Caffeine, a Risk Factor for Osteoarthritis and Longitudinal Bone Growth Inhibition
Abstract
1. Introduction
2. Osteoarthritis: Articular Cartilage Catabolism
3. Longitudinal Bone Growth Inhibition
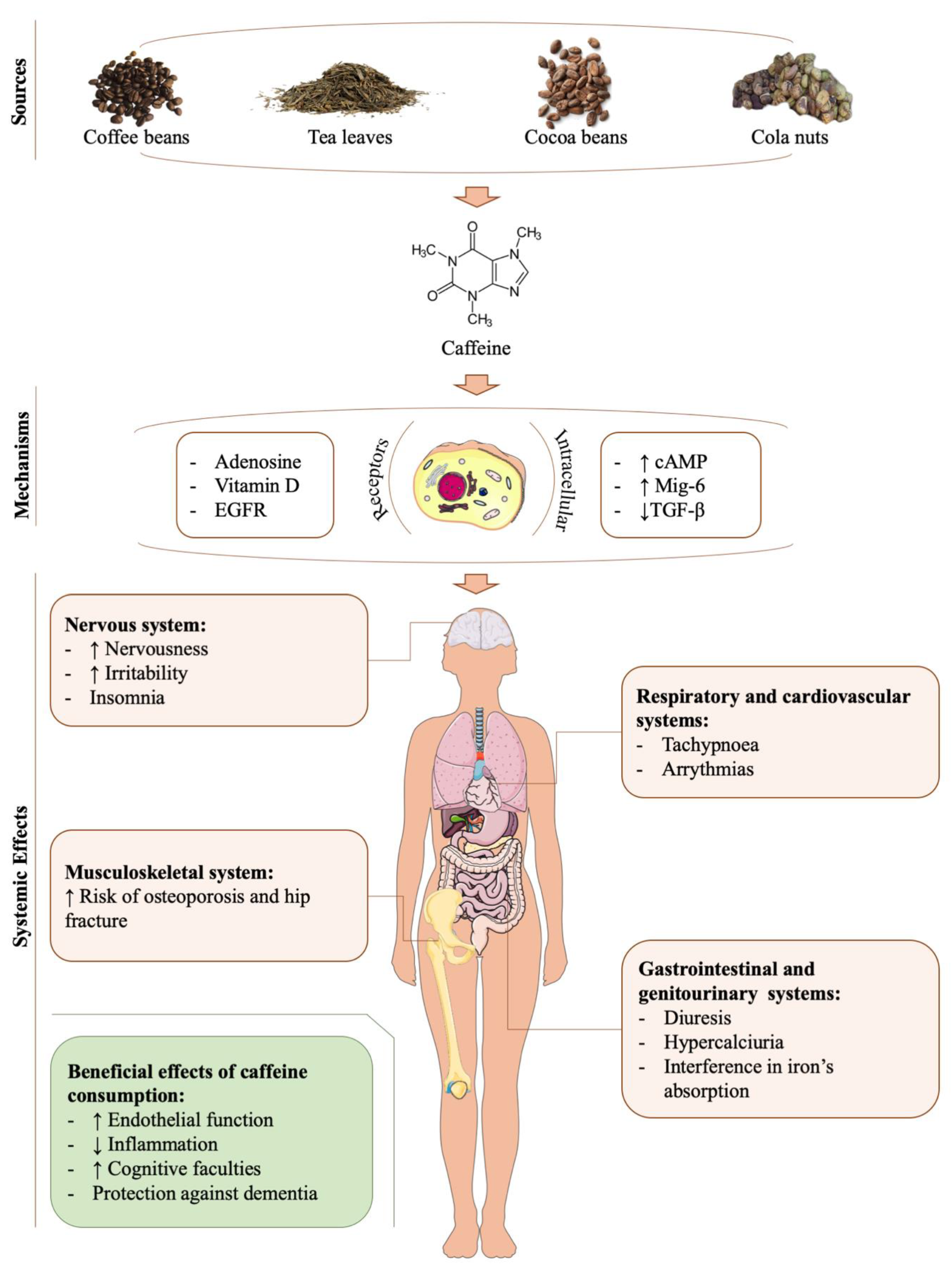
4. Caffeine
5. Caffeine’s Role in Cartilage-Related Disorders
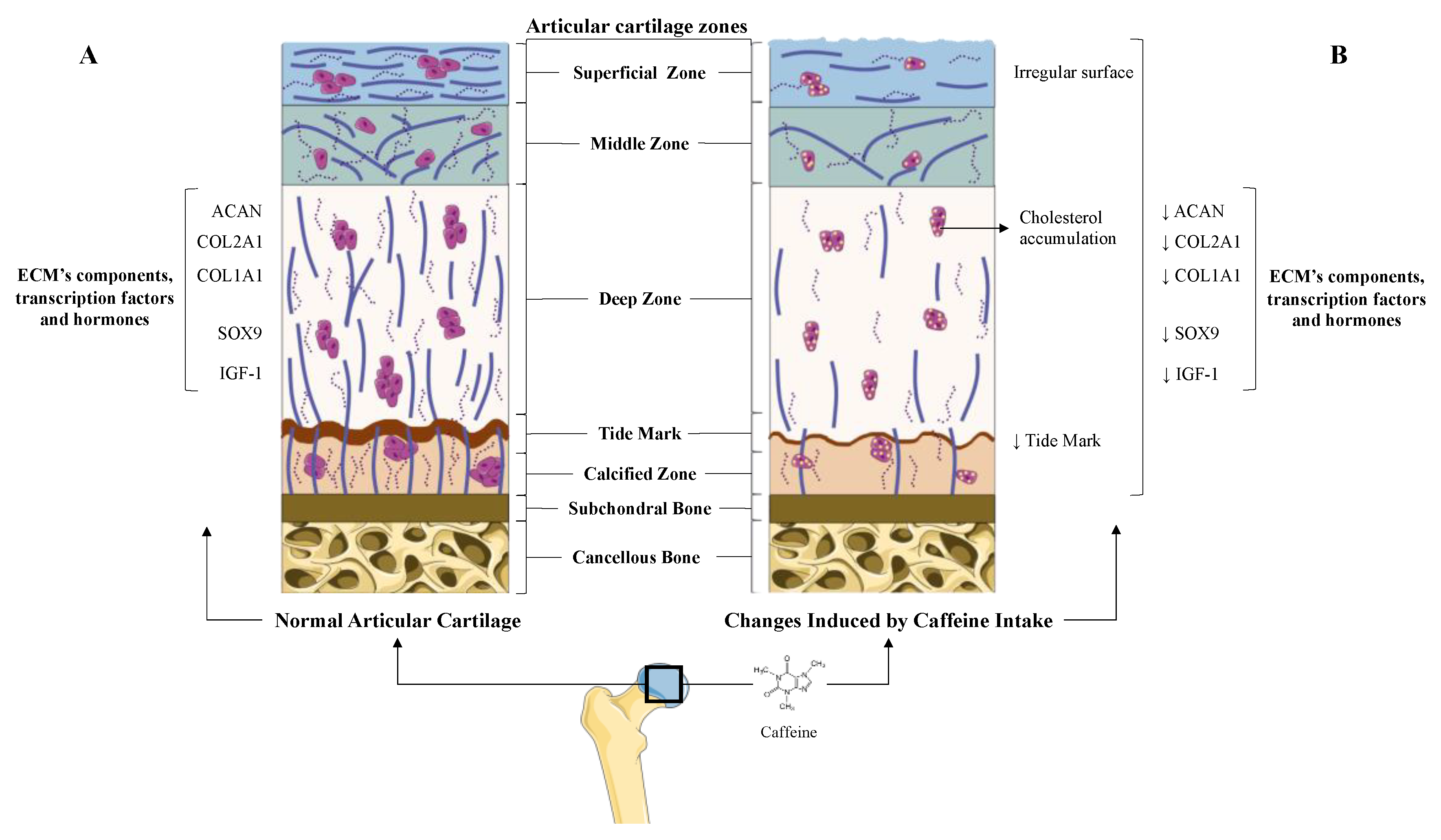
5.1. Caffeine’s Role in Articular Cartilage: Osteoarthritis
5.1.1. Prenatal Caffeine Exposure
5.1.2. Direct and Indirect Effect of Caffeine on Articular Chondrocytes
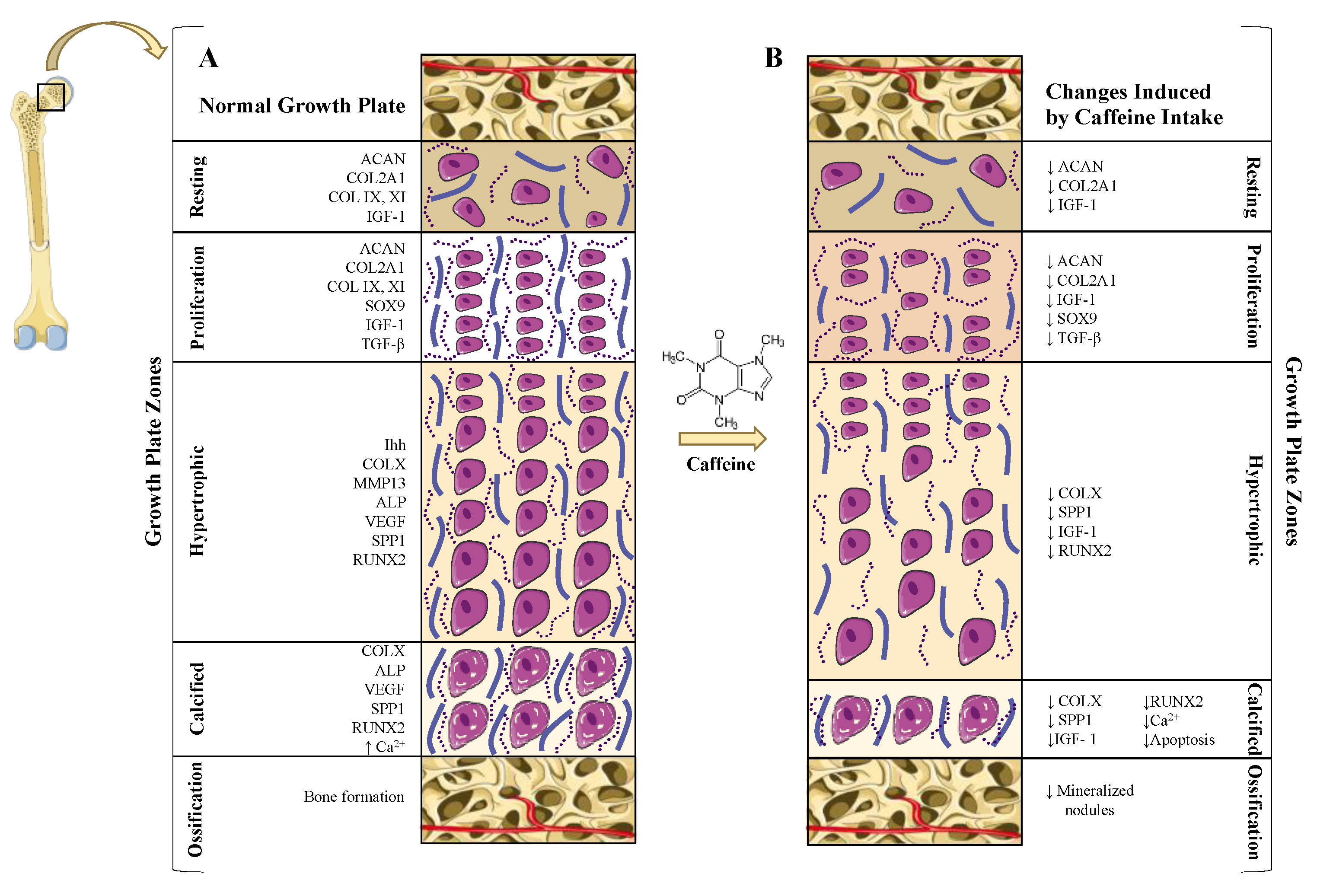
5.2. Caffeine Effects on the Growth Plate: Longitudinal Bone Growth Inhibition
5.2.1. Prenatal Caffeine Exposure
5.2.2. Direct and Indirect Effect of Caffeine on Growth Plate Chondrocytes
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- O’Neill, T.W.; McCabe, P.S.; McBeth, J. Update on the epidemiology, risk factors and disease outcomes of osteoarthritis. Best Pract. Res. Clin. Rheumatol. 2018, 32, 312–326. [Google Scholar] [CrossRef]
- Gómez, R.; Villalvilla, A.; Largo, R.; Gualillo, O.; Herrero-Beaumont, G. TLR4 signalling in osteoarthritis—Finding targets for candidate DMOADs. Nat. Rev. Rheumatol. 2015, 11, 159–170. [Google Scholar] [CrossRef]
- Litwic, A.; Edwards, M.H.; Dennison, E.M.; Cooper, C. Epidemiology and burden of osteoarthritis. Br. Med. Bull. 2013, 105, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Villalvilla, A.; da Silva, J.A.; Largo, R.; Gualillo, O.; Vieira, P.C.; Herrero-Beaumont, G.; Gómez, R. 6-Shogaol inhibits chondrocytes’ innate immune responses and cathepsin-K activity. Mol. Nutr. Food Res. 2014, 58, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef]
- Rahmati, M.; Nalesso, G.; Mobasheri, A.; Mozafari, M. Aging and osteoarthritis: Central role of the extracellular matrix. Ageing Res. Rev. 2017, 40, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, Y.; Grodzinsky, A.J. Cartilage diseases. Matrix Biol. 2018, 71–72, 51–69. [Google Scholar] [CrossRef] [PubMed]
- Van der Kraan, P.M.; van den Berg, W.B. Chondrocyte hypertrophy and osteoarthritis: Role in initiation and progression of cartilage degeneration? Osteoarthr. Cartil. 2012, 20, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Dreier, R. Hypertrophic differentiation of chondrocytes in osteoarthritis: The developmental aspect of degenerative joint disorders. Arthritis Res. Ther. 2010, 12, 216. [Google Scholar] [CrossRef]
- Mackie, E.J.; Ahmed, Y.A.; Tatarczuch, L.; Chen, K.-S.; Mirams, M. Endochondral ossification: How cartilage is converted into bone in the developing skeleton. Int. J. Biochem. Cell Biol. 2008, 40, 46–62. [Google Scholar] [CrossRef]
- De Hooge, A.S.K.; van de Loo, F.A.J.; Bennink, M.B.; Arntz, O.J.; Fiselier, T.J.W.; Franssen, M.J.A.M.; Joosten, L.A.B.; Van Lent, P.L.E.M.; Richards, C.D.; van den Berg, W.B. Growth plate damage, a feature of juvenile idiopathic arthritis, can be induced by adenoviral gene transfer of oncostatin M: A comparative study in gene-deficient mice. Arthritis Rheum. 2003, 48, 1750–1761. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, F.; Lazzeroni, P.; Sartori, C.; Street, M.E. Inflammatory Diseases and Growth: Effects on the GH-IGF Axis and on Growth Plate. Int. J. Mol. Sci. 2017, 18, 1878. [Google Scholar] [CrossRef] [PubMed]
- Buckwalter, J.A.; Mankin, H.J. Articular cartilage: Tissue design and chondrocyte-matrix interactions. Instr. Course Lect. 1998, 47, 477–486. [Google Scholar] [PubMed]
- Bang, C.H.; Kim, C.; Kim, J.H.; Choi, S.J.; Song, G.G.; Jung, J.H. Is knee osteoarthritis related to coffee drinking? A nationwide cross-sectional observational study. Clin. Rheumatol. 2019, 38, 817–825. [Google Scholar] [CrossRef]
- Pass, C.; MacRae, V.E.; Ahmed, S.F.; Farquharson, C. Inflammatory cytokines and the GH/IGF-I axis: Novel actions on bone growth. Cell Biochem. Funct. 2009, 27, 119–127. [Google Scholar] [CrossRef]
- Smink, J.J.; Buchholz, I.M.; Hamers, N.; van Tilburg, C.M.; Christis, C.; Sakkers, R.J.B.; de Meer, K.; van Buul-Offers, S.C.; Koedam, J.A. Short-term glucocorticoid treatment of piglets causes changes in growth plate morphology and angiogenesis. Osteoarthr. Cartil. 2003, 11, 864–871. [Google Scholar] [CrossRef][Green Version]
- Kronenberg, H.M. Developmental regulation of the growth plate. Nature 2003, 423, 332–336. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Sävendahl, L. Promoting growth in chronic inflammatory disease: Lessons from studies of the growth plate. Horm. Res. 2009, 72 (Suppl. 1), 42–47. [Google Scholar] [CrossRef]
- Ezri, J.; Marques-Vidal, P.; Nydegger, A. Impact of disease and treatments on growth and puberty of pediatric patients with inflammatory bowel disease. Digestion 2012, 85, 308–319. [Google Scholar] [CrossRef]
- Takahashi, M.; Saha, P.K.; Wehrli, F.W. Skeletal effects of short-term exposure to dexamethasone and response to risedronate treatment studied in vivo in rabbits by magnetic resonance micro-imaging and spectroscopy. J. Bone Miner. Metab. 2006, 24, 467–475. [Google Scholar] [CrossRef]
- Owen, H.C.; Roberts, S.J.; Ahmed, S.F.; Farquharson, C. Dexamethasone-induced expression of the glucocorticoid response gene lipocalin 2 in chondrocytes. Am. J. Physiol. Endocrinol. Metab. 2008, 294, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- MacRae, V.E.; Farquharson, C.; Ahmed, S.F. The pathophysiology of the growth plate in juvenile idiopathic arthritis. Rheumatology 2006, 45, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Lu, K.; Li, J.; Ni, Q.; Zhao, Z.; Magdalou, J.; Chen, L.; Wang, H. Prenatal caffeine exprosure increases adult female offspring rat’s susceptibility to osteoarthritis via low-functional programming of cartilage IGF-1 with histone acetylation. Toxicol. Lett. 2018, 295, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Shangguan, Y.; Jiang, H.; Pan, Z.; Xiao, H.; Tan, Y.; Tie, K.; Qin, J.; Deng, Y.; Chen, L.; Wang, H. Glucocorticoid mediates prenatal caffeine exposure-induced endochondral ossification retardation and its molecular mechanism in female fetal rats. Cell Death Dis. 2017, 8, e3157. [Google Scholar] [CrossRef]
- Tan, Y.; Liu, J.; Deng, Y.; Cao, H.; Xu, D.; Cu, F.; Lei, Y.; Magdalou, J.; Wu, M.; Chen, L.; et al. Caffeine-induced fetal rat over-exposure to maternal glucocorticoid and histone methylation of liver IGF-1 might cause skeletal growth retardation. Toxicol. Lett. 2012, 214, 279–287. [Google Scholar] [CrossRef]
- Luo, H.; Li, J.; Cao, H.; Tan, Y.; Magdalou, J.; Chen, L.; Wang, H. Prenatal caffeine exposure induces a poor quality of articular cartilage in male adult offspring rats via cholesterol accumulation in cartilage. Sci. Rep. 2015, 5, 17746. [Google Scholar] [CrossRef]
- Choi, H.; Choi, Y.; Kim, J.; Bae, J.; Roh, J. Longitudinal bone growth is impaired by direct involvement of caffeine with chondrocyte differentiation in the growth plate. J. Anat. 2017, 230, 117–127. [Google Scholar] [CrossRef]
- Cornelis, M.C.; Munafo, M.R. Mendelian Randomization Studies of Coffee and Caffeine Consumption. Nutrients 2018, 10, 1343. [Google Scholar] [CrossRef]
- Reis, A.M.S.; Raad, R.V.; de Melo Ocarino, N.; Serakides, R. In vitro effects of caffeine in growth cartilage of rats. Acta Ortop. Bras. 2013, 21, 307–309. [Google Scholar] [CrossRef]
- Nawrot, P.; Jordan, S.; Eastwood, J.; Rotstein, J.; Hugenholtz, A.; Feeley, M. Effects of caffeine on human health. Food Addit. Contam. 2003, 20, 1–30. [Google Scholar] [CrossRef]
- Wolde, T. Effects of caffeine on health and nutrition: A Review. Food Sci. Qual. Manag. 2014, 30, 59–65. [Google Scholar]
- Temple, J.L.; Bernard, C.; Lipshultz, S.E.; Czachor, J.D.; Westphal, J.A.; Mestre, M.A. The Safety of Ingested Caffeine: A Comprehensive Review. Front. Psychiatry 2017, 8, 80. [Google Scholar] [CrossRef] [PubMed]
- Tesch, A.M.; MacDonald, M.H.; Kollias-Baker, C.; Benton, H.P. Endogenously produced adenosine regulates articular cartilage matrix homeostasis: Enzymatic depletion of adenosine stimulates matrix degradation. Osteoarthr. Cartil. 2004, 12, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Barone, L.M.; Tassinari, M.S.; Bortell, R.; Owen, T.A.; Zerogian, J.; Gagne, K.; Stein, G.S.; Lian, J.B. Inhibition of induced endochondral bone development in caffeine-treated rats. J. Cell. Biochem. 1993, 52, 171–182. [Google Scholar] [CrossRef]
- Doepker, C.; Lieberman, H.R.; Smith, A.P.; Peck, J.D.; El-Sohemy, A.; Welsh, B.T. Caffeine: Friend or Foe? Annu. Rev. Food Sci. Technol. 2016, 7, 117–137. [Google Scholar] [CrossRef]
- Shechter, M.; Shalmon, G.; Scheinowitz, M.; Koren-Morag, N.; Feinberg, M.S.; Harats, D.; Sela, B.A.; Sharabi, Y.; Chouraqui, P. Impact of acute caffeine ingestion on endothelial function in subjects with and without coronary artery disease. Am. J. Cardiol. 2011, 107, 1255–1261. [Google Scholar] [CrossRef]
- de Oliveira Alves, A.; Weis, G.C.C.; Unfer, T.C.; Assmann, C.E.; Barbisan, F.; Azzolin, V.F.; Chitolina, B.; Duarte, T.; Ribeiro-Filho, E.E.; Duarte, M.M.M.F.; et al. Caffeinated beverages contribute to a more efficient inflammatory response: Evidence from human and earthworm immune cells. Food Chem. Toxicol. 2019, 134, 110809. [Google Scholar] [CrossRef]
- Saeed, M.; Naveed, M.; BiBi, J.; Ali Kamboh, A.; Phil, L.; Chao, S. Potential nutraceutical and food additive properties and risks of coffee: A comprehensive overview. Crit. Rev. Food Sci. Nutr. 2019, 59, 3293–3319. [Google Scholar] [CrossRef]
- Gavrieli, A.; Yannakoulia, M.; Fragopoulou, E.; Margaritopoulos, D.; Chamberland, J.P.; Kaisari, P.; Kavouras, S.A.; Mantzoros, C.S. Caffeinated Coffee Does Not Acutely Affect Energy Intake, Appetite, or Inflammation but Prevents Serum Cortisol Concentrations from Falling in Healthy Men. J. Nutr. 2011, 141, 703–707. [Google Scholar] [CrossRef]
- Paiva, C.L.R.S.; Beserra, B.T.S.; Reis, C.E.G.; Dorea, J.G.; Da Costa, T.H.M.; Amato, A.A. Consumption of coffee or caffeine and serum concentration of inflammatory markers: A systematic review. Crit. Rev. Food Sci. Nutr. 2019, 59, 652–663. [Google Scholar] [CrossRef]
- Tauler, P.; Martínez, S.; Moreno, C.; Monjo, M.; Martínez, P.; Aguiló, A. Effects of caffeine on the inflammatory response induced by a 15-km run competition. Med. Sci. Sports Exerc. 2013, 45, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.M.S.; Oliveira, K.P.; de Paula, I.H.F.; da Silva, A.P.; Tarragô, J.F.; de Melo Ocarino, N.; Serakides, R. Nonlinear effects of caffeine on the viability, synthesis and gene expression of chondrocytes from the offspring of rats treated during pregnancy. Acta Histochem. 2018, 120, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Fenster, L.; Eskenazi, B.; Windham, G.C.; Swan, S.H. Caffeine consumption during pregnancy and fetal growth. Am. J. Public Health 1991, 81, 458–461. [Google Scholar] [CrossRef] [PubMed]
- Konje, J.C.; Cade, J.E. Maternal caffeine intake during pregnancy and risk of fetal growth restriction: A large prospective observational study. BMJ 2008, 337, 1334–1338. [Google Scholar]
- Kuo, C.-L.; Liu, S.-T.; Chang, Y.-L.; Wu, C.-C.; Huang, S.-M. Zac1 regulates IL-11 expression in osteoarthritis. Oncotarget 2018, 9, 32478–32495. [Google Scholar] [CrossRef]
- Al-Arfaj, A.S. Radiographic osteoarthritis and serum cholesterol. Saudi Med. J. 2003, 24, 745–747. [Google Scholar]
- Zhuo, Q.; Yang, W.; Chen, J.; Wang, Y. Metabolic syndrome meets osteoarthritis. Nat. Rev. Rheumatol. 2012, 8, 729–737. [Google Scholar] [CrossRef]
- Villalvilla, A.; Gómez, R.; Largo, R.; Herrero-Beaumont, G. Lipid transport and metabolism in healthy and osteoarthritic cartilage. Int. J. Mol. Sci. 2013, 14, 20793–20808. [Google Scholar] [CrossRef]
- Patil, A.S.; Sable, R.B.; Kothari, R.M. Role of insulin-like growth factors (IGFs), their receptors and genetic regulation in the chondrogenesis and growth of the mandibular condylar cartilage. J. Cell. Physiol. 2012, 227, 1796–1804. [Google Scholar] [CrossRef]
- Walmsley, M.; Butler, D.M.; Marinova-Mutafchieva, L.; Feldmann, M. An anti-inflammatory role for interleukin-11 in established murine collagen-induced arthritis. Immunology 1998, 95, 31–37. [Google Scholar] [CrossRef]
- Stannus, O.; Jones, G.; Cicuttini, F.; Parameswaran, V.; Quinn, S.; Burgess, J.; Ding, C. Circulating levels of IL-6 and TNF-α are associated with knee radiographic osteoarthritis and knee cartilage loss in older adults. Osteoarthr. Cartil. 2010, 18, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Sayer, A.A.; Poole, J.; Cox, V.; Kuh, D.; Hardy, R.; Wadsworth, M.; Cooper, C. Weight from birth to 53 years: A longitudinal study of the influence on clinical hand osteoarthritis. Arthritis Rheum. 2003, 48, 1030–1033. [Google Scholar] [CrossRef] [PubMed]
- Jordan, K.M.; Syddall, H.; Dennison, E.M.; Cooper, C.; Arden, N.K. Birthweight, vitamin D receptor gene polymorphism, and risk of lumbar spine osteoarthritis. J. Rheumatol. 2005, 32, 678–683. [Google Scholar] [PubMed]
- Allen, D.B. Growth suppression by glucocorticoid therapy. Endocrinol. Metab. Clin. North Am. 1996, 25, 699–717. [Google Scholar] [CrossRef]
- Zhang, X.; Siclari, V.A.; Lan, S.; Zhu, J.; Koyama, E.; Dupuis, H.L.; Enomoto-Iwamoto, M.; Beier, F.; Qin, L. The critical role of the epidermal growth factor receptor in endochondral ossification. J. Bone Miner. Res. 2011, 26, 2622–2633. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.F.; Shen, C.-L.; Pollack, S.; Aloia, J.F.; Yeh, J.K. Adrenocorticotropin evokes transient elevations in intracellular free calcium ([Ca2+]i) and increases basal [Ca2+]i in resting chondrocytes through a phospholipase C-dependent mechanism. Endocrinology 2005, 146, 3123–3132. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guillán-Fresco, M.; Franco-Trepat, E.; Alonso-Pérez, A.; Jorge-Mora, A.; López-Fagúndez, M.; Pazos-Pérez, A.; Gualillo, O.; Gómez, R. Caffeine, a Risk Factor for Osteoarthritis and Longitudinal Bone Growth Inhibition. J. Clin. Med. 2020, 9, 1163. https://doi.org/10.3390/jcm9041163
Guillán-Fresco M, Franco-Trepat E, Alonso-Pérez A, Jorge-Mora A, López-Fagúndez M, Pazos-Pérez A, Gualillo O, Gómez R. Caffeine, a Risk Factor for Osteoarthritis and Longitudinal Bone Growth Inhibition. Journal of Clinical Medicine. 2020; 9(4):1163. https://doi.org/10.3390/jcm9041163
Chicago/Turabian StyleGuillán-Fresco, María, Eloi Franco-Trepat, Ana Alonso-Pérez, Alberto Jorge-Mora, Miriam López-Fagúndez, Andrés Pazos-Pérez, Oreste Gualillo, and Rodolfo Gómez. 2020. "Caffeine, a Risk Factor for Osteoarthritis and Longitudinal Bone Growth Inhibition" Journal of Clinical Medicine 9, no. 4: 1163. https://doi.org/10.3390/jcm9041163
APA StyleGuillán-Fresco, M., Franco-Trepat, E., Alonso-Pérez, A., Jorge-Mora, A., López-Fagúndez, M., Pazos-Pérez, A., Gualillo, O., & Gómez, R. (2020). Caffeine, a Risk Factor for Osteoarthritis and Longitudinal Bone Growth Inhibition. Journal of Clinical Medicine, 9(4), 1163. https://doi.org/10.3390/jcm9041163







