Valorization of Biodiesel Byproduct Crude Glycerol for the Production of Bioenergy and Biochemicals
Abstract
1. Introduction
2. Impurities in Crude Glycerol and Hurdles to Use
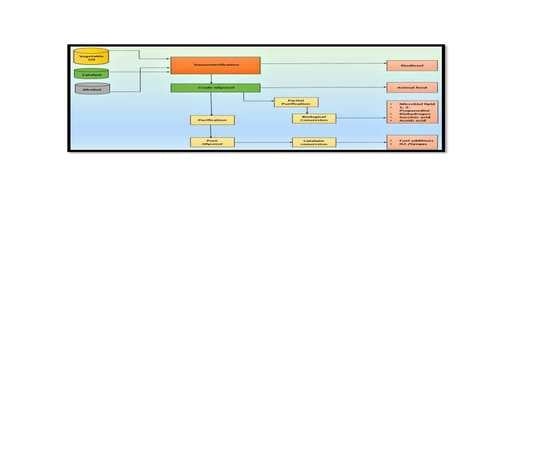
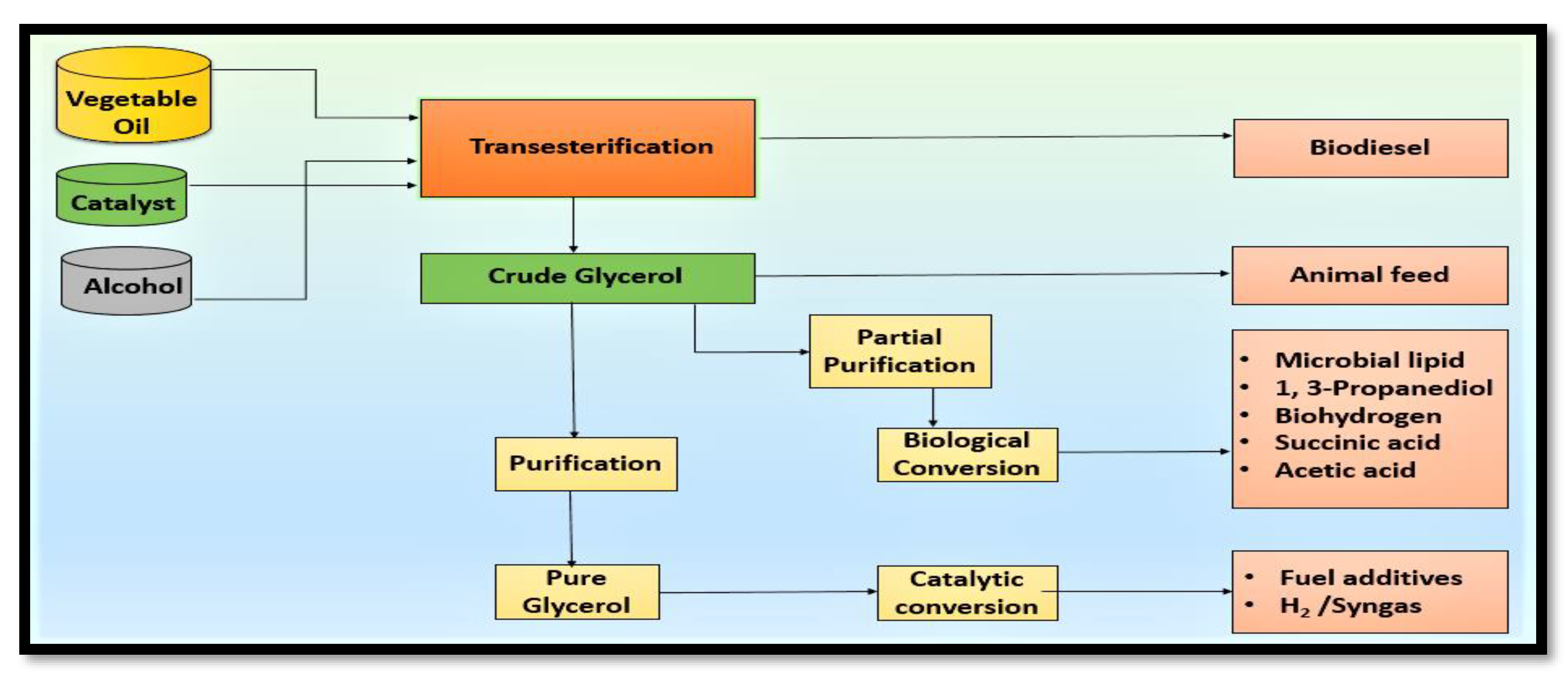
3. Technologies Studied for Value Addition of Glycerol
3.1. Chemo Catalytic Conversion
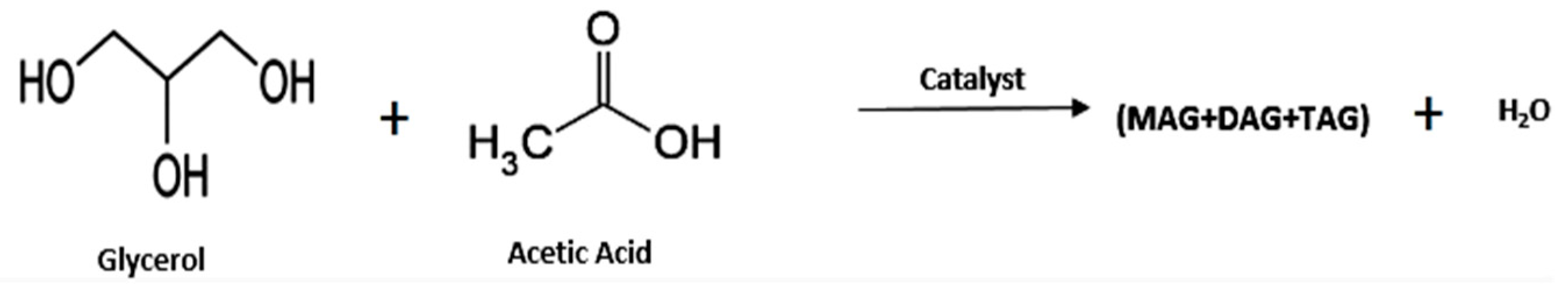
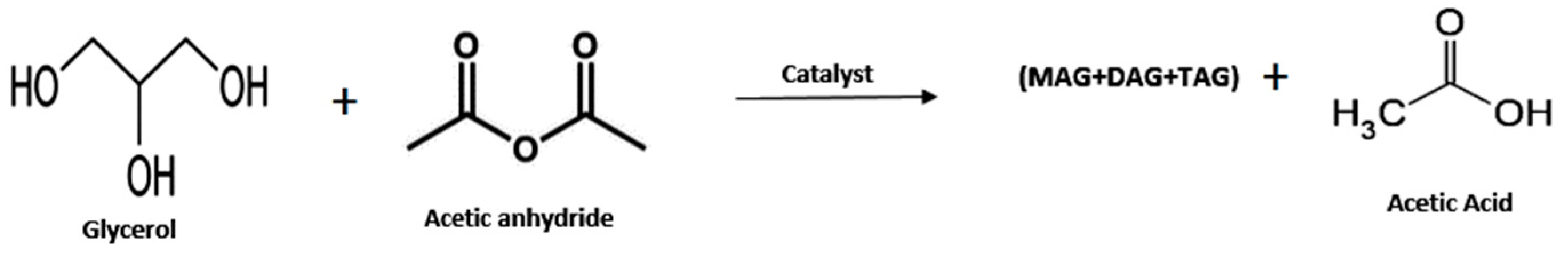
3.1.1. Oxidative Conversion of Glycerol to Fuel Additives
- 1.
- Glycerol Esters (Acetin)
- 2.
- Glycerol Ethers
- 3.
- Glycerol Formal
3.1.2. Hydrogen or Syngas Production from Glycerol
3.2. Direct Use or Minimal Treatment Products
3.3. Biological Conversion of Glycerol to Value-Added Chemicals
3.3.1. Microbial Lipids
3.3.2. Constraints for the Commercial Production of Microbial Lipid from Crude Glycerol
3.3.3. Production of 1, 3-Propanediol
3.3.4. Microbial Hydrogen Production
3.3.5. Succinic Acid
3.3.6. Citric Acid
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Inglesi-Lotz, R. The impact of renewable energy consumption to economic growth: A panel data application. Energy Econ. 2016, 53, 58–63. [Google Scholar] [CrossRef]
- Panwar, N.L.; Kaushik, S.C.; Kothari, S. Role of renewable energy sources in environmental protection: A review. Renew. Sustain. Energy Rev. 2011, 15, 1513–1524. [Google Scholar] [CrossRef]
- Van Gerpen, J. Biodiesel processing and production. Fuel Process. Technol. 2005, 86, 1097–1107. [Google Scholar] [CrossRef]
- Yousuf, A. Biodiesel from lignocellulosic biomass–Prospects and challenges. Waste Manag. 2012, 32, 2061–2067. [Google Scholar] [CrossRef]
- Cho, H.U.; Park, J.M. Biodiesel production by various oleaginous microorganisms from organic wastes. Bioresour. Technol. 2018, 256, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Yaakob, Z.; Mohammad, M.; Alherbawi, M.; Alam, Z.; Sopian, K. Overview of the production of biodiesel from waste cooking oil. Renew. Sustain. Energy Rev. 2013, 18, 184–193. [Google Scholar] [CrossRef]
- Pagliaro, M.; Rossi, M. Glycerol: Properties and production. In Future Glycerol; Royal Society of Chemistry’s: Cambridge, UK, 2010; pp. 20–21. [Google Scholar]
- Trchounian, K.; Trchounian, A. Hydrogen production from glycerol by Escherichia coli and other bacteria: An overview and perspectives. Appl. Energy 2015, 156, 174–184. [Google Scholar] [CrossRef]
- Bohon, M.D.; Metzger, B.A.; Linak, W.P.; King, C.J.; Roberts, W.L. Glycerol combustion and emissions. Proc. Combust. Inst. 2011, 33, 2717–2724. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Q.; Yang, W.Z.; Huo, W.J.; Dong, K.H.; Huang, Y.X.; Yang, X.M.; He, D.C. Effects of glycerol on lactation performance, energy balance and metabolites in early lactation Holstein dairy cows. Anim. Feed Sci. Technol. 2009, 151, 12–20. [Google Scholar] [CrossRef]
- Luo, X.; Ge, X.; Cui, S.; Li, Y. Value-added processing of crude glycerol into chemicals and polymers. Bioresour. Technol. 2016, 215, 144–154. [Google Scholar] [CrossRef]
- Yang, F.; Hanna, M.A.; Sun, R. Value-added uses for crude glycerol--a byproduct of biodiesel production. Biotechnol. Biofuels 2012, 5, 13. [Google Scholar] [CrossRef]
- Garlapati, V.K.; Shankar, U.; Budhiraja, A. Bioconversion technologies of crude glycerol to value added industrial products. Biotechnol. Rep. (Amst.) 2016, 9, 9–14. [Google Scholar] [CrossRef]
- Khanna, S.; Goyal, A.; Moholkar, V.S. Microbial conversion of glycerol: Present status and future prospects. Crit. Rev. Biotechnol. 2012, 32, 235–262. [Google Scholar] [CrossRef]
- Uprety, B.K.; Chaiwong, W.; Ewelike, C.; Rakshit, S.K. Biodiesel production using heterogeneous catalysts including wood ash and the importance of enhancing byproduct glycerol purity. Energy Convers. Manag. 2016, 115, 191–199. [Google Scholar] [CrossRef]
- Uprety, B.K.; Dalli, S.S.; Rakshit, S.K. Bioconversion of crude glycerol to microbial lipid using a robust oleaginous yeast Rhodosporidium toruloides ATCC 10788 capable of growing in the presence of impurities. Energy Convers. Manag. 2017, 135, 117–128. [Google Scholar] [CrossRef]
- Thompson, J.C.; He, B.B. Characterization of crude glycerol from biodiesel production from multiple feedstocks. Appl. Eng. Agric. 2006, 22, 261–265. [Google Scholar] [CrossRef]
- Hansen, C.F.; Hernandez, A.; Mullan, B.P.; Moore, K.; Trezona-Murray, M.; King, R.H.; Pluske, J.R. A chemical analysis of samples of crude glycerol from the production of biodiesel in Australia, and the effects of feeding crude glycerol to growing-finishing pigs on performance, plasma metabolites and meat quality at slaughter. Anim. Prod. Sci. 2009, 49, 154–161. [Google Scholar] [CrossRef]
- Tan, H.W.; Aziz, A.R.A.; Aroua, M.K. Glycerol production and its applications as a raw material: A review. Renew. Sustain. Energy Rev. 2013, 27, 118–127. [Google Scholar] [CrossRef]
- Kumar, L.R.; Yellapu, S.K.; Tyagi, R.D.; Zhang, X. A review on variation in crude glycerol composition, bio-valorization of crude and purified glycerol as carbon source for lipid production. Bioresour. Technol. 2019, 293, 122155. [Google Scholar] [CrossRef]
- Rodrigues, A.; Bordado, J.C.; Santos, R.G.D. Upgrading the glycerol from biodiesel production as a source of energy carriers and chemicals—A technological review for three chemical pathways. Energies 2017, 10, 1817. [Google Scholar] [CrossRef]
- Javani, A.; Hasheminejad, M.; Tahvildari, K.; Tabatabaei, M. High quality potassium phosphate production through step-by-step glycerol purification: A strategy to economize biodiesel production. Bioresour. Technol. 2012, 104, 788–790. [Google Scholar] [CrossRef] [PubMed]
- Isahak, W.; Ismail, M.; Yarmo, M.A.; Jahim, J.M.; Salimon, J. Purification of crude glycerol from transesterification RBD palm oil over homogeneous and heterogeneous catalysts for the biolubricant preparation. J. Appl. Sci. (Faisalabad) 2010, 10, 2590–2595. [Google Scholar] [CrossRef]
- Manosak, R.; Limpattayanate, S.; Hunsom, M. Sequential-refining of crude glycerol derived from waste used-oil methyl ester plant via a combined process of chemical and adsorption. Fuel Process. Technol. 2011, 92, 92–99. [Google Scholar] [CrossRef]
- Maleta, V.N.; Kiss, A.A.; Taran, V.M.; Maleta, B.V. Understanding process intensification in cyclic distillation systems. Chem. Eng. Process. Process Intensif. 2011, 50, 655–664. [Google Scholar] [CrossRef]
- Maleta, B.V.; Shevchenko, A.; Bedryk, O.; Kiss, A.A. Pilot-scale studies of process intensification by cyclic distillation. AIChE J. 2015, 61, 2581–2591. [Google Scholar] [CrossRef]
- Kiss, A.A.; Ignat, R.M. Enhanced methanol recovery and glycerol separation in biodiesel production—DWC makes it happen. Appl. Energy 2012, 99, 146–153. [Google Scholar] [CrossRef]
- Pyle, D.J.; Garcia, R.A.; Wen, Z. Producing docosahexaenoic acid (DHA)-rich algae from biodiesel-derived crude glycerol: Effects of impurities on DHA production and algal biomass composition. J. Agric. Food Chem. 2008, 56, 3933–3939. [Google Scholar] [CrossRef]
- Santibáñez, C.; Varnero, M.T.; Bustamante, M. Residual glycerol from biodiesel manufacturing, waste or potential source of bioenergy: A review. Chil. J. Agric. Res. 2011, 71, 469–475. [Google Scholar] [CrossRef]
- Hu, S.; Luo, X.; Wan, C.; Li, Y. Characterization of crude glycerol from biodiesel plants. J. Agric. Food Chem. 2012, 60, 5915–5921. [Google Scholar] [CrossRef]
- Nda-Umar, U.I.; Ramli, I.; Taufiq-Yap, Y.H.; Muhamad, E.N. An overview of recent research in the conversion of glycerol into biofuels, fuel additives and other bio-based chemicals. Catalysts 2019, 9, 15. [Google Scholar] [CrossRef]
- García, J.I.; García-Marín, H.; Pires, E. Glycerol based solvents: Synthesis, properties and applications. Green Chem. 2014, 16, 1007–1033. [Google Scholar] [CrossRef]
- Rahmat, N.; Abdullah, A.Z.; Mohamed, A.R. Recent progress on innovative and potential technologies for glycerol transformation into fuel additives: A critical review. Renew. Sustain. Energy Rev. 2010, 14, 987–1000. [Google Scholar] [CrossRef]
- Farinha, J.; Caiado, M.; Castanheiro, J. Valorisation of Glycerol into Biofuel Additives over Heterogeneous Catalysts; Formatex Research Center: Badajoz, Spain, 2013. [Google Scholar]
- Gonçalves, V.L.; Pinto, B.P.; Silva, J.C.; Mota, C.J. Acetylation of glycerol catalyzed by different solid acids. Catal. Today 2008, 133, 673–677. [Google Scholar] [CrossRef]
- Dosuna-Rodríguez, I.; Gaigneaux, E.M. Glycerol acetylation catalysed by ion exchange resins. Catal. Today 2012, 195, 14–21. [Google Scholar] [CrossRef]
- Liao, X.; Zhu, Y.; Wang, S.-G.; Li, Y. Producing triacetylglycerol with glycerol by two steps: Esterification and acetylation. Fuel Process. Technol. 2009, 90, 988–993. [Google Scholar] [CrossRef]
- Zhou, L.; Nguyen, T.-H.; Adesina, A.A. The acetylation of glycerol over amberlyst-15: Kinetic and product distribution. Fuel Process. Technol. 2012, 104, 310–318. [Google Scholar] [CrossRef]
- Balaraju, M.; Nikhitha, P.; Jagadeeswaraiah, K.; Srilatha, K.; Prasad, P.S.S.; Lingaiah, N. Acetylation of glycerol to synthesize bioadditives over niobic acid supported tungstophosphoric acid catalysts. Fuel Process. Technol. 2010, 91, 249–253. [Google Scholar] [CrossRef]
- Di Serio, M.; Casale, L.; Tesser, R.; Santacesaria, E. New process for the production of glycerol tert-butyl ethers. Energy Fuels 2010, 24, 4668–4672. [Google Scholar] [CrossRef]
- Usai, E.; Gualdi, E.; Solinas, V.; Battistel, E. Simultaneous enzymatic synthesis of FAME and triacetyl glycerol from triglycerides and methyl acetate. Bioresour. Technol. 2010, 101, 7707–7712. [Google Scholar] [CrossRef]
- Morales, G.; Paniagua, M.; Melero, J.A.; Vicente, G.; Ochoa, C. Sulfonic acid-functionalized catalysts for the valorization of glycerol via transesterification with methyl acetate. Ind. Eng. Chem. Res. 2011, 50, 5898–5906. [Google Scholar] [CrossRef]
- Testa, M.L.; La Parola, V.; Mesrar, F.; Ouanji, F.; Kacimi, M.; Ziyad, M.; Liotta, L.F. Use of Zirconium Phosphate-Sulphate as Acid Catalyst for Synthesis of Glycerol-Based Fuel Additives. Catalysts 2019, 9, 148. [Google Scholar] [CrossRef]
- Sánchez, J.A.; Hernández, D.L.; Moreno, J.A.; Mondragón, F.; Fernández, J.J. Alternative carbon based acid catalyst for selective esterification of glycerol to acetylglycerols. Appl. Catal. A Gen. 2011, 405, 55–60. [Google Scholar] [CrossRef]
- Sun, J.; Tong, X.; Yu, L.; Wan, J. An efficient and sustainable production of triacetin from the acetylation of glycerol using magnetic solid acid catalysts under mild conditions. Catal. Today 2016, 264, 115–122. [Google Scholar] [CrossRef]
- Cornejo, A.; Barrio, I.; Campoy, M.; Lázaro, J.; Navarrete, B. Oxygenated fuel additives from glycerol valorization. Main production pathways and effects on fuel properties and engine performance: A critical review. Renew. Sustain. Energy Rev. 2017, 79, 1400–1413. [Google Scholar] [CrossRef]
- Behr, A.; Eilting, J.; Irawadi, K.; Leschinski, J.; Lindner, F. Improved utilisation of renewable resources: New important derivatives of glycerol. Green Chem. 2008, 10, 13–30. [Google Scholar] [CrossRef]
- Jaecker-Voirol, A.; Durand, I.; Hillion, G.; Delfort, B.; Montagne, X. Glycerin for new biodiesel formulation. Oil Gas Sci. Technol. Rev. IFP 2008, 63, 395–404. [Google Scholar] [CrossRef]
- Mangourilos, V.; Bombos, D.; Juganaru, T.; Bolocan, I.; Bombos, M.; Ciuparu, D. Etherification of glycerol with isobutene on Amberlyst 35 ion exchange resin catalyst in presence of a cationic emulsifier. Rev. Chim. 2009, 60, 1338–1342. [Google Scholar]
- Behr, A.; Obendorf, L. Development of a process for the acid-catalyzed etherification of glycerine and isobutene forming glycerine tertiary butyl ethers. Eng. Life Sci. 2002, 2, 185–189. [Google Scholar] [CrossRef]
- Huang, R.; Kim, E.Y. Catalytic synthesis of glycerol tert-butyl ethers as fuel additives from the biodiesel by-product glycerol. J. Chem. 2015, 2015. [Google Scholar] [CrossRef]
- Maksimov, A.L.; Nekhaev, A.I.; Ramazanov, D.N.; Arinicheva, Y.A.; Dzyubenko, A.A.; Khadzhiev, S.N. Preparation of high-octane oxygenate fuel components from plant-derived polyols. Pet. Chem. 2011, 51, 61–69. [Google Scholar] [CrossRef]
- Mota, C.J.A.; da Silva, C.X.A.; Rosenbach Jr, N.; Costa, J.; da Silva, F. Glycerin derivatives as fuel additives: The addition of glycerol/acetone ketal (solketal) in gasolines. Energy Fuels 2010, 24, 2733–2736. [Google Scholar] [CrossRef]
- Suriyaprapadilok, N.; Kitiyanan, B. Synthesis of solketal from glycerol and its reaction with benzyl alcohol. Energy Procedia 2011, 9, 63–69. [Google Scholar] [CrossRef]
- Nanda, M.R.; Zhang, Y.; Yuan, Z.; Qin, W.; Ghaziaskar, H.S.; Xu, C.C. Catalytic conversion of glycerol for sustainable production of solketal as a fuel additive: A review. Renew. Sustain. Energy Rev. 2016, 56, 1022–1031. [Google Scholar] [CrossRef]
- Silva, P.H.R.; Gonçalves, V.L.C.; Mota, C.J.A. Glycerol acetals as anti-freezing additives for biodiesel. Bioresour. Technol. 2010, 101, 6225–6229. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, E.A.; D’Angelo, M.A.; Comelli, R.A. Hydrogen production from glycerol on Ni/Al2O3 catalyst. Int. J. Hydrog. Energy 2010, 35, 5902–5907. [Google Scholar] [CrossRef]
- Aguado-Deblas, L.; Estevez, R.; Russo, M.; La Parola, V.; Bautista, F.M.; Testa, M.L. Microwave-Assisted Glycerol Etherification Over Sulfonic Acid Catalysts. Materials 2020, 13, 1584. [Google Scholar] [CrossRef]
- Karinen, R.S.; Krause, A.O.I. New biocomponents from glycerol. Appl. Catal. A Gen. 2006, 306, 128–133. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, B.; Yi, C.; Lei, Z.; Xu, J. Etherification of glycerol with isobutylene to produce oxygenate additive using sulfonated peanut shell catalyst. Ind. Eng. Chem. Res. 2010, 49, 12399–12404. [Google Scholar] [CrossRef]
- Royon, D.; Locatelli, S.; Gonzo, E.E. Ketalization of glycerol to solketal in supercritical acetone. J. Supercrit. Fluids 2011, 58, 88–92. [Google Scholar] [CrossRef]
- Da Silva, M.J.; de Oliveira Guimaraes, M.; Julio, A.A. A highly regioselective and solvent-free Sn (II)-catalyzed glycerol ketals synthesis at room temperature. Catal. Lett. 2015, 145, 769–776. [Google Scholar] [CrossRef]
- Yang, J.; Li, N.; Ma, W.J.; Zhou, J.H.; Sun, H.Z. Synthesis of Solketal with Catalyst Sulfonic Acid Resin; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2014; pp. 176–179. [Google Scholar]
- Avasthi, K.S.; Reddy, R.N.; Patel, S. Challenges in the production of hydrogen from glycerol—A biodiesel byproduct via steam reforming process. Procedia Eng. 2013, 51, 423–429. [Google Scholar] [CrossRef]
- Adhikari, S.; Fernando, S.D.; Haryanto, A. Hydrogen production from glycerin by steam reforming over nickel catalysts. Renew. Energy 2008, 33, 1097–1100. [Google Scholar] [CrossRef]
- Wang, W. Thermodynamic analysis of glycerol partial oxidation for hydrogen production. Fuel Process. Technol. 2010, 91, 1401–1408. [Google Scholar] [CrossRef]
- Vaidya, P.D.; Rodrigues, A.E. Glycerol reforming for hydrogen production: A review. Chem. Eng. Technol. Ind. Chem. Plant Equip. Proc. Eng. Biotechnol. 2009, 32, 1463–1469. [Google Scholar] [CrossRef]
- Luo, N.; Zhao, X.; Cao, F.; Xiao, T.; Fang, D. Thermodynamic study on hydrogen generation from different glycerol reforming processes. Energy Fuels 2007, 21, 3505–3512. [Google Scholar] [CrossRef]
- Huber, G.W.; Cortright, R.D.; Dumesic, J.A. Renewable alkanes by aqueous-phase reforming of biomass-derived oxygenates. Angew. Chem. Int. Ed. 2004, 43, 1549–1551. [Google Scholar] [CrossRef]
- Davda, R.R.; Shabaker, J.W.; Huber, G.W.; Cortright, R.D.; Dumesic, J.A. A review of catalytic issues and process conditions for renewable hydrogen and alkanes by aqueous-phase reforming of oxygenated hydrocarbons over supported metal catalysts. Appl. Catal. B Environ. 2005, 56, 171–186. [Google Scholar] [CrossRef]
- Lin, Y.-C. Catalytic valorization of glycerol to hydrogen and syngas. Int. J. Hydrog. Energy 2013, 38, 2678–2700. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, X.; Azmat, M.U.; Xu, W.; Ren, J.; Wang, Y.; Lu, G. Hydrogen production by aqueous-phase reforming of glycerol over Ni-B catalysts. Int. J. Hydrog. Energy 2012, 37, 227–234. [Google Scholar] [CrossRef]
- Iriondo, A.; Cambra, J.F.; Güemez, M.B.; Barrio, V.L.; Requies, J.; Sánchez-Sánchez, M.C.; Navarro, R.M. Effect of ZrO2 addition on Ni/Al2O3 catalyst to produce H2 from glycerol. Int. J. Hydrog. Energy 2012, 37, 7084–7093. [Google Scholar] [CrossRef]
- Dou, B.; Dupont, V.; Rickett, G.; Blakeman, N.; Williams, P.T.; Chen, H.; Ding, Y.; Ghadiri, M. Hydrogen production by sorption-enhanced steam reforming of glycerol. Bioresour. Technol. 2009, 100, 3540–3547. [Google Scholar] [CrossRef] [PubMed]
- Kamonsuangkasem, K.; Therdthianwong, S.; Therdthianwong, A. Hydrogen production from yellow glycerol via catalytic oxidative steam reforming. Fuel Process. Technol. 2013, 106, 695–703. [Google Scholar] [CrossRef]
- Tuza, P.V.; Manfro, R.L.; Ribeiro, N.F.P.; Souza, M.M.V.M. Production of renewable hydrogen by aqueous-phase reforming of glycerol over Ni-Cu catalysts derived from hydrotalcite precursors. Renew. Energy 2013, 50, 408–414. [Google Scholar] [CrossRef]
- Liu, Y.; Farrauto, R.; Lawal, A. Autothermal reforming of glycerol in a dual layer monolith catalyst. Chem. Eng. Sci. 2013, 89, 31–39. [Google Scholar] [CrossRef]
- Dauenhauer, P.J.; Salge, J.R.; Schmidt, L.D. Renewable hydrogen by autothermal steam reforming of volatile carbohydrates. J. Catal. 2006, 244, 238–247. [Google Scholar] [CrossRef]
- Xu, D.; Wang, S.; Hu, X.; Chen, C.; Zhang, Q.; Gong, Y. Catalytic gasification of glycine and glycerol in supercritical water. Int. J. Hydrog. Energy 2009, 34, 5357–5364. [Google Scholar] [CrossRef]
- Johnson, R.B. The treatment of ketosis with glycerol and propylene glycol. Cornell Vet. 1954, 44, 6–21. [Google Scholar]
- Cottrill, B.; Berry, P.; Smith, C. Opportunities and Implications of Using Co-Products from Biofuel Production as Feeds for Livestock; Citeseer: Princeton, NJ, USA, 2007. [Google Scholar]
- Drackley, J.K. Opportunities for glycerol use in dairy diets. In Proceedings of the Four-State Dairy Nutrition and Management Conference, Dubuque, Iowa, 11–12 June 2008; p. 113. [Google Scholar]
- Bodarski, R.; Wertelecki, T.; Bommer, F.; Gosiewski, S. The changes of metabolic status and lactation performance in dairy cows under feeding TMR with glycerin [glycerol] supplement at periparturient period. Electron. J. Pol. Agric. Univ. Ser. Anim. Husb. 2005, 84, 1–9. [Google Scholar]
- Swiatkiewicz, S.; Koreleski, J. Effect of crude glycerin level in the diet of laying hens on egg performance and nutrient utilization. Poult. Sci. 2009, 88, 615–619. [Google Scholar] [CrossRef]
- McLea, L.; Ball, M.E.E.; Kilpatrick, D.; Elliott, C. The effect of glycerol inclusion on broiler performance and nutrient digestibility. Br. Poult. Sci. 2011, 52, 368–375. [Google Scholar] [CrossRef]
- Hampy, K.R.; Kellogg, D.W.; Coffey, K.P.; Kegley, E.B.; Caldwell, J.D.; Lee, M.S.; Akins, M.S.; Reynolds, J.L.; Moore, J.C.; Southern, K.D. Glycerol as a supplemental energy source for meat goats. AAES Res. Ser. 2008, 553, 63–64. [Google Scholar]
- Kholif, A.E. Glycerol use in dairy diets: A systemic review. Anim. Nutr. 2019, 5, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Liu, L.; Ye, X.P. Acrolein Production from Crude Glycerol in Sub-and Super-Critical Water. J. Am. Oil Chem. Soc. 2013, 90, 601–610. [Google Scholar] [CrossRef]
- San Kong, P.; Aroua, M.K.; Daud, W.M.A.W. Conversion of crude and pure glycerol into derivatives: A feasibility evaluation. Renew. Sustain. Energy Rev. 2016, 63, 533–555. [Google Scholar] [CrossRef]
- Kamzolova, S.V.; Fatykhova, A.R.; Dedyukhina, E.G.; Anastassiadis, S.G.; Golovchenko, N.P.; Morgunov, I.G. Citric acid production by yeast grown on glycerol-containing waste from biodiesel industry. Food Technol. Biotechnol. 2011, 49, 65–74. [Google Scholar]
- Steinig, G.H.; Livingston, A.G.; Stuckey, D.C. Bioconversion of hydrophobic compounds in a continuous closed-gas-loop bioreactor: Feasibility assessment and epoxide production. Biotechnol. Bioeng. 2000, 70, 553–563. [Google Scholar] [CrossRef]
- Wallace, S.; Balskus, E.P. Opportunities for merging chemical and biological synthesis. Curr. Opin. Biotechnol. 2014, 30, 1–8. [Google Scholar] [CrossRef]
- Uprety, B.K.; Reddy, J.V.; Dalli, S.S.; Rakshit, S.K. Utilization of microbial oil obtained from crude glycerol for the production of polyol and its subsequent conversion to polyurethane foams. Bioresour. Technol. 2017, 235, 309–315. [Google Scholar] [CrossRef]
- Samavi, M.; Uprety, B.K.; Rakshit, S. Bioconversion of Poplar Wood Hemicellulose Prehydrolysate to Microbial Oil Using Cryptococcus curvatus. Appl. Biochem. Biotechnol. 2019, 189, 626–637. [Google Scholar] [CrossRef]
- Zhang, D.; Feng, X.; Zhou, Z.; Zhang, Y.; Xu, H. Economical production of poly (γ-glutamic acid) using untreated cane molasses and monosodium glutamate waste liquor by Bacillus subtilis NX-2. Bioresour. Technol. 2012, 114, 583–588. [Google Scholar] [CrossRef]
- Da Silva, G.P.; Mack, M.; Contiero, J. Glycerol: A promising and abundant carbon source for industrial microbiology. Biotechnol. Adv. 2009, 27, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Block, D.E.; Mills, D.A. Simultaneous consumption of pentose and hexose sugars: An optimal microbial phenotype for efficient fermentation of lignocellulosic biomass. Appl. Microbiol. Biotechnol. 2010, 88, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Dharmadi, Y.; Murarka, A.; Gonzalez, R. Anaerobic fermentation of glycerol by Escherichia coli: A new platform for metabolic engineering. Biotechnol. Bioeng. 2006, 94, 821–829. [Google Scholar] [CrossRef]
- Karamerou, E.E.; Webb, C. Cultivation modes for microbial oil production using oleaginous yeasts—A review. Biochem. Eng. J. 2019, 151, 107322. [Google Scholar] [CrossRef]
- Uprety, B.K.; Venkatesagowda, B.; Rakshit, S.K. Current prospects on production of microbial lipid and other value-added products using crude glycerol obtained from biodiesel industries. Bioenergy Res. 2017, 10, 1117–1137. [Google Scholar] [CrossRef]
- Beopoulos, A.; Cescut, J.; Haddouche, R.; Uribelarrea, J.-L.; Molina-Jouve, C.; Nicaud, J.-M. Yarrowia lipolytica as a model for bio-oil production. Prog. Lipid Res. 2009, 48, 375–387. [Google Scholar] [CrossRef]
- Thevenieau, F.; Nicaud, J.-M. Microorganisms as sources of oils. OCL 2013, 20, D603. [Google Scholar] [CrossRef]
- Sun, X.-M.; Ren, L.-J.; Zhao, Q.-Y.; Ji, X.-J.; Huang, H. Enhancement of lipid accumulation in microalgae by metabolic engineering. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2019, 1864, 552–566. [Google Scholar] [CrossRef]
- Scott, S.A.; Davey, M.P.; Dennis, J.S.; Horst, I.; Howe, C.J.; Lea-Smith, D.J.; Smith, A.G. Biodiesel from algae: Challenges and prospects. Curr. Opin. Biotechnol. 2010, 21, 277–286. [Google Scholar] [CrossRef]
- Garay, L.A.; Boundy-Mills, K.L.; German, J.B. Accumulation of high-value lipids in single-cell microorganisms: A mechanistic approach and future perspectives. J. Agric. Food Chem. 2014, 62, 2709–2727. [Google Scholar] [CrossRef]
- Liang, Y.; Cui, Y.; Trushenski, J.; Blackburn, J.W. Converting crude glycerol derived from yellow grease to lipids through yeast fermentation. Bioresour. Technol. 2010, 101, 7581–7586. [Google Scholar] [CrossRef]
- Fakas, S.; Papanikolaou, S.; Galiotou-Panayotou, M.; Komaitis, M.; Aggelis, G. Biochemistry and Biotechnology of Single Cell Oil; University of Patras: Patras, Greece, 2008; pp. 38–60. [Google Scholar]
- Martínez, E.J.; Raghavan, V.; González-Andrés, F.; Gómez, X. New biofuel alternatives: Integrating waste management and single cell oil production. Int. J. Mol. Sci. 2015, 16, 9385–9405. [Google Scholar] [CrossRef] [PubMed]
- Ratledge, C.; Wynn, J.P. The biochemistry and molecular biology of lipid accumulation in oleaginous microorganisms. Adv. Appl. Microbiol. 2002, 51, 1–52. [Google Scholar] [PubMed]
- Huang, C.; Chen, X.-F.; Xiong, L.; Ma, L.-L.; Chen, Y. Single cell oil production from low-cost substrates: The possibility and potential of its industrialization. Biotechnol. Adv. 2013, 31, 129–139. [Google Scholar] [CrossRef]
- Uprety, B.K.; Rakshit, S.K. Compositional shift in fatty acid profiles of lipids obtained from oleaginous yeasts upon the addition of essential oil from Citrus sinensis L. Appl. Biochem. Biotechnol. 2017, 183, 1158–1172. [Google Scholar] [CrossRef] [PubMed]
- Uprety, B.K.; Rakshit, S.K. Use of essential oils from various plants to change the fatty acids profiles of lipids obtained from oleaginous yeasts. J. Am. Oil Chem. Soc. 2018, 95, 135–148. [Google Scholar] [CrossRef]
- Suutari, M.; Priha, P.; Laakso, S. Temperature shifts in regulation of lipids accumulated byLipomyces starkeyi. J. Am. Oil Chem. Soc. 1993, 70, 891–894. [Google Scholar] [CrossRef]
- Duarte, S.H.; de Andrade, C.C.P.; Ghiselli, G.; Maugeri, F. Exploration of Brazilian biodiversity and selection of a new oleaginous yeast strain cultivated in raw glycerol. Bioresour. Technol. 2013, 138, 377–381. [Google Scholar] [CrossRef]
- Fakas, S.; Papanikolaou, S.; Batsos, A.; Galiotou-Panayotou, M.; Mallouchos, A.; Aggelis, G. Evaluating renewable carbon sources as substrates for single cell oil production by Cunninghamella echinulata and Mortierella isabellina. Biomass Bioenergy 2009, 33, 573–580. [Google Scholar] [CrossRef]
- Ardi, M.S.; Aroua, M.K.; Hashim, N.A. Progress, prospect and challenges in glycerol purification process: A review. Renew. Sustain. Energy Rev. 2015, 42, 1164–1173. [Google Scholar] [CrossRef]
- Tang, S.; Boehme, L.; Lam, H.; Zhang, Z. Pichia pastoris fermentation for phytase production using crude glycerol from biodiesel production as the sole carbon source. Biochem. Eng. J. 2009, 43, 157–162. [Google Scholar] [CrossRef]
- Gao, Z.; Ma, Y.; Wang, Q.; Zhang, M.; Wang, J.; Liu, Y. Effect of crude glycerol impurities on lipid preparation by Rhodosporidium toruloides yeast 32489. Bioresour. Technol. 2016, 218, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Mothes, G.; Schnorpfeil, C.; Ackermann, J.U. Production of PHB from crude glycerol. Eng. Life Sci. 2007, 7, 475–479. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Fakas, S.; Fick, M.; Chevalot, I.; Galiotou-Panayotou, M.; Komaitis, M.; Marc, I.; Aggelis, G. Biotechnological valorisation of raw glycerol discharged after bio-diesel (fatty acid methyl esters) manufacturing process: Production of 1,3-propanediol, citric acid and single cell oil. Biomass Bioenergy 2008, 32, 60–71. [Google Scholar] [CrossRef]
- Liu, L.-P.; Hu, Y.; Lou, W.-Y.; Li, N.; Wu, H.; Zong, M.-H. Use of crude glycerol as sole carbon source for microbial lipid production by oleaginous yeasts. Appl. Biochem. Biotechnol. 2017, 182, 495–510. [Google Scholar] [CrossRef]
- Samavi, M.; Rakshit, S. Utilization of Microbial Oil from Poplar Wood Hemicellulose Prehydrolysate for the Production of Polyol Using Chemo-enzymatic Epoxidation. Biotechnol. Bioprocess Eng. 2020, 25, 1–9. [Google Scholar]
- Drozdzynska, A.; Leja, K.; Czaczyk, K. Biotechnological production of 1,3-propanediol from crude glycerol. Biotechnol. J. Biotechnol. Comput. Biol. Bionanotechnol. 2011, 92–100. [Google Scholar]
- Xu, Y.; Shang, S.; Huang, J. Crystallization behavior of poly (trimethylene terephthalate)-poly (ethylene glycol) segmented copolyesters/multi-walled carbon nanotube nanocomposites. Polym. Test. 2010, 29, 1007–1013. [Google Scholar] [CrossRef]
- González-Pajuelo, M.; Meynial-Salles, I.; Mendes, F.; Soucaille, P.; Vasconcelos, I. Microbial conversion of glycerol to 1,3-propanediol: Physiological comparison of a natural producer, Clostridium butyricum VPI 3266, and an engineered strain, Clostridium acetobutylicum DG1 (pSPD5). Appl. Environ. Microbiol. 2006, 72, 96–101. [Google Scholar] [CrossRef]
- Drożdżyńska, A.; Pawlicka, J.; Kubiak, P.; Kośmider, A.; Pranke, D.; Olejnik-Schmidt, A.; Czaczyk, K. Conversion of glycerol to 1,3-propanediol by Citrobacter freundii and Hafnia alvei—Newly isolated strains from the Enterobacteriaceae. New Biotechnol. 2014, 31, 402–410. [Google Scholar] [CrossRef]
- Saxena, R.K.; Anand, P.; Saran, S.; Isar, J. Microbial production of 1,3-propanediol: Recent developments and emerging opportunities. Biotechnol. Adv. 2009, 27, 895–913. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, G.P.; De Lima, C.J.B.; Contiero, J. Production and productivity of 1,3-propanediol from glycerol by Klebsiella pneumoniae GLC29. Catal. Today 2015, 257, 259–266. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, S.; Wang, Y.; Fang, B. Key enzymes catalyzing glycerol to 1,3-propanediol. Biotechnol. Biofuels 2016, 9, 57. [Google Scholar] [PubMed]
- Samul, D.; Leja, K.; Grajek, W. Impurities of crude glycerol and their effect on metabolite production. Ann. Microbiol. 2014, 64, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Chatzifragkou, A.; Papanikolaou, S.; Dietz, D.; Doulgeraki, A.I.; Nychas, G.-J.E.; Zeng, A.-P. Production of 1,3-propanediol by Clostridium butyricum growing on biodiesel-derived crude glycerol through a non-sterilized fermentation process. Appl. Microbiol. Biotechnol. 2011, 91, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Jun, S.-A.; Moon, C.; Kang, C.-H.; Kong, S.W.; Sang, B.-I.; Um, Y. Microbial fed-batch production of 1,3-propanediol using raw glycerol with suspended and immobilized Klebsiella pneumoniae. Appl. Biochem. Biotechnol. 2010, 161, 491–501. [Google Scholar] [CrossRef]
- Trchounian, A. Mechanisms for hydrogen production by different bacteria during mixed-acid and photo-fermentation and perspectives of hydrogen production biotechnology. Crit. Rev. Biotechnol. 2015, 35, 103–113. [Google Scholar] [CrossRef]
- Maintinguer, S.I.; Hatanaka, R.R.; De Oliveira, J.E. Glycerol as a raw material for hydrogen production. In Biofuels-Status and Perspective; InTech: Rijeka, Croatia, 2015. [Google Scholar]
- Hallenbeck, P.C. Fermentative hydrogen production: Principles, progress, and prognosis. Int. J. Hydrog. Energy 2009, 34, 7379–7389. [Google Scholar] [CrossRef]
- Mangayil, R.; Karp, M.; Santala, V. Bioconversion of crude glycerol from biodiesel production to hydrogen. Int. J. Hydrog. Energy 2012, 37, 12198–12204. [Google Scholar] [CrossRef]
- Lo, Y.-C.; Chen, X.-J.; Huang, C.-Y.; Yuan, Y.-J.; Chang, J.-S. Dark fermentative hydrogen production with crude glycerol from biodiesel industry using indigenous hydrogen-producing bacteria. Int. J. Hydrog. Energy 2013, 38, 15815–15822. [Google Scholar] [CrossRef]
- Pott, R.W.M.; Howe, C.J.; Dennis, J.S. Photofermentation of crude glycerol from biodiesel using Rhodopseudomonas palustris: Comparison with organic acids and the identification of inhibitory compounds. Bioresour. Technol. 2013, 130, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Chookaew, T.; Prasertsan, P.; Ren, Z.J. Two-stage conversion of crude glycerol to energy using dark fermentation linked with microbial fuel cell or microbial electrolysis cell. New Biotechnol. 2014, 31, 179–184. [Google Scholar] [CrossRef]
- Pinazo, J.M.; Domine, M.E.; Parvulescu, V.; Petru, F. Sustainability metrics for succinic acid production: A comparison between biomass-based and petrochemical routes. Catal. Today 2015, 239, 17–24. [Google Scholar] [CrossRef]
- Succinic Acid: Market by Type (Biobased, Petro-based), Application (Polyurethane, Resins, Coatings &Pigments, Pharmaceuticals, Plasticizers, Food & Beverage, PBS/PBST, Solvents & Lubricants, De-Icer Solutions, Personal Care, and Others), and by Region—Global Forecast to 2021; Markets and Markets Research Private Ltd.: Pune, India, July 2016; p. 208. Available online: https://www.marketsandmarkets.com/Market-Reports/succinic-acid-market-402.html (accessed on 16 April 2020).
- Byun, M.Y.; Kim, J.S.; Baek, J.H.; Park, D.-W.; Lee, M.S. Liquid-Phase Hydrogenation of Maleic Acid over Pd/Al2O3 Catalysts Prepared via Deposition-Precipitation Method. Energies 2019, 12, 284. [Google Scholar] [CrossRef]
- Guettler, M.V.; Rumler, D.; Jain, M.K. Actinobacillus succinogenes sp. nov., a novel succinic-acid-producing strain from the bovine rumen. Int. J. Syst. Evol. Microbiol. 1999, 49, 207–216. [Google Scholar] [CrossRef]
- Lee, P.; Lee, S.; Hong, S.; Chang, H. Isolation and characterization of a new succinic acid-producing bacterium, Mannheimia succiniciproducens MBEL55E, from bovine rumen. Appl. Microbiol. Biotechnol. 2002, 58, 663–668. [Google Scholar] [PubMed]
- Lee, P.C.; Lee, S.Y.; Chang, H.N. Kinetic study on succinic acid and acetic acid formation during continuous cultures of Anaerobiospirillum succiniciproducens grown on glycerol. Bioprocess Biosyst. Eng. 2010, 33, 465–471. [Google Scholar] [CrossRef]
- Lin, S.K.C.; Du, C.; Koutinas, A.; Wang, R.; Webb, C. Substrate and product inhibition kinetics in succinic acid production by Actinobacillus succinogenes. Biochem. Eng. J. 2008, 41, 128–135. [Google Scholar] [CrossRef]
- Scholten, E.; Renz, T.; Thomas, J. Continuous cultivation approach for fermentative succinic acid production from crude glycerol by Basfia succiniciproducens DD1. Biotechnol. Lett. 2009, 31, 1947. [Google Scholar] [CrossRef]
- Gao, C.; Yang, X.; Wang, H.; Rivero, C.P.; Li, C.; Cui, Z.; Qi, Q.; Lin, C.S.K. Robust succinic acid production from crude glycerol using engineered Yarrowia lipolytica. Biotechnol. Biofuels 2016, 9, 179. [Google Scholar] [CrossRef]
- Kang, Z.; Du, L.; Kang, J.; Wang, Y.; Wang, Q.; Liang, Q.; Qi, Q. Production of succinate and polyhydroxyalkanoate from substrate mixture by metabolically engineered Escherichia coli. Bioresour. Technol. 2011, 102, 6600–6604. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Hong, S.H.; Lee, S.H.; Park, S.J. Fermentative production of chemicals that can be used for polymer synthesis. Macromol. Biosci. 2004, 4, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.; Matos, M.; Roca, C.; Reis, M.A.M. Succinic acid production from glycerol by Actinobacillus succinogenes using dimethylsulfoxide as electron acceptor. New Biotechnol. 2014, 31, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Lotfy, W.A.; Ghanem, K.M.; El-Helow, E.R. Citric acid production by a novel Aspergillus niger isolate: II. Optimization of process parameters through statistical experimental designs. Bioresour. Technol. 2007, 98, 3470–3477. [Google Scholar] [CrossRef]
- Levinson, W.E.; Kurtzman, C.P.; Kuo, T.M. Characterization of Yarrowia lipolytica and related species for citric acid production from glycerol. Enzyme Microb. Technol. 2007, 41, 292–295. [Google Scholar] [CrossRef]
- Kamzolova, S.V.; Shishkanova, N.V.; Il’Chenko, A.P.; Sal’nikova, I.V.; Arzumanov, T.E.; Finogenova, T.V. Biosynthesis of citric and isocitric acids by Yarrowia lipolytica N1, at various concentrations of oxygen and iron in the culture medium. Appl. Biochem. Microbiol. 1997, 33, 359–363. [Google Scholar]
- Imandi, S.B.; Bandaru, V.R.; Somalanka, S.R.; Garapati, H.R. Optimization of medium constituents for the production of citric acid from byproduct glycerol using Doehlert experimental design. Enzyme Microb. Technol. 2007, 40, 1367–1372. [Google Scholar] [CrossRef]
- Yalcin, S.K.; Bozdemir, M.T.; Ozbas, Z.Y. Citric acid production by yeasts: Fermentation conditions, process optimization and strain improvement. Curr. Res.Technol. Educ. Top. Appl. Microbiol. Microb. Biotechnol. 2010, 9, 1374–1382. [Google Scholar]
- Rywinska, A.; Rymowicz, W.; Zarowska, B.; Wojtatowicz, M. Biosynthesis of citric acid from glycerol by acetate mutants of Yarrowia lipolytica in fed-batch fermentation. Food Technol. Biotechnol. 2009, 47, 1–6. [Google Scholar]
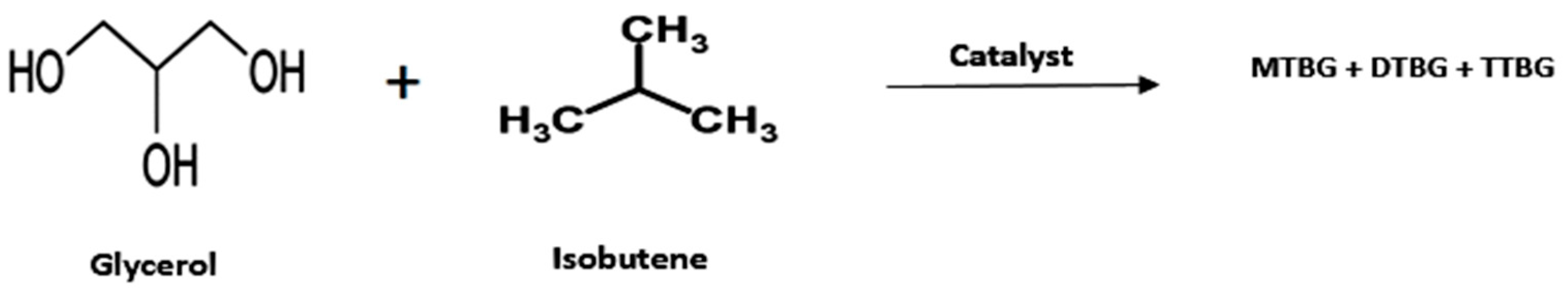
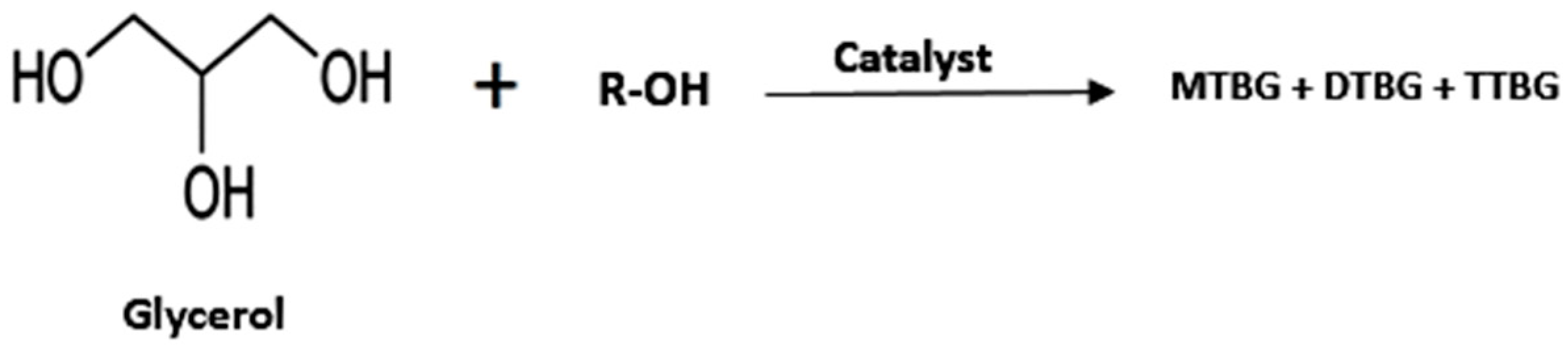
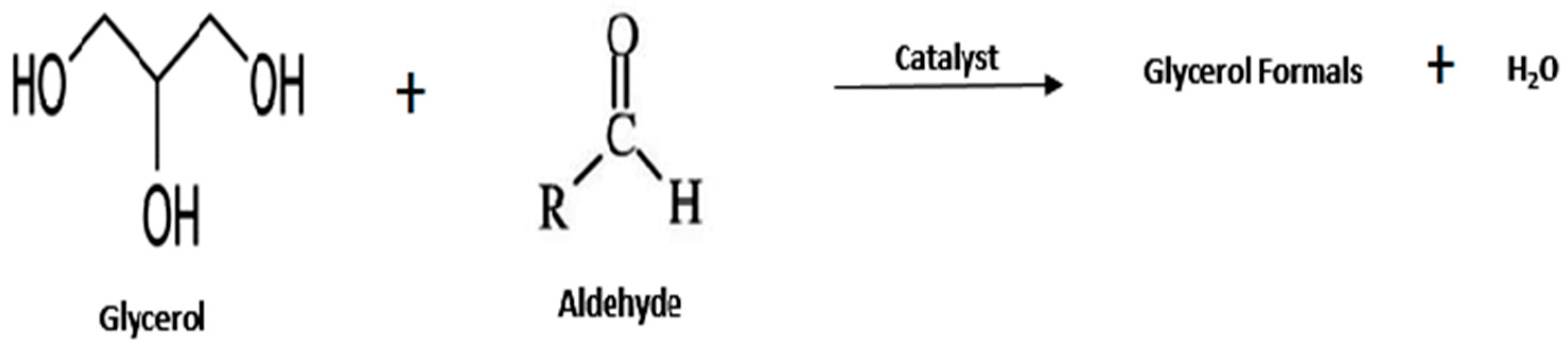

| Entry | Reactant | Catalyst | Operating Condition | % Conv. (glycerol) | Yield (%) | Ref. |
|---|---|---|---|---|---|---|
| 1 | Acetic acid | Catalyst Free | MR acetic acid to glycerol 6:1, 378 K, 4 h | 74 | 2 1 | [37] |
| 2 | Acetic acid | Zr3(PO4)2(SO4)3 | Catalyst 5 wt%, MR acetic acid to glycerol 3:1, 105 °C, 3 h | 100 | 53 1 | [43] |
| 3 | Acetic acid | Zr4(PO4)2(SO4)5 | Catalyst 5 wt%, MR acetic acid to glycerol 3:1, 105 °C, 3 h | 100 | 48 1 | [43] |
| 4 | Acetic acid | Zr (SO4)2 | Catalyst 5 wt%, MR acetic acid to Glycerol 3:1, 105 °C, 3 h | 60 | NA | [43] |
| 5 | Acetic acid | Amberlyst-15 | Catalyst 0.2 mol%, MR acetic acid to glycerol 3:1, room temp, 30 min | 97 | 90 1 | [35] |
| 6 | Acetic acid | TAC-673 Catalyst | Catalyst 5 wt%, MR acetic acid to glycerol 9:1, 378 K, 4 h | >99 | 17 1 | [57] |
| 7 | Acetic anhydride | H-Beta zeolite | Catalyst 2.0 mmol acid sites, MR acetic a hydride to glycerol 4:1, 393 K, 2 h | 94 | 43 1 | [56] |
| 8 | Acetic anhydride | Fe-Sn-Ti (SO4 2−)-400 | Catalyst 0.05 g, glycerol 1.5 g, acetic anhydride 8.39 g, 80 °C, 30 min | 100 | 99 1 | [45] |
| 9 | Acetic anhydride | Amberlyst-15 | Catalyst 0.05 g, glycerol 1.5 g, acetic anhydride 8.39 g, 80 °C, 30 min | 99 | 99 1 | [45] |
| 10 | Acetic anhydride | HZSM-5 | Catalyst 0.05 g, glycerol 1.5 g, acetic anhydride 8.39 g, 80 °C, 30 min | 99 | 24 1 | [45] |
| 11 | Tert Butyl alcohol (TBA) | Amberlyst-15 | Catalyst 7.5 wt%, TBA/glycerol molar ratio 8, 70 °C, 5–8 h | 97 | 30.3 2 | [51] |
| 12 | Tert Butyl alcohol (TBA) | SiO2-SO3H | Catalyst 5 wt%, TBA/glycerol = 2 mol/mol, 30 min, 130 °C | 78 | 24 2 | [58] |
| 13 | Isobutene (IB) | p-toluene sulfonic acid | Catalyst 2.16 wt%, IB/glycerol = 4 mol/mol, 5 h, 90 °C, 1.4 bar | 89 | 47 2 | [50] |
| 14 | Isobutene (IB) | Amberlyst-15 | Catalyst 1 g, IB/glycerol = 4 mol/mol, 7 h, 80 °C, 15 bar | >95 | 97 2 | [59] |
| 15 | Isobutene (IB) | Sulfonated peanut shell | Catalyst 6 wt%, IB/glycerol = 4 mol/mol, 2 h, 70 °C, 15 bar | 100 | 92 2 | [60] |
| 16 | Acetone | Amberlyst-36 | Acetone to glycerol ratio 4:1, 25 °C, 500 psi | 100 | 96 3 | [55] |
| 17 | Acetone | catalyst free | Reaction at super critical condition, 508 K, 8 MPa, 240 min | 28 | 80 3 | [61] |
| 18 | Acetone | SnCl2 | Catalyst: 1 wt%, acetone/glycerol = 8:1 | 78 | 76 3 | [62] |
| 19 | Acetone | DT-851 sulfonic acid resin | Catalyst: 5%, acetone/glycerol: 20:1, 58 °C, 10 bar | 95 | 99 3 | [63] |
| 20 | Benzalde-hyde | K10 montmorillonite | Benzaldehyde/glycerol: 1.1:1, 40 °C, 6 h | 83 | 99 3 | [63] |
| Entry | Reforming Technology | System of Operation | Glycerol/Water | Catalyst | Temp (°C) | Pressure | H2 Yield (%) | XG (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Steam reforming | Fixed bed reactor | 1:16 | Ni/Al2O3 | 600–700 | atm | 75–100 | 100 | [57] |
| 2 | Steam reforming | Fixed bed reactor | 1:9 | Ni, Pt, Pt–Ni with γ- and La2O3 | 500–600 | 0.4 MPa | 90 | 100 | [73] |
| 3 | Steam reforming | Fixed bed reactor | 1:3 | Ni/Al2O3 | 400–700 | atm | 80 | 100 | [74] |
| 4 | Partial oxidation reforming | Fixed bed reactor | 1:3, 1:6, 1:9 | Ni/CeZrO2/Al2O3 | 550–650 | atm | 67–69 | 40–70 | [75] |
| 5 | Aqueous phase reforming | Fixed bed reactor | 1:3 | AP Ni, Raney Ni | 225 | 2.76 MPa | 50–100 | 100 | [72] |
| 6 | Aqueous phase reforming | Fixed bed reactor | 1:3 | Ni, Ni5Cu, Ni10 Cu, Ni20Cu | 250–270 | 38–52 atm | 80–90 | 60 | [76] |
| 7 | Auto thermal Reforming | Fixed bed reactor | 80 wt% glycerol and 20 wt% D.I. water | BASF Pt. and Rh/Pt. double-layer monolith | 600–700 | atm | 75 | 100 | [77] |
| 8 | Auto thermal Reforming | Fixed bed reactor | 1:3 | Rh Ce/γ-Al2O3 | 900–1200 | atm | 79 | 100 | [78] |
| 9 | Super critical water reforming | Fixed bed reactor | 1:3 | Na2CO3 | 380–500 | 25 MPa | 60 | 100 | [79] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosamia, N.M.; Samavi, M.; Uprety, B.K.; Rakshit, S.K. Valorization of Biodiesel Byproduct Crude Glycerol for the Production of Bioenergy and Biochemicals. Catalysts 2020, 10, 609. https://doi.org/10.3390/catal10060609
Kosamia NM, Samavi M, Uprety BK, Rakshit SK. Valorization of Biodiesel Byproduct Crude Glycerol for the Production of Bioenergy and Biochemicals. Catalysts. 2020; 10(6):609. https://doi.org/10.3390/catal10060609
Chicago/Turabian StyleKosamia, Niravkumar Mahendrasinh, Mahdieh Samavi, Bijaya Kumar Uprety, and Sudip Kumar Rakshit. 2020. "Valorization of Biodiesel Byproduct Crude Glycerol for the Production of Bioenergy and Biochemicals" Catalysts 10, no. 6: 609. https://doi.org/10.3390/catal10060609
APA StyleKosamia, N. M., Samavi, M., Uprety, B. K., & Rakshit, S. K. (2020). Valorization of Biodiesel Byproduct Crude Glycerol for the Production of Bioenergy and Biochemicals. Catalysts, 10(6), 609. https://doi.org/10.3390/catal10060609