Exogenous Melatonin Improves Salt Tolerance by Mitigating Osmotic, Ion, and Oxidative Stresses in Maize Seedlings
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Growth Conditions and Salt Treatment
2.2. Biomass and Leaf Area
2.3. Chlorophyll, Gas Exchange Parameters, and Instantaneous Water Use Efficiency (WUEi)
2.4. Leaf Relative Water Content, Water Potential, Osmotic Potential, and Turgor Pressure
2.5. Sucrose, Fructose, and Proline Levels
2.6. Na+ and K+ Contents
2.7. Hydrogen Peroxide and Malondialdehyde
2.8. Activities of Enzymatic and Nonenzymatic Antioxidant
2.9. The Contents of Endogenous Melatonin
2.10. Statistical Analysis
3. Results
3.1. Effect of Salt and Melatonin Treatments on Plant Growth
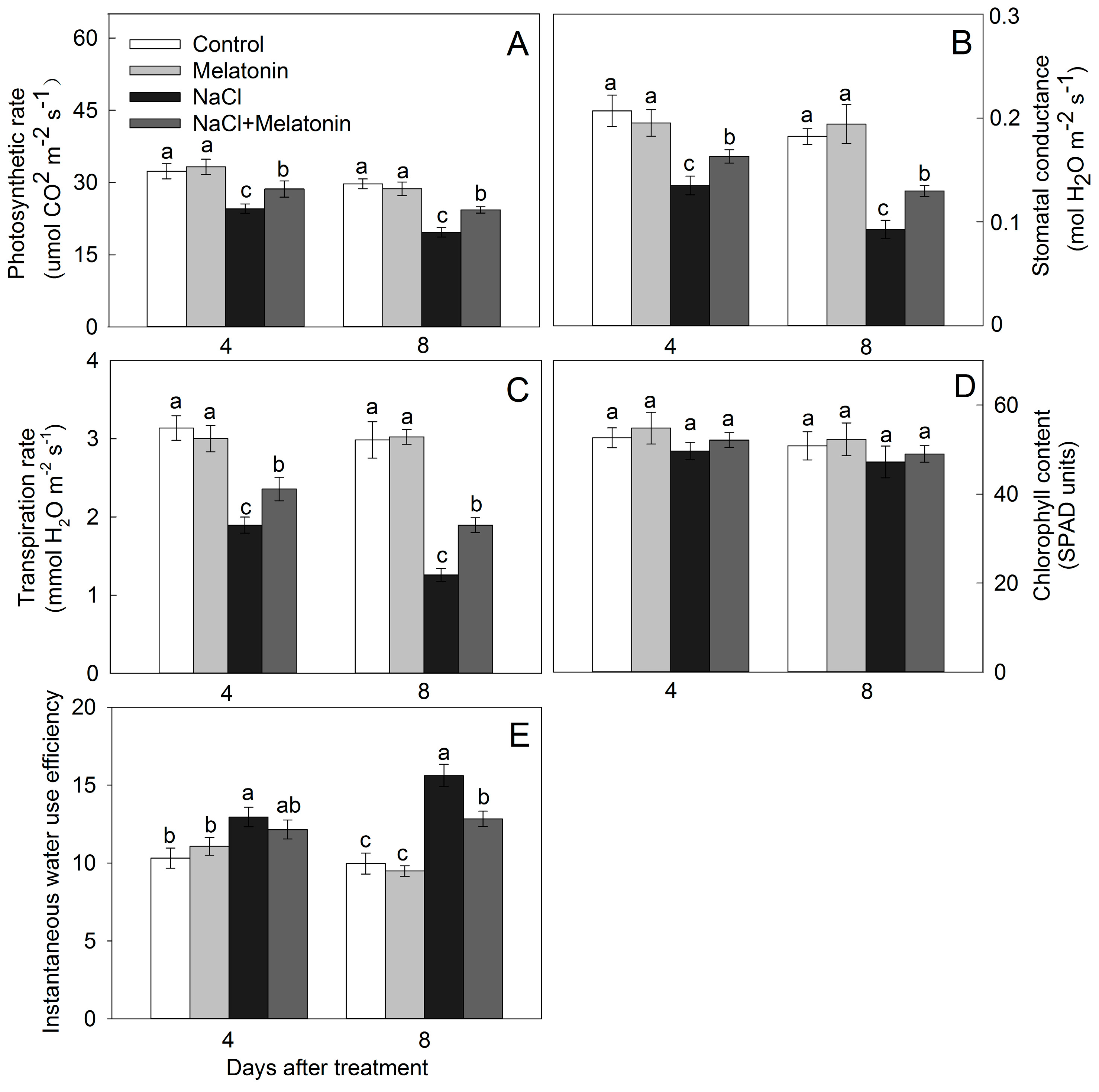
3.2. Effect of Salt and Melatonin Treatments on Photosynthetic Rate and Chlorophyll Content
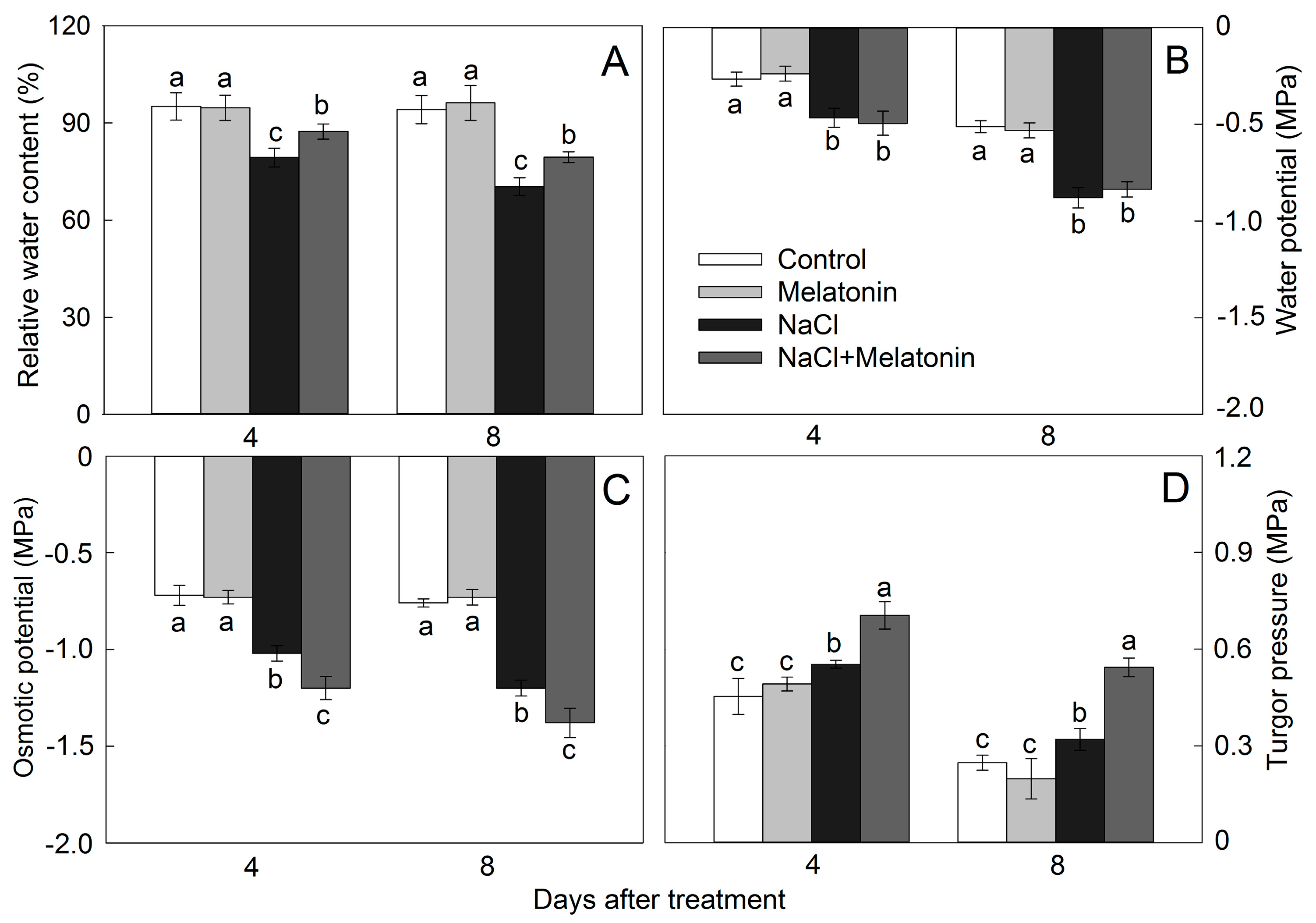
3.3. Effect of Salt and Melatonin Treatments on Leaf Water Status
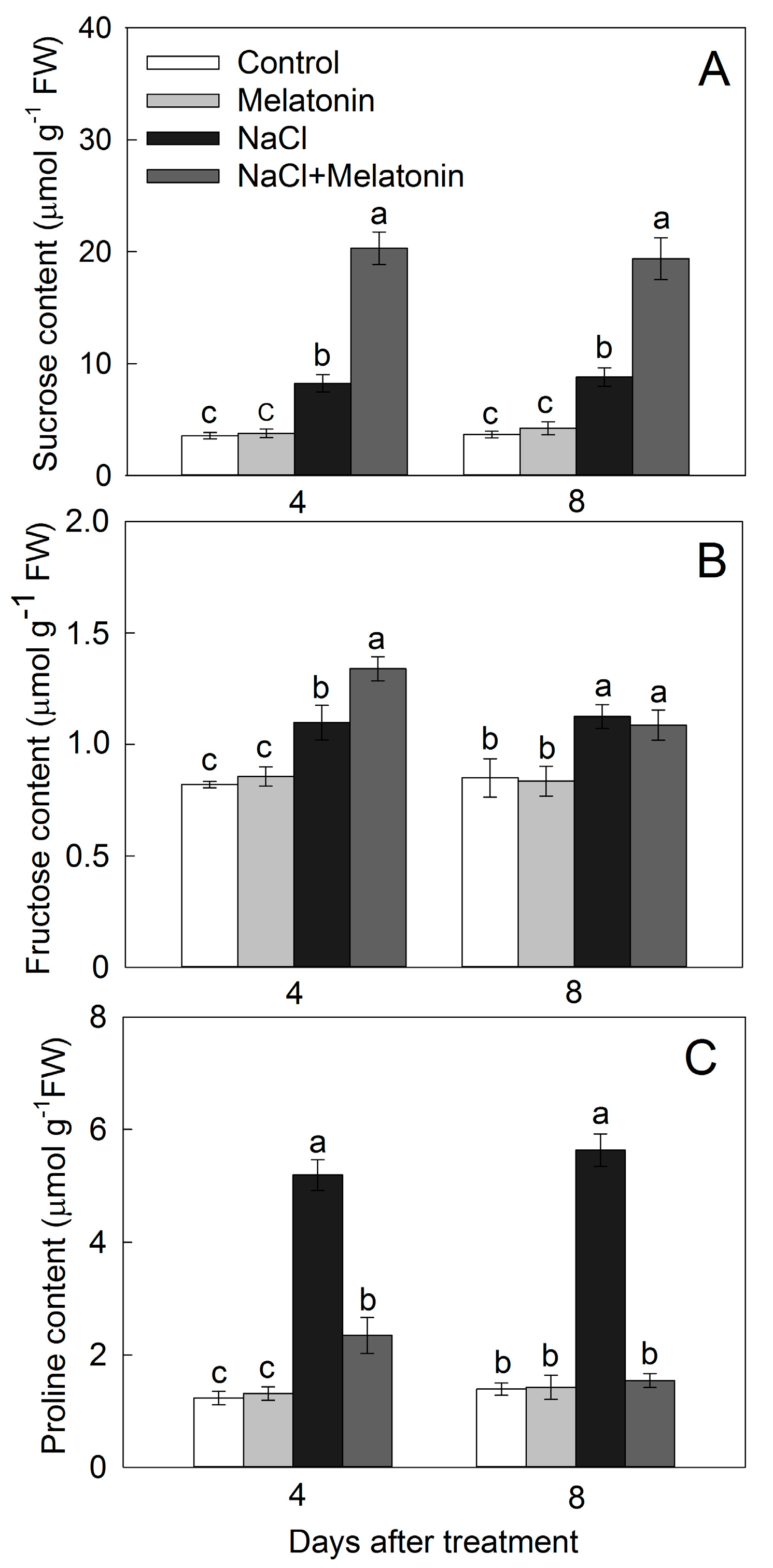
3.4. Effect of Salt and Melatonin Treatments on Soluble Sugar and Proline Contents
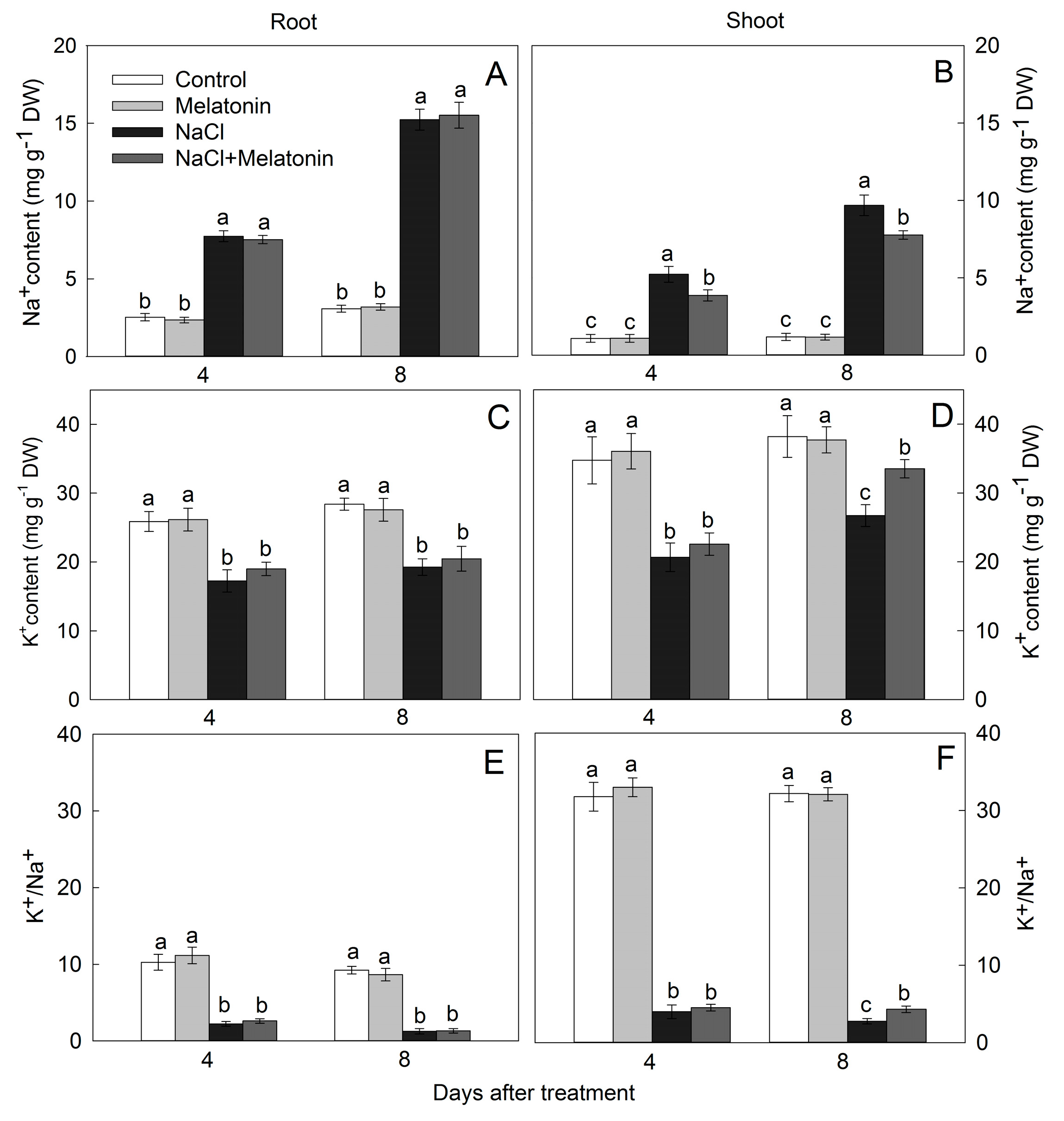
3.5. Effect of Salt and Melatonin Treatments on Na+ and K+ Accumulation
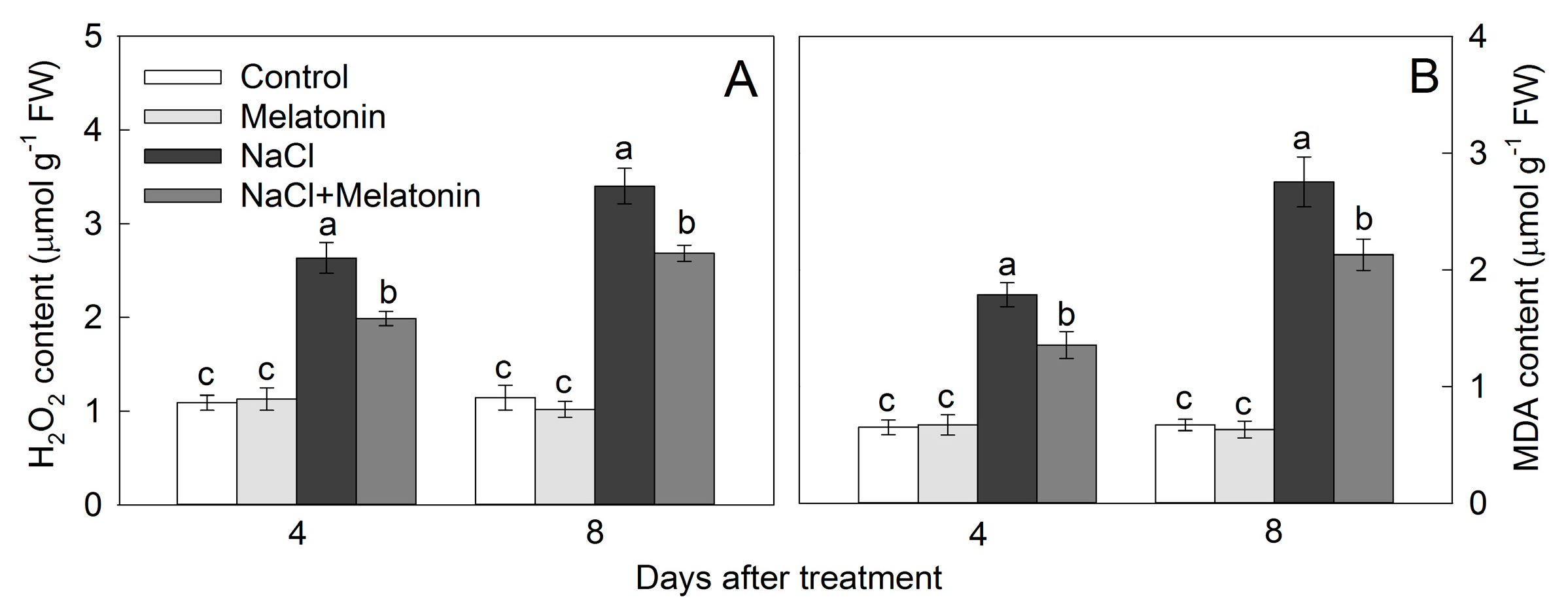
3.6. Effect of Salt and Melatonin Treatments on Leaf Hydrogen Peroxide (H2O2) and Malondialdehyde (MDA) Contents
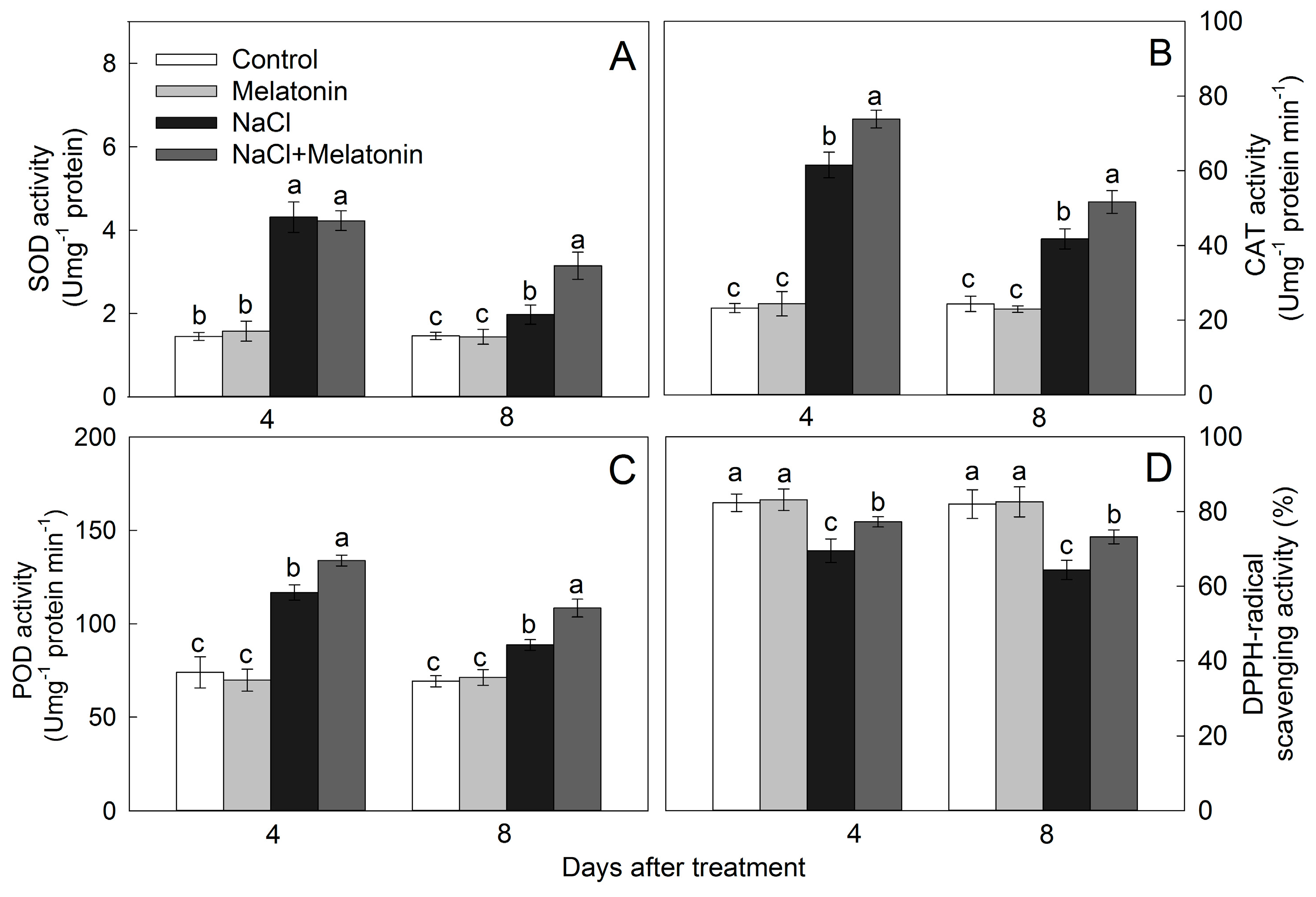
3.7. Effect of Salt and Melatonin Treatments on Enzymatic and Nonenzymatic Antioxidant Ability
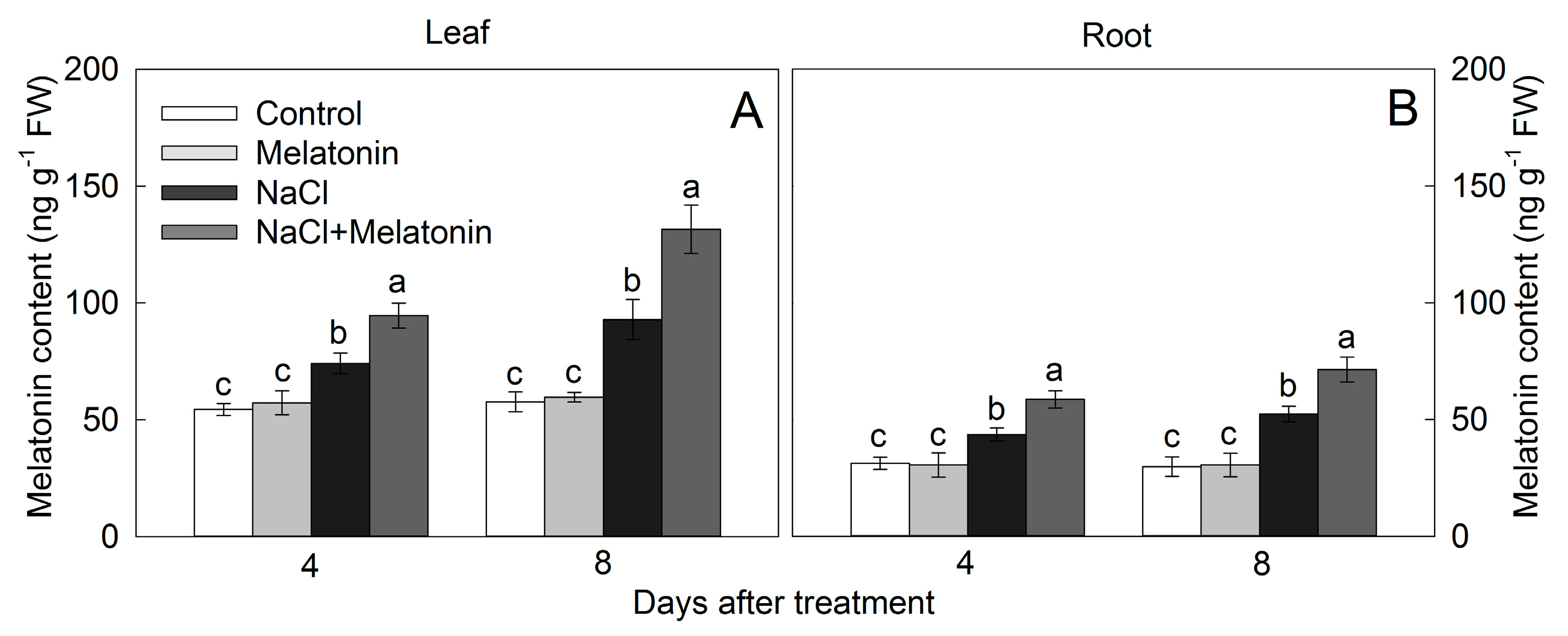
3.8. Effect of Salt and Melatonin Treatment on Endogenous Melatonin Contents
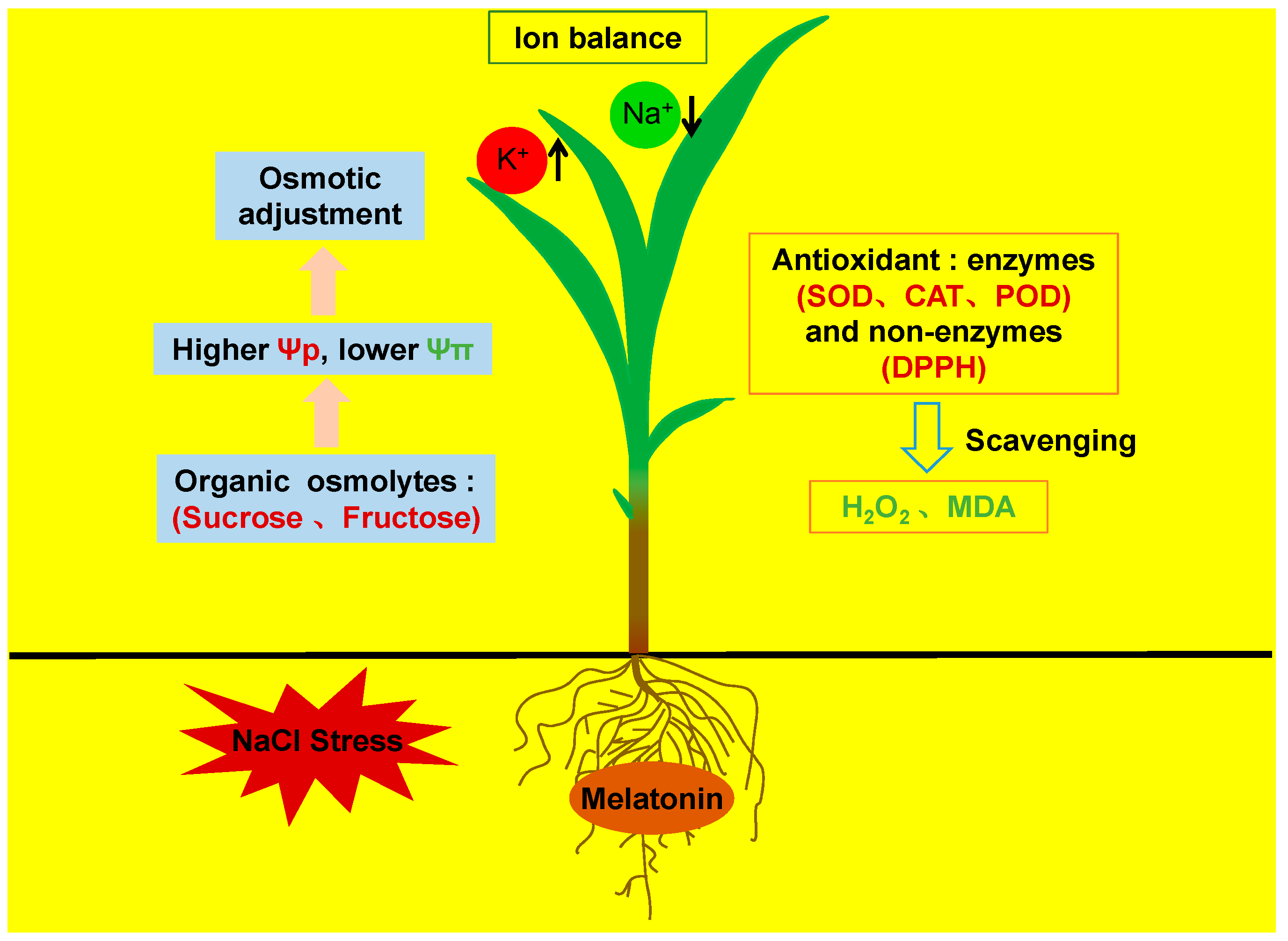
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: A new plant hormone and/or a plant master regulator? Trends Plant. Sci. 2018, 24, 38–48. [Google Scholar] [CrossRef]
- Chen, Y.E.; Mao, J.J.; Sun, L.Q.; Huang, B.; Ding, C.B.; Gu, Y.; Liao, J.Q.; Hu, C.; Zhang, Z.W.; Yuan, S. Exogenous melatonin enhances salt stress tolerance in maize seedlings by improving antioxidant and photosynthetic capacity. Physiol. Plantarum 2018, 164, 349–363. [Google Scholar] [CrossRef]
- Wang, P.; Sun, X.; Li, C.; Wei, Z.; Liang, D.; Ma, F. Long-term exogenous application of melatonin delays drought-induced leaf senescence in apple. J. Pineal Res. 2013, 54, 292–302. [Google Scholar] [CrossRef]
- Zhang, N.; Sun, Q.; Zhang, H.; Cao, Y.; Weeda, S.; Ren, S.; Guo, Y.D. Roles of melatonin in abiotic stress resistance in plants. J. Exp. Bot. 2015, 66, 647. [Google Scholar] [CrossRef] [PubMed]
- Kaya, A.; Doganlar, Z.B. Melatonin improves the multiple stress tolerance in pepper (Capsicum annuum). Sci. Hortic. 2019, 256. [Google Scholar] [CrossRef]
- Li, X.; Wei, J.P.; Scott, E.R.; Liu, J.W.; Guo, S.; Li, Y.; Zhang, L.; Han, W.Y. Exogenous melatonin alleviates cold stress by promoting antioxidant defense and redox homeostasis in camellia sinensis L. Molecules 2018, 23, 165. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Yin, L.; Wang, B.; Ke, Q.; Deng, X.; Wang, S. Melatonin promotes plant growth by increasing nitrogen uptake and assimilation under nitrogen deficient condition in winter wheat. Plant. Physiol. Bioch. 2019, 139, 342–349. [Google Scholar] [CrossRef]
- Wang, M.; Duan, S.; Zhou, Z.; Chen, S.; Wang, D. Foliar spraying of melatonin confers cadmium tolerance in Nicotiana tabacum L. Ecotox. Environ. Safe. 2019, 170, 68–76. [Google Scholar] [CrossRef]
- Wei, Z.; Li, C.; Gao, T.; Zhang, Z.; Liang, B.; Lv, Z.; Zou, Y.; Ma, F. Melatonin increases the performance of Malus hupehensis after UV-B exposure. Plant. Physiol. Bioch. 2019, 139, 630–641. [Google Scholar] [CrossRef]
- Arora, D.; Bhatla, S.C. Melatonin and nitric oxide regulate sunflower seedling growth under salt stress accompanying differential expression of Cu/Zn SOD and Mn SOD. Free Radic. Biol. Med. 2017, 106, 315–328. [Google Scholar] [CrossRef]
- Hwang, O.J.; Back, K. Melatonin deficiency confers tolerance to multiple abiotic stresses in rice via decreased brassinosteroid levels. Int. J. Mol. Sci. 2019, 20, 5173. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, B.; Cui, Y.; Yin, Y. Melatonin application confers enhanced salt tolerance by regulating Na+ and Cl− accumulation in rice. Plant. Growth Regul. 2017, 83, 1–14. [Google Scholar] [CrossRef]
- Wang, L.Y.; Liu, J.L.; Wang, W.X.; Sun, Y. Exogenous melatonin improves growth and photosynthetic capacity of cucumber under salinity-induced stress. Photosynthetica 2016, 54, 19–27. [Google Scholar] [CrossRef]
- Wei, W.; Li, Q.T.; Chu, Y.N.; Reiter, R.J.; Yu, X.M.; Zhu, D.H.; Zhang, W.K.; Ma, B.; Lin, Q.; Zhang, J.S. Melatonin enhances plant growth and abiotic stress tolerance in soybean plants. J. Exp. Bot. 2015, 66, 695. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhao, H.; Cao, K.; Hu, L.; Du, T.; Baluška, F.; Zou, Z. Beneficial roles of melatonin on redox regulation of photosynthetic electron transport and synthesis of D1 protein in tomato seedlings under salt stress. Front. Plant. Sci. 2016, 7, 1823. [Google Scholar] [CrossRef]
- Jiang, C.; Cui, Q.; Feng, K.; Xu, D.; Li, C.; Zheng, Q. Melatonin improves antioxidant capacity and ion homeostasis and enhances salt tolerance in maize seedlings. Acta Physiol. Plant. 2016, 38, 1–9. [Google Scholar] [CrossRef]
- Li, H.; Chang, J.; Chen, H.; Wang, Z.; Gu, X.; Wei, C.; Zhang, Y.; Ma, J.; Yang, J.; Zhang, X. Exogenous melatonin confers salt stress tolerance to watermelon by improving photosynthesis and redox homeostasis. Front. Plant. Sci. 2017, 8, 295. [Google Scholar] [CrossRef]
- Liang, C.; Zheng, G.; Li, W.; Wang, Y.; Hu, B.; Wang, H.; Wu, H.; Qian, Y.; Zhu, X.G.; Tan, D.X. Melatonin delays leaf senescence and enhances salt stress tolerance in rice. J. Pineal Res. 2015, 59, 91–101. [Google Scholar] [CrossRef]
- Zhang, H.J.; Zhang, N.; Yang, R.C.; Wang, L.; Sun, Q.Q.; Li, D.B.; Cao, Y.Y.; Weeda, S.; Zhao, B.; Ren, S. Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA₄ interaction in cucumber (Cucumis sativus L.). J. Pineal Res. 2015, 57, 269–279. [Google Scholar] [CrossRef]
- Ke, Q.; Ye, J.; Wang, B.; Ren, J.; Yin, L.; Deng, X.; Wang, S. Melatonin mitigates salt stress in wheat seedlings by modulating polyamine metabolism. Front. Plant. Sci. 2018, 9, 914. [Google Scholar] [CrossRef]
- Dąbrowski, P.; Kalaji, M.H.; Baczewska, A.H.; Pawluśkiewicz, B.; Mastalerczuk, G.; Borawska-Jarmułowicz, B.; Paunov, M.; Goltsev, V. Delayed chlorophyll a fluorescence, MR 820, and gas exchange changes in perennial ryegrass under salt stress. J. Lumin. 2017, 183, 322–333. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Govindjee; Bosa, K.; Kościelniak, J.; Żuk-Gołaszewska, K. Effects of salt stress on photosystem II efficiency and CO2 assimilation of two Syrian barley landraces. Environ. Exp. Bot. 2011, 73, 64–72. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant. Biol. 2008, 59, 651. [Google Scholar] [CrossRef]
- Chen, D.; Yin, L.; Deng, X.; Wang, S. Silicon increases salt tolerance by influencing the two-phase growth response to salinity in wheat (Triticum aestivum L.). Acta Physiol. Plant. 2014, 36, 2531–2535. [Google Scholar] [CrossRef]
- Yin, L.; Wang, S.; Tanaka, K.; Oka, M. Application of silicon improves salt tolerance through ameliorating osmotic and ionic stresses in the seedling of Sorghum bicolor. Acta Physiol. Plant. 2013, 35, 3099–3107. [Google Scholar] [CrossRef]
- Kostopoulou, Z.; Therios, I.; Roumeliotis, E.; Kanellis, A.K.; Molassiotis, A. Melatonin combined with ascorbic acid provides salt adaptation in Citrus aurantium L. seedlings. Plant Physiol. Bioch. 2015, 86, 155–165. [Google Scholar] [CrossRef]
- Wang, A.Y.; Li, Y.; Zhang, C.Q. QTL mapping for stay-green in maize (Zea mays). Can. J. Plant. Sci. 2012, 92, 249–256. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant. Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Storey, R. Salt tolerance, ion relations and the effect of root medium on the response of citruos to salinity. Aust. J. Plant. Physiol. 1995, 22, 101–114. [Google Scholar]
- Loreto, F.; Velikova, V. Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant. Physiol. 2001, 127, 1781–1787. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophysic. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Beauchamp, C.O.; Fridovich, I. Isozymes of superoxide dismutase from wheat germ. BBA-Protein Structure 1973, 317, 50–64. [Google Scholar] [CrossRef]
- Hamurcu, M.; Sekmen, A.H.; Turkan, İ.; Gezgin, S.; Demiral, T. Induced anti-oxidant activity in soybean alleviates oxidative stress under moderate boron toxicity. Plant. Growth Regul. 2013, 70, 217–226. [Google Scholar] [CrossRef]
- Kochba, J.; Lavee, S.; Spiegelroy, P. Differences in peroxidase activity and isoenzymes in embryogenic ane non-embryogenic ‘Shamouti’ orange ovular callus lines. Plant. Cell Physiol. 1977, 18, 463–467. [Google Scholar] [CrossRef]
- Wang, S.; Xie, B.; Yin, L.; Duan, L.; Li, Z.; Eneji, A.E.; Tsuji, W.; Tsunekawa, A. Increased UV-B radiation affects the viability, reactive oxygen species accumulation and antioxidant enzyme activities in maize (Zea mays L.) pollen. Photochem. Photobiol. 2010, 86, 110–116. [Google Scholar] [CrossRef]
- Byeon, Y.; Back, K. Melatonin synthesis in rice seedlings in vivo is enhanced at high temperatures and under dark conditions due to increased serotonin N-acetyltransferase and N-acetylserotonin methyltransferase activities. J. Pineal Res. 2014, 56, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhao, B.; Zhang, H.J.; Weeda, S.; Yang, C.; Yang, Z.C.; Ren, S.; Guo, Y.D. Melatonin promotes water-stress tolerance, lateral root formation, and seed germination in cucumber (Cucumis sativus L.). J. Pineal Res. 2012, 54, 15–23. [Google Scholar] [CrossRef]
- Volkmar, K.M.; Hu, Y.; Steppuhn, H. Physiological responses of plants to salinity: A review. Can. J. Plant. Sci. 1998, 78, 19–27. [Google Scholar] [CrossRef]
- Ye, J.; Wang, S.; Deng, X.; Yin, L.; Xiong, B.; Wang, X. Melatonin increased maize (Zea mays L.) seedling drought tolerance by alleviating drought-induced photosynthetic inhibition and oxidative damage. Acta Physiol. Plant. 2016, 38, 1–13. [Google Scholar] [CrossRef]
- Su, X.; Fan, X.; Shao, R.; Guo, J.; Wang, Y.; Yang, J.; Guo, L. Physiological and iTRAQ-based proteomic analyses reveal that melatonin alleviates oxidative damage in maize leaves exposed to drought stress. Plant. Physiol. Bioch. 2019, 142, 263–274. [Google Scholar] [CrossRef]
- Yang, C.; Chong, J.; Li, C.; Kim, C.; Shi, D.; Wang, D. Osmotic adjustment and ion balance traits of an alkali resistant halophyte Kochia sieversiana during adaptation to salt and alkali conditions. Plant. Soil 2007, 294, 263–276. [Google Scholar] [CrossRef]
- Lee, G.; Carrow, R.N.; Duncan, R.R.; Eiteman, M.A.; Rieger, M.W. Synthesis of organic osmolytes and salt tolerance mechanisms in Paspalum vaginatum. Environ. Exp. Bot. 2008, 63, 19–27. [Google Scholar] [CrossRef]
- Shi, H.; Qian, Y.; Tan, D.X.; Reiter, R.J.; He, C. Melatonin induces the transcripts of CBF/DREB1s and their involvement in both abiotic and biotic stresses in Arabidopsis. J. Pineal Res. 2015, 59, 334. [Google Scholar] [CrossRef] [PubMed]
- Kishor, P.; Hong, Z.; Miao, G.H.; Hu, C.; Verma, D. Overexpression of [delta]-pyrroline-5-carboxylate synthetase increases proline production and confers osmotolerance in transgenic plants. Plant. Physiol. 1995, 108, 1387–1394. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Madan, S.; Nainawatee, H.S.; Jain, R.K.; Chowdhury, J.B. Proline and proline metabolising enzymes in in-vitro selected nacl-tolerant brassica junceal L. under salt stress. Ann. Bot. 1995, 76, 51–57. [Google Scholar] [CrossRef]
- Nayyar, H.; Walia, D.P. Water stress induced proline accumulation in contrasting wheat genotypes as affected by calcium and abscisic acid. Biol. Plantarum 2003, 46, 275–279. [Google Scholar] [CrossRef]
- Lacerda, C.F.D.; Cambraia, J.; Oliva, M.A.; Ruiz, H.A.; Prisco, J.T.N. Solute accumulation and distribution during shoot and leaf development in two sorghum genotypes under salt stress. Environ. Exp. Bot. 2003, 49, 107–120. [Google Scholar] [CrossRef]
- Lutts, S.; Majerus, V.; Kinet, J.M. NaCl effects on proline metabolism in rice (Oryza sativa) seedlings. Physiol. Plantarum 2010, 105, 450–458. [Google Scholar] [CrossRef]
- Zhu, J.K. Regulation of ion homeostasis under salt stress. Curr. Opin. in Plant. Biol. 2003, 6, 441–445. [Google Scholar] [CrossRef]
- Shabala, S.; Cuin, T.A. Potassium transport and plant salt tolerance. Physiol. Plantarum 2008, 133, 651–669. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, P.; Wei, Z.; Liang, D.; Liu, C.; Yin, L.; Jia, D.; Fu, M.; Ma, F. The mitigation effects of exogenous melatonin on salinity-induced stress in Malus hupehensis. J. Pineal Res. 2012, 53, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Zhao, Y.; Yu, X.; Kiprotich, F.; Han, H.; Guan, R.; Shen, W. Nitric oxide is required for melatonin-enhanced tolerance against salinity stress in rapeseed (Brassica napus L.) seedlings. Int. J. Mol. Sci. 2018, 19, 1912. [Google Scholar] [CrossRef]
- Li, C.; Liang, B.; Chang, C.; Wei, Z.; Zhou, S.; Ma, F. Exogenous melatonin improved potassium content in Malus under different stress conditions. J. Pineal Res. 2016, 61, 218–229. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Tansley. Oxygen processing in photosynthesis: Regulation and signalling. New Phytol. 2010, 146, 359–388. [Google Scholar] [CrossRef]
- Meng, J.F.; Xu, T.F.; Wang, Z.Z.; Fang, Y.L.; Xi, Z.M.; Zhang, Z.W. The ameliorative effects of exogenous melatonin on grape cuttings under water-deficient stress: Antioxidant metabolites, leaf anatomy, and chloroplast morphology. J. Pineal Res. 2015, 57, 200–212. [Google Scholar] [CrossRef]
- Zhao, H.; Ye, L.; Wang, Y.; Zhou, X.; Yang, J.; Wang, J.; Cao, K.; Zou, Z. Melatonin increases the chilling tolerance of chloroplast in cucumber seedlings by regulating photosynthetic electron flux and the ascorbate-glutathione cycle. Front. Plant. Sci. 2016, 7, 1814. [Google Scholar] [CrossRef]
- Tan, D.X.; Hardeland, R.; Manchester, L.C.; Korkmaz, A.; Ma, S.; Rosales-Corral, S.; Reiter, R.J. Functional roles of melatonin in plants, and perspectives in nutritional and agricultural science. J. Exp. Bot. 2012, 63, 577–597. [Google Scholar] [CrossRef]
- Shi, H.; Chan, Z. The cysteine2/histidine2-type transcription factor ZINC FINGER OF ARABIDOPSIS THALIANA 6-activated C-REPEAT-BINDING FACTOR pathway is essential for melatonin-mediated freezing stress resistance in Arabidopsis. J. Pineal Res. 2014, 57, 185–191. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, J.; Ye, J.; Yin, L.; Li, G.; Deng, X.; Wang, S. Exogenous Melatonin Improves Salt Tolerance by Mitigating Osmotic, Ion, and Oxidative Stresses in Maize Seedlings. Agronomy 2020, 10, 663. https://doi.org/10.3390/agronomy10050663
Ren J, Ye J, Yin L, Li G, Deng X, Wang S. Exogenous Melatonin Improves Salt Tolerance by Mitigating Osmotic, Ion, and Oxidative Stresses in Maize Seedlings. Agronomy. 2020; 10(5):663. https://doi.org/10.3390/agronomy10050663
Chicago/Turabian StyleRen, Jianhong, Jun Ye, Lina Yin, Gouxia Li, Xiping Deng, and Shiwen Wang. 2020. "Exogenous Melatonin Improves Salt Tolerance by Mitigating Osmotic, Ion, and Oxidative Stresses in Maize Seedlings" Agronomy 10, no. 5: 663. https://doi.org/10.3390/agronomy10050663
APA StyleRen, J., Ye, J., Yin, L., Li, G., Deng, X., & Wang, S. (2020). Exogenous Melatonin Improves Salt Tolerance by Mitigating Osmotic, Ion, and Oxidative Stresses in Maize Seedlings. Agronomy, 10(5), 663. https://doi.org/10.3390/agronomy10050663







