Author Contributions
Conceptualization F.T. and S.P.; Methodology F.T. and S.P.; Formal Analysis F.T. and M.P.; Investigation, F.T. and M.P.; Data Curation F.T.; Writing—Original Draft Preparation F.T.; Writing—Review & Editing M.P. and G.D.; Visualization G.S., P.V.F., L.A.M., F.G., A.F., G.F., A.V., S.E.T.; Supervision, S.P.; Project Administration, S.P., C.V. and A.B. All authors meet the ICMJE Recommendations for authorship credit. All authors have read and approved the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors have contributed equally. The authors had full access to the data and take full responsibility for its integrity. All authors have read and agree to the manuscript as written. For this retrospective analysis, it was not necessary to request authorization from the ethics committee. The contents of this paper are consistent with the principles of the Declaration of Helsinki in the latest version.
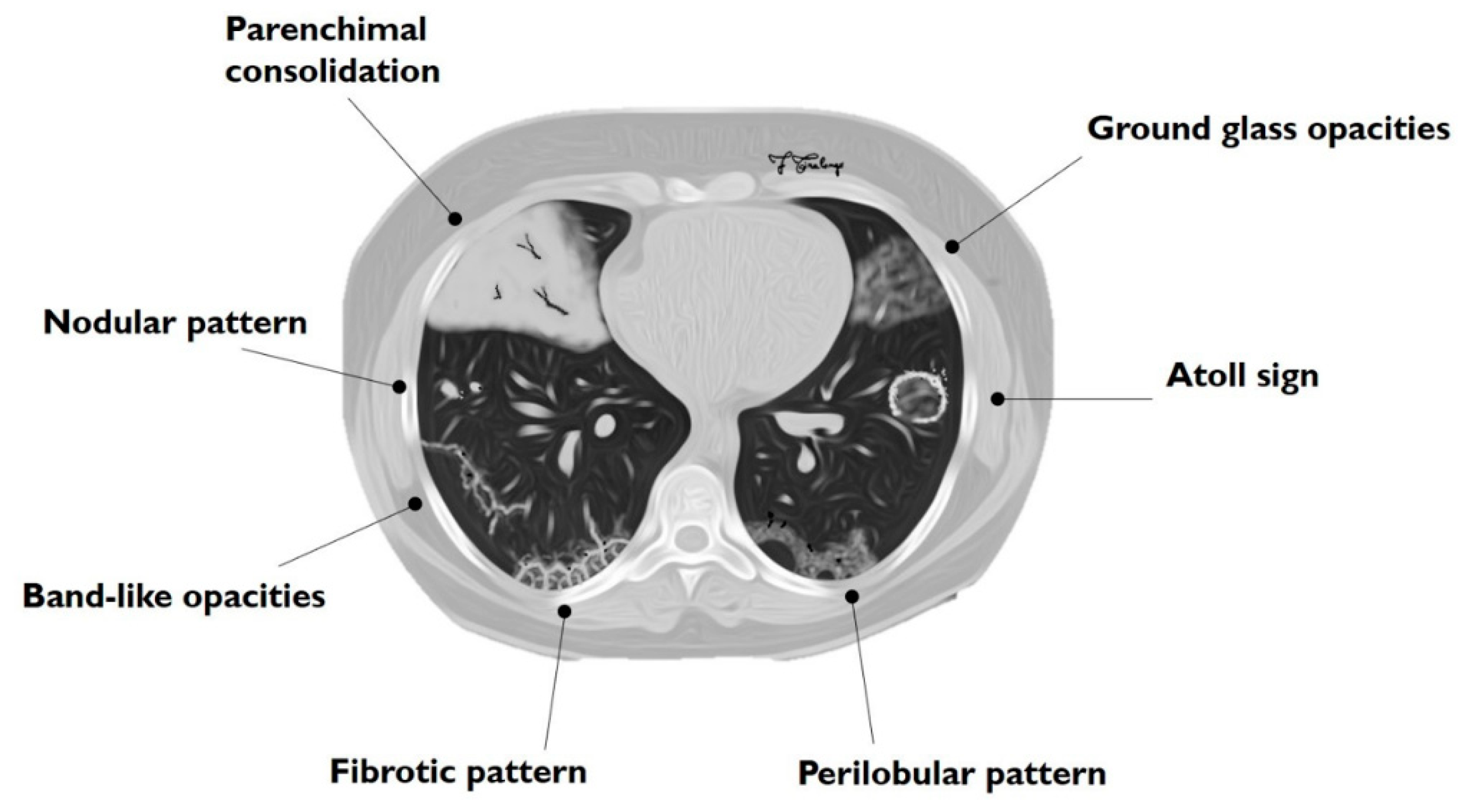
Figure 1.
Schematic drawing of main imaging patterns of cryptogenic organizing pneumonia (COP).
Figure 1.
Schematic drawing of main imaging patterns of cryptogenic organizing pneumonia (COP).
Figure 2.
Typical pattern: multifocal and asymmetrical parenchymal consolidations (arrowheads), with peripheral distribution. These lesions may reproduce an air bronchogram sign in the context.
Figure 2.
Typical pattern: multifocal and asymmetrical parenchymal consolidations (arrowheads), with peripheral distribution. These lesions may reproduce an air bronchogram sign in the context.
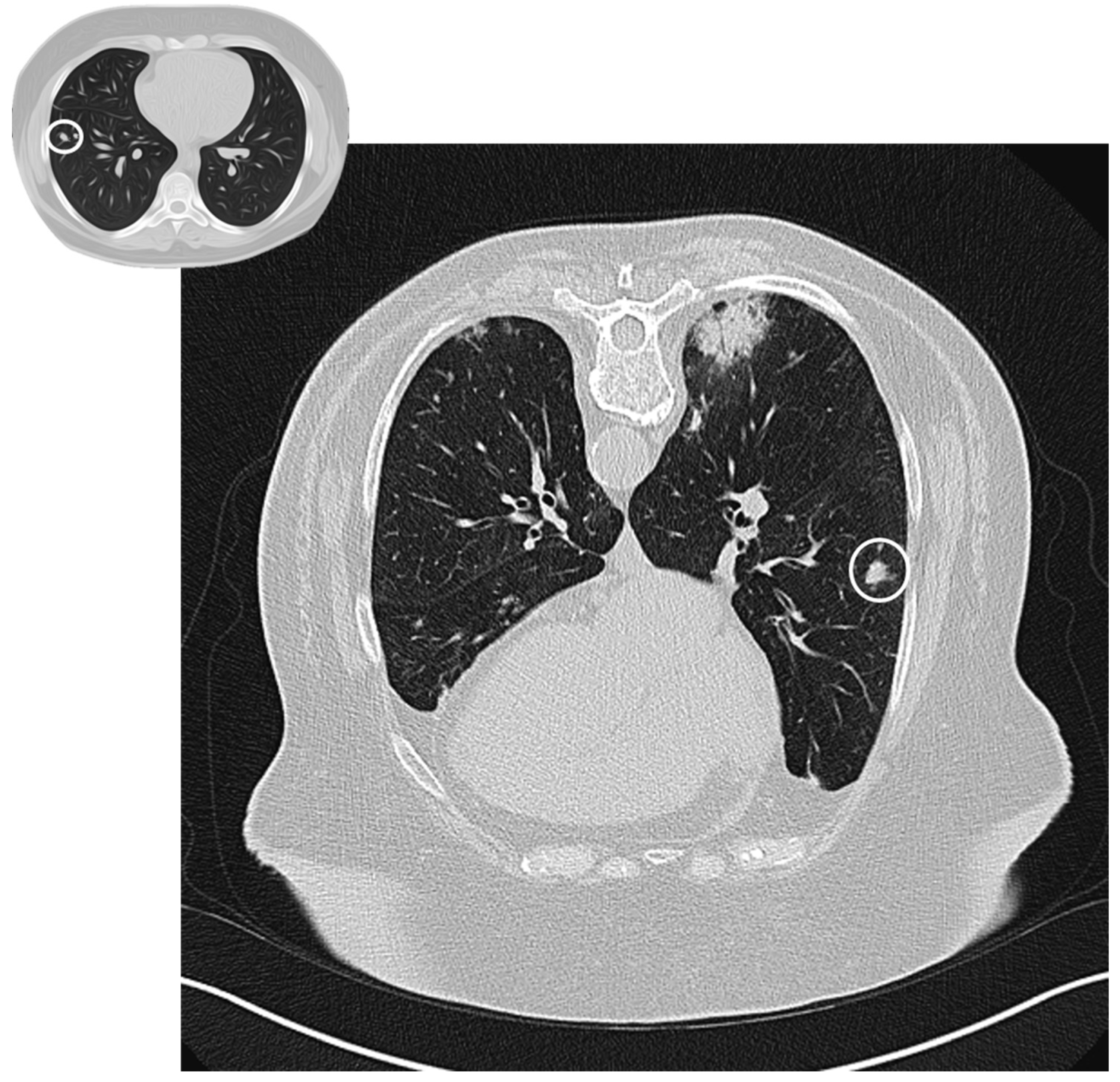
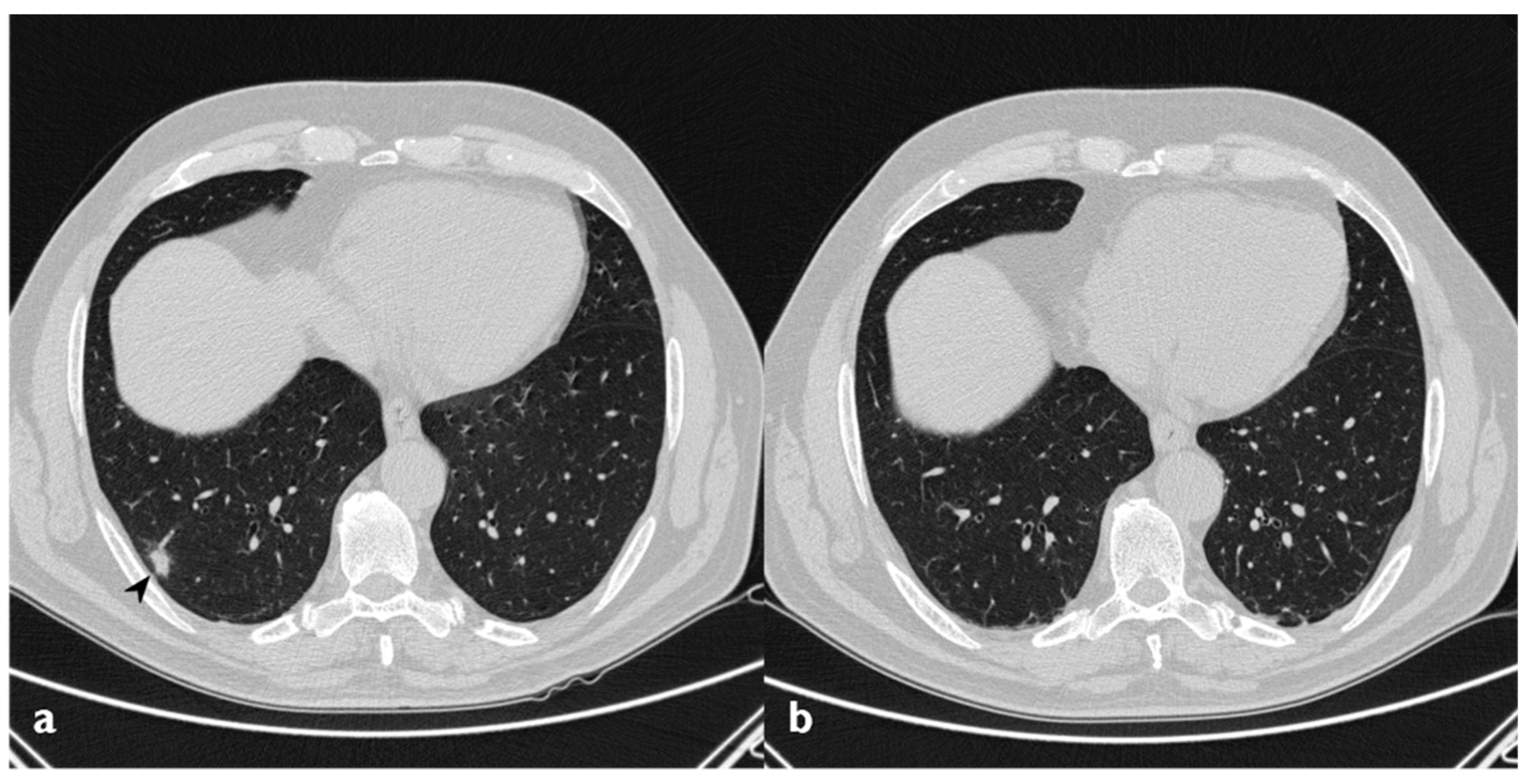
Figure 3.
Nodular pattern: peripheral solid nodule (white circles) in a patient affected by COP.
Figure 3.
Nodular pattern: peripheral solid nodule (white circles) in a patient affected by COP.
Figure 4.
Atoll or reverse halo sign: areas of ground glass opacity surrounded by a ring- or a crescent-shaped consolidation (white arrowheads), which are clearly depicted in the right upper lobe (a) and in the right lower lobe (b).
Figure 4.
Atoll or reverse halo sign: areas of ground glass opacity surrounded by a ring- or a crescent-shaped consolidation (white arrowheads), which are clearly depicted in the right upper lobe (a) and in the right lower lobe (b).
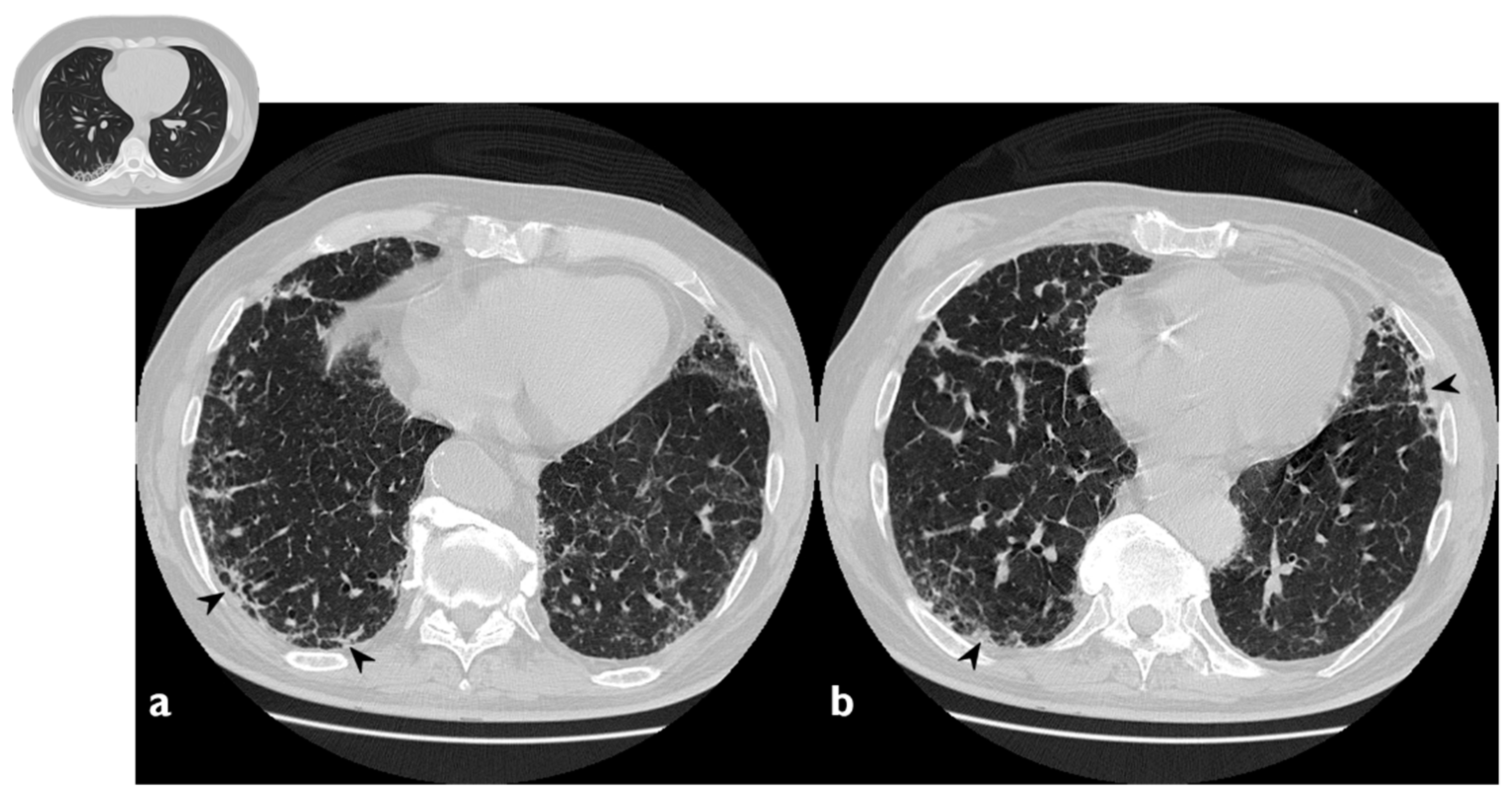
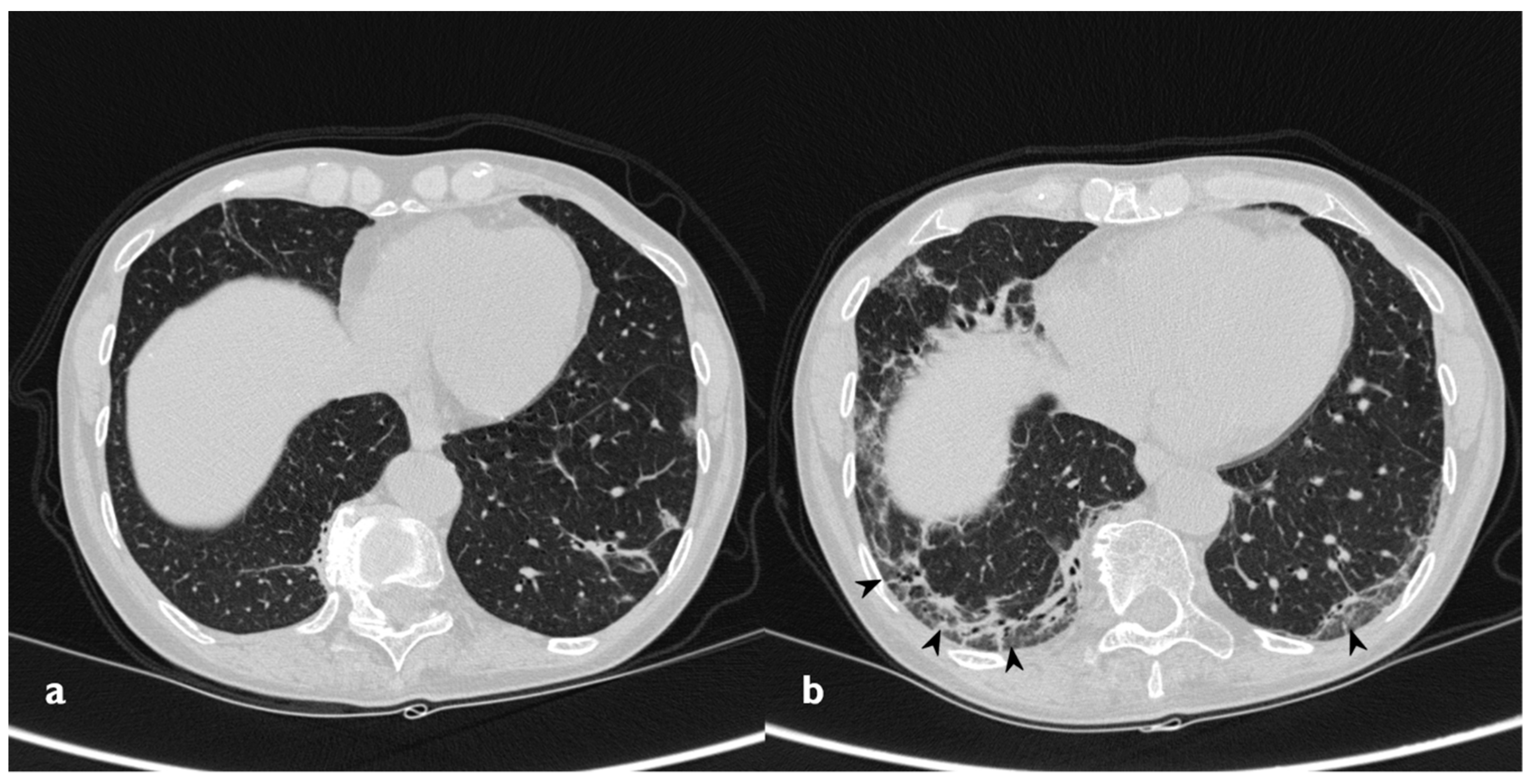
Figure 5.
Fibrotic pattern: bilateral sub-pleural reticulations (arrowheads) and architectural distortion, clearly visible in peripheral regions of right lung (a) and left lung (b).
Figure 5.
Fibrotic pattern: bilateral sub-pleural reticulations (arrowheads) and architectural distortion, clearly visible in peripheral regions of right lung (a) and left lung (b).
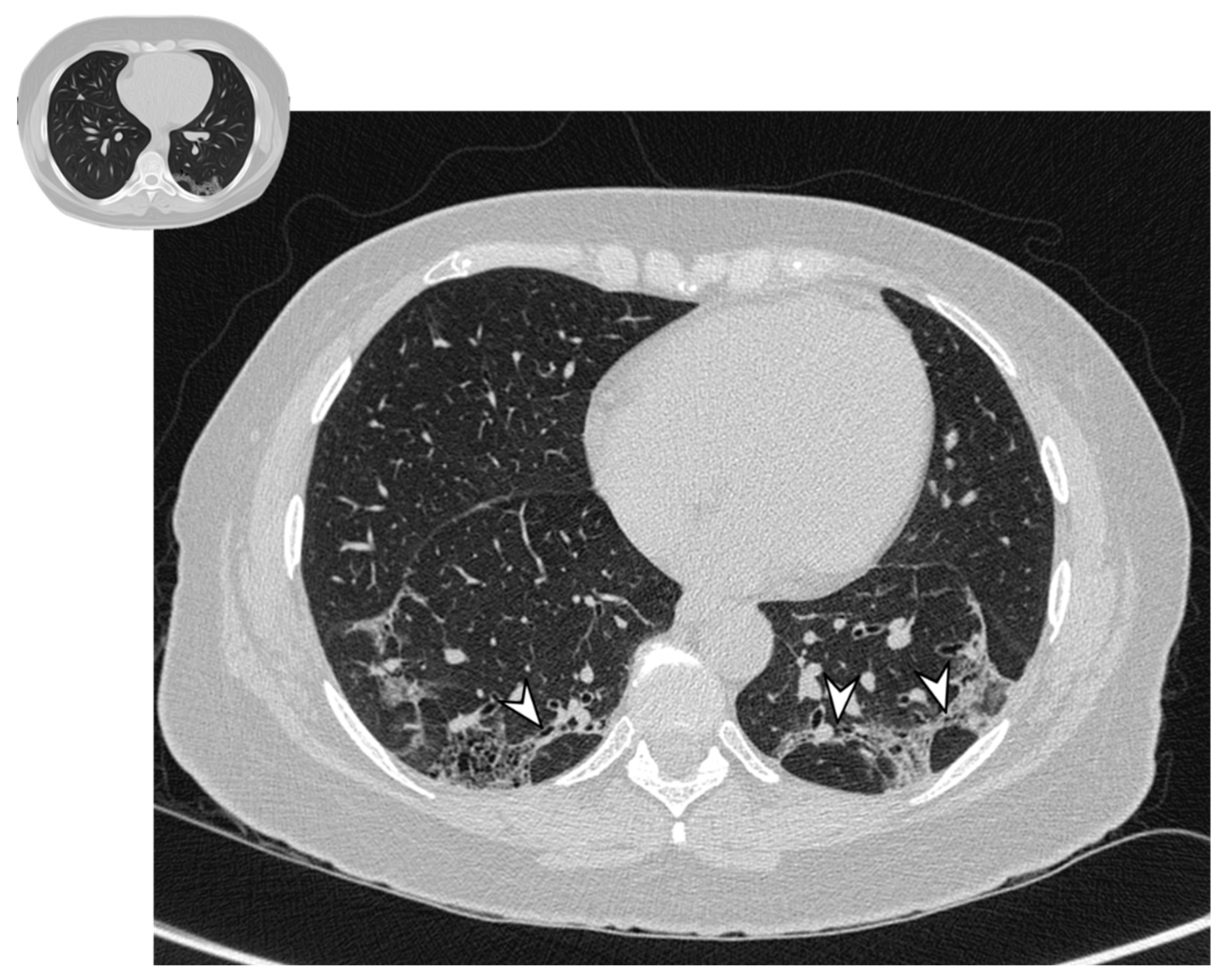
Figure 6.
Perilobular pattern: arcade-like bands of parenchymal consolidation (white arrowheads) with blurred borders and thickening of the interlobular septa with a reticular pattern.
Figure 6.
Perilobular pattern: arcade-like bands of parenchymal consolidation (white arrowheads) with blurred borders and thickening of the interlobular septa with a reticular pattern.
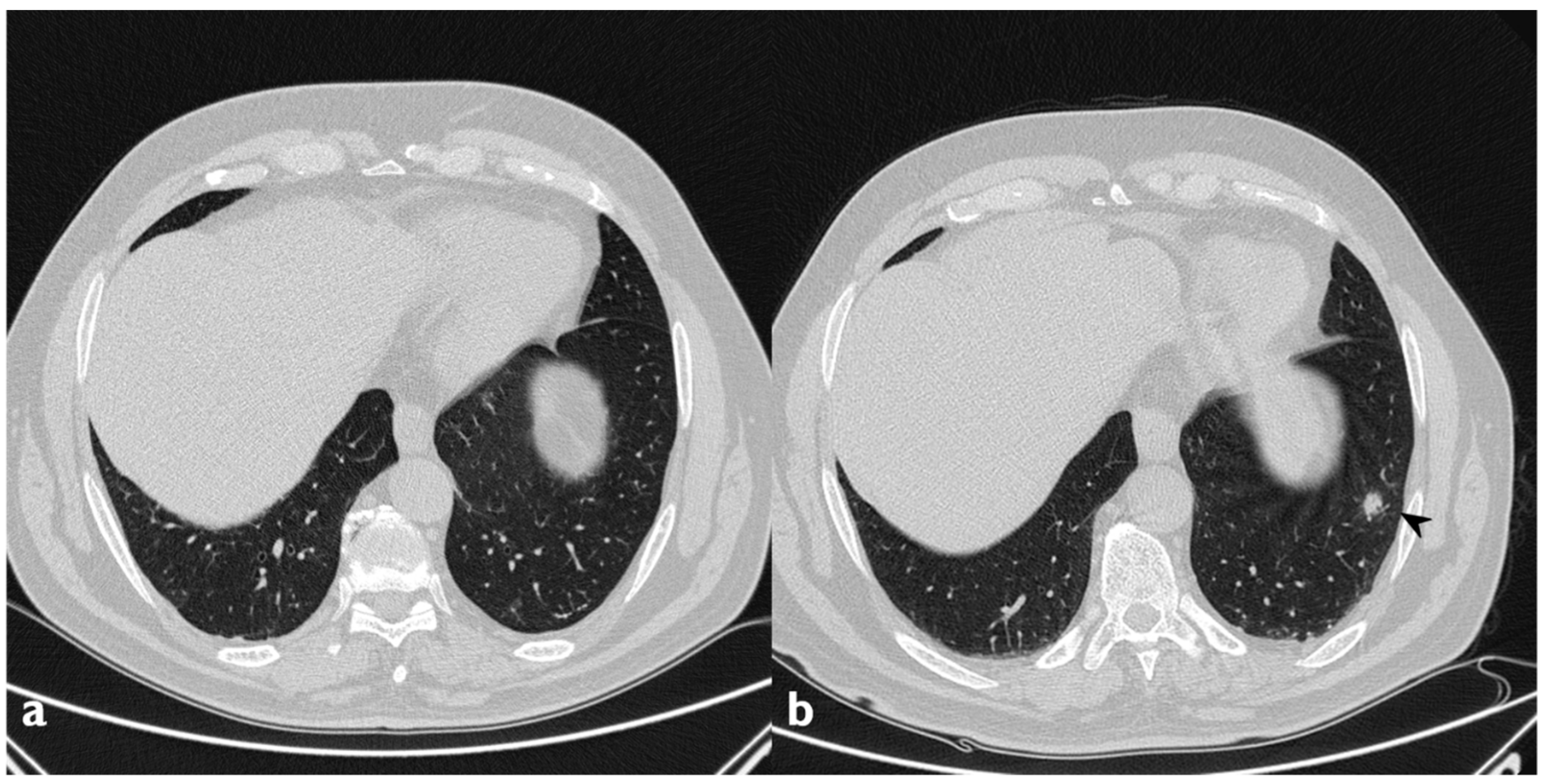
Figure 7.
Linear and band-like opacities: sub-pleural curvilinear bands, parallel to the pleural (arrowheads) in a patient with COP.
Figure 7.
Linear and band-like opacities: sub-pleural curvilinear bands, parallel to the pleural (arrowheads) in a patient with COP.
Figure 8.
On the left: baseline HRCT (a); subpleural consolidation in the right lower lobe. On the right (b): HRCT after 11 months of steroid therapy shows a disappearance of the consolidation.
Figure 8.
On the left: baseline HRCT (a); subpleural consolidation in the right lower lobe. On the right (b): HRCT after 11 months of steroid therapy shows a disappearance of the consolidation.
Figure 9.
Migratory infiltrates. On the left (a): baseline HRCT; subpleural consolidation in the right lower lobe (white arrowhead). On the right (b): HRCT after 13 months of steroid therapy; the consolidations have disappeared in the first site, appearing in the left lower lobe (arrowhead).
Figure 9.
Migratory infiltrates. On the left (a): baseline HRCT; subpleural consolidation in the right lower lobe (white arrowhead). On the right (b): HRCT after 13 months of steroid therapy; the consolidations have disappeared in the first site, appearing in the left lower lobe (arrowhead).
Figure 10.
On the left (a): baseline HRCT; left parenchymal consolidations with air bronchogram sign in the context (arrowheads). On the right (b): HRCT after steroid treatment; new ground-glass areas (arrowheads) have appeared in the site of consolidations.
Figure 10.
On the left (a): baseline HRCT; left parenchymal consolidations with air bronchogram sign in the context (arrowheads). On the right (b): HRCT after steroid treatment; new ground-glass areas (arrowheads) have appeared in the site of consolidations.
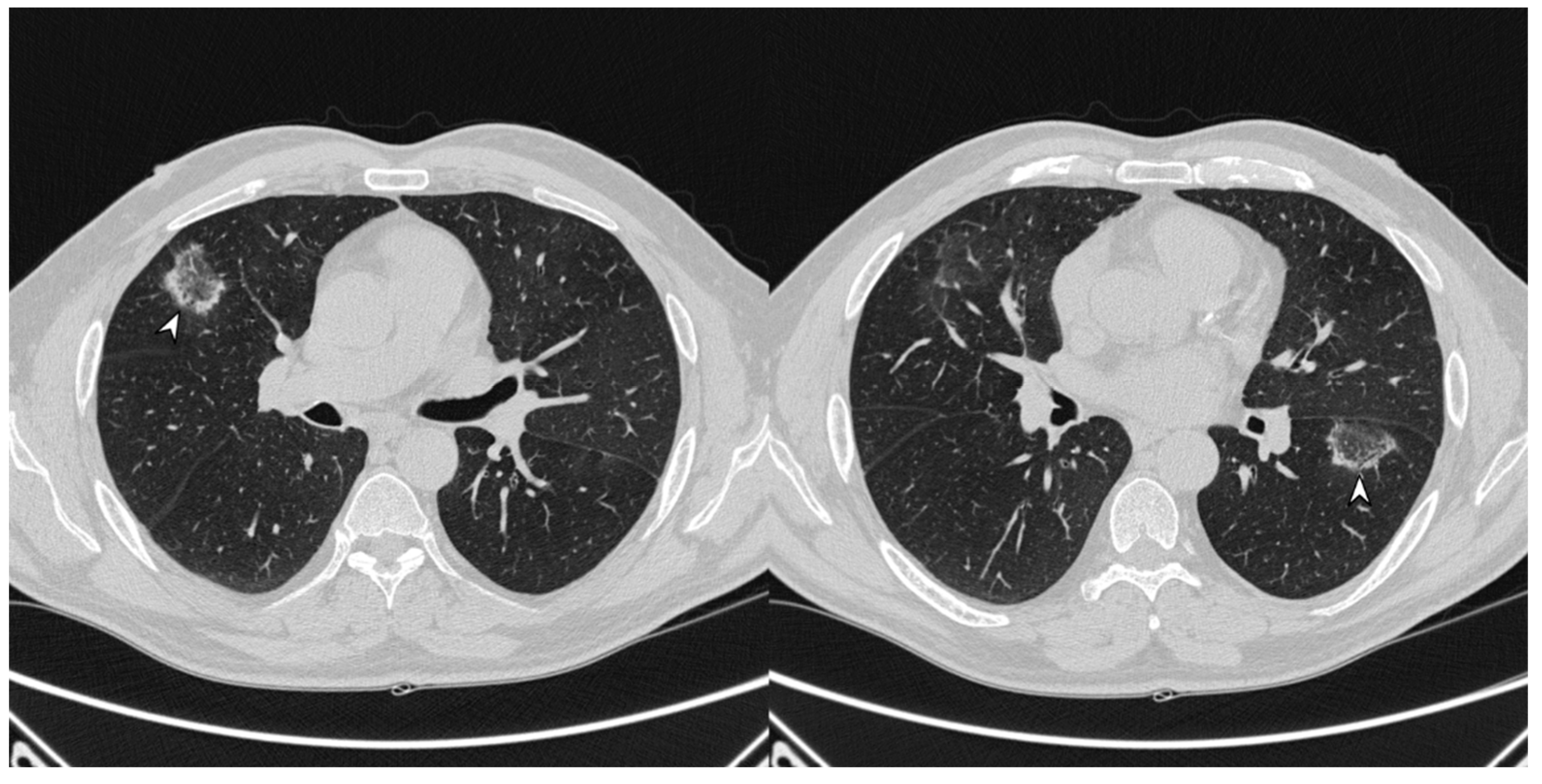
Figure 11.
On the left (a): baseline HRCT; peripheral solid nodule in the right lower lobe (arrowheads). On the right (b): HRCT after oral corticosteroid (OCS) therapy; the previous nodule is no longer present.
Figure 11.
On the left (a): baseline HRCT; peripheral solid nodule in the right lower lobe (arrowheads). On the right (b): HRCT after oral corticosteroid (OCS) therapy; the previous nodule is no longer present.
Figure 12.
On the left (a): baseline HRCT. On the right (b): follow-up HRCT; a new nodule (arrowhead) appeared in the left lower lobe.
Figure 12.
On the left (a): baseline HRCT. On the right (b): follow-up HRCT; a new nodule (arrowhead) appeared in the left lower lobe.
Figure 13.
Presence of atoll signs (white arrowheads) in a patient with COP, which were still recognizable despite steroid therapy.
Figure 13.
Presence of atoll signs (white arrowheads) in a patient with COP, which were still recognizable despite steroid therapy.
Figure 14.
On the left: axial view. On the right: sagittal view. Perilobular pattern: the presence of arcade-like bands with blurred borders and thickening of the interlobular septa—resembling a Roman Arch (arrowheads), despite steroid therapy.
Figure 14.
On the left: axial view. On the right: sagittal view. Perilobular pattern: the presence of arcade-like bands with blurred borders and thickening of the interlobular septa—resembling a Roman Arch (arrowheads), despite steroid therapy.
Figure 15.
Persistence of band-like opacities (arrowheads) despite 13 months of steroid treatment.
Figure 15.
Persistence of band-like opacities (arrowheads) despite 13 months of steroid treatment.
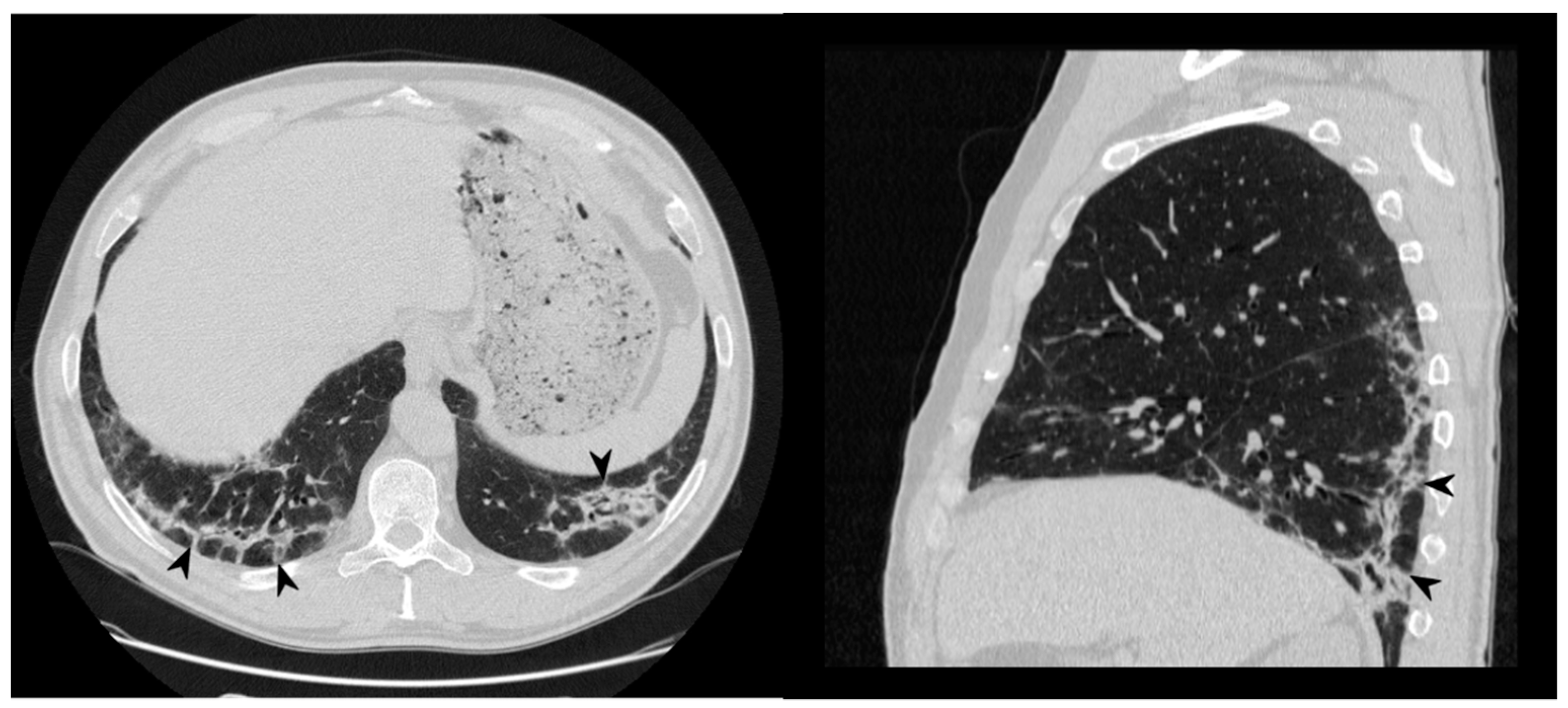
Figure 16.
Fibrosing evolution. On the left (a): baseline HRCT. On the right (b): HRCT after 11 months of steroid therapy; new signs of fibrosis-sub-pleural basal reticulations and architectural distortion (arrowheads).
Figure 16.
Fibrosing evolution. On the left (a): baseline HRCT. On the right (b): HRCT after 11 months of steroid therapy; new signs of fibrosis-sub-pleural basal reticulations and architectural distortion (arrowheads).
Table 1.
Patterns of COP.
Table 1.
Patterns of COP.
| Typical Pattern | Atypical Patterns |
|---|
| Multiple alveolar opacities | Nodular pattern |
| | Perilobular pattern |
| | Crazy paving pattern |
| | Progressive fibrosis COP |
| | Atoll sign |
| | Linear and band-like opacities |
Table 2.
Demographic data of our population study.
Table 2.
Demographic data of our population study.
| Variables | Patients Number (%) |
|---|
| N° of patients | 22 |
| Age (in years) | 58, range 25–84 |
| Sex | 15 F (68.1%)/6 M (27.2%) |
| Smoking | 5 (22.7%) |
| Ex-smokers | 3 (60%) |
| Currently smokers | 2 (40%) |
| Chemotherapy | 2 (9%) |
| Radiotherapy | 3 (13.6%) |
| Rheumatoid Arthritis | 1 (4.5%) |
Table 3.
Clinical and functional features. FVC: forced vital capacity, FEV1: Forced expiratory volume in 1 second, DLCO: diffusion lung carbon monoxide.
Table 3.
Clinical and functional features. FVC: forced vital capacity, FEV1: Forced expiratory volume in 1 second, DLCO: diffusion lung carbon monoxide.
| Symptoms | Patients Number (%) |
|---|
| Dyspnea | 12 (54.5%) |
| Cough | 12 (54.5%) |
| Dry | 10 (83.3%) |
| Productive | 2 (16.7%) |
| Weight loss | 1 (4.5%) |
| Weakness | 4 (18.2%) |
| Pleural effusion | 2 (9.1%) |
| Fever | 3 (13.6%) |
| Chest pain | 1 (4.5%) |
| Crackles | 4 (18.2%) |
| Inspiratory squawks | 1 (4.5%) |
| Expiratory wheeze | 2 (9.1%) |
| PFT | Values |
| FVC (L) | 2.9 ± 1.0 |
| FVC (%) | 95 ± 24.8 |
| FEV1 (L) | 3.2 ± 3.5 |
| FEV1 (%) | 93.8 ± 23.3 |
| FEV/FVC | 93.4 ± 12.8 |
| DLCO (%) | 84.5 ± 13.6 |
Table 4.
Most common HRCT findings at baseline.
Table 4.
Most common HRCT findings at baseline.
| HRCT Findings | Number of Patients (%) |
|---|
| Consolidation | 18 (81.8%) |
| GG opacities | 15 (68.1%) |
| Atoll sign | 4 (18.1%) |
| Nodular pattern | 5 (22.7%) |
| Perilobular pattern | 3 (13.6%) |
| Linear and band-like opacities | 3 (13.6%) |
| Mediastinum lymphadenomegaly | 4 (18.1%) |
Table 5.
Evolution of the 18 patients who presented consolidations.
Table 5.
Evolution of the 18 patients who presented consolidations.
| Evolution of Consolidations | Number of Patients (%) |
|---|
| Complete resolution | 11 (61.1) |
| Complete resolution + new ones | 3 (16.6) |
| Partial resolution | 2 (11.1) |
| No resolution | 2 (11.1) |
Table 6.
Evolution of the 15 patients who presented ground-glassopacities.
Table 6.
Evolution of the 15 patients who presented ground-glassopacities.
| Evolution of Ground-Glass Opacities | Number of Patients (%) |
|---|
| Complete resolution | 9 (60) |
| No resolution | 6 (40) |
| New ones | 2 |
Table 7.
Evolution of the 5 patients with a nodular pattern.
Table 7.
Evolution of the 5 patients with a nodular pattern.
| Evolution of Nodular Pattern | Number of Patients (%) |
|---|
| Complete resolution | 3 (60) |
| Partial resolution | 2 (40) |
| No resolution | 1 (20) |
| New ones | 1 |
Table 8.
Evolution of the 4 patients with atoll sign.
Table 8.
Evolution of the 4 patients with atoll sign.
| Evolution of Atoll Sign | Number of Patients (%) |
|---|
| Complete resolution | 3 (75) |
| No resolution | 1 (25) |
Table 9.
Evolution of the 3 patients with perilobular pattern.
Table 9.
Evolution of the 3 patients with perilobular pattern.
| Evolution of Perilobular Pattern | Number of Patients (%) |
|---|
| Complete resolution | 0 |
| No resolution | 3 (100) |
Table 10.
Evolution of the 3 patients with linear and band-like opacities.
Table 10.
Evolution of the 3 patients with linear and band-like opacities.
| Evolution of Linear and Band-Like Opacities | Number of Patients (%) |
|---|
| Complete resolution | 2 (67) |
| No resolution | 1 (33) |
| New ones | 1 |
Table 11.
Different kinds of evolution of the 21 patients who received steroid treatment.
Table 11.
Different kinds of evolution of the 21 patients who received steroid treatment.
| Evolution | Number of Patients (%) |
|---|
| Regression of radiological findings: | 17 (80) |
| Complete (no maintenance therapy) | 2 (9.5) |
| Significant relapse | 3 (14.2) |
| Fibrosing evolution | 2 (9.5) |