Recovery of Phytochemicals via Electromagnetic Irradiation (Microwave-Assisted-Extraction): Betalain and Phenolic Compounds in Perspective
Abstract
:1. Introduction
2. Food Color Compounds
- Carotenoids, 40 carbon atoms possessing terpenoids, are derived from the condensation of geranylgeranyl-PP molecules [29]. They are lipid-soluble, basically found in cyanobacteria, algae, plants, some fungi and bacteria, and are produced intracellularly by bioproduction of microorganisms [29,30]. Based on their chemical structure, they can be classified as hydrocarbon carotenoids and xanthophylls, and their extraction can easily be performed with nonpolar solvents [29].
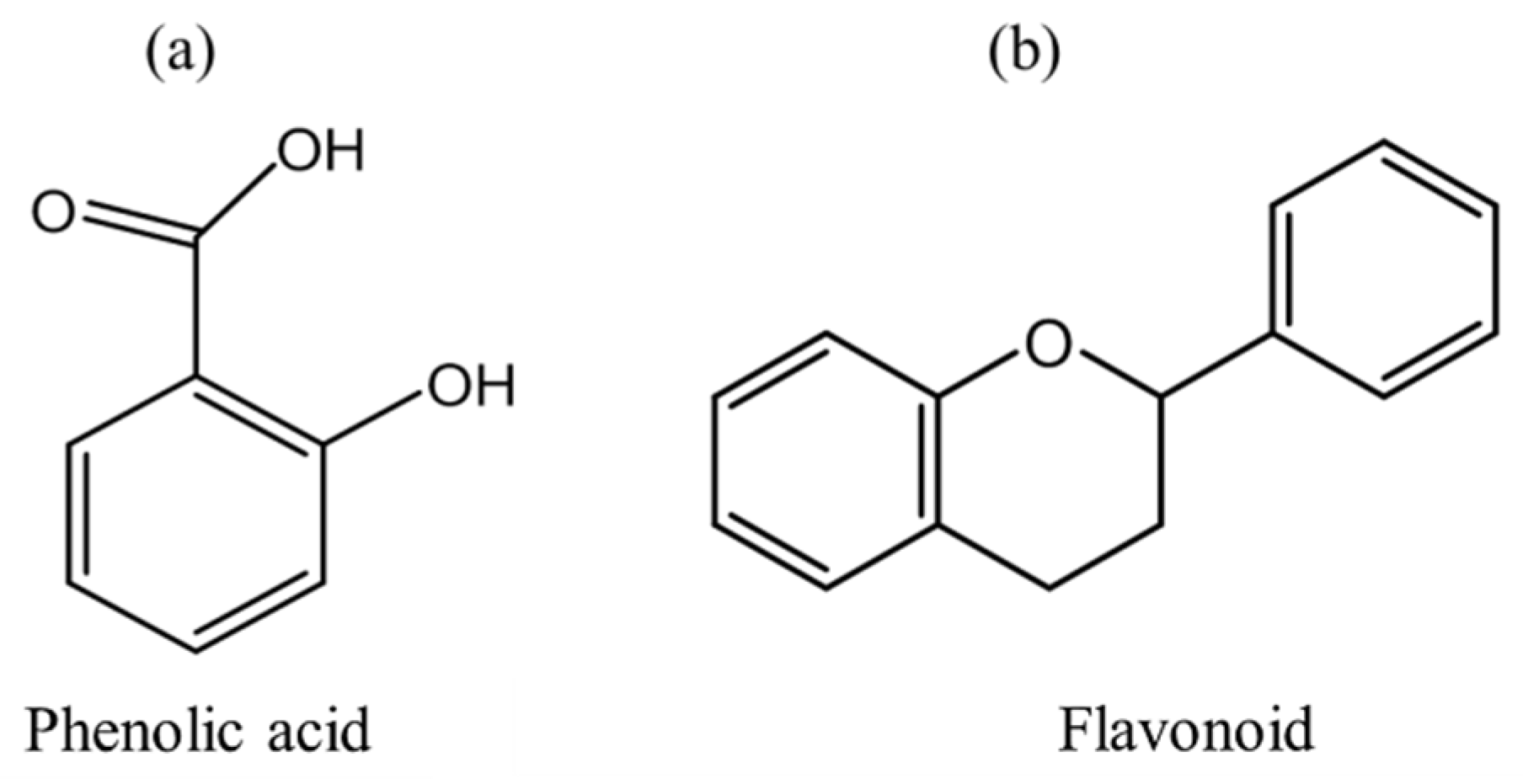
- Flavonoids are water-soluble compounds, consisting of 15 carbon atoms (C6-C3-C6) and belong to the class of phenylpropanoids [21,31,32]. Flavonoids furnish intense color, texture, and taste in fruits and flowers, stretching to a wide range of fruit and vegetable parts mostly leaves, flowers, and skin of the fruits [17,27]. The color variety and classification differ according to the structural groups such as hydroxyl, methyl, glucosyl, and acyl [32]. Anthocyanins (glycosylated and acylated) are groups of flavonoids derived from phenylalanine conferring coloration from pale yellow to blue with respect to pH changes [32]. Anthocyanins possess several therapeutic properties as they strongly exhibit free radical scavenging capacity [17]. They are abundantly found in berries, blackcurrant, and other purple color giving fruits and vegetables with host taste attributes [17,28].
- Both anthocyanin and betalain (betacyanin) have UV protectable ability for host plant tissues [28]. Betalains have a wider range of pH (3–7) with yellow to red coloration within that range though less stable to temperature and light exposure as compared to anthocyanin [33]. Slavov and coworkers [34] investigated the color pattern resulting from a mixture of betalain and anthocyanin-rich fruit juices accompanying their functional properties as their coexistence has never been explored.
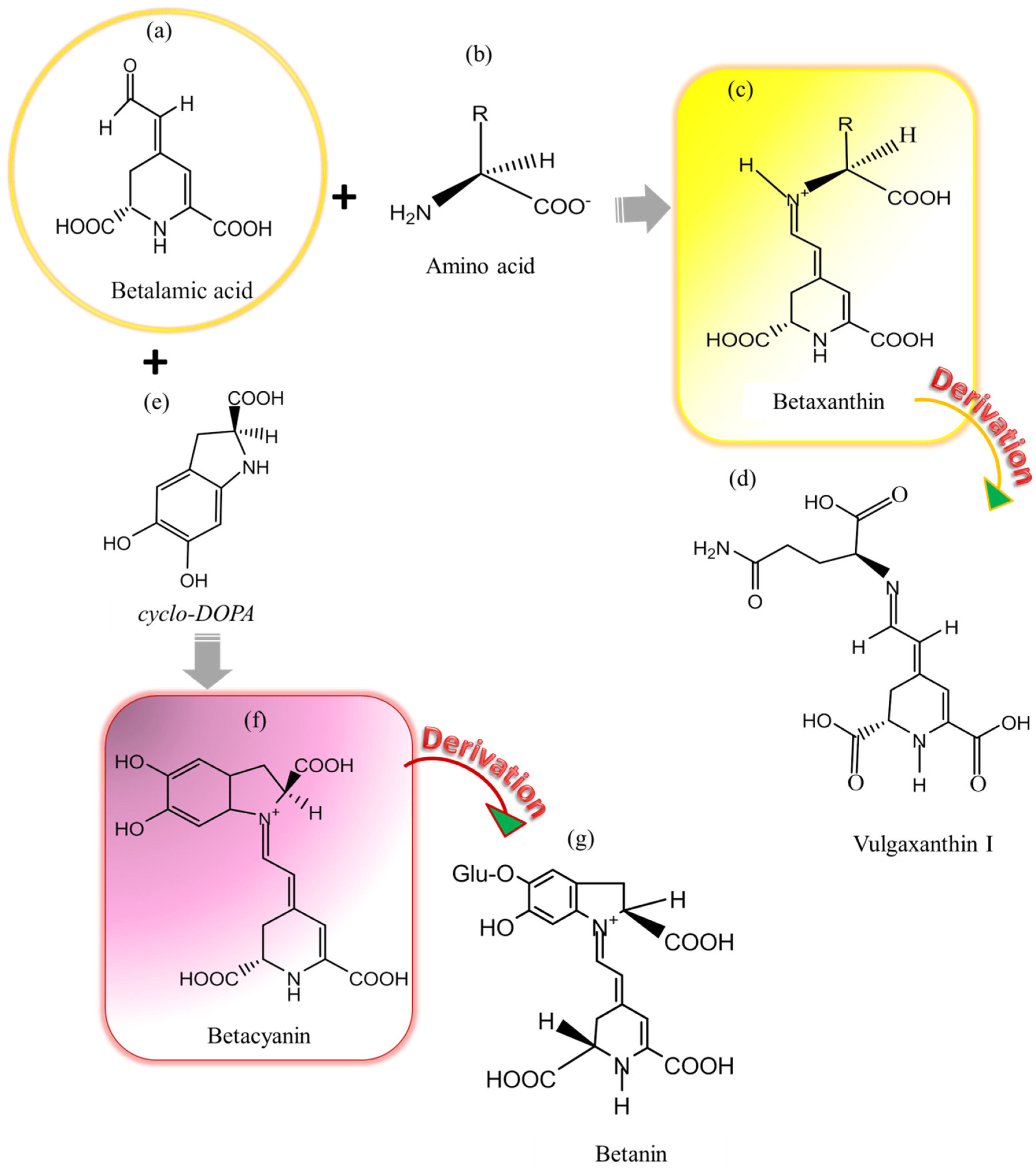
2.1. Betalains
2.2. Phenolic Compounds
3. Generalities of Solid-Liquid Extraction
4. Emerging Technology
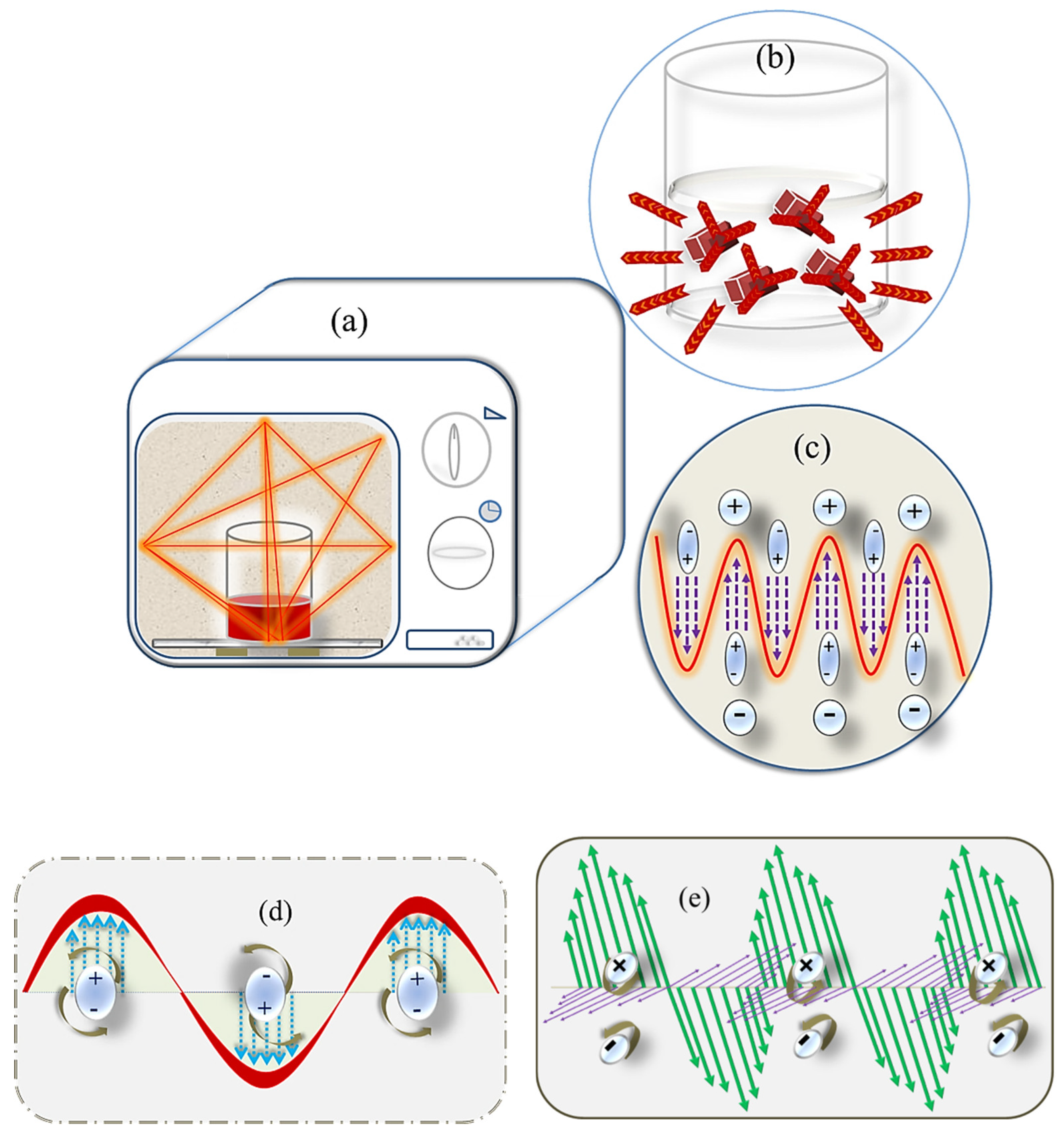
4.1. Microwave Irradiation
4.2. Mathematical Terms
4.3. Microwave-Assisted Extraction (MAE) of Betalains and Phenolic Compounds
5. Other Applications
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zaukuu, J.L.Z.; Bodor, Z.; Vitalis, F.; Zsom-Muha, V.; Kovacs, Z. Near infrared spectroscopy as a rapid method for detecting paprika powder adulteration with corn flour. Acta Period. Technol. 2019, 50, 346–352. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.I.; Giridhar, P. Plant betalains: Chemistry and biochemistry. Phytochemistry 2015, 117, 267–295. [Google Scholar] [CrossRef] [PubMed]
- Choo, W.S. Betalains: Application in functional foods. In Bioactive Molecules in Food, Reference Series in Phytochemistry; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer: Cham, Switzerland, 2019; pp. 1471–1498. [Google Scholar]
- Slimen, I.B.; Najar, T.; Abderrabba, M. Chemical and antioxidant properties of betalains. J. Agric. Food Chem. 2017, 65, 675–689. [Google Scholar] [CrossRef] [PubMed]
- Hussain, E.A.; Sadiq, Z.; Zia-Ul-Haq, M. Betalains: Biomolecular Aspects, 1st ed.; Springer International Publishing, AG: Cham, Switzerland, 2018; pp. 1–187. [Google Scholar]
- Rodriguez-Amaya, D.B. Betalains. Encycl. Food Chem. 2019, 35–39. [Google Scholar] [CrossRef]
- Maran, J.P.; Priya, B. Multivariate statistical analysis and optimization of ultrasound-assisted extraction of natural pigments from waste red beet stalks. J. Food Sci. Technol. 2016, 53, 792–799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.; Ganesapillai, M.; Gnanasundaram, N. Optimizaton of extraction of betalain pigments from beta vulgaris peels by microwave pretreatment. In IOP Conference Series: Materials Science and Engineering, Proceedings of the 14th ICSET-2017, School of Advanced Sciences, VIT University, Vellore, India, 2–3 May 2017; IOP Publishing: Bristol, UK, 2017; Volume 263. [Google Scholar]
- Zin, M.M.; Márki, E.; Bánvölgyi, S. Conventional extraction of betalain compounds from beetroot peels with aqueous ethanol solvent. Acta Aliment. 2020, 49, 163–169. [Google Scholar] [CrossRef]
- Costa, A.P.D.; Hermes, V.S.; Rios, A.D.O.; Flores, S.H. Minimally processed beetroot waste as an alternative source to obtain functional ingredients. J. Food Sci. Technol. 2017, 54, 2050–2058. [Google Scholar] [CrossRef] [PubMed]
- Vulić, J.J.; Ćebović, T.N.; Čanadanović, V.M.; Ćetković, G.S.; Djilas, S.M.; Čanadanović-Brunet, J.M.; Velićanski, A.S.; Cvetković, D.D.; Tumbas, V.T. Antiradical, antimicrobial and cytotoxic activities of commercial beetroot pomace. Food Funct. 2013, 4, 713–721. [Google Scholar] [CrossRef]
- Bucur, L.; Ţarălungă, G.; Schroder, V. The betalains content and antioxidant capacity of red beet (Beta vulgaris L. subsp. vulgaris) root. Farmacia 2016, 64, 198–201. [Google Scholar]
- Székely, D.; Szalóki-Dorkó, L.; Stéger-Máté, M.; Szabó-Nótin, B.; Ivanics, J.; Monspart-Sényi, J. Distribution of antioxidant components in roots of different red beets (Beta vulgaris L.) cultivars. Acta Aliment. 2014, 43, 164–171. [Google Scholar] [CrossRef] [Green Version]
- Bastos, E.L.; Gonçalves, L.C.P. Microwave-Assisted extraction of betalains. In Water Extraction of Bioactive Compounds: From Plants to Drug Development; González, H.D., Muñoz, M.J.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 245–267. [Google Scholar]
- Sawicki, T.; Bączek, N.; Wiczkowski, W. Betalain profile, content and antioxidant capacity of red beetroot dependent on the genotype and root part. J. Funct. Foods 2016, 27, 249–261. [Google Scholar] [CrossRef]
- Gallo, M.; Ferracane, R.; Graziani, G.; Ritieni, A.; Fogliano, V. Microwave assisted extraction of phenolic compounds from four different spices. Molecules 2010, 15, 6365–6374. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.; Navas, M.J.; Jiménez-Moreno, A.M.; Asuero, A.G. Anthocyanin pigments: Importance, sample preparation and extraction. In Phenolic Compounds: Natural Sources, Importance and Applications; Soto-Hernández, M., Palma-Tenango, M., García-Mateos, R., Eds.; BoD—Books on Demand: Norderstedt, Germany, 2017; pp. 117–152. [Google Scholar]
- Li, H.; Deng, Z.; Wu, T.; Liu, R.; Loewen, S.; Tsao, R. Microwave-assisted extraction of phenolics with maximal antioxidant activities in tomatoes. Food Chem. 2012, 130, 928–936. [Google Scholar] [CrossRef]
- Proestos, C.; Komaitis, M. Application of microwave-assisted extraction to the fast extraction of plant phenolic compounds. LWT Food Sci. Technol. 2008, 41, 652–659. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, J.; Fan, J.; Clark, J.; Shen, P.; Li, Y.; Zhang, C. Microwave assisted extraction of phenolic compounds from four economic brown macroalgae species and evaluation of their antioxidant activities and inhibitory effects on α-amylase, α-glucosidase, pancreatic lipase and tyrosinase. Food Res. Int. 2018, 113, 288–297. [Google Scholar] [CrossRef]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Rocchetti, G.; Blasi, F.; Montesano, D.; Ghisoni, S.; Marcotullio, M.C.; Sabatini, S.; Cossignani, L.; Lucini, L. Impact of conventional/non-conventional extraction methods on the untargeted phenolic profile of Moringa oleifera leaves. Food Res. Int. 2018, 115, 319–327. [Google Scholar] [CrossRef]
- Seoane, P.R.; Flórez-Fernández, N.; Piñeiro, E.C.; González, H.D. Microwave-assisted water extraction. In Water Extraction of Bioactive Compounds: From Plants to Drug Development; González, H.D., Muñoz, M.J.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 163–198. [Google Scholar]
- Cardoso-Ugarte, G.A.; Sosa-Morales, M.E.; Ballard, T.; Liceaga, A.; San Martín-González, M.F. Microwave-Assisted extraction of betalains from red beet (Beta vulgaris). LWT Food Sci. Technol. 2014, 59, 276–282. [Google Scholar] [CrossRef]
- Aberoumand, A.A. Review article on edible pigments properties and sources as natural biocolorants in foodstuff and food industry. World J. Dairy Food Sci. 2011, 6, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Renard, C. Extraction of bioactives from fruit and vegetables: State of the art and perspectives. LWT Food Sci. Technol. 2018, 93, 390–395. [Google Scholar] [CrossRef]
- Delgado-Vargas, F.; Jiménez, A.R.; Paredes-López, O. Natural pigments: Carotenoids, anthocyanins, and betalains—Characteristics, biosynthesis, processing, and stability. Crit. Rev. Food Sci. Nutr. 2010, 40. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Carle, R. Functional properties of anthocyanins and betalains in plants, food, and in human nutrition. Trends Food Sci. Technol. 2004, 15, 19–38. [Google Scholar] [CrossRef]
- Merhan, O. The biochemistry and antioxidant properties of carotenoids. In Carotenoids; Cvetkovic, D., Nikolic, G., Eds.; IntechOpen: London, UK, 2017; pp. 51–66. [Google Scholar]
- Urnau, L.; Colet, R.; Soares, V.F.; Franceschi, E.; Valduga, E.; Steffens, C. Extraction of carotenoids from Xanthophyllomyces dendrorhous using ultrasound-assisted and chemical cell disruption methods. Can. J. Chem. Eng. 2018, 96, 1377–1381. [Google Scholar] [CrossRef]
- Sperry, J.B.; Smith, A.B., III. Chemical synthesis of diverse phenolic compounds isolated from olive oils. Olives Olive Oil Health Dis. Prev. 2010, 1439–1464. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of plant pigments: Anthocyanins, betalains and carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef]
- Strack, D.; Vogt, T.; Schliemann, W. Recent advances in betalain research. Phytochemistry 2003, 62, 247–269. [Google Scholar] [CrossRef]
- Slavov, A.; Karagyozov, V.; Denev, P.; Kratchanova, M.; Kratchanov, C. Antioxidant activity of red beet juices obtained after microwave and thermal pretreatments. Czech J. Food Sci. 2013, 31, 139–147. [Google Scholar] [CrossRef] [Green Version]
- Nieves, J.W. Alternative therapy through nutrients and nutraceuticals. In Osteoporosis, 4th ed.; Marcus, R., Feldman, D., Dempster, D.W., Luckey, M., Cauley, J.A., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 1739–1749. [Google Scholar]
- Shetty, M.J.; Geethalekshmi, P.R.; Mini, C. Natural pigments as potential food colourants: A Review. Trends Biosci. 2017, 10, 4057–4064. [Google Scholar]
- Esquivel, P. Betalains. In Handbook on Natural Pigments in Food and Beverages: Industrial Applications for Improving Food Color; Carle, R., Schweiggert, R.M., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; pp. 81–99. [Google Scholar]
- Miguel, M. Betalains in some species of the amaranthaceae family: A Review. Antioxidants 2018, 7, 53. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.J.; An, D.; Nguyen, C.T.T.; Patil, B.S.; Kim, J.; Yoo, K.S. Betalain and betaine composition of greenhouse-or field-produced beetroot (Beta vulgaris L.) and inhibition of HepG2 cell proliferation. J. Agric. Food Chem. 2014, 62, 1324–1331. [Google Scholar] [CrossRef]
- Cai, Y.Z.; Sun, M.; Corke, H. Characterization and application of betalain pigments from plants of the Amaranthaceae. Trends Food Sci. Technol. 2005, 16, 370–376. [Google Scholar] [CrossRef]
- Mikołajczyk-Bator, K.; Pawlak, S. The effect of thermal treatment on antioxidant capacity and pigment contents in separated betalain fractions. Acta Sci. Pol. Technol. Aliment. 2016, 15, 257–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonçalves, L.C.P.; Di Genova, B.M.; Dörr, F.A.; Pinto, E.; Bastos, E.L. Effect of dielectric microwave heating on the color and antiradical capacity of betanin. J. Food Eng. 2013, 118, 49–55. [Google Scholar] [CrossRef] [Green Version]
- Nemzer, B.; Pietrzkowski, Z.; Spórna, A.; Stalica, P.; Thresher, W.; Michałowski, T.; Wybraniec, S. Betalainic and nutritional profiles of pigment-enriched red beet root (Beta vulgaris L.) dried extracts. Food Chem. 2011, 127, 42–53. [Google Scholar] [CrossRef]
- Zin, M.M.; Márki, E.; Bánvölgyi, S. Evaluation of reverse osmosis membranes in concentration of beetroot peel extract. Period. Polytech. Chem. Eng. 2020, 64, 340–348. [Google Scholar] [CrossRef]
- Bazaria, B.; Kumar, P. Optimization of spray drying parameters for beetroot juice powder using response surface methodology (RSM). J. Saudi Soc. Agric. Sci. 2018, 17, 408–415. [Google Scholar] [CrossRef] [Green Version]
- Junqueira-Goncalves, M.P.; Cardoso, L.P.; Pinto, M.S.; Pereira, R.M.; Soares, N.F.; Miltz, J. Irradiated beetroot extract as a colorant for cream cheese. Radiat. Phys. Chem. 2011, 80, 114–118. [Google Scholar] [CrossRef]
- Sivakumar, V.J.; Anna, J.L.; Vijayeeswarri, J.; Swaminathan, G. Ultrasound assisted enhancement in natural dye extraction from beetroot for industrial applications and natural dyeing of leather. Ultrason. Sonochem. 2009, 16, 782–789. [Google Scholar] [CrossRef]
- Delia, S.C.; Chávez, G.M.; Frank, M.L.M.; Araceli, S.G.P.; Irais, A.L.; Franco, A.A. Spray drying microencapsulation of betalain rich extracts from Escontria chiotilla and Stenocereus queretaroensis fruits using cactus mucilage. Food Chem. 2018, 272, 715–722. [Google Scholar] [CrossRef]
- Otálora, M.C.; Carriazo, J.G.; Iturriaga, L.; Osorio, C.; Nazareno, M.A. Encapsulating betalains from Opuntia ficus-indica fruits by ionic gelation: Pigment chemical stability during storage of beads. Food Chem. 2016, 202, 373–382. [Google Scholar] [CrossRef]
- Hidalgo, A.; Brandolini, A.; Čanadanović-Brunet, J.; Ćetković, G.; Šaponjac, V.T. Microencapsulates and extracts from red beetroot pomace modify antioxidant capacity, heat damage and colour of pseudocereals-enriched einkorn water biscuits. Food Chem. 2018, 268, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Swanson, B.G. Tannins, and polyphenols. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Cambridge, MA, USA, 2003; pp. 5729–5733. [Google Scholar]
- Rodríguez-Rojo, S.; Visentin, A.; Maestri, D.; Cocero, M.J. Assisted extraction of rosemary antioxidants with green solvents. J. Food Eng. 2012, 109, 98–103. [Google Scholar] [CrossRef] [Green Version]
- Flórez, N.; Conde, E.; Domínguez, H. Microwave assisted water extraction of plant compounds. J. Chem. Technol. Biotechnol. 2015, 90, 590–607. [Google Scholar] [CrossRef]
- Wang, L.; Qin, P.; Hu, Y. Study on the microwave-assisted extraction of polyphenols from tea. Front. Chem. Eng. China 2010, 4, 307–313. [Google Scholar] [CrossRef]
- Vinatoru, M.; Mason, T.J.; Calinescu, I. Ultrasonically assisted extraction (UAE) and microwave assisted extraction (MAE) of functional compounds from plant materials. TrAC Trends Anal. Chem. 2017, 97, 159–178. [Google Scholar] [CrossRef]
- Chong, P.H.; Yusof, Y.A.; Aziz, M.G.; Mohd Nazli, N.; Chin, N.L.; Syed Muhammad, S.K. Evaluation of solvent extraction of Amaranth betacyanins using multivariate analysis. Int. Food Res. J. 2014, 21, 1569–1573. [Google Scholar]
- López, N.; Puértolas, E.; Condón, S.; Raso, J.; Alvarez, I. Enhancement of the extraction of betanine from red beetroot by pulsed electric fields. J. Food Eng. 2009, 90, 60–66. [Google Scholar] [CrossRef]
- Latorre, M.E.; Bonelli, P.R.; Rojas, A.M.; Gerschenson, L.N. Microwave inactivation of red beet (Beta vulgaris L. var. conditiva) peroxidase and polyphenoloxidase and the effect of radiation on vegetable tissue quality. J. Food Eng. 2012, 109, 676–684. [Google Scholar] [CrossRef]
- Ohlsson, T.; Bengtsson, N. Minimal Processing Technologies in the Food Industry, 1st ed.; CRC Press: New York, NY, USA, 2002; pp. 4–29. [Google Scholar]
- Casazza, A.A.; Aliakbarian, B.; Mantegna, S.; Cravotto, G.; Perego, P. Extraction of phenolics from Vitis vinifera wastes using non-conventional techniques. J. Food Eng. 2010, 100, 50–55. [Google Scholar] [CrossRef]
- Gharekhani, M.; Ghorbani, M.; Rasoulnejad, N. Microwave-Assisted extraction of phenolic and flavonoid compounds from Eucalyptus camaldulensis Dehn leaves as compared with ultrasound-assisted extraction. Lat. Am. Appl. Res. 2012, 42, 305–310. [Google Scholar]
- Hayat, K.; Hussain, S.; Abbas, S.; Farooq, U.; Ding, B.; Xia, S.; Jia, C.; Zhang, X.; Xia, W. Optimized microwave-assisted extraction of phenolic acids from citrus mandarin peels and evaluation of antioxidant activity In Vitro. Sep. Purif. Technol. 2009, 70, 63–70. [Google Scholar] [CrossRef]
- Melgar, B.; Dias, M.I.; Barros, L.; Ferrerira, I.C.F.R.; Rodriguez-Lopez, A.D.; Garcia-Castello, E.M. Ultrasound and microwave assisted extraction of Opuntia fruit peels biocompounds: Optimization and comparison using RSM-CCD. Molecules 2019, 24, 3618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rafiee, Z.; Jafari, S.M.; Alami, M.; Khomeiri, M. Microwave-Assisted extraction of phenolic compounds from olive leaves; a comparison with maceration. J. Anim. Plant Sci. 2011, 21, 738–745. [Google Scholar]
- Kaderides, K.; Papaoikonomou, L.; Serafim, M.; Goula, A.M. Microwave-Assisted extraction of phenolics from pomegranate peels: Optimization, kinetics, and comparison with ultrasounds extraction. Chem. Eng. Process. 2019, 137, 1–11. [Google Scholar] [CrossRef]
- Medina-Torres, N.; Ayora-Talavera, T.; Espinosa-Andrews, H.; Sánchez-Contreras, A.; Pacheco, N. Ultrasound assisted extraction for the recovery of phenolic compounds from vegetable sources. Agronomy 2017, 7, 47. [Google Scholar] [CrossRef]
- Ballard, T.S.; Mallikarjunan, P.; Zhou, K.; O’Keefe, S. Microwave-Assisted extraction of phenolic antioxidant compounds from peanut skins. Food Chem. 2010, 120, 1185–1192. [Google Scholar] [CrossRef]
- Jokić, S.; Cvjetko, M.; Božić, D.; Fabek, S.; Toth, N.; Vorkapić-Furač, J.; Redovniković, I.R. Optimisation of microwave-assisted extraction of phenolic compounds from broccoli and its antioxidant activity. Int. J. Food Sci. Technol. 2012, 47, 2613–2619. [Google Scholar] [CrossRef]
- Pérez-Serradilla, J.A.; Luque de Castro, M.D. Microwave-Assisted extraction of phenolic compounds from wine lees and spray-drying of the extract. Food Chem. 2011, 124, 1652–1659. [Google Scholar] [CrossRef]
- Pinela, J.; Prieto, M.A.; Carvalho, A.M.; Barreiro, M.F.; Oliveira, M.B.P.P.; Barros, L.; Ferreira, I.C.F.R. Microwave-Assisted extraction of phenolic acids and flavonoids and production of antioxidant ingredients from tomato: A nutraceutical-oriented optimization study. Sep. Purif. Technol. 2016, 164, 114–124. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Li, D.; Liu, C.; Zhang, Y. Optimized microwave-assisted extraction of total phenolics (TP) from Ipomoea batatas leaves and its antioxidant activity. Innov. Food Sci. Emerg. Technol. 2011, 12, 282–287. [Google Scholar] [CrossRef]
- Thirugnanasambandham, K.; Sivakumar, V. Microwave assisted extraction process of betalain from dragon fruit and its antioxidant activities. J. Saudi Soc. Agric. Sci. 2017, 16, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.N.; Zhang, J.J.; Li, Y.; Meng, X.; Li, H.B. Microwave-Assisted extraction of phenolic compounds from melastoma sanguineum fruit: Optimization and identification. Molecules 2018, 23, 2498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreres, F.; Grosso, C.; Gil-Izquierdo, A.; Valentão, P.; Mota, A.T.; Andrade, P.B. Optimization of the recovery of high-value compounds from pitaya fruit by-products using microwave-assisted extraction. Food Chem. 2017, 230, 463–474. [Google Scholar] [CrossRef]
- Pollini, L.; Rocchi, R.; Cossignani, L.; Mañes, J.; Compagnone, D.; Blasi, F. Phenol profiling and nutraceutical potential of Lycium spp. leaf extracts obtained with ultrasound and microwave assisted techniques. Antioxidants 2019, 8, 260. [Google Scholar] [CrossRef] [Green Version]
- Inglett, G.E.; Rose, D.J.; Chen, D.; Stevenson, D.G.; Biswas, A. Phenolic content and antioxidant activity of extracts from whole buckwheat (Fagopyrum esculentum Möench) with or without microwave irradiation. Food Chem. 2010, 119, 1216–1219. [Google Scholar] [CrossRef]
- Nazeri, M.A.; Zain, N.M. Effect of different operating parameters on extraction of active compounds from pitaya peel by microwave assisted extraction (MAE). J. Teknol. (Sci. Eng.) 2018, 80, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Simsek, M.; Sumnu, G.; Sahin, S. Microwave assisted extraction of phenolic compounds from sour cherry pomace. Sep. Sci. Technol. 2012, 47, 1248–1254. [Google Scholar] [CrossRef]
- Shao, P.; He, J.; Sun, P.; Zhao, P. Analysis of conditions for microwave-assisted extraction of total water-soluble flavonoids from Perilla frutescens leaves. J. Food Sci. Technol. 2012, 49, 66–73. [Google Scholar] [CrossRef] [Green Version]
- Ravichandran, K.; Saw, N.M.M.T.; Mohdaly, A.A.A.; Gabr, A.M.M.; Kastell, A.; Riedel, H.; Cai, Z.; Knorr, D.; Smetanska, I. Impact of processing of red beet on betalain content and antioxidant activity. Food Res. Int. 2013, 50, 670–675. [Google Scholar] [CrossRef]
- Pedroza, M.A.; Amendola, D.; Maggi, L.; Zalacain, A.; De Faveri, D.M.; Spigno, G. Microwave-Assisted extraction of phenolic compounds from dried waste grape skins. Int. J. Food Eng. 2015, 11, 359–370. [Google Scholar] [CrossRef]
- Abolhasani, A.; Barzegar, M.; Sahari, M.A. Effect of gamma irradiation on the extraction yield, antioxidant, and antityrosinase activities of pistachio green hull extract. Radiat. Phys. Chem. 2017, 144, 373–378. [Google Scholar] [CrossRef]
- Da Silva, H.R.P.; da Silva, C.; Bolanho, B.C. Ultrasonic-Assisted extraction of betalains from red beet (Beta vulgaris L.). J. Food Process Eng. 2018, 41, 1–6. [Google Scholar] [CrossRef]
- Laqui-Vilca, C.; Aguilar-Tuesta, S.; Mamani-Navarro, W.; Montaño-Bustamante, J.; Condezo-Hoyos, L. Ultrasound-Assisted optimal extraction and thermal stability of betalains from colored quinoa (Chenopodium quinoa Willd) hulls. Ind. Crop. Prod. 2018, 90, 60–66. [Google Scholar] [CrossRef]
- Liu, B.; Ma, Y.; Liu, Y.; Yang, Z.; Zhang, L. Ultrasonic-Assisted extraction and antioxidant activity of flavonoids from Adinandra nitida leaves. Trop. J. Pharm. Res. 2013, 12, 1045–1051. [Google Scholar] [CrossRef] [Green Version]
- Loginova, K.V.; Lebovka, N.I.; Vorobiev, E. Pulsed electric field assisted aqueous extraction of colorants from red beet. J. Food Eng. 2011, 106, 127–133. [Google Scholar] [CrossRef]
- Nayak, C.A.; Chethana, S.; Rastogi, N.K.; Raghavarao, K.S.M.S. Enhanced mass transfer during solid-liquid extraction of gamma-irradiated red beetroot. Radiat. Phys. Chem. 2006, 75, 173–178. [Google Scholar] [CrossRef]
- Ramli, N.S.; Ismail, P.; Rahmat, A. Influence of conventional and ultrasonic-assisted extraction on phenolic contents, betacyanin contents, and antioxidant capacity of red dragon fruit (Hylocereus polyrhizus). Sci. World J. 2014, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Zvitov, R.; Nussinovitch, A. Low DC electrification of gel-plant tissue “sandwiches” facilitates extraction and separation of substances from Beta vulgaris beetroots. Food Hydrocoll. 2005, 19, 997–1004. [Google Scholar] [CrossRef]
- Wizi, J.; Wang, L.; Hou, X.; Tao, Y.; Ma, B.; Yang, Y. Ultrasound-Microwave assisted extraction of natural colorants from sorghum husk with different solvents. Ind. Crop. Prod. 2018, 120, 203–213. [Google Scholar] [CrossRef]
- Destandau, E.; Michel, T.; Elfakir, C. Microwave-Assisted extraction. In Natural Product Extraction: Principles and Applications; Rostagno, M.A., Prado, J.M., Eds.; The Royal Society of Chemistry: London, UK, 2013; pp. 113–156. [Google Scholar]
- Figura, L.O.; Teixeira, A.A. Food Physics: Physical Properties—Measurement and Applications; Springer: Berlin/Heidelberg, Germany, 2007; pp. 380–386. [Google Scholar]
- Filly, A.; Fernandez, X.; Minuti, M.; Visinoni, F.; Cravotto, G.; Chemat, F. Solvent-Free microwave extraction of essential oil from aromatic herbs: From laboratory to pilot and industrial scale. Food Chem. 2014, 150, 193–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryynänen, S. The electromagnetic properties of food materials: A review of the basic principles. J. Food Eng. 1995, 26, 409–429. [Google Scholar] [CrossRef]
- Hu, B.; Wang, H.; He, L.; Li, Y.; Li, C.; Zhang, Z.; Liu, Y.; Zhou, K.; Zhang, Q.; Liu, A.; et al. A method for extracting oil from cherry seed by ultrasonic-microwaveassisted aqueous enzymatic process and evaluation of its quality. J. Chromatogr. A 2019, 1587, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Benmoussa, H.; Elfalleh, W.; He, S.; Romdhane, M.; Benhamou, A.; Chawech, R. Microwave hydrodiffusion and gravity for rapid extraction of essential oil from Tunisian cumin (Cuminum cyminum L.) seeds: Optimization by response surface methodology. Ind. Crop. Prod. 2018, 124, 633–642. [Google Scholar] [CrossRef]
- Bagherian, H.; Ashtiani, F.Z.; Fouladitajar, A.; Mohtashamy, M. Comparisons between conventional, microwave- and ultrasound-assisted methods for extraction of pectin from grapefruit. Chem. Eng. Process. Process Intensif. 2011, 50, 1237–1243. [Google Scholar] [CrossRef]
- Moradi, S.; Fazlali, A.; Hamedi, H. Microwave-Assisted hydro-distillation of essential oil from rosemary: Comparison with traditional distillation. Avicenna J. Med. Biotechnol. 2018, 10, 22–28. [Google Scholar]
- Benmoussa, H.; Farhat, A.; Romdhane, M.; Bouajila, J. Enhanced solvent-free microwave extraction of Foeniculum vulgare Mill. essential oil seeds using double walled reactor. Arab. J. Chem. 2019, 12, 3863–3870. [Google Scholar] [CrossRef]
- Chen, Q.; Dong, W.; Wei, C.; Hu, R.; Long, Y. Combining integrated ultrasonic-microwave technique with ethanol to maximise extraction of green coffee oil from Arabica coffee beans. Ind. Crop. Prod. 2020, 151, 112405. [Google Scholar] [CrossRef]
- Jiang, Y.; Bai, X.; Lang, S.; Zhao, Y.; Liu, C.; Yu, L. Optimization of ultrasonic-microwave assisted alkali extraction of arabinoxylan from the corn bran using response surface methodology. Int. J. Biol. Macromol. 2019, 128, 452–458. [Google Scholar] [CrossRef]
- Chemat, F.; Perino-Issartier, S.; Petitcolas, E.; Fernandez, X. “In situ” extraction of essential oils by use of Dean-Stark glassware and a Vigreux column inside a microwave oven: A procedure for teaching green analytical chemistry. Anal. Bioanal. Chem. 2012, 404, 679–682. [Google Scholar] [CrossRef]
- Mullapudi, V.B.K.; Chandrasekaran, K.; Venkateswarlu, G.; Karunasagar, D. Development of a simple and rapid microwave-assisted extraction method using very dilute solutions of perchloric acid and hydrogen peroxide for the multi-elemental analysis of food materials by ICP-OES: A green analytical method. Microchemical 2019, 146, 807–817. [Google Scholar] [CrossRef]
- Gravador, R.S.; Harrison, S.M.; Monahan, F.J.; Gkarane, V.; Farmer, L.J.; Brunton, N.P. Validation of a rapid microwave-assisted extraction method and GC-FID quantification of total branched chain fatty acids in lamb subcutaneous adipose tissue. J. Food Sci. 2018, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raikos, V.; McDonagh, A.; Ranawana, V.; Duthie, G. Processed beetroot (Beta vulgaris L.) as a natural antioxidant in mayonnaise: Effects on physical stability, texture and sensory attributes. Food Sci. Hum. Wellness 2016, 5, 191–198. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.H.; Liu, T.T.; Yang, L.; Zu, Y.G.; Chen, X.; Zhang, L.; Zhang, Y.; Zhao, C. Ionic liquid-based microwave-assisted extraction of essential oil and biphenyl cyclooctene lignans from Schisandra chinensis Baill fruits. J. Chromatogr. A 2011, 1218, 8573–8580. [Google Scholar] [CrossRef] [PubMed]
- López-Hortas, L.; Conde, E.; Falqué, E.; Domínguez, H. Flowers of Ulex europaeus L.-Comparing two extraction techniques (MHG and distillation). C. R. Chim. 2016, 19, 718–725. [Google Scholar] [CrossRef]
- Farajzadeh, M.A.; Sohrabi, S.H.; Mohebbi, A.; Mogaddam, M.R.A. Combination of a modified quick, easy, cheap, efficient, rugged, and safe extraction method with a deep eutectic solvent based microwave-assisted dispersive liquid–liquid microextraction: Application in extraction and preconcentration of multiclass pesticide residues in tomato samples. J. Sep. Sci. 2019, 42, 1273–1280. [Google Scholar]
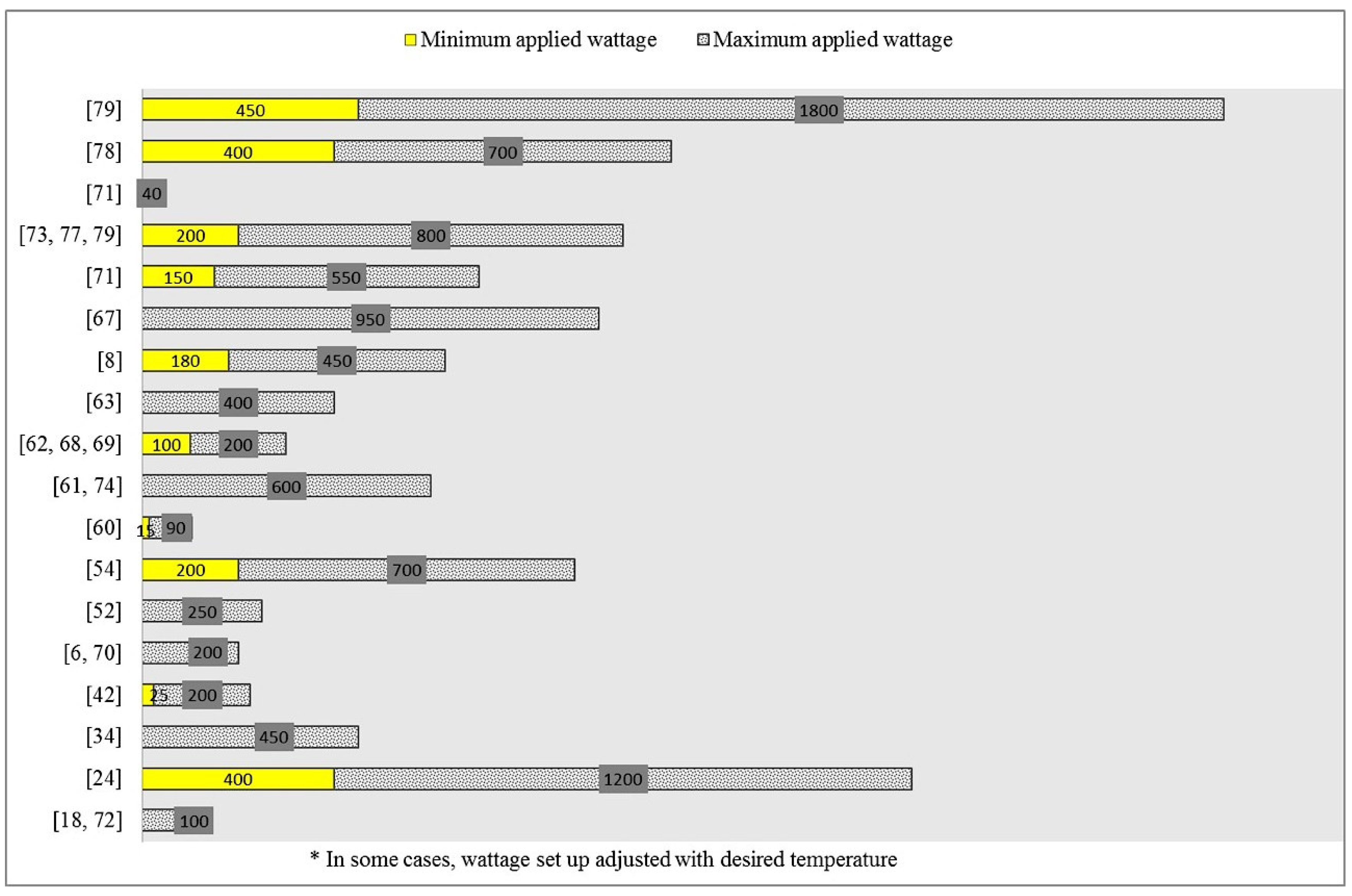
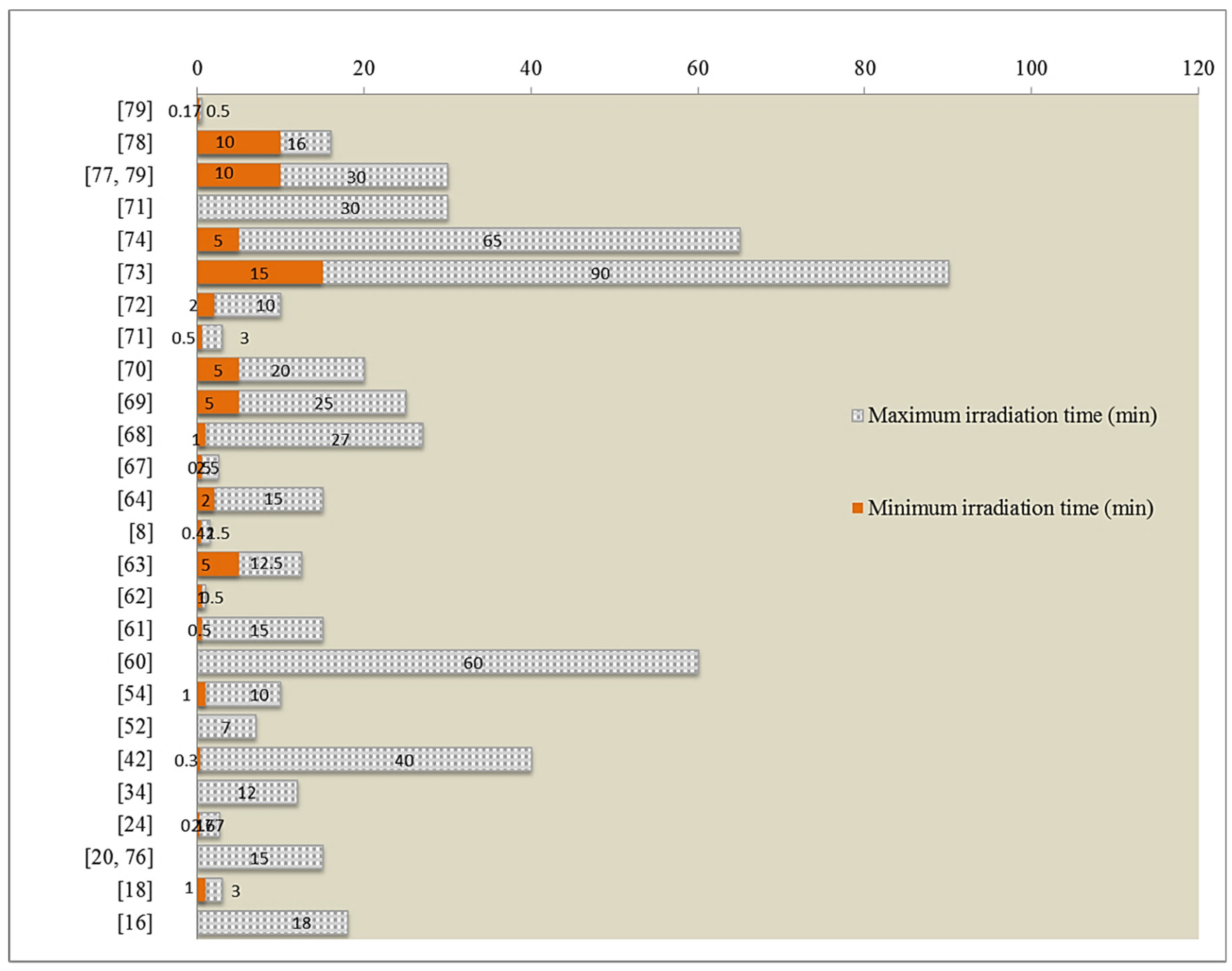
| Group | Compound/Product | Colour | Code | Common Sources |
|---|---|---|---|---|
| - | Curcumin, turmeric oleorisin | Yellow-orange | E100 | Turmeric |
| - | Riboflavin (vitamin B2), riboflavin-5′-phosphate | Yellow-orange | E101(i), E101(ii) | Eggs, green vegetables, milk and other dairy products, meat, mushroom, almond |
| - | Riboflavin-5-sodium phosphate | Yellow | E106 | Eggs, organ meats (kidney and liver), lean meat, milk, green vegetables |
| - | Carminic acid | Magenta-red/ crimson | E120 | Cochineal insect |
| - | Chlorophyll | Green | E140, E141 | Grass, alfalfa leaf, Tagetes erecta (marigold flowers), spinach |
| - | Caramel | Brown | E150 | Modified sugar |
| - | Vegetable carbon | Black | E153 | Vegetables |
| Carotenoid | α-carotene, β-carotene, γ-carotene | Orange-red, red, yellow, amber, brown | E160a | Carrot, pumpkin, apricot, sweet potato, beans, spinach, kale, collard greens, papaya, bell peppers, tomatoes, green leafy vegetables |
| Carotenoid | Annatto | Orange-red | E160b | Fruit of the achiote tree |
| Carotenoid | Paprika oleoresin | Red | E160c | Capsicum annuum or Capsicum frutescens |
| Carotenoid | Lycopene | Bright to deep red | E160d | Solanum lycopersicum (tomatoes), watermelon |
| Carotenoid | β-Apo-8′-carotenal | Orange to orange-red | E160e | Spinach, citrus fruits |
| Carotenoid | Lutein | Orange-red to yellow | E161b | Green leafy vegetables and fruits, yellowish flowers |
| Carotenoid | Canthaxanthin | Violet | E161g | Mushroom, crustaceans, fish, eggs |
| Nitrogen containing compound | Betalains/ betanin | Red violet to yellow (pH < 3 (more reddish), pH > 7 (more yellowish)) | E162 | Mangosteen, beetroot, dragonfruit, red cabbage, swiss chard, Opuntia |
| Phenolic | Anthocyanin | Dark purple (pH 1 (red), pH 4–5 (colorless), pH < 7 (purple), pH < 8 (deep blue), pH < 12 (yellow/brown)) | E163 | Black currant, berries, cherry, plum, grape, red cabbage, Opuntia, hibiscus, rose, onion |
| Carotenoid | Saffron | Yellow-orange-red | E164 | Crocus sativus |
| Solvent | Loss Tangent | Dielectric Constant (ε′) | Dielectric Loss (ε″) | Reference |
|---|---|---|---|---|
| water | 0.123 | 80.4 | 9.8892 | [23,53,58] |
| ethanol | 0.941 | 25.7 | 24.1837 | [23,53] |
| methanol | 0.659 | 32.7 | 21.5493 | [23,53] |
| acetone | 0.054 | 20.6 | 1.1124 | [53] |
| Raw Material | Solvent | Product | Process Optimum Conditions | Reference | ||
|---|---|---|---|---|---|---|
| Results | Time | Power | ||||
| Red beetroot peel | acidified water | betanin | 229.26 mg/L (predicted by RSM) | 0.95 min | 224.61 W | [8] |
| ethanol | 472.11 mg/L (predicted by RSM) | 1.25 min | 384.25 W | |||
| Red beetroot | pure water | betaxanthin | 1.25 mg/g of freeze dried red beet | 2.2 min/ 1.7 min | 400 W, 100% nominal | [24] |
| acidified water | betacyanin | 1.88 mg/g of freeze dried red beet | ||||
| Red beetroot | pure water | betacyanin | 52.2 mg/g of fresh matter | 3 min x 4 times | 450 W | [34] |
| betaxanthin | 42.8 mg/g of fresh matter | |||||
| Opuntia fruit peel | 34.6% methanol | betalain | 201.6 mg/g of extract | 2.5 min | 400 W | [63] |
| Dragon fruit peel | pure water | betalain | 9 mg/L (predicted by RSM) | 8 min | 100 W | [72] |
| White-fleshed red pitaya peel | pure water | betacyanin | 1.66 mg/g of dry extract | 5 min | 600 W | [74] |
| Raw Materials | Extraction | Analytical Method | Process Optimum Conditions | Reference | ||
|---|---|---|---|---|---|---|
| Results | Time | Power | ||||
| Coriandrum sativum (spice) | 50% ethanol | FC | 0.82 mg GAE/g of DW | 18 min | 200 W, 50% nominal | [16] |
| Cinnamomum zeylanicum (spice) | 16.8 mg GAE/g of DW | |||||
| Cuminum cyminum (spice) | 11.6 mg GAE/g of DW | |||||
| Crocus sativus (spice) | 29.4 mg GAE/g of DW | |||||
| Lessonia trabeculate (Brown algae) | 70% methanol | FC | 0.74 mg GAE/g of DW | 15 min | intermittent | [20] |
| Lessonia nigrecens (Brown algae) | 1.07 mg GAE/g of DW | |||||
| Ascophyllum nodosum (Brown algae) | 1.4 mg GAE/g of DW | |||||
| Laminaria japonica (Brown algae) | 0.73 mg GAE/g of DW | |||||
| Rosemary | 96% ethanol | FC | 0.9 mg GAE/g of fresh leaf | 7 min | 250 W (intermittent) | [52] |
| pure water | 0.1 mg GAE/g of fresh leaf | |||||
| Grape seed | methanol | FC | 67.88 mg GAE/g of DW | 60 min | 60 W | [60] |
| Grape skin | 7.33 mg GAE/g of DW | |||||
| Eucalyptus leaf | 50% ethanol | FC | 76.6 mg GAE/g of fresh leaf | 5 min | 600 W (intermittent) | [61] |
| Olive leaf | 50% ethanol | FC | 88.3 mg TAE/g of DW | 15 min | intermittent | [64] |
| Peanut skin | 30% ethanol | FC | 143.6 mg GAE/g of skins (predicted by RSM) | 0.5 min | 950 W, 90% nominal | [67] |
| Broccoli | 72.06% methanol | FC | 21.39 mg GAE/g of DW | 16.94 min | 159.33 W | [68] |
| Wine lee | 75% ethanol (acidified) | FC | 36.4 mg GAE/g of lee extract powder | 17 min | 200 W | [69] |
| (Ipomoea Batatas) Sweet potato leaf | 53% ethanol | FC | 61.26 mg GAE/g DW | 2.05 min | 302 W | [71] |
| Melastoma sanguineum Fruit | 31.33% ethanol | FC | 39.02 mg GAE/g of DW | 45 min | 500 W | [73] |
| Lycium spp. leaf | pure methanol | FC | 6.65 mg GAE/g of DW | 30 min | 40 W | [75] |
| Buckwheat | 50% ethanol | FC | 18.5 mg GAE/g of buckwheat | 15 min | - | [76] |
| Pitaya peel | pure water | FC | 5.8 mg GAE/g of extract | 20 min | 400 W | [77] |
| Sour cherry pomace | 50% ethanol | FC | 14.14 mg GAE/g of DW | 12 min | 700 W | [78] |
| Sources | Methods | Advantages | References |
|---|---|---|---|
| Sorghum husks | Ultrasonic-microwave assisted extraction of colorant (UMAE) | Higher in thermal stabilities and yield percent (3.6 times) with high contents of apigeninidin and luteolinidin than conventional shaking | [90] |
| Aromatic herbs | Enhanced solvent free microwave-assisted extraction (ESFMAE) | ESFMAE increased in oxygenated compound content which was more odoriferous than monoterpene hydrocarbons | [93] |
| Cherry seeds | Ultrasonic-microwave-assisted aqueous enzymatic extraction (UMAAEE) | Compared to Soxhlet extraction, oil by UMAAEE possessed superior physicochemical properties and higher content of bioactive constituents | [95] |
| Tunisian cumin (Cuminum cyminum L.) seeds | Microwave hydrodiffusion and gravity extraction (MHGE) | MHGE successfully improved the EO yield with high amount of oxygenated compounds in shorter extraction time, less electrical consumption, lower carbon dioxide emissions, and smaller volume of waste water | [96] |
| Rosemary plants | Microwave hydro-distillation (MHD) | MHD was superior in terms of saving energy and extraction time compared to hydro-distillation | [98] |
| Foeniculum vulgare Mill. seeds | Enhanced solvent free microwave-assisted extraction using double walled reactor (ESFMAE) | ESFMAE method was faster, cleaner, less cost and energy usage, and better essential oil composition than hydro-distillation method | [99] |
| Arabica coffee beans | Ultrasonic-microwave assisted extraction of green coffee oil (UMAE) | Extraction yields of two diterpenes (cafestol and kahweol) by UMAE were significantly higher than that of solvent method | [100] |
| Corn brans | Ultrasonic-microwave assisted alkali extraction of arabinoxylan (UMAAE) | By UMAAE, water-unextractable arabinoxylan (WUAX) showed good DPPH radical scavenging activity and strong Fe2+ chelating activity | [101] |
| Schisandra chinensis Baill fruits | Ionic liquid-based microwave-assisted extraction (ILMAE) | ILMAE method shortened the energy consumption time, improved the extraction efficiency of lignans as to reflux extraction | [106] |
| Flowers of Ulex europaeus L. | Microwave hydrodiffusion and gravity extraction (MHGE) | MHGE allowed an efficient water removal from the material, and could be suitable for extraction of antioxidant rich aromatic compounds | [107] |
| Tomatoes | Deep eutectic solvent-based microwave-assisted dispersive liquid–liquid microextraction preconcentration of multiclass pesticide residues in tomato samples (DES-MWA–DLLME) | DES-MWA–DLLME represented good repeatability, high (enrichment factors) EFs, low (limit of detection) LODs | [108] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zin, M.M.; Anucha, C.B.; Bánvölgyi, S. Recovery of Phytochemicals via Electromagnetic Irradiation (Microwave-Assisted-Extraction): Betalain and Phenolic Compounds in Perspective. Foods 2020, 9, 918. https://doi.org/10.3390/foods9070918
Zin MM, Anucha CB, Bánvölgyi S. Recovery of Phytochemicals via Electromagnetic Irradiation (Microwave-Assisted-Extraction): Betalain and Phenolic Compounds in Perspective. Foods. 2020; 9(7):918. https://doi.org/10.3390/foods9070918
Chicago/Turabian StyleZin, Moh Moh, Chukwuka Bethel Anucha, and Szilvia Bánvölgyi. 2020. "Recovery of Phytochemicals via Electromagnetic Irradiation (Microwave-Assisted-Extraction): Betalain and Phenolic Compounds in Perspective" Foods 9, no. 7: 918. https://doi.org/10.3390/foods9070918