Abstract
Since the 1980s, medicinal effects have been documented in scientific studies with the related Basidiomycota mushrooms Agaricus blazei Murill (AbM), Hericium erinaceus (HE) and Grifola frondosa (GF) from Brazilian and Eastern traditional medicine. Special focus has been on their antitumor effects, but the mushrooms’ anti-inflammatory and antiallergic properties have also been investigated. The antitumor mechanisms were either direct tumor attack, e.g., apoptosis and metastatic suppression, or indirect defense, e.g., inhibited tumor neovascularization and T helper cell (Th) 1 immune response. The anti-inflammatory mechanisms were a reduction in proinflammatory cytokines, oxidative stress and changed gut microbiota, and the antiallergic mechanism was amelioration of a skewed Th1/Th2 balance. Since a predominant Th2 milieu is also found in cancer, which quite often is caused by a local chronic inflammation, the three conditions—tumor, inflammation and allergy—seem to be linked. Further mechanisms for HE were increased nerve and beneficial gut microbiota growth, and oxidative stress regulation. The medicinal mushrooms AbM, HE and GF appear to be safe, and can, in fact, increase longevity in animal models, possibly due to reduced tumorigenesis and oxidation. This article reviews preclinical and clinical findings with these mushrooms and the mechanisms behind them.
1. Introduction
Agaricus blazei Murill (AbM) is an edible mushroom of the Basidomycota family, growing freely in the coastal Piedade rain forest area outside of São Paulo, Brazil. It was defined in 1967 by the Belgian botanist Paull Heinemann, who named it after the American mycologist William Murill [1]. AbM has mainly been used by the local population as a food ingredient, but also as a remedy against a wide range of diseases, in particular against infection and cancer [2]. In 1965, spores from AbM were brought to Japan, where artificial cultivation and large-scale production was undertaken. At the same time, AbM became the subject of extensive medical research, showing notable immunomodulating and tumoricidal effects, primarily in vitro [1,2]. The fruiting body of AbM is rich in β-glucans, and the biological effect of AbM has originally been attributed to the immunomodulating effect of this polysaccharide, mainly through activation of the innate immune system [3]. β-glucans are chains of D-glucose linked by β-type glycosidic bonds [4], and the main structure of ß-glucans in AbM is a β- (1–3) linked backbone with (1–6) linked side branches. Their molecular weight varies from a few kDa to several thousand kDa, and their biologic effects vary with their structure, molecular weight, and tertiary conformation [5]. In addition, AbM contains metabolic substances which exhibit cytotoxic effects, such as the steroid blazein, the lipid ergosterol, its derivate agarol, and the fenolhydrazine containing compound agaritine [6,7,8]. Although several mushrooms in the Basidiomycetes family have similar functions to those examined here, the related Agaricus blazei Murill (AbM), Hericium erinaceus (HE) and Grifola frondosa (GF) mushrooms were focused on in this review because they are components of AndosanTM, which has been extensively investigated in clinical studies.
HE and GF are also rich in β-glucans, and they have both been shown to have immunomodulating and antitumor effects [9,10]. HE (“lion’s mane”) and GF (“hen of the woods”) grow on hardwood trees in America and Asia and are both widely consumed, especially in the Far East, because of their nutritional qualities and perceived health benefits. In addition to glycoproteins and polysaccharides, HE contains a number of metabolic substances, in particular the aromatic compounds hericerins and erinacines, which have been shown to have a function as a nerve growth factor [11]. Research on GF has particularly focused on a proteoglycan, called the D-fraction, which has been shown to have immunomodulatory, cytotoxic and antioxidant effects in vitro [12]. HE and GF are minor ingredients (15% and 3%, respectively) in the AbM-based mushroom product AndosanTM (ACE Co. Ltd. produced for Immunopharma, Gifu-ken, Japan) which first was shown to have significant anti-infective effects in mouse models of bacterial sepsis [13,14], and later to have antiallergic [15] and antitumor effects [16] as well.
Chronic inflammatory conditions per se or infections giving rise to a chronic inflammatory response tend to predispose cancer development. Examples are: hepatitis B and C infection and hepatocellular carcinoma [17], pancreatitis and pancreatic cancer [18], atrophic gastritis and gastric cancer [19], Helicobacter pylori infection and mucosa-associated lymphoid tissue (MALT) lymphoma, which usually regress after antibiotic treatment [20], bladder infection with Schistosomia hematobium and bladder cancer [21], prostatitis and prostatic cancer [22], and inflammatory bowel disease and colorectal cancer [23,24]. Hence, there is a linkage between inflammation and tumorigenesis.
An increased T helper cell (Th) 2 response promotes allergy and asthma development. Th2 is also dominant in the cancer environment [25], where a T helper cell (Th) 1 immune response is essential for antitumor activity [26]. Since the Th1/Th2 balance is inversely linked [27], one may speculate whether there is a link between allergy/asthma and cancer. This seems to be controversial; while one study found increased risk of colorectal cancer in allergic disease [28], another study did not find any association between allergy and risk for breast or prostate cancer [29]. Moreover, since allergy and asthma can be regarded as topic inflammation induced by different allergens, e.g., in the eyes and nose for pollen, in the throat or skin for food components, and in the lungs for inhalants, these atopic conditions could also predispose for cancer. In fact, a recent meta-analysis could not rule out a possible association between atopy and malignancy of lung, skin and oesophagus [30]. The hygiene hypothesis has also established a link between protection by early presence of bacterial and parasitic infections against allergy and asthma development in childhood [31]. An interesting twist in this respect is our previous finding of increased allergy among tuberculosis (TB) and leprosy patients relative to their healthy controls [32,33], which was explained by a reduced defense against TB infection, i.e., low Th1 response, in approximately 10% of individuals, who normally contract TB after exposure to M. tuberculosis [33].
The aim of this paper was therefore to review preclinical and clinical studies on the antitumor, anti-inflammatory, and antiallergic effects of AbM and its related medicinal mushrooms GF and HE. Selection criteria were inclusion of PubMed/Medline indexed articles on the above in vivo results with these Basidiomycetes mushrooms, and exclusion of articles on findings in other areas.
2. Material and Methods
2.1. Antitumor Effects of ABM-Preclinical Studies
Since the early 1990s, there has been a host of studies on preclinical antitumor effects of AbM extracts in rodent models (Table 1). At first, fibrosarcoma was studied in a double grafted mouse model, and both a polysaccharide–protein complex and lipid fraction of AbM were found to inhibit tumor growth after oral and intraperitoneal administration [34,35,36]. This was thought to be due to immunological- and ergosterol-mediated inhibited neovascularization of tumor. However, Delmanto et al. [37] suggested that the mechanism for the observed antitumor effect of AbM was the mushroom’s antimutagenic effect, as demonstrated by its reduction in cyclophosphamide-induced micronuclei in the bone marrow and blood cells of the treated mice. The antitumor and antimetastatic effect of AbM polysaccharide in the fibrosarcoma model was confirmed after intratumor injection in 2005 [38], oral administration of AbM hot water extract in 2016 [39], and fermented AbM mycelia in 2018 [40]. The mechanism in the latter study was suggested to be an increase in cluster of differentiation (CD) 4+ and CD8+ T cells and a reduction in CD19+ B cells [40], restoring the balance between cellular and humoral immunity.

Table 1.
Antitumor effects of Agaricus blazei Murill—Preclinical studies.
One study also found that AbM extract, combined with marine phospholipid to increase its uptake, suppressed growth of myeloma cells in a mouse model [50]. Another study reported the inhibition of prostate cancer in a mouse model by a β-glucan-enriched AbM froth fraction by means of apoptosis and antiangiogenesis [49]. Niu et al. [47,48] performed extensive studies in mouse models for melanoma and sarcoma and showed that a low molecular weight (LMW) AbM polysaccharide could reduce lung metastasis of the former and growth and metastasis of the latter. Mechanisms for reduced tumor growth were an antiangiogenetic effect [47], and for the antimetastatic effect, modulation was mediated by metalloproteinase-9 and nm23-H1 [48], a metastatic suppressor [51]. On the other hand, Ziolotto et al. found no effect on colon carcinogenesis of AbM given orally to Wistar rats [46]. This is, however, in contrast to findings referred to below. Wu et al. [43,44] showed that AbM extract inhibited growth of colon cancer, hepatoma and melanoma in severe combined immunodeficient (SCID) mice and increased their life span in a dose-dependent manner. The finding of AbM (combined with chitosan N-acetyl glucosamine) inhibiting hepatoma cell growth in SCID mice was confirmed later [41]. Pinto et al. [45] observed that a β-glucan-rich AbM preparation reduced growth of Ehrlich tumor after intratumor injection, increased blood levels of IFNγ, CD4+ T cells and macrophages (MΦ), and reduced interleukin (IL)-10, resulting in immune cell migration to tumor and cytokine switch.
In a study of murine leukemia, an AbM extract reduced liver and spleen sizes, increased interferon (IFN)γ, IL-1β, IL-6 and reduced IL-4 levels [42]. The authors related this to increased CD3+ and CD19+ cells and reduced MΦ. Moreover, ex vivo experiments demonstrated that the AndosanTM extract (containing mainly AbM, see above) had a cytotoxic effect on primary myeloma cells, and also on myeloma and leukemia cell lines in vitro, probably caused by cell cycle arrest [52]. One study found an anticancer effect of an ergosterol derivative (Agarol) from AbM in a xenografted carcinoma murine model due to apoptosis [7]. We have reported that the addition of AndosanTM to drinking water protected mice against tumorigenesis of intestinal adenocarcinoma, which develops spontaneously from polyposis in A/J Min mice with a deletion in the apc gene [16]. The mechanism was immunomodulatory, as shown by increased IL-12 levels, and the growth inhibition of tumor cells by the induction of apoptosis. Recently, Sovrani et al. [53] reported that AbM exobiopolymers inhibited solid Walker 256 tumors in rats due to increased nitric oxide (NO-) production by peritoneal MΦ.
In summary, the mechanisms for the AbM-induced antitumor effects in rodent models were the following: inhibition of tumor neovascularization [35,36,47,49], antimutagenesis [37], modulation of metalloproteinase and a metastatic suppressor [48], Th1 immune cell migration to tumor [45], apoptosis [7,16,49,52], and increased NO- production by MΦ [53].
2.2. Antitumor Effects of AbM—Clinical Studies
In 1994, it was reported that treatment with AbM had an inhibitory effect on malignant cells in patients with acute non-lymphoblastic leukaemia [54] (Table 2). Ten years later, Ahn et al. [55] treated 100 patients with gynecological cancer who received chemotherapy with add-on AbM extract, and found that this, in contrast to add-on placebo, increased natural killer (NK) cell activity and improved quality of life (QoL) [55].

Table 2.
Antitumor effects of Agaricus blazei Murill–Clinical studies.
In a pilot study with AndosanTM, in the treatment of five patients with chronic hepatitis C virus (HCV) infection for a week, a slight, but statistically non-significant, reduction in serum HCV load was observed [58]. In addition, there was a surprising finding: microarray analysis of their peripheral leukocytes showed an increased expression of genes inducing apoptosis and inhibition of cell division, which is related to tumor defense [58]. In a recent placebo-controlled and randomized clinical study by Tangen et al. [56], AndosanTM extract was given orally for seven weeks as adjuvant treatment to half of 40 multiple myeloma patients undergoing high-dose chemotherapy with autologous stem cell transplantation (ASCT). The immunomodulatory findings were an increased number of plasmacytoid dendritic cells (pDC) and T regulatory cells (Tregs) in the blood, increased serum levels of IL-1ra (receptor antagonist), IL-5 and IL-7, and enhanced expression of immunoglobulin genes, Killer Immunoglobulin Receptor (KIR) genes and human leukocyte (HLA) genes in the bone marrow in the AndosanTM group. Although time with intravenous (i.v.) antibiotics during aplasia was 1.5 days shorter, and time to second line treatment and overall survival (OS) was 6 and 3.3 months longer, respectively, no statistically significant clinical impact of AndosanTM was detected at follow-up after 4 years. At a second follow-up after 5.7 years, mean OS was 79.0 months (95% CI: 61.5–96.5 months) in the AndosanTM group against 65.8 months (95% confidence interval (CI): 50.5–80.9 months) in the placebo group (mean observation time = 67.7 months) (p = 0.16)(Figure 1). Moreover, mean time to second line treatment was 47.3 months (95% CI: 30.5–64.0 months) in the AndosanTM group and 38.0 months (95% CI: 25.1–50.9 months) (median observation time 3.5 years)(p = 0.39) (unpublished data).
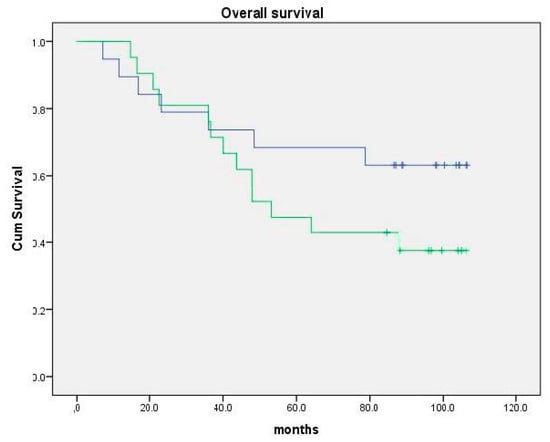
Figure 1.
Follow-up of overall survival (OS) of patients included in the clinical study: AndosanTM as adjuvant treatment in MM patients receiving autologous stem cell transplantation (ASCT) [56]. Upper curve: AndosanTM group (n = 19). Median OS = 79.0 months (95% CI 61.5–96.5 months). Lower curve: Placebo group (n = 21). Median OS = 65.8 months (95% CI 50.5–80.9 months) Median observation time 67.7 months. p = 0.16. Previously unpublished result.
There is one clinical study on prostate cancer with an AbM extract (Senseiro), which found prolonged doubling time of prostate specific antigen (PSA) levels. However, since it was not correlated with testosterone production and placebo controls were lacking, the authors concluded with no significant effect of AbM [57].
2.3. Antitumor Effects of GF
Studies of the anti-tumor effects of GF polysaccharides began in 1985, when the repression of implanted Meth A sarcoma was found in a mouse model. This was due to the stimulation of adaptive immunity, i.e., an increase in spleen cells [59] and cytotoxic T cells, as well as MΦ [60] (Table 3). A GF (fraction D) β-glucan had an antitumor effect on colon carcinoma implanted in mice, which was related to the establishment of Th1 dominance in a population that was Th2-dominant due to carcinoma [25]. A year later, this GF β-glucan was tested in 10 cancer patients (with lung, lingual, breast, gastric, or liver cancer) and reported to inhibit the progression of metastasis and reduce the expression of tumor markers (carcinoembryonic antigen (CEA), cancer antigen (CA)15-3, and CA19-9). This was primarily caused by an increase in NK cell activity and Th1 response and, inversely, a reduction in Th2 activity [61]. The same group at Kobe Pharmaceutical University, Japan, confirmed that the GF β-glucan could reduce colon cancer growth in mice by inducing cell-mediated immunity and Th1 response [62,63]. They further showed that GF β-glucan both enhanced antitumor and antimetastatic effects of cisplatin and reduced nephrotoxicity [64].

Table 3.
Antitumor effect of Grifola fondosa (Human study).
A LMW protein fraction of GF inhibited implanted colon carcinoma in mice, which was thought to be due both to increased IL-1β, TNFα, and, surprisingly, IL-10. Furthermore, an increase was found in the Th1 cytokines IL-12 and IFNγ, and in activated MΦ, NK and dendritic cells [68]. A systemic antitumor response of the GF β-glucan in mice was caused by immunomodulation, including the activation of MΦ in Peyer’s patches and an increase in IFNγ [63]. Moreover, the antitumor effect of a GF extract on implanted kidney cancer has been shown in rats, due to immunomodulation and tumor necrosis [67]. Recently, a selenium-enriched GF polysaccharide has been reported to have an enhanced antitumor effect in hepatoma (hepatocellular carcinoma (Heps))-bearing mice by improved immune function [66]. Since the GF β-glucan also enhanced the antitumor activity of 5-Fluorouracil (5-FU) in addition to protecting against its side effects, GF β-glucan could be developed as an auxiliary substance for chemotherapeutic drugs [65].
To summarize the mechanisms behind the GF-induced antitumor effects, they were: increase in spleen cells, Th1 and cytotoxic T cells, activated MΦ (also in Peyers patches) and NK cell activity [25,59,60,62,64], enhanced effects and reduced side effects of cisplatin and 5-FU [63,65], and necrosis [67].
2.4. Antitumor Effects of HE
In a murine model with a xenografted human colon cancer cell line, Kim et al. [9] found that aqueous and aqueous/ethanol extracts of HE [69] injected intraperitoneally led to the regression of the tumors. This effect was associated with an increase in intraperitoneal MΦ and serum pro-inflammatory cytokines. Furthermore, increased expression of genes coding for vascular endothelial growth factor (VEGF), cyclooxygenase 2 (Cox 2) and 5-lipooxygenase (5-LOX) were noted [9]. It was shown that treatment with these extracts also inhibited migration of the cancer cells to the lungs by 66% and 69%, respectively [70]. In these animals, a reduced expression was found of the matrix metalloproteinases MMP-2 and MMP-9 in the cancer cells, possibly causing inhibition of migration and invasion. In another murine model with xenografted human liver cancer-, gastric cancer- and colon cancer cells Li et al. [71] found that two extracts from HE, called HTJ5 and HTJ5A, with a mixed content of aromatic compounds, dipeptides indoles and amino acids, also exhibited a considerable antitumor activity against all cell lines.
3. Anti-inflammatory Effects of AbM and HE
3.1. AbM
Animal data (Table 4).

Table 4.
Anti-inflammatory effect of Agaricus blazei Murill, Hericium erinaceus and Grifola frondosa-Preclinical studies.
AbM extract given orally has been found to reduce carcinogen-induced lung damage in rats due to the attenuation of pulmonary inflammation [74]. Interestingly, in a murine malaria model, it was shown that AbM extract counteracted the deteriorating consequence of cerebral malaria by reducing proinflammatory cytokines TNFα, IL-6 and IL-1β [73]. Very recently, another AbM extract was found to prevent non-alcoholic steatosis by reducing hepatic stress in mice [72].
Human data (Table 5).

Table 5.
Anti-inflammatory effect of Agaricus blazei Murill—Clinical studies.
In contrast to the proinflammatory effects of AndosanTM found on human monocytic and endothelial cells cultured in vitro and on whole blood cells ex vivo [87,88], a significant decrease in IL-1β, TNFα, IL-17 and IL-2 was observed in ten volunteers who ingested AndosanTM for 12 days, showing a predominantly anti-inflammatory effect in vivo [87] (Table 3). Furthermore, in eight healthy volunteers who took AndosanTM, increased preshedding expression of the adhesion molecules CD62L (L-selectin) was found, while expression of the adhesion molecules CD11b and CD11c remained unchanged and intracellular reactive oxygen species (iROS) decreased both in monocytes and granulocytes [86].
Twenty-one patients with ulcerative colitis (UC) (n = 10) and Crohn’s disease (CD) (n = 11) received AndosanTM orally, alone or in addition to standard medical treatment, for 12 days. In both groups, plasma levels of several proinflammatory cytokines and chemokines in lipopolysaccharide (LPS) stimulated blood ex vivo were reduced, as well as fecal content of the pro-inflammatory marker, calprotectin, in the UC patients [85]. In another clinical trial 50 patients with UC and 50 patients with CD were randomized to receive either 60 mL of AndosanTM or placebo for 21 days. Plasma levels of IL-5 (UC group) and IL-2 (CD group) were reduced in patients receiving AndosanTM [82], and patients in both groups enjoyed an improvement in clinical symptoms and quality of life [83,84]. When looking at IL-1ß, IL-6 and granulocyte-colony stimulating factor (G-CSF) combined in the patients with CD, the cytokine levels were significantly lower in the AndosanTM group. In addition, total fatigue was improved in both the patients with UC and CD. The difference found in the in vitro and in vivo effects of AndosanTM is intriguing, and the reason for this discrepancy has not been clarified. However, potentially absorbable LMW substances like flavonoids and other less defined substances [89,90,91] may contribute to the anti-inflammatory effect of AbM in vivo.
3.2. HE
Anti-inflammatory properties have also been attributed to HE (Table 4). This is shown for HE itself, and also for erinacine A from HE, both of which protected against brain-ischemia-induced neuronal cell death in rats [78]. The mechanism was the inhibition of inducible nitric oxide synthase (iNOS) and mitogen-activated protein kinases (MAPK), reduced proinflammatory cytokines and nerve growth properties of the mushroom [78]. Another study in rats found that the HE β-glucan did improve inflammatory bowel disease (IBD)-induced colonic mucosa changes, because it promoted the growth of beneficial gut bacteria, which may have improved host immunity by reducing the activation of myeloperoxidase (MPO), nuclear factor kappa B (NFKB) and T cells [77]. Moreover, a HE polysaccharide reportedly attenuated colitis in mice by regulating oxidative stress through inflammation-related signaling pathways, composition of gut microbiota, and maintenance of an intestinal barrier [76]. Most interestingly, a HE fraction has been found to increase longevity in aged and carcinoma-bearing mice by the induction of endogenous antioxidative enzymes [75].
In summary, the mechanisms behind the anti-inflammatory effects induced in vivo by AbM were: predominant decrease in proinflammatory cytokines in healthy individuals and IBD patients [82,87], increased shedding of the adhesion molecule leukocyte-selectin and reduced iROS [86]. Anti-inflammatory mechanisms induced by HE were: educed proinflammatory cytokines, inhibition of iNOS, increased nerve growth protecting against neuron death in brain ischemia [78], growth of beneficial gut microbiota protecting against IBD-induced mucosa damages and improving host immunity [77], regulation of oxidative stress through signaling pathways attenuating colitis [76], and endogenous antioxidative enzymes increasing longevity [75].
3.3. GF
Regarding GF, it has been shown that a polysaccharide given orally had a similar protective effect on non-alcoholic steato-hepatitis in rats as AbM had in mice above [66]. The mechanism was a beneficial regulation of gut microbiota [79]. Moreover, a fermented GF extract had anti-inflammatory effect in an endotoxin-induced uveitis model in rats [80], and a GF extract reduced colon ulceration in an IBD rat model through an antioxidative and anti-inflammatory mechanism [81].
4. Antiallergic Effects of AbM and GF Extracts
It was reported in 2006 [92] that an AbM extract inhibited induced anaphylactic reaction (and also passive immunization), i.e., ear-swelling, in a mouse model by means of a treatment effect on the mast cell reaction (Table 6). In another mouse model for induction of allergic asthma, an AbM extract given orally, reduced levels of specific immunoglobulin (Ig)E, IgG1 and bronchial eosinophils due to the amelioration of skewed Th1/Th2 balance [93]. The finding was confirmed the year after by us with AndosanTM in the similar ovalbumin (OVA)-induced allergic sensitization mouse model, where specific IgE and IgG1 were also reduced and Th1 response increased relative to Th2 response [15]. When this established OVA sensitization model for allergy in the mouse was employed again, it was found that the mechanism behind the reduced specific IgE and improved Th1/Th2 balance was MΦ cell activation by epithelial cells and AbM promotion of differentiation of naïve T cells to Th1 cells [94]. Recently, our group performed a placebo-controlled randomized clinical study in which blood donors with self-reported and specific IgE-confirmed birch allergy and asthma, given AndosanTM orally for 2 months before the pollen season, had less general allergy and asthma ailments and used less medication. This was due to reduced specific IgE levels and reduced mast cell sensitization, as demonstrated indirectly by the basophil activation test [95].

Table 6.
Anti-allergic Effects of Agaricus blazei Murill and Grifola frondosa (Human study).
Antiallergic effects have also been observed in mice after the oral administration of a GF polysaccharide or extract (Table 6), where atopic dermatitis-like skin lesions and mast cell degranulation were inhibited, respectively, owing to alleviated anaphylactic cutaneous response [96,97]. The authors found that this was caused by reduced IgE and mast cell infiltration, a cytokine expression ameliorating the Th1/Th2 imbalance [97], and a reduced type I allergic response by suppression of mast cell degranulation [96]. Accordingly, GF polysaccharides could be used as a novel therapeutic agent, replacing corticosteroids, or as a supplementary substance [97].
5. Safety of AbM, GF and HE
Three cases of severe hepatic dysfunction have been reported in cancer patients using an oral AbM extract [98] (Table 7). However, other causes of hepatic dysfunction could not be ruled out in these patients. Furthermore, a case of allergic chronic cheilitis possibly occurred following daily AbM intake for 6 months [99]. AbM has been found to inhibit cytochrome P-450 and the trans-membrane-efflux pump P-glycoprotein (P-gp) to a mild degree in vitro (similar to green tea) [100]. AbM should therefore, as a precaution, not be used together with drugs that are P-gp substrates, such as vinblastine, vincristine, digitoxin, cyclosporine, loperamide, verapamil, quinidine and others [101]. One report found negative genotoxicity tests with AbM extracts in rats [102]. In a toxicity study in rats ingesting AbM over 2 years, there was no carcinogenicity or other adverse health effects of AbM. Rather, significantly lower mortality was found among the male rats on AbM treatment [103]. There is a case report of occupational hypersensitivity pneumonitis to GF spore after work in a mushroom farm [104]. Interestingly, HE protein fraction has been found to increase longevity in aged mice by means of antioxidative mechanism [76].

Table 7.
Safety of Agaricus blazei Murill, Grifola frondosa and Hericium erinaceus (Human Studies).
6. Discussion
Here, we review preclinical and clinical studies with the three related medicinal mushrooms AbM, HE and GF, where all exhibited both antitumor, anti-inflammatory and antiallergic properties, except for HE that did not reveal antiallergic effects. The antitumor mechanisms were either direct tumor attack, i.e., apoptosis/necrosis, antimutagenesis, and metastatic suppression, or indirect defence, i.e., inhibited neovascularization, Th1 cytotoxic cell tumor migration and increased MΦ NO production, NK cell activation, and enhanced effects and reduced side effects of chemotherapeutic drugs. The anti-inflammatory mechanisms were decreased in proinflammatory cytokines, adhesion molecules, iROS and iNOS. Furthermore, for HE, increased nerve and beneficial gut microbiota growth, and oxidative stress regulation, were noted. The antiallergic mechanism was amelioration of the skewed Th1/Th2 balance found in allergy and asthma. Regarding safety, besides allergic reactions (AbM and GF), undocumented hepatotoxicity (AbM) in case reports, and advised caution with simultaneous use of drugs affecting the intestinal trans-membrane-efflux pump (AbM), these medicinal mushrooms appear to be safe and can, in fact, increase longevity in animal models by reducing tumorigenesis (AbM) or an antioxidative mechanism (HE).
Most animal studies with AbM have been performed with grounded powder or extracts of the fruiting body with high β-glucan contents, and probably with varying batch-to-batch quality. In early studies [38,59], tumor growth suppression by mushroom fruiting body extracts or isolated β-glucan was established by direct intratumor injections. However, it is well-documented that β-glucan is taken up actively from intestines of rodents and brought to the reticulo-endothelial (RE) system [106]. This may explain the proinflammatory and immunomodulating effects observed with AbM in animal models referred to above, including with AndosanTM [16] where IL-1β, monocyte chemoattractant protein-1 (MCP-1) and tumor necrosis factor α (TNFα) were increased in addition to IL-12. This is opposite to the anti-inflammatory effect of AndosanTM found in healthy individuals and IBD patients [82,87]. In humans, uptake of β-glucan, which are often large cellulose polysaccharides [106], has not been documented, but there are studies arguing that it occurs [107,108]. Although AndosanTM has been found to contain only small amounts of β-glucan [109], it has a clear proinflammatory effect in vitro [87,88]. Most probably, this is caused by specific binding to receptors on immune cells (i.e., toll-like receptor 2 (TLR2), dectin-1, complement receptor 3 (CR3)) [106] of β-glucan still present in this extract, which then must have a high affinity to these receptors. AndosanTM, which is made from the mushroom mycelium, is one of the commercial AbM products that has been most investigated and shows promise in clinical studies. The mycelium may contain other or more effective ingredients than the fruiting body.
In vivo, there are other LMW molecules in AbM and other mechanisms involved than those involved in vitro. Such LMW components of AbM with anti-inflammatory and antioxidative effects, are isoflavonoids and components from aqueous and alkaline extracts that may be absorbed through the intestinal mucosa in order to exert their effects [89,90,91]. This agrees with our previous observation that the antiallergic effect of AndosanTM in the mouse allergy model seemed to be due to LMW substances [15]. In cultures, the cells are exposed to the whole mushroom extract, in which β-glucans probably drive the proinflammatory response. The different results on intestinal tumorigenesis found in rodents by use of AbM fruiting body (no effect) [46] versus with an AbM-based mycelium extract (AndosanTM) (60% reduced tumor load), may be due to the different AbM preparations used.
The different effects of the various AbM extracts can be due to differences in AbM subspecies, the production process, including type of extraction method, and whether the fruiting body was used or the mycelia, as mentioned. This was demonstrated in a blinded comparison of five AbM extracts from major Japanese health food producers in the pneumococcal sepsis model in mice [110]. When given orally before bacterial challenge i.p., only one of the extracts, later called AndosanTM, significantly reduced bacteremia and increased survival rate as compared with the saline control. Hence, the different mushroom extracts, even from the same subspecies, may contain different constituents or the required effective concentrations thereof. Moreover, AndosanTM contains extracts and substances from three different mushrooms, AbM, HE and GF, which also may have a positive synergistic effect, but this is unknown.
In the randomized clinical trial with multiple myeloma patients, there seems to be a positive effect, with 1.3 years longer survival in the AndosanTM-treated patients. This suggestion should be addressed in a study that is properly powered.
In the A/J Min mice model for colorectal cancer, animals treated with AndosanTM had reduced intestinal tumor load, and also expressed less of the tumor-associated protease, legumain, in their intestines [16]. The fact that this enzyme is also pro-inflammatory in nature, demonstrates a link between inflammation and tumorigenesis. Moreover, AndosanTM also had a systemic proinflammatory effect in these mice, as shown by increased levels of proinflammatory cytokines, which is similar to the mushroom-extract-treated mice in a sepsis model [13]. Hence, AndosanTM has a general proinflammatory effect in mice, in contrast to humans, which is in agreement with the active intestinal uptake of β-glucan in mice [106]. However, this also varies in humans because, in cancer patients given increasing doses of a GF polysaccharide orally, there was a non-monotonic and fluctuating association with both immune-stimulatory and –inhibitory systemic effects [105]. In humans, the main results found with AndosanTM probably is due to β-glucan stimulation of Peyer’s patches in the gut-associated-lymphoid–tissue (GALT) [111], together with other less defined absorbable LMW substances such as flavonoids. However, the main active principle in AndosanTM still remains unknown.
Interestingly, AbM also had antitumor effect on implanted tumor in the very same animals where the antiallergic and antiasthmatic effect of the mushroom extract was shown [92]. At least from an immunological perspective, this illustrates a possible relationship between cancer and allergy.
7. Conclusions
This review on scientific in vivo findings with three of the most well-known medicinal mushrooms, AbM, HE and GF, shows that they possess valuable antitumor, anti-inflammatory and antiallergic properties. These should be investigated more thoroughly with quality-controlled and standardized mushroom extracts, or fractions thereof, in randomized clinical studies powered to show putative therapeutic effects. In this way, medicinal Basidomycota could become significant supplementary drugs in Western medicine.
Author Contributions
Conceptualization, G.H., J.-M.T., L.S.H.N.-M., and E.J.; methodology, G.H., J.-M.T., M.R.M., I.N., and E.J.; software, J.-M.T. and S.P.T.; validation, J.-M.T., F.M., I.N., and S.P.T.; formal analysis, G.H., J.-M.T., F.M., M.R.M., S.P.T. and E.J.; investigation, G.H., J.-M.T., F.M., M.R.M., L.S.H.N.-M., I.N., S.P.T., G.E.T. and E.J.; resources, G.H., L.S.H.N.-M., G.E.T. and E.J.; data curation, G.H., J.-M.T., F.M., M.R.M., I.N., S.P.T., and E.J.; resources, G.H., L.S.H.N.-M., G.E.T. and E.J.; writing—original draft preparation, G.H.; writing—review and editing, G.H., J.-M.T., F.M., M.R.M., L.S.H.N.-M., I.N., S.P.T., G.E.T. and E.J.; visualization, J.-M.T., M.R.M., S.P.T., E.J.; supervision, G.H., L.S.H.N.-M., G.E.T. and E.J.; project administration, G.H., L.S.H.N.-M., G.E.T., and E.J.; internal funding acquisition, G.H., L.S.H.N.-M., G.E.T., and E.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
Geir Hetland is a cofounder and shareholder of Immunopharma, Oslo, Norway. The other authors declare no commercial or financial conflict of interest. Immunopharma had no other role than providing AndosanTM free of charge for the studies referred to.
References
- Firenzuoli, F.; Gori, L.; Lombardo, G. The Medicinal Mushroom Agaricus blazei Murrill: Review of Literature and Pharmaco-Toxicological Problems. Evid. Based Complement Altern. Med. 2008, 5, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Fu, Z.; Han, C. The Medicinal Values of Culinary-Medicinal Royal Sun Mushroom (Agaricus blazei Murrill). Evid. Based Complement Altern. Med. 2013, 2013, 842619. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Biedron, R.; Tangen, J.M.; Maresz, K.; Hetland, G. Agaricus blazei Murill immunomodulating properties and health benefits. Funct. Food Health Dis. 2012, 2, 428–447. [Google Scholar] [CrossRef]
- Rop, O.; Mlcek, J.; Jurikova, T. Beta-glucans in higher fungi and their health effects. Nutr. Rev. 2009, 67, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Du, B.; Bian, Z.; Xu, B. Beta-glucans from edible and medicinal mushrooms: Characteristics, physiochemical and biological activities. J. Food Compos. Anal. 2015, 41, 165–173. [Google Scholar] [CrossRef]
- Itoh, H.; Ito, H.; Hibasami, H. Blazein of a New Steroid Isolated from Agaricus blazei Murrill (Himematsutake) Induces Cell Death and Morphological Change Indicative of Apoptotic Chromatin Condensation in Human Lung Cancer LU99 and Stomach Cancer KATO III Cells. Oncol. Rep. 2008, 20, 1359–1361. [Google Scholar]
- Shimizu, T.; Kawai, J.; Ouchi, K.; Kikuchi, H.; Osima, Y.; Hidemi, R. Agarol, an Ergosterol Derivative from Agaricus blazei, Induces Caspase-Independent Apoptosis in Human Cancer Cells. Int. J. Oncol. 2016, 48, 1670–1678. [Google Scholar] [CrossRef]
- Endo, M.; Beppu, H.; Akiyama, H.; Wakamatsu, K.; Ito, S.; Kawamoto, Y.; Shimpo, K.; Sumiya, T.; Koike, T.; Matsui, T. Agaritine Purified from Agaricus blazei Murrill Exerts Anti-Tumor Activity against Leukemic Cells. Biochim. Biophys. Acta 2010, 1800, 669–673. [Google Scholar] [CrossRef]
- Kim, S.P.; Kang, M.Y.; Kim, J.H.; Nam, S.H.; Friedman, M. Composition and mechanism of antitumor effects of Hericium erinaceus mushroom extracts in tumor-bearing mice. J. Agric. Food Chem. 2011, 28, 599861–599869. [Google Scholar] [CrossRef]
- Masuda, Y.; Inoue, H.; Ohta, H.; Miyake, A.; Konishi, M.; Nanba, H. Oral administration of soluble β-glucans extracted from Grifola frondosa induces systemic antitumor immune response and decreases immunosuppression in tumor-bearing mice. Int. J. Cancer 2013, 133, 108–119. [Google Scholar] [CrossRef]
- Friedman, M. Chemistry, Nutrition, and Health-Promoting Properties of Hericium erinaceus (Lion’s Mane) Mushroom Fruiting Bodies and Mycelia and Their Bioactive Compounds. J. Agric. Food Chem. 2015, 63, 7108–7123. [Google Scholar] [CrossRef] [PubMed]
- Klaus, A.; Kozarski, M.; Vunduk, J.; Todorovic, N.; JakovljeviĆ, D.; Zizak, Z.; Pavlović, V.; Levic, S.; Nikšić, M.; Van Griensven, L.J.L.D. Biological potential of extracts of the wild edible Basidiomycete mushroom Grifola frondosa. Food Res. Int. 2015, 67, 272–283. [Google Scholar] [CrossRef]
- Bernardshaw, S.; Johnson, E.; Hetland, G. An extract of the mushroom Agaricus blazei Murill administered orally protects against systemic Streptococcus pneumoniae infection in mice. Scand. J. Immunol. 2005, 62, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Bernardshaw, S.; Hetland, G.; Grinde, B.; Johnson, E. An extract of the mushroom Agaricus blazei Murill protects against lethal septicemia in a mouse model of fecal peritonitis. Shock 2006, 25, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Ellertsen, L.K.; Hetland, G. An extract of the medicinal mushroom Agaricus blazei Murill can protect against allergy. Clin. Mol. Allergy 2009, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Hetland, G.; Eide, D.M.; Tangen, J.M.; Haugen, M.H.; Mirlashari, M.R.; Paulsen, J.E. The Agaricus blazei-Based Mushroom Extract, Andosan™, Protects against Intestinal Tumorigenesis in the A/J Min/+ Mouse. PLoS ONE 2016, 11, e0167754. [Google Scholar] [CrossRef] [PubMed]
- Baecker, A.; Liu, X.; La Vecchia, C.; Zhang, Z.F. Worldwide incidence of hepatocellular carcinoma cases attributable to major risk factors. Eur. J. Cancer Prev. 2018, 27, 205–212. [Google Scholar] [CrossRef]
- McGuigan, A.; Kelly, P.; Turkington, R.C.; Jones, C.; Coleman, H.G.; McCain, R.S. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J. Gastroenterol. 2018, 24, 4846–4861. [Google Scholar] [CrossRef]
- Watari, J.; Chen, N.; Amenta, P.S.; Fukui, H.; Oshima, T.; Tomita, T.; Miwa, H.; Lim, K.J.; Das, K.M. Helicobacter pylori associated chronic gastritis, clinical syndromes, precancerous lesions, and pathogenesis of gastric cancer development. World J. Gastroenterol. 2014, 20, 5461–5573. [Google Scholar] [CrossRef]
- Morgner, A.; Bayerdörffer, E.; Neubauer, A.; Stolte, M. Gastric MALT lymphoma and its relationship to Helicobacter pylori infection: Management and pathogenesis of the disease. Microsc. Res. Tech. 2000, 48, 349–356. [Google Scholar] [CrossRef]
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Padala, S.A.; Barsouk, A. Epidemiology of Bladder Cancer. Med. Sci. 2020, 8, 15. [Google Scholar] [CrossRef]
- Perletti, G.; Monti, E.; Magri, V.; Cai, T.; Cleves, A.; Trinchieri, A.; Montanari, E. The association between prostatitis and prostate cancer. Systematic review and meta-analysis. Arch. Ital. Urol. Androl. 2017, 89, 259–265. [Google Scholar] [CrossRef]
- Canavan, C.; Abrams, K.R.; Mayberry, J. Meta-analysis: Colorectal and small bowel cancer risk in patients with Crohn’s disease. Aliment. Pharmacol. Ther. 2006, 23, 1097–1104. [Google Scholar] [CrossRef]
- Zhou, Q.; Shen, Z.F.; Wu, B.S.; Xu, C.B.; He, Z.Q.; Chen, T.; Shang, H.T.; Xie, C.F.; Huang, S.Y.; Chen, Y.G.; et al. Risk of Colorectal Cancer in Ulcerative Colitis Patients: A Systematic Review and Meta-Analysis. Gastroenterol. Res. Pract. 2019, 2019, 5363261. [Google Scholar] [CrossRef]
- Kodama, N.; Komuta, K.; Sakai, N.; Nanba, H. Effects of D-Fraction, a polysaccharide from Grifola frondosa on tumor growth involve activation of NK cells. Biol. Pharm. Bull. 2002, 25, 1647–1650. [Google Scholar] [CrossRef]
- Lu, X. Impact of IL-12 in Cancer. Curr. Cancer Drug Targets 2017, 17, 682–697. [Google Scholar] [CrossRef]
- Romagnani, S. Induction of TH1 and TH2 responses: A key role for the ‘natural’ immune response? Immunol. Today 1992, 13, 379–381. [Google Scholar] [CrossRef]
- Chou, W.Y.; Lai, P.Y.; Hu, J.M.; Hsu, C.H.; Chen, Y.C.; Tian, Y.F.; You, S.L.; Hsiao, C.W.; Chou, Y.C.; Sun, C.A. Association between atopic dermatitis and colorectal cancer risk: A nationwide cohort study. Medicine 2020, 99, e18530. [Google Scholar] [CrossRef]
- Jiang, X.; Dimou, N.L.; Zhu, Z.; Bonilla, C.; Lewis, S.J.; Lindström, S.; Kraft, P.; Tsilidis, K.K.; Martin, R.M. Allergy, asthma, and the risk of breast and prostate cancer: A Mendelian randomization study. Cancer Causes Control. 2020, 31, 273–282. [Google Scholar] [CrossRef]
- Muir, A.B.; Whelan, K.A.; Dougherty, M.K.; Aaron, B.; Navarre, B.; Aceves, S.S.; Dellon, E.S.; Jensen, E.T. The potential for malignancy from atopic disorders and allergic inflammation: A systematic review and meta-analysis. Clin. Exp. Allergy 2020, 50, 147–159. [Google Scholar] [CrossRef]
- Strachan, D.P. Hay fever, hygiene, and household size. BMJ 1989, 299, 1259–1260. [Google Scholar] [CrossRef] [PubMed]
- Ellertsen, L.K.; Wiker, H.G.; Egeberg, N.T.; Hetland, G. Allergic sensitisation in tuberculosis and leprosy patients. Int. Arch. Allergy Immunol. 2005, 138, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Ellertsen, L.K.; Storla, D.G.; Diep, L.M.; Brokstad, K.A.; Wiker, H.G.; Hetland, G. Allergic sensitisation in tuberculosis patients at the time of diagnosis and following chemotherapy. BMC Infect. Dis. 2009, 9, 100. [Google Scholar] [CrossRef] [PubMed]
- Itoh, H.; Ito, H.; Amano, H.; Noda, H. Inhibitory action of a (1-->6)-beta-D-glucan-protein complex (F III-2-b) isolated from Agaricus blazei Murill (“himematsutake”) on Meth A fibrosarcoma-bearing mice and its antitumor mechanism. Jpn. J. Pharmacol. 1994, 66, 265–271. [Google Scholar] [CrossRef]
- Ito, H.; Shimura, K.; Itoh, H.; Kawade, M. Antitumor effects of a new polysaccharide-protein complex (ATOM) prepared from Agaricus blazei (Iwade strain 101) “Himematsutake” and its mechanisms in tumor-bearing mice. Anticancer Res. 1997, 17, 277–284. [Google Scholar]
- Takaku, T.; Kimura, Y.; Okuda, H. Isolation of an antitumor compound from Agaricus blazei Murill and its mechanism of action. J. Nutr. 2001, 131, 1409–1413. [Google Scholar] [CrossRef]
- Delmanto, R.D.; de Lima, P.L.; Sugui, M.M.; da Eira, A.F.; Salvadori, D.M.; Speit, G.; Ribeiro, L.R. Antimutagenic effect of Agaricus blazei Murrill mushroom on the genotoxicity induced by cyclophosphamide. Mutat. Res. 2001, 496, 15–21. [Google Scholar] [CrossRef]
- Ebina, T.; Fujimiya, Y. Antitumor effect of a peptide-glucan preparation extracted from Agaricus blazei in a double-grafted tumor system in mice. Biotherapy 1998, 11, 259–265. [Google Scholar] [CrossRef]
- Bertéli, M.B.; Lopes, A.D.; Colla, I.M.; Linde, G.A.; Colauto, N.B. Agaricus subrufescens: Substratum nitrogen concentration and mycelial extraction method on antitumor activity. An. Acad. Bras. Ciências 2016, 88, 2239–2246. [Google Scholar] [CrossRef]
- Rubel, R.; Santa, H.S.D.; Dos Santos, L.F.; Fernandes, L.C.; Figueiredo, B.C.; Soccol, C.R. Immunomodulatory and Antitumoral Properties of Ganoderma lucidum and Agaricus brasiliensis (Agaricomycetes) Medicinal Mushrooms. Int. J. Med. Mushrooms 2018, 20, 393–403. [Google Scholar] [CrossRef]
- Yeh, M.Y.; Shang, H.S.; Lu, H.F.; Chou, J.; Yeh, C.; Chang, J.B.; Hung, H.F.; Kuo, W.L.; Wu, L.Y.; Chung, J.G. Chitosan oligosaccharides in combination with Agaricus blazei Murill extract reduces hepatoma formation in mice with severe combined immunodeficiency. Mol. Med. Rep. 2015, 12, 133–140. [Google Scholar] [CrossRef]
- Lin, J.G.; Fan, M.J.; Tang, N.Y.; Yang, J.S.; Hsia, T.C.; Lin, J.J.; Lai, K.C.; Wu, R.S.; Ma, C.Y.; Wood, W.G.; et al. An extract of Agaricus blazei Murill administered orally promotes immune responses in murine leukemia BALB/c mice in vivo. Integr. Cancer Ther. 2012, 11, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.F.; Chen, Y.L.; Lee, M.H.; Shih, Y.L.; Hsu, Y.M.; Tang, M.C.; Lu, H.F.; Tang, N.Y.; Yang, S.T.; Chueh, F.S.; et al. Effect of Agaricus blazei Murrill extract on HT-29 human colon cancer cells in SCID mice in vivo. In Vivo 2011, 25, 673–677. [Google Scholar] [PubMed]
- Wu, M.F.; Lu, H.F.; Hsu, Y.M.; Tang, M.C.; Chen, H.C.; Lee, C.S.; Yang, Y.Y.; Yeh, M.Y.; Chung, H.K.; Huang, Y.P.; et al. Possible reduction of hepatoma formation by Smmu 7721 cells in SCID mice and metastasis formation by B16F10 melanoma cells in C57BL/6 mice by Agaricus blazei murill extract. In Vivo 2011, 25, 399–404. [Google Scholar]
- Pinto, A.V.; Martins, P.R.; Romagnoli, G.G.; Campanelli, A.P.; Terezan, A.P.; Filho, E.R.; Ferreira da Eira, A.; Kaneno, R. Polysaccharide fraction of Agaricus brasiliensis avoids tumor-induced IL-10 production and changes the microenvironment of subcutaneous Ehrlich adenocarcinoma. Cell Immunol. 2009, 256, 27–38. [Google Scholar] [CrossRef]
- Ziliotto, L.; Pinheiro, F.; Barbisan, L.F.; Rodrigues, M.A. Screening for in vitro and in vivo antitumor activities of the mushroom Agaricus blazei. Nutr. Cancer 2009, 61, 245–250. [Google Scholar] [CrossRef]
- Niu, Y.C.; Liu, J.C.; Zhao, X.M.; Wu, X.X. A low molecular weight polysaccharide isolated from Agaricus blazei suppresses tumor growth and angiogenesis in vivo. Oncol. Rep. 2009, 21, 145–152. [Google Scholar]
- Niu, Y.C.; Liu, J.C.; Zhao, X.M.; Cao, J. A low molecular weight polysaccharide isolated from Agaricus blazei Murill (LMPAB) exhibits its anti-metastatic effect by down-regulating metalloproteinase-9 and up-regulating Nm23-H1. Am. J. Chin. Med. 2009, 37, 909–921. [Google Scholar] [CrossRef]
- Yu, C.H.; Kan, S.F.; Shu, C.H.; Lu, T.J.; Sun-Hwang, L.; Wang, P.S. Inhibitory mechanisms of Agaricus blazei Murill on the growth of prostate cancer in vitro and in vivo. J. Nutr. Biochem. 2009, 20, 753–764. [Google Scholar] [CrossRef]
- Murakawa, K.; Fukunaga, K.; Tanouchi, M.; Hosokawa, M.; Hossain, Z.; Takahashi, K. Therapy of Myeloma In Vivo Using Marine Phospholipid in Combination with Agaricus blazei Murill as an Immune Respond Activator. J. Oleo. Sci. 2007, 56, 179–188. [Google Scholar] [CrossRef][Green Version]
- Prabhu, V.V.; Siddikuzzaman Grace, V.M.; Guruvayoorappan, C. Targeting tumor metastasis by regulating Nm23 gene expression. Asian Pac. J. Cancer Prev. 2012, 13, 3539–3548. [Google Scholar] [CrossRef]
- Tangen, J.M.; Holien, T.; Mirlashari, M.R.; Misund, K.; Hetland, G. Cytotoxic Effect on Human Myeloma Cells and Leukemic Cells by the Agaricus blazei Murill Based Mushroom Extract, Andosan™. Biomed. Res. Int. 2017, 2017, 2059825. [Google Scholar] [CrossRef] [PubMed]
- Sovrani, V.; da Rosa, J.; Drewinski, M.P.; Colodi, F.G.; Tominaga, T.T.; Santa, H.S.D.; Rebeca, R. In Vitro and In Vivo Antitumoral Activity of Exobiopolymers from the Royal Sun Culinary-Medicinal Mushroom Agaricus brasiliensis (Agaricomycetes). Int. J. Med. Mushrooms 2017, 19, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Hui, T.; Guo, L.; Wang, J.; Ito, H.; Simura, K. Clinical observation on treatment of acute nonlymphocytic leukemia with Agaricus blazei Murill. J. Lanzhou Med. Coll. 1994, 20, 169–171. [Google Scholar]
- Ahn, W.-S.; Kim, D.-J.; Chae, G.-T.; Lee, J.-M.; Bae, S.-M.; Sin, J.-I.; Kim, Y.-W.; Namkoong, S.-E.; Lee, I.P. Natural killer cell activity and quality of life were improved by consumption of a mushroom extract, Agaricus blazei Murill Kyowa, in gynecological cancer patients undergoing chemotherapy. Int. J. Gynecol. Cancer 2004, 14, 589–594. [Google Scholar] [CrossRef]
- Tangen, J.M.; Tierens, A.; Caers, J.; Binsfeld, M.; Olstad, O.K.; Trøseid, A.M.; Wang, J.; Tjønnfjord, G.E.; Hetland, G. Immunomodulatory effects of the Agaricus blazei Murrill-based mushroom extract AndoSan in patients with multiple myeloma undergoing high dose chemotherapy and autologous stem cell transplantation: A randomized, double blinded clinical study. Biomed. Res. Int. 2015, 2015, 718539. [Google Scholar] [CrossRef]
- Yoshimura, K.; Kamoto, T.; Ogawa, O.; Matsui, S.; Tsuchiya, N.; Tada, H.; Murata, K.; Yoshimura, K.; Habuchi, T.; Fukushima, M. Medical mushrooms used for biochemical failure after radical treatment for prostate cancer: An open-label study. Int. J. Urol. 2010, 17, 548–554. [Google Scholar] [CrossRef]
- Grinde, B.; Hetland, G.; Johnson, E. Effects on gene expression and viral load of a medicinal extract from Agaricus blazei in patients with chronic hepatitis C infection. Int. Immunopharmacol. 2006, 6, 1311–1314. [Google Scholar] [CrossRef]
- Suzuki, I.; Itani, T.; Ohno, N.; Oikawa, S.; Sato, K.; Miyamzaki, T.; Yadomae, T. Effect of a polysaccharide fraction from Grifola frondosa on immune response in mice. J. Pharm. Dyn. 1985, 8, 217–226. [Google Scholar] [CrossRef]
- Takeyama, T.; Suzuki, I.; Ohno, N.; Oikawa, S.; Sato, K.; Ohsawa, M.; Yadomae, T. Host-mediated antitumor effect of grifolan NMF-5N, a polysaccharide obtained from Grifola frondosa. J. Pharmacobiodyn. 1987, 10, 644–651. [Google Scholar] [CrossRef]
- Kodama, N.; Komuta, K.; Nanba, H. Effect of Maitake (Grifola frondosa) D-Fraction on the activation of NK cells in cancer patients. J. Med. Food 2003, 6, 371–377. [Google Scholar] [CrossRef]
- Masuda, Y.; Matsumoto, A.; Toida, T.; Oikawa, T.; Ito, K.; Nanba, H. Characterization and antitumor effect of a novel polysaccharide from Grifola frondosa. J. Agric. Food Chem. 2009, 57, 10143–10149. [Google Scholar] [CrossRef]
- Masuda, Y.; Nakayama, Y.; Tanaka, A.; Naito, K.; Konishi, M. Antitumor activity of orally administered maitake α-glucan by stimulating antitumor immune response in murine tumor. PLoS ONE 2017, 12, e0173621. [Google Scholar] [CrossRef]
- Masuda, Y.; Inoue, M.; Miyata, A.; Mizuno, S.; Nanba, H. Maitake beta-glucan enhances therapeutic effect and reduces myelosupression and nephrotoxicity of cisplatin in mice. Int. Immunopharmacol. 2009, 9, 620–626. [Google Scholar] [CrossRef]
- Mao, G.H.; Zhang, Z.H.; Fei, F.; Ding, Y.Y.; Zhang, W.J.; Chen, H.; Ali, S.S.; Zhao, T.; Feng, W.W.; Wu, X.Y.; et al. Effect of Grifola frondosa polysaccharide on anti-tumor activity in combination with 5-Fu in Heps-bearing mice. Int. J. Biol. Macromol. 2019, 121, 930–935. [Google Scholar] [CrossRef]
- Mao, G.; Li, Q.; Deng, C.; Wang, Y.; Ding, Y.; Zhang, W.; Chen, Y.; Zhao, T.; Wei, F.; Yang, L.; et al. The synergism and attenuation effect of Selenium (Se)-enriched Grifola frondosa (Se)-polysaccharide on 5-Fluorouracil (5-Fu) in Heps-bearing mice. Int. J. Biol. Macromol. 2018, 107 Pt B, 2211–2216. [Google Scholar] [CrossRef]
- Vetchinkina, E.; Shirokov, A.; Bucharskaya, A.; Navolokin, N.; Prilepskii, A.; Burov, A.; Maslyakova, G.; Nikitina, V.E. Antitumor Activity of Extracts from Medicinal Basidiomycetes Mushrooms. Int. J. Med. Mushrooms 2016, 18, 955–964. [Google Scholar] [CrossRef]
- Kodama, N.; Mizuno, S.; Nanba, H.; Saito, N. Potential antitumor activity of a low-molecular-weight protein fraction from Grifola frondosa through enhancement of cytokine production. J. Med. Food 2010, 13, 20–30. [Google Scholar] [CrossRef]
- Kim, S.P.; Kang, M.Y.; Choi, Y.H.; Kim, J.H.; Nam, S.H.; Friedman, M. Mechanism of Hericium erinaceus (Yamabushitake) mushroom-induced apoptosis of U937 human monocytic leukemia cells. Food Funct. 2011, 2, 348–356. [Google Scholar] [CrossRef]
- Kim, S.P.; Nam, S.H.; Friedman, M. Hericium erinaceus (Lion’s Mane) mushroom extracts inhibit metastasis of cancer cells to the lung in CT-26 colon cancer-transplanted mice. J. Agric. Food Chem. 2013, 61, 4898–4904. [Google Scholar] [CrossRef]
- Li, G.; Yu, K.; Li, F.; Xu, K.; Li, J.; He, S.; Cao, S.; Tan, G.-S. Anticancer potential of Hericium erinaceus extracts against human gastrointestinal cancers. J. Ethnopharmacol. 2014, 153, 521–530. [Google Scholar] [CrossRef]
- Nakamura, A.; Zhu, Q.; Yokoyama, Y.; Kitamura, N.; Uchida, S.; Kumadaki, K.; Tsubota, K.; Watanabe, M. Agaricus brasiliensis KA21 May Prevent Diet-Induced Nash Through Its Antioxidant, Anti-Inflammatory, and Anti-Fibrotic Activities in the Liver. Foods 2019, 8, 546. [Google Scholar] [CrossRef]
- Val, C.H.; Brant, F.; Miranda, A.S.; Rodrigues, F.G.; Oliveira, B.C.L.; Dos Santos, E.A.; De Assis, D.R.R.; Esper, L.; Silva, B.C.; Rachid, M.A.; et al. Effect of mushroom Agaricus blazei on immune response and development of experimental cerebral malaria. Malar. J. 2015, 14, 311. [Google Scholar] [CrossRef]
- Croccia, C.; Lopes, A.J.; Pinto, L.F.R.; Sabaa-Srur, A.U.O.; Vaz, L.C.A.; Trotte, M.N.D.S.; Tessarollo, B.; Silva, A.C.; De Matos, H.J.; Nunes, R.A. Royal Sun Medicinal Mushroom Agaricus Brasiliensis (Higher Basidiomycetes) and the Attenuation of Pulmonary Inflammation Induced by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK). Int. J. Med. Mushrooms 2013, 15, 345–355. [Google Scholar] [CrossRef]
- Li, I.C.; Lee, L.Y.; Chen, Y.J.; Chou, M.Y.; Wang, M.F.; Chen, W.P.; Chen, Y.P.; Chen, C.C. Erinacine A-enriched Hericium erinaceus mycelia promotes longevity in Drosophila melanogaster and aged mice. PLoS ONE 2019, 14, e0217226. [Google Scholar] [CrossRef]
- Ren, Y.; Geng, Y.; Du, Y.; Wang, L.; Lu, Z.-M.; Xu, H.-Y.; Xu, G.-H.; Shi, J.-S.; Xu, Z.-H. Polysaccharide of Hericium Erinaceus Attenuates Colitis in C57BL/6 Mice via Regulation of Oxidative Stress, Inflammation-Related Signaling Pathways and Modulating the Composition of the Gut Microbiota. J. Nutr. Biochem. 2018, 57, 67–76. [Google Scholar] [CrossRef]
- Diling, C.; Xin, Y.; Chaoqun, Z.; Jian, Y.; Xiaocui, T.; Jun, C.; Ou, S.; Yizhen, X. Extracts from Hericium erinaceus relieve inflammatory bowel disease by regulating immunity and gut microbiota. Oncotarget 2017, 8, 85838–85857. [Google Scholar] [CrossRef]
- Lee, K.-F.; Chen, J.-H.; Teng, C.-C.; Shen, C.-H.; Hsieh, M.-C.; Lu, C.-C.; Lee, K.-C.; Lee, L.-Y.; Chen, W.-P.; Chen, C.-C.; et al. Protective effects of Hericium erinaceus mycelium and its isolated erinacine A against ischemia-injury-induced neuronal cell death via the inhibition of iNOS/p38 MAPK and nitrotyrosine. Int. J. Mol. Sci. 2014, 15, 15073–15089. [Google Scholar] [CrossRef]
- Li, X.; Zeng, F.; Huang, Y.; Liu, B. The Positive Effects of Grifola frondosa Heteropolysaccharide on NAFLD and Regulation of the Gut Microbiota. Int. J. Mol. Sci. 2019, 20, 5302. [Google Scholar] [CrossRef]
- Han, C.; Cui, B. Pharmacological and pharmacokinetic studies with agaricoglycerides, extracted from Grifola frondosa, in animal models of pain and inflammation. Inflammation 2012, 35, 1269–1275. [Google Scholar] [CrossRef]
- Lee, J.S.; Park, S.-Y.; Thapa, D.; Choi, M.K.; Chung, I.-M.; Park, Y.-J.; Yong, C.S.; Choi, H.G.; Kim, J.-A. Grifola frondosa water extract alleviates intestinal inflammation by suppressing TNF-alpha production and its signaling. Exp. Mol. Med. 2010, 42, 143–154. [Google Scholar] [CrossRef]
- Therkelsen, S.P.; Hetland, G.; Lyberg, T.; Lygren, I.; Johnson, E. Cytokine Levels after Consumption of a Medicinal Agaricus blazei Murill-Based Mushroom Extract, AndoSan™, in Patients with Crohn’s Disease and Ulcerative Colitis in a Randomized Single-Blinded Placebo-Controlled Study. Scand. J. Immunol. 2016, 84, 323–331. [Google Scholar] [CrossRef]
- Therkelsen, S.P.; Hetland, G.; Lyberg, T.; Lygren, I.; Johnson, E. Effect of a Medicinal Agaricus blazei Murill-Based Mushroom Extract, AndoSan™, on Symptoms, Fatigue and Quality of Life in Patients with Ulcerative Colitis in a Randomized Single-Blinded Placebo Controlled Study. PLoS ONE 2016, 11, e0150191. [Google Scholar] [CrossRef]
- Therkelsen, S.P.; Hetland, G.; Lyberg, T.; Lygren, I.; Johnson, E. Effect of the Medicinal Agaricus blazei Murill-Based Mushroom Extract, AndoSanTM, on Symptoms, Fatigue and Quality of Life in Patients with Crohn’s Disease in a Randomized Single-Blinded Placebo Controlled Study. PLoS ONE 2016, 11, e0159288. [Google Scholar] [CrossRef]
- Førland, D.T.; Johnson, E.; Saetre, L.; Lyberg, T.; Lygren, I.; Hetland, G. Effect of an extract based on the medicinal mushroom Agaricus blazei Murill on expression of cytokines and calprotectin in patients with ulcerative colitis and Crohn’s disease. Scand. J. Immunol. 2011, 73, 66–75. [Google Scholar] [CrossRef]
- Johnson, E.; Førland, D.T.; Hetland, G.; Sætre, L.; Olstad, O.K.; Lyberg, T. Effect of AndoSan™ on expression of adhesion molecules and production of reactive oxygen species in human monocytes and granulocytes in vivo. Scand. J. Gastroenterol. 2012, 47, 984–992. [Google Scholar] [CrossRef]
- Johnson, E.; Førland, D.T.; Saetre, L.; Bernardshaw, S.V.; Lyberg, T.; Hetland, G. Effect of an extract based on the medicinal mushroom Agaricus blazei murill on release of cytokines, chemokines and leukocyte growth factors in human blood ex vivo and in vivo. Scand. J. Immunol. 2009, 69, 242–250. [Google Scholar] [CrossRef]
- Bernardshaw, S.; Hetland, G.; Ellertsen, L.K.; Tryggestad, A.M.; Johnson, E. An extract of the medicinal mushroom Agaricus blazei Murill differentially stimulates production of pro-inflammatory cytokines in human monocytes and human vein endothelial cells in vitro. Inflammation 2005, 29, 147–153. [Google Scholar] [CrossRef]
- Oh, T.W.; Kim, Y.A.; Jang, W.J.; Byeon, J.I.; Ryu, C.H.; Kim, J.O.; Ha, Y.L. Semipurified fractions from the submerged-culture broth of Agaricus blazei Murill reduce blood glucose levels in streptozotocin-induced diabetic rats. J. Agric. Food Chem. 2010, 58, 4113–4119. [Google Scholar] [CrossRef]
- Padilha, M.M.; Avila, A.A.; Sousa, P.J.; Cardoso, L.G.; Perazzo, F.F.; Carvalho, J.C. Anti-inflammatory activity of aqueous and alkaline extracts from mushrooms (Agaricus blazei Murill). J. Med. Food 2009, 12, 359–364. [Google Scholar] [CrossRef]
- Izawa, S.; Inoue, Y. A screening system for antioxidants using thioredoxin-deficient yeast: Discovery of thermostable antioxidant activity from Agaricus blazei Murill. Appl. Microbiol. Biotechnol. 2004, 64, 537–542. [Google Scholar] [CrossRef]
- Choi, Y.H.; Yan, G.H.; Chai, O.H.; Choi, Y.H.; Zhang, X.; Lim, J.M.; Kim, J.H.; Lee, M.S.; Han, E.H.; Kim, H.T.; et al. Inhibitory effects of Agaricus blazei on mast cell-mediated anaphylaxis-like reactions. Biol. Pharm. Bull. 2006, 29, 1366–1371. [Google Scholar] [CrossRef]
- Takimoto, H.; Kato, H.; Kaneko, M.; Kumazawa, Y. Amelioration of skewed Th1/Th2 balance in tumor-bearing and asthma-induced mice by oral administration of Agaricus blazei extracts. Immunopharmacol. Immunotoxicol. 2008, 30, 747–760. [Google Scholar] [CrossRef]
- Bouike, G.; Nishitani, Y.; Shiomi, H.; Yoshida, M.; Azuma, T.; Hashimoto, T.; Kanazawa, K.; Mizuno, M. Oral Treatment with Extract of Agaricus blazei Murill Enhanced Th1 Response through Intestinal Epithelial Cells and Suppressed OVA-Sensitized Allergy in Mice. Evid. Based Complement Altern. Med. 2011, 2011, 1–11. [Google Scholar] [CrossRef]
- Mahmood, F.; Hetland, G.; Nentwich, I.; Mirlashari, M.R.; Ghiasvand, R.; Nissen-Meyer, L.S.H. Agaricus blazei-Based Mushroom Extract Supplementation to Birch Allergic Blood Donors: A Randomized Clinical Trial. Nutrients 2019, 11, 2339. [Google Scholar] [CrossRef]
- Kawai, J.; Mori, K.; Hirasawa, N. Grifola frondosa extract and ergosterol reduce allergic reactions in an allergy mouse model by suppressing the degranulation of mast cells. Biosci. Biotechnol. Biochem. 2019, 83, 2280–2287. [Google Scholar] [CrossRef]
- Park, H.S.; Hwang, Y.H.; Kim, M.K.; Hong, G.E.; Lee, H.J.; Nagappan, A.; Yumnam, S.; Kim, E.H.; Heo, J.D.; Lee, S.J.; et al. Functional Polysaccharides from Grifola Frondosa Aqueous Extract Inhibit Atopic Dermatitis-Like Skin Lesions in NC/Nga Mice. Biosci. Biotechnol. Biochem. 2015, 79, 147–154. [Google Scholar] [CrossRef]
- Mukai, H.; Watanabe, T.; Ando, M.; Katsumata, N. An alternative medicine, Agaricus blazei, may have induced severe hepatic dysfunction in cancer patients. Jpn. J. Clin. Oncol. 2006, 36, 808–810. [Google Scholar] [CrossRef]
- Suehiro, M.; Katoh, N.; Kishimoto, S. Cheilitis due to Agaricus blazei Murill mushroom extract. Contact Dermat. 2007, 56, 293–294. [Google Scholar] [CrossRef]
- Engdal, S.; Nilsen, O.G. In vitro inhibition of CYP3A4 by herbal remedies frequently used by cancer patients. Phytother. Res. 2009, 23, 906–912. [Google Scholar] [CrossRef]
- Shapiro, L.E.; Shear, N.H. Drug interactions: Proteins, pumps, and P-450s. J. Am. Acad. Dermatol. 2002, 47, 467–484. [Google Scholar] [CrossRef] [PubMed]
- Sumiya, T.; Ikeda, Y.; Broadmeadow, A.; May, K.; Pritchard, L.; Horne, C.; Burlinson, B. Himematsutake (Iwade Strain 101) extract (ABM-FD): Genetic toxicology and a 3-month dietary toxicity study in rats. Food Chem. Toxicol. 2008, 46, 1949–1959. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.P.; Kang, B.H.; Roh, J.K.; Kim, J.R. Lack of carcinogenicity of lyophilized Agaricus blazei Murill in a F344 rat two year bioassay. Food Chem. Toxicol. 2008, 46, 87–95. [Google Scholar] [CrossRef]
- Tanaka, H.; Tsunematsu, K.; Nakamura, N.; Suzuki, K.; Tanaka, N.; Takeya, I.; Saikai, T.; Abe, S. Successful treatment of hypersensitivity pneumonitis caused by Grifola frondosa (Maitake) mushroom using a HFA-BDP extra-fine aerosol. Intern. Med. 2004, 43, 737–740. [Google Scholar] [CrossRef][Green Version]
- Deng, G.; Lin, H.; Seidman, A.; Fornier, M.; D’Andrea, G.; Wesa, K.; Yeung, K.; Cunningham-Rundles, S.; Vickers, A.; Cassileth, B. A phase I/II trial of a polysaccharide extract from Grifola frondosa (Maitake mushroom) in breast cancer patients: Immunological effects. J. Cancer Res. Clin. Oncol. 2009, 135, 1215–1221. [Google Scholar] [CrossRef]
- Chan, G.C.; Chan, W.K.; Sze, D.M. The effects of beta-glucan on human immune and cancer cells. J. Hematol. Oncol. 2009, 2, 25. [Google Scholar] [CrossRef]
- Lehne, G.; Haneberg, B.; Gaustad, P.; Johansen, P.W.; Preus, H.; Abrahamsen, T.G. Oral administration of a new soluble branched beta-1,3-D-glucan is well tolerated and can lead to increased salivary concentrations of immunoglobulin A in healthy volunteers. Clin. Exp. Immunol. 2006, 143, 65–69. [Google Scholar] [CrossRef]
- Samuelsen, A.B.; Schrezenmeir, J.; Knutsen, S.H. Effects of orally administered yeast-derived beta-glucans: A review. Mol. Nutr. Food Res. 2014, 58, 183–193. [Google Scholar] [CrossRef]
- Berven, L.; Karppinen, P.; Hetland, G.; Samuelsen, A.B. The polar high molecular weight fraction of the Agaricus blazei Murill extract, AndoSan™, reduces the activity of the tumor-associated protease, legumain, in RAW 264.7 cells. J. Med. Food 2015, 18, 429–438. [Google Scholar] [CrossRef]
- Hetland, G.; Johnson, E.; Lyberg, T.; Bernardshaw, S.; Tryggestad, A.M.; Grinde, B. Effects of the medicinal mushroom Agaricus blazei Murill on immunity, infection and cancer. Scand. J. Immunol. 2008, 68, 363–370. [Google Scholar] [CrossRef]
- Batbayar, S.; Lee, D.H.; Kim, H.W. Immunomodulation of Fungal β-Glucan in Host Defense Signaling by Dectin-1. Biomol. Ther. 2012, 20, 433–445. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).