The Healthy Eating Plate Advice for Migraine Prevention: An Interventional Study
Abstract
1. Introduction
2. Materials and Methods
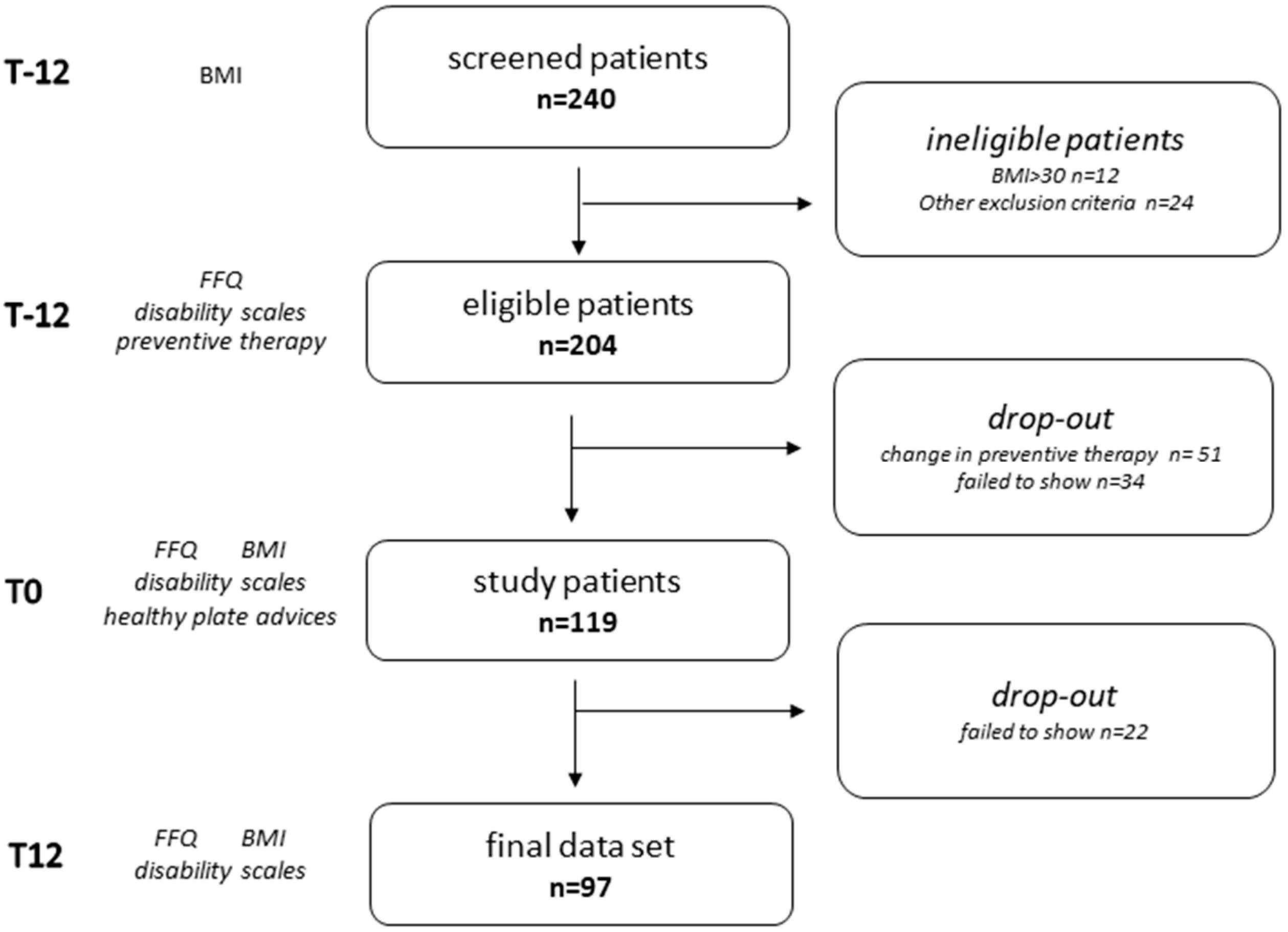
2.1. Study Design
2.2. Participant Selection
- Inclusion criteria: diagnosis of migraine with aura or migraine without aura, age >18-years-old.
- Exclusion criteria: BMI > 30, cancer, inflammatory bowel disease, celiac disease, type 1 diabetes, chronic renal insufficiency, and other neurological disorders.
2.3. Anthropometric Measurements
2.4. Migraine Attack Frequency and Disability Assessment
2.5. Dietary Assessment and Education
- At T-12, T0, and T12:
- Q1-WATER: “How many liters of water do you drink daily?”
- At T0
- Q2-EXPECTANCIES: “Do you think that a healthy diet can help improve headaches?”
- At T12
- Q3-SELF REPORTED ADHERENCE “Were you able to follow the healthy eating plate advice?”
- Q4-SUGAR: “Were you able to reduce daily sugar consumption?”
- Q5-SALT: “Were you able to reduce salt use as flavor enhancer?”
- Q6-EXERCISE: “Were you able to exercise at least 30 min a day”
- Q7-EXPERIENCE: “In your experience, do you think that the Healthy Eating Plate advice helped you improving headaches?”
2.6. Data Processing
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Feigin, V.; Abajobir, A.A.; Abate, K.H.; Abd-Allah, F.; Abdulle, A.M.; Abera, S.F.; Abyu, G.Y.; Ahmed, M.; Aichour, A.N.; Aichour, I.; et al. Global, regional, and national burden of neurological disorders during 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017, 16, 877–897. [Google Scholar] [CrossRef]
- Martin, V.T.; Vij, B. Diet and Headache: Part 1. Headache 2016, 56, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Rockett, F.C.; De Oliveira, V.R.; Castro, K.; Chaves, M.L.F.; Perla, A.D.S.; Perry, I.D. Dietary aspects of migraine trigger factors. Nutr. Rev. 2012, 70, 337–356. [Google Scholar] [CrossRef] [PubMed]
- Orr, S.L. Diet and nutraceutical interventions for headache management: A review of the evidence. Cephalalgia 2016, 36, 1112–1133. [Google Scholar] [CrossRef] [PubMed]
- Zaeem, Z.; Zhou, L.; Dilli, E. Headaches: A Review of the Role of Dietary Factors. Curr. Neurol. Neurosci. Rep. 2016, 16, 101. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Coppola, G.; Sirianni, G.; Di Lorenzo, G.; Bracaglia, M.; Di Lenola, D.; Siracusano, A.; Rossi, P.; Pierelli, F. Migraine improvement during short lasting ketogenesis: A proof-of-concept study. Eur. J. Neurol. 2015, 22, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Slavin, M.; Ailani, J. A Clinical Approach to Addressing Diet with Migraine Patients. Curr. Neurol. Neurosci. Rep. 2017, 17, 17. [Google Scholar] [CrossRef]
- Hajjarzadeh, S.; Mahdavi, R.; Shalilahmadi, D.; Nikniaz, Z. The association of dietary patterns with migraine attack frequency in migrainous women. Nutr. Neurosci. 2018, 1–7. [Google Scholar] [CrossRef]
- Mirzababaei, A.; Khorsha, F.; Togha, M.; Yekaninejad, M.S.; Okhovat, A.A.; Mirzaei, K. Associations between adherence to dietary approaches to stop hypertension (DASH) diet and migraine headache severity and duration among women. Nutr. Neurosci. 2018, 23, 335–342. [Google Scholar] [CrossRef]
- “The Nutrition Source”, “the Harvard T.H. Chan School of Public Health”. Available online: https://www.hsph.harvard.edu/nutritionsource/healthy-eating-plate (accessed on 27 May 2020).
- Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef]
- Silberstein, S.D.; Holland, S.; Freitag, F.; Dodick, D.W.; Argoff, C.; Ashman, E. Quality standards subcommittee of the american academy of neurology and the american headache society evidence-based guideline update: Pharmacologic treatment for episodic migraine prevention in adults: Table 1. Neurology 2012, 78, 1337–1345. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.S. Principles of Nutritional Assessment; Oxford University Press: Oxford, UK, 2005. [Google Scholar]
- D’Amico, D.; Grazzi, L.; Usai, S.; Raggi, A.; Leonardi, M.; Bussone, G. Disability in chronic daily headache: State of the art and future directions. Neurol. Sci. 2011, 32, S71–S76. [Google Scholar] [CrossRef] [PubMed]
- Marventano, S.; Mistretta, A.; Platania, A.; Galvano, F.; Grosso, G. Reliability and relative validity of a food frequency questionnaire for Italian adults living in Sicily, Southern Italy. Int. J. Food Sci. Nutr. 2016, 67, 857–864. [Google Scholar] [CrossRef] [PubMed]
- SINU-Italian Society of Human Nutrition. Use of Food Composition Databases For Nutritional Assessments. Available online: http://www.sinu.it/public/20141111_LARN_Porzioni.pdf (accessed on 27 May 2020).
- Vernieri, F.; Paolucci, M.; Altamura, C.; Pasqualetti, P.; Mastrangelo, V.; Pierangeli, G.; Cevoli, S.; D’Amico, D.; Grazzi, L. Onabotulinumtoxin-A in Chronic Migraine: Should Timing and Definition of Non-Responder Status Be Revised? Suggestions From a Real-Life Italian Multicenter Experience. Headache 2019, 59, 1300–1309. [Google Scholar] [CrossRef]
- Altamura, C.; Botti, G.; Paolucci, M.; Brunelli, N.; Cecchi, G.; Khazrai, M.; Vernieri, F. Promoting healthy eating can help preventing migraine: A real-life preliminary study. Neurol. Sci. 2018, 39, 155–156. [Google Scholar] [CrossRef]
- Razeghi Jahromi, S.; Ghorbani, Z.; Martelletti, P.; Lampl, C.; Togha, M. Association of diet and headache. J. Headache Pain 2019, 20, 106. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Pinto, A.; Ienca, R.; Coppola, G.; Sirianni, G.; Di Lorenzo, G.; Parisi, V.; Serrao, M.; Spagnoli, A.; Vestri, A.; et al. A Randomized Double-Blind, Cross-Over Trial of very Low-Calorie Diet in Overweight Migraine Patients: A Possible Role for Ketones? Nutrients 2019, 11, 1742. [Google Scholar] [CrossRef]
- Augustin, K.; Khabbush, A.; Williams, S.; Eaton, S.; Orford, M.; Cross, J.H.; Heales, S.J.R.; Walker, M.C.; Williams, R.S.B. Mechanisms of action for the medium-chain triglyceride ketogenic diet in neurological and metabolic disorders. Lancet Neurol. 2018, 17, 84–93. [Google Scholar] [CrossRef]
- Fitzgerald, K.C.; Tyry, T.; Salter, A.; Cofield, S.S.; Cutter, G.; Fox, R.; Marrie, R.A. Diet quality is associated with disability and symptom severity in multiple sclerosis. Neurology 2018, 90, E1–E11. [Google Scholar] [CrossRef]
- Petersson, S.D.; Philippou, E. Mediterranean diet, cognitive function, and dementia: A systematic review of the evidence. Adv. Nutr. 2016, 7, 889–904. [Google Scholar] [CrossRef]
- Gross, E.C.; Lisicki, M.; Fischer, D.; Sándor, P.S.; Schoenen, J. The metabolic face of migraine—From pathophysiology to treatment. Nat. Rev. Neurol. 2019, 15, 627–643. [Google Scholar] [CrossRef] [PubMed]
- Evcili, G. Early and Long Period Follow-up Results of Low-Glycemic Index Diet for Migraine Prophylaxis. Ağrı-J. Turkish Soc. Algol. 2018, 30, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Martins-Oliveira, M.; Akerman, S.; Holland, P.R.; Hoffmann, J.R.; Tavares, I.; Goadsby, P.J. Neuroendocrine signaling modulates specific neural networks relevant to migraine. Neurobiol. Dis. 2017, 101, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Kilic, K.; Karatas, H.; Dönmez-Demir, B.; Eren-Kocak, E.; Gursoy-Ozdemir, Y.; Can, A.; Petit, J.-M.; Magistretti, P.J.; Dalkara, T. Inadequate brain glycogen or sleep increases spreading depression susceptibility. Ann. Neurol. 2018, 83, 61–73. [Google Scholar] [CrossRef]
- Slavin, M.; Li, H.; Frankenfeld, C.; Cheskin, L.J. What is needed for evidence-based dietary recommendations for migraine: A call to action for nutrition and microbiome research. Headache 2019, 59, 1566–1581. [Google Scholar] [CrossRef]
| Item | Yes | No |
|---|---|---|
| Water per day ≥2 L | 1 | 0 |
| At least 2 servings of seasonal fresh fruits per day | 1 | 0 |
| At least 3 servings of vegetables per day | 1 | 0 |
| No more than 2 servings of milk/dairy per day | 1 | 0 |
| At least 8 servings per week of whole grains in the mean AND a ratio of whole grains/refined grain and potato consumption >1 | 1 | 0 |
| Having for breakfast whole grains cereals or biscuits at least 5 times per week | 1 | 0 |
| Less than 3 servings a day of fat dressing AND a ratio of healthy vegetable oils/partially hydrogenated oils and animal fats >3 | 1 | 0 |
| Having no more than 1 sweetened beverage (including packaged fruit juice) per week | 1 | 0 |
| Having 1–2 serving of nuts as a snack/day | 1 | 0 |
| Subtotal | ||
| Healthy protein sub-score | ||
| Cheese no more than 1 serving per week | 1 | 0 |
| 1 or 2 eggs per week | 1 | 0 |
| Fresh fish (including shellfish) at least 2 servings per week | 1 | 0 |
| Beans at least 2 servings per week | 1 | 0 |
| Poultry: 2 servings per week | 1 | 0 |
| Red meat or processed meat once a week or less | 1 | 0 |
| Protein subtotal | ||
| Protein Score | Protein subtotal/6 | |
| Total | Subtotal + Protein Score | |
| n = 97 | T-12 | T0 | T12 | p |
|---|---|---|---|---|
| Sex, F (%) | 84.5 | - | - | |
| Age, years (mean, SD) | 42.08 (12.93) | - | - | |
| BMI, kg/m2 (median, IQr) | 23.2 (5.30) | 23.21 (5.65) | 23.14 (5.18) | 0.009 |
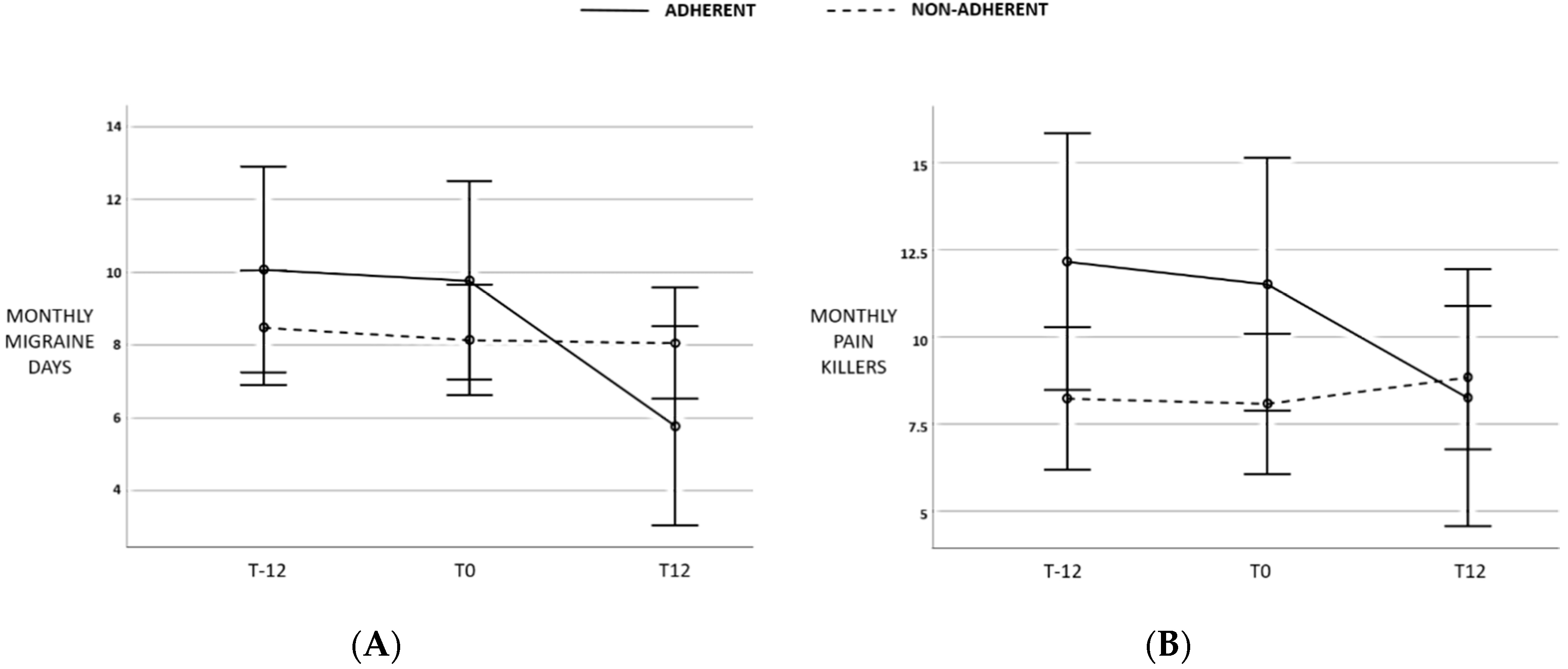
| Last month migraine days (median, IQr) | 7 (8) | 7 (7) | 6 (7.5) | n.s. |
| Last month pain killer intake (median, IQr) | 6 (8.5) | 6(8) | 6 (9,5) | n.s. |
| MIDAS A (median, IQr) | 18 (20) | 18 (19) | 15 (19.5) | 0.001 |
| MIDAS B (median, IQr) | 7 (2) | 7(3) | 7 (2) | n.s. |
| MIDAS score (median, IQr) | 14 (26) | 13 (22) | 15 (34.75) | n.s. |
| n = 97 (Median, IQr) | T-12 | T0 | T12 | p |
|---|---|---|---|---|
| Water intake, L/day | 1.00 (0.50) | 1.00 (0.5) | 2.00 (1.00) | <0.0001 |
| Fresh fruits, serving/week | 7 (10.5) | 7 (10) | 7 (8.5) | n.s. |
| Vegetables, serving/week | 11 (8) | 10 (8) | 10 (7) | n.s. |
| Milk and diary, serving/week | 4 (7.5) | 7 (8) | 6 (7) | n.s. |
| Whole grain cereals, serving/week | 1 (5.5) | 1 (6) | 3.5 (7) | 0.001 |
| Refined cereals and potatoes, serving/week | 12 (12) | 12 (11.5) | 9 (9) | <0.0001 |
| Whole grain breakfast, serving/week | 0 (3.5) | 0 (2) | 2 (6.5) | 0.002 |
| High-carb breakfast and snacks, serving/week | 2 (6) | 2 (7) | 2 (69 | n.s. |
| Vegetable oils, serving/week | 14 (6.25) | 14 (6) | 14 (7) | n.s. |
| Animal fat and margarine, serving/week | 0 (1) | 0 (1) | 0 (0) | n.s. |
| Cheese, serving/week | 4 (4) | 4 (5) | 4 (6) | n.s. |
| Eggs, serving/week | 1 (1) | 1 (1) | 1 (1) | n.s. |
| Legumes, serving/week | 1 (1) | 1 (1) | 1 (1) | n.s. |
| Fish, serving/week | 1 (1) | 1 (1) | 1 (1) | n.s. |
| White meat, serving/week | 2 (3) | 2 (3) | 2 (3) | n.s. |
| Red and processed meat, serving/week | 4 (3) | 4 (4) | 3 (2) | <0.0001 |
| Sweetened beverage, serving/week | 1 (4) | 1 (2) | 0 (2) | 0.006 |
| Nuts/serving/week | 1 (4) | 1 (4) | 1 (4) | n.s. |
| Alcohol/serving/week | 0 (2) | 0 (2) | 0 (2) | n.s. |
| Healthy Eating Plate score | 3.5 (1.33) | 4.67 (2.05) | 4.33 (1.84) | <0.0001 |
| Food Group (Median, IQr) | RESPONDERS (n = 33) | NON-RESPONDERS (n = 64) | p |
|---|---|---|---|
| Water intake, L/day | 0 (0.5) | 0.8 (0,5) | n.s. |
| Fresh fruits, serving/week | 0 (5) | 0 (3) | n.s. |
| Vegetables, serving/week | 14 (13) | 10 (17) | n.s. |
| Milk and diary, serving/week | 0 (1) | 0 (2) | n.s. |
| TOTAL WHOLE-GRAINs, serving/week | 6 (8) | 1.5 (5.75) | n.s. |
| TOTAL CARBs, serving/week | −4.5 (10) | −3 (12.75) | 0.015 |
| Vegetable oils, serving/week | 0 (10.25) | 0 (0.75) | n.s. |
| Animal fat and margarine, serving/week | 0 (1) | 0 (0) | n.s. |
| Red and processed meat, serving/week | −1.5 (3) | −0.5 (3) | 0.033 |
| HEALTHY PROTEINs, serving/week | −0.5 (7) | 0 (3) | n.s. |
| OTHER PROTEINs, serving/week | 1 (6.75) | 2 (6.75) | n.s. |
| Sweetened beverage, serving/week | −0.5 (1.25) | 0 (1) | n.s. |
| Nuts, serving/week | 0 (2) | 0 (1) | n.s. |
| Alcohol, serving/week | 0 (1.25) | 0 (1.75) | n.s. |
| B | Sign. | 95% C.I. | ||
|---|---|---|---|---|
| Lower Limit | Upper Limit | |||
| Age | −0.019 | 0.715 | −0.125 | 0.086 |
| Sex | 1.520 | 0.377 | −1.881 | 4.921 |
| BMI | −0.370 | 0.076 | −0.778 | 0.039 |
| Healthy Eating Plate score | −1.118 | 0.025 | −2.093 | −0.142 |
| Red and processed meat | −1.118 | 0.025 | −2.093 | −0.142 |
| TOTAL CARBS | 0.159 | 0.028 | 0.018 | 0.301 |
| Q6-EXERCISE | 0.680 | 0.592 | −1.830 | 3.190 |
| Preventive therapy | 1.956 | 0.130 | −0.589 | 4.501 |
| Constant | 4.401 | 0.384 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altamura, C.; Cecchi, G.; Bravo, M.; Brunelli, N.; Laudisio, A.; Di Caprio, P.; Botti, G.; Paolucci, M.; Khazrai, Y.M.; Vernieri, F. The Healthy Eating Plate Advice for Migraine Prevention: An Interventional Study. Nutrients 2020, 12, 1579. https://doi.org/10.3390/nu12061579
Altamura C, Cecchi G, Bravo M, Brunelli N, Laudisio A, Di Caprio P, Botti G, Paolucci M, Khazrai YM, Vernieri F. The Healthy Eating Plate Advice for Migraine Prevention: An Interventional Study. Nutrients. 2020; 12(6):1579. https://doi.org/10.3390/nu12061579
Chicago/Turabian StyleAltamura, Claudia, Gianluca Cecchi, Maria Bravo, Nicoletta Brunelli, Alice Laudisio, Paola Di Caprio, Giorgia Botti, Matteo Paolucci, Yeganeh Manon Khazrai, and Fabrizio Vernieri. 2020. "The Healthy Eating Plate Advice for Migraine Prevention: An Interventional Study" Nutrients 12, no. 6: 1579. https://doi.org/10.3390/nu12061579
APA StyleAltamura, C., Cecchi, G., Bravo, M., Brunelli, N., Laudisio, A., Di Caprio, P., Botti, G., Paolucci, M., Khazrai, Y. M., & Vernieri, F. (2020). The Healthy Eating Plate Advice for Migraine Prevention: An Interventional Study. Nutrients, 12(6), 1579. https://doi.org/10.3390/nu12061579





