Review: Cantilever-Based Sensors for High Speed Atomic Force Microscopy
Abstract
:1. Introduction
2. Generic Operations of the Microcantilever
3. Modes of Operations of Cantilever-Based AFM
4. Methods of Cantilever Detection
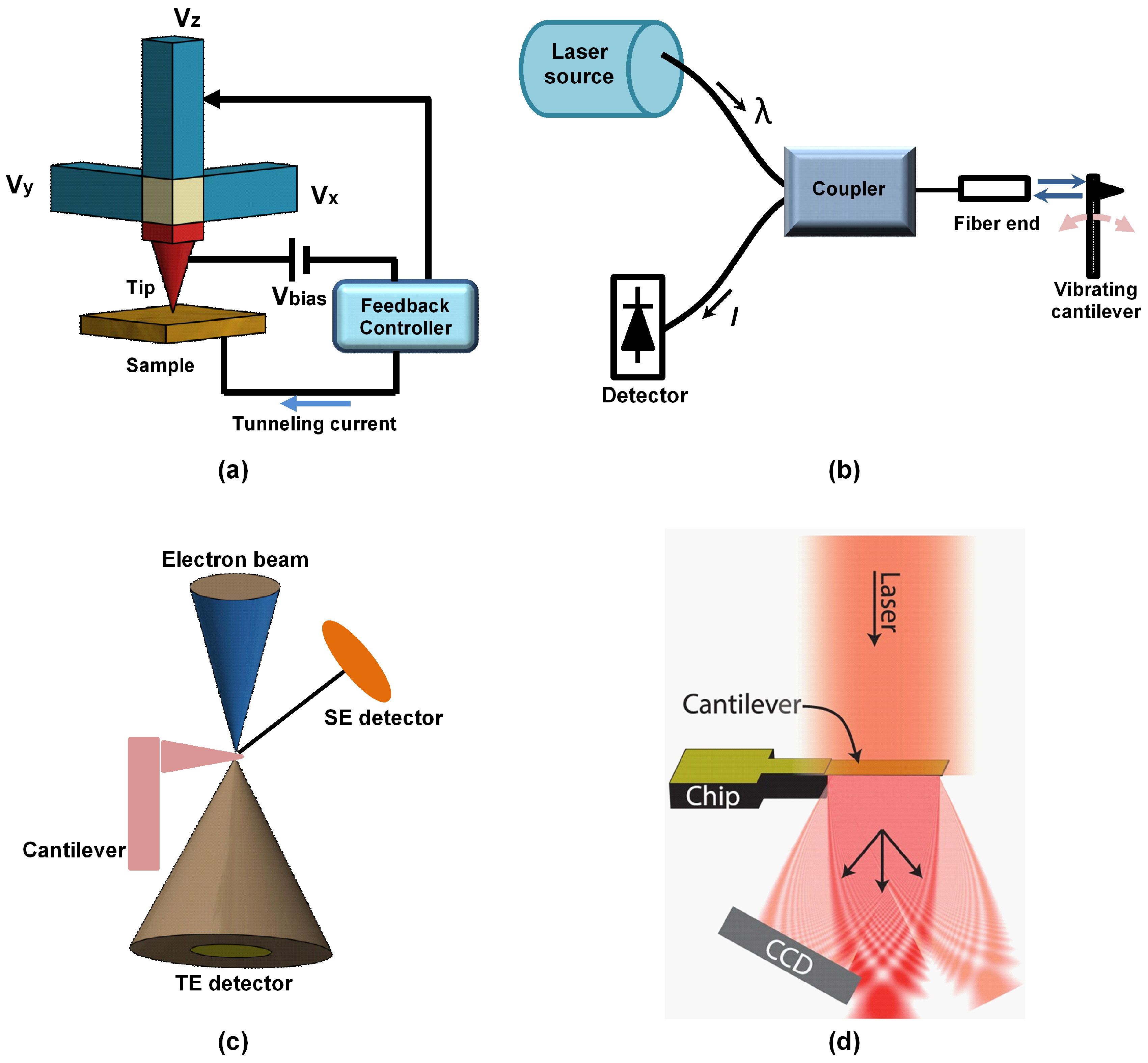
4.1. Electron Tunneling Method
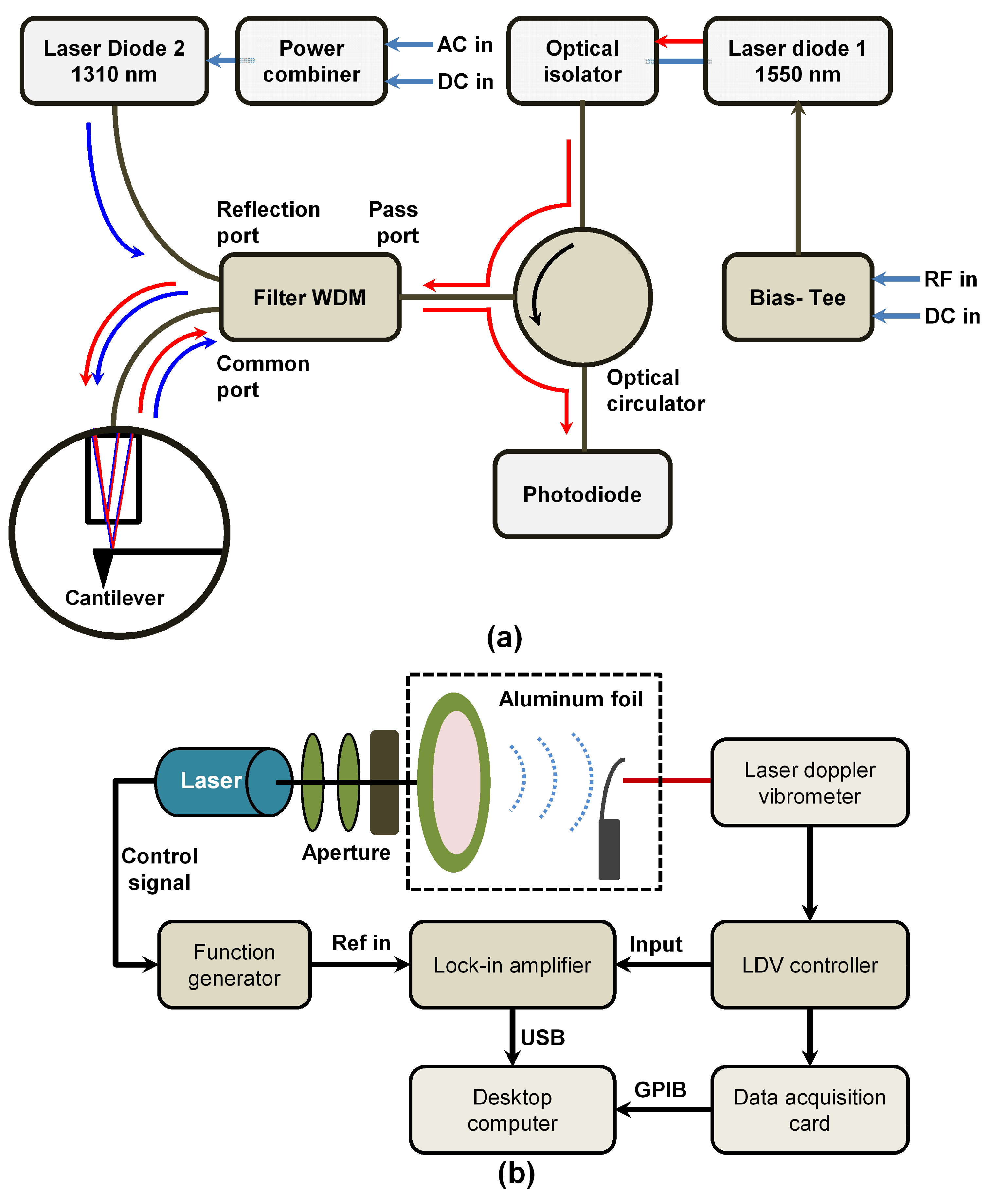
4.2. Interferometry Method
4.3. Electron Beam Detection Method
4.4. Optical Diffraction Grating
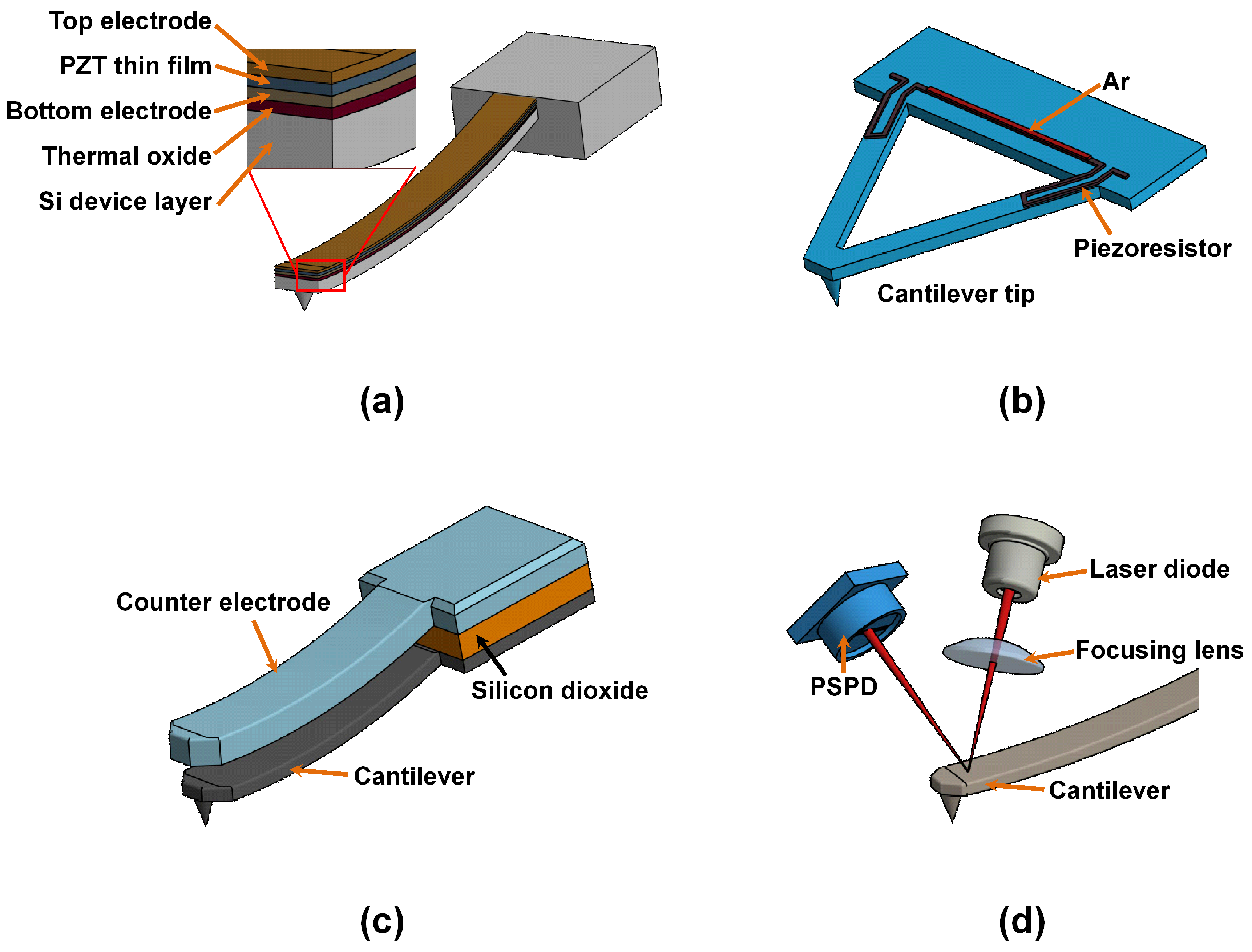
4.5. Piezoelectric Method
4.6. Piezoresistive Method
4.7. Capacitive Detection Scheme
4.8. Optical Lever Method
5. Microcantilever Excitation Methods
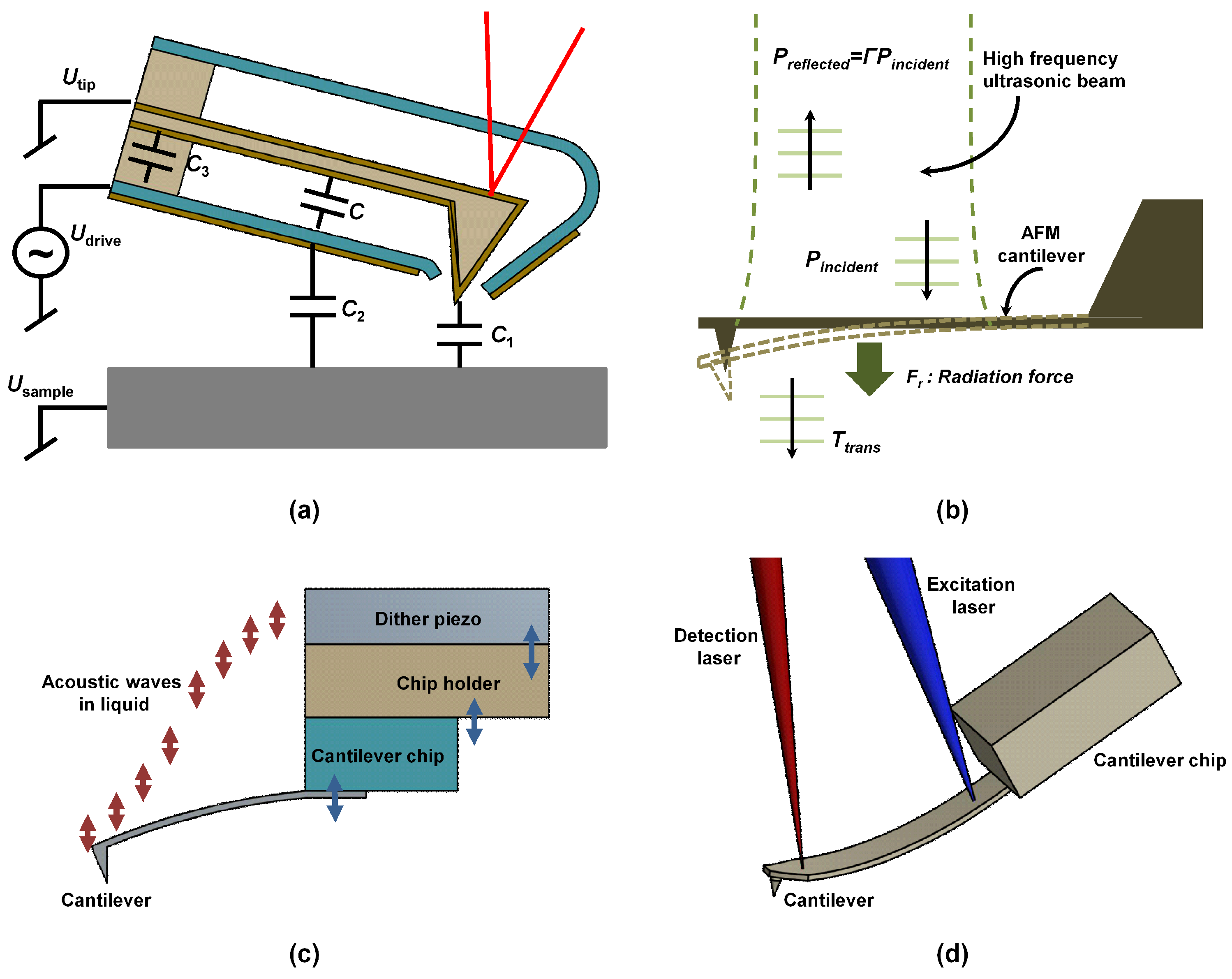
5.1. Magnetic Excitation
5.2. Brownian Motion
5.3. Sample Excitation
5.4. Electrostatic Actuation
5.5. Acoustic Radiation Pressure Method
5.6. Piezo-Acoustic Excitation
5.7. Photothermal Excitation
5.8. Optical Excitation
5.9. Laser Induced Photoacoustic Excitation
6. Fabrication, Modification, and Functionalization of AFM Microcantilevers
6.1. Fabrication
6.2. Microcantilever Tip Fabrication
6.3. AFM Microcantilever Modification
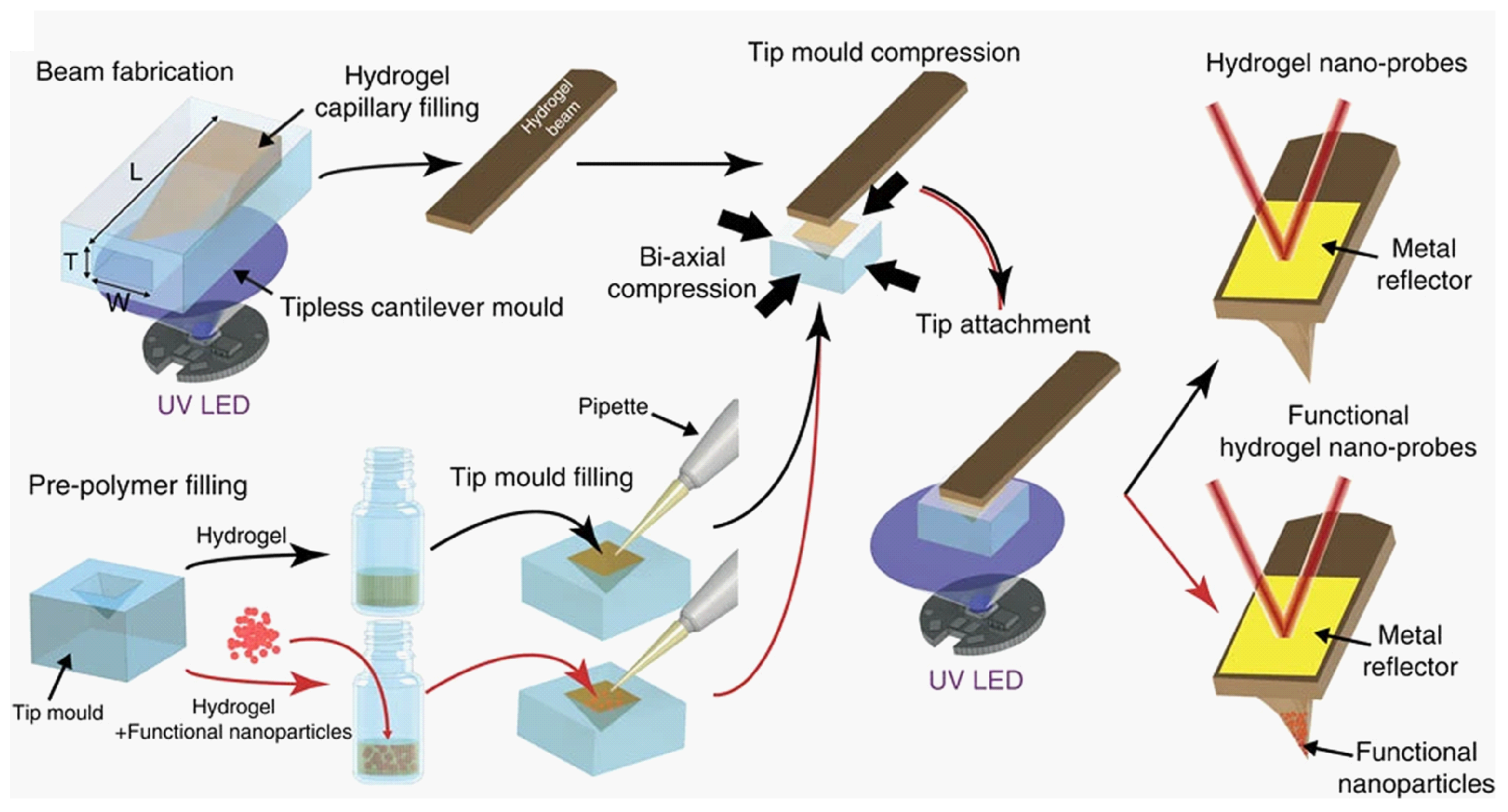
6.4. AFM Probe Functionalization
7. High-Speed Imaging
8. Microcantilever Sensors in AFM Applications
9. Conclusions and Prospects
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACM | Aperture Correction Microscopy |
| AFM | Atomic Force Microscope |
| APTES | Aminopropyltrietoxisilane |
| CM | Contact Mode |
| CMI | Contact Resonance Imaging |
| CMOS | Complementary Metal Oxide Semiconductor |
| EBID | Electron Beam Induced Deposition |
| EDC | 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide |
| FEIG | Field Emission Induced Growth |
| FIB | Focused Ion-Beam |
| FLIM | Fluorescence Lifetime Imaging Microscopy |
| FMM | Friction Mode Microscopy |
| FRET | Förster Resonance Energy Transfer |
| FWDM | Filter Wavelength Division Multiplexer |
| HSAFM | High-Speed Atomic Force Microscopy |
| IC | Integrated Circuits |
| KPFM | Kelvin Probe Force Microscopy |
| LSCM | Laser Scanning Confocal Microscopy |
| MEMS | Microelectromechanical Systems |
| MFFM | Multifrequency Force Microscopy |
| MFM | Magnetic Force Microscopy |
| MMM | Mechanical Mapping Mode |
| MWCNT | Multiwalled Carbon Nanotube |
| NCM | Non-Contact Mode |
| NHS | N-Hydroxysuccinimide |
| OBD | Optical Beam Deflection |
| OBP | Odorant Binding Proteins |
| OCLSM | Optical Confocal Laser Scanning Microscopy |
| OFM | Optical Fluorescent Microscopy |
| PDMS | Polydimethylsiloxane |
| PECVD | Plasma Enhanced Chemical Vapor Deposition |
| PI | Phase Imaging |
| TERS | Tip-Enhanced Raman Spectroscopy |
| SAM | Self-Assembly Monolayer |
| SEM | Scanning Electron Microscopy |
| SOI | Silicon-on-Insulator |
| SNOM | Scanning Near-Field Optical Microscopy |
| SRFM | Super-Resolution Fluorescence Microscopy |
| STEDM | Correlative Stimulated Emission Depletion Microscopy |
| STORM | Stochastic Optical Reconstruction Microscopy |
| TEM | Transmission Electron Microscopy |
| TIRFM | Total Internal Reflection Fluorescence Microscopy |
| VMM | Viscoelastic Mapping Microscopy |
References
- Kwon, T.; Gunasekaran, S.; Eom, K. Atomic force microscopy-based cancer diagnosis by detecting cancer-specific biomolecules and cells. Biochim. Biophys. Acta-Rev. Cancer 2019, 1871, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, F.S.; Šneideris, T.; Vendruscolo, M.; Knowles, T.P.J. Atomic force microscopy for single molecule characterisation of protein aggregation. Arch. Biochem. Biophys. 2019, 664, 134–148. [Google Scholar] [CrossRef] [PubMed]
- Valotteau, C.; Sumbul, F.; Rico, F. High-speed force spectroscopy: Microsecond force measurements using ultrashort cantilevers. Biophy. Rev. 2019, 11, 689–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasas, S.; Ruggeri, F.S.; Benadiba, C.; Maillard, C.; Stupar, P.; Tournu, H.; Dietler, G.; Longo, G. Detecting nanoscale vibrations as signature of life. Proc. Natl. Acad. Sci. USA 2015, 112, 378–381. [Google Scholar] [CrossRef] [Green Version]
- Guillaume-Gentil, O.; Potthoff, E.; Ossola, D.; Franz, C.M.; Zambelli, T.; Vorholt, J.A. Force-controlled manipulation of single cells: From AFM to FluidFM. Trends Biotechnol. 2014, 32, 381–388. [Google Scholar] [CrossRef]
- Beaussart, A.; El-Kirat-Chatel, S. Microbial adhesion and ultrastructure from the single-molecule to the single-cell levels by atomic force microscopy. Cell Surf. 2019, 5, 100031. [Google Scholar] [CrossRef]
- Kojima, T.; Husari, A.; Dieterle, M.P.; Fontaine, S.; Prucker, O.; Tomakidi, P.; Rühe, J. PnBA/PDMAA-based iron-loaded micropillars allow for discrete cell adhesion and analysis of actuation-related molecular responses. Adv. Mater. Interfaces 2020, 7, 1901806. [Google Scholar] [CrossRef]
- Dickinson, L.E.; Rand, D.R.; Tsao, J.; Eberle, W.; Gerecht, S. Endothelial cell responses to micropillar substrates of varying dimensions and stiffness. J. Biomed. Mater. Res. A 2012, 100, 1457–1466. [Google Scholar] [CrossRef] [Green Version]
- Doll, J.C.; Harjee, N.; Klejwa, N.; Kwon, R.; Coulthard, S.M.; Petzold, B.; Goodman, M.B.; Pruitt, B.L. SU-8 force sensing pillar arrays for biological measurements. Lab Chip 2009, 9, 1449–1454. [Google Scholar] [CrossRef] [Green Version]
- Luka, G.; Ahmadi, A.; Najjaran, H.; Alocilja, E.; DeRosa, M.; Wolthers, K.; Malki, A.; Aziz, H.; Althani, A.; Hoorfar, M. Microfluidics integrated biosensors: A leading technology towards lab-on-a-chip and sensing applications. Sensors 2015, 15, 30011–30031. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Ouyang, R.; Zhang, W.; Zhou, S.; Yang, Y.; Ji, Y.; Feng, K.; Liang, X.; Xiao, M.; Miao, Y. Single walled carbon nanotube sandwiched Ni-Ag hybrid nanoparticle layers for the extraordinary electrocatalysis toward glucose oxidation. Electrochim. Acta 2016, 188, 197–209. [Google Scholar] [CrossRef]
- Mi, S.; Xia, J.; Xu, Y.; Du, Z.; Sun, W. An integrated microchannel biosensor platform to analyse low density lactate metabolism in HepG2 cells in vitro. RSC Adv. 2019, 9, 9006–9013. [Google Scholar] [CrossRef] [Green Version]
- Drake, B.; Prater, C.B.; Weisenhorn, A.L.; Gould, S.A.C.; Albrecht, T.R.; Quate, C.F.; Cannell, D.S.; Hansma, H.G.; Hansma, P.K. Imaging crystals, polymers, and processes in water with the atomic force microscope. Science 1989, 243, 1586–1589. [Google Scholar] [CrossRef] [PubMed]
- Muller, D.J.; Dufrêne, Y.F. Atomic force microscopy: A nanoscopic window on the cell surface. Trends Cell Biol. 2011, 21, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Pillet, F.; Chopinet, L.; Formosa, C.; Dague, É. Atomic force microscopy and pharmacology: From microbiology to cancerology. Biochim. Biophys. Acta 2014, 1840, 1028–1050. [Google Scholar] [CrossRef] [PubMed]
- Moropoulou, A.; Zendri, E.; Ortiz, P.; Delegou, E.T.; Ntoutsi, I.; Balliana, E.; Becerra, J.; Ortiz, R. Scanning microscopy techniques as an assessment tool of materials and interventions for the protection of built cultural heritage. Scanning 2019. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Chen, X.; Cao, H.; Deng, C.; Cao, X.; Wang, P. A structural study of Escherichia coli cells using an in situ liquid chamber TEM technology. J. Anal. Methods Chem. 2015. [Google Scholar]
- Medalia, O.; Weber, I.; Frangakis, A.S.; Nicastro, D.; Gerisch, G.; Baumeister, W. Macromolecular architecture in eukaryotic cells visualized by cryoelectron tomography. Science 2002, 298, 1209–1213. [Google Scholar] [CrossRef] [Green Version]
- Leis, A.; Rockel, B.; Andrees, L.; Baumeister, W. Visualizing cells at the nanoscale. Trends Biochem. Sci. 2009, 34, 60–70. [Google Scholar] [CrossRef]
- Liv, N.; van Oosten Slingeland, D.S.; Baudoin, J.P.; Kruit, P.; Piston, D.W.; Hoogenboom, J.P. Electron microscopy of living cells during in situ fluorescence microscopy. ACS Nano 2016, 10, 265–273. [Google Scholar] [CrossRef] [Green Version]
- De Jonge, N.; Peckys, D.B. Live cell electron microscopy is probably impossible. ACS Nano 2016, 10, 9061–9063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennedy, E.; Nelson, E.M.; Tanaka, T.; Damiano, J.; Timp, G. Live bacterial physiology visualized with 5 nm resolution using scanning transmission electron microscopy. ACS Nano 2016, 10, 2669–2677. [Google Scholar] [CrossRef] [PubMed]
- Staunton, J.R.; Doss, B.L.; Lindsay, S.; Ros, R. Correlating confocal microscopy and atomic force indentation reveals metastatic cancer cells stiffen during invasion into collagen I matrices. Sci. Rep. 2016, 6, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newton, R.; Delguste, M.; Koehler, M.; Dumitru, A.C.; Laskowski, P.R.; Müller, D.J.; Alsteens, D. Combining confocal and atomic force microscopy to quantify single-virus binding to mammalian cell surfaces. Nat. Protoc. 2017, 12, 2275–2292. [Google Scholar] [CrossRef]
- Ramachandran, S.; Arce, F.T.; Patel, N.R.; Quist, A.P.; Cohen, D.A.; Lal, R. Structure and permeability of ion-channels by integrated AFM and waveguide TIRF microscopy. Sci. Rep. 2014, 4, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Christenson, W.; Yermolenko, I.; Plochberger, B.; Camacho-Alanis, F.; Ros, A.; Ugarova, T.P.; Ros, R. Combined single cell AFM manipulation and TIRFM for probing the molecular stability of multilayer fibrinogen matrices. Ultramicroscopy 2014, 136, 211–215. [Google Scholar] [CrossRef] [Green Version]
- Maki, K.; Han, S.W.; Hirano, Y.; Yonemura, S.; Hakoshima, T.; Adachi, T. Real-time TIRF observation of vinculin recruitment to stretched α-catenin by AFM. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Miranda, A.; Martins, M.; De Beule, P.A. Simultaneous differential spinning disk fluorescence optical sectioning microscopy and nanomechanical mapping atomic force microscopy. Rev. Sci. Instrum. 2015, 86, 093705. [Google Scholar] [CrossRef]
- Harke, B.; Chacko, J.V.; Haschke, H.; Canale, C.; Diaspro, A. A novel nanoscopic tool by combining AFM with STED microscopy. Opt. Nanoscopy 2012, 1, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Curry, N.; Ghézali, G.; Kaminski Schierle, G.S.; Rouach, N.; Kaminski, C.F. Correlative STED and atomic force microscopy on live astrocytes reveals plasticity of cytoskeletal structure and membrane physical properties during polarized migration. Front. Cell. Neurosci. 2017, 11, 104. [Google Scholar] [CrossRef]
- Cosentino, M.; Canale, C.; Bianchini, P.; Diaspro, A. AFM-STED correlative nanoscopy reveals a dark side in fluorescence microscopy imaging. Sci. Adv. 2019, 5, eaav8062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, D.; Micic, M.; Klymyshyn, N.; Suh, Y.D.; Lu, H.P. Correlated topographic and spectroscopic imaging beyond diffraction limit by atomic force microscopy metallic tip-enhanced near-field fluorescence lifetime microscopy. Rev. Sci. Instrum. 2003, 74, 3347–3355. [Google Scholar] [CrossRef]
- Micic, M.; Hu, D.; Suh, Y.D.; Newton, G.; Romine, M.; Lu, H.P. Correlated atomic force microscopy and fluorescence lifetime imaging of live bacterial cells. Colloids Surface B 2004, 34, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Odermatt, P.D.; Shivanandan, A.; Deschout, H.; Jankele, R.; Nievergelt, A.P.; Feletti, L.; Davidson, M.W.; Radenovic, A.; Fantner, G.E. High-resolution correlative microscopy: Bridging the gap between single molecule localization microscopy and atomic force microscopy. Nano Lett. 2015, 15, 4896–4904. [Google Scholar] [CrossRef]
- Hirvonen, L.M.; Cox, S. STORM without enzymatic oxygen scavenging for correlative atomic force and fluorescence superresolution microscopy. Methods Appl. Fluoresc. 2018, 6, 045002. [Google Scholar] [CrossRef]
- Gómez-Varela, A.I.; Stamov, D.R.; Miranda, A.; Alves, R.; Barata-Antunes, C.; Dambournet, D.; Drubin, D.G.; Paiva, S.; De Beule, P.A. Simultaneous co-localized super-resolution fluorescence microscopy and atomic force microscopy: Combined SIM and AFM platform for the life sciences. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Chan, K.A.; Kazarian, S.G. Tip-enhanced Raman mapping with top-illumination AFM. Nanotechnology 2011, 22, 175701. [Google Scholar] [CrossRef]
- Kumar, N.; Su, W.; Veselý, M.; Weckhuysen, B.M.; Pollard, A.J.; Wain, A.J. Nanoscale chemical imaging of solid–liquid interfaces using tip-enhanced Raman spectroscopy. Nanoscale 2018, 10, 1815–1824. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, R.; Zhu, Z.; Wang, J.; Tian, Q. Experimental research on the spectral response of tips for tip-enhanced raman spectroscopy. J. Opt. 2013, 15, 055006. [Google Scholar] [CrossRef]
- Ingham, J.; Craig, T.; Smith, C.I.; Varro, A.; Pritchard, D.M.; Barrett, S.D.; Martin, D.S.; Harrison, P.; Unsworth, P.; Kumar, J.D.; et al. Submicron infrared imaging of an oesophageal cancer cell with chemical specificity using an IR-FEL. Biomed. Phys. Eng. Express 2018, 5, 015009. [Google Scholar] [CrossRef]
- Halliwell, D.E.; Morais, C.L.; Lima, K.M.; Trevisan, J.; Siggel-King, M.R.; Craig, T.; Ingham, J.; Martin, D.S.; Heys, K.A.; Kyrgiou, M.; et al. Imaging cervical cytology with scanning near-field optical microscopy (SNOM) coupled with an IR-FEL. Sci. Rep. 2016, 6, 29494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Troian, B.; Boscolo, R.; Ricci, G.; Lazzarino, M.; Zito, G.; Prato, S.; Andolfi, L. Ultra-structural analysis of human spermatozoa by aperture scanning near-field optical microscopy. J. Biophotonics 2020, 13, e2418. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Lu, M.; Cao, J.; Lu, H.P. Manipulating protein conformations by single-molecule AFM-FRET nanoscopy. ACS Nano 2012, 6, 1221–1229. [Google Scholar] [CrossRef] [Green Version]
- Binnig, G.; Quate, C.F.; Gerber, C. Atomic force microscope. Phys. Rev. Lett. 1986, 56, 930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fritz, J.; Baller, M.K.; Lang, H.P.; Rothuizen, H.; Vettiger, P.; Meyer, E.; Güntherodt, H.J.; Gerber, C.; Gimzewski, J.K. Translating biomolecular recognition into nanomechanics. Science 2000, 288, 316–318. [Google Scholar] [CrossRef] [Green Version]
- Wan, Z.; Lee, S.; Koo, K.; Kim, K. Nanowire-based sensors for biological and medical applications. IEEE Trans. Nanobiosci. 2016, 15, 186–199. [Google Scholar]
- Pramanik, C.; Saha, H. Low pressure piezoresistive sensors for medical electronics applications. Mater. Manuf. Process. 2006, 21, 233–238. [Google Scholar] [CrossRef]
- Dong, Y.; Gao, W.; Zhou, Q.; Zheng, Y.; You, Z. Characterization of the gas sensors based on polymer-coated resonant microcantilevers for the detection of volatile organic compounds. Anal. Chim. Acta 2010, 671, 85–91. [Google Scholar] [CrossRef]
- Lavrik, N.V.; Sepaniak, M.J.; Datskos, P.G. Cantilever transducers as a platform for chemical and biological sensors. Rev. Sci. Instrum. 2004, 75, 2229–2253. [Google Scholar] [CrossRef]
- Bashir, R. BioMEMS: State-of-the-art in detection, opportunities and prospects. Adv. Drug Deliv. Rev. 2004, 56, 1565–1586. [Google Scholar] [CrossRef]
- Kenkel, S.; Mittal, S.; Bhargava, R. Closed-loop atomic force microscopy-infrared spectroscopic imaging for nanoscale molecular characterization. Nat. Commun. 2020, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, C. Cantilever-based biosensors. Anal. Bioanal. Chem. 2004, 379, 946–959. [Google Scholar] [CrossRef]
- Tomizawa, Y.; Dixit, K.; Daggett, D.; Hoshino, K. Biocompatible cantilevers for mechanical characterization of zebrafish embryos using image analysis. Sensors 2019, 19, 1506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brand, U.; Xu, M.; Doering, L.; Langfahl-Klabes, J.; Behle, H.; Bütefisch, S.; Ahbe, T.; Peiner, E.; Völlmeke, S.; Frank, T.; et al. Long slender piezo-resistive silicon microprobes for fast measurements of roughness and mechanical properties inside micro-holes with diameters below 100 μm. Sensors 2019, 19, 1410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markidou, A.; Shih, W.Y.; Shih, W.H. Soft-materials elastic and shear moduli measurement using piezoelectric cantilevers. Rev. Sci. Instrum. 2005, 76, 064302. [Google Scholar] [CrossRef] [Green Version]
- Oden, P.I.; Chen, G.Y.; Steele, R.A.; Warmack, R.J.; Thundat, T. Viscous drag measurements utilizing microfabricated cantilevers. Appl. Phys. Lett. 1996, 68, 3814–3816. [Google Scholar] [CrossRef]
- Berger, R.; Gerber, C.; Gimzewski, J.K.; Meyer, E.; Güntherodt, H.J. Thermal analysis using a micromechanical calorimeter. Appl. Phys. Lett. 1996, 69, 40–42. [Google Scholar] [CrossRef]
- Arakawa, E.T.; Lavrik, N.V.; Rajic, S.; Datskos, P.G. Detection and differentiation of biological species using microcalorimetric spectroscopy. Ultramicroscopy. 2003, 97, 459–465. [Google Scholar] [CrossRef]
- Cherian, S.; Thundat, T. Determination of adsorption-induced variation in the spring constant of a microcantilever. Appl. Phys. Lett. 2002, 80, 2219–2221. [Google Scholar] [CrossRef]
- Pei, J.; Tian, F.; Thundat, T. Novel glucose biosensor based on the microcantilever. Mater. Res. Soc. Symp. Proc. 2003, 776, 243–247. [Google Scholar] [CrossRef]
- Subramanian, A.; Oden, P.I.; Kennel, S.J.; Jacobson, K.B.; Warmack, R.J.; Thundat, T.; Doktycz, M.J. Glucose biosensing using an enzyme-coated microcantilever. Appl. Phys. Lett. 2002, 81, 385–387. [Google Scholar] [CrossRef]
- Hansen, K.M.; Thundat, T. Microcantilever biosensors. Methods 2005, 37, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.H.; Lee, C.Y.; Wang, Y.H.; Chen, H.J. Microcantilever-based weather station for temperature, humidity and flow rate measurement. Microsyst Technol. 2008, 14, 971–977. [Google Scholar] [CrossRef]
- Wang, J.; Wu, W.G.; Huang, Y.; Hao, Y.L. Microcantilever humidity sensor based on embedded nMOSFET with <100>-crystal-orientation channel. Proc. IEEE Sens. 2009, 50730009, 727–730. [Google Scholar]
- Priyadarisshini, B.; Sindhanaiselvi, D. Microcantilever as humidity sensor. In Proceedings of the Conference on Emerging Devices and Smart Systems, Tamilnadu, India, 2–3 March 2018. [Google Scholar]
- Strembicke, D.; Robinson, A.M.; Vermeulen, F.E.; Seto, M.; Brown, K.B. Humidity measurement using resonating CMOS microcantilever structures. Can. Conf. Electr. Comput. Eng. 1999, 3, 1658–1661. [Google Scholar]
- Steffens, C.; Manzoli, A.; Leite, F.L.; Fatibello, O.; Herrmann, P.S.P. Atomic force microscope microcantilevers used as sensors for monitoring humidity. Microelectron. Eng. 2014, 113, 80–85. [Google Scholar] [CrossRef]
- Wu, L.; Cheng, T.; Zhang, Q.C. A bi-material microcantilever temperature sensor based on optical readout. Meas. J. Int. Meas. Confed. 2012, 45, 1801–1806. [Google Scholar] [CrossRef]
- Egger, M.; Ohnesorge, F.; Weisenhorn, A.L.; Heyn, S.P.; Drake, B.; Prater, C.B.; Gould, S.A.C.; Hansma, P.K.; Gaub, H.E. Wet lipid-protein membranes imaged at submolecular resolution by atomic force microscopy. J. Struct. Biol. 1990, 103, 89–94. [Google Scholar] [CrossRef]
- Bianco, S.; Cocuzza, M.; Ferrero, S.; Giuri, E.; Piacenza, G.; Pirri, C.F.; Ricci, A.; Scaltrito, L.; Bich, D.; Merialdo, A.; et al. Silicon resonant microcantilevers for absolute pressure measurement. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. 2006, 24, 1803–1809. [Google Scholar] [CrossRef] [Green Version]
- Keskar, G.; Elliott, B.; Gaillard, J.; Skove, M.J.; Rao, A.M. Using electric actuation and detection of oscillations in microcantilevers for pressure measurements. Sens. Actuators Phys. 2008, 147, 203–209. [Google Scholar] [CrossRef]
- Albrecht, T.R.; Akamine, S.; Carver, T.E.; Quate, C.F. Microfabrication of cantilever styli for the atomic force microscope. J. Vac. Sci. Technol. A Vac. Surf. Film 1990, 8, 3386–3396. [Google Scholar] [CrossRef]
- Barnes, J.R.; Stephenson, R.J.; Welland, M.E.; Gerber, C.; Gimzewski, J.K. Photothermal spectroscopy with femtojoule sensitivity using a micromechanical device. Nature 1994, 372, 79–81. [Google Scholar] [CrossRef]
- Boisen, A.; Thaysen, J.; Jensenius, H.; Hansen, O. Environmental sensors based on micromachined cantilevers with integrated read-out. Ultramicroscopy 2000, 82, 11–16. [Google Scholar] [CrossRef]
- Singamaneni, S.; McConney, M.E.; LeMieux, M.C.; Jiang, H.; Enlow, J.O.; Bunning, T.J.; Naik, R.R.; Tsukruk, V.V. Polymer-silicon flexible structures for fast chemical vapor detection. Adv. Mater. 2007, 19, 4248–4255. [Google Scholar] [CrossRef]
- Huber, C.; Pina, M.P.; Morales, J.J.; Mehdaoui, A.A. A multiparameter gas-monitoring system combining functionalized and non-functionalized microcantilevers. Micromachines 2020, 11, 283. [Google Scholar] [CrossRef] [Green Version]
- Grogan, C.; Amarandei, G.; Lawless, S.; Pedreschi, F.; Lyng, F.; Benito-Lopez, F.; Raiteri, R.; Florea, L. Silicon microcantilever sensors to detect the reversible conformational change of a molecular switch, spiropyan. Sensors 2020, 20, 854. [Google Scholar] [CrossRef] [Green Version]
- Boisen, A.; Dohn, S.; Keller, S.S.; Schmid, S.; Tenje, M. Cantilever-like micromechanical sensors. Rep. Prog. Phys. 2011, 74, 036101. [Google Scholar] [CrossRef]
- Diksha, S.; Tripathi, N. Microcantilever: An efficient tool for biosensing applications. Int. J. Intell. Syst. Appl. 2017, 9, 63–74. [Google Scholar]
- Sepaniak, M.; Datskos, P.; Lavrik, N.; Tipple, C. Peer reviewed: Microcantilever transducers: A new approach in sensor technology. Anal. Chem. 2002, 74, 568A–575A. [Google Scholar] [CrossRef] [Green Version]
- McKendry, R.; Zhang, J.; Arntz, Y.; Strunz, T.; Hegner, M.; Lang, H.P.; Baller, M.K.; Certa, U.; Meyer, E.; Güntherodt, H.J.; et al. Multiple label-free biodetection and quantitative DNA-binding assays on a nanomechanical cantilever array. Proc. Natl. Acad. Sci. USA 2002, 99, 9783–9788. [Google Scholar] [CrossRef] [Green Version]
- Lange, D.; Hagleitner, C.; Hierlemann, A.; Brand, O.; Baltes, H. Cantilever arrays on a single chip: Mass-sensitive detection of volatile organic compounds. Anal. Chem. 2002, 74, 3084–3095. [Google Scholar] [CrossRef] [PubMed]
- Chow, E.M.; Yaralioglu, G.G.; Quate, C.F.; Kenny, T.W. Characterization of a two-dimensional cantilever array with through-wafer electrical interconnects. Appl. Phys. Lett. 2002, 80, 664–666. [Google Scholar] [CrossRef]
- Fagan, B.C.; Tipple, C.A.; Xue, Z.; Sepaniak, M.J.; Datskos, P.G. Modification of micro-cantilever sensors with sol-gels to enhance performance and immobilize chemically selective phases. Talanta 2000, 53, 599–608. [Google Scholar] [CrossRef]
- Long, Z.; Kou, L.; Sepaniak, M.J.; Hou, X. Recent advances in gas phase microcantileverbased sensing. Rev. Anal. Chem. 2013, 32, 135–158. [Google Scholar] [CrossRef]
- Patkar, R.; Vinchurkar, M.; Ashwin, M.; Adami, A.; Giacomozzi, F.; Lorenzelli, L.; Baghini, M.S.; Rao, V.R. Microcantilever based dual mode biosensor for agricultural applications. IEEE Sens. J. 2019. [Google Scholar] [CrossRef]
- Gopinath, P.G.; Anitha, V.R.; Mastani, S.A. Microcantilever based biosensor for disease detection applications. J. Med. Bioeng. 2015, 4, 307–311. [Google Scholar] [CrossRef]
- Cai, T. Theoretical Analysis of Torsionally Vibrating Microcantilevers for Chemical Sensor Applications in Viscous Liquids. Ph.D. Thesis, Marquette University, Milwaukee, WI, USA, 2013. [Google Scholar]
- Canavese, G.; Ricci, A.; Gazzadi, G.C.; Ferrante, I.; Mura, A.; Marasso, S.L.; Ricciardi, C. Resonating behaviour of nanomachined holed microcantilevers. Sci. Rep. 2015, 5, 1–6. [Google Scholar] [CrossRef]
- Sohi, A.N.; Nieva, P.M. Frequency response of curved bilayer microcantilevers with applications to surface stress measurement. J. Appl. Phys. 2016, 119, 044503. [Google Scholar] [CrossRef]
- Ejeian, F.; Etedali, P.; Mansouri-Tehrani, H.A.; Soozanipour, A.; Low, Z.X.; Asadnia, M.; Taheri-Kafrani, A.; Razmjou, A. Biosensors for wastewater monitoring: A review. Biosens. Bioelectron. 2018, 118, 66–79. [Google Scholar] [CrossRef]
- Cheulkar, L.N.; Sawant, V.B.; Mohite, S.S. Evaluating performance of thermally curled microcantilever RF MEMS switches. Mater. Today Proc. 2019, 27, 12–18. [Google Scholar] [CrossRef]
- Basha, S.J.; Krishna, M.H.S.; Praharsha, C.A.; Babu, P.H.; Karthikeya, V.; Srinivas, Y.; Lakshmi, D.R.; Rao, S. Microcantilever based RF MEMS switch for wireless communication. Microelectron. Solid State Electron. 2016, 5, 1–6. [Google Scholar]
- Choi, J.Y. RF MEMS switch using silicon cantilevers. In Proceedings of the EU-Korea Conference on Science and Technology, Berlin, Germany, 19 October 2008. [Google Scholar]
- Leitner, M.; Fantner, G.E.; Fantner, E.J.; Ivanova, K.; Ivanov, T.; Rangelow, I.; Ebner, A.; Rangl, M.; Tang, J.; Hinterdorfer, P. Single-molecule AFM characterization of individual chemically tagged DNA tetrahedra. ACS Nano 2011, 5, 7048–7054. [Google Scholar] [CrossRef] [PubMed]
- Shibata, M.; Uchihashi, T.; Ando, T.; Yasuda, R. Long-tip high-speed atomic force microscopy for nanometer-scale imaging in live cells. Sci. Rep. 2015, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Vashist, S.K.; Holthofer, H. Microcantilevers for sensing applications. Meas. Control 2010, 43, 84–88. [Google Scholar] [CrossRef]
- Severi, S.; Heck, J.; Chou, T.K.; Belov, N.; Park, J.S.; Harrar, D.; Jain, A.; Van Hoof, R.; Du Bois, B.; De Coster, J.; et al. CMOS-integrated poly-sige cantilevers with read/write system for probe storage device. In Proceedings of the International Conference on Solid-State Sensors, Actuators and Microsystems, Denver, CO, USA, 21–25 June 2009. [Google Scholar]
- Pinnaduwage, L.A.; Ji, H.F.; Thundat, T. Moore’s law in homeland defense: An integrated sensor platform based on silicon microcantilevers. IEEE Sens. J. 2005, 5, 774–785. [Google Scholar] [CrossRef]
- Praveenkumar, S.; Nath, S.S.; Dinesh, R.G.; Ramya, S.; Priya, M. Design optimization and simulation of micro-electro-mechanical system based solar energy harvester for low voltage applications. J. Renew. Sustain. Energy 2018, 10, 053503. [Google Scholar] [CrossRef]
- Gupta, R.; Rana, L.; Sharma, A.; Gupta, V.; Tomar, M. Fabrication of micro-cantilever and its theoretical validation for energy harvesting applications. Microsyst. Technol. 2019, 25, 4249–4256. [Google Scholar] [CrossRef]
- Park Systems, XE-70 High accuracy small sample SPM. In User’s Manual; Park Systems: Suwon, Korea, 2013.
- Meyer, G.; Amer, N.M. Novel optical approach to atomic force microscopy. Appl. Phys. Lett. 1988, 53, 1045–1047. [Google Scholar] [CrossRef]
- Alexander, S.; Hellemans, L.; Marti, O.; Schneir, J.; Elings, V.; Hansma, P.K.; Longmire, M.; Gurley, J. An atomic-resolution atomic-force microscope implemented using an optical lever. J. Appl. Phys. 1989, 65, 164–167. [Google Scholar] [CrossRef]
- Meyer, G.; Amer, N.M. Optical-beam-deflection atomic force microscopy: The NaCl (001) surface. Appl. Phys. Lett. 1990, 56, 2100–2101. [Google Scholar] [CrossRef]
- Putman, C.A.J.; De Grooth, B.G.; Van Hulst, N.F.; Greve, J. A detailed analysis of the optical beam deflection technique for use in atomic force microscopy. J. Appl. Phys. 1992, 72, 6–12. [Google Scholar] [CrossRef] [Green Version]
- Marrese, M.; Guarino, V.; Ambrosio, L. Atomic force microscopy: A powerful tool to address scaffold design in tissue engineering. J. Funct. Biomater. 2017, 8, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, Y.; Williams, C.C.; Wickramasinghe, H.K. Atomic force microscope–force mapping and profiling on a sub 100-Å scale. J. Appl. Phys. 1987, 61, 4723–4729. [Google Scholar] [CrossRef]
- Kodera, N.; Sakashita, M.; Ando, T. Dynamic proportional-integral-differential controller for high-speed atomic force microscopy. Rev. Sci. Instrum. 2006, 77, 083704. [Google Scholar] [CrossRef] [Green Version]
- Kodera, N.; Yamashita, H.; Ando, T. Active damping of the scanner for high-speed atomic force microscopy. Rev. Sci. Instrum. 2005, 76, 053708. [Google Scholar] [CrossRef] [Green Version]
- Wickramasinghe, H.K. Scanning probe microscopy: Current status and future trends. J. Vac. Sci. Technol. Vac. Surf. Film 1990, 8, 363–368. [Google Scholar] [CrossRef]
- Maver, U.; Velnar, T.; Gaberšček, M.; Planinšek, O.; Finšgar, M. Recent progressive use of atomic force microscopy in biomedical applications. TrAC Trends Anal. Chem. 2016, 80, 96–111. [Google Scholar] [CrossRef] [Green Version]
- Harcombe, D.M.; Ruppert, M.G.; Ragazzon, M.R.P.; Fleming, A.J. Higher-harmonic AFM Imaging with a high-bandwidth multifrequency lyapunov filter. In Proceedings of the International Conference on Advanced Intelligent Mechatronics (AIM), Munich, Germany, 3–7 July 2017. [Google Scholar]
- Shamsudhin, N.; Rothuizen, H.; Nelson, B.J.; Sebastian, A. Multi-frequency atomic force microscopy: A system-theoretic approach. IFAC Proc. Vol. 2014, 47, 7499–7504. [Google Scholar] [CrossRef] [Green Version]
- Krisenko, M.O.; Cartagena, A.; Raman, A.; Geahlen, R.L. Nanomechanical property maps of breast cancer cells as determined by multiharmonic atomic force microscopy reveal SYK-dependent changes in microtubule stability mediated by MAP1B. Biochemistry 2015, 54, 60–68. [Google Scholar] [CrossRef] [Green Version]
- Benaglia, S.; Gisbert, V.G.; Perrino, A.P.; Amo, C.A.; Garcia, R. Fast and high-resolution mapping of elastic properties of biomolecules and polymers with bimodal AFM. Nat. Protoc. 2018, 13, 2890–2907. [Google Scholar] [CrossRef]
- Dufrêne, Y.F.; Martínez-Martín, D.; Medalsy, I.; Alsteens, D.; Müller, D.J. Multiparametric imaging of biological systems by force-distance curve-based AFM. Nat. Methods 2013, 10, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Chawla, G.; Solares, S.D. Mapping of conservative and dissipative interactions in bimodal atomic force microscopy using open-loop and phase-locked-loop control of the higher eigenmode. Appl. Phys. Lett. 2011, 99, 2011–2014. [Google Scholar] [CrossRef]
- Chanmin Su, J.S.; Hu, Y.; Hu, S.; Ma, J. Method and Apparatus of Using Peak Force Tapping Mode to Measure Physical Properties of a Sample. U.S. Patent No. 8,650,660, 13 December 2008. [Google Scholar]
- Schillers, H.; Medalsy, I.; Hu, S.; Slade, A.L.; Shaw, J.E. PeakForce Tapping resolves individual microvilli on living cells. J. Mol. Recognit. 2016, 29, 95–101. [Google Scholar] [CrossRef] [Green Version]
- Al-Rekabi, Z.; Contera, S. Multifrequency AFM reveals lipid membrane mechanical properties and the effect of cholesterol in modulating viscoelasticity. Proc. Natl. Acad. Sci. USA 2018, 115, 2658–2663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Z.; Xu, R.; Ye, S.; Hussain, S.; Ji, W.; Cheng, P.; Li, Y.; Sugawara, Y.; Cheng, Z. High harmonic exploring on different materials in dynamic atomic force microscopy. Sci. China Technol. Sci. 2018, 61, 446–452. [Google Scholar] [CrossRef]
- Rother, J.; Nöding, H.; Mey, I.; Janshoff, A. Atomic force microscopy-based microrheology reveals significant differences in the viscoelastic response between malign and benign cell lines. Open Biol. 2014, 4, 140046. [Google Scholar] [CrossRef]
- Yang, Q.; Ma, Q.; Herum, K.M.; Wang, C.; Patel, N.; Lee, J.; Wang, S.; Yen, T.M.; Wang, J.; Tang, H.; et al. Array atomic force microscopy for real-time multiparametric analysis. Proc. Natl. Acad. Sci. USA 2019, 116, 5872–5877. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.J.; Ono, T.; Esashi, M. Capacitive resonant mass sensor with frequency demodulation detection based on resonant circuit. Appl. Phys. Lett. 2006, 88, 1–3. [Google Scholar] [CrossRef]
- Rogers, B.; Manning, L.; Sulchek, T.; Adams, J. Improving tapping mode atomic force microscopy with piezoelectric cantilevers. Ultramicroscopy 2004, 100, 267–276. [Google Scholar] [CrossRef]
- Dukic, M.; Winhold, M.; Schwalb, C.H.; Adams, J.D.; Stavrov, V.; Huth, M.; Fantner, G.E. Direct-write nanoscale printing of nanogranular tunnelling strain sensors for sub-micrometre cantilevers. Nat. Commun. 2016, 7, 1–7. [Google Scholar] [CrossRef]
- Itoh, T.; Suga, T. Piezoelectric sensor for detecting force gradients in atomic force microscopy. Jpn. J. Appl. Phys. 1994, 33, 334. [Google Scholar] [CrossRef]
- Ruiz-Díez, V.; Toledo, J.; Hernando-García, J.; Ababneh, A.; Seidel, H.; Sánchez-Rojas, J.L. A geometrical study on the roof tile-shaped modes in AIN-based piezoelectric microcantilevers as viscosity–density sensors. Sensors 2019, 19, 658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischeneder, M.; Oposich, M.; Schneider, M.; Schmid, U. Tuneable Q-factor of MEMS cantilevers with integrated piezoelectric thin films. Sensors 2018, 18, 3842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shekhawat, G.; Tark, S.H.; Dravid, V.P. MOSFET-embedded microcantilevers for measuring deflection in biomolecular sensors. Science 2006, 311, 1592–1595. [Google Scholar] [CrossRef] [Green Version]
- Wagner, R.; Woehl, T.J.; Keller, R.R.; Killgore, J.P. Detection of atomic force microscopy cantilever displacement with a transmitted electron beam. Appl. Phys. Lett. 2016, 109, 043111. [Google Scholar] [CrossRef]
- Hermans, R.I.; Dueck, B.; Ndieyira, J.W.; McKendry, R.A.; Aeppli, G. Optical diffraction for measurements of nano-mechanical bending. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Andreeva, N.V. Atomic force microscopy with interferometric method for detection of the cantilever displacement and its application for low-temperature studies. Ferroelectrics 2018, 525, 178–186. [Google Scholar] [CrossRef]
- Putman, C.A.J.; de Grooth, B.G.; van Hulst, N.F.; Greve, J. A theoretical comparison between interferometric and optical beam deflection technique for the measurement of cantilever displacement in AFM. Ultramicroscopy 1992, 42–44 Pt 2, 1509–1513. [Google Scholar] [CrossRef] [Green Version]
- Routley, B.; Fleming, A. High sensitivity interferometer for on-axis detection of AFM cantilever deflection. In Proceedings of the International Conference on Manipulation, Automation and Robotics at Small Scales, Paris, France, 18–22 July 2016. [Google Scholar]
- Von Schmidsfeld, A.; Nörenberg, T.; Temmen, M.; Reichling, M. Understanding interferometry for micro-cantilever displacement detection. Beilstein J. Nanotechnol. 2016, 7, 841–851. [Google Scholar] [CrossRef]
- Rugar, D.; Mamin, H.J.; Guethner, P. Improved fiber-optic interferometer for atomic force microscopy. Appl. Phys. Lett. 1989, 55, 2588–2590. [Google Scholar] [CrossRef]
- Yaralioglu, G.G.; Atalar, A.; Manalis, S.R.; Quate, C.F. Analysis and design of an interdigital cantilever as a displacement sensor. J. Appl. Phys. 1998, 83, 7405–7415. [Google Scholar] [CrossRef] [Green Version]
- Manalis, S.R.; Minne, S.C.; Atalar, A.; Quate, C.F. Interdigital cantilevers for atomic force microscopy. Appl. Phys. Lett. 1996, 69, 3944–3946. [Google Scholar] [CrossRef]
- Doll, J.C.; Pruitt, B.L. Design of piezoresistive versus piezoelectric contact mode scanning probes. J. Micromech. Microeng. 2010, 20, 095023. [Google Scholar] [CrossRef]
- Moore, S.I.; Ruppert, M.G.; Yong, Y.K. An optimization framework for the design of piezoelectric AFM cantilevers. Precis. Eng. 2019, 60, 130–142. [Google Scholar] [CrossRef]
- Ruppert, M.G.; Moore, S.I.; Zawierta, M.; Fleming, A.J.; Putrino, G.; Yong, Y.K. Multimodalatomic force microscopy with optimized higher eigenmode sensitivity using on-chip piezoelectric actuation and sensing. Nanotechnology 2019, 30, 085503. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, S.; Kathiresan, R.; Kobayashi, T.; Lee, C. Development of piezoelectric microcantilever flow sensor with wind-driven energy harvesting capability. Appl. Phys. Lett. 2012, 100, 2012–2015. [Google Scholar] [CrossRef] [Green Version]
- Brugger, J.; Buser, R.A.; de Rooij, N.F. Micromachined atomic force microprobe with integrated capacitive read-out. J. Micromech. Microeng. 1992, 2, 218–220. [Google Scholar] [CrossRef] [Green Version]
- Tortonese, M.; Yamada, H.; Barrett, R.C.; Quate, C.F. Atomic force microscopy using a piezoresistive cantilever. In Proceedings of the International Conference on Solid-State Sensors and Actuators, San Francisco, CA, USA, 24–27 June 1991. [Google Scholar]
- Gotszalk, T.; Linnemann, R.; Rangelow, I.W.; Dumania, P.; Grabiec, P.B. AFM with piezoresistive Wheatstone bridge cantilever: Noise performance and applications in contact and noncontact mode. Met. Microsys. Phys. Technol. Appl. 1996, 2780, 376–379. [Google Scholar]
- Harley, J.A.; Kenny, T.W. High-sensitivity piezoresistive cantilevers under 1000 Å thick. Appl. Phys. Lett. 1999, 75, 289–291. [Google Scholar] [CrossRef]
- Rangelow, I.W.; Skocki, S.; Dumania, P. Plasma etching for micromechanical sensor applications. Microelectron. Eng. 1994, 23, 365–368. [Google Scholar] [CrossRef]
- Tortonese, M.; Barrett, R.C.; Quate, C.F. Atomic resolution with an atomic force microscope using piezoresistive detection. Appl. Phys. Lett. 1993, 62, 834–836. [Google Scholar] [CrossRef]
- Oden, P.I.; Datskos, P.G.; Thundat, T.; Warmack, R.J. Uncooled thermal imaging using a piezoresistive microcantilever. Appl. Phys. Lett. 1996, 69, 3277–3279. [Google Scholar] [CrossRef]
- Thaysen, J.; Boisen, A.; Hansen, O.; Bouwstra, S. Atomic force microscopy probe with piezoresistive read-out and a highly symmetrical Wheatstone bridge arrangement. Sens. Actuators Phys. 2000, 83, 47–53. [Google Scholar] [CrossRef]
- Linnemann, R.; Gotszalk, T.; Hadjiiski, L.; Rangelow, I.W. Characterization of a cantilever with an integrated deflection sensor. Thin Solid Films 1995, 264, 159–164. [Google Scholar] [CrossRef]
- Yu, X.; Thaysen, J.; Hansen, O.; Boisen, A. Optimization of sensitivity and noise in piezoresistive cantilevers. J. Appl. Phys. 2002, 92, 6296–6301. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, P.A.; Thaysen, J.; Hansen, O.; Eriksen, S.C.; Boisen, A. Optimised cantilever biosensor with piezoresistive read-out. Ultramicroscopy 2003, 97, 371–376. [Google Scholar] [CrossRef]
- Thaysen, J. Cantilever for Bio-Chemical Sensing Integrated In a Microliquid Handling System. Ph.D. Thesis, Technical University of Denmark (DTU), Lyngby, Denmark, 1999. [Google Scholar]
- Lange, D.; Akiyama, T.; Hagleitner, C.; Tonin, A.; Hidber, H.R.; Niedermann, P.; Staufer, U.; De Rooij, N.F.; Brand, O.; Baltes, H. Parallel scanning AFM with on-chip circuitry in CMOS technology. In Proceedings of the International MEMS Conference on Micro Electro Mechanical Systems, Orlando, FL, USA, 21 January 1999. [Google Scholar]
- Thaysen, J.; Yalçinkaya, A.D.; Vettiger, P.; Menon, A. Polymer-based stress sensor with integrated readout. J. Phys. D Appl. Phys. 2002, 35, 2698–2703. [Google Scholar] [CrossRef]
- Xia, F.; Yang, C.; Wang, Y.; Youcef-Toumi, K.; Reuter, C.; Ivanov, T.; Holz, M.; Rangelow, I.W. Lights out! nano-scale topography imaging of sample surface in opaque liquid environments with coated active cantilever probes. Nanomaterials 2019, 9, 1013. [Google Scholar] [CrossRef] [Green Version]
- Goeders, K.M.; Colton, J.S.; Bottomley, L.A. Microcantilevers: Sensing chemical interactions via mechanical motion. Chem. Rev. 2008, 108, 522–542. [Google Scholar] [CrossRef]
- Britton, C.L., Jr.; Jones, R.L.; Oden, P.I.; Hu, Z.; Warmack, R.J.; Smith, S.F.; Bryan, W.L.; Rochelle, J.M. Multiple-input microcantilever sensors. Ultramicroscopy 2000, 82, 17–21. [Google Scholar] [CrossRef]
- Forsen, E.; Abadal, G.; Ghatnekar-Nilsson, S.; Teva, J.; Verd, J.; Sandberg, R.; Svendsen, W.; Perez-Murano, F.; Esteve, J.; Figueras, E.; et al. Ultrasensitive mass sensor fully integrated with complementary metal-oxide-semiconductor circuitry. Appl. Phys. Lett. 2005, 87, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Azcona, F.J.; Jha, A.; Yáñez, C.; Atashkhooei, R.; Royo, S. Microcantilever displacement measurement using a mechanically modulated optical feedback interferometer. Sensors 2016, 16, 997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, B.; Prater, C.B.; King, W.P. Lorentz force actuation of a heated atomic force microscope cantilever. Nanotechnology 2012, 23, 055709. [Google Scholar] [CrossRef] [PubMed]
- Revenko, I.; Proksch, R. Magnetic and acoustic tapping mode microscopy of liquid phase phospholipid bilayers and DNA molecules. J. Appl. Phys. 1999, 87, 526. [Google Scholar] [CrossRef] [Green Version]
- Florin, E.; Radmacher, M.; Fleck, B.; Gaub, H.E. Atomic force microscope with magnetic force modulation. Rev. Sci. Instrum. 1994, 65, 639–643. [Google Scholar] [CrossRef]
- Mazeran, P.E.; Loubet, J.L. Force modulation with a scanning force microscope: An analysis. Tribol. Lett. 1997, 3, 125–132. [Google Scholar] [CrossRef]
- Xu, X.; Raman, A. Comparative dynamics of magnetically, acoustically, and Brownian motion driven microcantilevers in liquids. J. Appl. Phys. 2007, 102, 034303. [Google Scholar] [CrossRef] [Green Version]
- Motamedi, R.; Wood-Adams, P.M. Influence of fluid cell design on the frequency response of afm microcantilevers in liquid media. Sensors 2008, 8, 5927–5941. [Google Scholar] [CrossRef] [Green Version]
- Carbone, G.; Pierro, E.; Soria, L. Microcantilever dynamics: Effect of Brownian excitation in liquids. In Proceedings of the SEM Annual Conference Exposition on Experimental and Applied Mechanics, Hyatt Regency Albuquerque, Albuquerque, New Mexico, 1–4 June 2009. [Google Scholar]
- Berg, J.; Briggs, G.A.D. Nonlinear dynamics of intermittent-contact mode atomic force microscopy. Phys. Rev. B 1997, 55, 14899–14908. [Google Scholar] [CrossRef]
- Salapaka, S.; Dahleh, M.; Mezić, I. On the dynamics of a harmonic oscillator undergoing impacts with a vibrating platform. Nonlinear Dyn. 2001, 24, 333–358. [Google Scholar] [CrossRef]
- Umeda, K.; Oyabu, N.; Kobayashi, K.; Hirata, Y.; Matsushige, K.; Yamada, H. High-resolution frequency-modulation atomic force microscopy in liquids using electrostatic excitation method. Appl. Phys. Express 2010, 3, 065205. [Google Scholar] [CrossRef]
- Desbiolle, B.X.E.; Furlan, G.; Schwartzberg, A.M.; Ashby, P.D.; Ziegler, D. Electrostatically actuated encased cantilevers. Beilstein J. Nanotechnol. 2018, 9, 1381–1389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Degertekin, F.L.; Hadimioglu, B.; Sulchek, T.; Quate, C.F. Actuation and characterization of atomic force microscope cantilevers in fluids by acoustic radiation pressure. Appl. Phys. Lett. 2001, 78, 1628–1630. [Google Scholar] [CrossRef]
- Chu, B.; Apfel, R.E. Acoustic radiation pressure produced by a beam of sound. J. Acoust. Soc. Am. 1982, 72, 1673–1687. [Google Scholar] [CrossRef]
- Kiracofe, D.; Raman, A. Quantitative force and dissipation measurements in liquids using piezo-excited atomic force microscopy: A unifying theory. Nanotechnology 2011, 22, 485502. [Google Scholar] [CrossRef] [Green Version]
- Rogers, B.; York, D.; Whisman, N.; Jones, M.; Murray, K.; Adams, J.D.; Sulchek, T.; Minne, S.C. Tapping mode atomic force microscopy in liquid with an insulated piezoelectric microactuator. Rev. Sci. Instrum. 2002, 73, 3242–3244. [Google Scholar] [CrossRef]
- Maali, A.; Hurth, C.; Cohen-Bouhacina, T.; Couturier, G.; Aimé, J.P. Improved acoustic excitation of atomic force microscope cantilevers in liquids. Appl. Phys. Lett. 2006, 88, 163504. [Google Scholar] [CrossRef] [Green Version]
- Carrasco, C.; Ares, P.; de Pablo, P.J.; Gómez-Herrero, J. Cutting down the forest of peaks in acoustic dynamic atomic force microscopy in liquid. Rev. Sci. Instrum. 2008, 79, 126106. [Google Scholar] [CrossRef]
- Rogers, B.; Sulchek, T.; Murray, K.; York, D.; Jones, M.; Manning, L.; Malekos, S.; Beneschott, B.; Adams, J.D.; Cavazos, H.; et al. High speed tapping mode atomic force microscopy in liquid using an insulated piezoelectric cantilever. Rev. Sci. Instrum. 2003, 74, 4683–4686. [Google Scholar] [CrossRef] [Green Version]
- Ilic, B.; Krylov, S.; Craighead, H.G. Theoretical and experimental investigation of optically driven nanoelectromechanical oscillators. J. Appl. Phys. 2010, 107, 034311. [Google Scholar] [CrossRef]
- Pedrak, R.; Ivanov, T.; Ivanova, K.; Gotszalk, T.; Abedinov, N.; Rangelow, I.W. Micromachined atomic force microscopy sensor with integrated piezoresistive sensor and thermal bimorph actuator for high-speed tapping-mode atomic force microscopy phase-imaging in higher eigenmodes. J. Vac. Sci. Technol. Microelectron. Nanometer Struct. Process. Meas. Phenom. 2003, 21, 3102–3107. [Google Scholar] [CrossRef]
- Angelov, T.; Roeser, D.; Ivanov, T.; Gutschmidt, S.; Sattel, T.; Rangelow, I.W. Thermo-mechanical transduction suitable for high-speed scanning probe imaging and lithography. Microelectron. Eng. 2016, 154, 1–7. [Google Scholar] [CrossRef]
- Michels, T.; Guliyev, E.; Klukowski, M.; Rangelow, I. Micromachined self-actuated piezoresistive cantilever for high speed SPM. Microelectron. Eng. 2012, 97, 265–268. [Google Scholar] [CrossRef]
- Nishida, S.; Kobayashi, D.; Kawakatsu, H.; Nishimori, Y. Photothermal excitation of a single-crystalline silicon cantilever for higher vibration modes in liquid. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. 2009, 27, 964–968. [Google Scholar] [CrossRef]
- Miyahara, Y.; Griffin, H.; Roy-Gobeil, A.; Belyansky, R.; Bergeron, H.; Bustamante, J.; Grutter, P. Optical excitation of atomic force microscopy cantilever for accurate spectroscopic measurements. EPJ Tech. Instrum. 2020, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Gao, N.; Zhao, D.; Jia, R.; Liu, D. Microcantilever actuation by laser induced photoacoustic waves. Sci. Rep. 2016, 6, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Ono, M.; Lange, D.; Brand, O.; Hagleitner, C.; Baltes, H. A complementary metal oxide semiconductor field effect transistor compatible atomic force microscopy tip fabrication process and integrated atomic force microscopy cantilevers fabricated with this process. Ultramicroscopy 2002, 91, 9–20. [Google Scholar] [CrossRef]
- Franks, W.; Lange, D.; Lee, S.; Hierlemann, A.; Spencer, N.; Baltes, H. Nanochemical surface analyzer in CMOS technology. Ultramicroscopy 2002, 91, 21–27. [Google Scholar] [CrossRef]
- Rangelow, I.W.; Grabiec, P.; Gotszalk, T.; Edinger, K. Piezoresistive SXM sensors. Surf. Interface Anal. 2002, 33, 59–64. [Google Scholar] [CrossRef]
- Miyahara, Y.; Deschler, M.; Fujii, T.; Watanabe, S.; Bleuler, H. Non-contact atomic force microscope with a PZT cantilever used for deflection sensing, direct oscillation and feedback actuation. Appl. Surf. Sci. 2002, 188, 450–455. [Google Scholar] [CrossRef]
- Bachels, T.; Schäfer, R. Microfabricated cantilever-based detector for molecular beam experiments. Rev. Sci. Instrum. 1998, 69, 3794–3797. [Google Scholar] [CrossRef]
- Chand, A.; Viani, M.B.; Schäffer, T.E.; Hansma, P.K. Microfabricated small metal cantilevers with silicon tip for atomic force microscopy. J. Microelectromech. Syst. 2000, 9, 112–116. [Google Scholar] [CrossRef]
- Viani, M.B.; Schäffer, T.E.; Chand, A.; Rief, M.; Gaub, H.E.; Hansma, P.K. Small cantilevers for force spectroscopy of single molecules. J. Appl. Phys. 1999, 86, 2258–2262. [Google Scholar] [CrossRef]
- Du, B.; Xu, X.; He, J.; Guo, K.; Huang, W.; Zhang, F.; Zhang, M.; Wang, Y. In-fiber collimator-based fabry-perot interferometer with enhanced vibration sensitivity. Sensors 2019, 19, 435. [Google Scholar] [CrossRef] [Green Version]
- Sidler, K. Fabrication and Characterization of SU-8 Cantilevers with Integrated Tips Designed for Dip-Pen Nanolithography. Master’s Thesis, ETH, Eidgenössische Technische Hochschule Zürich, Mechanical Engineering, Zürich, Switzerland, 2006. [Google Scholar]
- Hosseini, N.; Neuenschwander, M.; Peric, O.; Andany, S.H.; Adams, J.D.; Fantner, G.E. Integration of sharp silicon nitride tips into high-speed SU8 cantilevers in a batch fabrication process. Beilstein J. Nanotechnol. 2019, 10, 2357–2363. [Google Scholar] [CrossRef]
- Martin-Olmos, C.; Rasool, H.I.; Weiller, B.H.; Gimzewski, J.K. Graphene MEMS: AFM probe performance improvement. ACS Nano 2013, 7, 4164–4170. [Google Scholar] [CrossRef]
- Genolet, G.; Despont, M.; Vettiger, P.; Anselmetti, D.; De Rooij, N.F. All-photoplastic, soft cantilever cassette probe for scanning force microscopy. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. 2000, 18, 617–620. [Google Scholar] [CrossRef] [Green Version]
- Adams, J.D.; Erickson, B.W.; Grossenbacher, J.; Brugger, J.; Nievergelt, A.; Fantner, G.E. Harnessing the damping properties of materials for high-speed atomic force microscopy. Nat. Nanotechnol. 2016, 11, 147–151. [Google Scholar] [CrossRef] [Green Version]
- Kramer, R.C.; Verlinden, E.J.; Angeloni, L.; Van Den Heuvel, A.; Fratila-Apachitei, L.E.; Van Der Maarel, S.M.; Ghatkesar, M.K. Multiscale 3D-printing of microfluidic AFM cantilevers. Lab Chip 2020, 20, 311–319. [Google Scholar] [CrossRef] [Green Version]
- Zenhausern, F.; Adrian, M.; Ten Heggeler-Bordier, B.; Ardizzoni, F.; Descouts, P. Enhanced imaging of biomolecules with electron beam deposited tips for scanning force microscopy. J. Appl. Phys. 1993, 73, 7232–7237. [Google Scholar] [CrossRef]
- Akiyama, K.; Eguchi, T.; An, T.; Fujikawa, Y.; Yamada-Takamura, Y.; Sakurai, T.; Hasegawa, Y. Development of a metal-tip cantilever for noncontact atomic force microscopy. Rev. Sci. Instrum. 2005, 76, 033705. [Google Scholar] [CrossRef]
- Tay, A.B.H.; Thong, J.T.L. Fabrication of super-sharp nanowire atomic force microscope probes using a field emission induced growth technique. Rev. Sci. Instrum. 2004, 75, 3248–3255. [Google Scholar] [CrossRef]
- Dremov, V.; Fedoseev, V.; Fedorov, P.; Grebenko, A. Fast and reliable method of conductive carbon nanotube-probe fabrication for scanning probe microscopy. Rev. Sci. Instrum. 2015, 86, 053703. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Song, J.; Kim, S.O.; Kim, S.; Lee, W.; Jackman, J.A.; Kim, D.; Cho, N.J.; Lee, J. Multifunctional hydrogel nano-probes for atomic force microscopy. Nat. Commun. 2016, 7, 1–14. [Google Scholar] [CrossRef]
- Imran, A.B.; Esaki, K.; Gotoh, H.; Seki, T.; Ito, K.; Sakai, Y.; Takeoka, Y. Extremely stretchable thermosensitive hydrogels by introducing slide-ring polyrotaxane cross-linkers and ionic groups into the polymer network. Nat. Commun. 2014, 5, 1–8. [Google Scholar] [CrossRef]
- Gou, M.; Qu, X.; Zhu, W.; Xiang, M.; Yang, J.; Zhang, K.; Wei, Y.; Chen, S. Bio-inspired detoxification using 3d-printed hydrogel nanocomposites. Nat. Commun. 2014, 5, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Purcell, B.P.; Lobb, D.; Charati, M.B.; Dorsey, S.M.; Wade, R.J.; Zellars, K.N.; Doviak, H.; Pettaway, S.; Logdon, C.B.; Shuman, J.A.; et al. Injectable and bioresponsive hydrogels for on-demand matrix metalloproteinase inhibition. Nat. Mater. 2014, 13, 653–661. [Google Scholar] [CrossRef] [Green Version]
- Ivanovska, I.L.; Miranda, R.; Carrascosa, J.L.; Wuite, G.J.L.; Schmidt, C.F. Discrete fracture patterns of virus shells reveal mechanical building blocks. Proc. Natl. Acad. Sci. USA 2011, 108, 12611–12616. [Google Scholar] [CrossRef] [Green Version]
- Rosario, R.; Mutharasan, R. Piezoelectric excited millimeter sized cantilever sensors for measuring gas density changes. Sens. Actuators B Chem. 2014, 192, 99–104. [Google Scholar] [CrossRef]
- Zhao, L.; Xu, L.; Zhang, G.; Jiang, Z.; Zhao, Y.; Wang, J.; Wang, X.; Liu, Z. In-situ measurement of fluid density rapidly using a vibrating piezoresistive microcantilever sensor without resonance occurring. IEEE Sens. J. 2014, 14, 645–650. [Google Scholar] [CrossRef]
- Bircher, B.A.; Duempelmann, L.; Renggli, K.; Lang, H.P.; Gerber, C.; Bruns, N.; Braun, T. Real-time viscosity and mass density sensors requiring microliter sample volume based on nanomechanical resonators. Anal. Chem. 2013, 85, 8676–8683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boudjiet, M.T.; Cuisset, V.; Pellet, C.; Bertrand, J.; Dufour, I. Preliminary results of the feasibility of hydrogen detection by the use of uncoated silicon microcantilever-based sensors. Int. J. Hydrogen Energy 2014, 39, 20497–20502. [Google Scholar] [CrossRef] [Green Version]
- Boudjiet, M.T.; Bertrand, J.; Mathieu, F.; Nicu, L.; Mazenq, L.; Leichle, T.; Heinrich, S.M.; Pellet, C.; Dufour, I. Geometry optimization of uncoated silicon microcantilever-based gas density sensors. Sens. Actuators Chem. 2015, 208, 600–607. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Ning, X.; Park, B.; Boons, G.J.; Xu, B. Simple, clickable protocol for atomic force microscopy tip modification and its application for trace ricin detection by recognition imaging. Langmuir 2009, 25, 2860–2864. [Google Scholar] [CrossRef] [Green Version]
- Arafat, A.; Schroën, K.; de Smet, L.C.; Sudhölter, E.J.; Zuilhof, H. Tailor-made functionalization of silicon nitride surfaces. J. Am. Chem. Soc. 2004, 126, 8600–8601. [Google Scholar] [CrossRef]
- Daza, R.; Colchero, L.; Corregidor, D.; Elices, M.; Guinea, G.V.; Rojo, F.J.; Pérez-Rigueiro, J. Functionalization of atomic force microscopy cantilevers and tips by activated vapour silanization. Appl. Surf. Sci. 2019, 484, 1141–1148. [Google Scholar] [CrossRef]
- Volcke, C.; Gandhiraman, R.P.; Gubala, V.; Doyle, C.; Fonder, G.; Thiry, P.A.; Cafolla, A.A.; James, B.; Williams, D.E. Plasma functionalization of AFM tips for measurement of chemical interactions. J. Colloid Interface Sci. 2010, 348, 322–328. [Google Scholar] [CrossRef]
- Carrascosa, L.G.; Moreno, M.; Álvare, M.; Lechuga, L.M. Nanomechanical biosensors: A new sensing tool. TrAC Trends Anal. Chem. 2006, 25, 196–206. [Google Scholar] [CrossRef]
- Ito, T.; Ibrahim, S.; Grabowska, I. Chemical-force microscopy for materials characterization. TrAC-Trends Anal. Chem. 2010, 29, 225–233. [Google Scholar] [CrossRef]
- Smith, D.A.; Connell, S.D.; Robinson, C.; Kirkham, J. Chemical force microscopy: Applications in surface characterisation of natural hydroxyapatite. Anal. Chim. Acta 2003, 479, 39–57. [Google Scholar] [CrossRef]
- Shchukin, D.G.; Möhwald, H. Smart nanocontainers as depot media for feedback active coatings. Chem. Commun. 2011, 47, 8730. [Google Scholar] [CrossRef] [PubMed]
- Vinogradova, O.I.; Yakubov, G.E. Dynamic effects on force measurements Lubrication and the atomic force microscope. Langmuir 2003, 19, 1227–1234. [Google Scholar] [CrossRef]
- Alonso, J.L.; Goldmann, W.H. Feeling the forces: Atomic force microscopy in cell biology. Life Sci. 2003, 72, 2553–2560. [Google Scholar] [CrossRef]
- Gillies, G.; Prestidge, C.A.; Attard, P. An AFM study of the deformation and nanorheology of cross-linked PDMS droplets. Langmuir 2002, 18, 1674–1679. [Google Scholar] [CrossRef]
- Gillies, G.; Prestidge, C.A. Interaction forces, deformation and nano-rheology of emulsion droplets as determined by colloid probe AFM. Adv. Colloid Interface Sci. 2004, 108–109, 197–205. [Google Scholar] [CrossRef]
- Ma, C.D.; Acevedo-Velez, C.; Wang, C.; Gellman, S.H.; Abbott, N.L. Interaction of the hydrophobic tip of an atomic force microscope with oligopeptides immobilized using short and long tethers. Langmuir 2016, 32, 2985–2995. [Google Scholar] [CrossRef]
- Pfreundschuh, M.; Alsteens, D.; Wieneke, R.; Zhang, C.; Coughlin, S.R.; Tampe, R.; Kobilka, B.K.; Müller, D.J. Identifying and quantifying two ligand-binding sites while imaging native human membrane receptors by AFM. Nat. Commun. 2015, 6, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Litvinov, R.I.; Shuman, H.; Bennett, J.S.; Weisel, J.W. Binding strength and activation state of single fibrinogen-integrin pairs on living cells. Proc. Natl. Acad. Sci. USA 2002, 99, 7426–7431. [Google Scholar] [CrossRef] [Green Version]
- Friedrichs, J.; Legate, K.R.; Schubert, R.; Bharadwaj, M.; Werner, C.; Müller, D.J.; Benoit, M. A practical guide to quantify cell adhesion using single-cell force spectroscopy. Methods 2013, 60, 169–178. [Google Scholar] [CrossRef]
- Zhang, X.; Wojcikiewicz, E.; Moy, V. Force spectroscopy of the leukocyte function-associated antigen-1/intercellular adhesion molecule-1 interaction. Biophys. J. 2002, 83, 2270–2279. [Google Scholar] [CrossRef] [Green Version]
- Fleming, A.J. Dual-stage vertical feedback for high-speed scanning probe microscopy. IEEE Trans. Control Syst. Technol. 2011, 19, 156–165. [Google Scholar] [CrossRef] [Green Version]
- Fantner, G.E.; Schitter, G.; Kindt, J.H.; Ivanov, T.; Ivanova, K.; Patel, R.; Holten-Andersen, N.; Adams, J.; Thurner, P.J.; Rangelow, I.W.; et al. Components for high speed atomic force microscopy. Ultramicroscopy 2006, 106, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Fantner, G.E.; Hegarty, P.; Kindt, J.H.; Schitter, G.; Cidade, G.A.G.; Hansma, P.K. Data acquisition system for high speed atomic force microscopy. Rev. Sci. Instrum. 2005, 76, 026118. [Google Scholar] [CrossRef] [Green Version]
- Ando, T.; Uchihashi, T.; Fukuma, T. High-speed atomic force microscopy for nano-visualization of dynamic biomolecular processes. Prog. Surf. Sci. 2008, 83, 337–437. [Google Scholar] [CrossRef] [Green Version]
- Alunda, B.O.; Lee, Y.J.; Park, S. A novel two-axis parallel-kinematic high-speed piezoelectric scanner for atomic force microscopy. J. Korean Phys. Soc. 2016, 69, 691–696. [Google Scholar] [CrossRef]
- Yang, C.; Yan, J.; Dukic, M.; Hosseini, N.; Zhao, J.; Fantner, G.E. Design of a high-bandwidth tripod scanner for high speed atomic force microscopy. Scanning 2016, 38, 889–900. [Google Scholar] [CrossRef] [Green Version]
- Adams, J.D.; Nievergelt, A.; Erickson, B.W.; Yang, C.; Dukic, M.; Fantner, G.E. High-speed imaging upgrade for a standard sample scanning atomic force microscope using small cantilevers. Rev. Sci. Instrum. 2014, 85, 093702. [Google Scholar] [CrossRef]
- Leitner, M.; Fantner, G.E.; Fantner, E.J.; Ivanova, K.; Ivanov, T.; Rangelow, I.; Ebner, A.; Rangl, M.; Tang, J.; Hinterdorfer, P. Increased imaging speed and force sensitivity for bio-applications with small cantilevers using a conventional AFM setup. Micron. 2012, 43, 1399–1407. [Google Scholar] [CrossRef]
- Alunda, B.O.; Otieno, L.O.; Chepkoech, M.; Byeon, C.C.; Lee, Y.J. Comparative study of trans-linear and trans-impedance readout circuits for optical beam deflection sensors in atomic force microscopy. J. Korean Phys. Soc. 2019, 74, 88–93. [Google Scholar] [CrossRef]
- Fukuma, T.; Kimura, M.; Kobayashi, K.; Matsushige, K.; Yamada, H. Development of low noise cantilever deflection sensor for multienvironment frequency-modulation atomic force microscopy. Rev. Sci. Instrum. 2005, 76, 053704. [Google Scholar] [CrossRef] [Green Version]
- Enning, R.; Ziegler, D.; Nievergelt, A.; Friedlos, R.; Venkataramani, K.; Stemmer, A. A high frequency sensor for optical beam deflection atomic force microscopy. Rev. Sci. Instrum. 2011, 82, 043705. [Google Scholar] [CrossRef] [PubMed]
- Fukuma, T. Wideband low-noise optical beam deflection sensor with photothermal excitation for liquid-environment atomic force microscopy. Rev. Sci. Instrum. 2009, 80, 023707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ando, T.; Kodera, N.; Uchihashi, T.; Miyagi, A.; Nakakita, R.; Yamashita, H.; Matada, K. High-speed atomic force microscopy for capturing dynamic behavior of protein molecules at work. e-J. Surf. Sci. Nanotechnol. 2005, 3, 384–392. [Google Scholar] [CrossRef]
- Tabak, F.C.; Disseldorp, E.C.M.; Wortel, G.H.; Katan, A.J.; Hesselberth, M.B.S.; Oosterkamp, T.H.; Frenken, J.W.M.; van Spengen, W.M. MEMS-based fast scanning probe microscopes. Ultramicroscopy 2010, 110, 599–604. [Google Scholar] [CrossRef]
- Richter, C.; Weinzierl, P.; Krause, O.; Engl, W.; Penzkofer, C.; Irmer, B.; Sulzbach, T. Microfabricated ultrashort cantilever probes for high speed AFM. In Proceedings of the International Society for Optics and Photonics, Prague, Czech Republic, 5 May 2011. [Google Scholar]
- Ando, T.; Kodera, N.; Takai, E.; Maruyama, D.; Saito, K.; Toda, A. A high-speed atomic force microscope for studying biological macromolecules. Proc. Natl. Acad. Sci. USA 2001, 98, 12468–12472. [Google Scholar] [CrossRef] [Green Version]
- Kodera, N.; Yamamoto, D.; Ishikawa, R.; Ando, T. Video imaging of walking myosin v by high-speed atomic force microscopy. Nature 2010, 468, 72–76. [Google Scholar] [CrossRef] [Green Version]
- Rico, F.; Gonzalez, L.; Casuso, I.; Puig-Vidal, M.; Scheuring, S. High-speed force spectroscopy unfolds titin at the velocity of molecular dynamics simulations. Science 2013, 342, 741–743. [Google Scholar] [CrossRef] [Green Version]
- Ando, T. High-speed atomic force microscopy and its future prospects. Biophys. Rev. 2018, 10, 285–292. [Google Scholar] [CrossRef] [Green Version]
- Heath, G.R.; Scheuring, S. Advances in high-speed atomic force microscopy (HS-AFM) reveal dynamics of transmembrane channels and transporters. Curr. Opin. Struct. Biol. 2019, 57, 93–102. [Google Scholar] [CrossRef] [Green Version]
- Koehler, M.; Fis, A.; Gruber, H.J.; Hinterdorfer, P. AFM-based force spectroscopy guided by recognition imaging: A new mode for mapping and studying interaction sites at low lateral density. Methods Protoc. 2019, 2, 6. [Google Scholar] [CrossRef] [Green Version]
- Noy, A.; Friddle, R.W. Practical single molecule force spectroscopy: How to determine fundamental thermodynamic parameters of intermolecular bonds with an atomic force microscope. Methods 2013, 60, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Helenius, J.; Heisenberg, C.P.; Gaub, H.E.; Muller, D.J. Single-cell force spectroscopy. J. Cell Sci. 2008, 121, 1785–1791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butt, H.J.; Cappella, B.; Kappl, M. Force measurements with the atomic force microscope: Technique, interpretation and applications. Surf. Sci. Rep. 2005, 59, 1–152. [Google Scholar] [CrossRef] [Green Version]
- Ando, T.; Uchihashi, T.; Scheuring, S. Filming biomolecular processes by high-speed atomic force microscopy. Chem. Rev. 2014, 114, 3120–3188. [Google Scholar] [CrossRef] [PubMed]
- Humphris, A.D.L.; Miles, M.J.; Hobbs, J.K. A mechanical microscope: High-speed atomic force microscopy. Appl. Phys. Lett. 2005, 86, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Siewny, M.G.W.; Edwards, D.T.; Sanders, A.W.; Perkins, T.T. Hidden dynamics in the unfolding of individual bacteriorhodopsin proteins. Science (80) 2017, 355, 945–950. [Google Scholar] [CrossRef] [Green Version]
- Matusovsky, O.S.; Mansson, A.; Persson, M.; Cheng, Y.S.; Rassier, D.E. High-speed AFM reveals subsecond dynamics of cardiac thin filaments upon Ca2+ activation and heavy meromyosin binding. Proc. Natl. Acad. Sci. USA 2019, 116, 16384–16393. [Google Scholar] [CrossRef] [Green Version]
- Frutos-Puerto, S.; Miró, C.; Pinilla-Gil, E. Nafion-protected sputtered-bismuth screen-printed electrode for on-site voltammetric measurements of Cd (II) and Pb (II) in natural water samples. Sensors 2019, 19, 279. [Google Scholar] [CrossRef] [Green Version]
- Muenchen, D.K.; Martinazzo, J.; de Cezaro, A.M.; Rigo, A.A.; Brezolin, A.N.; Manzoli, A.; de Lima Leite, F.; Steffens, C.; Steffens, J. Pesticide detection in soil using biosensors and nanobiosensors. Biointerface Res. Appl. 2016, 6, 6. [Google Scholar]
- Rotake, D.; Darji, A.D. Heavy metal ion detection in water using MEMs based sensor. Mater. Today Proc. 2018, 5, 1530–1536. [Google Scholar] [CrossRef]
- Rigo, A.A.; Cezaro, A.M.D.; Muenchen, D.K.; Martinazzo, J.; Brezolin, A.N.; Hoehne, L.; Steffens, C. Cantilever nanobiosensor based on the enzyme urease for detection of heavy metals. Braz. J. Chem. Eng. 2019, 36, 1429–1437. [Google Scholar]
- Muenchen, D.K.; Martinazzo, J.; Brezolin, A.N.; de Cezaro, A.M.; Rigo, A.A.; Mezarroba, M.N.; Manzoli, A.; De Lima Leite, F.; Steffens, J.; Steffens, C. Cantilever Functionalization using peroxidase extract of low cost for glyphosate detection. Appl. Biochem. Biotechnol. 2018, 186, 1061–1073. [Google Scholar] [CrossRef]
- SoltanRezaee, M.; Bodaghi, M. Simulation of an electrically actuated cantilever as a novel biosensor. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sutter, M.; Faulkner, M.; Aussignargues, C.; Paasch, B.C.; Barrett, S.; Kerfeld, C.A.; Liu, L.N. Visualization of bacterial microcompartment facet assembly using high-speed atomic force microscopy. Nano Lett. 2016, 16, 1590–1595. [Google Scholar] [CrossRef] [PubMed]
- Possas-Abreu, M.; Rousseau, L.; Ghassemi, F.; Lissorgues, G.; Habchi, M.; Scorsone, E.; Cal, K.; Persaud, K. Biomimetic diamond MEMS sensors based on odorant-binding proteins: Sensors validation through an autonomous electronic system. In Proceedings of the International Symposium on Olfaction and Electronic Nose (ISOEN), Montreal, QC, Canada, 28–31 May 2017. [Google Scholar]
- Liu, X.; Wang, L.; Zhao, J.; Zhu, Y.; Yang, J.; Yang, F. Enhanced binding efficiency of microcantilever biosensor for the detection of Yersinia. Sensors 2019, 19, 3326. [Google Scholar] [CrossRef] [Green Version]
- Bertke, M.; Xu, J.; Setiono, A.; Kirsch, I.; Uhde, E.; Peiner, E. Fabrication of a microcantilever-based aerosol detector with integrated electrostatic on-chip ultrafine particle separation and collection. Micromech. Microeng. 2020, 30, 014001. [Google Scholar] [CrossRef]
- Guillaume-Gentil, O.; Grindberg, R.V.; Kooger, R.; Dorwling-Carter, L.; Martinez, V.; Ossola, D.; Pilhofer, M.; Zambelli, T.; Vorholt, J.A. Tunable single-cell Extraction for molecular analyses. Cell 2016, 166, 506–516. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, Y.; Zhang, J.; Yang, J. Development of microcantilever sensors for liver cancer detection. Adv. Cancer Prev. 2016, 1, 1000103. [Google Scholar] [CrossRef]
- Kamble, C.; Panse, M.S. Microdevices for low-level acetone gas sensing using tungsten trioxides. IETE J. Res. 2019, 1–8. [Google Scholar] [CrossRef]
- Kim, H.; Hoshi, M.; Iijima, M.; Kuroda, S.; Nakamura, C. Development of a universal method for the measurement of binding affinities of antibody drugs towards a living cell based on AFM force spectroscopy. Anal. Methods 2020, 12, 2922–2927. [Google Scholar] [CrossRef]
- Korayem, M.H.; Heidary, K.; Rastegar, Z. The head and neck cancer (HN-5) cell line properties extraction by AFM. J. Biol. Eng. 2020, 14, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
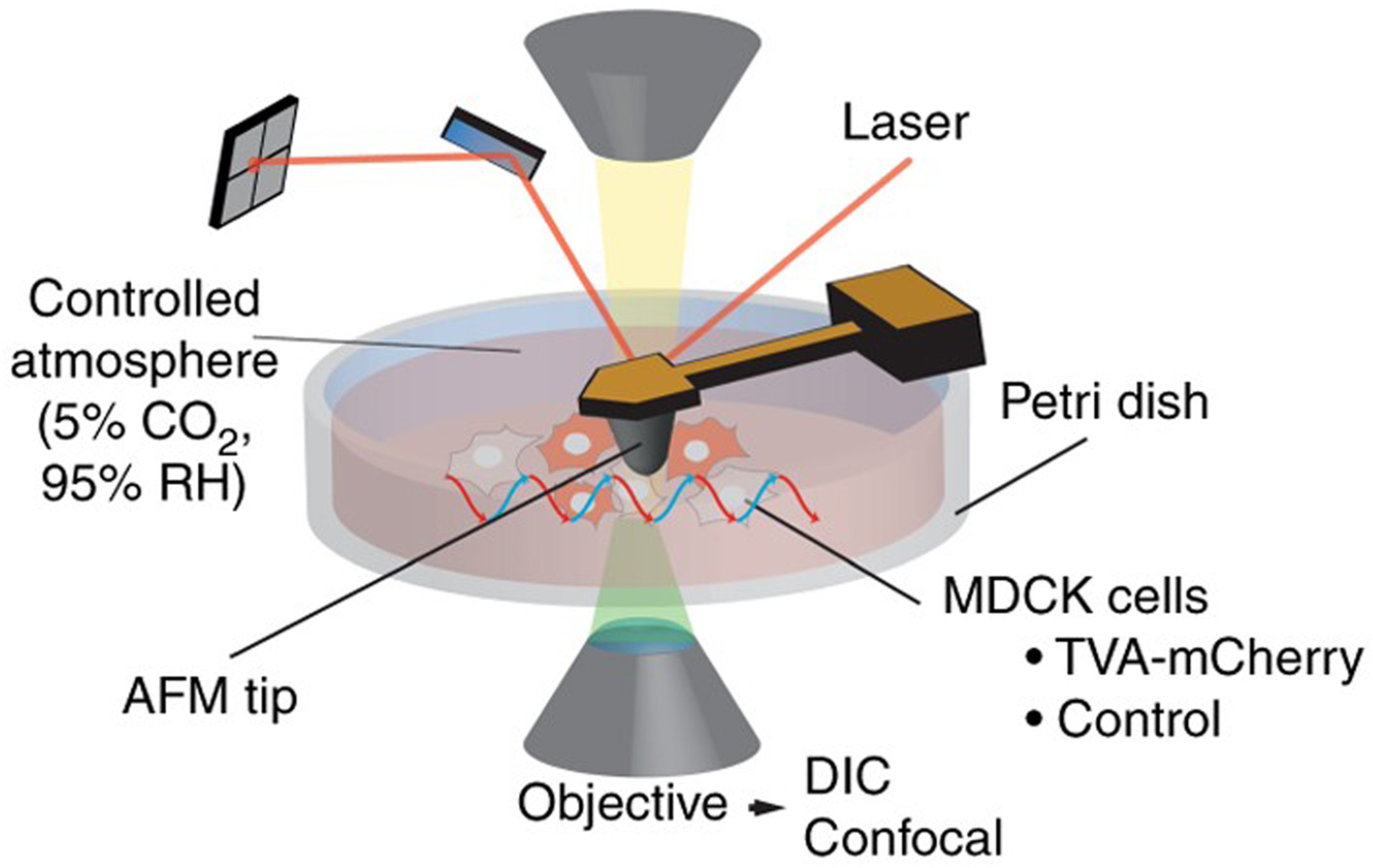
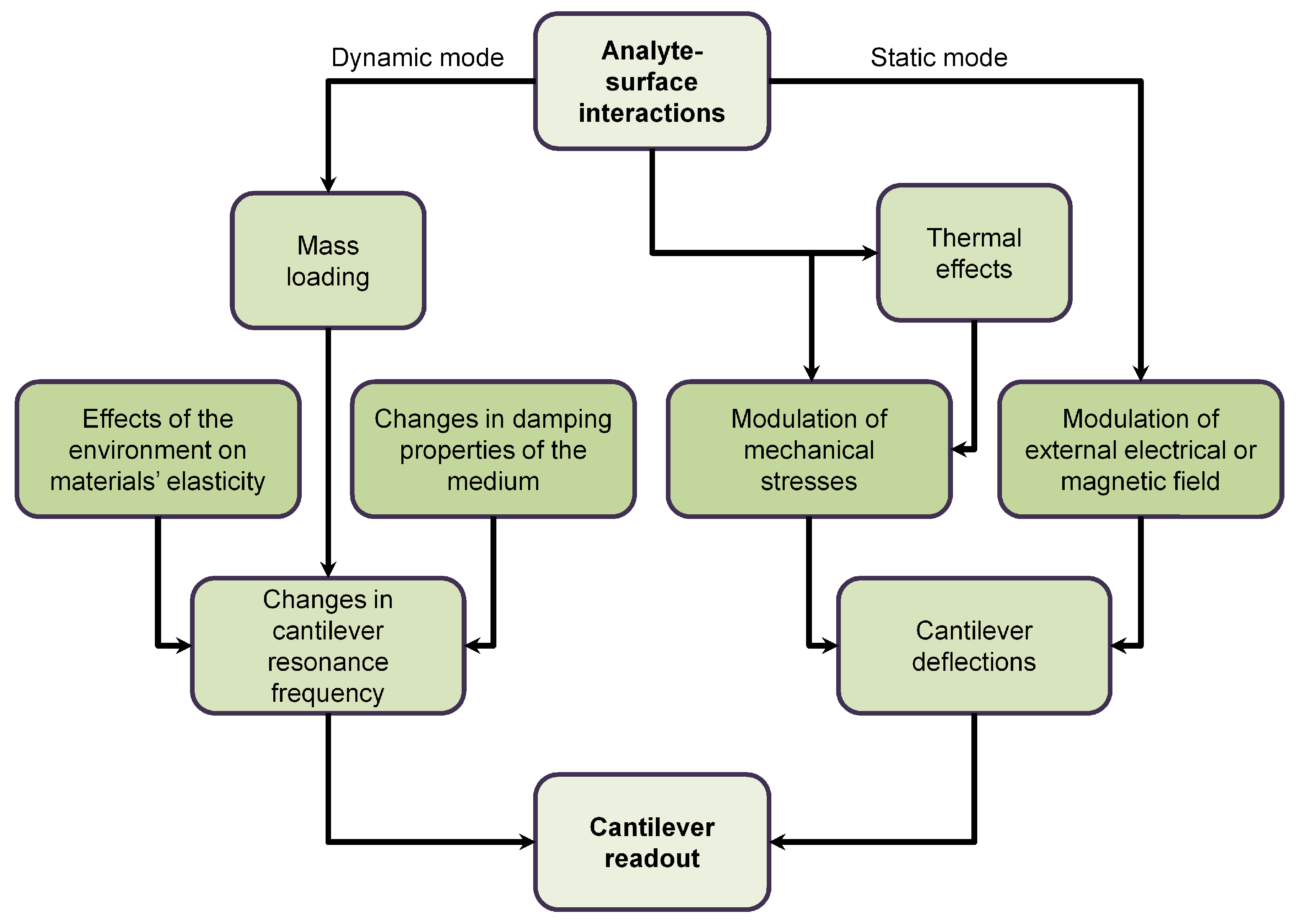
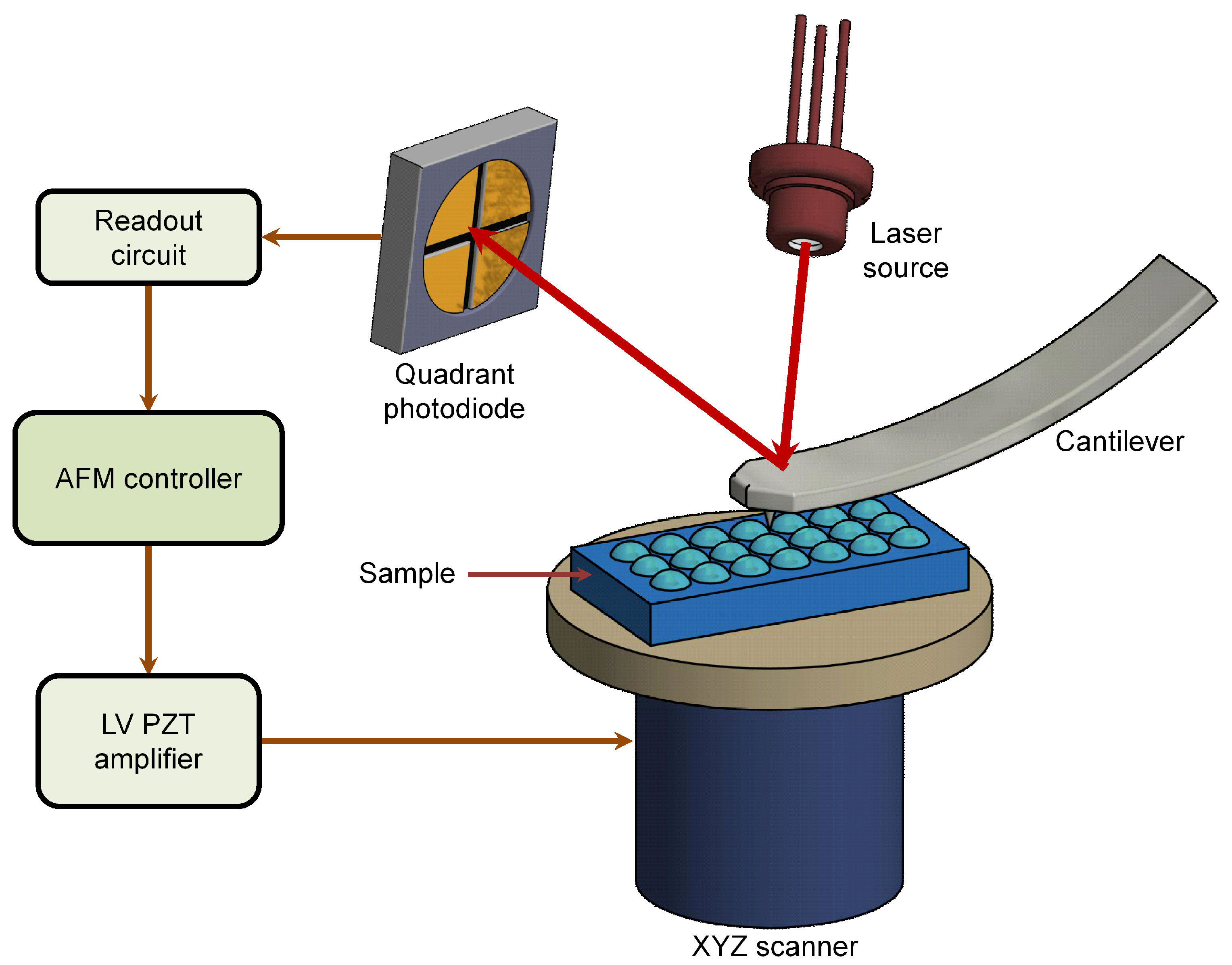
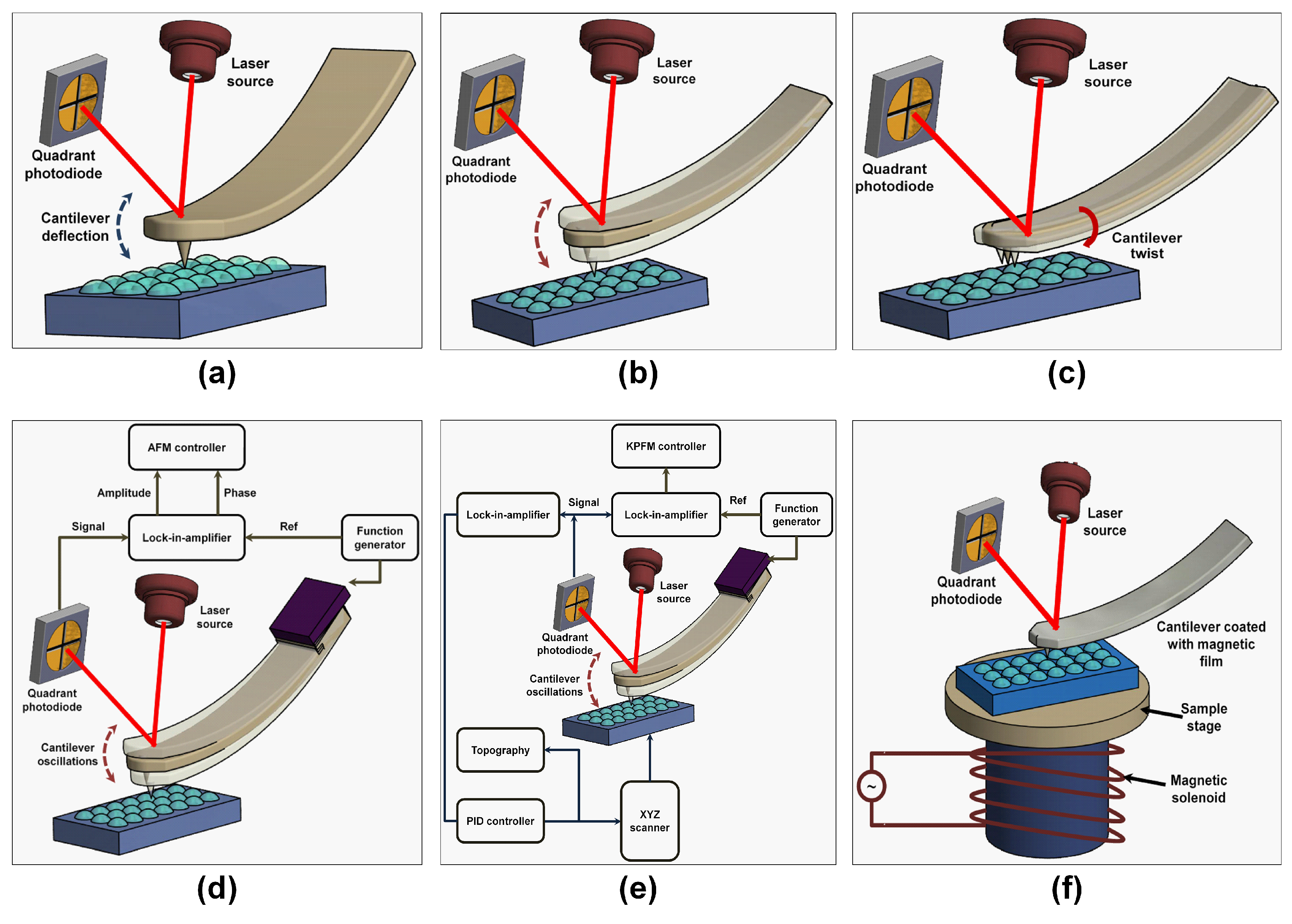
| S/No | Areas of Application | Examples |
|---|---|---|
| 1 | Biomedical applications [86,87] | Biosensors (DNA, antibodies |
| proteins, viruses, and microorganisms) | ||
| Diagnostics | ||
| pH sensors | ||
| 2 | High frequency resonators [88,89,90] | Chemical sensors |
| 3 | Food production and safety [91] | Detection of heavy metals in water |
| To detect concentrations of herbicides | ||
| Changes in pH | ||
| 4 | RF switching [92,93,94] | Broadband switches |
| Switches for wireless communication | ||
| 5 | Atomic force microscopy [3,95,96] | Live cells |
| Reaction processes of DNA | ||
| Biomolecules | ||
| 6 | Environmental monitoring [97] | Temperature detection |
| Humidity detection | ||
| Heat changes | ||
| 7 | Read and write storage devices [98] | Storage devices |
| 8 | Home land security [99] | Detection of terrorism weapons |
| Explosives detection | ||
| Monitor missile storage and maintenance needs | ||
| 9 | Energy [100,101] | Energy harvesters |
| Property | MLCT-E | AC40 | AC10 | AC7 |
|---|---|---|---|---|
| Shape | V-shaped | Rectangle | Rectangle | Rectangle |
| Length (m) | 140 | 38 | 8 | 6 |
| Width (m) | 18 | 16 | 2 | 2 |
| Thickness (nm) | 600 | 180 | 130 | 130 |
| K (pN/nm) | 112 | 102 | 87 | 592 |
| in liquid (kHz) | 7 | 31 | 431 | 1231 |
| Q-factor in liquid | 1.7 | 1.6 | 0.8 | 0.7 |
| (pNs/m) | 4.59 | 0.82 | 0.03 | 0.05 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alunda, B.O.; Lee, Y.J. Review: Cantilever-Based Sensors for High Speed Atomic Force Microscopy. Sensors 2020, 20, 4784. https://doi.org/10.3390/s20174784
Alunda BO, Lee YJ. Review: Cantilever-Based Sensors for High Speed Atomic Force Microscopy. Sensors. 2020; 20(17):4784. https://doi.org/10.3390/s20174784
Chicago/Turabian StyleAlunda, Bernard Ouma, and Yong Joong Lee. 2020. "Review: Cantilever-Based Sensors for High Speed Atomic Force Microscopy" Sensors 20, no. 17: 4784. https://doi.org/10.3390/s20174784






