OMV Vaccines and the Role of TLR Agonists in Immune Response
Abstract
1. Introduction
2. Toll-like Receptors (TLR)
3. PAMPs on OMVs
4. Engagement of Innate and Adaptive Immune Response Induced upon Activation by PAMPs
5. OMVs Compared to Classical Vaccines
6. Discussion
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| OMV | Outer Membrane Vesicle |
| LPS | Lipopolysaccharide |
| TLR | Toll Like Receptor |
| PAMP | Pathogen-Associated Molecular Pattern |
| PRR | Pattern Recognition Receptor |
| GMMA | Generalized Modules for Membrane Antigens |
| DAMP | Damage-Associated Molecular Pattern |
| RLR | RIG-I-Like Receptor |
| NLR | NOD-Like Receptor |
| TIR | Toll/interleukin-1 receptor |
| IL | interleukin |
| IFN | interferon |
| MHC | major histocompatibility complex |
| DC | dendritic cell |
| fmOMV | OMV with attenuated endotoxicity |
| nOMV | native Outer Membrane Vesicle |
| OMPC | Outer Membrane Protein Complex |
| LBP | LPS Binding Protein |
| MD2 | Myeloid Differentation 2 |
References
- Schwechheimer, C.; Kuehn, M.J. Outer-membrane vesicles from Gram-negative bacteria: Biogenesis and functions. Nat. Rev. Microbiol. 2015, 13, 605–619. [Google Scholar] [CrossRef] [PubMed]
- Wensink, J.; Witholt, B. Conversion of Free Lipoprotein to the Murein-Bound Form. Eur. J. Biochem. 1981, 117, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Bohuszewicz, O.; Liu, J.; Low, H.H. Membrane remodelling in bacteria. J. Struct. Biol. 2016, 196, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Roier, S.; Zingl, F.G.; Cakar, F.; Durakovic, S.; Kohl, P.; Eichmann, T.O.; Klug, L.; Gadermaier, B.; Weinzerl, K.; Prassl, R.; et al. A novel mechanism for the biogenesis of outer membrane vesicles in Gram-negative bacteria. Nat. Commun. 2016, 7, 10515. [Google Scholar] [CrossRef] [PubMed]
- Sutterlin, H.A.; Shi, H.; May, K.L.; Miguel, A.; Khare, S.; Huang, K.C.; Silhavy, T.J. Disruption of lipid homeostasis in the Gram-negative cell envelope activates a novel cell death pathway. Proc. Natl. Acad. Sci. USA 2016, 113, E1565–E1574. [Google Scholar] [CrossRef]
- McBroom, A.J.; Kuehn, M.J. Release of outer membrane vesicles by Gram-negative bacteria is a novel envelope stress response. Mol. Microbiol. 2007, 63, 545–558. [Google Scholar] [CrossRef]
- Gerritzen, M.J.H.; Martens, D.E.; Uittenbogaard, J.P.; Wijffels, R.H.; Stork, M. Sulfate depletion triggers overproduction of phospholipids and the release of outer membrane vesicles by Neisseria meningitidis. Sci. Rep. 2019, 9, 4716. [Google Scholar] [CrossRef]
- Kaparakis-Liaskos, M.; Ferrero, R.L. Immune modulation by bacterial outer membrane vesicles. Nat. Rev. Immunol. 2015, 15, 375–387. [Google Scholar] [CrossRef]
- Reidl, J. Outer Membrane Vesicle Biosynthesis in Salmonella: Is There More to Gram-Negative Bacteria? mBio 2016, 7, e01282-16. [Google Scholar] [CrossRef]
- Elhenawy, W.; Bording-Jorgensen, M.; Valguarnera, E.; Haurat, M.F.; Wine, E.; Feldman, M.F. LPS Remodeling Triggers Formation of Outer Membrane Vesicles in Salmonella. mBio 2016, 7, e00940-16. [Google Scholar] [CrossRef]
- Athman, J.J.; Wang, Y.; McDonald, D.J.; Boom, W.H.; Harding, C.V.; Wearsch, P.A. Bacterial Membrane Vesicles Mediate the Release of Mycobacterium tuberculosis Lipoglycans and Lipoproteins from Infected Macrophages. J. Immunol. 2015, 195, 1044–1053. [Google Scholar] [CrossRef]
- Mashburn-Warren, L.M.; Whiteley, M. Special delivery: Vesicle trafficking in prokaryotes. Mol. Microbiol. 2006, 61, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.Y.; Choi, D.S.; Kim, K.P.; Gho, Y.S. Proteomics in gram-negative bacterial outer membrane vesicles. Mass Spectrom. Rev. 2008, 27, 535–555. [Google Scholar] [CrossRef] [PubMed]
- Bonnington, K.E.; Kuehn, M.J. Protein selection and export via outer membrane vesicles. Biochim. Biophys. Acta 2014, 1843, 1612–1619. [Google Scholar] [CrossRef] [PubMed]
- Unal, C.M.; Schaar, V.; Riesbeck, K. Bacterial outer membrane vesicles in disease and preventive medicine. Semin. Immunopathol. 2011, 33, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Horstman, A.L.; Kuehn, M.J. Enterotoxigenic Escherichia coli secretes active heat-labile enterotoxin via outer membrane vesicles. J. Biol. Chem. 2000, 275, 12489–12496. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D.; Chaudhuri, K. Association of cholera toxin with Vibrio cholerae outer membrane vesicles which are internalized by human intestinal epithelial cells. FEBS Lett. 2011, 585, 1357–1362. [Google Scholar] [CrossRef] [PubMed]
- Lindmark, B.; Rompikuntal, P.K.; Vaitkevicius, K.; Song, T.; Mizunoe, Y.; Uhlin, B.E.; Guerry, P.; Wai, S.N. Outer membrane vesicle-mediated release of cytolethal distending toxin (CDT) from Campylobacter jejuni. BMC Microbiol. 2009, 9, 220. [Google Scholar] [CrossRef]
- Pettit, R.K.; Judd, R.C. Characterization of naturally elaborated blebs from serum-susceptible and serum-resistant strains of Neisseria gonorrhoeae. Mol. Microbiol. 1992, 6, 723–728. [Google Scholar] [CrossRef]
- Schooling, S.R.; Beveridge, T.J. Membrane Vesicles: An Overlooked Component of the Matrices of Biofilms. J. Bacteriol. 2006, 188, 5945–5957. [Google Scholar] [CrossRef]
- Palsdottir, H.; Remis, J.P.; Schaudinn, C.; O‘Toole, E.; Lux, R.; Shi, W.; McDonald, K.L.; Costerton, J.W.; Auer, M. Three-dimensional macromolecular organization of cryofixed Myxococcus xanthus biofilms as revealed by electron microscopic tomography. J. Bacteriol. 2009, 191, 2077–2082. [Google Scholar] [CrossRef] [PubMed]
- Kadurugamuwa, J.L.; Beveridge, T.J. Virulence factors are released from Pseudomonas aeruginosa in association with membrane vesicles during normal growth and exposure to gentamicin: A novel mechanism of enzyme secretion. J. Bacteriol. 1995, 177, 3998–4008. [Google Scholar] [CrossRef] [PubMed]
- Fulsundar, S.; Harms, K.; Flaten, G.E.; Johnsen, P.J.; Chopade, B.A.; Nielsen, K.M. Gene transfer potential of outer membrane vesicles of Acinetobacter baylyi and effects of stress on vesiculation. Appl. Environ. Microbiol. 2014, 80, 3469–3483. [Google Scholar] [CrossRef]
- Renelli, M.; Matias, V.; Lo, R.Y.; Beveridge, T.J. DNA-containing membrane vesicles of Pseudomonas aeruginosa PAO1 and their genetic transformation potential. Microbiology 2004, 150, 2161–2169. [Google Scholar] [CrossRef]
- Kuehn, M.J.; Kesty, N.C. Bacterial outer membrane vesicles and the host-pathogen interaction. Genes Dev. 2005, 19, 2645–2655. [Google Scholar] [CrossRef]
- Beveridge, T.J. Structures of gram-negative cell walls and their derived membrane vesicles. J. Bacteriol. 1999, 181, 4725–4733. [Google Scholar] [CrossRef] [PubMed]
- Dowling, D.J.; Sanders, H.; Cheng, W.K.; Joshi, S.; Brightman, S.; Bergelson, I.; Pietrasanta, C.; van Haren, S.D.; van Amsterdam, S.; Fernandez, J.; et al. A Meningococcal Outer Membrane Vesicle Vaccine Incorporating Genetically Attenuated Endotoxin Dissociates Inflammation from Immunogenicity. Front. Immunol. 2016, 7, 562. [Google Scholar] [CrossRef]
- Bottero, D.; Gaillard, M.E.; Zurita, E.; Moreno, G.; Martinez, D.S.; Bartel, E.; Bravo, S.; Carriquiriborde, F.; Errea, A.; Castuma, C.; et al. Characterization of the immune response induced by pertussis OMVs-based vaccine. Vaccine 2016, 34, 3303–3309. [Google Scholar] [CrossRef]
- Pritsch, M.; Ben-Khaled, N.; Chaloupka, M.; Kobold, S.; Berens-Riha, N.; Peter, A.; Liegl, G.; Schubert, S.; Hoelscher, M.; Löscher, T.; et al. Comparison of Intranasal Outer Membrane Vesicles with Cholera Toxin and Injected MF59C.1 as Adjuvants for Malaria Transmission Blocking Antigens AnAPN1 and Pfs48/45. J. Immunol. Res. 2016, 2016, 3576028. [Google Scholar] [CrossRef]
- Tan, K.; Li, R.; Huang, X.; Liu, Q. Outer Membrane Vesicles: Current Status and Future Direction of These Novel Vaccine Adjuvants. Front. Microbiol. 2018, 9, 783. [Google Scholar] [CrossRef]
- Berlanda Scorza, F.; Colucci, A.M.; Maggiore, L.; Sanzone, S.; Rossi, O.; Ferlenghi, I.; Pesce, I.; Caboni, M.; Norais, N.; Di Cioccio, V.; et al. High yield production process for Shigella outer membrane particles. PLoS ONE 2012, 7, e35616. [Google Scholar] [CrossRef]
- McMahon, K.J.; Castelli, M.E.; García Vescovi, E.; Feldman, M.F. Biogenesis of outer membrane vesicles in Serratia marcescens is thermoregulated and can be induced by activation of the Rcs phosphorelay system. J. Bacteriol. 2012, 194, 3241–3249. [Google Scholar] [CrossRef] [PubMed]
- Kulp, A.J.; Sun, B.; Ai, T.; Manning, A.J.; Orench-Rivera, N.; Schmid, A.K.; Kuehn, M.J. Genome-Wide Assessment of Outer Membrane Vesicle Production in Escherichia coli. PLoS ONE 2015, 10, e0139200. [Google Scholar] [CrossRef] [PubMed]
- Micoli, F.; Rondini, S.; Alfini, R.; Lanzilao, L.; Necchi, F.; Negrea, A.; Rossi, O.; Brandt, C.; Clare, S.; Mastroeni, P.; et al. Comparative immunogenicity and efficacy of equivalent outer membrane vesicle and glycoconjugate vaccines against nontyphoidal Salmonella. Proc. Natl. Acad. Sci. USA 2018, 115, 10428–10433. [Google Scholar] [CrossRef]
- Rossi, O.; Caboni, M.; Negrea, A.; Necchi, F.; Alfini, R.; Micoli, F.; Saul, A.; MacLennan, C.A.; Rondini, S.; Gerke, C. Toll-Like Receptor Activation by Generalized Modules for Membrane Antigens from Lipid A Mutants of Salmonella enterica Serovars Typhimurium and Enteritidis. Clin. Vaccine Immunol. 2016, 23, 304–314. [Google Scholar] [CrossRef] [PubMed]
- De Benedetto, G.; Alfini, R.; Cescutti, P.; Caboni, M.; Lanzilao, L.; Necchi, F.; Saul, A.; MacLennan, C.A.; Rondini, S.; Micoli, F. Characterization of O-antigen delivered by Generalized Modules for Membrane Antigens (GMMA) vaccine candidates against nontyphoidal Salmonella. Vaccine 2017, 35, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Schager, A.E.; Dominguez-Medina, C.C.; Necchi, F.; Micoli, F.; Goh, Y.S.; Goodall, M.; Flores-Langarica, A.; Bobat, S.; Cook, C.N.L.; Arcuri, M.; et al. IgG Responses to Porins and Lipopolysaccharide within an Outer Membrane-Based Vaccine against Nontyphoidal Salmonella Develop at Discordant Rates. mBio 2018, 9, e02379-17. [Google Scholar] [CrossRef]
- Koeberling, O.; Ispasanie, E.; Hauser, J.; Rossi, O.; Pluschke, G.; Caugant, D.A.; Saul, A.; MacLennan, C.A. A broadly-protective vaccine against meningococcal disease in sub-Saharan Africa based on generalized modules for membrane antigens (GMMA). Vaccine 2014, 32, 2688–2695. [Google Scholar] [CrossRef]
- Gerke, C.; Colucci, A.M.; Giannelli, C.; Sanzone, S.; Vitali, C.G.; Sollai, L.; Rossi, O.; Martin, L.B.; Auerbach, J.; Di Cioccio, V.; et al. Production of a Shigella sonnei Vaccine Based on Generalized Modules for Membrane Antigens (GMMA), 1790GAHB. PLoS ONE 2015, 10, e0134478. [Google Scholar] [CrossRef]
- Launay, O.; Lewis, D.J.M.; Anemona, A.; Loulergue, P.; Leahy, J.; Sciré, A.S.; Maugard, A.; Marchetti, E.; Zancan, S.; Huo, Z.; et al. Safety Profile and Immunologic Responses of a Novel Vaccine Against Shigella sonnei Administered Intramuscularly, Intradermally and Intranasally: Results From Two Parallel Randomized Phase 1 Clinical Studies in Healthy Adult Volunteers in Europe. EBioMedicine 2017, 22, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Launay, O.; Ndiaye, A.G.W.; Conti, V.; Loulergue, P.; Sciré, A.S.; Landre, A.M.; Ferruzzi, P.; Nedjaai, N.; Schütte, L.D.; Auerbach, J.; et al. Booster Vaccination With GVGH Shigella sonnei 1790GAHB GMMA Vaccine Compared to Single Vaccination in Unvaccinated Healthy European Adults: Results From a Phase 1 Clinical Trial. Front. Immunol. 2019, 10, 335. [Google Scholar] [CrossRef] [PubMed]
- Van de Waterbeemd, B.; Streefland, M.; van der Ley, P.; Zomer, B.; van Dijken, H.; Martens, D.; Wijffels, R.; van der Pol, L. Improved OMV vaccine against Neisseria meningitidis using genetically engineered strains and a detergent-free purification process. Vaccine 2010, 28, 4810–4816. [Google Scholar] [CrossRef] [PubMed]
- Koeberling, O.; Giuntini, S.; Seubert, A.; Granoff, D.M. Meningococcal outer membrane vesicle vaccines derived from mutant strains engineered to express factor H binding proteins from antigenic variant groups 1 and 2. Clin. Vaccine Immunol. 2009, 16, 156–162. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Watkins, H.C.; Rappazzo, C.G.; Higgins, J.S.; Sun, X.; Brock, N.; Chau, A.; Misra, A.; Cannizzo, J.P.B.; King, M.R.; Maines, T.R.; et al. Safe Recombinant Outer Membrane Vesicles that Display M2e Elicit Heterologous Influenza Protection. Mol. Ther. 2017, 25, 989–1002. [Google Scholar] [CrossRef]
- Scaria, P.V.; Rowe, C.G.; Chen, B.B.; Muratova, O.V.; Fischer, E.R.; Barnafo, E.K.; Anderson, C.F.; Zaidi, I.U.; Lambert, L.E.; Lucas, B.J.; et al. Outer membrane protein complex as a carrier for malaria transmission blocking antigen Pfs230. NPJ Vaccines 2019, 4, 24. [Google Scholar] [CrossRef]
- Grandi, A.; Tomasi, M.; Zanella, I.; Ganfini, L.; Caproni, E.; Fantappiè, L.; Irene, C.; Frattini, L.; Isaac, S.J.; König, E.; et al. Synergistic Protective Activity of Tumor-Specific Epitopes Engineered in Bacterial Outer Membrane Vesicles. Front. Oncol. 2017, 7, 253. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef]
- Kumar, H.; Kawai, T.; Akira, S. Toll-like receptors and innate immunity. Biochem. Biophys. Res. Commun. 2009, 388, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.V.; Nusslein-Volhard, C. Information for the dorsal-ventral pattern of the Drosophila embryo is stored as maternal mRNA. Nature 1984, 311, 223–227. [Google Scholar] [CrossRef]
- Jin, M.S.; Lee, J.O. Structures of TLR-ligand complexes. Curr. Opin. Immunol. 2008, 20, 414–419. [Google Scholar] [CrossRef]
- Mahla, R.S.; Reddy, M.C.; Prasad, D.V.R.; Kumar, H. Sweeten PAMPs: Role of Sugar Complexed PAMPs in Innate Immunity and Vaccine Biology. Front. Immunol. 2013, 4, 248. [Google Scholar] [CrossRef]
- Blasius, A.L.; Beutler, B. Intracellular toll-like receptors. Immunity 2010, 32, 305–315. [Google Scholar] [CrossRef]
- Poltorak, A.; He, X.; Smirnova, I.; Liu, M.Y.; Van Huffel, C.; Du, X.; Birdwell, D.; Alejos, E.; Silva, M.; Galanos, C.; et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: Mutations in Tlr4 gene. Science 1998, 282, 2085–2088. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.Y.; Nan, X.; Jin, M.S.; Youn, S.J.; Ryu, Y.H.; Mah, S.; Han, S.H.; Lee, H.; Paik, S.G.; Lee, J.O. Recognition of lipopeptide patterns by Toll-like receptor 2-Toll-like receptor 6 heterodimer. Immunity 2009, 31, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Gewirtz, A.T.; Navas, T.A.; Lyons, S.; Godowski, P.J.; Madara, J.L. Cutting edge: Bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J. Immunol. 2001, 167, 1882–1885. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, F.; Smith, K.D.; Ozinsky, A.; Hawn, T.R.; Yi, E.C.; Goodlett, D.R.; Eng, J.K.; Akira, S.; Underhill, D.M.; Aderem, A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 2001, 410, 1099–1103. [Google Scholar] [CrossRef]
- Hemmi, H.; Takeuchi, O.; Kawai, T.; Kaisho, T.; Sato, S.; Sanjo, H.; Matsumoto, M.; Hoshino, K.; Wagner, H.; Takeda, K.; et al. A Toll-like receptor recognizes bacterial DNA. Nature 2000, 408, 740–745. [Google Scholar] [CrossRef]
- Hidmark, A.; von Saint Paul, A.; Dalpke, A.H. Cutting edge: TLR13 is a receptor for bacterial RNA. J. Immunol. 2012, 189, 2717–2721. [Google Scholar] [CrossRef]
- Gerritzen, M.J.H.; Martens, D.E.; Wijffels, R.H.; van der Pol, L.; Stork, M. Bioengineering bacterial outer membrane vesicles as vaccine platform. Biotechnol. Adv. 2017, 35, 565–574. [Google Scholar] [CrossRef]
- Olguín, Y.; Villalobos, P.; Carrascosa, L.G.; Young, M.; Valdez, E.; Lechuga, L.; Galindo, R. Detection of flagellin by interaction with human recombinant TLR5 immobilized in liposomes. Anal. Bioanal. Chem. 2013, 405, 1267–1281. [Google Scholar] [CrossRef]
- Fitzgerald, K.A.; Kagan, J.C. Toll-like Receptors and the Control of Immunity. Cell 2020, 180, 1044–1066. [Google Scholar] [CrossRef] [PubMed]
- Yarovinsky, F.; Zhang, D.; Andersen, J.F.; Bannenberg, G.L.; Serhan, C.N.; Hayden, M.S.; Hieny, S.; Sutterwala, F.S.; Flavell, R.A.; Ghosh, S.; et al. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science 2005, 308, 1626–1629. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.S.; Kim, S.E.; Heo, J.Y.; Lee, M.E.; Kim, H.M.; Paik, S.G.; Lee, H.; Lee, J.O. Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell 2007, 130, 1071–1082. [Google Scholar] [CrossRef] [PubMed]
- Park, B.S.; Song, D.H.; Kim, H.M.; Choi, B.S.; Lee, H.; Lee, J.O. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature 2009, 458, 1191–1195. [Google Scholar] [CrossRef] [PubMed]
- Ohto, U.; Ishida, H.; Shibata, T.; Sato, R.; Miyake, K.; Shimizu, T. Toll-like Receptor 9 Contains Two DNA Binding Sites that Function Cooperatively to Promote Receptor Dimerization and Activation. Immunity 2018, 48, 649–658.e4. [Google Scholar] [CrossRef] [PubMed]
- Vasselon, T.; Detmers, P.A.; Charron, D.; Haziot, A. TLR2 recognizes a bacterial lipopeptide through direct binding. J. Immunol. 2004, 173, 7401–7405. [Google Scholar] [CrossRef]
- Raetz, C.R.; Reynolds, C.M.; Trent, M.S.; Bishop, R.E. Lipid A modification systems in gram-negative bacteria. Annu. Rev. Biochem. 2007, 76, 295–329. [Google Scholar] [CrossRef]
- Trent, M.S.; Stead, C.M.; Tran, A.X.; Hankins, J.V. Diversity of endotoxin and its impact on pathogenesis. J. Endotoxin Res. 2006, 12, 205–223. [Google Scholar] [CrossRef]
- Clementz, T.; Bednarski, J.J.; Raetz, C.R. Function of the htrB high temperature requirement gene of Escherichia coli in the acylation of lipid A: HtrB catalyzed incorporation of laurate. J. Biol. Chem. 1996, 271, 12095–12102. [Google Scholar] [CrossRef]
- Clementz, T.; Zhou, Z.; Raetz, C.R. Function of the Escherichia coli msbB gene, a multicopy suppressor of htrB knockouts, in the acylation of lipid A. Acylation by MsbB follows laurate incorporation by HtrB. J. Biol. Chem. 1997, 272, 10353–10360. [Google Scholar] [CrossRef]
- Bishop, R.E.; Kim, S.H.; El Zoeiby, A. Role of lipid A palmitoylation in bacterial pathogenesis. J. Endotoxin Res. 2005, 11, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, C.M.; Ribeiro, A.A.; McGrath, S.C.; Cotter, R.J.; Raetz, C.R.; Trent, M.S. An outer membrane enzyme encoded by Salmonella typhimurium lpxR that removes the 3′-acyloxyacyl moiety of lipid A. J. Biol. Chem. 2006, 281, 21974–21987. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, K.; Teramoto, M.; Tatsui, R.; Amamoto, S. Lipid A 3′-O-deacylation by Salmonella outer membrane enzyme LpxR modulates the ability of lipid A to stimulate Toll-like receptor 4. Biochem. Biophys. Res. Commun. 2012, 428, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Hsu, F.F.; Turk, J.; Groisman, E.A. The PmrA-regulated pmrC gene mediates phosphoethanolamine modification of lipid A and polymyxin resistance in Salmonella enterica. J. Bacteriol. 2004, 186, 4124–4133. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.; Six, D.A.; Roland, K.L.; Liu, Q.; Gu, L.; Reynolds, C.M.; Wang, X.; Raetz, C.R.; Curtiss, R., 3rd. Salmonella synthesizing 1-dephosphorylated [corrected] lipopolysaccharide exhibits low endotoxic activity while retaining its immunogenicity. J. Immunol. 2011, 187, 412–423. [Google Scholar] [CrossRef]
- Anandan, A.; Vrielink, A. Structure and function of lipid A–modifying enzymes. Ann. N. Y. Acad. Sci. 2020, 1459, 19–37. [Google Scholar] [CrossRef]
- Vorachek-Warren, M.K.; Carty, S.M.; Lin, S.; Cotter, R.J.; Raetz, C.R. An Escherichia coli mutant lacking the cold shock-induced palmitoleoyltransferase of lipid A biosynthesis: Absence of unsaturated acyl chains and antibiotic hypersensitivity at 12 degrees C. J. Biol. Chem. 2002, 277, 14186–14193. [Google Scholar] [CrossRef]
- Needham, B.D.; Carroll, S.M.; Giles, D.K.; Georgiou, G.; Whiteley, M.; Trent, M.S. Modulating the innate immune response by combinatorial engineering of endotoxin. Proc. Natl. Acad. Sci. USA 2013, 110, 1464–1469. [Google Scholar] [CrossRef]
- Gioannini, T.L.; Teghanemt, A.; Zhang, D.; Coussens, N.P.; Dockstader, W.; Ramaswamy, S.; Weiss, J.P. Isolation of an endotoxin-MD-2 complex that produces Toll-like receptor 4-dependent cell activation at picomolar concentrations. Proc. Natl. Acad. Sci. USA 2004, 101, 4186–4191. [Google Scholar] [CrossRef]
- Akashi, S.; Ogata, H.; Kirikae, F.; Kirikae, T.; Kawasaki, K.; Nishijima, M.; Shimazu, R.; Nagai, Y.; Fukudome, K.; Kimoto, M.; et al. Regulatory roles for CD14 and phosphatidylinositol in the signaling via toll-like receptor 4-MD-2. Biochem. Biophys. Res. Commun. 2000, 268, 172–177. [Google Scholar] [CrossRef]
- Rossi, O.; Pesce, I.; Giannelli, C.; Aprea, S.; Caboni, M.; Citiulo, F.; Valentini, S.; Ferlenghi, I.; MacLennan, C.A.; D’Oro, U.; et al. Modulation of endotoxicity of Shigella generalized modules for membrane antigens (GMMA) by genetic lipid A modifications: Relative activation of TLR4 and TLR2 pathways in different mutants. J. Biol. Chem. 2014, 289, 24922–24935. [Google Scholar] [CrossRef] [PubMed]
- Leitner, D.R.; Feichter, S.; Schild-Prüfert, K.; Rechberger, G.N.; Reidl, J.; Schild, S. Lipopolysaccharide modifications of a cholera vaccine candidate based on outer membrane vesicles reduce endotoxicity and reveal the major protective antigen. Infect. Immun. 2013, 81, 2379–2393. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Keiser, P.B.; Biggs-Cicatelli, S.; Moran, E.E.; Schmiel, D.H.; Pinto, V.B.; Burden, R.E.; Miller, L.B.; Moon, J.E.; Bowden, R.A.; Cummings, J.F.; et al. A phase 1 study of a meningococcal native outer membrane vesicle vaccine made from a group B strain with deleted lpxL1 and synX, over-expressed factor H binding protein, two PorAs and stabilized OpcA expression. Vaccine 2011, 29, 1413–1420. [Google Scholar] [CrossRef]
- Keiser, P.B.; Gibbs, B.T.; Coster, T.S.; Moran, E.E.; Stoddard, M.B.; Labrie, J.E., 3rd; Schmiel, D.H.; Pinto, V.; Chen, P.; Zollinger, W.D. A phase 1 study of a group B meningococcal native outer membrane vesicle vaccine made from a strain with deleted lpxL2 and synX and stable expression of opcA. Vaccine 2010, 28, 6970–6976. [Google Scholar] [CrossRef]
- Fransen, F.; Boog, C.J.; van Putten, J.P.; van der Ley, P. Agonists of Toll-Like Receptors 3, 4, 7, and 9 Are Candidates for Use as Adjuvants in an Outer Membrane Vaccine against Neisseria meningitidis Serogroup B. Infect. Immun. 2007, 75, 5939–5946. [Google Scholar] [CrossRef]
- Opal, S.M.; Palardy, J.E.; Chen, W.H.; Parejo, N.A.; Bhattacharjee, A.K.; Cross, A.S. Active immunization with a detoxified endotoxin vaccine protects against lethal polymicrobial sepsis: Its use with CpG adjuvant and potential mechanisms. J. Infect. Dis. 2005, 192, 2074–2080. [Google Scholar] [CrossRef]
- Bauman, S.J.; Kuehn, M.J. Purification of outer membrane vesicles from Pseudomonas aeruginosa and their activation of an IL-8 response. Microbes Infect. 2006, 8, 2400–2408. [Google Scholar] [CrossRef] [PubMed]
- Maggiore, L.; Yu, L.; Omasits, U.; Rossi, O.; Dougan, G.; Thomson, N.R.; Saul, A.; Choudhary, J.S.; Gerke, C. Quantitative proteomic analysis of Shigella flexneri and Shigella sonnei Generalized Modules for Membrane Antigens (GMMA) reveals highly pure preparations. Int. J. Med. Microbiol. 2016, 306, 99–108. [Google Scholar] [CrossRef]
- Nussenzweig, M.C.; Steinman, R.M. Contribution of dendritic cells to stimulation of the murine syngeneic mixed leukocyte reaction. J. Exp. Med. 1980, 151, 1196–1212. [Google Scholar] [CrossRef] [PubMed]
- Duthie, M.S.; Windish, H.P.; Fox, C.B.; Reed, S.G. Use of defined TLR ligands as adjuvants within human vaccines. Immunol. Rev. 2011, 239, 178–196. [Google Scholar] [CrossRef]
- Gnjatic, S.; Sawhney, N.B.; Bhardwaj, N. Toll-like receptor agonists: Are they good adjuvants? Cancer J. 2010, 16, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Turley, S.J.; Inaba, K.; Garrett, W.S.; Ebersold, M.; Unternaehrer, J.; Steinman, R.M.; Mellman, I. Transport of peptide-MHC class II complexes in developing dendritic cells. Science 2000, 288, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Blander, J.M.; Medzhitov, R. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature 2006, 440, 808–812. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, E.S.; Ebersold, M.; Garrett, W.; Pypaert, M.; Mellman, I. Activation of lysosomal function during dendritic cell maturation. Science 2003, 299, 1400–1403. [Google Scholar] [CrossRef]
- Nair-Gupta, P.; Baccarini, A.; Tung, N.; Seyffer, F.; Florey, O.; Huang, Y.; Banerjee, M.; Overholtzer, M.; Roche, P.A.; Tampé, R.; et al. TLR signals induce phagosomal MHC-I delivery from the endosomal recycling compartment to allow cross-presentation. Cell 2014, 158, 506–521. [Google Scholar] [CrossRef]
- Kaisho, T.; Akira, S. Toll-like receptors as adjuvant receptors. Biochim. Biophys. Acta 2002, 1589, 1–13. [Google Scholar] [CrossRef]
- Ismail, S.; Hampton, M.B.; Keenan, J.I. Helicobacter pylori Outer Membrane Vesicles Modulate Proliferation and Interleukin-8 Production by Gastric Epithelial Cells. Infect. Immun. 2003, 71, 5670–5675. [Google Scholar] [CrossRef]
- Edwards, A.D.; Diebold, S.S.; Slack, E.M.C.; Tomizawa, H.; Hemmi, H.; Kaisho, T.; Akira, S.; Reis e Sousa, C. Toll-like receptor expression in murine DC subsets: Lack of TLR7 expresion of CD8α+ DC correlates with unresponsiveness to imidazoquinolines. Eur. J. Immunol. 2003, 33, 827–833. [Google Scholar] [CrossRef]
- Hornung, V.; Rothenfusser, S.; Britsch, S.; Krug, A.; Jahrsdörfer, B.; Giese, T.; Endres, S.; Hartmann, G. Quantitative Expression of Toll-Like Receptor 1–10 mRNA in Cellular Subsets of Human Peripheral Blood Mononuclear Cells and Sensitivity to CpG Oligodeoxynucleotides. J. Immunol. 2002, 168, 4531–4537. [Google Scholar] [CrossRef]
- Merad, M.; Sathe, P.; Helft, J.; Miller, J.; Mortha, A. The Dendritic Cell Lineage: Ontogeny and Function of Dendritic Cells and Their Subsets in the Steady State and the Inflamed Setting. Annu. Rev. Immunol. 2013, 31, 563–604. [Google Scholar] [CrossRef]
- Anderson, D.A.; Murphy, K.M. Chapter Four - Models of dendritic cell development correlate ontogeny with function. In Advances in Immunology; Alt, F., Ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 143, pp. 99–119. [Google Scholar]
- Schulz, O.; Diebold, S.S.; Chen, M.; Näslund, T.I.; Nolte, M.A.; Alexopoulou, L.; Azuma, Y.-T.; Flavell, R.A.; Liljeström, P.; Reis e Sousa, C. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature 2005, 433, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Browne, E.P. Regulation of B-cell responses by Toll-like receptors. Immunology 2012, 136, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Visintin, A.; Mazzoni, A.; Spitzer, J.H.; Wyllie, D.H.; Dower, S.K.; Segal, D.M. Regulation of Toll-Like Receptors in Human Monocytes and Dendritic Cells. J. Immunol. 2001, 166, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Schreibelt, G.; Tel, J.; Sliepen, K.H.E.W.J.; Benitez-Ribas, D.; Figdor, C.G.; Adema, G.J.; de Vries, I.J.M. Toll-like receptor expression and function in human dendritic cell subsets: Implications for dendritic cell-based anti-cancer immunotherapy. Cancer Immunol. Immunother. 2010, 59, 1573–1582. [Google Scholar] [CrossRef]
- Komai-Koma, M.; Jones, L.; Ogg, G.S.; Xu, D.; Liew, F.Y. TLR2 is expressed on activated T cells as a costimulatory receptor. Proc. Natl. Acad. Sci. USA 2004, 101, 3029–3034. [Google Scholar] [CrossRef]
- Kokkinopoulos, I.; Jordan, W.J.; Ritter, M.A. Toll-like receptor mRNA expression patterns in human dendritic cells and monocytes. Mol. Immunol. 2005, 42, 957–968. [Google Scholar] [CrossRef] [PubMed]
- Wesch, D.; Beetz, S.; Oberg, H.H.; Marget, M.; Krengel, K.; Kabelitz, D. Direct costimulatory effect of TLR3 ligand poly(I:C) on human gamma delta T lymphocytes. J. Immunol. 2006, 176, 1348–1354. [Google Scholar] [CrossRef]
- Reynolds, J.M.; Martinez, G.J.; Chung, Y.; Dong, C. Toll-like receptor 4 signaling in T cells promotes autoimmune inflammation. Proc. Natl. Acad. Sci. USA 2012, 109, 13064–13069. [Google Scholar] [CrossRef]
- Bekeredjian-Ding, I.; Jego, G. Toll-like receptors--sentries in the B-cell response. Immunology 2009, 128, 311–323. [Google Scholar] [CrossRef]
- Crellin, N.K.; Garcia, R.V.; Hadisfar, O.; Allan, S.E.; Steiner, T.S.; Levings, M.K. Human CD4+ T cells express TLR5 and its ligand flagellin enhances the suppressive capacity and expression of FOXP3 in CD4+CD25+ T regulatory cells. J. Immunol. 2005, 175, 8051–8059. [Google Scholar] [CrossRef]
- De Marcken, M.; Dhaliwal, K.; Danielsen, A.C.; Gautron, A.S.; Dominguez-Villar, M. TLR7 and TLR8 activate distinct pathways in monocytes during RNA virus infection. Sci. Signal. 2019, 12, eaaw1347. [Google Scholar] [CrossRef] [PubMed]
- Caron, G.; Duluc, D.; Frémaux, I.; Jeannin, P.; David, C.; Gascan, H.; Delneste, Y. Direct Stimulation of Human T Cells via TLR5 and TLR7/8: Flagellin and R-848 Up-Regulate Proliferation and IFN-γ Production by Memory CD4+ T Cells. J. Immunol. 2005, 175, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Babu, S.; Blauvelt, C.P.; Kumaraswami, V.; Nutman, T.B. Cutting Edge: Diminished T Cell TLR Expression and Function Modulates the Immune Response in Human Filarial Infection. J. Immunol. 2006, 176, 3885–3889. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.; Sun, T.; Yu, X.-H.; Yang, Y.-X.; Yeo, A.E.T. The effects of TLR activation on T-cell development and differentiation. Clin. Dev. Immunol. 2012, 2012, 836485. [Google Scholar] [CrossRef] [PubMed]
- Conroy, H.; Marshall, N.A.; Mills, K.H. TLR ligand suppression or enhancement of Treg cells? A double-edged sword in immunity to tumours. Oncogene 2008, 27, 168–180. [Google Scholar] [CrossRef]
- Keijzer, C.; van der Zee, R.; van Eden, W.; Broere, F. Treg inducing adjuvants for therapeutic vaccination against chronic inflammatory diseases. Front. Immunol. 2013, 4, 245. [Google Scholar] [CrossRef]
- Toussi, D.N.; Massari, P. Immune Adjuvant Effect of Molecularly-defined Toll-Like Receptor Ligands. Vaccines (Basel) 2014, 2, 323–353. [Google Scholar] [CrossRef]
- Kreuk, L.S.; Koch, M.A.; Slayden, L.C.; Lind, N.A.; Chu, S.; Savage, H.P.; Kantor, A.B.; Baumgarth, N.; Barton, G.M. B cell receptor and Toll-like receptor signaling coordinate to control distinct B-1 responses to both self and the microbiota. Elife 2019, 8, e47015. [Google Scholar] [CrossRef]
- Pasare, C.; Medzhitov, R. Control of B-cell responses by Toll-like receptors. Nature 2005, 438, 364–368. [Google Scholar] [CrossRef]
- Steinhagen, F.; Kinjo, T.; Bode, C.; Klinman, D.M. TLR-based immune adjuvants. Vaccine 2011, 29, 3341–3355. [Google Scholar] [CrossRef]
- Boland, G.; Beran, J.; Lievens, M.; Sasadeusz, J.; Dentico, P.; Nothdurft, H.; Zuckerman, J.N.; Genton, B.; Steffen, R.; Loutan, L.; et al. Safety and immunogenicity profile of an experimental hepatitis B vaccine adjuvanted with AS04. Vaccine 2004, 23, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Wille-Reece, U.; Flynn, B.J.; Loré, K.; Koup, R.A.; Kedl, R.M.; Mattapallil, J.J.; Weiss, W.R.; Roederer, M.; Seder, R.A. HIV Gag protein conjugated to a Toll-like receptor 7/8 agonist improves the magnitude and quality of Th1 and CD8+ T cell responses in nonhuman primates. Proc. Natl. Acad. Sci. USA 2005, 102, 15190–15194. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; McLaughlin, C.; Weizer, A.; Su, Z.; Boczkowski, D.; Dannull, J.; Vieweg, J.; Gilboa, E. Injection of Immature Dendritic Cells into Adjuvant-Treated Skin Obviates the Need for Ex Vivo Maturation. J. Immunol. 2003, 171, 6275–6282. [Google Scholar] [CrossRef] [PubMed]
- Mancini, F.; Monaci, E.; Lofano, G.; Torre, A.; Bacconi, M.; Tavarini, S.; Sammicheli, C.; Arcidiacono, L.; Galletti, B.; Laera, D.; et al. One Dose of Staphylococcus aureus 4C-Staph Vaccine Formulated with a Novel TLR7-Dependent Adjuvant Rapidly Protects Mice through Antibodies, Effector CD4+ T Cells, and IL-17A. PLoS ONE 2016, 11, e0147767. [Google Scholar] [CrossRef] [PubMed]
- Schulze, H.J.; Cribier, B.; Requena, L.; Reifenberger, J.; Ferrándiz, C.; Garcia Diez, A.; Tebbs, V.; McRae, S. Imiquimod 5% cream for the treatment of superficial basal cell carcinoma: Results from a randomized vehicle-controlled phase III study in Europe. Br. J. Dermatol. 2005, 152, 939–947. [Google Scholar] [CrossRef]
- Lebwohl, M.; Dinehart, S.; Whiting, D.; Lee, P.K.; Tawfik, N.; Jorizzo, J.; Lee, J.H.; Fox, T.L. Imiquimod 5% cream for the treatment of actinic keratosis: Results from two phase III, randomized, double-blind, parallel group, vehicle-controlled trials. J. Am. Acad. Dermatol. 2004, 50, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Feyerabend, S.; Stevanovic, S.; Gouttefangeas, C.; Wernet, D.; Hennenlotter, J.; Bedke, J.; Dietz, K.; Pascolo, S.; Kuczyk, M.; Rammensee, H.G.; et al. Novel multi-peptide vaccination in Hla-A2+ hormone sensitive patients with biochemical relapse of prostate cancer. Prostate 2009, 69, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Smorlesi, A.; Papalini, F.; Orlando, F.; Donnini, A.; Re, F.; Provinciali, M. Imiquimod and S-27609 as adjuvants of DNA vaccination in a transgenic murine model of HER2/neu-positive mammary carcinoma. Gene Ther. 2005, 12, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.; O’Neill, D.W.; Nonaka, D.; Hardin, E.; Chiriboga, L.; Siu, K.; Cruz, C.M.; Angiulli, A.; Angiulli, F.; Ritter, E.; et al. Immunization of malignant melanoma patients with full-length NY-ESO-1 protein using TLR7 agonist imiquimod as vaccine adjuvant. J. Immunol. 2008, 181, 776–784. [Google Scholar] [CrossRef]
- Kim, O.Y.; Hong, B.S.; Park, K.S.; Yoon, Y.J.; Choi, S.J.; Lee, W.H.; Roh, T.Y.; Lötvall, J.; Kim, Y.K.; Gho, Y.S. Immunization with Escherichia coli outer membrane vesicles protects bacteria-induced lethality via Th1 and Th17 cell responses. J. Immunol. 2013, 190, 4092–4102. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-J.; Kwon, H.-I.; Kim, E.-H.; Park, S.-J.; Lee, S.-H.; Choi, Y.K.; Kim, S.-H. Assessment of mOMV adjuvant efficacy in the pathogenic H1N1 influenza virus vaccine. Clin. Exp. Vaccine Res. 2014, 3, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.Y.; Kim, C.U.; Bae, E.H.; Seo, S.H.; Jeong, D.G.; Yoon, S.W.; Chang, K.T.; Kim, Y.S.; Kim, S.H.; Kim, D.J. Outer membrane vesicles harboring modified lipid A moiety augment the efficacy of an influenza vaccine exhibiting reduced endotoxicity in a mouse model. Vaccine 2017, 35, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Holst, J.; Martin, D.; Arnold, R.; Huergo, C.C.; Oster, P.; O’Hallahan, J.; Rosenqvist, E. Properties and clinical performance of vaccines containing outer membrane vesicles from Neisseria meningitidis. Vaccine 2009, 27 (Suppl. 2), B3–B12. [Google Scholar] [CrossRef] [PubMed]
- Frasch, C.E. Production and control of Neisseria meningitidis vaccines. Adv. Biotechnol. Process. 1990, 13, 123–145. [Google Scholar]
- Durand, V.; MacKenzie, J.; de Leon, J.; Mesa, C.; Quesniaux, V.; Montoya, M.; Le Bon, A.; Wong, S.Y.C. Role of lipopolysaccharide in the induction of type I interferon-dependent cross-priming and IL-10 production in mice by meningococcal outer membrane vesicles. Vaccine 2009, 27, 1912–1922. [Google Scholar] [CrossRef]
- Schild, S.; Nelson, E.J.; Camilli, A. Immunization with Vibrio cholerae Outer Membrane Vesicles Induces Protective Immunity in Mice. Infect. Immun. 2008, 76, 4554–4563. [Google Scholar] [CrossRef]
- Alaniz, R.C.; Deatherage, B.L.; Lara, J.C.; Cookson, B.T. Membrane Vesicles Are Immunogenic Facsimiles of Salmonella typhimurium That Potently Activate Dendritic Cells, Prime B and T Cell Responses, and Stimulate Protective Immunity In Vivo. J. Immunol. 2007, 179, 7692–7701. [Google Scholar] [CrossRef]
- Whitmire, W.M.; Garon, C.F. Specific and nonspecific responses of murine B cells to membrane blebs of Borrelia burgdorferi. Infect. Immun. 1993, 61, 1460–1467. [Google Scholar] [CrossRef]
- Aoki, M.; Kondo, M.; Nakatsuka, Y.; Kawai, K.; Oshima, S. Stationary phase culture supernatant containing membrane vesicles induced immunity to rainbow trout Oncorhynchus mykiss fry syndrome. Vaccine 2007, 25, 561–569. [Google Scholar] [CrossRef]
- Arigita, C.; Kersten, G.F.; Hazendonk, T.; Hennink, W.E.; Crommelin, D.J.; Jiskoot, W. Restored functional immunogenicity of purified meningococcal PorA by incorporation into liposomes. Vaccine 2003, 21, 950–960. [Google Scholar] [CrossRef]
- Marini, A.; Rossi, O.; Aruta, M.G.; Micoli, F.; Rondini, S.; Guadagnuolo, S.; Delany, I.; Henderson, I.R.; Cunningham, A.F.; Saul, A.; et al. Contribution of factor H-Binding protein sequence to the cross-reactivity of meningococcal native outer membrane vesicle vaccines with over-expressed fHbp variant group 1. PLoS ONE 2017, 12, e0181508. [Google Scholar] [CrossRef] [PubMed]
- Raeven, R.H.; van der Maas, L.; Tilstra, W.; Uittenbogaard, J.P.; Bindels, T.H.; Kuipers, B.; van der Ark, A.; Pennings, J.L.; van Riet, E.; Jiskoot, W.; et al. Immunoproteomic Profiling of Bordetella pertussis Outer Membrane Vesicle Vaccine Reveals Broad and Balanced Humoral Immunogenicity. J. Proteome Res. 2015, 14, 2929–2942. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, M.E.; Bottero, D.; Errea, A.; Ormazábal, M.; Zurita, M.E.; Moreno, G.; Rumbo, M.; Castuma, C.; Bartel, E.; Flores, D.; et al. Acellular pertussis vaccine based on outer membrane vesicles capable of conferring both long-lasting immunity and protection against different strain genotypes. Vaccine 2014, 32, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Muralinath, M.; Kuehn, M.J.; Roland, K.L.; Curtiss, R. Immunization with Salmonella enterica Serovar Typhimurium-Derived Outer Membrane Vesicles Delivering the Pneumococcal Protein PspA Confers Protection against Challenge with Streptococcus pneumoniae. Infect. Immun. 2011, 79, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Wang, S.; Yao, Y.; Xia, Y.; Yang, X.; Li, K.; Sun, P.; Liu, C.; Sun, W.; Bai, H.; et al. Employing Escherichia coli-derived outer membrane vesicles as an antigen delivery platform elicits protective immunity against Acinetobacter baumannii infection. Sci. Rep. 2016, 6, 37242. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Valentine, J.L.; Huang, C.-J.; Endicott, C.E.; Moeller, T.D.; Rasmussen, J.A.; Fletcher, J.R.; Boll, J.M.; Rosenthal, J.A.; Dobruchowska, J.; et al. Outer membrane vesicles displaying engineered glycotopes elicit protective antibodies. Proc. Natl. Acad. Sci. USA 2016, 113, E3609–E3618. [Google Scholar] [CrossRef]
- Bartolini, E.; Ianni, E.; Frigimelica, E.; Petracca, R.; Galli, G.; Berlanda Scorza, F.; Norais, N.; Laera, D.; Giusti, F.; Pierleoni, A.; et al. Recombinant outer membrane vesicles carrying Chlamydia muridarum HtrA induce antibodies that neutralize chlamydial infection in vitro. J. Extracell. Vesicles 2013, 2, 20181. [Google Scholar] [CrossRef]
- Fantappiè, L.; de Santis, M.; Chiarot, E.; Carboni, F.; Bensi, G.; Jousson, O.; Margarit, I.; Grandi, G. Antibody-mediated immunity induced by engineered Escherichia coli OMVs carrying heterologous antigens in their lumen. J. Extracell. Vesicles 2014, 3, 24015. [Google Scholar] [CrossRef]
- Chen, D.J.; Osterrieder, N.; Metzger, S.M.; Buckles, E.; Doody, A.M.; DeLisa, M.P.; Putnam, D. Delivery of foreign antigens by engineered outer membrane vesicle vaccines. Proc. Natl. Acad. Sci. USA 2010, 107, 3099–3104. [Google Scholar] [CrossRef]
- Valguarnera, E.; Feldman, M.F. Glycoengineered Outer Membrane Vesicles as a Platform for Vaccine Development. Methods Enzymol. 2017, 597, 285–310. [Google Scholar]
- Price, N.L.; Goyette-Desjardins, G.; Nothaft, H.; Valguarnera, E.; Szymanski, C.M.; Segura, M.; Feldman, M.F. Glycoengineered Outer Membrane Vesicles: A Novel Platform for Bacterial Vaccines. Sci. Rep. 2016, 6, 24931. [Google Scholar] [CrossRef]
- Fransen, F.; Stenger, R.M.; Poelen, M.C.M.; van Dijken, H.H.; Kuipers, B.; Boog, C.J.P.; van Putten, J.P.M.; van Els, C.A.C.M.; van der Ley, P. Differential effect of TLR2 and TLR4 on the immune response after immunization with a vaccine against Neisseria meningitidis or Bordetella pertussis. PLoS ONE 2010, 5, e15692. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Przysiecki, C.; Flanagan, E.; Bello-Irizarry, S.N.; Ionescu, R.; Muratova, O.; Dobrescu, G.; Lambert, L.; Keister, D.; Rippeon, Y.; et al. Sustained high-titer antibody responses induced by conjugating a malarial vaccine candidate to outer-membrane protein complex. Proc. Natl. Acad. Sci. USA 2006, 103, 18243–18248. [Google Scholar] [CrossRef] [PubMed]
- Latz, E.; Franko, J.; Golenbock, D.T.; Schreiber, J.R. Haemophilus influenzae Type b-Outer Membrane Protein Complex Glycoconjugate Vaccine Induces Cytokine Production by Engaging Human Toll-Like Receptor 2 (TLR2) and Requires the Presence of TLR2 for Optimal Immunogenicity. J. Immunol. 2004, 172, 2431–2438. [Google Scholar] [CrossRef]
- Bishop, R.E. Polymorphic Regulation of Outer Membrane Lipid A Composition. mBio 2016, 7, e01903-16. [Google Scholar] [CrossRef]
- Rivero-Calle, I.; Raguindin, P.F.; Gómez-Rial, J.; Rodriguez-Tenreiro, C.; Martinón-Torres, F. Meningococcal Group B Vaccine For The Prevention Of Invasive Meningococcal Disease Caused By Neisseria meningitidis Serogroup B. Infect. Drug Resist. 2019, 12, 3169–3188. [Google Scholar] [CrossRef]
- Obiero, C.W.; Ndiaye, A.G.W.; Sciré, A.S.; Kaunyangi, B.M.; Marchetti, E.; Gone, A.M.; Schütte, L.D.; Riccucci, D.; Auerbach, J.; Saul, A.; et al. A Phase 2a Randomized Study to Evaluate the Safety and Immunogenicity of the 1790GAHB Generalized Modules for Membrane Antigen Vaccine against Shigella sonnei Administered Intramuscularly to Adults from a Shigellosis-Endemic Country. Front. Immunol. 2017, 8, 1884. [Google Scholar] [CrossRef]
- Li, Y.P.; Yu, S.L.; Huang, Z.J.; Huang, J.; Pan, J.; Feng, X.; Zhang, X.G.; Wang, J.H.; Wang, J. An impaired inflammatory cytokine response to gram-negative LPS in human neonates is associated with the defective TLR-mediated signaling pathway. J. Clin. Immunol. 2015, 35, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Grabiec, A.; Meng, G.; Fichte, S.; Bessler, W.; Wagner, H.; Kirschning, C.J. Human but not murine toll-like receptor 2 discriminates between tri-palmitoylated and tri-lauroylated peptides. J. Biol. Chem. 2004, 279, 48004–48012. [Google Scholar] [CrossRef] [PubMed]
- Jurk, M.; Heil, F.; Vollmer, J.; Schetter, C.; Krieg, A.M.; Wagner, H.; Lipford, G.; Bauer, S. Human TLR7 or TLR8 independently confer responsiveness to the antiviral compound R-848. Nat. Immunol. 2002, 3, 499. [Google Scholar] [CrossRef]
- Bauer, S.; Kirschning, C.J.; Häcker, H.; Redecke, V.; Hausmann, S.; Akira, S.; Wagner, H.; Lipford, G.B. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc. Natl. Acad. Sci. USA 2001, 98, 9237–9242. [Google Scholar] [CrossRef] [PubMed]
- Chuang, T.H.; Lee, J.; Kline, L.; Mathison, J.C.; Ulevitch, R.J. Toll-like receptor 9 mediates CpG-DNA signaling. J. Leukoc. Biol. 2002, 71, 538–544. [Google Scholar] [PubMed]
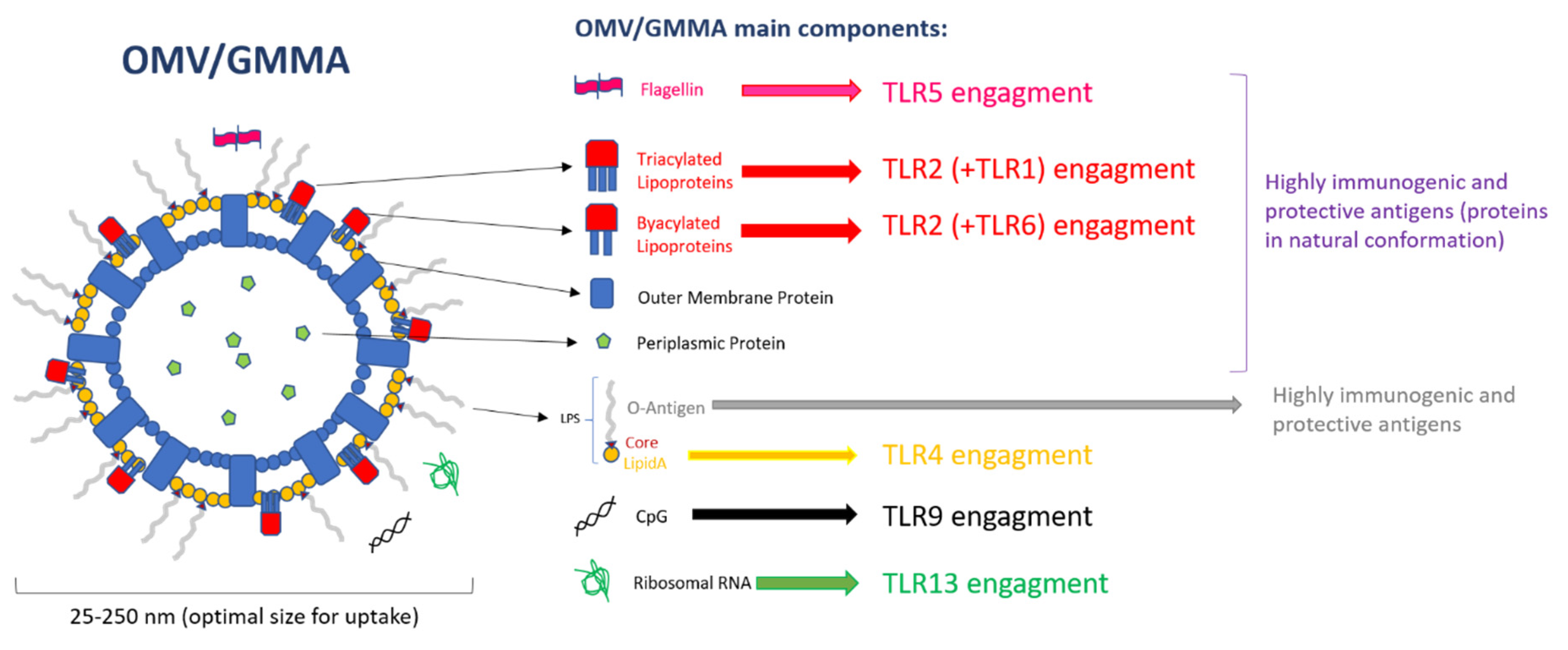
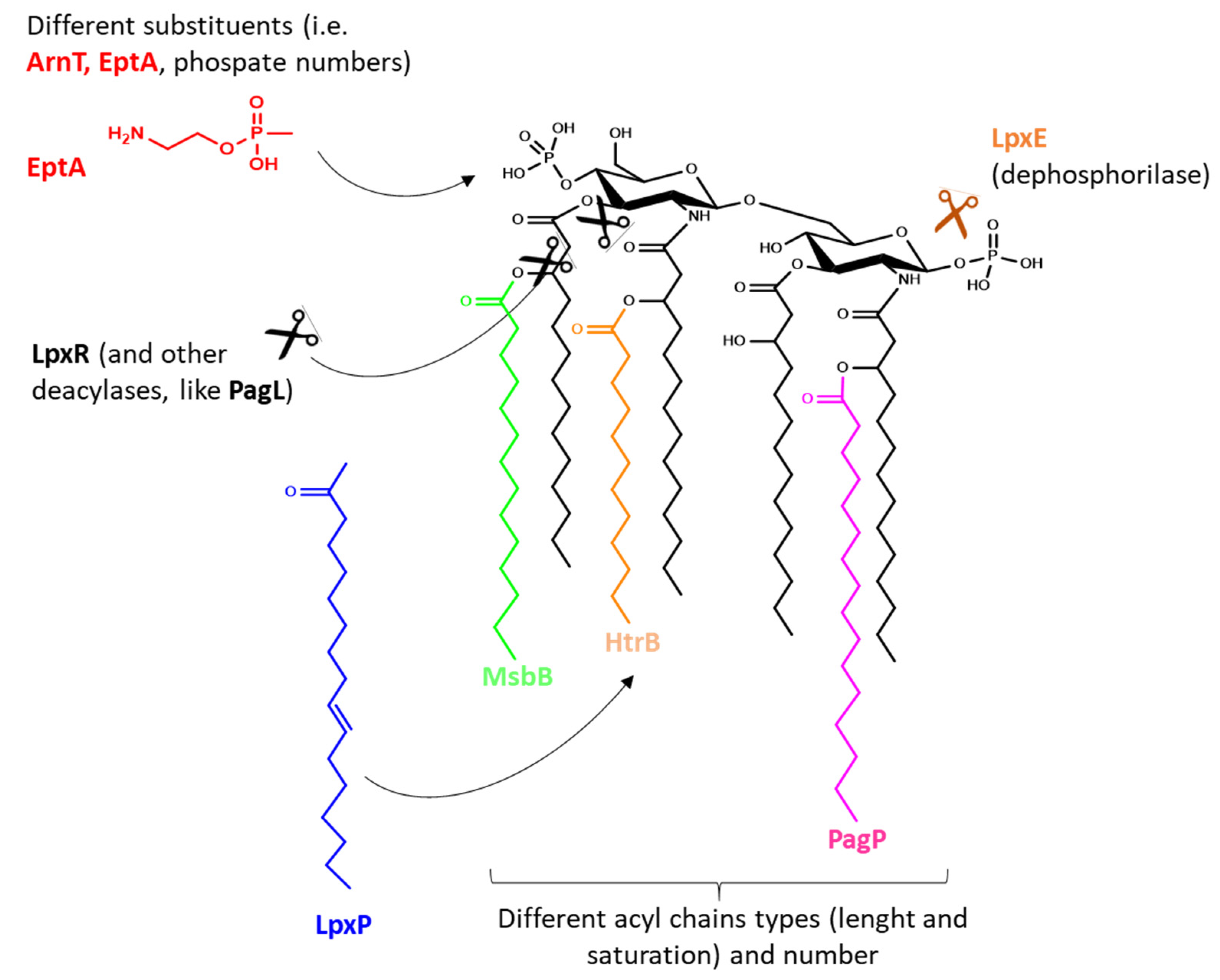
| TLR Type | Absent/Low Expression | Medium/High Expression |
|---|---|---|
| TLR2 (+TLR1) | plasmacytoid DCs, B cells * [103] | monocytes [104], myeloid DCs [105], T cells [106] |
| TLR2 (+TLR6) | monocytes [107], plasmacytoid DCs, B cells * [103] | myeloid DCs [105], T cells [106] |
| TLR3 | monocytes [104], plasmacytoid DCs | CD8a+ myeloid DCs [105], CD138+ B cells [103], T cells [108] |
| TLR4 | plasmacytoid DCs | monocytes [104], myeloid DCs [105], CD138+ B cells [103], T cells [109] |
| TLR5 | plasmacytoid DCs, B cells | monocytes [107], myeloid DCs [105], B cells [110], T cells [111] |
| TLR7 | monocytes [112], plasmacytoid DCs, myeloid DCs, B cells [103], T cells [113] | |
| TLR8 | plasmacytoid DCs | monocytes [112], myeloid DCs [105], B cells [103], T cells [113] |
| TLR9 | monocytes [107], plasmacytoid DCs, B cells [103], T cells [114] |
| OMVs | Classical Vaccines | |
|---|---|---|
| Purified PorA on OMV | Purified PorA | [141] |
| Salmonella Typhimurium OMV expressing PspA in the lumen | Recombinant PspA | [145] |
| E. coli OMVs expressing Omp22 of A. baumannii | Recombinant Omp22 adjuvanted with Alum | [146] |
| OMVs over-expressing fHbp | Recombinant fHbp | [38,142] |
| TdapOMVsBp | Whole cell B. pertussis vaccine and acellular vaccines | [28] |
| OMVs from B. pertussis B1917 | Whole cell B. pertussis vaccine and acellular vaccines | [143] |
| S. Typhimurium and S. Enteritidis GMMA | S. Typhimurium and S. Enteritidis O-antigen conjugated to CRM197 and formulated on Alhydrogel | [34] |
| glyOMVs displaying O-polysaccharides from F. tularensis | Native F. tularensis LPS | [147] |
| E. coli OMV expressing Chlamydia rHtrA | Purified Chlamydia rHtrA | [148] |
| E. coli OMVs carrying different heterologous antigens in their lumen | Purified heterologous antigens | [149] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mancini, F.; Rossi, O.; Necchi, F.; Micoli, F. OMV Vaccines and the Role of TLR Agonists in Immune Response. Int. J. Mol. Sci. 2020, 21, 4416. https://doi.org/10.3390/ijms21124416
Mancini F, Rossi O, Necchi F, Micoli F. OMV Vaccines and the Role of TLR Agonists in Immune Response. International Journal of Molecular Sciences. 2020; 21(12):4416. https://doi.org/10.3390/ijms21124416
Chicago/Turabian StyleMancini, Francesca, Omar Rossi, Francesca Necchi, and Francesca Micoli. 2020. "OMV Vaccines and the Role of TLR Agonists in Immune Response" International Journal of Molecular Sciences 21, no. 12: 4416. https://doi.org/10.3390/ijms21124416
APA StyleMancini, F., Rossi, O., Necchi, F., & Micoli, F. (2020). OMV Vaccines and the Role of TLR Agonists in Immune Response. International Journal of Molecular Sciences, 21(12), 4416. https://doi.org/10.3390/ijms21124416







