Distribution of Lactoferrin Is Related with Dynamics of Neutrophils in Bacterial Infected Mice Intestine
Abstract
1. Introduction
2. Results
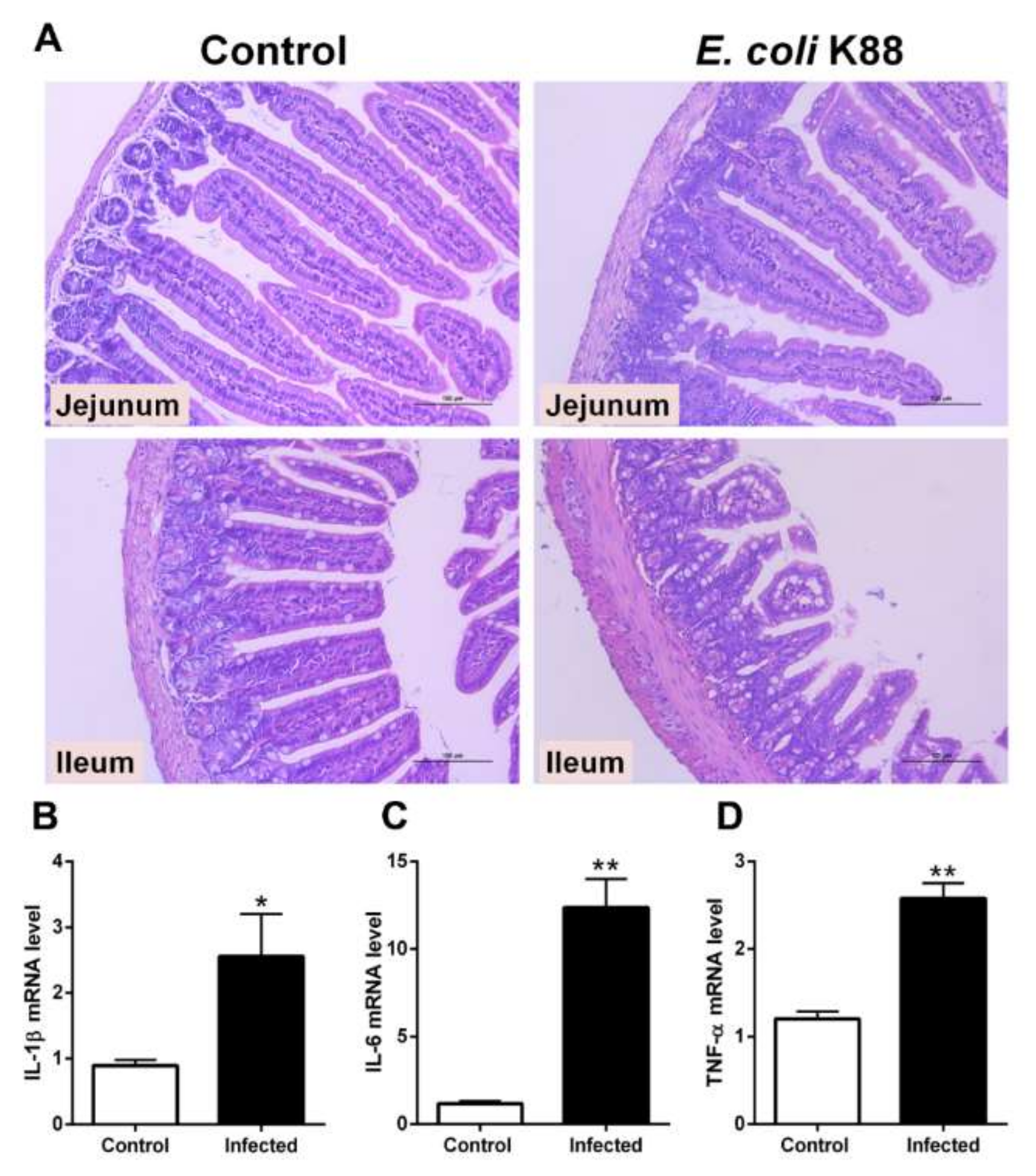
2.1. Establishment of a Murine Model of E. coli K88 Challenge
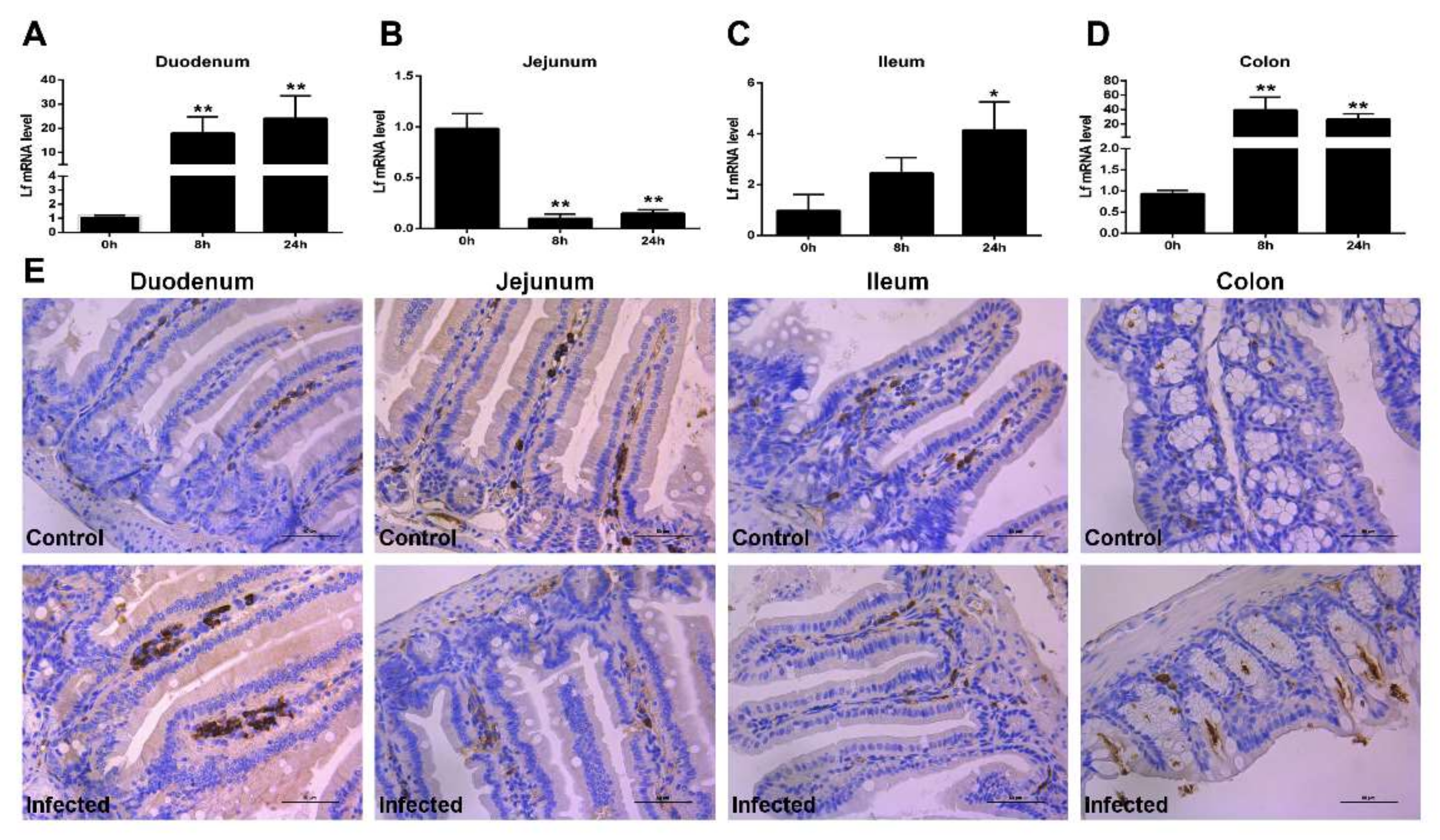
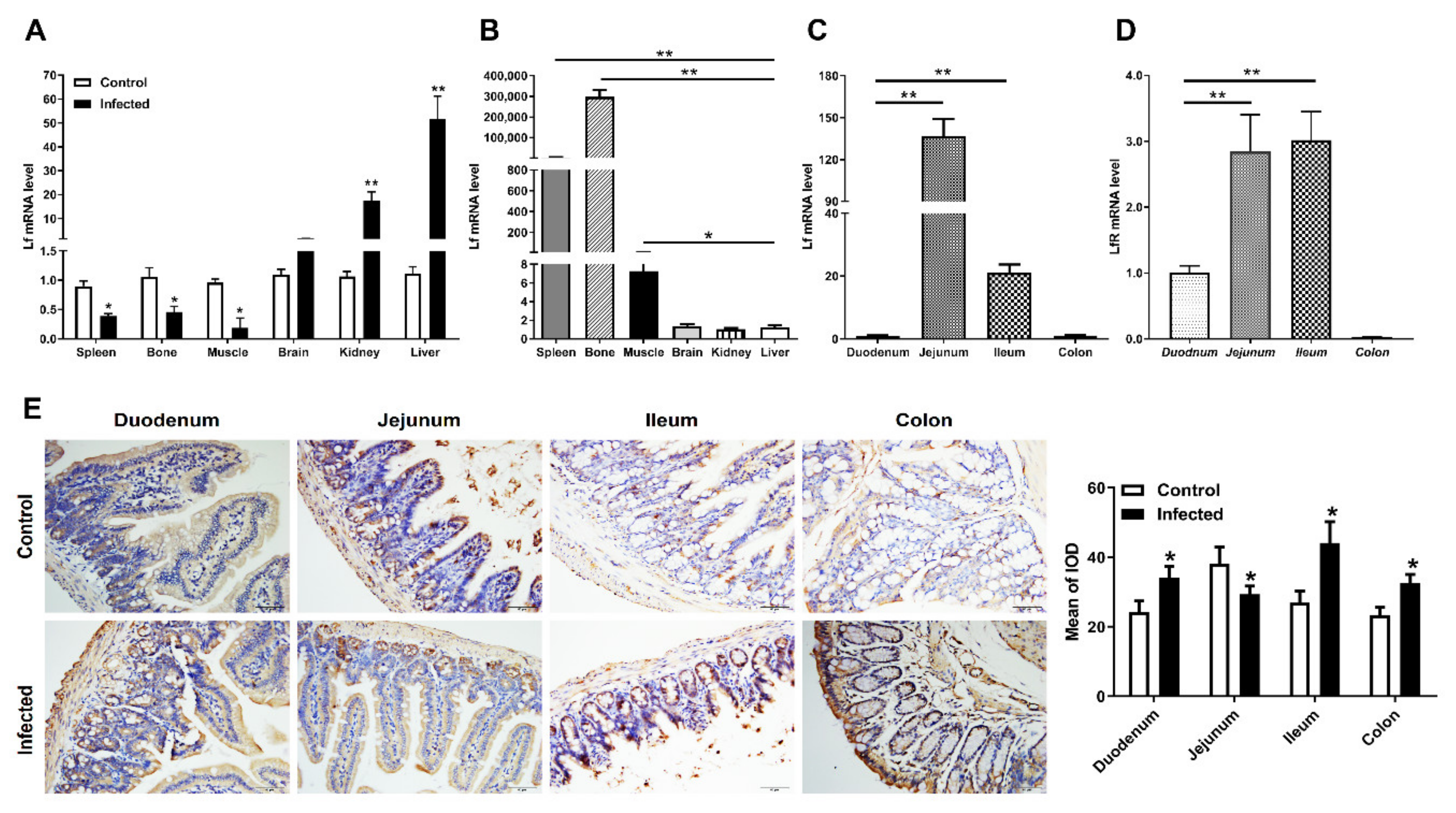
2.2. Expression of Lf in the Intestine of E. coli K88-Infected Mice
2.3. Distribution of Lf and Neutrophils in the Intestine of E. coli K88-Infected Mice
3. Discussion
4. Materials and Methods
4.1. Animals and Bacteria
4.2. Animal Infections
4.3. RT-PCR
4.4. Immunohistochemistry (IHC) Staining
4.5. Hematoxylin and Eosin (H&E) Staining
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Baker, H.M.; Baker, E.N. A structural perspective on lactoferrin function. Biochem. Cell Biol. 2012, 90, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Brock, J.H. Lactoferrin--50 years on. Biochem. Cell Biol. 2012, 90, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.B.; Iigo, M.; Yamauchi, K.; Suzui, M.; Tsuda, H. Lactoferrin: An alternative view of its role in human biological fluids. Biochem. Cell Biol. 2012, 90, 279–306. [Google Scholar] [CrossRef] [PubMed]
- Jenssen, H.; Hancock, R.E. Antimicrobial properties of lactoferrin. Biochimie 2009, 91, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Legrand, D. Overview of lactoferrin as a natural immune modulator. J. Pediatr. 2016, 173, S10–S15. [Google Scholar] [CrossRef] [PubMed]
- Wada, Y.; Lönnerdal, B. Bioactive peptides derived from human milk proteins--mechanisms of action. J. Nutr. Biochem. 2014, 25, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Drago-Serrano, M.E.; Campos-Rodríguez, R.; Carrero, J.C.; de la Garza, M. Lactoferrin: Balancing ups and downs of inflammation due to microbial infections. Int. J. Mol. Sci. 2017, 18, 501. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tu, Y.; Han, F.; Xu, Z.; Wang, J. Developmental gene expression of lactoferrin and effect of dietary iron on gene regulation of lactoferrin in mouse mammary gland. J. Dairy Sci. 2005, 88, 2065–2071. [Google Scholar] [CrossRef]
- åbrink, M.; Larsson, E.; Gobl, A.; Hellman, L. Expression of lactoferrin in the kidney: Implications for innate immunity and iron metabolism. Kidney Int. 2000, 57, 2004–2010. [Google Scholar] [CrossRef] [PubMed]
- Pearl, C.A.; Roser, J.F. Lactoferrin expression and secretion in the stallion epididymis. Reprod. Biol. 2014, 14, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Legrand, D. Lactoferrin, a key molecule in immune and inflammatory processes. Biochem. Cell Biol. 2012, 90, 252–268. [Google Scholar] [CrossRef] [PubMed]
- Black, R.E. Epidemiology of travelers’ diarrhea and relative importance of various pathogens. Rev. Infect. Dis. 1990, 1, S73–S79. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Roy, B.; Khan, S.; Septer, S.; Umar, S. Microbiome, metabolome and inflammatory bowel disease. Microorganisms 2016, 4, 20. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Chen, S.; Yin, J.; Duan, J.; Li, T.; Liu, G.; Feng, Z.; Tan, B.; Yin, Y.; Wu, G. Dietary Dietary arginine supplementation of mice alters the microbial population and activates intestinal innate immunity. J. Nutr. 2014, 144, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Li, F.; Tan, B.; Liu, G.; Kong, X.; Hardwidge, P.R.; Yin, Y. Enterotoxigenic Escherichia coli infection induces intestinal epithelial cell autophagy. Vet. Microbiol. 2014, 171, 160–164. [Google Scholar] [CrossRef]
- Embleton, N.D.; Berrington, J.E.; McGuire, W.; Stewart, C.J.; Cummings, S.P. Lactoferrin: Antimicrobial activity and therapeutic potential. Semin. Fetal Neonat. M. 2013, 18, 143–149. [Google Scholar] [CrossRef]
- Wisgrill, L.; Wessely, I.; Spittler, A.; Förster-Waldl, E.; Berger, A.; Sadeghi, K. Human lactoferrin attenuates the proinflammatory response of neonatal monocyte-derived macrophages. Clin. Exp. Immunol. 2018, 192, 315–324. [Google Scholar] [CrossRef]
- Mayeur, S.; Spahis, S.; Pouliot, Y.; Levy, E. Lactoferrin, a pleiotropic protein in health and disease. Antioxid. Redox Signal. 2016, 24, 813–836. [Google Scholar] [CrossRef]
- Fournier, B.M.; Parkos, C.A. The role of neutrophils during intestinal inflammation. Mucosal. Immunol. 2012, 5, 354–366. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
| Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| Lf | CCCTACAAACTGCGACCTGT | CACGACTGCTACCGCATAGT |
| LfR | GTGCAGTGTGGAGACTTTGC | CAATGGAGAAGTCAGGGCCA |
| IL-1β | AGTTGACGGACCCCAAAAG | TTTGAAGCTGGATGCTCTCAT |
| IL-6 | AGTCCTTCCTACCCCAATTTCC | GGTCTTGGTCCTTAGCCACT |
| TNF-α | GCTCTTCTGTCTACTGAACTTCGG | ATGATCTGAGTGTGAGGGTCTGG |
| GAPDH | CCGCATCTTCTTGTGCAGTG | CAAATCCGTTCACACCGACC |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, L.; Wang, Z.-J.; Ye, G.; Tang, X.-Y.; Zhang, Y.-Y.; Kong, J.-X.; Du, H.-H. Distribution of Lactoferrin Is Related with Dynamics of Neutrophils in Bacterial Infected Mice Intestine. Molecules 2020, 25, 1496. https://doi.org/10.3390/molecules25071496
Liang L, Wang Z-J, Ye G, Tang X-Y, Zhang Y-Y, Kong J-X, Du H-H. Distribution of Lactoferrin Is Related with Dynamics of Neutrophils in Bacterial Infected Mice Intestine. Molecules. 2020; 25(7):1496. https://doi.org/10.3390/molecules25071496
Chicago/Turabian StyleLiang, Li, Zhen-Jie Wang, Guang Ye, Xue-You Tang, Yuan-Yuan Zhang, Jing-Xia Kong, and Hua-Hua Du. 2020. "Distribution of Lactoferrin Is Related with Dynamics of Neutrophils in Bacterial Infected Mice Intestine" Molecules 25, no. 7: 1496. https://doi.org/10.3390/molecules25071496
APA StyleLiang, L., Wang, Z.-J., Ye, G., Tang, X.-Y., Zhang, Y.-Y., Kong, J.-X., & Du, H.-H. (2020). Distribution of Lactoferrin Is Related with Dynamics of Neutrophils in Bacterial Infected Mice Intestine. Molecules, 25(7), 1496. https://doi.org/10.3390/molecules25071496





