Consumption of Biscuits with a Beverage of Mulberry or Barley Leaves in the Afternoon Prevents Dinner-Induced High, but Not Low, Increases in Blood Glucose among Young Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Test Sessions
2.3. Anthropometric Measurements
2.4. Dietary Intake Assessments
2.5. Blood Glucose Evaluations
2.6. Physical Activity Assessments
2.7. Circadian Rhythms
2.8. Nutritional Information for Snacks and Dinner Meals
2.9. Statistical Analyses
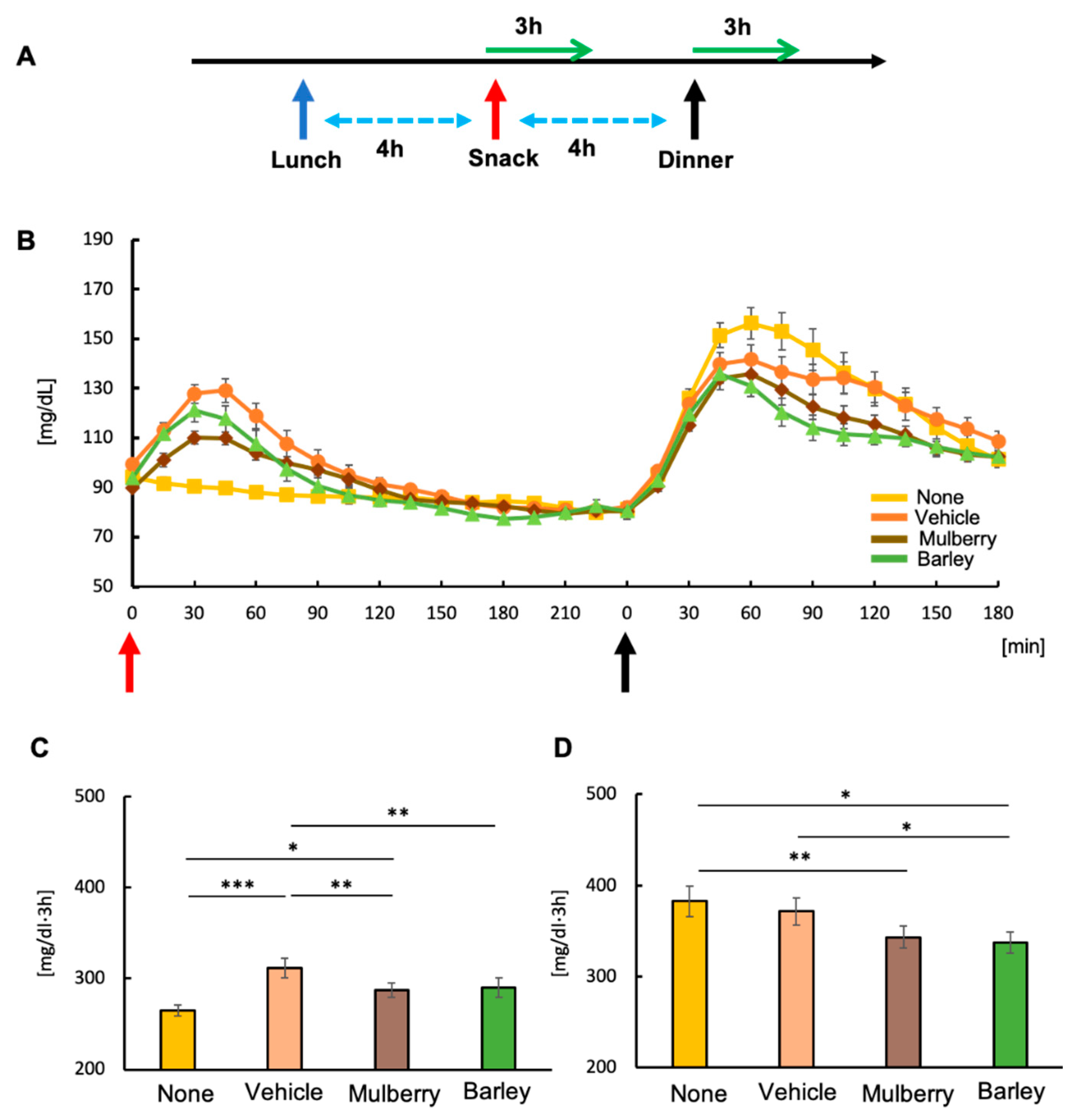
3. Results
3.1. Intake of Biscuits Increased Postprandial Blood Glucose Levels
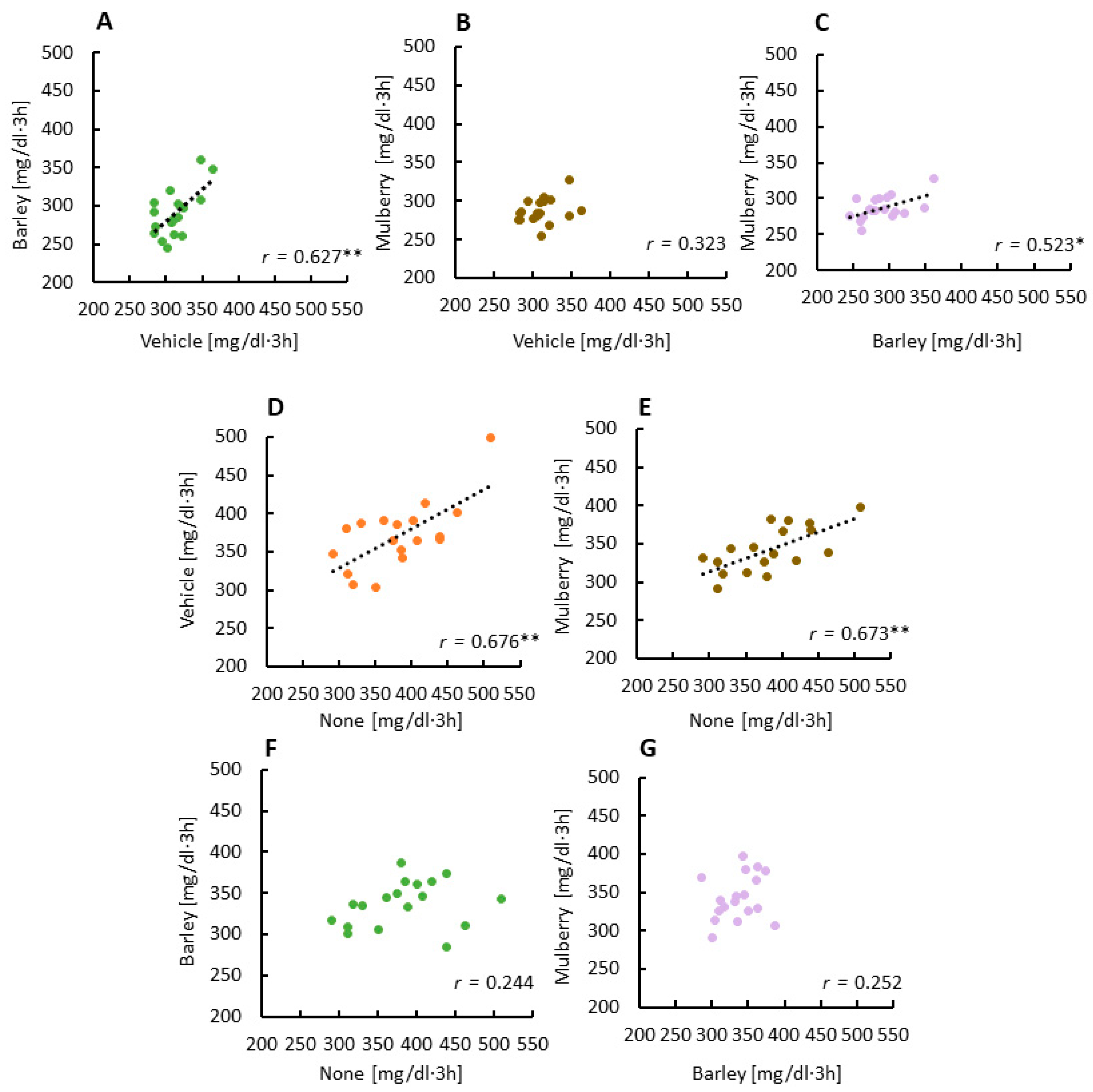
3.2. Correlations between Glucose AUCs Revealed a Second Meal Effect by the Barley Leaves
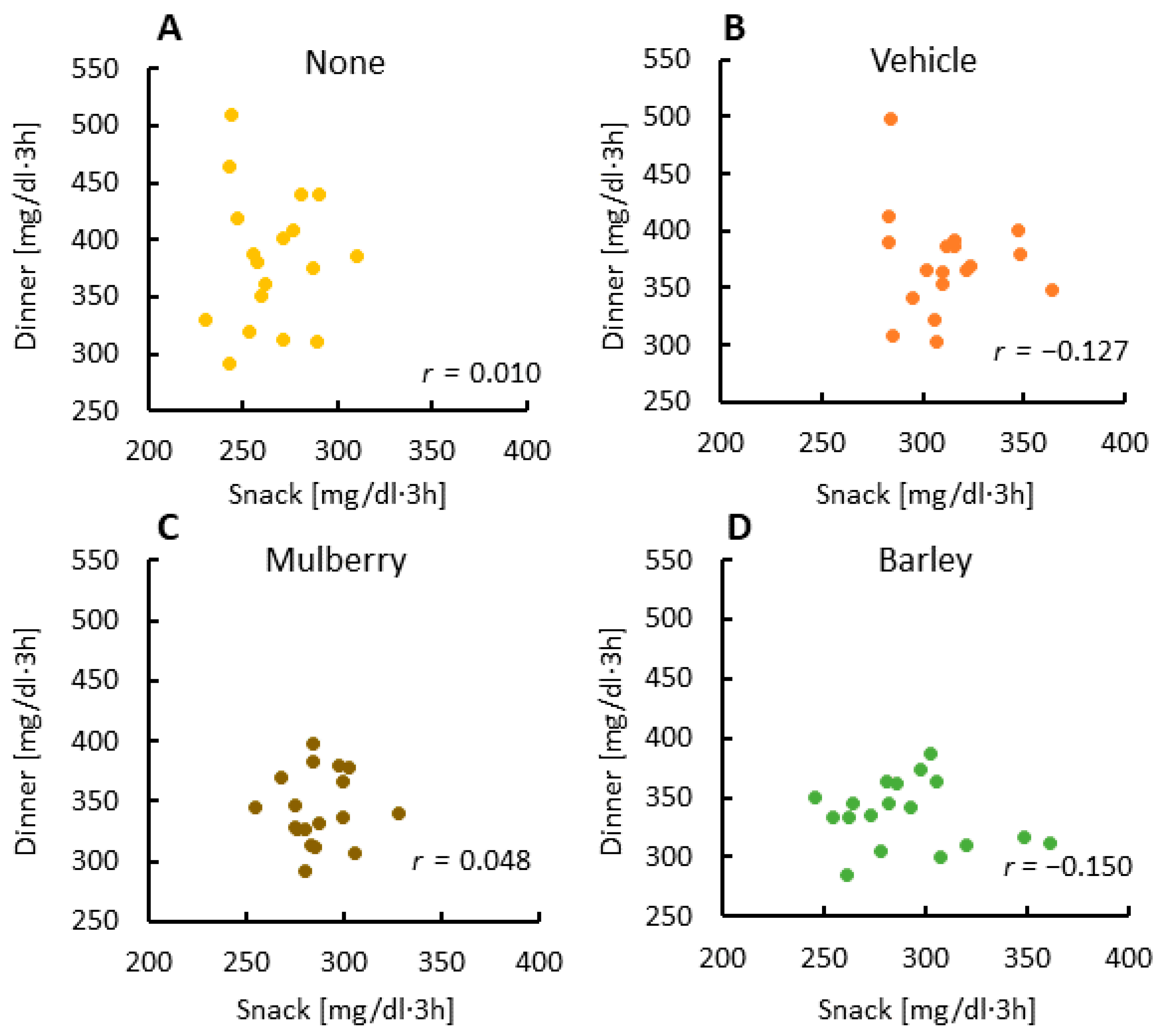
3.3. There Was No Relationship between Glucose AUCs at Snack and Dinner Times
3.4. Young Adults with Healthy Body Weights and Preferences for Evenings Had Higher Glucose Levels at Dinner Time
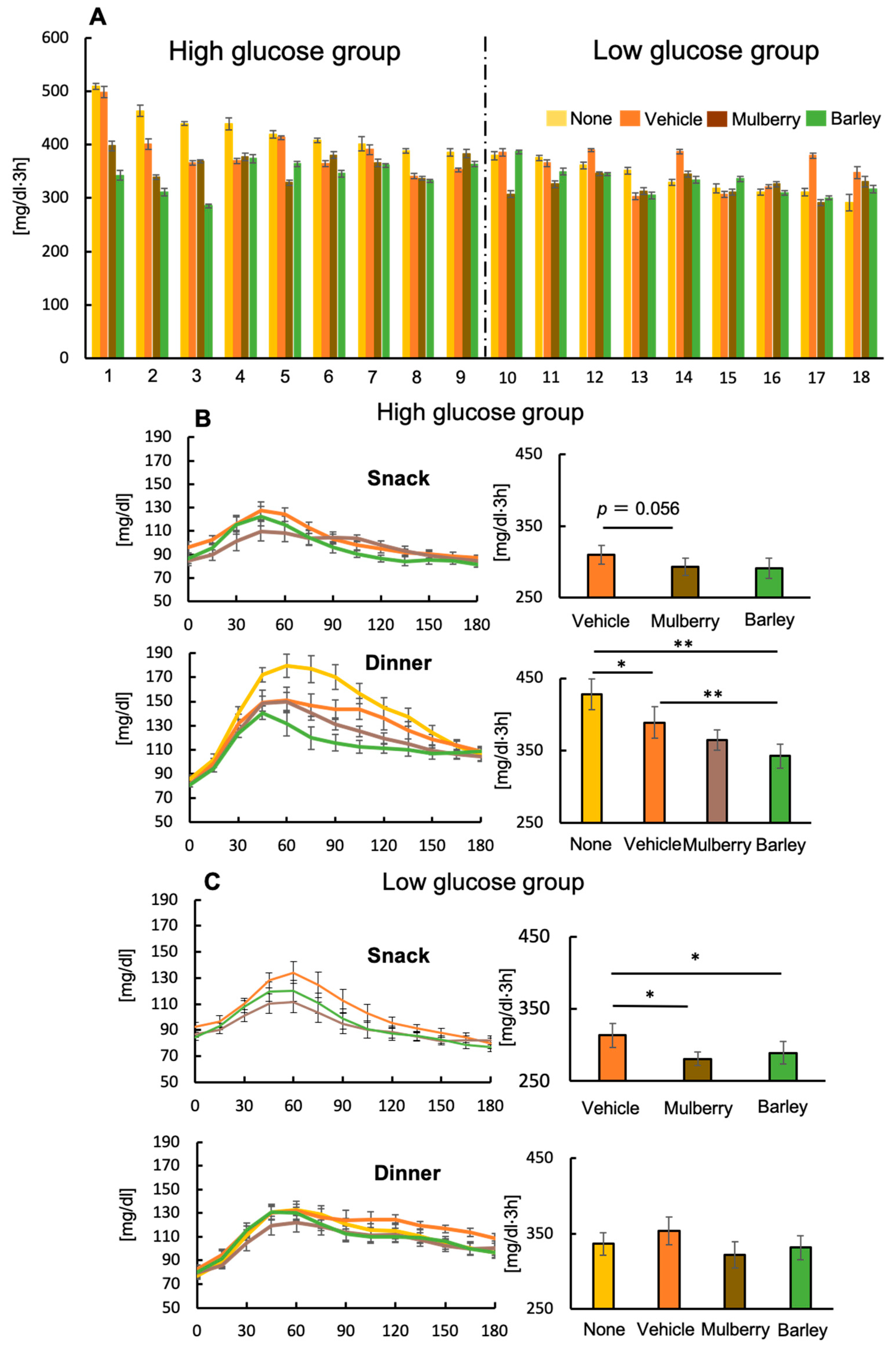
3.5. A Second Meal Effect Was Observed at Dinner Time for Individuals with High Glucose Levels
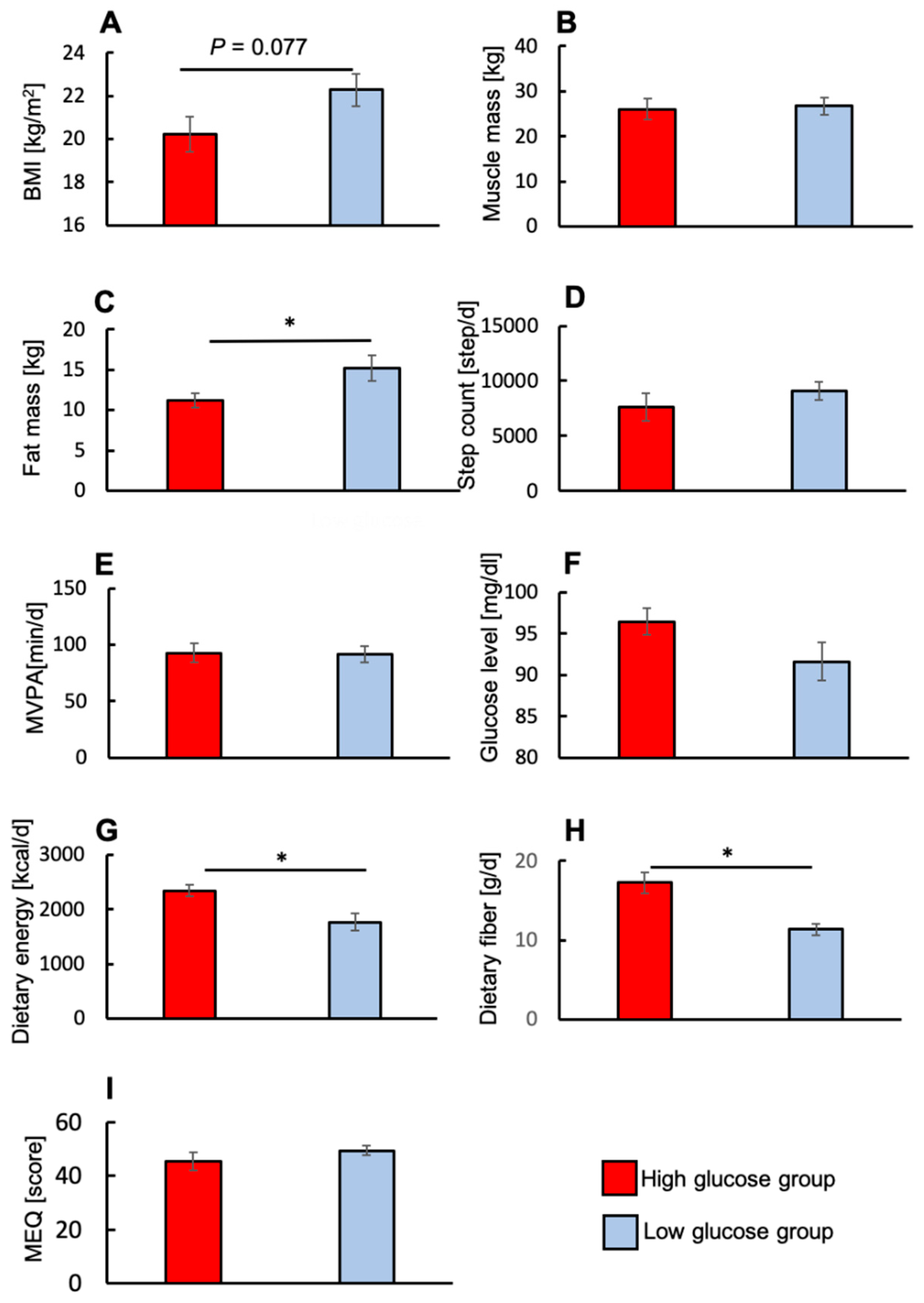
3.6. Differences in Physiological Factors between the High- and Low-Glucose Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bi, H.; Gan, Y.; Yang, C.; Chen, Y.; Tong, X.; Lu, Z. Breakfast skipping and the risk of type 2 diabetes: A meta-analysis of observational studies. Public Health Nutr. 2015, 18, 3013–3019. [Google Scholar] [CrossRef] [PubMed]
- Nas, A.; Mirza, N.; Hagele, F.; Kahlhofer, J.; Keller, J.; Rising, R.; Kufer, T.A.; Bosy-Westphal, A. Impact of breakfast skipping compared with dinner skipping on regulation of energy balance and metabolic risk. Am. J. Clin. Nutr. 2017, 105, 1351–1361. [Google Scholar] [CrossRef] [PubMed]
- Liljeberg, H.G.; Akerberg, A.K.; Bjorck, I.M. Effect of the glycemic index and content of indigestible carbohydrates of cereal-based breakfast meals on glucose tolerance at lunch in healthy subjects. Am. J. Clin. Nutr. 1999, 69, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, A.C.; Ostman, E.M.; Granfeldt, Y.; Bjorck, I.M. Effect of cereal test breakfasts differing in glycemic index and content of indigestible carbohydrates on daylong glucose tolerance in healthy subjects. Am. J. Clin. Nutr. 2008, 87, 645–654. [Google Scholar] [CrossRef]
- Chen, M.J.; Jovanovic, A.; Taylor, R. Utilizing the second-meal effect in type 2 diabetes: Practical use of a soya-yogurt snack. Diabetes Care 2010, 33, 2552–2554. [Google Scholar] [CrossRef]
- Imai, S.; Kajiyama, S.; Hashimoto, Y.; Nitta, A.; Miyawaki, T.; Matsumoto, S.; Ozasa, N.; Tanaka, M.; Kajiyama, S.; Fukui, M. Consuming snacks mid-afternoon compared with just after lunch improves mean amplitude of glycaemic excursions in patients with type 2 diabetes: A randomized crossover clinical trial. Diabetes Metab. 2018, 44, 482–487. [Google Scholar] [CrossRef]
- Saad, A.; Dalla Man, C.; Nandy, D.K.; Levine, J.A.; Bharucha, A.E.; Rizza, R.A.; Basu, R.; Carter, R.E.; Cobelli, C.; Kudva, Y.C.; et al. Diurnal pattern to insulin secretion and insulin action in healthy individuals. Diabetes 2012, 61, 2691–2700. [Google Scholar] [CrossRef]
- Kessler, K.; Hornemann, S.; Petzke, K.J.; Kemper, M.; Kramer, A.; Pfeiffer, A.F.; Pivovarova, O.; Rudovich, N. The effect of diurnal distribution of carbohydrates and fat on glycaemic control in humans: A randomized controlled trial. Sci. Rep. 2017, 7, 44170. [Google Scholar] [CrossRef]
- Kajiyama, S.; Imai, S.; Hashimoto, Y.; Yamane, C.; Miyawaki, T.; Matsumoto, S.; Ozasa, N.; Tanaka, M.; Kajiyama, S.; Fukui, M. Divided consumption of late-night-dinner improves glucose excursions in young healthy women: A randomized cross-over clinical trial. Diabetes Res. Clin. Pract. 2018, 136, 78–84. [Google Scholar] [CrossRef]
- Imai, S.; Kajiyama, S.; Hashimoto, Y.; Yamane, C.; Miyawaki, T.; Ozasa, N.; Tanaka, M.; Fukui, M. Divided consumption of late-night-dinner improves glycemic excursions in patients with type 2 diabetes: A randomized cross-over clinical trial. Diabetes Res. Clin. Pract. 2017, 129, 206–212. [Google Scholar] [CrossRef]
- Butt, M.S.; Nazir, A.; Sultan, M.T.; Schroën, K. Morus alba L. nature’s functional tonic. Trends Food Sci. Technol. 2008, 19, 505–512. [Google Scholar] [CrossRef]
- Gao, R.; Wang, Y.; Wu, Z.; Ming, J.; Zhao, G. Interaction of barley beta-glucan and tea polyphenols on glucose metabolism in streptozotocin-induced diabetic rats. J. Food Sci. 2012, 77, H128–H134. [Google Scholar] [CrossRef]
- Weickert, M.O.; Pfeiffer, A.F.H. Impact of Dietary Fiber Consumption on Insulin Resistance and the Prevention of Type 2 Diabetes. J. Nutr. 2018, 148, 7–12. [Google Scholar] [CrossRef]
- Wang, R.; Li, Y.; Mu, W.; Li, Z.; Sun, J.; Wang, B.; Zhong, Z.; Luo, X.; Xie, C.; Huang, Y. Mulberry leaf extract reduces the glycemic indexes of four common dietary carbohydrates. Medicine 2018, 97, e11996. [Google Scholar] [CrossRef]
- Riche, D.M.; Riche, K.D.; East, H.E.; Barrett, E.K.; May, W.L. Impact of mulberry leaf extract on type 2 diabetes (Mul-DM): A randomized, placebo-controlled pilot study. Complement. Ther. Med. 2017, 32, 105–108. [Google Scholar] [CrossRef]
- Dikeman, C.L.; Murphy, M.R.; Fahey, G.C., Jr. Dietary fibers affect viscosity of solutions and simulated human gastric and small intestinal digesta. J. Nutr. 2006, 136, 913–919. [Google Scholar] [CrossRef]
- Garcia, A.L.; Otto, B.; Reich, S.C.; Weickert, M.O.; Steiniger, J.; Machowetz, A.; Rudovich, N.N.; Mohlig, M.; Katz, N.; Speth, M.; et al. Arabinoxylan consumption decreases postprandial serum glucose, serum insulin and plasma total ghrelin response in subjects with impaired glucose tolerance. Eur. J. Clin. Nutr. 2007, 61, 334–341. [Google Scholar] [CrossRef]
- Umin UMIN Clinicalinic Trials registry (UMIN-CTR). Available online: www.umin.ac.jp/ctr/z (accessed on 28 May 2020).
- Wakai, K. A review of food frequency questionnaires developed and validated in Japan. J. Epidemiol. 2009, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bollwein, J.; Diekmann, R.; Kaiser, M.J.; Bauer, J.M.; Uter, W.; Sieber, C.C.; Volkert, D. Distribution but not amount of protein intake is associated with frailty: A cross-sectional investigation in the region of Nurnberg. Nutr. J. 2013, 12, 109. [Google Scholar] [CrossRef] [PubMed]
- Horne, J.A.; Ostberg, O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int. J. Chronobiol. 1976, 4, 97–110. [Google Scholar] [PubMed]
- Takahashi, M.; Ozaki, M.; Kang, M.I.; Sasaki, H.; Fukazawa, M.; Iwakami, T.; Lim, P.J.; Kim, H.K.; Aoyama, S.; Shibata, S. Effects of Meal Timing on Postprandial Glucose Metabolism and Blood Metabolites in Healthy Adults. Nutrients 2018, 10, 1763. [Google Scholar] [CrossRef] [PubMed]
- Murakami, I.; Sakuragi, T.; Uemura, H.; Menda, H.; Shindo, M.; Tanaka, H. Significant effect of a pre-exercise high-fat meal after a 3-day high-carbohydrate diet on endurance performance. Nutrients 2012, 4, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.N.; Kwon, Y.I.; Jang, H.D. Mulberry leaf extract reduces postprandial hyperglycemia with few side effects by inhibiting alpha-glucosidase in normal rats. J. Med. Food 2011, 14, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Wang, M.; Liu, C.; Zhao, H.; Zhao, B. Mulberry leaf reduces inflammation and insulin resistance in type 2 diabetic mice by TLRs and insulin Signalling pathway. BMC Complement. Altern. Med. 2019, 19, 326. [Google Scholar] [CrossRef] [PubMed]
- Casiraghi, M.C.; Garsetti, M.; Testolin, G.; Brighenti, F. Post-prandial responses to cereal products enriched with barley beta-glucan. J. Am. Coll. Nutr. 2006, 25, 313–320. [Google Scholar] [CrossRef]
- Jozefczuk, J.; Malikowska, K.; Glapa, A.; Stawinska-Witoszynska, B.; Nowak, J.K.; Bajerska, J.; Lisowska, A.; Walkowiak, J. Mulberry leaf extract decreases digestion and absorption of starch in healthy subjects-A randomized, placebo-controlled, crossover study. Adv. Med. Sci. 2017, 62, 302–306. [Google Scholar] [CrossRef]
- Nitta, A.; Imai, S.; Kajiyama, S.; Miyawaki, T.; Matsumoto, S.; Ozasa, N.; Kajiyama, S.; Hashimoto, Y.; Tanaka, M.; Fukui, M. Impact of different timing of consuming sweet snack on postprandial glucose excursions in healthy women. Diabetes Metab. 2019, 45, 369–374. [Google Scholar] [CrossRef]
- Ando, T.; Nakae, S.; Usui, C.; Yoshimura, E.; Nishi, N.; Takimoto, H.; Tanaka, S. Effect of diurnal variations in the carbohydrate and fat composition of meals on postprandial glycemic response in healthy adults: A novel insight for the second-meal phenomenon. Am. J. Clin. Nutr. 2018, 108, 332–342. [Google Scholar] [CrossRef]
- Liljeberg, H.; Bjorck, I. Effects of a low-glycaemic index spaghetti meal on glucose tolerance and lipaemia at a subsequent meal in healthy subjects. Eur. J. Clin. Nutr. 2000, 54, 24–28. [Google Scholar] [CrossRef]
- Lattimer, J.M.; Haub, M.D. Effects of dietary fiber and its components on metabolic health. Nutrients 2010, 2, 1266–1289. [Google Scholar] [CrossRef] [PubMed]
- Maki, K.C.; Davidson, M.H.; Witchger, M.S.; Dicklin, M.R.; Subbaiah, P.V. Effects of high-fiber oat and wheat cereals on postprandial glucose and lipid responses in healthy men. Int. J. Vitam. Nutr. Res. 2007, 77, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Lightowler, H.J.; Henry, C.J. Glycemic response of mashed potato containing high-viscocity hydroxypropylmethylcellulose. Nutr. Res. 2009, 29, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Wolever, T.M.; Bentum-Williams, A.; Jenkins, D.J. Physiological modulation of plasma free fatty acid concentrations by diet. Metabolic implications in nondiabetic subjects. Diabetes Care 1995, 18, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Roden, M.; Price, T.B.; Perseghin, G.; Petersen, K.F.; Rothman, D.L.; Cline, G.W.; Shulman, G.I. Mechanism of free fatty acid-induced insulin resistance in humans. J. Clin. Investig. 1996, 97, 2859–2865. [Google Scholar] [CrossRef]
- Miyamoto, J.; Watanabe, K.; Taira, S.; Kasubuchi, M.; Li, X.; Irie, J.; Itoh, H.; Kimura, I. Barley beta-glucan improves metabolic condition via short-chain fatty acids produced by gut microbial fermentation in high fat diet fed mice. PLoS ONE 2018, 13, e0196579. [Google Scholar] [CrossRef]
- Sheng, Y.; Liu, J.; Zheng, S.; Liang, F.; Luo, Y.; Huang, K.; Xu, W.; He, X. Mulberry leaves ameliorate obesity through enhancing brown adipose tissue activity and modulating gut microbiota. Food Funct. 2019, 10, 4771–4781. [Google Scholar] [CrossRef]
- Mandaliya, D.K.; Seshadri, S. Short Chain Fatty Acids, pancreatic dysfunction and type 2 diabetes. Pancreatology 2019, 19, 280–284. [Google Scholar] [CrossRef]
- Hernandez, M.A.G.; Canfora, E.E.; Jocken, J.W.E.; Blaak, E.E. The Short-Chain Fatty Acid Acetate in Body Weight Control and Insulin Sensitivity. Nutrients 2019, 11, 1943. [Google Scholar] [CrossRef]
- Drucker, D.J.; Nauck, M.A. The incretin system: Glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006, 368, 1696–1705. [Google Scholar] [CrossRef]
- Kalyani, R.R.; Tra, Y.; Egan, J.M.; Ferrucci, L.; Brancati, F. Hyperglycemia is associated with relatively lower lean body mass in older adults. J. Nutr. Health Aging 2014, 18, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Park, S.M. Association of muscle mass and fat mass with insulin resistance and the prevalence of metabolic syndrome in Korean adults: A cross-sectional study. Sci. Rep. 2018, 8, 2703. [Google Scholar] [CrossRef] [PubMed]
- Osonoi, Y.; Mita, T.; Osonoi, T.; Saito, M.; Tamasawa, A.; Nakayama, S.; Someya, Y.; Ishida, H.; Kanazawa, A.; Gosho, M.; et al. Morningness-eveningness questionnaire score and metabolic parameters in patients with type 2 diabetes mellitus. Chronobiol. Int. 2014, 31, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.H.; Yun, C.H.; Ahn, J.H.; Suh, S.; Cho, H.J.; Lee, S.K.; Yoo, H.J.; Seo, J.A.; Kim, S.G.; Choi, K.M.; et al. Evening chronotype is associated with metabolic disorders and body composition in middle-aged adults. J. Clin. Endocrinol. Metab. 2015, 100, 1494–1502. [Google Scholar] [CrossRef] [PubMed]
| Meal Time | None | Vehicle | Mulberry | Barley |
|---|---|---|---|---|
| Lunch | 12:48 ± 0:10 | 12:53 ± 0:08 | 12:53 ± 0:11 | 12:59 ± 0:10 |
| Snack | - | 16:56 ± 0:08 | 16:56 ± 0:11 | 17:03 ± 0:09 |
| Dinner | 20:54 ± 0:11 | 20:59 ± 0:09 | 20:58 ± 0:10 | 21:06 ± 0:09 |
| Nutrients | Mulberry | Barley |
|---|---|---|
| Energy (kcal) | 11.3 | 10.7 |
| Protein (g) | 0.7 | 0.8 |
| Fat (g) | 0.2 | 0.2 |
| Total Carbohydrate (g) | 1.7 | 1.5 |
| Sugar (g) | 0.6 | 0.3 |
| Dietary fiber (g) | 1.1 | 1.2 |
| Nutrients | Biscuit | Dinner |
|---|---|---|
| Energy (kcal) | 156.6 | 752.0 |
| Protein (g) | 3.0 | 22.3 |
| Fat (g) | 4.7 | 33.6 |
| Total Carbohydrate (g) | 25.9 | 91.4 |
| Sugar (g) | 25.2 | 87.3 |
| Dietary fiber (g) | 0.7 | 4.1 |
| Baseline | After Two Weeks | |
|---|---|---|
| Age (y) | 23.1 ± 0.4 | |
| Height (cm) | 166.1 ± 2.1 | |
| Weight (kg) | 59.3 ± 2.5 | 59.0 ± 2.4 |
| BMI (kg/m2) | 21.2 ± 0.6 | 21.1 ± 0.5 |
| Fat (%) | 21.8 ± 1.5 | 20.8 ± 1.5 |
| Muscle mass (kg) | 25.8 ± 1.4 | 25.7 ± 1.4 |
| Fat mass (kg) | 12.8 ± 0.9 | 13.2 ± 1.0 |
| Step count (steps/d) | 9100.6 ± 817.8 | 8973.3 ± 673.4 |
| MVPA (min/d) | 89.5 ± 7.8 | 90.3 ± 6.2 |
| MEQ | 47.8 ±1.8 | |
| Energy intakes (kcal/d) (1) | 2036.4 ± 122.6 | |
| Dietary fiber intakes (g/d) (1) | 14.0 ± 1.1 | |
| Snack | Dinner | ||||||
|---|---|---|---|---|---|---|---|
| Vehicle | Mulberry | Barley | None | Vehicle | Mulberry | Barley | |
| Glucose level (mg/dL) | −0.34 | 0.07 | −0.37 | 0.54 * | 0.24 | 0.49 * | 0.25 |
| BMI (kg/m2) | 0.00 | −0.13 | −0.07 | −0.56 * | −0.43 (1) | −0.40 | −0.10 |
| Muscle mass (kg) | −0.07 | −0.02 | −0.24 | −0.24 | −0.44 (2) | 0.45 (3) | 0.28 |
| Fat mass(kg) | −0.15 | −0.19 | −0.2 | −0.43 | −0.23 (4) | −0.45 (5) | 0.00 |
| Step count (steps/d) | 0.08 | −0.20 | 0.26 | −0.11 | 0.36 | −0.20 | 0.24 |
| MVPA (min/d) | −0.33 | −0.09 | 0.37 | 0.02 | 0.52 * | 0.05 | 0.41 |
| MEQ (score) | 0.14 | 0.09 | 0.21 | −0.48 (6) | −0.60 * | −0.56 * | −0.24 |
| Dietary energy(kcal/d) | 0.18 | 0.20 | 0.38 | 0.32 | 0.49 | 0.02 | 0.32 |
| Dietary fiber (g/d) | −0.13 | −0.07 | 0.06 | 0.42 | 0.39 | 0.09 | 0.36 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuwahara, M.; Kim, H.-K.; Ozaki, M.; Nanba, T.; Chijiki, H.; Fukazawa, M.; Okubo, J.; Mineshita, Y.; Takahashi, M.; Shibata, S. Consumption of Biscuits with a Beverage of Mulberry or Barley Leaves in the Afternoon Prevents Dinner-Induced High, but Not Low, Increases in Blood Glucose among Young Adults. Nutrients 2020, 12, 1580. https://doi.org/10.3390/nu12061580
Kuwahara M, Kim H-K, Ozaki M, Nanba T, Chijiki H, Fukazawa M, Okubo J, Mineshita Y, Takahashi M, Shibata S. Consumption of Biscuits with a Beverage of Mulberry or Barley Leaves in the Afternoon Prevents Dinner-Induced High, but Not Low, Increases in Blood Glucose among Young Adults. Nutrients. 2020; 12(6):1580. https://doi.org/10.3390/nu12061580
Chicago/Turabian StyleKuwahara, Mai, Hyeon-Ki Kim, Mamiho Ozaki, Takuya Nanba, Hanako Chijiki, Mayuko Fukazawa, Jin Okubo, Yui Mineshita, Masaki Takahashi, and Shigenobu Shibata. 2020. "Consumption of Biscuits with a Beverage of Mulberry or Barley Leaves in the Afternoon Prevents Dinner-Induced High, but Not Low, Increases in Blood Glucose among Young Adults" Nutrients 12, no. 6: 1580. https://doi.org/10.3390/nu12061580
APA StyleKuwahara, M., Kim, H.-K., Ozaki, M., Nanba, T., Chijiki, H., Fukazawa, M., Okubo, J., Mineshita, Y., Takahashi, M., & Shibata, S. (2020). Consumption of Biscuits with a Beverage of Mulberry or Barley Leaves in the Afternoon Prevents Dinner-Induced High, but Not Low, Increases in Blood Glucose among Young Adults. Nutrients, 12(6), 1580. https://doi.org/10.3390/nu12061580





