231. https://doi.org/10.3390/ijms21176231
Shi H, Lo T-H, Ma D, Condor B, Lesmana B, Parungao RJ, Tsai KH-Y, Kim S, Chen H-T, Silveira PA, et al. Dihydrotestosterone (DHT) Enhances Wound Healing of Major Burn Injury by Accelerating Resolution of Inflammation in Mice. International Journal of Molecular Sciences. 2020; 21(17):6231. https://doi.org/10.3390/ijms21176231
Chicago/Turabian StyleShi, Huaikai, Tsun-Ho Lo, Duncan Ma, Brenton Condor, Brian Lesmana, Roxanne J Parungao, Kevin H.-Y. Tsai, Sarah Kim, Hsiao-Ting Chen, Pablo A Silveira, and et al. 2020. "Dihydrotestosterone (DHT) Enhances Wound Healing of Major Burn Injury by Accelerating Resolution of Inflammation in Mice" International Journal of Molecular Sciences 21, no. 17: 6231. https://doi.org/10.3390/ijms21176231
APA StyleShi, H., Lo, T.-H., Ma, D., Condor, B., Lesmana, B., Parungao, R. J., Tsai, K. H.-Y., Kim, S., Chen, H.-T., Silveira, P. A., Li, Z., Cooper, M. S., Simanainen, U., Handelsman, D. J., Maitz, P. K., & Wang, Y. (2020). Dihydrotestosterone (DHT) Enhances Wound Healing of Major Burn Injury by Accelerating Resolution of Inflammation in Mice. International Journal of Molecular Sciences, 21(17), 6231. https://doi.org/10.3390/ijms21176231






