The Possibility of Suppression of Increased Postprandial Blood Glucose Levels by Gamma-Polyglutamic Acid-Rich Natto in the Early Phase after Eating: A Randomized Crossover Pilot Study
Abstract
1. Introduction
2. Materials and Methods
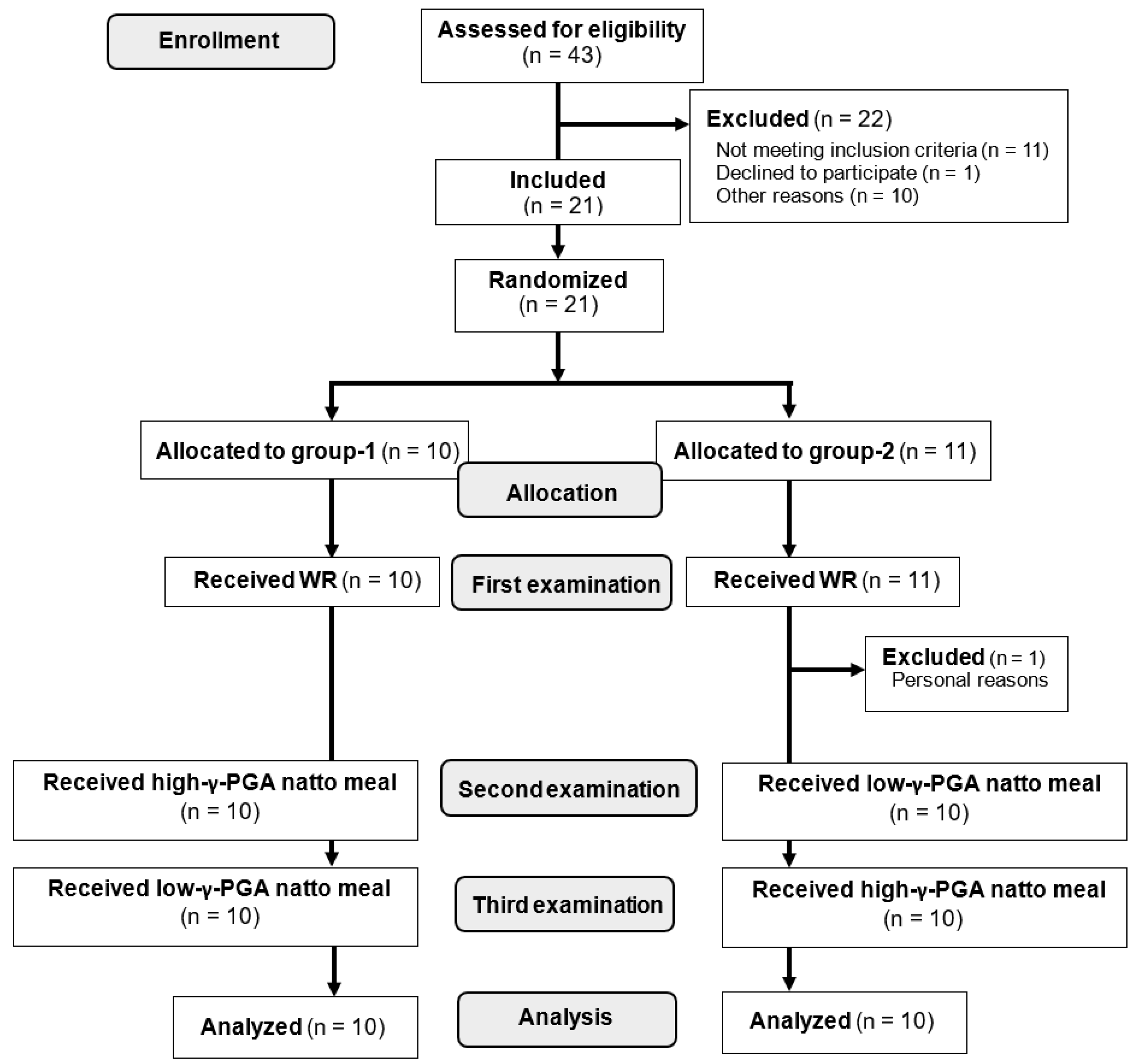
2.1. Participants
2.2. Study Protocol
2.3. Clinical Measurements
2.4. Analysis of Blood Biochemical Parameters
2.5. Questionnaire Survey
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
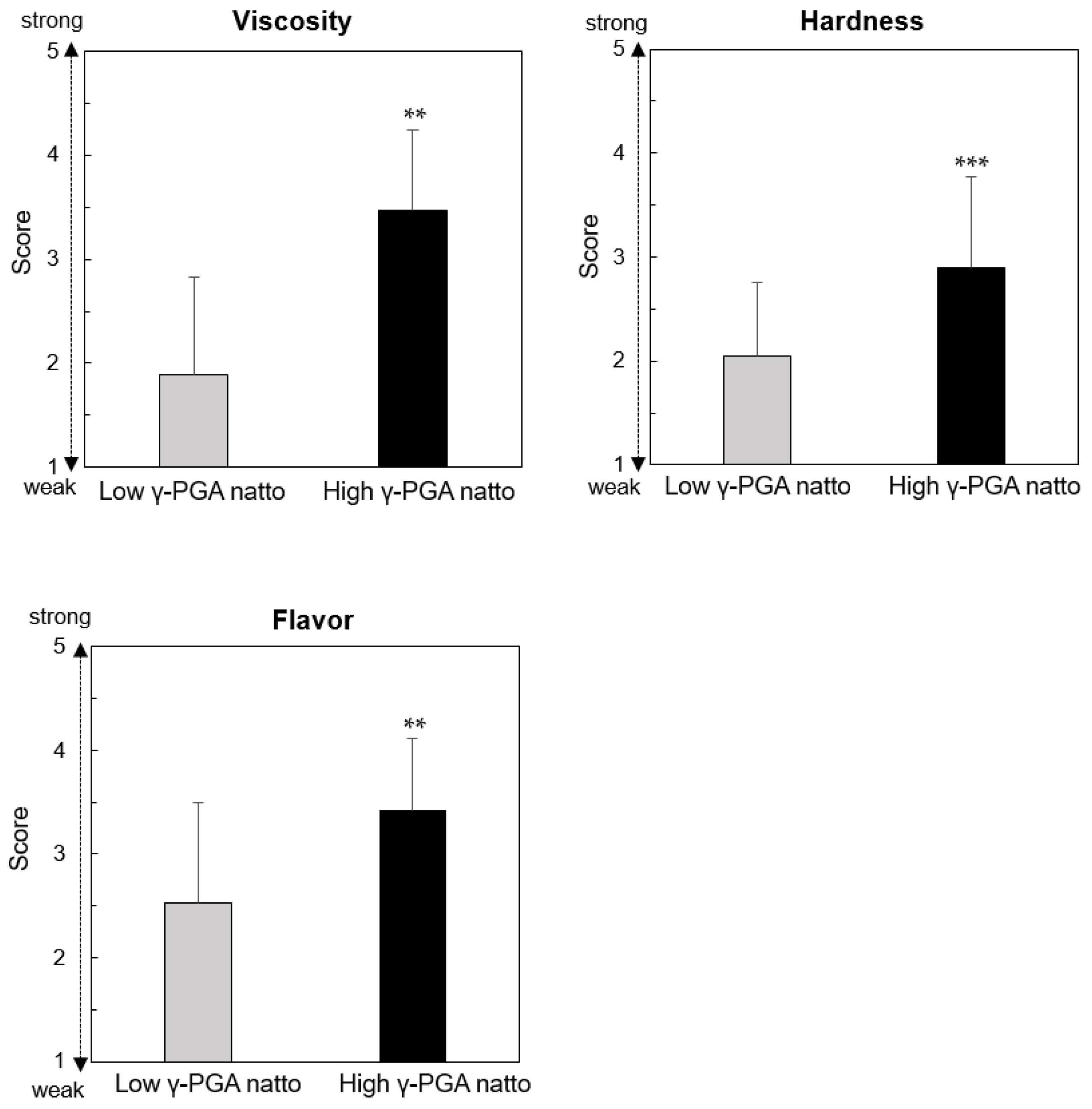
3.2. Sensory Evaluations of Natto Used in the Present Study
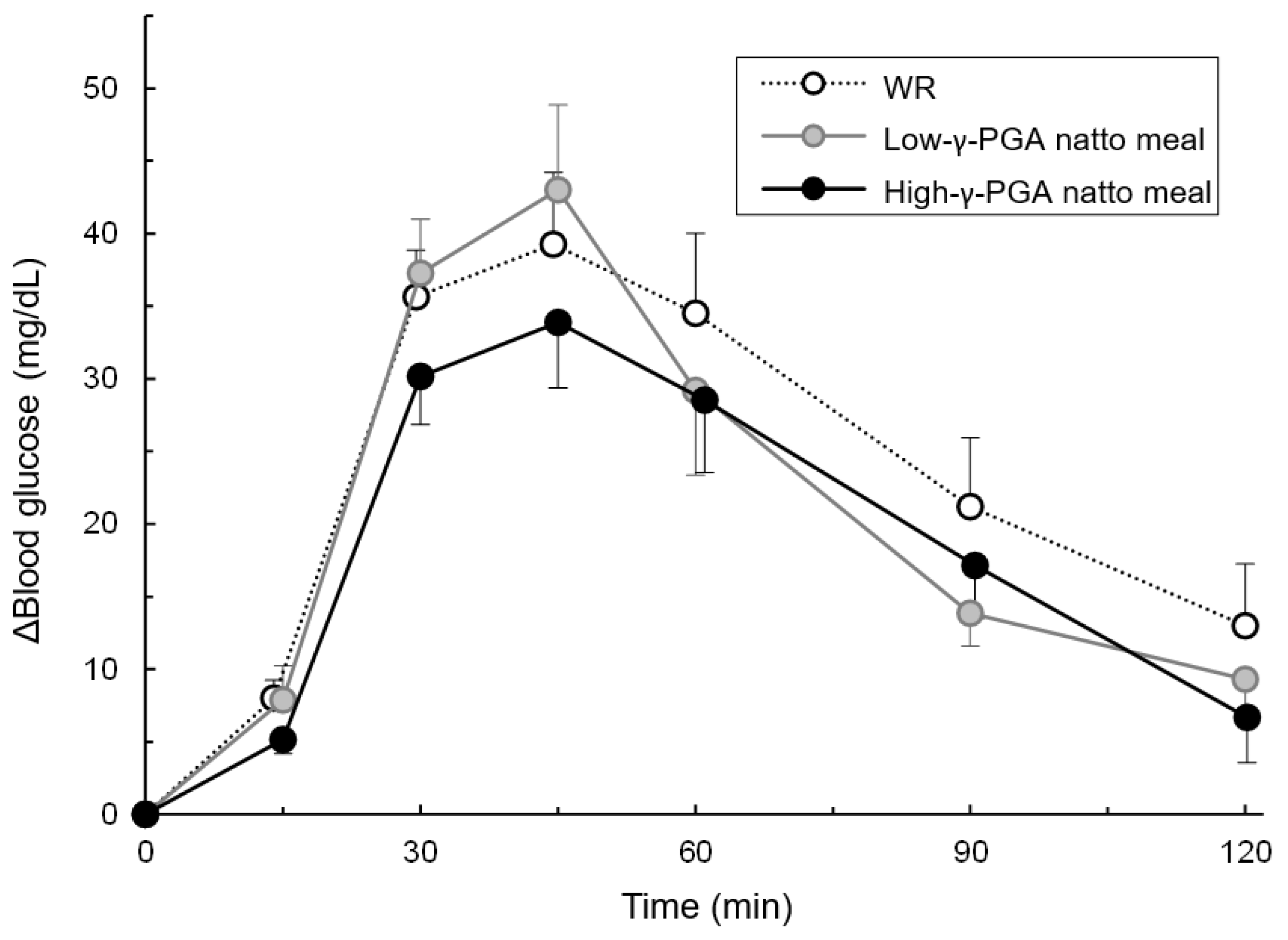
3.3. Comparison of Blood Glucose’s IAUC between the Test Meals at Each Time Point
3.4. Changes in Blood Glucose Levels Over Time Following Each Test Meal Loading
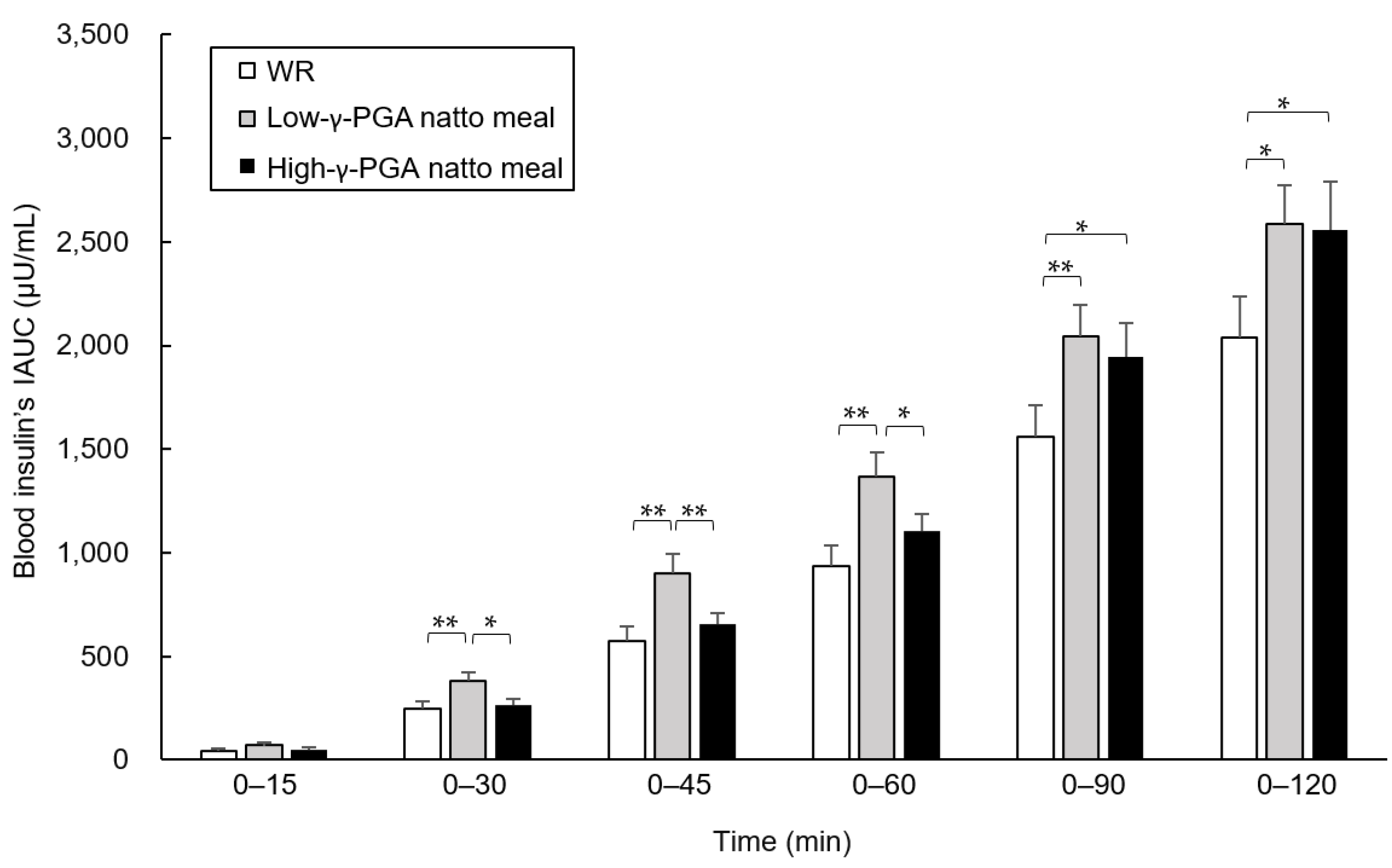
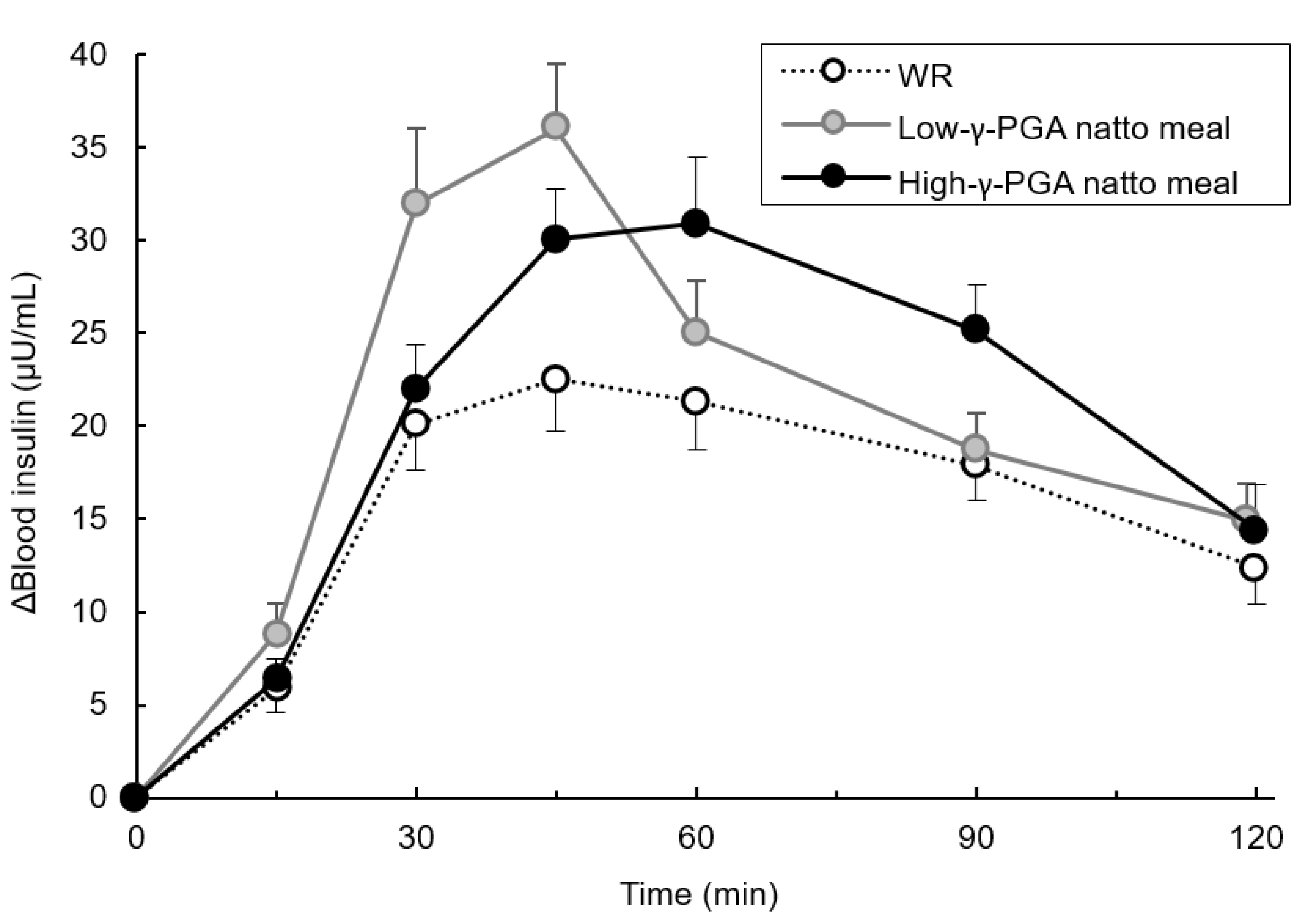
3.5. Comparison of blood insulin’s IAUC between Test Meals at Each Time Point
3.6. Changes in Blood Insulin Levels Over Time Following Each Test Meal Loading
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. World Health Statistics 2016: Monitoring Health for the SDG. Available online: http://www.who.int/gho/publications/world_health_statistics/2016/en/ (accessed on 13 February 2020).
- Suzuki, N.; Goto, Y.; Ota, H.; Kito, K.; Mano, F.; Joo, E.; Ikeda, K.; Inagaki, N.; Nakayama, T. Characteristics of the Japanese diet described in epidemiologic publications: A qualitative systematic review. J. Nutr. Sci. Vitaminol. 2018, 64, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Tada, N.; Maruyama, C.; Koba, S.; Tanaka, H.; Birou, S.; Teramoto, T.; Sasaki, J. Japanese dietary lifestyle and cardiovascular disease. J. Atheroscler. Thromb. 2011, 18, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Nagata, C.; Wada, K.; Tamura, T.; Konishi, K.; Goto, Y.; Koda, S.; Kawachi, T.; Tsuji, M.; Nakamura, K. Dietary soy and natto intake and cardiovascular disease mortality in Japanese adults: The Takayama study. Am. J. Clin. Nutr. 2017, 105, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Katsuyama, H.; Ideguchi, S.; Fukunaga, M.; Fukunaga, T.; Saijoh, K.; Sunami, S. Promotion of bone formation by fermented soybean (Natto) intake in premenopausal women. J. Nutr. Sci. Vitaminol. 2004, 50, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Nozue, M.; Shimazu, T.; Sasazuki, S.; Charvat, H.; Mori, N.; Mutoh, M.; Sawada, N.; Iwasaki, M.; Yamaji, T.; Inoue, M.; et al. Fermented soy product intake is inversely associated with the development of high blood pressure: The Japan public health center-based prospective study. J. Nutr. 2017, 1471, 1749–1756. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Itoh, Y. Characterization of a generalized transducing phage of poly-(gamma)-glutamic acid-producing bacillus subtilis and its application for analysis of Tn917-LTV1 insertional mutants defective in poly-(gamma)-glutamic acid production. Appl. Environ. Microbiol. 1997, 63, 4087–4089. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.H.; Kwak, M.S.; Sung, M.H.; Kim, S.H.; Kim, M.H.; Chang, M.J. High-molecular-weight poly-gamma-glutamate protects against hypertriglyceridemic effects of a high-fructose diet in rat. J. Microbiol. Biotechnol. 2013, 23, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Tanimoto, H.; Mori, M.; Motoki, M.; Torii, K.; Kadowaki, M.; Noguchi, T. Natto mucilage containing poly-gamma-glutamic acid increases soluble calcium in the rat small intestine. Biosci. Biotechnol. Biochem. 2001, 65, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Ishimaru, K.; Osaki, N.; Sawada, K. Blood Sugar Concentration Rise Inhibitor. Available online: https://patents.google.com/patent/JP2012144485A/en (accessed on 13 February 2020).
- Karmaker, S.; Saha, T.K.; Yoshikawa, Y.; Sakurai, H. Amelioration of hyperglycemia and metabolic syndromes in type 2 diabetic KKA(y) mice by poly (gamma-glutamic acid) oxovanadium(IV) complex. Chem. Med. Chem. 2007, 2, 1607–1612. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, A.; Yamanaka-Okumura, H.; Nishida, Y.; Yamamoto, H.; Taketani, Y.; Takeda, E. Natto and viscous vegetables in a Japanese style meal suppress postprandial glucose and insulin responses. Asia Pac. J. Clin. Nutr. 2008, 17, 663–668. [Google Scholar] [PubMed]
- Ishikawa, A.; Kishi, M.; Yamaguchi, K. Effect of intake of natto and soybeans on postprandial blood glucose levels in healthy adults. SEIKATSU EISEI 2009, 53, 257–260. [Google Scholar]
- Kanno, A.; Takamatsu, H. Determination of γ-polyglutamic acid in “natto”using cetyltrimethylammonium bromide. Nippon Shokuhin Kagaku Gakkai-Shi 1995, 42, 878–886. [Google Scholar] [CrossRef]
- Regand, A.; Chowdhury, Z.; Tosh, S.M.; Wolever, T.M.S.; Wood, P. The molecular weight, solubility and viscosity of oat beta-glucan affect human glycemic response by modifying starch digestibility. Food Chem. 2011, 129, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Würsch, P.; Pi-Sunyer, F.X. The role of viscous soluble fiber in the metabolic control of diabetes. A review with special emphasis on cereals rich in beta-glucan. Diabetes Care 1997, 20, 1774–1780. [Google Scholar]
- Taniguchi-Fukatsu, A.; Yamanaka-Okumura, H.; Naniwa-Kuroki, Y.; Nishida, Y.; Yamamoto, H.; Taketani, Y.; Takeda, E. Natto and viscous vegetables in a Japanese-style breakfast improved insulin sensitivity, lipid metabolism and oxidative stress in overweight subjects with impaired glucose tolerance. Br. J. Nutr. 2012, 107, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Tamura, M.; Hori, S.; Inose, A.; Kobori, M. Effects of γ-polyglutamic acid on blood glucose and caecal short chain fatty acids in adult male mice. Food Nutr. Sci. 2020, 11, 8–22. [Google Scholar]
- Zhang, T.; Li, C. Mechanisms of amino acid-stimulated insulin secretion in congenital hyperinsulinism. Acta Biochim. Biophys. Sin. 2013, 45, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.Y.; Liu, Q.B.; Yang, H.Y.; Tang, H.Y.; Yang, J.G. Study on the improvement of natto-production process. Adv. J. Food Sci. Technol. 2015, 7, 704–708. [Google Scholar] [CrossRef]

| WR | Low-γ-PGA Natto Meal | High-γ-PGA Natto Meal | ||||
|---|---|---|---|---|---|---|
| Packaged rice | 150 g | Packaged rice | 150 g | Packaged rice | 150 g | |
| Low-γ-PGA natto | 40 g | High-γ-PGA natto | 40 g | |||
| Water | 150 g | Water | 150 g | Water | 150 g | |
| Energy (kcal) | 210 | 286 | 288 | |||
| Protein (g) | 2.9 | 10.2 | 10.3 | |||
| Fat (g) | 0 | 3.8 | 3.8 | |||
| Carbohydrate (g) | 48.5 | 53.1 | 53.3 | |||
| γ-PGA (mg) | 0 | 33.6 | 269.9 | |||
| Characteristic | Value | ||
|---|---|---|---|
| Male/female (number of persons) | 12/8 | ||
| Age (years) | 37.7 | ± | 16.1 |
| BMI (kg/m2) | 21.4 | ± | 1.7 |
| FPG (mg/dL) | 91.3 | ± | 6.4 |
| HbA1c (%) | 5.5 | ± | 0.2 |
| Insulin (µU/mL) | 5.6 | ± | 2.3 |
| HOMA-IR | 1.3 | ± | 0.5 |
| Consumption frequency of natto (number of persons, (%)) | |||
| Every day | 2 | (10.0) | |
| Almost every day | 3 | (15.0) | |
| 3–4 times per week | 4 | (20.0) | |
| 1–2 times per week | 6 | (30.0) | |
| Less than once per week | 5 | (25.0) | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araki, R.; Fujie, K.; Yuine, N.; Watabe, Y.; Maruo, K.; Suzuki, H.; Hashimoto, K. The Possibility of Suppression of Increased Postprandial Blood Glucose Levels by Gamma-Polyglutamic Acid-Rich Natto in the Early Phase after Eating: A Randomized Crossover Pilot Study. Nutrients 2020, 12, 915. https://doi.org/10.3390/nu12040915
Araki R, Fujie K, Yuine N, Watabe Y, Maruo K, Suzuki H, Hashimoto K. The Possibility of Suppression of Increased Postprandial Blood Glucose Levels by Gamma-Polyglutamic Acid-Rich Natto in the Early Phase after Eating: A Randomized Crossover Pilot Study. Nutrients. 2020; 12(4):915. https://doi.org/10.3390/nu12040915
Chicago/Turabian StyleAraki, Risa, Keiko Fujie, Nanako Yuine, Yuta Watabe, Kazushi Maruo, Hiroaki Suzuki, and Koichi Hashimoto. 2020. "The Possibility of Suppression of Increased Postprandial Blood Glucose Levels by Gamma-Polyglutamic Acid-Rich Natto in the Early Phase after Eating: A Randomized Crossover Pilot Study" Nutrients 12, no. 4: 915. https://doi.org/10.3390/nu12040915
APA StyleAraki, R., Fujie, K., Yuine, N., Watabe, Y., Maruo, K., Suzuki, H., & Hashimoto, K. (2020). The Possibility of Suppression of Increased Postprandial Blood Glucose Levels by Gamma-Polyglutamic Acid-Rich Natto in the Early Phase after Eating: A Randomized Crossover Pilot Study. Nutrients, 12(4), 915. https://doi.org/10.3390/nu12040915





