Abstract
Colorectal cancer (CRC) is a fatal disease caused by the uncontrolled propagation and endurance of atypical colon cells. A person’s lifestyle and eating pattern have significant impacts on the CRC in a positive and/or negative way. Diet-derived phytochemicals modulate the microbiome as well as targeting colon cancer stem cells (CSCs) that are found to offer significant protective effects against CRC, which were organized in an appropriate spot on the paper. All information on dietary phytochemicals, gut microbiome, CSCs, and their influence on CRC were accessed from the various databases and electronic search engines. The effectiveness of CRC can be reduced using various dietary phytochemicals or modulating microbiome that reduces or inverses the progression of a tumor as well as CSCs, which could be a promising and efficient way to reduce the burden of CRC. Phytochemicals with modulation of gut microbiome continue to be auspicious investigations in CRC through noticeable anti-tumorigenic effects and goals to CSCs, which provides new openings for cancer inhibition and treatment.
1. Introduction
Colorectal cancer (CRC) is one of the most fatal diseases and foremost causes of death globally, representing the third most common malignancy. The American Cancer Society estimated that the rough sum of CRC incidences in the United States in 2018 alone was 97,220 (colon cancer), and 43,030 (rectal cancer), which had a great influence on curative care, which exceeded $17 billion in the medical care system [1]. The CRC develops (70%) via a serious transformation of specific morphological traits, denoted as adenoma to a carcinoma sequence [2]. About 30% of CRC cases are caused due to hereditary disorder, often connected with familial adenomatous polyposis and/or hereditary non-polyposis [3]. Chronic inflammatory bowel diseases (IBD) or family history of CRC are the primary causes of CRC [4]. In economically developed countries, the mortality connected to CRC is greater than the economically developing nations, and it affects over a million people annually [5]. Several epidemiological studies have also shown different risk factors to CRC including age, family history, IBD, obesity, smoking, lack of exercise, alcohol consumption, and diet [6]. Unfortunately, the present treatments are inadequate, owing to its effective treatment, and besides, have various side effects, chemo-resistance, and recurrence of the illness.
The growing oncogenic study provides awareness about the malignancies in humans that could have a history of stem cell diseases. Rendering to the cancer stem cell (CSC) study, CRC originates from a minor portion of tumor cells in the colon that demonstrate self-renewal, pluripotency, and can recruit and sustain tumor development [7]. The cancer-developing cells or CSC were initially recognized in blood cancer, which is copious in most of the hard tumors, especially in CRC. The smaller fraction of CSC can develop the spread of tumorous tissues, in analog to target tissues that produce effective histological units, and organs. CSCs are generally tumor-initiating, self-renewal, long-lasting cells that divide asymmetrically and harvest aggressively thriving cancer progenitor cells. These cells are resistant to cytotoxic conditions, divide into the manifold, and create endless copies, characterizing clinically relevant CRC development [8]. Nowadays, the prevalence of CRC is increasing even in historically low-risk nations, including Korea, Japan, China, and Eastern Europe. A high-frequency rate of CRC has been reported in these topographical areas, which is due to the outcomes of western diets, microbiota alterations in the gut, and cancer-causing dietary components [2,9]. Being overweight and obesity are also recognized risk factors of CRC. High consumption of red meat and reduced intake of fruits and vegetables are additional key factors to the increase f the menace of CRC [10].
To alleviate the effects of CRC and understanding the colon CSCs proliferation, there is an urgent requirement to develop an innovative and safer drug for treating CRC and preventing CSCs growth. Recently, diet-derived phytochemicals or bioactive compounds have the potential to reduce the effects of CRC that upsurge many interests among researchers [7,11]. Recently, the impact of phytochemicals in decreasing the risk of CRC and the connection with CSCs are well-documented in the literature [12,13,14]. The actions of bioactive compounds are varied depending on distinct chemicals by targeting diverse pathways and beneficial to human health. Various preclinical investigations have been examined related to anti-cancer activities of phytocompounds in CRC models. The results suggest several novel compounds such as apigenin, betanin, α, and β-carotene, diallyl sulfide, ethyl gallate, gallic acid, resveratrol, quercetin, luteolin, silymarin [15,16]. These compounds are harmless and can be employed in synergistic treatment to decrease cancer cell growth via chemotherapeutic mediators [15].
The microbiome is comprised of the main inhabitants in the human gut, comprising of 100 trillion microbes with diverse actions that maintain the integrity of a healthy colon [17]. Undigested dietary residues in the colonic lumen are the prime energy sources for the gut microbiota, which digest those dietary residues, resulting in the formation of several active metabolites with favorable functions. Imbalance of gut microbiota or dysbiosis can lead to several pathologies, including infectious diseases, gastrointestinal cancers, inflammatory bowel disease, and even obesity and diabetes. Dysbiosis may cause chronic inflammation, recognized as one of the prime causes of CRC. Earlier, our publications have also summarized the functions of gut microbiota, particularly, short-chain fatty acid synthesis with their benefits to the hosts in regulating various diseases such as diabetes, cardiovascular diseases, and cancer [18,19,20]. Dietary interventions or the consumption of phytochemicals is the beneficial component, which has been proved as effective in treating CRC [21,22,23,24,25,26,27,28]. Taking this into account, we aimed to review in-depth analysis of various diet-derived phytochemicals mediating the gut microbiome and its role in CRC prevention and treatment. In addition, we intend to review the dietary phytochemical interventions targeting colon CSCs on CRC prevention.
2. Diet-Derived Phytochemicals Modulate the Gut Microbiome
Earlier studies suggested the gut microbiota (Bacteroides fragilis, Escherichia coli strain NC101, Desulfovibrio, Helicobacter hepaticus, Clostridium ramosum, Fusobacterium, Campylobacter, Prevotella, etc.) in humans play a significant role and alter the immune function through pro-carcinogenic markers resulting in the etiology of CRC [20]. Altering the immune system in the gut normally enhances tumor microhabitats, and inflammation, ensuing the CRC development [19]. In recent research has also recommended genetically reformed colon bacteria, which are beneficial and are currently employed in experimental cases that outcomes are promising [29]. Furthermore, they can be greatly beneficial to the host as probiotics that inhibit CRC through alterations of microbiota and colon environment.
The consumption of natural products produces essential bioeffects in the body through multifaceted relations with gut microbiota [30,31]. Natural phytochemicals normally have fiber-rich glycosides that exist as complex molecules with the properties of lower bioavailability and lesser solubility [32]. The nature of the phytochemicals could be altered during microbial fermentation in the colon, ensuring high quantities of various byproducts with greater pharmacological activity [33]. Numerous metabolites that derived from gut microbiota may further be subject to various enzymatic cleavage by methylation, glucuronidation, glycination, or sulfation in the hepatocytes, which are then trafficked into the tissues and finally excreted into the gut [32,34]. Gut microbiota converts glucuronides to aglycones by β-glucuronidases, which can be immediately reabsorbed in the colon. Thus, the synthesis of microbial β-glucuronidase and its enterohepatic passage have possible steps to extend the holding period of phytochemicals in the host [32,34]. Rising data suggested the dietary phytometabolites derived from gut microbiota, which are capable of enhancing the bioavailability, antioxidant properties, detoxification of xenobiotics, and prebiotics function [34,35]. Furthermore, these compounds can eliminate gut pathogenic organisms, reduce oxidative DNA damage and pro-inflammatory mediators, and thus regulate normal cell division and apoptosis [36,37]. The effects of phytochemicals on gut microbiota and their anti-inflammatory effects are presented in Table 1.

Table 1.
Effects of phytochemicals on gut microbiota and their anti-inflammatory effects.
2.1. Polyphenols
Polyphenols are one of the prime classes of chemicals in plants, extensively studied for their health-promoting properties [38,39,40]. Human diets contain varieties of polyphenols and have significant protective activities against various cancer types. Scavenging of free radicals and reducing oxidative stress are the key mechanisms by which a polyphenol can achieve [38]. Several studies confirmed the actions of polyphenols on CRC inhibition, which often interconnected with the relationship of gut microbiota [41,42,43]. For instance, an animal study was conducted related to cranberry polyphenols on Akkermansia (mucin-degrading bacterium), which protected the host from obesity, diabetes, and gut inflammation. In this study, the mice were administered with high fat and sugar diet and cranberry extract (CE) (200 mg/kg/day) for eight weeks, and the various gut microbiota were analyzed by the methods of 16S rRNA and 454 pyrosequencing. The outcomes of the study revealed the administration of CE reduced body weight, visceral fat obesity, triglyceride accumulation, and inflammation, and elevated antioxidant properties and insulin sensitivity. Furthermore, the metagenomics study of CE treatment exhibited an increased percentage of Akkermansia [42].
The anti-carcinogenic properties of the gut microbiota are generally attributed based on the two properties, (a) either by improving the host’s immune system or (b) by generating the metabolites, which can interfere with the pathways involving CRC formation. A study demonstrated that the presence of amines, bile acids, and high consumption of meat can reduce some bacterial growth such as Clostridium, which inhibits the development of CRC [43]. By using the alimentary metabolites, gut microbiota produces biologically active short-chain fatty acids. The Rosburia faecis and Eubacterium rectale group of bacteria can normally produce the butyrate, which involves reducing cell apoptosis and diversity [41]. A study showed the polyphenol metabolites modulated microbiota that directly restricted the growth/proliferation of CRC [44]. Another study has also related intestinal metabolites, quercetin, chlorogenic, and caffeic acids to interfering in cyclooxygenase-2 expression resulting in the prevention of DNA damage in the colon [45]. The polyphenols-mediated gut microbiota changes are a potential technique for inhibiting colon cancer, although insufficient trials have been piloted, in which, wine [46], blueberry [47], and cocoa [48] displayed a bifidogenic outcome.
2.2. Flavonoids
Flavonoids are mainly present in fruits, vegetables, seeds, and various beverages such as tea, coffee, and red wine. Several medicinal herbs are amongst the richest sources of flavonoids. They are grouped into the following sub-classes-flavonols (quercetin, rutin), flavanols (catechin, epicatechin, and epigallocatechin), flavones (luteolin, apigenin), anthocyanidins (malvidin, cyanidin, and delphinidin), isoflavones (daidzein, genistein, glycetin, and formanantine), and flavanones (naringenin, hesperetin) [70,71]. A hypothesis stated that the presence of beneficial phytochemicals in diets attributes an anticancer property to the respective food. The flavonoids present in the food prevent CRC development by exerting various mechanisms: alleviating DNA damage, reducing the effects of gene mutation, regulation of phase I, and phase II enzymes via modulation in cell signaling pathways, suppressing oncogene expression, and regulating inflammatory responses [72,73,74,75,76]. In a recent clinical trial, a flavonoid mixture of 20 mg apigenin along with 20 mg epigallocatechin gallate was given to CRC patients daily for long-term interventions that showed the reduction of CRC relapse [77]. The greater quantities of polymeric flavonoids and the non-absorbed flavonoids passed into the colon region where they underwent breakdown and gut microbiota facilitate converting these flavonoids into simple phenolic acids [78].
The digestion of flavonoids is often mediated by gut microbiota, which is a similar pattern to other phytochemicals. Gut microbiota facilitates converting a large group of flavonoids into simple active metabolites (aromatic catabolites and small phenolic acids) by oxidation and demethylation [14,79]. These active products augment physiological activity and perform various roles in the regulation of the host’s immune system. One best instance for the gut microbiota-mediated metabolite is daidzein-isoflavones, which serves various benefits to the host. Daidzein is found in numerous plants and predominantly occurs in soybeans; daidzein is transformed by bacterial flora into the most active compound equol. In vitro and clinical trials showed that equol is more bioactive than daidzein (food precursor), and the biological effect is significantly improved in patients who produced equol after isoflavone consumption [80]. This result strongly suggested that gut microbiota aid a pivotal function in regulating the biological effects of ingested phytochemicals.
We recognize that the impacts of the gut bacterium on the flavonoids and the effects of flavonoids on the gut microbiota are bidirectional. Flavonoids can change the organization and roles of gut microbiota, and similarly, gut microbiota can enhance the flavonoid breakdown. A case pilot study with 178 elderly people showed the habitual diet, which contributed to bacterial alterations resulted in the improvement of frailty and inflammation [81]. Another fascinating study revealed that 15 women with a two-month dietary intervention connected to alterations of gut microbiota including, Gammaproteobacteria and Erysipelotrichi [82]. A study on the impacts of grape extract (GE) on experimental animals showed the reduction of the Firmicutes-to-Bacteroidetes ratio and an increasing of Akkermansia muciniphila. Supplementation of GE along with gut microbiota significantly reduced inflammatory response and improved insulin sensitivity. These findings offered noteworthy support in favor of colonic bacteria and their substantial role in facilitating the flavonoids on health impacts, which reduced inflammatory response as well as improved the metabolic function. Another interesting clinical study demonstrated that the feeding stable isotope-labeled anthocyanins were ingested by gut microbiota, which yielded high quantities of diverse active metabolites [17,83]. These colonic bioactive phytometabolites exert greater anti-inflammatory functions and maintain vascular integrity when compared to the normal colonic metabolites [84]. This statement complements the belief of the effect of increased activities of phytochemicals on host health, which are the utmost prospective study related to gut microbiota.
3. Colon CSCs and their Tumorigenic Effects
Over the last decade, the development CSC model has progressively recognized as an account for cancer propagation and recurrent. The CSC model was initially established for hematological malignancy and in recent years, many investigators validated it for other solid tumors, including colon CSC [85,86,87]. This model proposed a salient feature of the CSCs: minor populace of colonic cells, greater strength, capacity to recruit distinct metastases, capable of self-renewal, becoming metastatic heterogeneous tumors, and more resistant to various therapies [85]. During an asymmetric division, these multipotent cells generate populace cells without any control measures contributing to tumorigenesis. Loss of cell replicative control usually leads to an increased count of cells like embryonic stem cells that lead to tumor growth [87]. These stem cells and their offspring can harbor an astonishing number of inconsistent cells based on the DNA mutations, which may contribute heterogeneous tumors and carcinogenesis [88].
Colon cancer primarily increases through abnormal directions of the Wnt/β-catenin pathway, either activating mutations in β-catenin or disabling mutations in the β-catenin regulator, adenomatous polyposis coli (APC). This mechanism provides irregular deposition and stimulation of a β-catenin/transcription factor T-cell factor 4 (Tcf4) in the nucleus, which targets c-MYC resulting in the prevention of p21CIP1/WAF1 expression [86]. Notch and Hedgehog (Hh) pathways have also presented to be intricate in the maintenance of the self-renewal in either a normal stem cell or colon CSC [87]. The Wnt pathway contributes to CSC proliferation through the prevention of GSK-3β, phosphorylation of β-catenin, endorses its translocation to the nucleus, and activates Tcf4 [89]. Animal trials have also confirmed that activated β-catenin spread to the cell and become malignant [90].
Various researches confirmed that the intestinal markers contributed to characterizing and distinguishing normal colon stem cells from colon CSC [91,92]. Normal colon stem cells are identified by various markers such as Msi-1, Hes1, integrins α2, and β1 subunits, EphB receptors, Bmi-1, Lgr5, and Aldh1, whereas colon CSC is recognized by CD44, CDD133, CD166, CD34, CD24, ESA, LGR5, CD29, nuclear β–catenin, EpCAM, CD49f and Aldh1 [91,92,93]. Colon CSC markers are often used as prognostic indicators that help eliminate colon CSCs. The list of the disease model, markers, and the mechanism associated with the findings presented in Table 2. Several genes and their multiple signaling pathways have been identified in normal and colon CSC. Inconsistency of these cellular signaling triggers anomalous transformation, tumorigenesis, resulting in cancer. The major pathways, Notch, Hh, and Wnt/β-catenin participate in the maintenance of the self- renewal of both SCs and CSCs, where Hh is a glycoprotein, involved in the pro-survival pathways; Notch and Wnt/β-catenin involve in the self-renewal [89].

Table 2.
Tumorigenic effects of colon cancer stem cells (CSCs).
4. Effect of Diet-Derived Phytochemicals on the CSCs
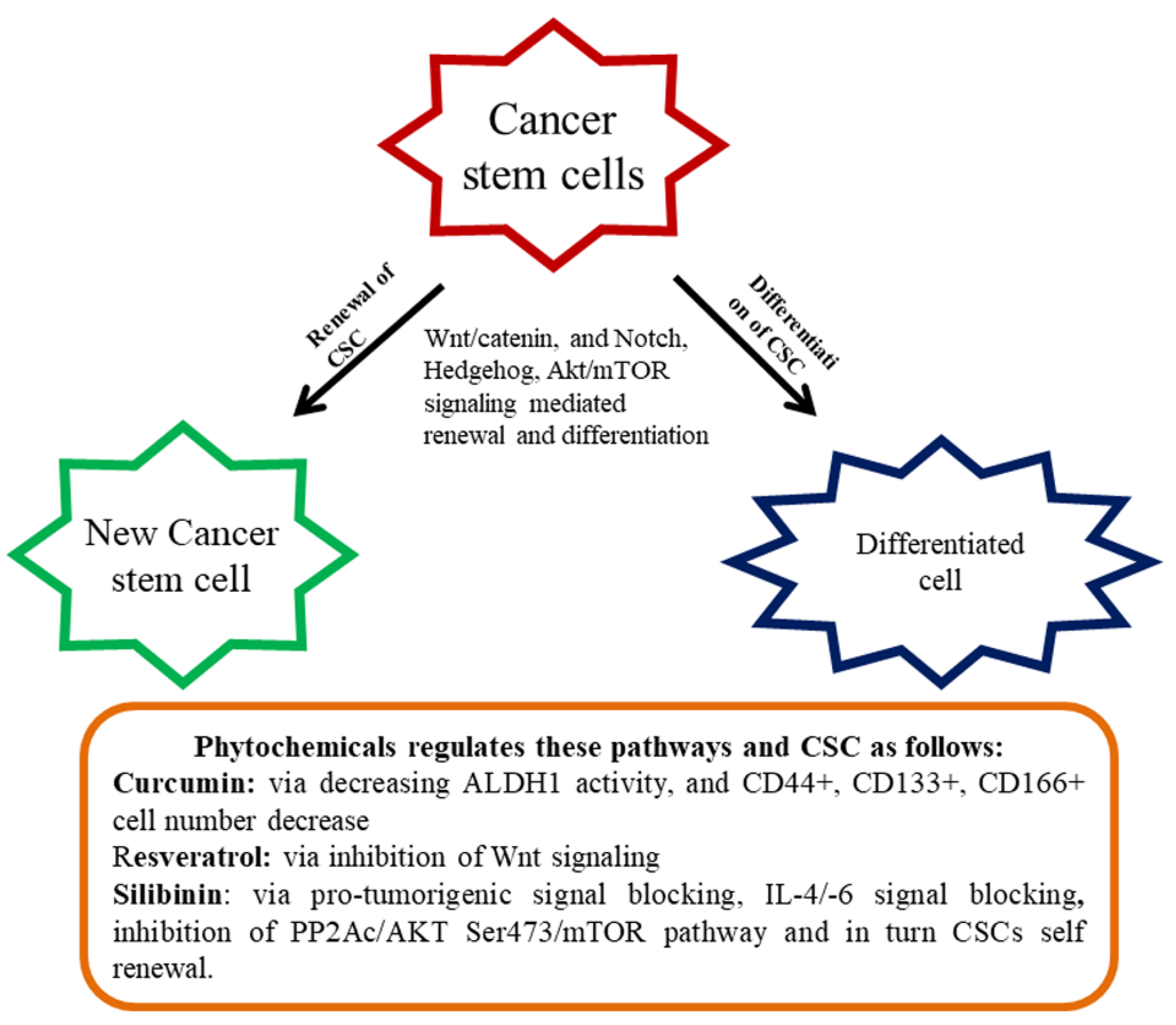
Signal transduction pathways, namely, Hh, Wnt/β-catenin, and Notch, contribute to a variety of usual stem cells and provide striking strategies to CSC [101,102]. Irregular cascade signaling of Wnt/β-catenin causes the majority of malignancy in most individuals [103]. Preclinical investigations have been undertaken to find small molecules, which are capable of distracting the pathway of Wnt/β-catenin [104,105]. Based on the findings, monoclonal antibodies and siRNA are promising blockers against the Wnt1/2 pathway [104,105]. However, targeting Wnt1/2 is still a primitive stage and no beneficial mediators have yet been permitted for patient practice until today [106]. Numerous bioactive chemicals have been studied in inhibiting the above-stated pathways. For example, Corn lily-derived cyclopamine that targeted hedgehog signaling [107]. Epigallocatechin gallate (EGCG) inhibited Wnt/β-catenin signaling and was found to influence CSC self-renewal and invasive abilities [108,109]. Retinoic acid is an active molecule derived from vitamin A, can downregulate the Notch signaling, and differentiate CSCs or reduce their development [110]. The Akt/mTOR signaling pathway is one of the significant pathways intricate in the CSC. This CSC existence and invasion of the stimulation of Akt/mTOR is very decisive. Declining motility and apoptosis commencement of CSC occurs repetitively, owing to Akt deterrence [35] (Figure 1).
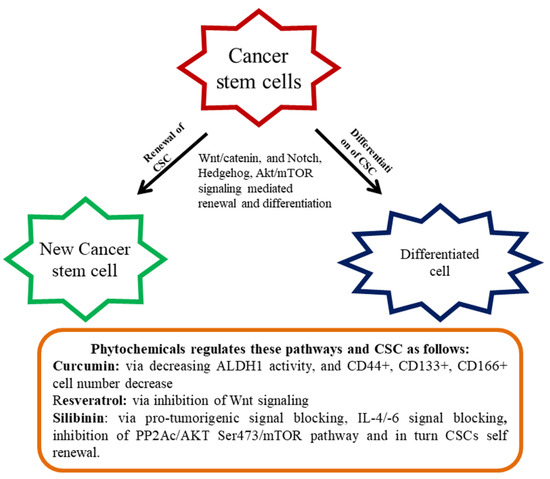
Figure 1.
Renewal and differentiation of cancer stem cells (CSC). Diet-derived phytochemicals generally attenuate various signaling mediated renewal and differentiation and thereby regulate CSC proliferation.
The anti-cancer effect of polyphenols is normally achieved by the inhibition of tumor cell proliferation, and stimulation of caspase-3-dependent apoptosis via the Akt/mTOR pathway [111]. An assortment of the investigation suggested that polyphenols and flavonoids can affect various CSCs and inhibit proliferation and thus the outcomes exhibited phytochemicals are promising anti-cancer agents targeting CSCs [112]. There are several colon CSCs markers with varying functions comprising a cluster of differentiation 44 (CD44, a receptor of hyaluronic acid), CD133 (unidentified), CD166 (fixative substances), and aldehyde dehydrogenase-1 (Aldh-1 an enzyme). In tumors, CD133 is recognized as a colon cancer-originating cell. The markers of CD166 along with CD4435 or CD24/CD29 identified the populace of colorectal CSC. Aldh1 is also accepted as a novel indicator of CSCs in humans. Curcumin contributed to the control of colon CSCs and standardized the several markers of CRC stem cells. It reduced Aldh1, CD44+, CD133+, CD166+ cell numbers, and enhanced apoptosis in tumors [113]. In another study, curcumin-enhanced G2/M phase arrested and downregulated β-catenin expression [114].
An interesting study on the Sasa quelpaertensis extract (SQE) showed the induction of CSC variation and inhibited Wnt signaling. SQE contains high quantities of polyphenol, including p-coumaric acid and tricin that inhibited the renewal and differentiation of CSC [115]. In this study associated with colon, HCT116, and HT29 CSCs were labeled with respective markers (CD133+ and CD44+) and introduced into the nude mice to develop the CRC. The nude mice were administered with the SQE extract (300 mg/kg b.w) that reduced signaling of CSC marker expression and Wnt/β-catenin, as well as the hypoxia-inducible factor-1α [115]. Resveratrol, a renowned phytochemical present in several dietary sources inhibited the effect on colon CSCs through the hindrance of Wnt signaling [116]. Ellagic acid is an active principle of walnut displayed to inhibit CRC by regulating the colon CSCs [117]. Silibinin is another imperative phytochemical revealed to regulate colon CSCs via blocking of pro-tumorigenic signaling, including, IL-4/IL-6 [118]. By overwhelming the PP2Ac/AKT Ser473/ mTOR pathway, silibinin impeded colon CSCs self-renewal [92]. In an interesting study connected to the colon CSC, cinnamic acid found to reduce the CSC markers connected with HT-29 colon cancer cells [119].
5. The Anti-Tumorigenic Potential of Phytochemicals through Various Molecular Goals in Colon CSC
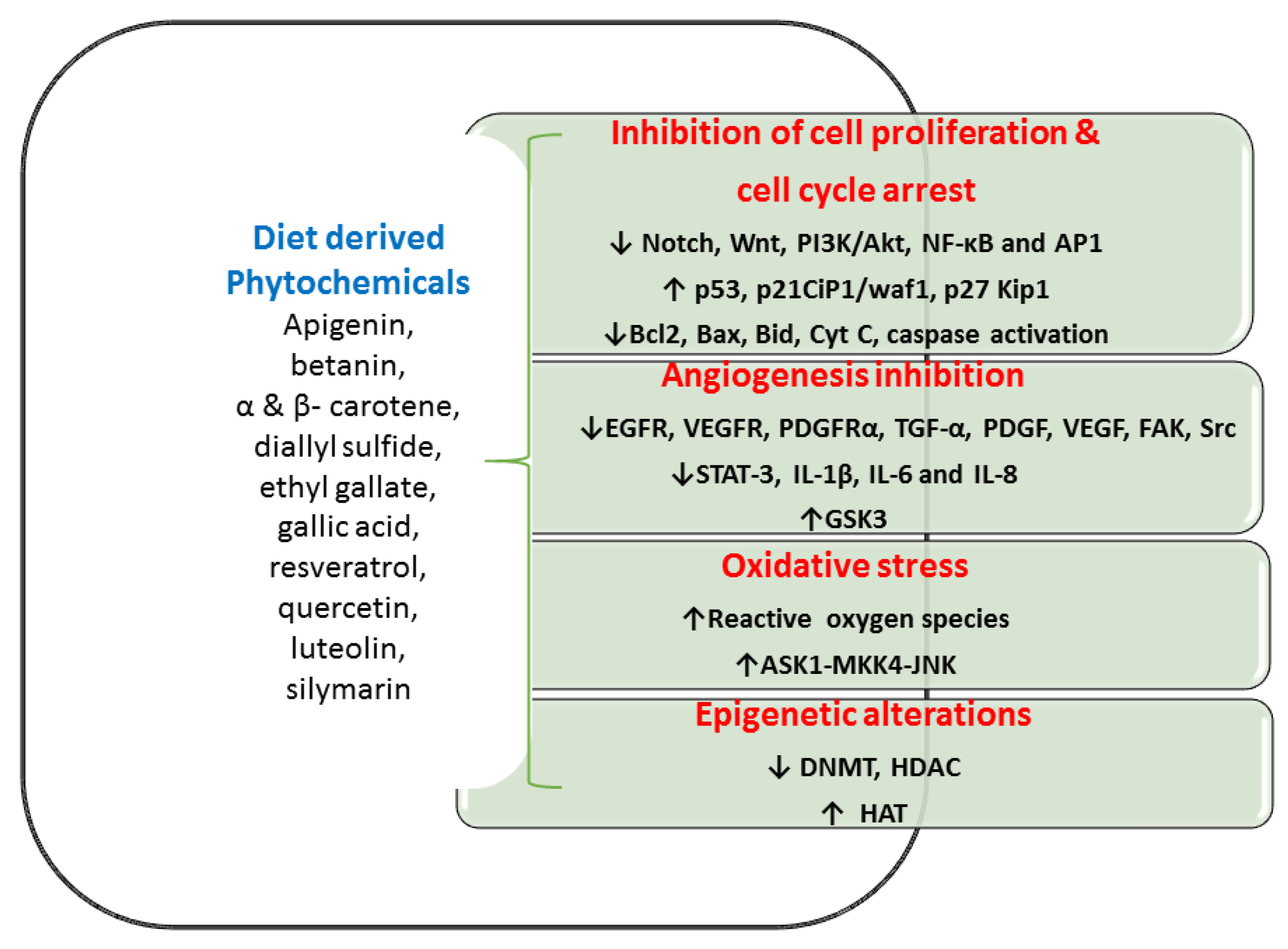
Globally, diet-derived phytochemicals lead to reduced CRC incidences. For incidence, Mediterranean people generally have a low prevalence of CRC, because of the high consumption of olive oil and tomato [120]. Both olive oil and tomato have phytochemical-rich dietary materials that can reduce CRC in Mediterranean individuals [120]. Various in vitro and in vivo studies showed that phytochemicals inhibit cell propagation, differentiation, angiogenesis, and anti-apoptotic activities in the colon (Figure 2 and Table 3). These diet-derived phytochemicals offered a significant success rate in numerous medical trials of CRC individuals [121,122,123]. A beneficial efficacy of diet-derived phytochemicals in the CRC management especially targeting of CSCs increases greater interests among researchers [124,125]. Antitumor effects of diet-derived phytochemicals are presented via four molecular targets as below.
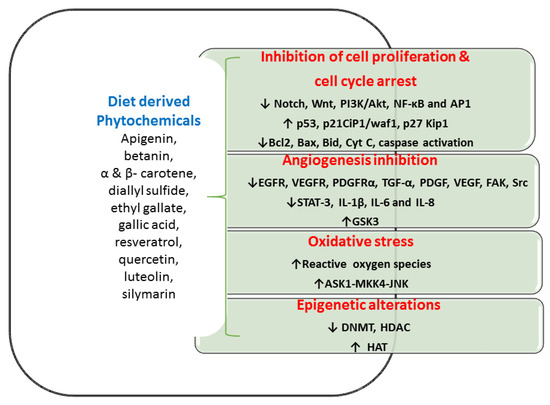
Figure 2.
Various in vitro and in vivo studies showed the phytochemicals inhibit cell propagation, differentiation, angiogenesis, and anti-apoptotic activities in the colon. Abbreviation: Akt- serine/threonine-specific protein kinase; AP1—Activator protein 1; ASK1—apoptosis signal-regulating kinase 1; Bax—bcl-2-like protein; Bcl 2-B-cell lymphoma 2; Bid—BH3 Interacting Domain Death Agonist; CIP1/waf1—cyclin-dependent kinase inhibitor 1; Cyt C—cytochrome C; DNMT—DNA methyltransferase; EGFR—epidermal growth factor receptor; FAK—Focal adhesion kinase; GSK3- glycogen synthase kinase-3; HAT—histone acetyltransferases; HDAC—histone deacetylase; IL- interleukin; JNK—c-Jun N-terminal kinases; Kip1—kinesin-like protein1; MKK4—mitogen-activated protein kinase kinase 4; NF-κB—nuclear factor kappa-B; PDGF—platelet-derived growth factor; PDGFRα—platelet-derived growth factor receptor A; PI3K—Phosphoinositide 3-kinases; SrC—protooncogene c; STAT3—signal transducer and activator of transcription 3; TGFα—Transforming Growth Factor-alpha; VEGF—vascular endothelial growth factor; VEGFR—vascular endothelial growth factor receptor.

Table 3.
List of phytochemicals and their anti-tumorigenic effect on colon CSC.
5.1. Inhibition of Cell Multiplication and Cell Cycle Progression
Colon CSCs have the ability of proliferation and metastatic effect with atypical maintenance of numerous signaling pathways, accountable for malignancy. Diet-derived phytocompounds that are connected to multiple signalings, such as PI3K/Akt, Hh, Wnt, and Notch could be beneficial healing approaches in managing CSCs induced malignancy. The unusual stimulus of NF-κB signaling normally accelerates malignant cell proliferation that averts apoptosis [130]. Phytocompounds contribute to the initiation of this apoptosis, prevent cell division with cell cycle growth, and hence phytocompounds are a great attractive drug candidature for tumor therapy. Various cancer models connected with phytochemicals that have established with the upregulation of proapoptotic proteins (Bax, and Cyt C), triggers caspase cascade, and cleavage of poly (ADP-Ribose) polymerase and thus regulates cancer development [105,131]. Diet-derived phytochemicals such as curcumin, EGCG, and lycopene demonstrated an ability to increase apoptosis via induction of p53-dependent Bax, upregulating p21waf1/Cip1, and p27Kip1 CDK inhibitors and thus repressed the normal cell cycle [132,133].
Likewise, isothiocyanates exhibited a reduction in the incidence of CRC through elevated apoptosis, cessation of the cell cycle, and self-renewal of CSCs [15]. Curcumin, gingerol, EGCG, and resveratrol inhibited the signaling of Notch, Wnt signaling, β-catenin/TCF transcription as well as targets to avert CSC self-renewal [134,135]. Sulforaphane is generally acquired from broccoli, which is effective in preventing colon CSCs proliferation through modulation of multiple signaling pathways, comprising PI3K-Akt, NF-κB, Hh, Wnt/β-catenin [136,137].
5.2. Inhibition of Angiogenesis Mechanism
Angiogenesis supports CRC initiation, development, and metastasis and its suppression provides a fascinating strategy for the treatment of CRC. Diet-derived phytochemicals reduce angiogenesis through several pathways. Curcumin gingerol, and EGCG inhibited Wnt signaling with various receptors of the epidermal growth factor (EGFR), vascular endothelial growth factors (VEGFR-1, VEGFR-2, and VEGFR-3) and downregulated IL-1β, IL-6, and IL-8 and thus these compounds inhibited chemoresistance, angiogenesis, and invasion [138]. Experiments validated that dose-dependent manners of curcumin prevented interleukin from the gut and inhibited angiogenesis and CSCs stimulation [138]. EGCG impeded angiogenesis and growth of the tumors through the activation of receptors of EGFR and platelet-derived growth factor receptor-α (PDGFRα) [139]. Studies established that capsaicin inhibited CRC-provoked angiogenesis through the reduction of the STAT-3 facilitated downstream mechanism [140]. Isoflavones also suppressed Wnt signaling by augmenting glycogen synthase kinase expression, fixes with β-catenin resulting in elevated phosphorylation, and successively decreased CRC development [141].
5.3. Oxidative Stress and Anti-Tumorigenic Effect
Investigators established that CSCs in many tumor cells contain a negligible concentration of reactive oxygen species (ROS) and these quantities are dynamic for preserving normal stem cell functions [142]. These ROS conservations in normal cells as well as CSCs are greatly important. The beneficial outcome of elevated ROS eradicates CSCs, which can be one of the vital goals for CRC treatment. Hence, the increased ROS plays as “double-edged sword”, which is not only an illness maker but also as a missile in tumor treatment. Curcumin has contradictory roles in hunting and creating ROS, and however, the consumption of dietary curcumin possesses a potential anticancer activity. Curcumin-induced ROS generation and their oxidative stress that largely induced cell apoptosis in HT29 cell lines through the activation of signaling cascade ASK1-MKK4-JNK [143]. Studies revealed the twin function of lycopene as a ROS scavenger and creator based on its dose-dependent manner. Ribeiro et al. [144] established oxidative stress in the HT29 cell line, resulting in functional DNA impairment, which was greatly secured by lycopene (1-3 μM); however, DNA damage is amplified while lycopene treatment in higher concentrations (4-10 μM). Capsaicin-stimulated apoptosis in human CRC cell lines, which is connected with an upsurge production of ROS and disruption of membrane potential in mitochondria [145].
5.4. Epigenetic Alterations
There are three enzymes viz., DNA methyltransferases (DNMTs), histone acetyltransferases (HATs), and histone deacetylases (HDACs) play an energetic function in chromatin organization and direction of transcription. HAT activity is connected to dynamic chromatin in transcription, while, DNMTs and HDACs induce silencing of the gene. The disparity of DNA methylation and histone acetylation/deacetylation often contributes to cancer. Multiple signaling pathways in CRC comprise Wnt/β-catenin, Hh, Notch, and TGF-β/BMP provide self-renewal, and variation in stem cells that are regularly modulated by epigenetic mechanisms. The mechanism of HDAC inhibition is an extensive platform of anti-tumorigenic effects comprising cell cycle arrest, apoptosis, and cell differentiation that have fascinated new consideration as possible anticancer candidates. Various researchers have recommended that curcumin, phenyl isothiocyanate, EGCG have anti-tumorigenic properties that are possibly mediated through an epigenetic mechanism by DNMTs and HATs inhibition [146,147].
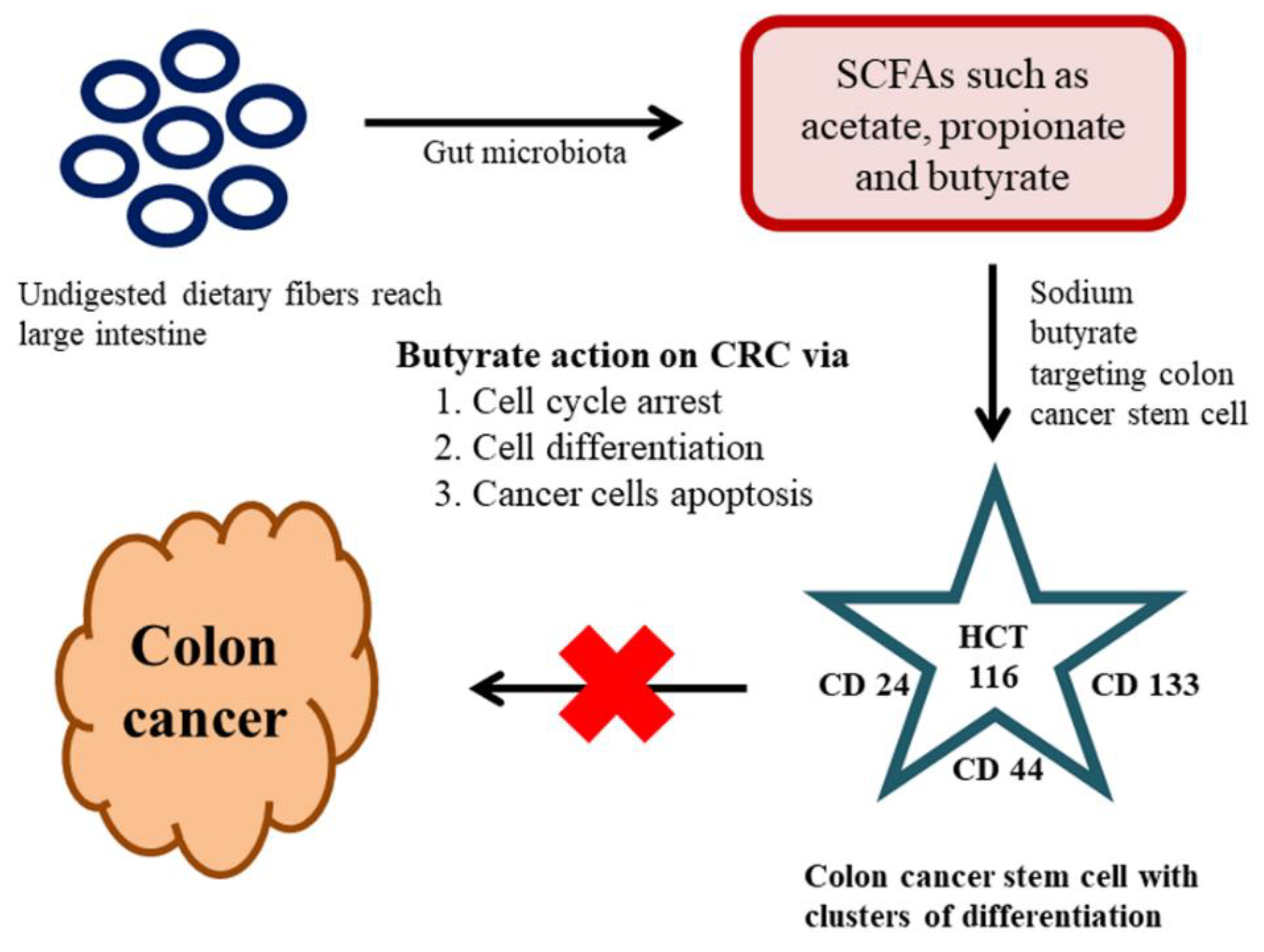
6. Effect of the Gut Microbiome on Colon CSCs and CRC
The key factors contributing to CRC are colon CSCs and diet, which is a renowned and significant environmental factor connected to CRC. The metabolites from gut microbiota have the potential to be either tumorigenic or anti-tumorigenic agents. Intestinal microbiota produces short-chain fatty acids (SCFAs) from the dietary fibers through fermentation in the host. SCFAs are aliphatic carbon-based acids, in which the most abundant SCFAs are acetate (C2), propionate (C3), and butyrate (C4) [148]. These SCFAs are shown to exert numerous beneficial effects on the host’s energy metabolism. The dietary fibers reach into the large intestine without undergoing any course of digestion; the reason is owing to the absence of dietary digestive enzymes in the upper intestinal tract. The gut microbiota present in the large intestine is accountable for the breakdown of these dietary fibers into active metabolites [149]. One noteworthy beneficial effect of SCFAs with over the host immune system is butyrate. Butyrate is a metabolic product of dietary fiber and resistant starch by the bacterial action (Faecalibacterium prausnitzii and Eubacterium rectale) in the colonic lumen. The literature claimed that the butyrate can induce G1 phase-cell cycle arrest, cell differentiation, and apoptosis in CRC [150]. As we stated earlier only a small populace of cells is accountable for the initial generation of malignancy cells and referred to as CSCs. The method of traditional malignancy therapy is proven ineffective against these CSCs. The CSC holds a specific cluster of differentiation (CD) markers on their surface, hence targeting those CD markers may be the best way to target the CSCs [151]. In a recent study on the effects of butyrate on the colon CSCs, sodium butyrate (NaB) employed in CRC stem cells (human) type HCT116. The investigation was carried out by analyzing the expression profiles of a definite marker for CRC stem cells, including CD24, CD133, and CD44. These results revealed that the SCFA-NaB had variable impacts on HCT116 stem cells (CD24, CD44, and CD133). The results still varied bestowing on the concentration of NaB and incubation time. Overall, this study offers some interesting information on NaB and whether it is possible to develop it as a novel therapeutic drug targeting cancer stem cells [152] (Figure 3).
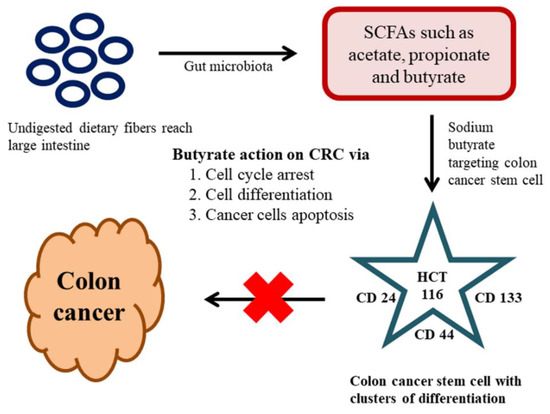
Figure 3.
Effect of the gut microbiome on colon cancer stemm cells (CSCs) and colorectal cancer (CRC).
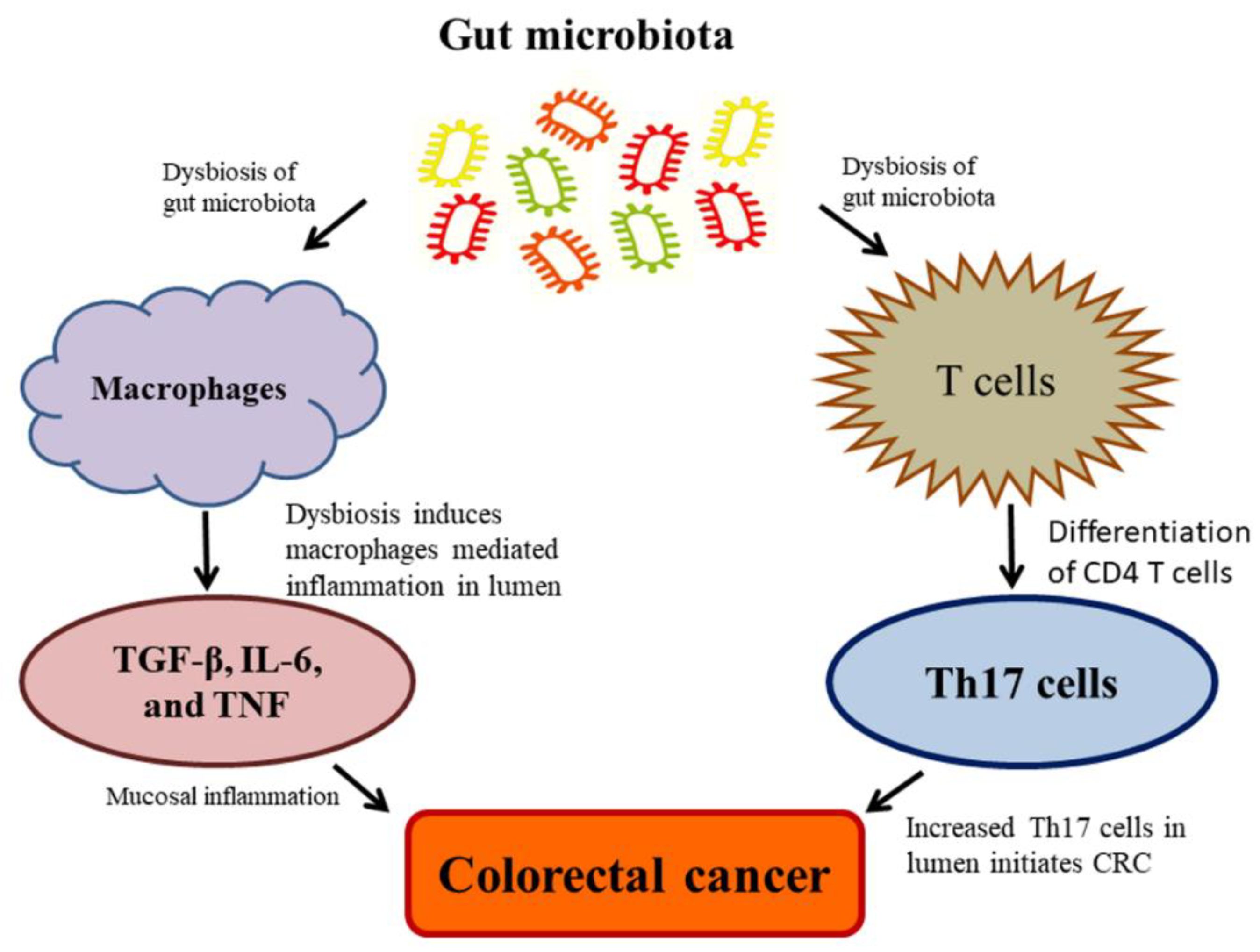
Currently, the studies on the complexity of microbiota associated with CRC revealed that microbiota unconditionally influences CRC at high risk due to the range and complexity of the gut microbiota. Despite the contemporary debate, regarding whether alterations in the microbiota give rise to colon carcinogenesis, in which, some noteworthy explanations have been made to recommend a causative function of the gut microbiota in the CRC. The rodent model investigations employed as natural, chemically-induced, or genetically predisposed CRC, which revealed the enhancing tumorigenic properties of microbiota and their effects on the development of CRC [97,100]. These tumorigenicity effects are attributed to inflammation, which plays as a cancer inducer in animals. Dysbiosis has also detected during the exposure of subsequent radiation in animals, which represented the vulnerability of the microbiota; additionally, dysbiosis may play as a pilot and facilitate the formation of CRC [153] (Figure 4). Eubiosis is referred to as the balance microbiome status maintaining healthy human body conditions [154]. Upon the impaired eubiosis, macrophages generally produce TGF-β, IL-6, and TNF; and T cells produce the pro-inflammatory Th17 cells by the differentiation of CD4 T cells; and thus, cause an adaptive immune response. The Th17 cell is abundant in the mucosal inflammation, which leads to CRC development [155]. The commensal bacteria, Clostridia species, can promote the overproduction of Th17 cells, leads to increased IL-17 generation in the epithelial cells. It is well established that Th17 acted as a driving force for the initiation of CRC Min-mouse models exposing the animals to enterotoxigenic Bacteroides fragilis [156] (Figure 4). These findings suggest that the inflammatory process plays a pivotal role among the gut microbiota and CRC. The pathogenic bacteria stimulate cancer formation through diverse mechanisms, including (a) dysbiosis and inflammation induced by a microorganism-associated molecular pattern (MAMP) triggering toll-like receptor (TLR) and additional pattern recognition receptors (PRR); (b) detrimental effects are intervened by bacterial toxins such as colibactin and CDT, and (c) acetaldehydes and nitrosamines by activating toxins through metabolic activities [157].
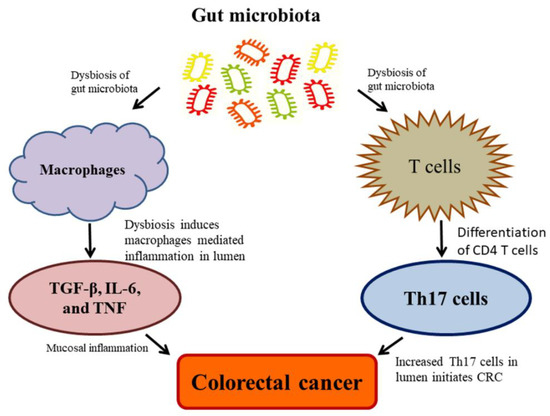
Figure 4.
Dysbiosis of gut microbiota causes a high risk of colorectal cancer (CRC).
Nevertheless, the stimulus of the innate immunity achieved by toll-like receptor and agonists of the NOD-like receptor has been established as possible innate immunity and increase of anti-tumor activity [158]. Taking this into account, these explanations recommend an ambiguous role of the gut microbiota in carcinogenesis that may be reliant on the grade and mechanism. Microbial diversity is considerably poorer in tumor tissues matched with noncancerous tissues, proposing that a more appropriate microhabitat occurs in the vicinity to gut tissue. In CRC patients, the higher abundances of Erysipelotrichaceae, Prevotellaceae, Coriobacteriaceae, Lactobacillales, Fusobacterium, Porphyromonas, Peptostreptococcus, Mogibacterium, Escherichia- Shigella, Prevotella and lower loads of Bifidobacterium, Faecalibacterium, Blautia, Staphylococcus, and Bacillus were found [156,157,158]. These observations recommend certain bacteria may well compete in the converted niche and disclose novel steps in which the microbiota influences CRC development.
Gut Microbiome Regulates Wnt/β-catenin Signaling Pathways
Adult CSC has the properties of self-renewal and targeting for cancer-originating mutation [159]. Elevated mutations in colon CSC ensuing the changes in variation/plasticity and site of the stem cell/propagation are the most represented primary sign for colon tumorigenesis [160]. The initiation of colon tumorigenesis is frequently determined by mutations in the Wnt signaling. Wnt is generally a secreted signaling protein. Conversely, the loss of function of adenomatous polyposis coli or gain of function of β-catenin causes the balance of unrestricted β-catenin that provides abnormal Wnt signaling leads to tumorigenesis [161]. Notably, mutation triggering of the Wnt pathway in G-protein-coupled receptor (Lgr5+) cells contributes to intestinal tumors with high competence compared to other colonic cell tumors [162]. According to the CSC hypothesis, the populace of colon cells can propagate tumor generation, measured as multipotent resulting in the cell of cancer [162,163]. Current data shows that multiple CSC hierarchies occur in the colon, facilitate cell fate in the account for various extrinsic factors including, diet, inflammation, and body anxiety [164]. Additionally, a function of diet in the maintenance of colon CSC has also described [165].
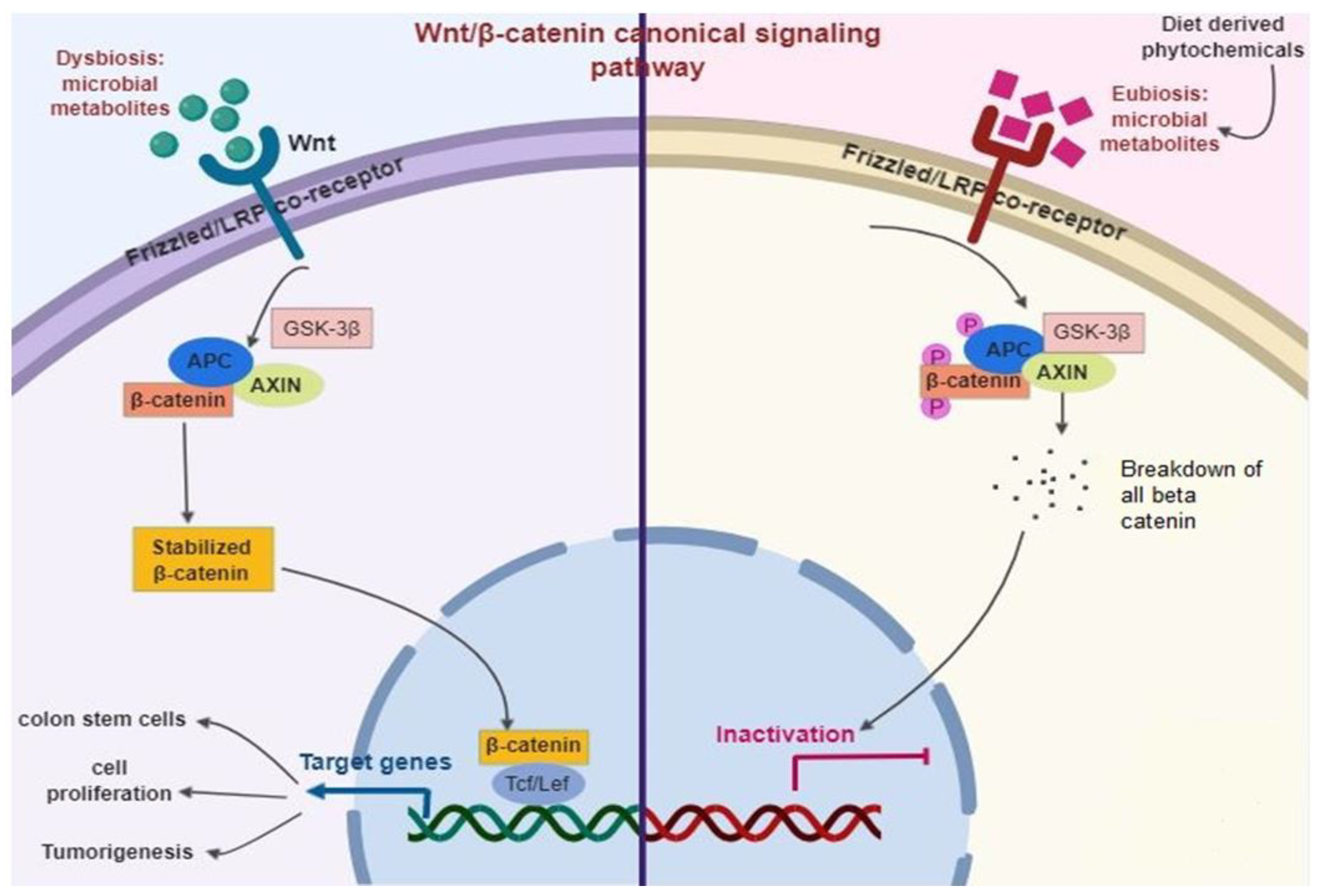
The Wnt signaling generally occurs in an upstream of the β-catenin pathway [166] (Figure 5). Briefly, Wnt ligands largely fix with the complex of the Frizzled/LRP co-receptor, which triggers the canonical pathway. Axin, a Wnt signaling inhibitor protein is employed to the cell membrane, resulting in the inactivation of the adenomatous polyposis coli complex succeeding in the equilibrium of β-catenin. When Wnt is triggered, β-catenin is instantly soothed, allowing transfer to the nucleus and fixes with T cell factor and eventually elicits the expression of target genes. Among them, Leucine-rich repeat-containing Lgr5+ genes participated in stem cell proliferation [167].
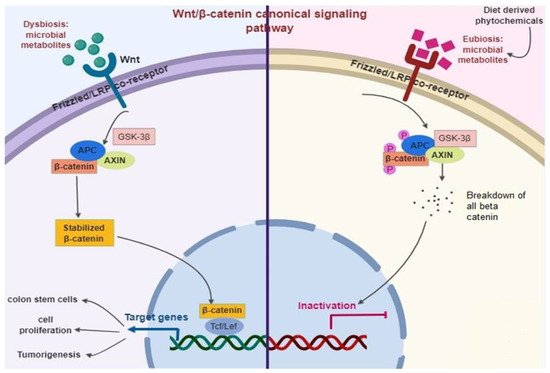
Figure 5.
Diet-derived phytochemicals stabilize the microbiome status (Eubiosis) that inhibits Wnt/β-catenin signaling pathways successively prevent intestinal infection and inflammation.
The adenomatous polyposis coli is normally a tumor-suppressor protein that is mutated in almost 80% of CRC. Thus, the stimulation of Wnt/β-catenin is a primary biomarker of colitis-related CRC [168]. Diet-derived phytochemicals balance the microbiome status (Eubiosis), which inhibits Wnt/β-catenin signaling pathways and successively prevent intestinal infection and inflammation [96,169].
7. The Triangular Relationship between Phytochemicals, Gut Microbiome, and CSCs
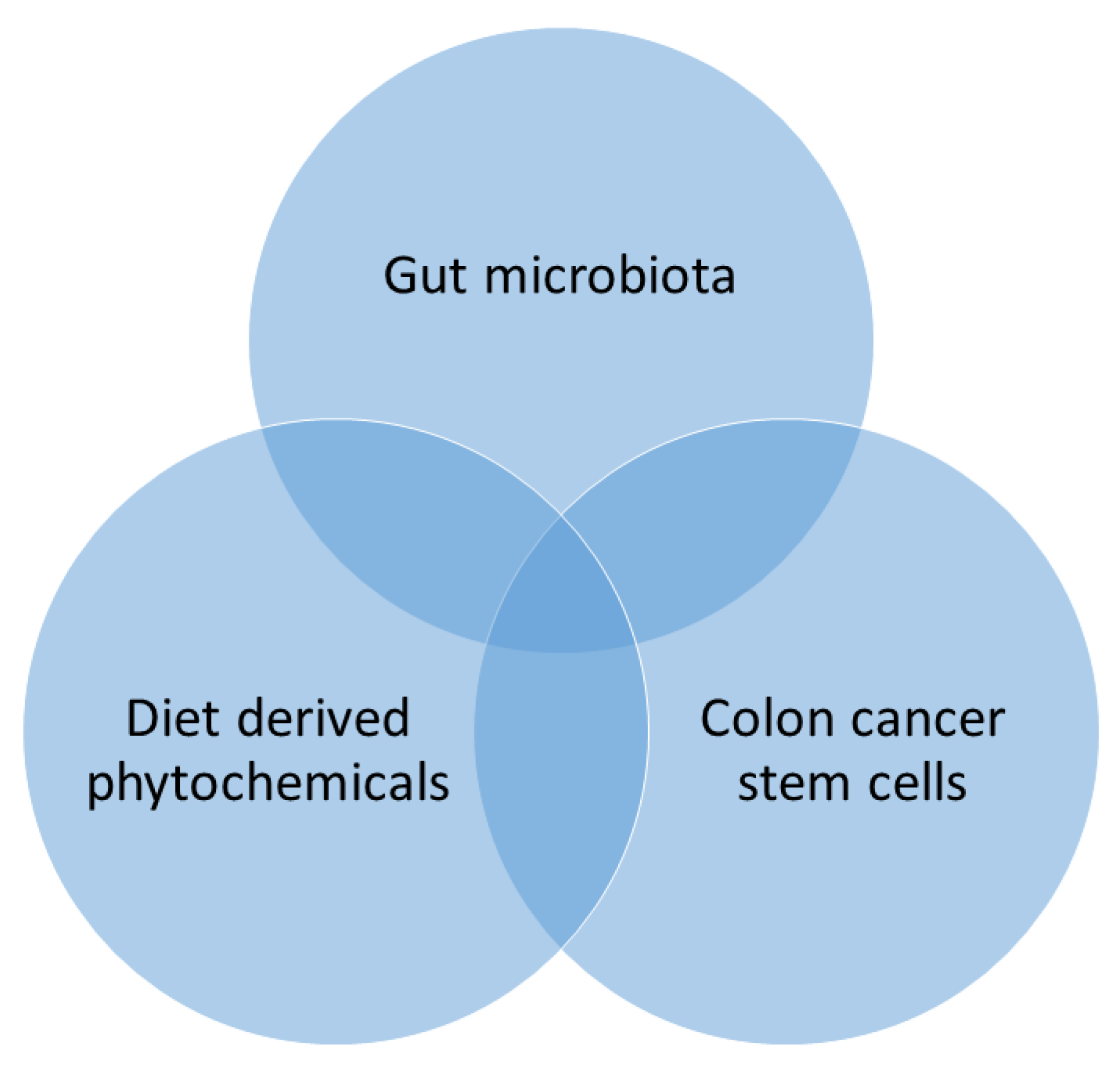
Gut microbiota is chiefly affected by the dietary phytochemicals that can disturb its physiological relations in the host [170]. Through their alimentary canal route, phytochemicals are digested by colonic bacteria and produce several by-products [171]. These phytochemicals are rich in various active principles comprising polyphenols and flavonoids that upsurge the Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria [30,171], which alters the pH of the colon environment and maintains the balance of the colonic microbiome [172]. Therefore, the effect of colonic bacteria on the dietary phytochemicals targeting dietary intervention which may contribute to host well-being [171]. The phytochemicals facilitate colonic bacteria, which may influence as adjuvants to treat cancer, obesity, diabetes, and chronic inflammatory diseases and prove as potentially prophylactics and candidates for the treatment of these diseases [30,171]. Furthermore, diet-derived phytochemical modulates colonic microbiota that targeting CSCs recognized as capable of decreasing the burden of CRC by triangle relationship (Figure 6).
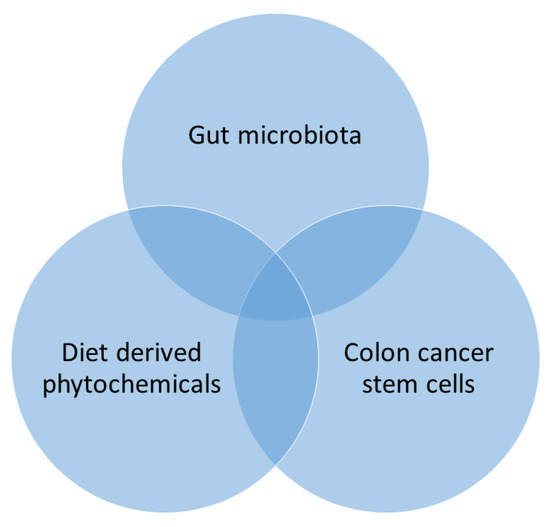
Figure 6.
Triangular relationship between phytochemicals, gut microbiome, and cancer stem cells (CSCs).
The accumulating data put forward to the etiology of CRC, which is linked through the actions of colonic bacteria not only because of the pro-carcinogenic actions of particular pathogens but also other bacterial communities, especially their metabolome [173]. The multipotent colon CSCs undergo self-renewal during the asymmetric cell division and produce a populace of transit magnifying cells in CRC [174]. These cells undergo migration, proliferation, and differentiation to produce mature tumors and cancer progenitors. Uncontrolled proliferation or cell division of CSCs can repopulate [175].
Several phytochemicals including cinnamic acid, curcumin, EGCG, lycopene, quercetin, resveratrol, silibinin have been described to interfere with various regulatory pathways in the preservation of CSCs or to modify the CSC phenotype [12]. Notch, Hedgehog, and Wnt/β-catenin signaling pathways are the central signaling pathways, and they are involving in the self-renewal and differentiation of CSCs [102]. Thus, synergistic activities are anticipated when the CSC-directing phytochemicals and modulating colonic bacteria. By considering the above facts, CSCs influence a significant function in the tumor formation, targeting various signaling pathways and involves in the cancer development that may gain much interest in the field of cancer prevention via phytochemicals modulated colonic microbiota [176]. It is clearly understood that diet-derived phytochemicals undergo various alteration in the colonic bacteria and vice versa, various phytochemicals could regulate the colonic CSCs has also found to modify the gut microbiota population through triangular rapport, which may benefit to the host in combating the CRC.
8. Conclusions
CRC remains a significant threat to human society. Several investigations have elucidated the actions of several phytochemicals on the colon carcinogenesis via regulating several pathways, new insights into the relationships among the phytochemicals and colonic bacteria seem interesting and promising. Phytochemicals are a concoction of various bioactive compounds directing various cell signaling pathways that altered gut microbiota composition. This may support to destroying malignant cells with minor risks of emerging drug resistance. Dietary phytochemicals, or bioactive compounds and their analogs offer the advance of better-quality drugs that may ultimately provide the resolution to eradicate CSCs. These bioactive compounds would reinforce gut microbiota and combat against dreaded CRC. Dietary phytochemical-induced gut microbiota continues to be an encouraging and dynamic research niche in the upcoming days with evident anti-tumorigenesis effects and goals of abolishing the CSCs; propose novel opportunities for CRC prevention and treatment.
Author Contributions
All authors conceived and designed the review; K.G. and M.J. wrote the paper and B.X. critically revised and improved the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study is jointly supported by two research grants R201714 and R201914 from Beijing Normal University-Hong Kong Baptist University United International College, Zhuhai, China.
Acknowledgments
The authors sincerely thank the faculty members and technical staff in the Food Science and Technology Programme, Beijing Normal University-Hong Kong Baptist University United International College, Zhuhai, China who provided a great arrangement of support and cooperation in the study.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
| Akt | serine/threonine-specific protein kinase |
| Aldh1 | aldehyde Dehydrogenase 1 |
| AOM | azoxymethane |
| AP1 | activator protein 1 |
| APC | adenomatous polyposis coli |
| ApcMin | adenomatous polyposis coli/ multiple intestinal neoplasia |
| ASK1 | apoptosis signal-regulating kinase 1 |
| b.w. | body weight |
| Bax | bcl-2-like protein |
| Bcl 2 | B-cell lymphoma 2 |
| Bid | BH3 Interacting domain death agonist |
| Caco-2 | human epithelial colorectal adenocarcinoma cells |
| CD | cluster of differentiation |
| Cdx2 | human caudal type homeobox 2 |
| CIP1/waf1 | cyclin-dependent kinase inhibitor 1 |
| c-MYC | myc protein |
| CRC | colorectal cancer |
| CSCs | colon cancer stem cells |
| Cyt C | cytochrome C |
| DLK1 | delta like non-canonical notch ligand 1 |
| DMH | 1,2-Dimethylhydrazine |
| DNA | deoxyribonucleic acid |
| DNMT | DNA methyltransferase |
| DSS | dextran sodium sulfate |
| EGCG | Epigallocatechin-3-gallate |
| EGFR | epidermal growth factor receptor |
| EpCAM | epithelial cell adhesion molecule |
| EphB | ephrin B |
| ESA | epithelial surface antigen |
| FAK | Focal adhesion kinase |
| GPx | glutathione peroxidase |
| GSK3 | glycogen synthase kinase-3 |
| HAT | histone acetyltransferases |
| HDAC | histone deacetylase |
| HFD | high-fat diet |
| Hh | hedgehog |
| HT-29 | human colorectal adenocarcinoma cells |
| IBD | chronic inflammatory bowel disease |
| IEC lines | intestinal epithelial cell lines |
| IL | interleukin |
| iNOS | inducible nitric oxide synthase |
| JNK | c-Jun N-terminal kinases |
| Kip1 | kinesin-like protein1 |
| LDLR | low-density lipoprotein receptor |
| LGR5 | leucine-rich repeat-containing G protein-coupled receptor 5 |
| LPS | lipopolysaccharides |
| MAM-GlcUA | methyl azoxy methanol-beta-D-glucosiduronic acid |
| MAPK | mitogen-activated protein kinase |
| MKK4 | mitogen-activated protein kinase kinase 4 |
| mRNA | messenger ribonucleic acid |
| MRP | Multidrug resistance-associated protein |
| mTOR | mammalian target of rapamycin |
| NF-κB | nuclear factor kappa-B |
| NO | nitric oxide |
| Nod | nucleotide-binding oligomerization domain-containing protein |
| PDGF | platelet-derived growth factor |
| PDGFRα | platelet-derived growth factor receptor A |
| PI3K | Phosphoinositide 3-kinases |
| PP2Ac | Protein phosphatase 2A homologs, catalytic domain |
| siRNA | Small interfering RNA |
| SOD | superoxide dismutase |
| SrC | protooncogene c |
| STAT3 | signal transducer and activator of transcription 3 |
| Tcf4 | T-cell factor 4 |
| TGFα | Transforming Growth Factor-alpha |
| TLR-4 | Toll-like receptor 4 |
| TNF-α | tumor necrosis factor-alpha |
| VEGF | vascular endothelial growth factor |
| VEGFR | vascular endothelial growth factor receptor |
References
- Tarver, T. Cancer Facts & Figures 2012. American Cancer Society (ACS). J. Consum. Health. Internet. 2012, 16, 366–367. [Google Scholar] [CrossRef]
- Jemal, A.; Center, M.M.; DeSantis, C.; Ward, E.M. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol. Biomarkers Preven. 2010, 19, 1893–1907. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.; Xu, X.; Liu, T. Hereditary nonpolyposis colorectal cancer and cancer syndromes: Recent basic and clinical discoveries. J. Oncol. 2018, 2018, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Jung, Y.S.; Park, J.H.; Park, D.I.; Sohn, C.I. Association between family history of colorectal cancer and the risk of metachronous colorectal neoplasia following polypectomy in patients aged <50 years. J. Gastroenterol. Hepatol. 2018, 34, 383–389. [Google Scholar] [CrossRef]
- Sahni, M.; Goel, A.; Pande, P.; Kumar, K. Multiple gastrointestinal cancers in a single patient—A rare clinical entity. Indian, J. Surg. Oncol. 2018, 9, 633–635. [Google Scholar] [CrossRef]
- Ganesan, K.; Sukalingam, K.; Xu, B. Impact of consumption of repeatedly heated cooking oils on the incidence of various cancers- A critical review. Crit. Rev. Food Sci. Nutr. 2017, 1–18. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Molecular targets of vitexin and isovitexin in cancer therapy: A critical review. Ann. N.Y. Acad. Sci. 2017, 1401, 102–113. [Google Scholar] [CrossRef]
- Hatano, Y.; Fukuda, S.; Hisamatsu, K.; Hirata, A.; Hara, A.; Tomita, H. Multifaceted interpretation of colon cancer stem cells. Int. J. Mol. Sci. 2017, 18, 1446. [Google Scholar] [CrossRef]
- Whalen, K.A.; McCullough, M.; Flanders, W.D.; Hartman, T.J.; Judd, S.; Bostick, R.M. Paleolithic and Mediterranean diet pattern scores and risk of incident, sporadic colorectal adenomas. Am. J. Epidemiol. 2014, 180, 1088–1097. [Google Scholar] [CrossRef]
- Conlon, M.; Bird, A. The impact of diet and lifestyle on gut microbiota and human health. Nutrients 2014, 7, 17–44. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Yusoff, M.M.; Maniam, G.P.; Ichwan, S.J.A.; Soundharrajan, I.; Govindan, N. Nutraceuticals as potential therapeutic agents for colon cancer: A review. Acta Pharma. Sin. B 2014, 4, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Scarpa, E.-S.; Ninfali, P. Phytochemicals as innovative therapeutic tools against cancer stem cells. Int. J. Mol. Sci. 2015, 16, 15727–15742. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Maoka, T.; Das, S.K.; Kanazawa, K.; Horinaka, M.; Wakada, M.; Satomi, Y.; Nishino, H.; Sakai, T. Halocynthiaxanthin and Peridinin sensitize colon cancer cell lines to tumor necrosis factor-related apoptosis-inducing ligand. Mol. Cancer Res. 2007, 5, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Chang, H.-W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Translat. Med. 2017, 15, 73. [Google Scholar] [CrossRef]
- Singh, S.V.; Singh, K. Cancer chemoprevention with dietary isothiocyanates mature for clinical translational research. Carcinogenesis 2012, 33, 1833–1842. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Kao, C.-L.; Liu, C.-M. The cancer prevention, anti-inflammatory and anti-oxidation of bioactive phytochemicals targeting the TLR4 signaling pathway. Int. J. Mol. Sci. 2018, 19, 2729. [Google Scholar] [CrossRef]
- Czank, C.; Cassidy, A.; Zhang, Q.; Morrison, D.J.; Preston, T.; Kroon, P.A.; Botting, N.P.; Kay, C.D. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: A 13C-tracer study. Am. J. Clin. Nutr. 2013, 97, 995–1003. [Google Scholar] [CrossRef]
- Jayachandran, M.; Xiao, J.; Xu, B. A Critical review on health promoting benefits of edible mushrooms through gut microbiota. Int. J. Mol. Sci. 2017, 18, 1934. [Google Scholar] [CrossRef]
- Ganesan, K.; Chung, S.K.; Vanamala, J.; Xu, B. Causal relationship between diet-induced gut microbiota changes and diabetes: A novel strategy to transplant Faecalibacterium prausnitzii in preventing diabetes. Int. J. Mol. Sci. 2018, 19, 3720. [Google Scholar] [CrossRef]
- Ganesan, K.; Guo, S.; Fayyaz, S.; Zhang, G.; Xu, B. Targeting programmed Fusobacterium nucleatum Fap2 for colorectal cancer therapy. Cancers 2019, 11, 1592. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Anti-diabetic effects and mechanisms of dietary polysaccharides. Molecules 2019, 24, 2556. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Xu, B. Anti-obesity effects of medicinal and edible mushrooms. Molecules 2018, 23, 2880. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Jayachandran, M.; Xu, B. A critical review on hepatoprotective effects of bioactive food components. Crit. Rev. Food Sci. Nutrit. 2017, 58, 1165–1229. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. A critical review on polyphenols and health benefits of black soybeans. Nutrients 2017, 9, 455. [Google Scholar] [CrossRef]
- Ganesan, K.; Mickymaray, S.; Alfaiz, F.A.; Thatchinamoorthi, R.; Aboody, M.S.A.; Xu, B. Immunomodulatory and antineoplastic efficacy of common spices and their connection with phenolic antioxidants. Bioact. Compd. Health Dis. 2020, 3, 15–31. [Google Scholar] [CrossRef]
- Islam, T.; Ganesan, K.; Xu, B. New insight into mycochemical profiles and antioxidant potential of edible and medicinal mushrooms: A review. Int. J. Med. Mushrooms 2019, 21, 237–251. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Telomerase inhibitors from natural products and their anticancer potential. Int. J. Mol. Sci. 2017, 19, 13. [Google Scholar] [CrossRef]
- Sukalingam, K.; Ganesan, K.; Xu, B. Trianthema portulacastrum L. (giant pigweed): Phytochemistry and pharmacological properties. Phytochem. Rev. 2017, 16, 461–478. [Google Scholar] [CrossRef]
- Abreu, M.T.; Peek, R.M. Gastrointestinal malignancy and the microbiome. Gastroenterol. 2014, 146, 1534–1546.e1533. [Google Scholar] [CrossRef]
- Eid, H.M.; Wright, M.L.; Anil Kumar, N.V.; Qawasmeh, A.; Hassan, S.T.S.; Mocan, A.; Nabavi, S.M.; Rastrelli, L.; Atanasov, A.G.; Haddad, P.S. Significance of microbiota in obesity and metabolic diseases and the modulatory potential by medicinal plant and food ingredients. Front. Pharmacol. 2017, 8, 387. [Google Scholar] [CrossRef]
- Espín, J.C.; González-Sarrías, A.; Tomás-Barberán, F.A. The gut microbiota: A key factor in the therapeutic effects of (poly)phenols. Biochem. Pharmacol. 2017, 139, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Bohn, T.; McDougall, G.J.; Alegría, A.; Alminger, M.; Arrigoni, E.; Aura, A.-M.; Brito, C.; Cilla, A.; El, S.N.; Karakaya, S.; et al. Mind the gap-deficits in our knowledge of aspects impacting the bioavailability of phytochemicals and their metabolites-a position paper focusing on carotenoids and polyphenols. Mol. Nutr. Food Res. 2015, 59, 1307–1323. [Google Scholar] [CrossRef] [PubMed]
- Marín, L.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Bioavailability of dietary polyphenols and gut microbiota metabolism: Antimicrobial properties. BioMed Res. Int. 2015, 2015, 1–18. [Google Scholar] [CrossRef]
- Louis, P.; Hold, G.L.; Flint, H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 2014, 12, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Ozdal, T.; Sela, D.A.; Xiao, J.; Boyacioglu, D.; Chen, F.; Capanoglu, E. The reciprocal interactions between polyphenols and gut microbiota and effects on bioaccessibility. Nutrients 2016, 8, 78. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxidants Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed]
- Etxeberria, U.; Fernández-Quintela, A.; Milagro, F.I.; Aguirre, L.; Martínez, J.A.; Portillo, M.P. Impact of polyphenols and polyphenol-rich dietary sources on gut microbiota composition. J. Agric. Food Chem. 2013, 61, 9517–9533. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Polyphenol-rich dry common beans (Phaseolus vulgaris L.) and their health benefits. Int. J. Mol. Sci. 2017, 18, 2331. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Polyphenol-rich lentils and their health promoting effects. Int. J. Mol. Sci. 2017, 18, 2390. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. A critical review on phytochemical profile and health promoting effects of mung bean ( Vigna radiata ). Food Sci. Hum. Wellness 2018, 7, 11–33. [Google Scholar] [CrossRef]
- Dueñas, M.; Cueva, C.; Muñoz-González, I.; Jiménez-Girón, A.; Sánchez-Patán, F.; Santos-Buelga, C.; Moreno-Arribas, M.; Bartolomé, B. Studies on modulation of gut microbiota by wine polyphenols: From isolated cultures to omic approaches. Antioxidants 2015, 4, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Anhê, F.F.; Roy, D.; Pilon, G.; Dudonné, S.; Matamoros, S.; Varin, T.V.; Garofalo, C.; Moine, Q.; Desjardins, Y.; Levy, E.; et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 2014, 64, 872–883. [Google Scholar] [CrossRef] [PubMed]
- Pahle, J.; Menzel, L.; Niesler, N.; Kobelt, D.; Aumann, J.; Rivera, M.; Walther, W. Rapid eradication of colon carcinoma by Clostridium perfringens enterotoxin suicidal gene therapy. BMC Cancer 2017, 17, 129. [Google Scholar] [CrossRef] [PubMed]
- Duda-Chodak, A.; Tarko, T.; Satora, P.; Sroka, P. Interaction of dietary compounds, especially polyphenols, with the intestinal microbiota: A review. Eur. J. Nutr. 2015, 54, 325–341. [Google Scholar] [CrossRef]
- Miene, C.; Weise, A.; Glei, M. Impact of polyphenol metabolites produced by colonic microbiota on expression of COX-2 and GSTT2 in human colon cells (LT97). Nutrit. Cancer 2011, 63, 653–662. [Google Scholar] [CrossRef]
- Barron, C.C.; Moore, J.; Tsakiridis, T.; Pickering, G.; Tsiani, E. Inhibition of human lung cancer cell proliferation and survival by wine. Cancer Cell Int. 2014, 14, 6. [Google Scholar] [CrossRef]
- Yi, W.; Fischer, J.; Krewer, G.; Akoh, C.C. Phenolic compounds from blueberries can inhibit colon cancer cell proliferation and induce apoptosis. J. Agric. Food Chem. 2005, 53, 7320–7329. [Google Scholar] [CrossRef]
- Martín, M.; Goya, L.; Ramos, S. Preventive effects of cocoa and cocoa antioxidants in colon cancer. Diseases 2016, 4, 6. [Google Scholar] [CrossRef]
- Esposito, D.; Damsud, T.; Wilson, M.; Grace, M.H.; Strauch, R.; Li, X.; Lila, M.A.; Komarnytsky, S. Black currant anthocyanins attenuate weight gain and improve glucose metabolism in diet-induced obese mice with intact, but not disrupted, gut microbiome. J. Agric. Food Chem. 2015, 63, 6172–6180. [Google Scholar] [CrossRef]
- Wu, T.; Tang, Q.; Yu, Z.; Gao, Z.; Hu, H.; Chen, W.; Zheng, X.; Yu, T. Inhibitory effects of sweet cherry anthocyanins on the obesity development in C57BL/6 mice. Int. J. Food Sci. Nutr. 2013, 65, 351–359. [Google Scholar] [CrossRef]
- Remely, M.; Ferk, F.; Sterneder, S.; Setayesh, T.; Roth, S.; Kepcija, T.; Noorizadeh, R.; Rebhan, I.; Greunz, M.; Beckmann, J.; et al. EGCG prevents high fat diet-induced changes in gut microbiota, decreases of dna strand breaks, and changes in expression and dna methylation of Dnmt1 and MLH1 in C57BL/6J male mice. Oxid. Med. Cell. Longev. 2017, 2017, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Zhang, X.; Miao, Y.; Cao, J.; Wu, Z.; Weng, P. The modulatory effect of (-)-epigallocatechin 3-O-(3-O-methyl) gallate (EGCG3″Me) on intestinal microbiota of high fat diet-induced obesity mice model. Food Res. Int. 2017, 92, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Unno, T.; Sakuma, M.; Mitsuhashi, S. Effect of dietary supplementation of (-)-epigallocatechin gallate on gut microbiota and biomarkers of colonic fermentation in rats. J. Nutr. Sci. Vitaminol. 2014, 60, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Porras, D.; Nistal, E.; Martínez-Flórez, S.; Pisonero-Vaquero, S.; Olcoz, J.L.; Jover, R.; González-Gallego, J.; García-Mediavilla, M.V.; Sánchez-Campos, S. Protective effect of quercetin on high-fat diet-induced non-alcoholic fatty liver disease in mice is mediated by modulating intestinal microbiota imbalance and related gut-liver axis activation. Free Rad. Biol. Med. 2017, 102, 188–202. [Google Scholar] [CrossRef]
- Etxeberria, U.; Arias, N.; Boqué, N.; Macarulla, M.T.; Portillo, M.P.; Martínez, J.A.; Milagro, F.I. Reshaping faecal gut microbiota composition by the intake of trans-resveratrol and quercetin in high-fat sucrose diet-fed rats. J. Nutr. Biochem. 2015, 26, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Roldán-Marín, E.; Krath, B.N.; Poulsen, M.; Binderup, M.-L.; Nielsen, T.H.; Hansen, M.; Barri, T.; Langkilde, S.; Pilar Cano, M.; Sánchez-Moreno, C.; et al. Effects of an onion by-product on bioactivity and safety markers in healthy rats. Br. J. Nutr. 2009, 102, 1574. [Google Scholar] [CrossRef]
- Le Sage, F.; Meilhac, O.; Gonthier, M.-P. Anti-inflammatory and antioxidant effects of polyphenols extracted from Antirhea borbonica medicinal plant on adipocytes exposed to Porphyromonas gingivalis and Escherichia coli lipopolysaccharides. Pharmacol. Res. 2017, 119, 303–312. [Google Scholar] [CrossRef]
- Pu, P.; Wang, X.-A.; Salim, M.; Zhu, L.-H.; Wang, L.; Chen, K.-J.; Xiao, J.-F.; Deng, W.; Shi, H.-W.; Jiang, H.; et al. Baicalein, a natural product, selectively activating AMPKα2 and ameliorates metabolic disorder in diet-induced mice. Mol. Cell. Endocrinol. 2012, 362, 128–138. [Google Scholar] [CrossRef]
- Lai, Y.-S.; Chen, W.-C.; Ho, C.-T.; Lu, K.-H.; Lin, S.-H.; Tseng, H.-C.; Lin, S.-Y.; Sheen, L.-Y. Garlic essential oil protects against obesity-triggered nonalcoholic fatty liver disease through modulation of lipid metabolism and oxidative stress. J. Agric. Food Chem. 2014, 62, 5897–5906. [Google Scholar] [CrossRef]
- Neyrinck, A.M.; Alligier, M.; Memvanga, P.B.; Névraumont, E.; Larondelle, Y.; Préat, V.; Cani, P.D.; Delzenne, N.M. Curcuma longa extract associated with white pepper lessens high fat diet-induced inflammation in subcutaneous adipose tissue. PLoS ONE 2013, 8, e81252. [Google Scholar] [CrossRef]
- Ghosh, S.S.; Bie, J.; Wang, J.; Ghosh, S. Oral supplementation with non-absorbable antibiotics or Curcumin attenuates Western diet-induced atherosclerosis and glucose intolerance in LDLR−/− Mice – role of intestinal permeability and macrophage activation. PLoS ONE 2014, 9, e108577. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ghosh, S.S.; Ghosh, S. Curcumin improves intestinal barrier function: Modulation of intracellular signaling, and organization of tight junctions. Am. J. Physiol. Cell Physiol. 2017, 312, C438–C445. [Google Scholar] [CrossRef] [PubMed]
- Päivärinta, E.; Niku, M.; Maukonen, J.; Storvik, M.; Heiman-Lindh, A.; Saarela, M.; Pajari, A.-M.; Mutanen, M. Changes in intestinal immunity, gut microbiota, and expression of energy metabolism–related genes explain adenoma growth in bilberry and cloudberry-fed Apc Min mice. Nutr. Res. 2016, 36, 1285–1297. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Sun, J.; Xia, S.; Tang, X.; Shi, Y.; Le, G. Effects of resveratrol on gut microbiota and fat storage in a mouse model with high-fat-induced obesity. Food Funct. 2014, 5, 1241. [Google Scholar] [CrossRef] [PubMed]
- Dao, T.-M.A.; Waget, A.; Klopp, P.; Serino, M.; Vachoux, C.; Pechere, L.; Drucker, D.J.; Champion, S.; Barthélemy, S.; Barra, Y.; et al. Resveratrol increases glucose induced GLP-1 secretion in mice: A mechanism which contributes to the glycemic control. PLoS ONE 2011, 6, e20700. [Google Scholar] [CrossRef]
- Nøhr, M.K.; Kroager, T.P.; Sanggaard, K.W.; Knudsen, A.D.; Stensballe, A.; Enghild, J.J.; Ølholm, J.; Richelsen, B.; Pedersen, S.B. SILAC-MS based characterization of LPS and Resveratrol induced changes in adipocyte proteomics – Resveratrol as ameliorating factor on LPS induced changes. PLoS ONE 2016, 11, e0159747. [Google Scholar] [CrossRef]
- Korsholm, A.; Kjær, T.; Ornstrup, M.; Pedersen, S. Comprehensive metabolomic analysis in blood, urine, fat, and muscle in men with metabolic syndrome: A randomized, placebo-controlled clinical trial on the effects of Resveratrol after four months’ treatment. Int. J. Mol. Sci. 2017, 18, 554. [Google Scholar] [CrossRef]
- Tung, Y.-C.; Lin, Y.-H.; Chen, H.-J.; Chou, S.-C.; Cheng, A.-C.; Kalyanam, N.; Ho, C.-T.; Pan, M.-H. Piceatannol exerts anti-obesity effects in C57BL/6 mice through modulating adipogenic proteins and gut microbiota. Molecules 2016, 21, 1419. [Google Scholar] [CrossRef]
- Hijona, E.; Aguirre, L.; Pérez-Matute, P.; Villanueva-Millán, M.J.; Mosqueda-Solis, A.; Hasnaoui, M.; Nepveu, F.; Senard, J.M.; Bujanda, L.; Aldámiz-Echevarría, L.; et al. Limited beneficial effects of piceatannol supplementation on obesity complications in the obese Zucker rat: Gut microbiota, metabolic, endocrine, and cardiac aspects. J. Physiol. Biochem. 2016, 72, 567–582. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Ethnobotanical studies on folkloric medicinal plants in Nainamalai, Namakkal District, Tamil Nadu, India. Trend. Phytochem. Res. 2017, 1, 153–168. [Google Scholar]
- Wang, W.; VanAlstyne, P.C.; Irons, K.A.; Chen, S.; Stewart, J.W.; Birt, D.F. Individual and interactive effects of apigenin analogs on G2/M cell-cycle arrest in human colon carcinoma cell lines. Nutr. Cancer 2004, 48, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Aron, P.M.; Kennedy, J.A. Flavan-3-ols: Nature, occurrence and biological activity. Mol. Nutr. Food Res. 2008, 52, 79–104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Jayachandran, M.; Ganesan, K.; Xu, B. Black truffle aqueous extract attenuates oxidative stress and inflammation in STZ-induced hyperglycemic rats via Nrf2 and NF-κB pathways. Front. Pharmacol. 2018, 9, 1257. [Google Scholar] [CrossRef] [PubMed]
- Jayachandran, M.; Zhang, T.; Ganesan, K.; Xu, B.; Chung, S.S.M. Isoquercetin ameliorates hyperglycemia and regulates key enzymes of glucose metabolism via insulin signaling pathway in streptozotocin-induced diabetic rats. Eur. J. Pharmacol. 2018, 829, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Jayachandran, M.; Wu, Z.; Ganesan, K.; Khalid, S.; Chung, S.M.; Xu, B. Isoquercetin upregulates antioxidant genes, suppresses inflammatory cytokines and regulates AMPK pathway in streptozotocin-induced diabetic rats. Chem.Biol. Int. 2019, 303, 62–69. [Google Scholar] [CrossRef]
- Hoensch, H.; Groh, B.; Edler, L.; Kirch, W. Prospective cohort comparison of flavonoid treatment in patients with resected colorectal cancer to prevent recurrence. World J. Gastroenterol. 2008, 14, 2187. [Google Scholar] [CrossRef]
- Murota, K.; Nakamura, Y.; Uehara, M. Flavonoid metabolism: The interaction of metabolites and gut microbiota. Biosci. Biotechnol. Biochem. 2018, 82, 600–610. [Google Scholar] [CrossRef]
- Clavel, T.; Henderson, G.; Alpert, C.A.; Philippe, C.; Rigottier-Gois, L.; Dore, J.; Blaut, M. Intestinal bacterial communities that produce active estrogen-like compounds enterodiol and enterolactone in humans. Appl. Environ. Microbiol. 2005, 71, 6077–6085. [Google Scholar] [CrossRef]
- Setchell, K.D.R.; Brown, N.M.; Lydeking-Olsen, E. The clinical importance of the metabolite Equol—A clue to the effectiveness of soy and its isoflavones. J. Nutr. 2002, 132, 3577–3584. [Google Scholar] [CrossRef]
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O’Connor, E.M.; Cusack, S.; Harris, H.M.B.; Coakley, M.; Lakshminarayanan, B.; O’Sullivan, O.; et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012, 488, 178–184. [Google Scholar] [CrossRef]
- Spencer, M.D.; Hamp, T.J.; Reid, R.W.; Fischer, L.M.; Zeisel, S.H.; Fodor, A.A. Association between composition of the human gastrointestinal microbiome and development of fatty liver with choline deficiency. Gastroenterology 2011, 140, 976–986. [Google Scholar] [CrossRef] [PubMed]
- De Ferrars, R.M.; Czank, C.; Zhang, Q.; Botting, N.P.; Kroon, P.A.; Cassidy, A.; Kay, C.D. The pharmacokinetics of anthocyanins and their metabolites in humans. Br. J. Pharmacol. 2014, 171, 3268–3282. [Google Scholar] [CrossRef]
- Gil-Cardoso, K.; Ginés, I.; Pinent, M.; Ardévol, A.; Blay, M.; Terra, X. Effects of flavonoids on intestinal inflammation, barrier integrity and changes in gut microbiota during diet-induced obesity. Nutr. Res. Rev. 2016, 29, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.A. Colon cancer stem cells: Implications in carcinogenesis. Front. Biosci. 2011, 16, 1651. [Google Scholar] [CrossRef] [PubMed]
- Van de Wetering, M.; Sancho, E.; Verweij, C.; de Lau, W.; Oving, I.; Hurlstone, A.; van der Horn, K.; Batlle, E.; Coudreuse, D.; Haramis, A.-P.; et al. The β-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 2002, 111, 241–250. [Google Scholar] [CrossRef]
- Ricci-Vitiani, L.; Fabrizi, E.; Palio, E.; De Maria, R. Colon cancer stem cells. J. Mol. Med. 2009, 87, 1097–1104. [Google Scholar] [CrossRef]
- Zheng, S.; Xin, L.; Liang, A.; Fu, Y. Cancer stem cell hypothesis: A brief summary and two proposals. Cytotechnology 2012, 65, 505–512. [Google Scholar] [CrossRef][Green Version]
- Liu, S.; Dontu, G.; Wicha, M.S. Mammary stem cells, self-renewal pathways, and carcinogenesis. Breast Cancer Res. 2005, 7, 86–95. [Google Scholar] [CrossRef]
- Zito, G.; Saotome, I.; Liu, Z.; Ferro, E.G.; Sun, T.Y.; Nguyen, D.X.; Bilguvar, K.; Ko, C.J.; Greco, V. Spontaneous tumour regression in keratoacanthomas is driven by Wnt/retinoic acid signalling cross-talk. Nat. Commun. 2014, 5, 3543. [Google Scholar] [CrossRef]
- Munro, M.J.; Wickremesekera, S.K.; Peng, L.; Tan, S.T.; Itinteang, T. Cancer stem cells in colorectal cancer: A review. J. Clin. Pathol. 2017, 71, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-Y.; Chang, C.-C.; Chiang, C.-C.; Chen, W.-M.; Hung, S.-C. Silibinin suppresses the maintenance of colorectal cancer stem-like cells by inhibiting PP2A/AKT/mTOR pathways. J. Cell. Biochem. 2011, 113, 1733–1743. [Google Scholar] [CrossRef] [PubMed]
- Van der Gun, B.T.F.; Melchers, L.J.; Ruiters, M.H.J.; de Leij, L.F.M.H.; McLaughlin, P.M.J.; Rots, M.G. EpCAM in carcinogenesis: The good, the bad or the ugly. Carcinogenesis 2010, 31, 1913–1921. [Google Scholar] [CrossRef] [PubMed]
- Uronis, J.M.; Mühlbauer, M.; Herfarth, H.H.; Rubinas, T.C.; Jones, G.S.; Jobin, C. Modulation of the intestinal microbiota alters colitis-associated colorectal cancer susceptibility. PLoS ONE 2009, 4, e6026. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Wang, K.; Mucida, D.; Stewart, C.A.; Schnabl, B.; Jauch, D.; Taniguchi, K.; Yu, G.-Y.; Österreicher, C.H.; Hung, K.E.; et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature 2012, 491, 254–258. [Google Scholar] [CrossRef]
- Schwabe, R.F.; Jobin, C. The microbiome and cancer. Nat. Rev. Cancer 2013, 13, 800–812. [Google Scholar] [CrossRef]
- Vannucci, L.; Stepankova, R.; Kozakova, H.; Fiserova, A.; Rossmann, P.; Tlaskalova-Hogenova, H. Colorectal carcinogenesis in germ-free and conventionally reared rats: Different intestinal environments affect the systemic immunity. Int. J. Oncol. 2008, 32, 609–617. [Google Scholar] [CrossRef]
- Li, K.; Xing, S.; Wang, M.; Peng, Y.; Dong, Y.; Li, X. Anticomplement and antimicrobial activities of flavonoids from Entada phaseoloides. Nat. Prod. Commun. 2012, 7, 1934578X1200700715. [Google Scholar] [CrossRef]
- Chen, G.Y.; Shaw, M.H.; Redondo, G.; Nunez, G. The innate immune receptor Nod1 protects the intestine from inflammation-induced tumorigenesis. Cancer Res. 2008, 68, 10060–10067. [Google Scholar] [CrossRef]
- Klimesova, K.; Kverka, M.; Zakostelska, Z.; Hudcovic, T.; Hrncir, T.; Stepankova, R.; Rossmann, P.; Ridl, J.; Kostovcik, M.; Mrazek, J.; et al. Altered gut microbiota promotes colitis-associated cancer in IL-1 receptor–associated Kinase M–deficient mice. Inflamm. Bowel Dis. 2013, 19, 1266–1277. [Google Scholar] [CrossRef]
- Ma, Y.; Ren, Y.; Han, E.Q.; Li, H.; Chen, D.; Jacobs, J.J.; Gitelis, S.; O’Keefe, R.J.; Konttinen, Y.T.; Yin, G.; et al. Inhibition of the Wnt-β-catenin and Notch signaling pathways sensitizes osteosarcoma cells to chemotherapy. Biochem. Biophys. Res. Commun. 2013, 431, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Koury, J.; Zhong, L.; Hao, J. Targeting signaling pathways in cancer stem cells for cancer treatment. Stem Cells Int. 2017, 2017, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Brittan, M.; Wright, N.A. Gastrointestinal stem cells. J. Pathol. 2002, 197, 492–509. [Google Scholar] [CrossRef] [PubMed]
- Deonarain, M.P.; Kousparou, C.A.; Epenetos, A.A. Antibodies targeting cancer stem cells: A new paradigm in immunotherapy? mAbs 2009, 1, 12–25. [Google Scholar] [CrossRef]
- Tan, M.L.; Ooi, J.P.; Ismail, N.; Moad, A.I.H.; Muhammad, T.S.T. Programmed cell death pathways and current antitumor targets. Pharma. Res. 2009, 26, 1547–1560. [Google Scholar] [CrossRef] [PubMed]
- Kahn, M. Can we safely target the WNT pathway? Nat. Rev. Drug Discover. 2014, 13, 513–532. [Google Scholar] [CrossRef]
- Chen, J.K. Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened. Genes Dev. 2002, 16, 2743–2748. [Google Scholar] [CrossRef]
- Bar, E.E.; Chaudhry, A.; Lin, A.; Fan, X.; Schreck, K.; Matsui, W.; Piccirillo, S.; Vescovi, A.L.; DiMeco, F.; Olivi, A.; et al. Cyclopamine-mediated Hedgehog pathway inhibition depletes stem-like cancer cells in glioblastoma. Stem Cells 2007, 25, 2524–2533. [Google Scholar] [CrossRef]
- Oh, S.; Gwak, J.; Park, S.; Yang, C.S. Green tea polyphenol EGCG suppresses Wnt/β-catenin signaling by promoting GSK-3β- and PP2A-independent β-catenin phosphorylation/degradation. BioFactors 2014, 40, 586–595. [Google Scholar] [CrossRef]
- Hong, O.-Y.; Noh, E.-M.; Jang, H.-Y.; Lee, Y.-R.; Lee, B.K.; Jung, S.H.; Kim, J.-S.; Youn, H.J. Epigallocatechin gallate inhibits the growth of MDA-MB-231 breast cancer cells via inactivation of the β-catenin signaling pathway. Oncol. Lett. 2017, 14, 441–446. [Google Scholar] [CrossRef]
- Castillo-Pichardo, L.; Dharmawardhane, S.F. Grape polyphenols inhibit Akt/mammalian target of rapamycin signaling and potentiate the effects of Gefitinib in breast cancer. Nutr. Cancer 2012, 64, 1058–1069. [Google Scholar] [CrossRef] [PubMed]
- Abdal Dayem, A.; Choi, H.; Yang, G.-M.; Kim, K.; Saha, S.; Cho, S.-G. The anti-cancer effect of polyphenols against breast cancer and cancer stem cells: Molecular mechanisms. Nutrients 2016, 8, 581. [Google Scholar] [CrossRef] [PubMed]
- Nautiyal, J.; Banerjee, S.; Kanwar, S.S.; Yu, Y.; Patel, B.B.; Sarkar, F.H.; Majumdar, A.P.N. Curcumin enhances dasatinib-induced inhibition of growth and transformation of colon cancer cells. Int. J. Cancer 2010, 128, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, A.S.; Marlow, B.P.; Gupta, N.; Narayan, S. β-Catenin-mediated transactivation and cell–cell adhesion pathways are important in curcumin (diferuylmethane)-induced growth arrest and apoptosis in colon cancer cells. Oncogene 2002, 21, 8414–8427. [Google Scholar] [CrossRef]
- Min, S.; Lim, J.; Kim, H.; Kim, S.-J.; Kim, Y. Sasa quelpaertensis leaf extract inhibits colon cancer by regulating cancer cell stemness in vitro and in vivo. Int.J. Mol. Sci. 2015, 16, 9976–9997. [Google Scholar] [CrossRef]
- Dandawate, P.; Padhye, S.; Ahmad, A.; Sarkar, F.H. Novel strategies targeting cancer stem cells through phytochemicals and their analogs. Drug Deliv. Translat. Res. 2012, 3, 165–182. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, Y.-S.; Lee, J.; Heo, S.; Lee, K.; Choi, S.-W.; Kim, Y. Walnut phenolic extract and its bioactive compounds suppress colon cancer cell growth by regulating colon cancer stemness. Nutrients 2016, 8, 439. [Google Scholar] [CrossRef]
- Kumar, S.; Raina, K.; Agarwal, C.; Agarwal, R. Silibinin strongly inhibits the growth kinetics of colon cancer stem cell-enriched spheroids by modulating interleukin 4/6-mediated survival signals. Oncotarget 2014, 5, 4972–4989. [Google Scholar] [CrossRef]
- Soltanian, S.; Riahirad, H.; Pabarja, A.; Jafari, E.; Khandani, B.K. Effect of Cinnamic acid and FOLFOX in diminishing side population and downregulating cancer stem cell markers in colon cancer cell line HT-29. DARU J. Pharma. Sci. 2018, 26, 19–29. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G. Adherence to Mediterranean diet and risk of cancer: A systematic review and meta-analysis of observational studies. Int. J. Cancer 2014, 135, 1884–1897. [Google Scholar] [CrossRef]
- Epstein, J.; Sanderson, I.R.; MacDonald, T.T. Curcumin as a therapeutic agent: The evidence from in vitro, animal and human studies. Br. J. Nutr. 2010, 103, 1545–1557. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Kong, D.; Wang, Z.; Bao, B.; Hillman, G.G.; Sarkar, F.H. Attenuation of multi-targeted proliferation-linked signaling by 3,3′-diindolylmethane (DIM): From bench to clinic. Mutat. Res. Rev. Mutat. Res. 2011, 728, 47–66. [Google Scholar] [CrossRef] [PubMed]
- Ezzat, S.; Hegazy, A.; Amer, A.M.; Kamel, G.; El-Alfy, T. Isolation of biologically active constituents from Moringa peregrina (Forssk.) Fiori. (family: Moringaceae) growing in Egypt. Pharmacogn. Mag. 2011, 7, 109. [Google Scholar] [CrossRef]
- Sharma, N.; Samarakoon, K.; Gyawali, R.; Park, Y.-H.; Lee, S.-J.; Oh, S.; Lee, T.-H.; Jeong, D. Evaluation of the antioxidant, anti-inflammatory, and anticancer activities of Euphorbia hirta ethanolic extract. Molecules 2014, 19, 14567–14581. [Google Scholar] [CrossRef] [PubMed]
- Mongre, R.K.; Sodhi, S.S.; Ghosh, M.; Kim, J.H.; Kim, N.; Park, Y.H.; Kim, S.J.; Heo, Y.J.; Sharma, N.; Jeong, D.K. The novel inhibitor BRM270 downregulates tumorigenesis by suppression of NF-κB signaling cascade in MDR-induced stem like cancer-initiating cells. Int. J. Oncol. 2015, 46, 2573–2585. [Google Scholar] [CrossRef][Green Version]
- Enkhbat, T.; Nishi, M.; Yoshikawa, K.; Jun, H.; Tokunaga, T.; Takasu, C.; Kashihara, H.; Ishikawa, D.; Tominaga, M.; Shimada, M. Epigallocatechin-3-gallate enhances radiation sensitivity in colorectal cancer cells through Nrf2 activation and autophagy. Anticancer Res. 2018, 38, 6247–6252. [Google Scholar] [CrossRef]
- Tomasello, G.; Mazzola, M.; Bosco, V.; Tomasello, G.; Damiani, P.; Sinagra, E.; Carini, F. Intestinal dysbiosis and hormonal neuroendocrine secretion in the fibromyalgic patient. Biomed. Papers 2018, 162, 258–262. [Google Scholar] [CrossRef]
- Darband, S.G.; Kaviani, M.; Yousefi, B.; Sadighparvar, S.; Pakdel, F.G.; Attari, J.A.; Mohebbi, I.; Naderi, S.; Majidinia, M. Quercetin: A functional dietary flavonoid with potential chemo-preventive properties in colorectal cancer. J. Cell. Physiol. 2018, 233, 6544–6560. [Google Scholar] [CrossRef]
- Bernkopf, D.B.; Daum, G.; Brückner, M.; Behrens, J. Sulforaphane inhibits growth and blocks Wnt/β-catenin signaling of colorectal cancer cells. Oncotarget 2018, 9, 33982–33994. [Google Scholar] [CrossRef]
- O’Gorman, D.M.; Cotter, T.G. Molecular signals in anti-apoptotic survival pathways. Leukemia 2001, 15, 21–34. [Google Scholar] [CrossRef]
- Neergheen, V.S.; Bahorun, T.; Taylor, E.W.; Jen, L.-S.; Aruoma, O.I. Targeting specific cell signaling transduction pathways by dietary and medicinal phytochemicals in cancer chemoprevention. Toxicology 2010, 278, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Mukhtar, H. Multitargeted therapy of cancer by green tea polyphenols. Cancer Lett. 2008, 269, 269–280. [Google Scholar] [CrossRef]
- Meeran, S.M. Cell cycle control as a basis for cancer chemoprevention through dietary agents. Front. Biosci. 2008, 13, 2191. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Toume, K.; Arai, M.A.; Sadhu, S.K.; Ahmed, F.; Ishibashi, M. Calotropin: A cardenolide from Calotropis gigantea that inhibits Wnt signaling by increasing casein kinase 1α in colon cancer cells. Chem. BioChem. 2014, 15, 872–878. [Google Scholar] [CrossRef]
- Shakibaei, M.; Mobasheri, A.; Lueders, C.; Busch, F.; Shayan, P.; Goel, A. Curcumin enhances the effect of chemotherapy against colorectal cancer cells by inhibition of NF-κB and Src protein kinase signaling pathways. PLoS ONE 2013, 8, e57218. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Ganapathy, S.; Srivastava, R.K. Sulforaphane enhances the therapeutic potential of TRAIL in prostate cancer orthotopic model through regulation of apoptosis, metastasis, and angiogenesis. Clin. Cancer Res. 2008, 14, 6855–6866. [Google Scholar] [CrossRef] [PubMed]
- Suppipat, K.; Park, C.S.; Shen, Y.; Zhu, X.; Lacorazza, H.D. Sulforaphane induces cell cycle arrest and apoptosis in acute lymphoblastic leukemia cells. PLoS ONE 2012, 7, e51251. [Google Scholar] [CrossRef]
- Sung, B.; Prasad, S.; Yadav, V.R.; Aggarwal, B.B. Cancer cell signaling pathways targeted by spice-derived nutraceuticals. Nutr. Cancer 2012, 64, 173–197. [Google Scholar] [CrossRef]
- Shankar, S.; Marsh, L.; Srivastava, R.K. EGCG inhibits growth of human pancreatic tumors orthotopically implanted in Balb C nude mice through modulation of FKHRL1/FOXO3a and neuropilin. Mol. Cell. Biochem. 2012, 372, 83–94. [Google Scholar] [CrossRef]
- Chakraborty, S.; Adhikary, A.; Mazumdar, M.; Mukherjee, S.; Bhattacharjee, P.; Guha, D.; Choudhuri, T.; Chattopadhyay, S.; Sa, G.; Sen, A.; et al. Capsaicin-induced activation of p53-SMAR1 auto-regulatory loop down-regulates VEGF in non-small cell lung cancer to restrain angiogenesis. PLoS ONE 2014, 9, e99743. [Google Scholar] [CrossRef]
- Amado, N.G.; Fonseca, B.F.; Cerqueira, D.M.; Neto, V.M.; Abreu, J.G. Flavonoids: Potential Wnt/beta-catenin signaling modulators in cancer. Life Sci. 2011, 89, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Diehn, M.; Cho, R.W.; Lobo, N.A.; Kalisky, T.; Dorie, M.J.; Kulp, A.N.; Qian, D.; Lam, J.S.; Ailles, L.E.; Wong, M.; et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature 2009, 458, 780–783. [Google Scholar] [CrossRef] [PubMed]
- Park, W.; Amin, A.R.M.R.; Chen, Z.G.; Shin, D.M. New Perspectives of curcumin in cancer prevention. Cancer Prev. Res. 2013, 6, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, H.S.; Guerrero, J.M.M.; Briviba, K.; Rechkemmer, G.; Schuchmann, H.P.; Schubert, H. Cellular uptake of carotenoid-loaded oil-in-water emulsions in colon carcinoma cells in vitro. J. Agric. Food Chem. 2006, 54, 9366–9369. [Google Scholar] [CrossRef]
- Yang, K.; Pyo, J.; Kim, G.-Y.; Yu, R.; Han, I.; Ju, S.; Kim, W.; Kim, B.-S. Capsaicin induces apoptosis by generating reactive oxygen species and disrupting mitochondrial transmembrane potential in human colon cancer cell lines. Cell. Mol. Biol. Lett. 2009, 14, 497–510. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Park, B.; Goel, A.; Aggarwal, B.B. Epigenetic changes induced by curcumin and other natural compounds. Genes Nutr. 2011, 6, 93–108. [Google Scholar] [CrossRef]
- Thakur, V.S.; Deb, G.; Babcook, M.A.; Gupta, S. Plant phytochemicals as epigenetic modulators: Role in cancer chemoprevention. AAPS J. 2013, 16, 151–163. [Google Scholar] [CrossRef]
- Den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Zhang, L.; Mu, C.; He, X.; Su, Y.; Mao, S.; Zhang, J.; Smidt, H.; Zhu, W. Effects of dietary fibre source on microbiota composition in the large intestine of suckling piglets. FEMS Microbiol. Lett. 2016, 363, fnw138. [Google Scholar] [CrossRef]
- Matthews, G.M.; Howarth, G.S.; Butler, R.N. Short-chain fatty acids induce apoptosis in colon cancer cells associated with changes to intracellular redox state and glucose metabolism. Chemotherapy 2012, 58, 102–109. [Google Scholar] [CrossRef]
- Mambet, C.; Chivu-Economescu, M.; Matei, L.; Necula, L.G.; Dragu, D.L.; Bleotu, C.; Diaconu, C.C. Murine models based on acute myeloid leukemia-initiating stem cells xenografting. World J. Stem Cells 2018, 10, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Taussig, D.; Cheng, W.-H.; Johnson, L.; Hakkak, R. Butyrate inhibits cancerous HCT116 colon cell proliferation but to a lesser extent in noncancerous NCM460 colon cells. Nutrients 2017, 9, 25. [Google Scholar] [CrossRef]
- Ritchie, L.E.; Taddeo, S.S.; Weeks, B.R.; Lima, F.; Bloomfield, S.A.; Azcarate-Peril, M.A.; Zwart, S.R.; Smith, S.M.; Turner, N.D. Space environmental factor impacts upon murine colon microbiota and mucosal homeostasis. PLoS ONE 2015, 10, e0125792. [Google Scholar] [CrossRef] [PubMed]
- Hooks, K.B.; O’Malley, M.A. Dysbiosis and Its Discontents. mBio 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.I.; Atarashi, K.; Manel, N.; Brodie, E.L.; Shima, T.; Karaoz, U.; Wei, D.; Goldfarb, K.C.; Santee, C.A.; Lynch, S.V.; et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 2009, 139, 485–498. [Google Scholar] [CrossRef]
- Dejea, C.; Wick, E.; Sears, C.L. Bacterial oncogenesis in the colon. Future Microbiol. 2013, 8, 445–460. [Google Scholar] [CrossRef]
- Fukata, M.; Arditi, M. The role of pattern recognition receptors in intestinal inflammation. Mucosal Immunol. 2013, 6, 451–463. [Google Scholar] [CrossRef]
- Garaude, J.; Kent, A.; van Rooijen, N.; Blander, J.M. Simultaneous targeting of Toll- and Nod-like receptors induces effective tumor-specific immune responses. Sci Translat. Med. 2012, 4, 120ra116. [Google Scholar] [CrossRef]
- Clevers, H. The cancer stem cell: Premises, promises and challenges. Nat. Med. 2011, 17, 313–319. [Google Scholar] [CrossRef]
- Blokzijl, F.; de Ligt, J.; Jager, M.; Sasselli, V.; Roerink, S.; Sasaki, N.; Huch, M.; Boymans, S.; Kuijk, E.; Prins, P.; et al. Tissue-specific mutation accumulation in human adult stem cells during life. Nature 2016, 538, 260–264. [Google Scholar] [CrossRef]
- Polakis, P. Wnt signaling in cancer. Cold Spring Harbor Perspectives in Biology 2012, 4, a008052. [Google Scholar] [CrossRef] [PubMed]
- Seidel, D.V.; Azcárate-Peril, M.A.; Chapkin, R.S.; Turner, N.D. Shaping functional gut microbiota using dietary bioactives to reduce colon cancer risk. Semin. Cancer Biol. 2017, 46, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Rycaj, K.; Tang, D.G. Cell-of-origin of cancer versus cancer stem cells: Assays and interpretations. Cancer Res. 2015, 75, 4003–4011. [Google Scholar] [CrossRef] [PubMed]
- Tetteh, P.W.; Farin, H.F.; Clevers, H. Plasticity within stem cell hierarchies in mammalian epithelia. Trends Cell Biol. 2015, 25, 100–108. [Google Scholar] [CrossRef]
- Beyaz, S.; Mana, M.D.; Roper, J.; Kedrin, D.; Saadatpour, A.; Hong, S.-J.; Bauer-Rowe, K.E.; Xifaras, M.E.; Akkad, A.; Arias, E.; et al. High-fat diet enhances stemness and tumorigenicity of intestinal progenitors. Nature 2016, 531, 53–58. [Google Scholar] [CrossRef]
- Haegebarth, A.; Clevers, H. Wnt Signaling, Lgr5, and stem cells in the intestine and skin. Am. J. Pathol. 2009, 174, 715–721. [Google Scholar] [CrossRef]
- Barker, N.; Clevers, H. Leucine-rich repeat-containing G-protein-coupled receptors as markers of adult stem cells. Gastroenterology 2010, 138, 1681–1696. [Google Scholar] [CrossRef]
- Claessen, M.M.H.; Vleggaar, F.P.; Schipper, M.; Oldenburg, B.; Offerhaus, J.; Siersema, P.D. Wnt-pathway activation in early IBD-associated colorectal carcinogenesis: A biomarker for colonic surveillance. Gastroenterology 2008, 134, A-132. [Google Scholar] [CrossRef]
- Sauceda, A.E.Q.; Pacheco-Ordaz, R.; Ayala-Zavala, J.F.; Mendoza, A.H.; González-Córdova, A.F.; Vallejo-Galland, B.; González-Aguilar, G.A. Impact of fruit dietary fibers and polyphenols on modulation of the human gut microbiota. In Fruit and Vegetable Phytochemicals; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 405–422. [Google Scholar] [CrossRef]
- Pyra, K.A.; Saha, D.C.; Reimer, R.A. Prebiotic fiber increases hepatic acetyl CoA carboxylase phosphorylation and suppresses glucose-dependent insulinotropic polypeptide secretion more effectively when used with metformin in obese rats. J. Nutr. 2012, 142, 213–220. [Google Scholar] [CrossRef]
- Laparra, J.M.; Sanz, Y. Interactions of gut microbiota with functional food components and nutraceuticals. Pharmacol. Res. 2010, 61, 219–225. [Google Scholar] [CrossRef]
- Scott, K.P.; Gratz, S.W.; Sheridan, P.O.; Flint, H.J.; Duncan, S.H. The influence of diet on the gut microbiota. Pharmacol. Res. 2013, 69, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.L.; Scott, A.J.; Pouncey, A.L.; Marchesi, J.; Kinross, J.; Teare, J. Colorectal carcinogenesis: An archetype of gut microbiota–host interaction. ecancermedicalscience 2018, 12. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.J.; Spradling, A.C. Stem cells and niches: Mechanisms that promote stem cell maintenance throughout life. Cell 2008, 132, 598–611. [Google Scholar] [CrossRef] [PubMed]
- Frank, N.Y.; Schatton, T.; Frank, M.H. The therapeutic promise of the cancer stem cell concept. J. Clin. Investig. 2010, 120, 41–50. [Google Scholar] [CrossRef]
- Domingo-Domenech, J.; Vidal, S.J.; Rodriguez-Bravo, V.; Castillo-Martin, M.; Quinn, S.A.; Rodriguez-Barrueco, R.; Bonal, D.M.; Charytonowicz, E.; Gladoun, N.; de la Iglesia-Vicente, J.; et al. Suppression of acquired docetaxel resistance in prostate cancer through depletion of Notch- and Hedgehog-dependent tumor-initiating cells. Cancer Cell. 2012, 22, 373–388. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).