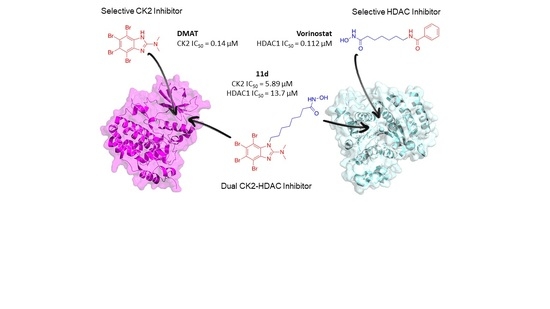
Multitarget Anticancer Agents Based on Histone Deacetylase and Protein Kinase CK2 Inhibitors
Abstract
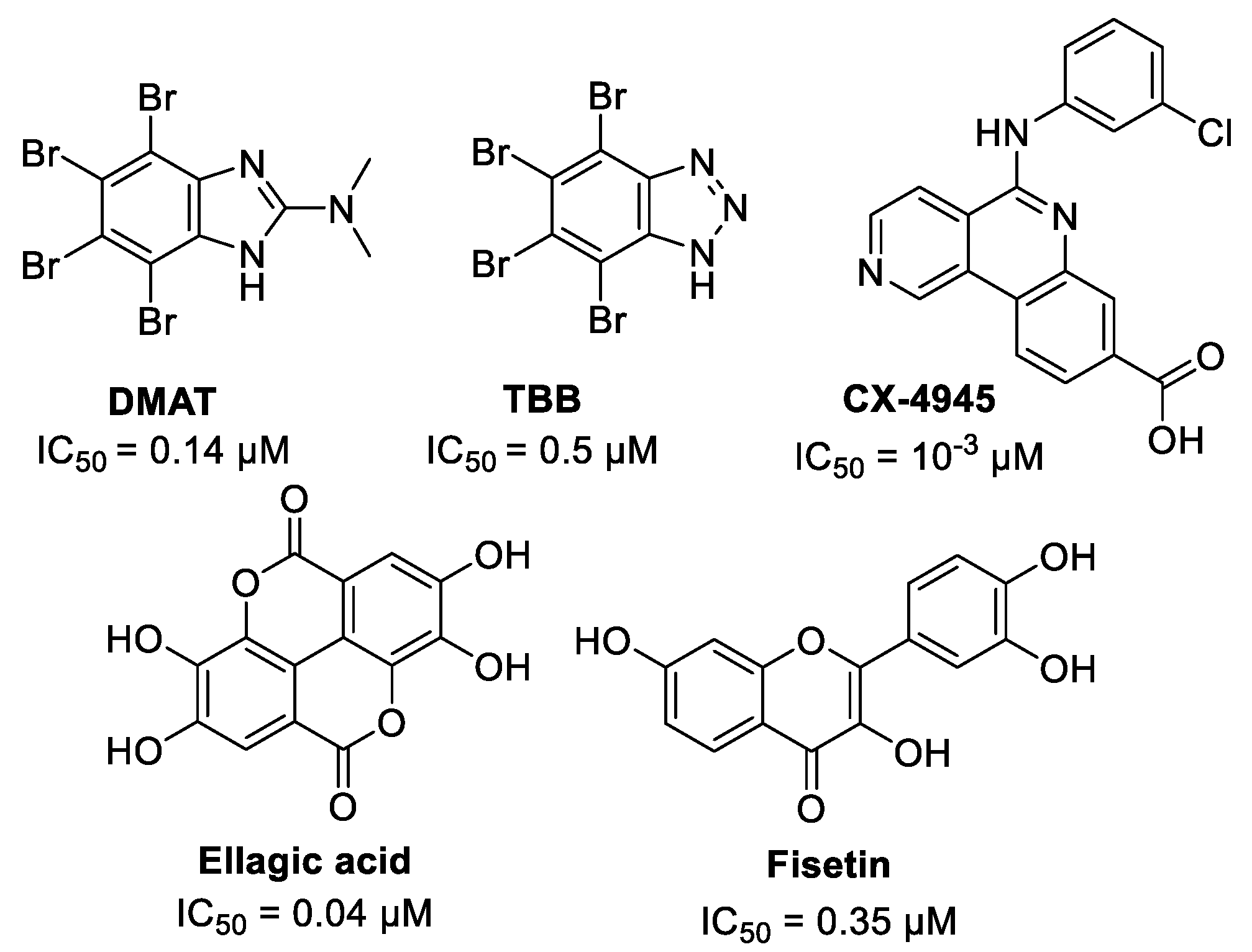
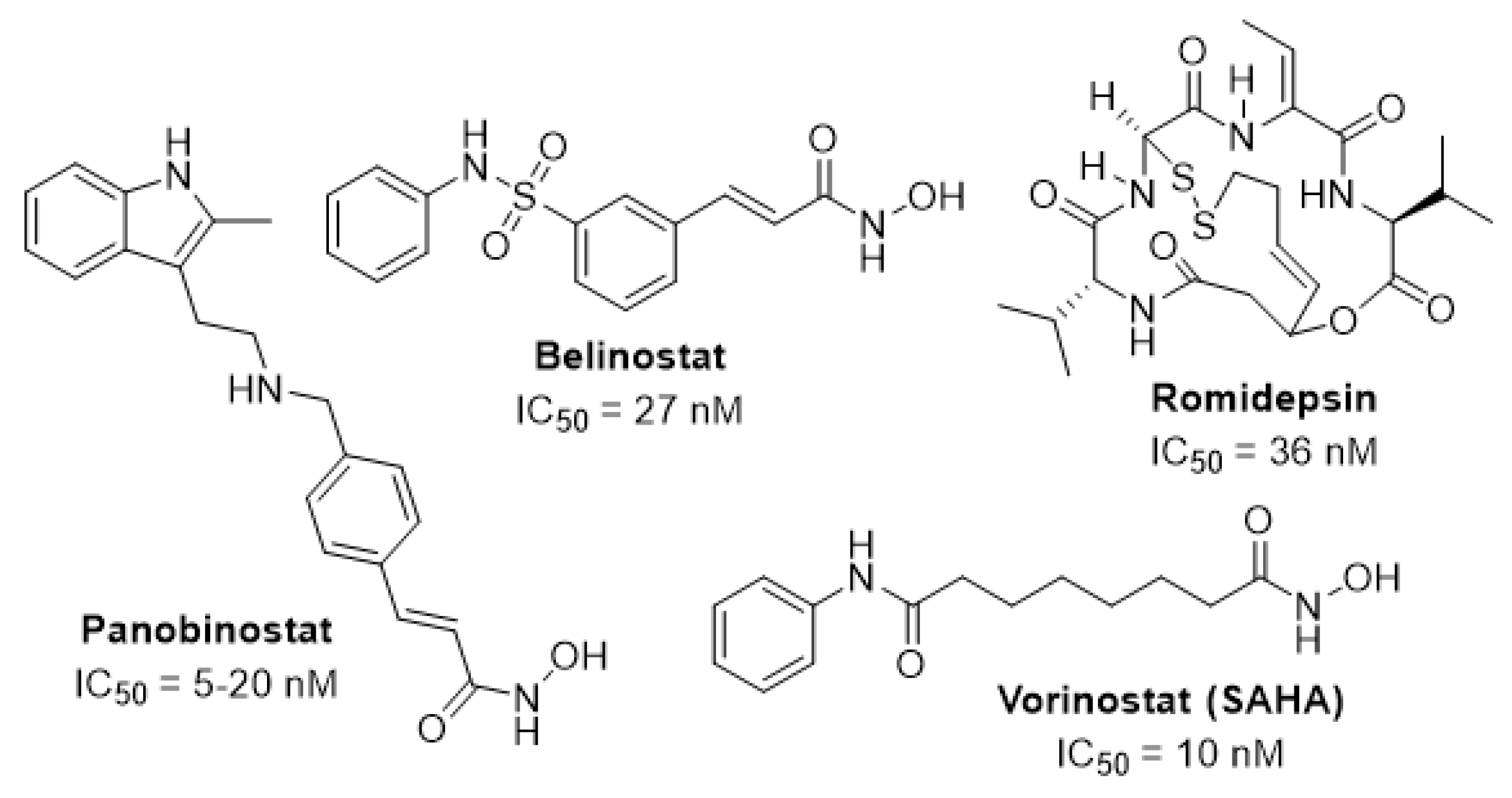
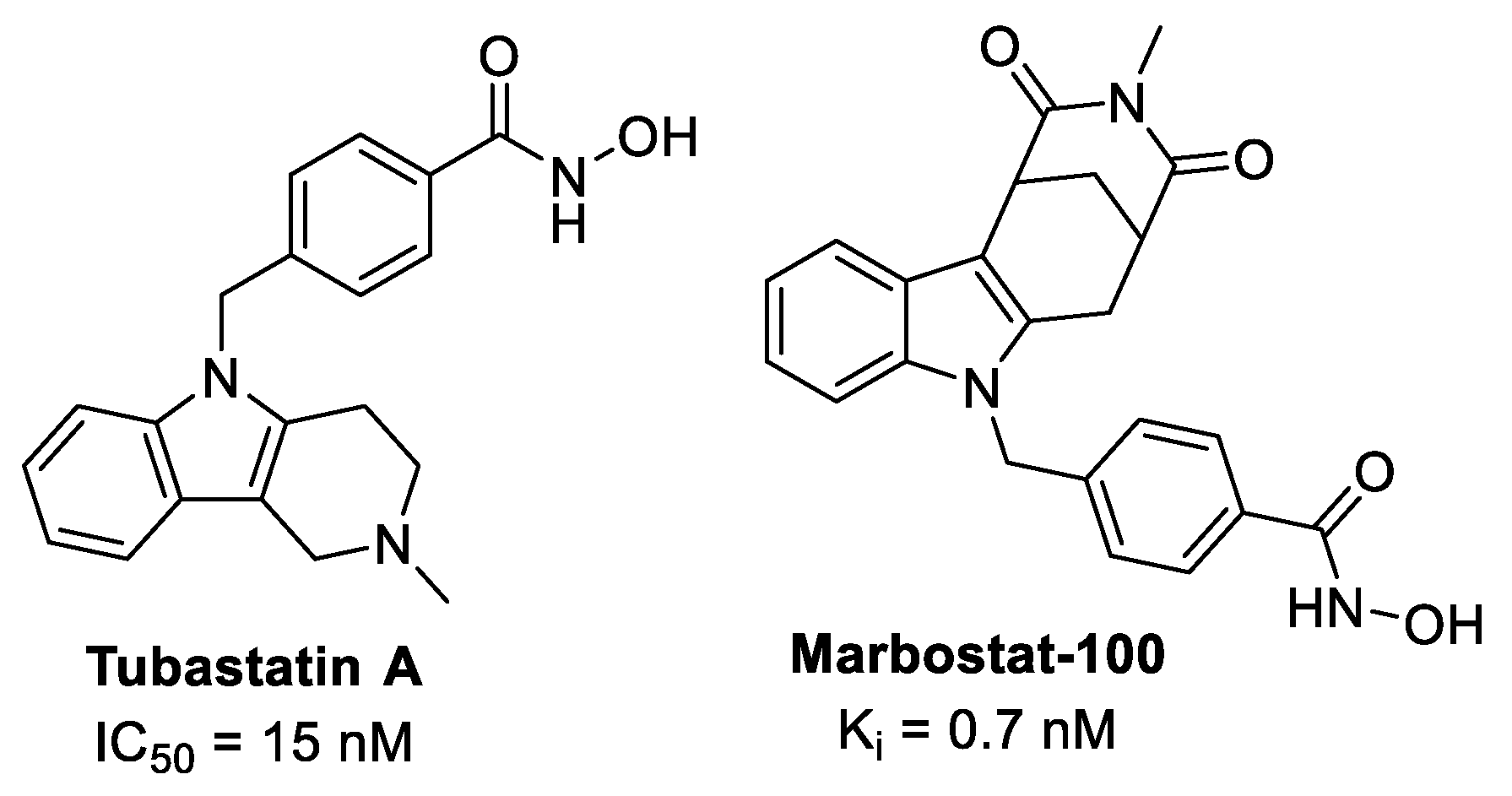
1. Introduction
2. Results and Discussion
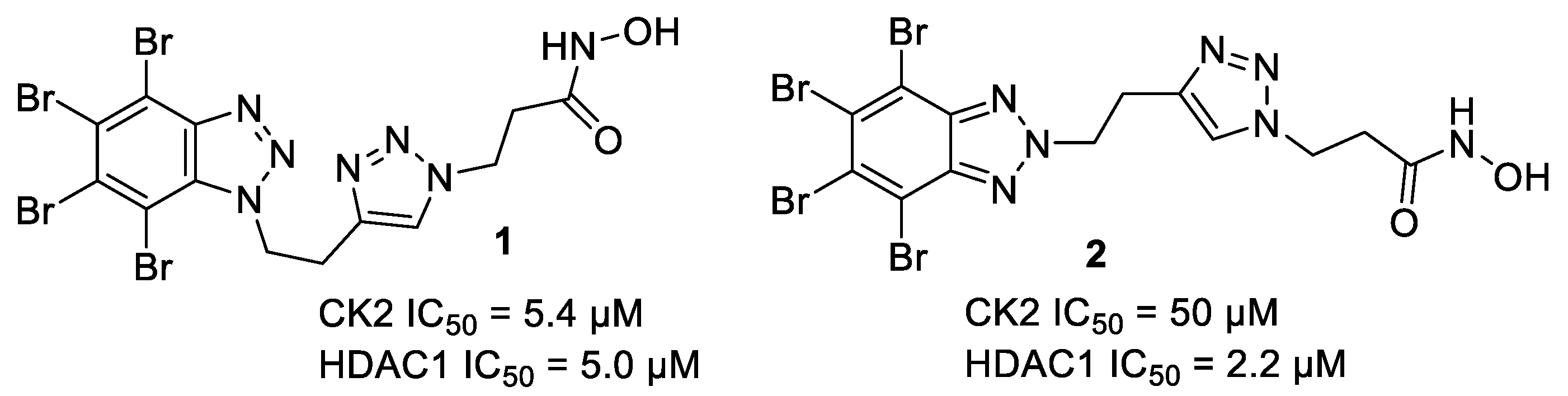
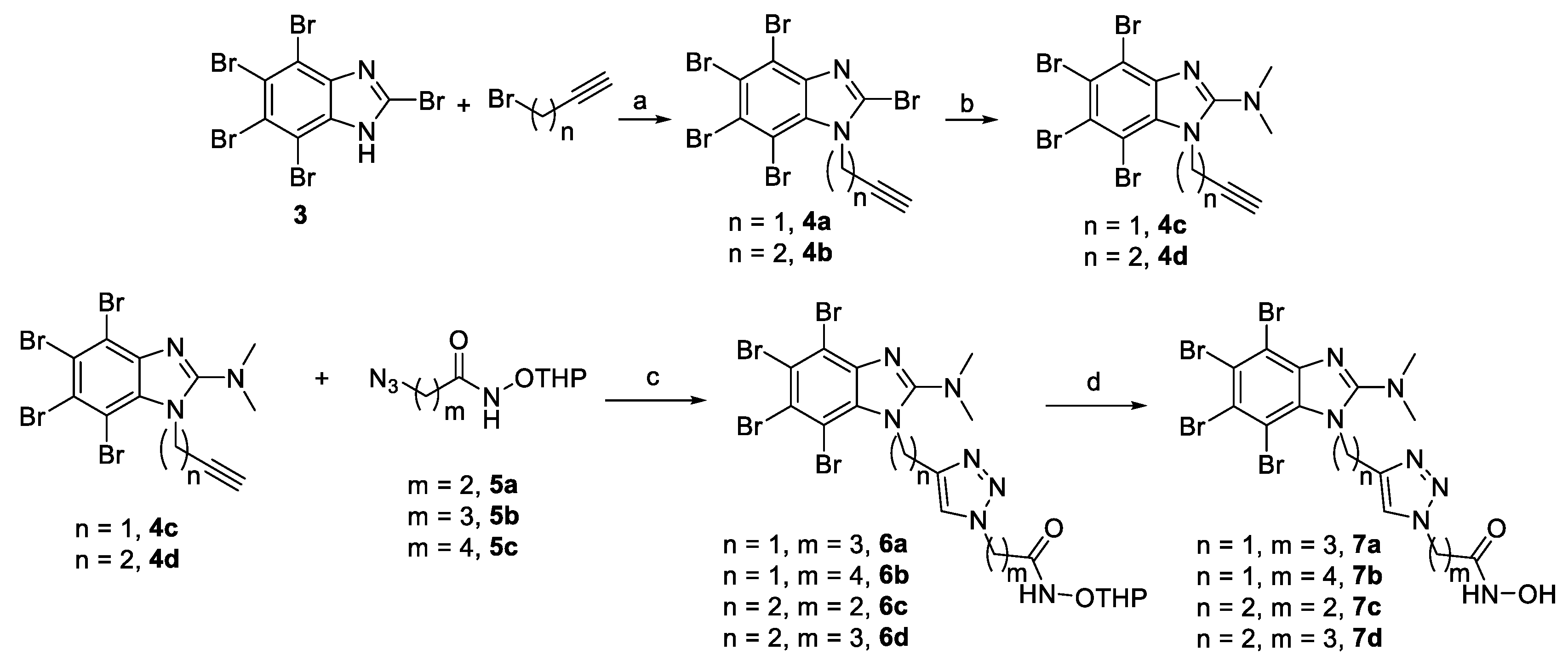
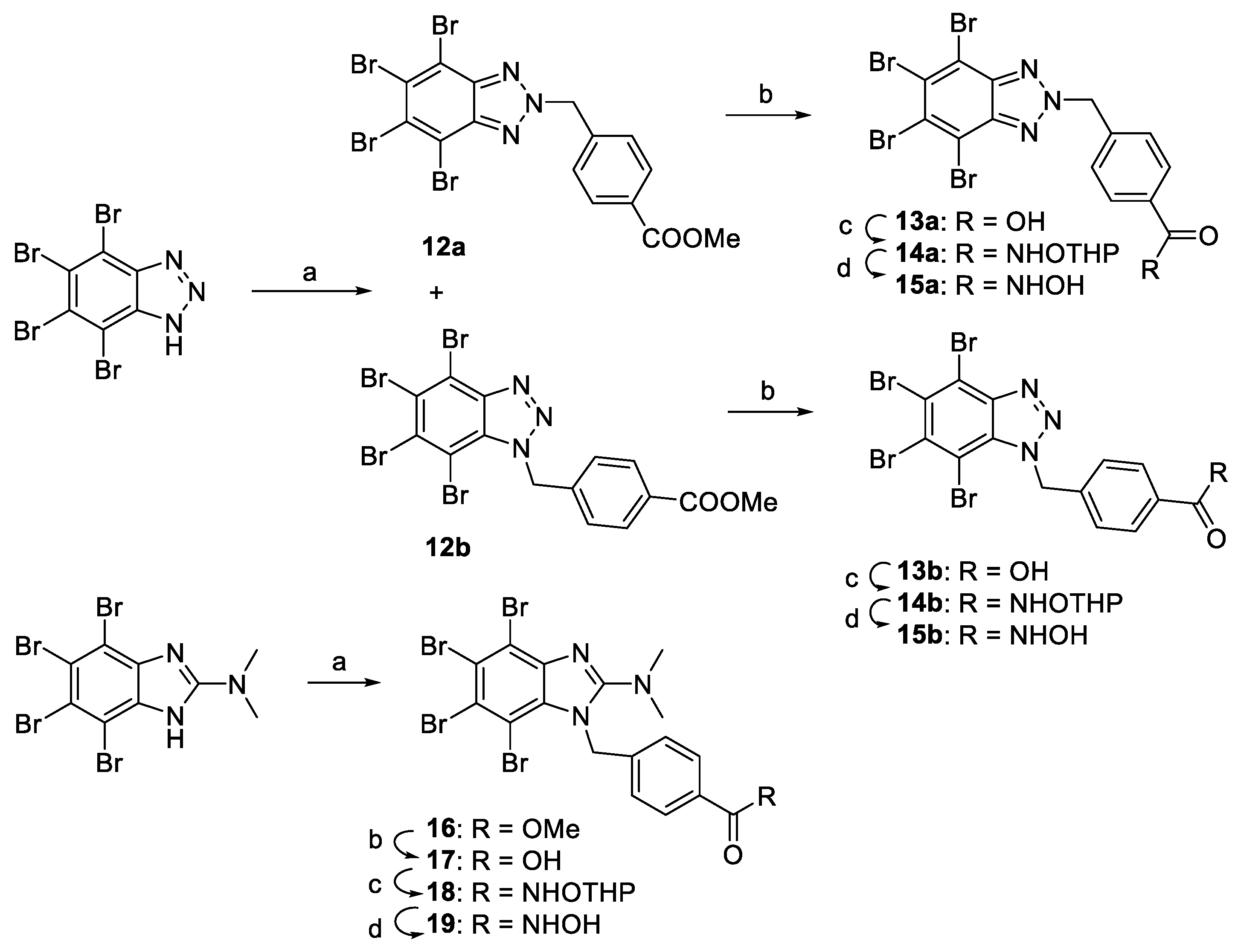
2.1. Chemistry
2.2. Enzymatic Inhibitory Evaluation
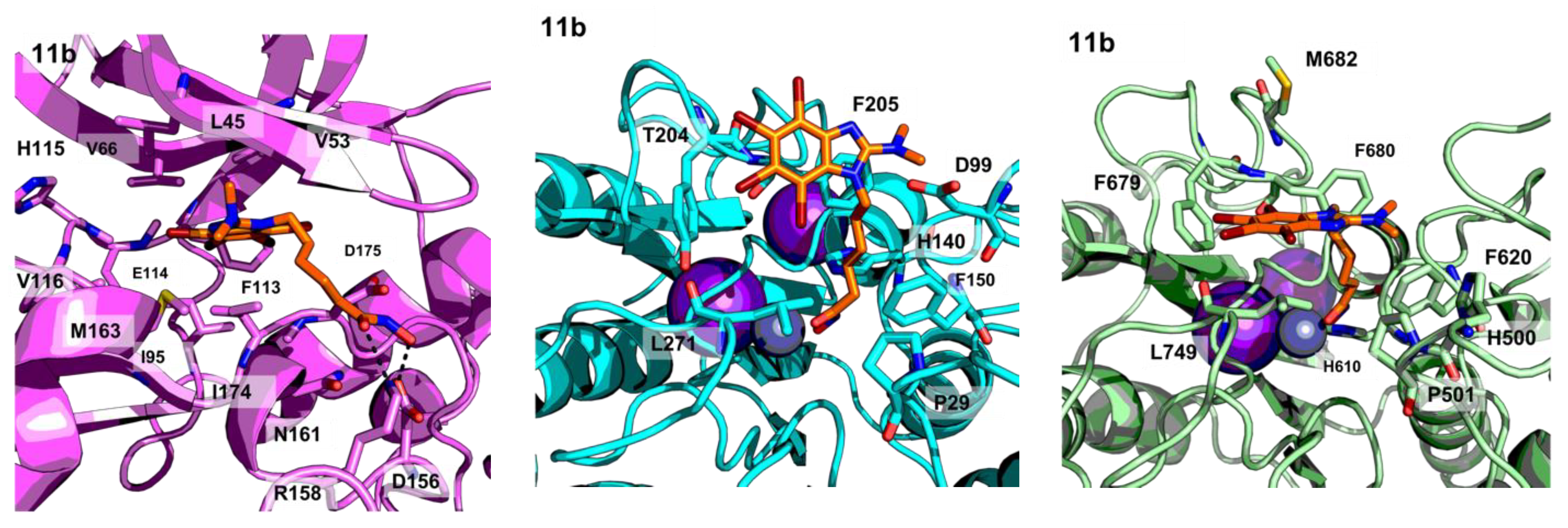
2.3. Molecular Modelling
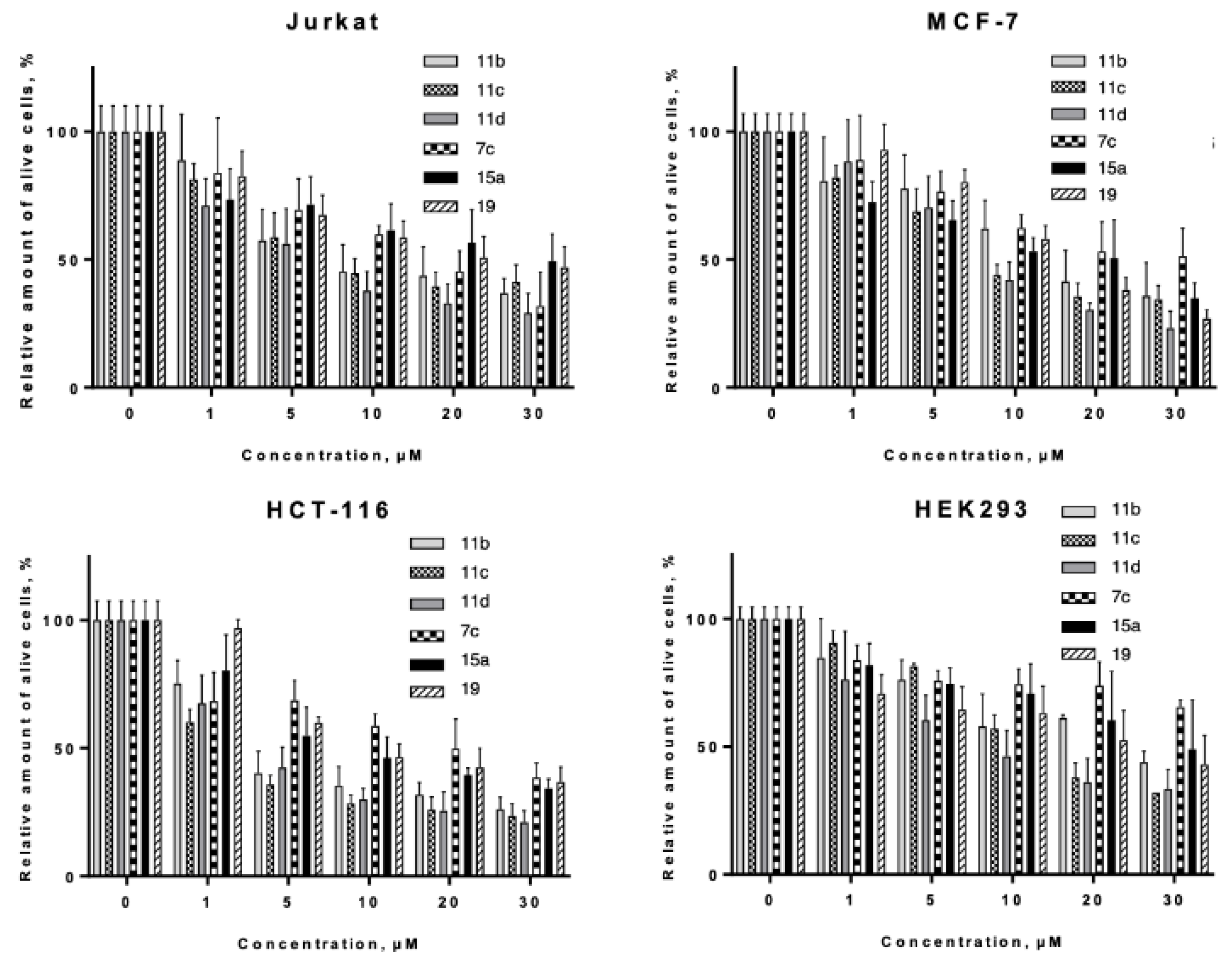
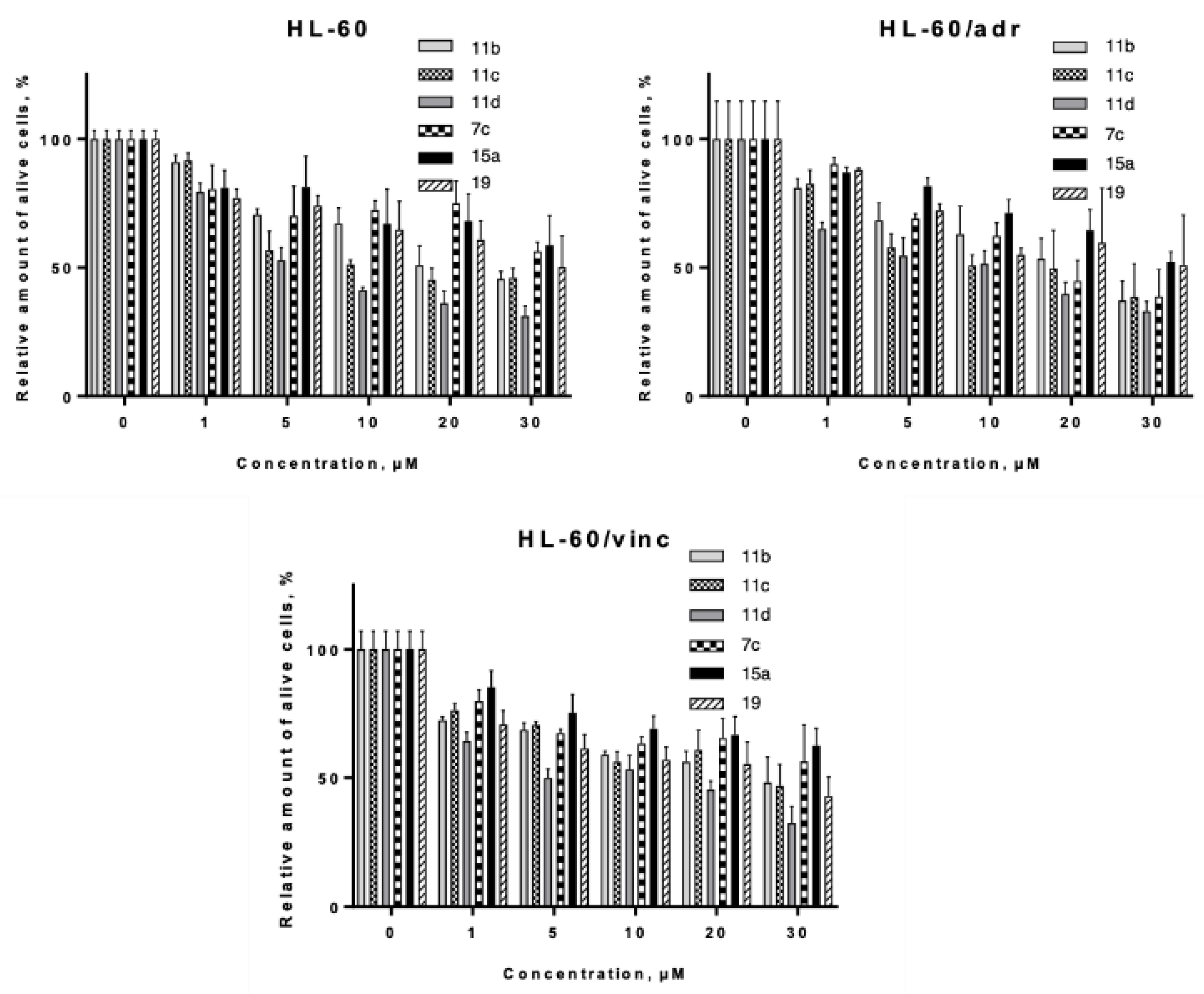
2.4. In Vitro Evaluation
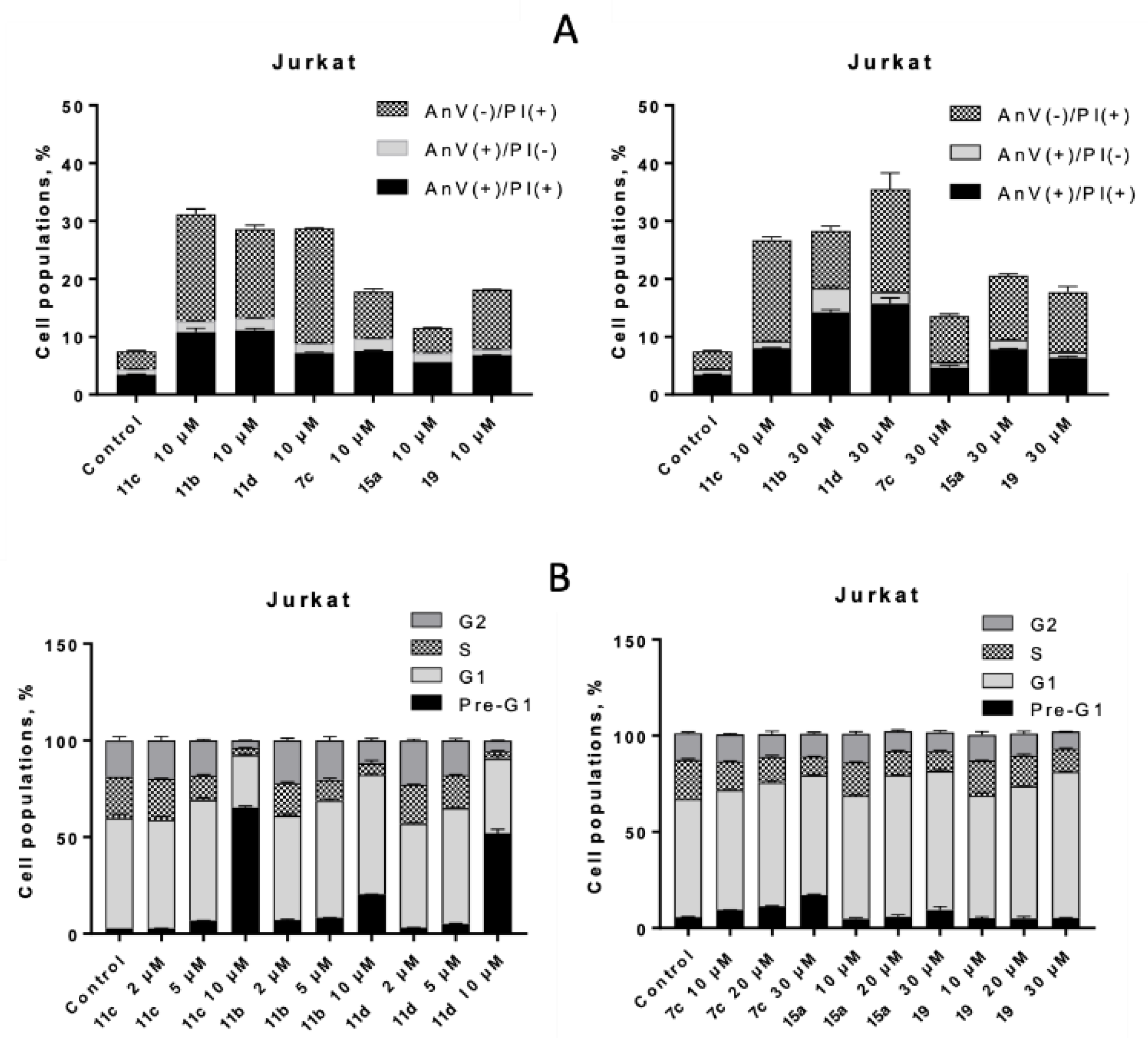
2.4.1. Proapoptotic Activity Against Tumor and Pseudonormal Cell Lines.
2.4.2. Impact of ROS on Circumvention of Cancer Drug Resistance
3. Materials and Methods
3.1. Chemistry
- 2,4,5,6,7-Pentabromo-1-(prop-2-yn-1-yl)-1H-benzo[d]imidazole (4a)
- 2,4,5,6,7-Pentabromo-1-(but-3-yn-1-yl)-1H-benzo[d]imidazole (4b)
- 4,5,6,7-tetrabromo-N,N-dimethyl-1-(prop-2-yn-1-yl)-1H-benzo[d]imidazol-2-amine (4c)
- 4,5,6,7-Tetrabromo-1-(but-3-yn-1-yl)-N,N-dimethyl-1H-benzo[d]imidazol-2-amine (4d)
- 4-(4-((4,5,6,7-Tetrabromo-2-(dimethylamino)-1H-benzo[d]imidazol-1-yl)methyl)-1H-1,2,3-triazol-1-yl)-N-((tetrahydro-2H-pyran-2-yl)oxy)butanamide (6a)
- 5-(4-((4,5,6,7-Tetrabromo-2-(dimethylamino)-1H-benzo[d]imidazol-1-yl)methyl)-1H-1,2,3-triazol-1-yl)-N-((tetrahydro-2H-pyran-2-yl)oxy)pentanamide (6b)
- 3-(4-(2-(4,5,6,7-Tetrabromo-2-(dimethylamino)-1H-benzo[d]imidazol-1-yl)ethyl)-1H-1,2,3-triazol-1-yl)-N-((tetrahydro-2H-pyran-2-yl)oxy)propanamide (6c)
- 4-(4-(2-(4,5,6,7-Tetrabromo-2-(dimethylamino)-1H-benzo[d]imidazol-1-yl)ethyl)-1H-1,2,3-triazol-1-yl)-N-((tetrahydro-2H-pyran-2-yl)oxy)butanamide (6d)
- N-Hydroxy-4-(4-((4,5,6,7-tetrabromo-2-(dimethylamino)-1H-benzo[d]imidazol-1-yl)methyl)-1H-1,2,3-triazol-1-yl)butanamide (7a)
- N-Hydroxy-5-(4-((4,5,6,7-tetrabromo-2-(dimethylamino)-1H-benzo[d]imidazol-1-yl)methyl)-1H-1,2,3-triazol-1-yl)pentanamide (7b)
- N-Hydroxy-3-(4-(2-(4,5,6,7-tetrabromo-2-(dimethylamino)-1H-benzo[d]imidazol-1-yl)ethyl)-1H-1,2,3-triazol-1-yl)propanamide (7c)
- N-Hydroxy-4-(4-(2-(4,5,6,7-tetrabromo-2-(dimethylamino)-1H-benzo[d]imidazol-1-yl)ethyl)-1H-1,2,3-triazol-1-yl)butanamide (7d)
- Methyl 5-(4,5,6,7-tetrabromo-2-(dimethylamino)-1H-benzo[d]imidazol-1-yl)pentanoate (8a).
- Ethyl 6-(4,5,6,7-tetrabromo-2-(dimethylamino)-1H-benzo[d]imidazol-1-yl)hexanoate (8b)
- Ethyl 7-(4,5,6,7-tetrabromo-2-(dimethylamino)-1H-benzo[d]imidazol-1-yl)heptanoate (8c)
- Methyl 8-(4,5,6,7-tetrabromo-2-(dimethylamino)-1H-benzo[d]imidazol-1-yl)octanoate (8d)
- 5-(4,5,6,7-Tetrabromo-2-(dimethylamino)-1H-benzo[d]imidazol-1-yl)pentanoic acid (9a)
- 6-(4,5,6,7-Tetrabromo-2-(dimethylamino)-1H-benzo[d]imidazol-1-yl)hexanoic acid (9b)
- 7-(4,5,6,7-Tetrabromo-2-(dimethylamino)-1H-benzo[d]imidazol-1-yl)heptanoic acid (9c)
- 8-(4,5,6,7-Tetrabromo-2-(dimethylamino)-1H-benzo[d]imidazol-1-yl)octanoic acid (9d)
- 5-(4,5,6,7-Tetrabromo-2-(dimethylamino)-1H-benzo[d]imidazol-1-yl)-N-((tetrahydro-2H-pyran-2-yl)oxy)pentanamide (10a)
- 6-(4,5,6,7-Tetrabromo-2-(dimethylamino)-1H-benzo[d]imidazol-1-yl)-N-((tetrahydro-2H-pyran-2-yl)oxy)hexanamide (10b)
- 7-(4,5,6,7-Tetrabromo-2-(dimethylamino)-1H-benzo[d]imidazol-1-yl)-N-((tetrahydro-2H-pyran-2-yl)oxy)heptanamide (10c)
- 8-(4,5,6,7-Tetrabromo-2-(dimethylamino)-1H-benzo[d]imidazol-1-yl)-N-((tetrahydro-2H-pyran-2-yl)oxy)octanamide (10d)
- N-Hydroxy-5-(4,5,6,7-tetrabromo-2-(dimethylamino)-1H-benzo[d]imidazol-1-yl)pentanamide (11a)
- N-Hydroxy-6-(4,5,6,7-tetrabromo-2-(dimethylamino)-1H-benzo[d]imidazol-1-yl)hexanamide (11b)
- N-Hydroxy-7-(4,5,6,7-tetrabromo-2-(dimethylamino)-1H-benzo[d]imidazol-1-yl)heptanamide (11c)
- N-Hydroxy-8-(4,5,6,7-tetrabromo-2-(dimethylamino)-1H-benzo[d]imidazol-1-yl)octanamide (11d)
- Methyl 4-((tetrabromo-2H-benzo[d][1,2,3]triazol-2-yl)methyl)benzoate (12a) and methyl 4-((tetrabromo-1H-benzo[d][1,2,3]triazol-1-yl)methyl)benzoate (12b)
- 4-((Tetrabromo-2H-benzo[d][1,2,3]triazol-2-yl)methyl)benzoic acid (13a)
- 4-((Tetrabromo-1H-benzo[d][1,2,3]triazol-1-yl)methyl)benzoic acid (13b)
- 4-((Tetrabromo-2H-benzo[d][1,2,3]triazol-2-yl)methyl)-N-((tetrahydro-2H-pyran-2-yl)oxy)benzamide (14a)
- 4-((Tetrabromo-1H-benzo[d][1,2,3]triazol-1-yl)methyl)-N-((tetrahydro-2H-pyran-2-yl)oxy)benzamide (14b)
- N-Hydroxy-4-((tetrabromo-2H-benzo[d][1,2,3]triazol-2-yl)methyl)benzamide (15a)
- N-Hydroxy-4-((tetrabromo-1H-benzo[d][1,2,3]triazol-1-yl)methyl)benzamide (15b)
- Methyl 4-((4,5,6,7-tetrabromo-2-(dimethylamino)-1H-benzo[d]imidazol-1-yl)methyl)benzoate (16)
- 4-((4,5,6,7-Tetrabromo-2-(dimethylamino)-1H-benzo[d]imidazol-1-yl)methyl)-N-((tetrahydro-2H-pyran-2-yl)oxy)benzamide (18)
- N-Hydroxy-4-((4,5,6,7-tetrabromo-2-(dimethylamino)-1H-benzo[d]imidazol-1-yl)methyl)benzamide (19)
3.2. Molecular Modelling
3.3. Biological Assays
3.4. Cytotoxicity Studies
3.5. Apoptosis Analysis
3.6. Cell Cycle Analysis
3.7. Studies of the Functional Status of Mitochondria
3.8. DCFDA and DHE Assays
3.9. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ATP | adenosine triphosphate |
| CK2 | Protein kinase 2 |
| BSA | Bovine Serum Albumin |
| CuAAC | Cu (I)-catalyzed alkyne azide cycloaddition |
| DCFDA | 2′,7′ –dichlorofluorescin diacetate |
| DCM | dichloromethane |
| DMAT | 2-dimethylamino-4,5,6,7-tetrabromo-1H-benzimidazole |
| DMF | dimethylformamide |
| DMSO | dimethyl sulfoxide |
| EDCI | 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide |
| EGFR | epidermal growth factor receptor |
| FACS | Fluorescence-activated cell sorting |
| FITC | Fluorescein isothiocyanate |
| HAT | histone acetyl transferase |
| HDAC | histone deacetylase |
| HER2 | human epidermal growth factor receptor 2 |
| HOBt | 1-hydroxybenzotriazole |
| JAK2 | Janus kinase 2 |
| LC50 | lethal concentration 50 |
| LMD | molecular dynamics |
| MDR | multi-drug resistance |
| MTD | multitarget drugs |
| MW | microwave |
| NMM | N-Methylmorpholine |
| OTHP | 2-tetrahydropyranyl |
| PI3K | osfoinositol 3-quinasa |
| RMSD | root-mean-square deviation |
| ROS | reactive oxygen species |
| RT | room temperature |
| SAR | structure activity relationship |
| SRM | surface recognition moiety |
| TBB | tetrabromobenzotriazole |
| TBI | tetrabromobenzimidazole |
| TBTA | Tris(benzyltriazolyl)methylamine |
| THF | Tetrahydrofuran |
| TsOH | p-Toluenesulfonic acid |
| ZBG | Zinc binding group |
References
- Sawyers, C.L. Mixing cocktails. Nature 2007, 449, 993–996. [Google Scholar] [CrossRef]
- Zimmermann, G.R.; Lehár, J.; Keith, C.T. Multi-target therapeutics: When the whole is greater than the sum of the parts. Drug Discov. Today 2007, 12, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Kwak, E.L.; Clark, J.W.; Chabner, B. Targeted Agents: The Rules of Combination. Clin. Cancer Res. 2007, 13, 5232–5237. [Google Scholar] [CrossRef] [PubMed]
- Simon, F. The trouble with making combination drugs. Nat. Rev. Drug Discov. 2006, 5, 881–882. [Google Scholar] [CrossRef] [PubMed]
- Morphy, R.; Rankovic, Z. Designed Multiple Ligands. An Emerging Drug Discovery Paradigm. J. Med. Chem. 2005, 48, 6523–6543. [Google Scholar] [CrossRef]
- Boyle, N.M.O.; Meegan, M.J. Designed Multiple Ligands for Cancer Therapy. Curr. Med. Chem. 2011, 18, 4722–4737. [Google Scholar] [CrossRef]
- Proschak, E.; Stark, H.; Merk, D. Polypharmacology by Design: A Medicinal Chemist’s Perspective on Multitargeting Compounds. J. Med. Chem. 2019, 62, 420–444. [Google Scholar] [CrossRef]
- Jonathan Fray, M.; Bish, G.; Brown, A.D.; Fish, P.V.; Stobie, A.; Wakenhut, F.; Whitlock, G.A. N-(1,2-Diphenylethyl)piperazines: A new class of dual serotonin/noradrenaline reuptake inhibitor. Bioorg. Med. Chem. Lett. 2006, 16, 4345–4348. [Google Scholar] [CrossRef]
- Neumeyer, J.L.; Peng, X.; Knapp, B.I.; Bidlack, J.M.; Lazarus, L.H.; Salvadori, S.; Trapella, C.; Balboni, G. New Opioid Designed Multiple Ligand from Dmt-Tic and Morphinan Pharmacophores. J. Med. Chem. 2006, 49, 5640–5643. [Google Scholar] [CrossRef][Green Version]
- Bornot, A.; Bauer, U.; Brown, A.; Firth, M.; Hellawell, C.; Engkvist, O. Systematic Exploration of Dual-Acting Modulators from a Combined Medicinal Chemistry and Biology Perspective. J. Med. Chem. 2013, 56, 1197–1210. [Google Scholar] [CrossRef]
- Costantino, L.; Barlocco, D. Designed Multiple Ligands: Basic Research vs. Clinical Outcomes. Curr. Med. Chem. 2012, 19, 3353–3387. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, A.; Bolognesi, M.L.; Minarini, A.; Rosini, M.; Tumiatti, V.; Recanatini, M.; Melchiorre, C. Multi-target-Directed Ligands To Combat Neurodegenerative Diseases. J. Med. Chem. 2008, 51, 347–372. [Google Scholar] [CrossRef] [PubMed]
- Bolognesi, M.L. Harnessing Polypharmacology with Medicinal Chemistry. ACS Med. Chem. Lett. 2019, 10, 273–275. [Google Scholar] [CrossRef] [PubMed]
- Lolli, G.; Pinna, L.A.; Battistutta, R. Structural Determinants of Protein Kinase CK2 Regulation by Autoinhibitory Polymerization. ACS Chem. Biol. 2012, 7, 1158–1163. [Google Scholar] [CrossRef]
- Litchfield, D.W. Protein kinase CK2: Structure, regulation and role in cellular decisions of life and death. Biochem. J. 2003, 369, 1–15. [Google Scholar] [CrossRef]
- Cozza, G.; Meggio, F.; Moro, S. The Dark Side of Protein Kinase CK2 Inhibition. Curr. Med. Chem. 2011, 18, 2867–2884. [Google Scholar] [CrossRef]
- St-Denis, N.A.; Litchfield, D.W. Protein Kinase CK2 in Health and Disease. Cell. Mol. Life Sci. 2009, 66, 1817–1829. [Google Scholar] [CrossRef]
- Ahmad, K.A.; Wang, G.; Unger, G.; Slaton, J.; Ahmed, K. Protein kinase CK2—A key suppressor of apoptosis. Adv. Enzym. Regul. 2008, 48, 179–187. [Google Scholar] [CrossRef]
- Guerra, B.; Issinger, O.G. Protein Kinase CK2 in Human Diseases. Curr. Med. Chem. 2008, 15, 1870–1886. [Google Scholar] [CrossRef]
- Chua, M.M.; Ortega, E.C.; Sheikh, A.; Lee, M.; Abdul-Rassoul, H.; Hartshorn, L.K.; Dominguez, I. CK2 in Cancer: Cellular and Biochemical Mechanisms and Potential Therapeutic Target. Pharmaceuticals 2017, 10, 18. [Google Scholar] [CrossRef]
- Unger, G.M.; Davis, A.T.; Ahmed, J.W.; Ahmed, K. Protein Kinase CK2 as Regulator of Cell Survival: Implications for Cancer Therapy. Curr. Cancer Drug Targets 2004, 4, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Cozza, G.; Moro, S. Kinase CK2 Inhibition: An Update. Curr. Med. Chem. 2013, 20, 671–693. [Google Scholar] [CrossRef] [PubMed]
- Cozza, G.; Girardi, C.; Ranchio, A.; Lolli, G.; Sarno, S.; Orzeszko, A.; Kazimierczuk, Z.; Battistutta, R.; Ruzzene, M.; Pinna, L.A. Cell-permeable dual inhibitors of protein kinases CK2 and PIM-1: Structural features and pharmacological potential. Cell. Mol. Life Sci. 2014, 71, 3173–3185. [Google Scholar] [CrossRef] [PubMed]
- Cozza, G. The Development of CK2 Inhibitors: From Traditional Pharmacology to in Silico Rational Drug Design. Pharmaceuticals 2017, 10, 26. [Google Scholar] [CrossRef]
- Battistutta, R. Protein Kinase CK2 in Health and Disease. Cell. Mol. Life Sci. 2009, 66, 1868–1889. [Google Scholar] [CrossRef]
- Sarno, S.; Moro, S.; Meggio, F.; Zagotto, G.; Dal Ben, D.; Ghisellini, P.; Battistutta, R.; Zanotti, G.; Pinna, L.A. Toward the rational design of protein kinase casein kinase-2 inhibitors. Pharmacol. Therapeut. 2002, 93, 159–168. [Google Scholar] [CrossRef]
- Raaf, J.; Guerra, B.; Neundorf, I.; Bopp, B.; Issinger, O.-G.; Jose, J.; Pietsch, M.; Niefind, K. First Structure of Protein Kinase CK2 Catalytic Subunit with an Effective CK2β-Competitive Ligand. ACS Chem. Biol. 2013, 8, 901–907. [Google Scholar] [CrossRef]
- Prudent, R.; Cochet, C. New Protein Kinase CK2 Inhibitors: Jumping out of the Catalytic Box. Chem. Biol. 2009, 16, 112–120. [Google Scholar] [CrossRef]
- Swider, R.; Masłyk, M.; Zapico, J.M.; Coderch, C.; Panchuk, R.; Skorokhyd, N.; Schnitzler, A.; Niefind, K.; de Pascual-Teresa, B.; Ramos, A. Synthesis, biological activity and structural study of new benzotriazole-based protein kinase CK2 inhibitors. RSC Adv. 2015, 5, 72482–72494. [Google Scholar] [CrossRef]
- Purwin, M.; Hernández-Toribio, J.; Coderch, C.; Panchuk, R.; Skorokhyd, N.; Filipiak, K.; de Pascual-Teresa, B.; Ramos, A. Design and synthesis of novel dual-target agents for HDAC1 and CK2 inhibition. RSC Adv. 2016, 6, 66595–66608. [Google Scholar] [CrossRef]
- Pastor, M.; Zapico, J.M.; Coderch, C.; Maslyk, M.; Panchuk, R.; de Pascual-Teresa, B.; Ramos, A. From a MMP2/CK2 multitarget approach to the identification of potent and selective MMP13 inhibitors. Org. Biomol. Chem. 2019, 17, 916–929. [Google Scholar] [CrossRef]
- Darden, T.; York, D.; Pedersen, L. Particle mesh Ewald: An N⋅log(N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]
- Sekiguchi, Y.; Nakaniwa, T.; Kinoshita, T.; Nakanishi, I.; Kitaura, K.; Hirasawa, A.; Tsujimoto, G.; Tada, T. Structural insight into human CK2alpha in complex with the potent inhibitor ellagic acid. Bioorgan. Med. Chem. Lett. 2009, 19, 2920–2923. [Google Scholar] [CrossRef] [PubMed]
- Pierre, F.; Chua, P.C.; O’Brien, S.E.; Siddiqui-Jain, A.; Bourbon, P.; Haddach, M.; Michaux, J.; Nagasawa, J.; Schwaebe, M.K.; Stefan, E.; et al. Discovery and SAR of 5-(3-Chlorophenylamino)benzo[c][2,6]naphthyridine-8-carboxylic Acid (CX-4945), the First Clinical Stage Inhibitor of Protein Kinase CK2 for the Treatment of Cancer. J. Med. Chem. 2011, 54, 635–654. [Google Scholar] [CrossRef] [PubMed]
- Battistutta, R.; Cozza, G.; Pierre, F.; Papinutto, E.; Lolli, G.; Sarno, S.; O’Brien, S.E.; Siddiqui-Jain, A.; Haddach, M.; Anderes, K.; et al. Unprecedented Selectivity and Structural Determinants of a New Class of Protein Kinase CK2 Inhibitors in Clinical Trials for the Treatment of Cancer. Biochemistry 2011, 50, 8478–8488. [Google Scholar] [CrossRef] [PubMed]
- Witt, O.; Deubzer, H.E.; Milde, T.; Oehme, I. HDAC family: What are the cancer relevant targets? Cancer Lett. 2009, 277, 8–21. [Google Scholar] [CrossRef]
- Marks, P.A.; Rifkind, R.A.; Richon, V.M.; Breslow, R.; Miller, T.; Kelly, W.K. Histone deacetylases and cancer: Causes and therapies. Nat. Rev. Cancer 2001, 1, 194–202. [Google Scholar] [CrossRef]
- Gräff, J.; Tsai, L.-H. The Potential of HDAC Inhibitors as Cognitive Enhancers. Annu. Rev. Pharmacol. 2013, 53, 311–330. [Google Scholar] [CrossRef]
- Andrews, K.T.; Haque, A.; Jones, M.K. HDAC inhibitors in parasitic diseases. Immunol. Cell Biol. 2012, 90, 66–77. [Google Scholar] [CrossRef]
- Rotili, D.; Simonetti, G.; Savarino, A.; Palamara, A.T.; Migliaccio, A.R.; Mai, A. Non-Cancer Uses of Histone Deacetylase Inhibitors: Effects on Infectious Diseases and β-Hemoglobinopathies+. Curr. Top. Med. Chem. 2009, 9, 272–291. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Hanson, M.A.; Buklijas, T.; Low, F.M.; Beedle, A.S. Epigenetic mechanisms that underpin metabolic and cardiovascular diseases. Nat. Rev. Endocrinol. 2009, 5, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Gregoretti, I.; Lee, Y.-M.; Goodson, H.V. Molecular Evolution of the Histone Deacetylase Family: Functional Implications of Phylogenetic Analysis. J. Mol. Biol. 2004, 338, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Haberland, M.; Montgomery, R.L.; Olson, E.N. The many roles of histone deacetylases in development and physiology: Implications for disease and therapy. Nat. Rev. Genet. 2009, 10, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Lehrmann, H.; Pritchard, L.L.; Harel-Bellan, A. Histone acetyltransferases and deacetylases in the control of cell proliferation and differentiation. Adv. Cancer Res. 2002, 86, 41–65. [Google Scholar]
- Lane, A.A.; Chabner, B.A. Histone Deacetylase Inhibitors in Cancer Therapy. J. Clin. Oncol. 2009, 27, 5459–5468. [Google Scholar] [CrossRef]
- Minucci, S.; Pelicci, P.G. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat. Rev. Cancer 2006, 6, 38–51. [Google Scholar] [CrossRef]
- Pontiki, E.; Hadjipavlou-Litina, D. Histone deacetylase inhibitors (HDACIs). Structure—Activity relationships: History and new QSAR perspectives. Med. Res. Rev. 2012, 32, 1–165. [Google Scholar] [CrossRef]
- Paris, M.; Porcelloni, M.; Binaschi, M.; Fattori, D. Histone Deacetylase Inhibitors: From Bench to Clinic. J. Med. Chem. 2008, 51, 1505–1529. [Google Scholar] [CrossRef]
- Schobert, R.; Biersack, B. Multimodal HDAC Inhibitors with Improved Anticancer Activity. Curr. Cancer Drug Targets 2018, 18, 39–56. [Google Scholar] [CrossRef]
- Hesham, H.; Lasheen, D.; Abouzid, K. Chimeric HDAC inhibitors: Comprehensive review on the HDAC-based strategies developed to combat cancer. Med. Res. Rev. 2018, 38, 2058–2109. [Google Scholar] [CrossRef]
- Tang, C.; Li, C.; Zhang, S.; Hu, Z.; Wu, J.; Dong, C.; Huang, J.; Zhou, H.-B. Novel Bioactive Hybrid Compound Dual Targeting Estrogen Receptor and Histone Deacetylase for the Treatment of Breast Cancer. J. Med. Chem. 2015, 58, 4550–4572. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Li, J.; Inks, E.S.; Chou, C.J.; Jia, Y.; Chu, X.; Li, X.; Xu, W.; Zhang, Y. Design, Synthesis, and Antitumor Evaluation of Novel Histone Deacetylase Inhibitors Equipped with a Phenylsulfonylfuroxan Module as a Nitric Oxide Donor. J. Med. Chem. 2015, 58, 4325–4338. [Google Scholar] [CrossRef] [PubMed]
- Ai, T.; Cui, H.; Chen, L. Multi-Targeted Histone Deacetylase Inhibitors in Cancer Therapy. Curr. Med. Chem. 2012, 19, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.-Y. Multi-targeted hybrids based on HDAC inhibitors for anti-cancer drug discovery. Arch. Pharm. Res. 2012, 35, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Guerrant, W.; Patil, V.; Canzoneri, J.C.; Oyelere, A.K. Dual Targeting of Histone Deacetylase and Topoisomerase II with Novel Bifunctional Inhibitors. J. Med. Chem. 2012, 55, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Petrelli, R.; Gao, G.; Wilson, D.J.; McLean, G.T.; Jayaram, H.N.; Sham, Y.Y.; Pankiewicz, K.W. Dual inhibitors of inosine monophosphate dehydrogenase and histone deacetylase based on a cinnamic hydroxamic acid core structure. Bioorgan. Med. Chem. 2010, 18, 5950–5964. [Google Scholar] [CrossRef]
- Tavera-Mendoza, L.E.; Quach, T.D.; Dabbas, B.; Hudon, J.; Liao, X.; Palijan, A.; Gleason, J.L.; White, J.H. Incorporation of histone deacetylase inhibition into the structure of a nuclear receptor agonist. Proc. Nat. Acad. Sci. USA 2008, 105, 8250–8255. [Google Scholar] [CrossRef]
- Nishiguchi, G.A.; Rico, A.; Tanner, H.; Aversa, R.J.; Taft, B.R.; Subramanian, S.; Setti, L.; Burger, M.T.; Wan, L.; Tamez, V.; et al. Design and Discovery of N-(2-Methyl-5′-morpholino-6′-((tetrahydro-2H-pyran-4-yl)oxy)-[3′,-bipyridin]-5-yl)-3-(trifluoromethyl)benzamide (RAF709): A Potent, Selective, and Efficacious RAF Inhibitor Targeting RAS Mutant Cancers. J. Med. Chem. 2017, 60, 4869–4881. [Google Scholar] [CrossRef]
- Ling, Y.; Liu, J.; Qian, J.; Meng, C.; Guo, J.; Gao, W.; Xiong, B.; Ling, C.; Zhang, Y. Recent Advances in Multi-target Drugs Targeting Protein Kinases and Histone Deacetylases in Cancer Therapy. Curr. Med. Chem. 2020, 27, 1–24. [Google Scholar] [CrossRef]
- Chu-Farseeva, Y.Y.; Mustafa, N.; Poulsen, A.; Tan, E.C.; Yen, J.J.Y.; Chng, W.J.; Dymock, B.W. Design and synthesis of potent dual inhibitors of JAK2 and HDAC based on fusing the pharmacophores of XL019 and vorinostat. Eur. J. Med. Chem. 2018, 158, 593–619. [Google Scholar] [CrossRef]
- Luan, Y.; Li, J.; Bernatchez, J.A.; Li, R. Kinase and Histone Deacetylase Hybrid Inhibitors for Cancer Therapy. J. Med. Chem. 2018, 62, 3171–3183. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Zhai, H.-X.; Wang, J.; Forrester, J.; Qu, H.; Yin, L.; Lai, C.-J.; Bao, R.; Qian, C. Discovery of 7-(4-(3-Ethynylphenylamino)-7-methoxyquinazolin-6-yloxy)-N-hydroxyheptanamide (CUDC-101) as a Potent Multi-Acting HDAC, EGFR, and HER2 Inhibitor for the Treatment of Cancer. J. Med. Chem. 2010, 53, 2000–2009. [Google Scholar] [CrossRef] [PubMed]
- Mahboobi, S.; Dove, S.; Sellmer, A.; Winkler, M.; Eichhorn, E.; Pongratz, H.; Ciossek, T.; Baer, T.; Maier, T.; Beckers, T. Design of Chimeric Histone Deacetylase- and Tyrosine Kinase-Inhibitors: A Series of Imatinib Hybrides as Potent Inhibitors of Wild-Type and Mutant BCR-ABL, PDGF-Rβ, and Histone Deacetylases. J. Med. Chem. 2009, 52, 2265–2279. [Google Scholar] [CrossRef] [PubMed]
- Khan, D.H.; He, S.; Yu, J.; Winter, S.; Cao, W.; Seiser, C.; Davie, J.R. Protein kinase CK2 regulates the dimerization of histone deacetylase 1 (HDAC1) and HDAC2 during mitosis. J. Biol. Chem. 2013, 288, 16518–16528. [Google Scholar] [CrossRef]
- Pluemsampant, S.; Safronova, O.S.; Nakahama, K.-I.; Morita, I. Protein kinase CK2 is a key activator of histone deacetylase in hypoxia-associated tumors. Int. J. Cancer 2008, 122, 333–341. [Google Scholar] [CrossRef]
- Thirumurugan, P.; Matosiuk, D.; Jozwiak, K. Click Chemistry for Drug Development and Diverse Chemical–Biology Applications. Chem. Rev. 2013, 113, 4905–4979. [Google Scholar] [CrossRef]
- Andrzejewska, M.; Pagano, M.A.; Meggio, F.; Brunati, A.M.; Kazimierczuk, Z. Polyhalogenobenzimida-zoles: Synthesis and Their inhibitory activity against casein kinases. Bioorgan. Med. Chem. 2003, 11, 3997–4002. [Google Scholar] [CrossRef]
- Świder, R.; Masłyk, M.; Martín-Santamaría, S.; Ramos, A.; de Pascual-Teresa, B. Multisite-directed inhibitors of protein kinase CK2: New challenges. Mol. Cell. Biochem. 2011, 356, 117–119. [Google Scholar] [CrossRef]
- Porter, N.J.; Osko, J.D.; Diedrich, D.; Kurz, T.; Hooker, J.M.; Hansen, F.K.; Christianson, D.W. Histone Deacetylase 6-Selective Inhibitors and the Influence of Capping Groups on Hydroxamate-Zinc Denticity. J. Med. Chem. 2018, 61, 8054–8060. [Google Scholar] [CrossRef]
- Yuan, Y.-G.; Peng, Q.-L.; Gurunathan, S. Combination of palladium nanoparticles and tubastatin-A potentiates apoptosis in human breast cancer cells: A novel therapeutic approach for cancer. Int. J. Nanomed. 2017, 12, 6503–6520. [Google Scholar] [CrossRef]
- Sellmer, A.; Stangl, H.; Beyer, M.; Grünstein, E.; Leonhardt, M.; Pongratz, H.; Eichhorn, E.; Elz, S.; Striegl, B.; Jenei-Lanzl, Z.; et al. Marbostat-100 Defines a New Class of Potent and Selective Antiinflammatory and Antirheumatic Histone Deacetylase 6 Inhibitors. J. Med. Chem. 2018, 61, 3454–3477. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Dong, G.; Li, H.; Liu, N.; Zhang, W.; Sheng, C. Discovery of Janus Kinase 2 (JAK2) and Histone Deacetylase (HDAC) Dual Inhibitors as a Novel Strategy for the Combinational Treatment of Leukemia and Invasive Fungal Infections. J. Med. Chem. 2018, 61, 6056–6074. [Google Scholar] [CrossRef] [PubMed]
- Wiley, R.H.; Hussung, K.F. Halogenated benzotriazoles. J. Am. Chem. Soc. 1957, 79, 4395–4400. [Google Scholar] [CrossRef]
- Pagano, M.A.; Andrzejewska, M.; Ruzzene, M.; Sarno, S.; Cesaro, L.; Bain, J.; Elliott, M.; Meggio, F.; Kazimierczuk, Z.; Pinna, L.A. Optimization of Protein Kinase CK2 Inhibitors Derived from 4,5,6,7-Tetrabromobenzimidazole. J. Med. Chem. 2004, 47, 6239–6247. [Google Scholar] [CrossRef] [PubMed]
- Battistutta, R.; De Moliner, E.; Sarno, S.; Zanotti, G.; Pinna, L.A. Structural features underlying selective inhibition of protein kinase CK2 by ATP site-directed tetrabromo-2-benzotriazole. Protein Sci. 2001, 10, 2200–2206. [Google Scholar] [CrossRef]
- Battistutta, R.; Mazzorana, M.; Cendron, L.; Bortolato, A.; Sarno, S.; Kazimierczuk, Z.; Zanotti, G.; Moro, S.; Pinna, L.A. The ATP-Binding Site of Protein Kinase CK2 Holds a Positive Electrostatic Area and Conserved Water Molecules. Chembiochem 2007, 8, 1804–1809. [Google Scholar] [CrossRef]
- Battistutta, R.; Mazzorana, M.; Sarno, S.; Kazimierczuk, Z.; Zanotti, G.; Pinna, L.A. Inspecting the structure-activity relationship of protein kinase CK2 inhibitors derived from tetrabromo-benzimidazole. Chem. Biol. 2005, 12, 1211–1219. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Bernhart, E.; Stuendl, N.; Kaltenegger, H.; Windpassinger, C.; Donohue, N.; Leithner, A.; Lohberger, B. Histone deacetylase inhibitors vorinostat and panobinostat induce G1 cell cycle arrest and apoptosis in multidrug resistant sarcoma cell lines. Oncotarget 2017, 8, 77254–77267. [Google Scholar] [CrossRef]
- Garraway, L.A.; Jänne, P.A. Circumventing Cancer Drug Resistance in the Era of Personalized Medicine. Cancer Discov. 2012, 2, 214. [Google Scholar] [CrossRef]
- Borgo, C.; Ruzzene, M. Role of protein kinase CK2 in antitumor drug resistance. J. Exp. Clin. Canc. Res. 2019, 38, 287. [Google Scholar] [CrossRef] [PubMed]
- Pizzi, M.; Piazza, F.; Agostinelli, C.; Fuligni, F.; Benvenuti, P.; Mandato, E.; Casellato, A.; Rugge, M.; Semenzato, G.; Pileri, S.A. Protein kinase CK2 is widely expressed in follicular, Burkitt and diffuse large B-cell lymphomas and propels malignant B-cell growth. Oncotarget 2015, 6, 6544–6552. [Google Scholar] [CrossRef]
- Korynevska, A.; Heffeter, P.; Matselyukh, B.; Elbling, L.; Micksche, M.; Stoika, R.; Berger, W. Mechanisms underlying the anticancer activities of the angucycline landomycin E. Biochem. Pharmacol. 2007, 74, 1713–1726. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ojeda, F.J.; Gomez-Llorente, C.; Aguilera, C.M.; Gil, A.; Rupérez, A.I. Impact of 3-Amino-1,2,4-Triazole (3-AT)-Derived Increase in Hydrogen Peroxide Levels on Inflammation and Metabolism in Human Differentiated Adipocytes. PLoS ONE 2016, 11, e0152550. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.J.; Millard, C.J.; Riley, A.M.; Robertson, N.S.; Wright, L.C.; Godage, H.Y.; Cowley, S.M.; Jamieson, A.G.; Potter, B.V.L.; Schwabe, J.W.R. Insights into the activation mechanism of class I HDAC complexes by inositol phosphates. Nat. Commun. 2016, 7, 11262. [Google Scholar] [CrossRef]
- Hai, Y.; Christianson, D.W. Histone deacetylase 6 structure and molecular basis of catalysis and inhibition. Nat. Chem. Biol. 2016, 12, 741. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, X.; Wu, Y.-D.; Wiest, O. Inhibition and Mechanism of HDAC8 Revisited. J. Am. Chem. Soc. 2014, 136, 11636–11643. [Google Scholar] [CrossRef]
- Sastry, G.M.; Adzhigirey, M.; Day, T.; Annabhimoju, R.; Sherman, W. Protein and ligand preparation: Parameters, protocols, and influence on virtual screening enrichments. J. Comput. Aid. Mol. Des. 2013, 27, 221–234. [Google Scholar] [CrossRef]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra Precision Glide: Docking and Scoring Incorporating a Model of Hydrophobic Enclosure for Protein−Ligand Complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef]
- Case, D.A.; Betz, R.M.; Cerutti, D.S.; Cheatham, T.E., III; Darden, T.A.; Duke, R.E.; Giese, T.J.; Gohlke, H.; Goetz, A.W.; Homeyer, N.; et al. AMBER 2016; University of California: San Francisco, CA, USA, 2016. [Google Scholar]
- Li, P.; Merz, K.M. MCPB.py: A Python Based Metal Center Parameter Builder. J. Chem. Inf. Model. 2016, 56, 599–604. [Google Scholar] [CrossRef]
- Torras, J.; Maccarrone, M.; Dainese, E. Molecular dynamics study on the Apo- and Holo-forms of 5-lipoxygenase. Biotechnol. Appl. Bioc. 2018, 65, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Maier, J.A.; Martinez, C.; Kasavajhala, K.; Wickstrom, L.; Hauser, K.E.; Simmerling, C. ff14SB: Improving the Accuracy of Protein Side Chain and Backbone Parameters from ff99SB. J. Chem. Theory Comput. 2015, 11, 3696–3713. [Google Scholar] [CrossRef] [PubMed]
- Yoneya, M.; Berendsen, H.J.C.; Hirasawa, K. A Non-Iterative Matrix Method for Constraint Molecular Dynamics Simulations. Mol. Simulat. 1994, 13, 395–405. [Google Scholar] [CrossRef]
- Cheatham, T.E., III; Miller, J.L.; Fox, T.; Darden, T.A.; Kollman, P.A. Molecular Dynamics Simulations on Solvated Biomolecular Systems: The Particle Mesh Ewald Method Leads to Stable Trajectories of DNA, RNA, and Proteins. J. Am. Chem. Soc. 1995, 117, 4193–4194. [Google Scholar] [CrossRef]
- Roe, D.R.; Cheatham, T.E. PTRAJ and CPPTRAJ: Software for Processing and Analysis of Molecular Dynamics Trajectory Data. J. Chem Theory Comput. 2013, 9, 3084–3095. [Google Scholar] [CrossRef]
- Walker, P.R.; Kwast-Welfeld, J.; Gourdeau, H.; Leblanc, J.; Neugebauer, W.; Sikorska, M. Relationship between Apoptosis and the Cell Cycle in Lymphocytes: Roles of Protein Kinase C, Tyrosine Phosphorylation, and AP1. Exp. Cell Res. 1993, 207, 142–151. [Google Scholar] [CrossRef]



| Compound | HDAC1 | HDAC6 | CK2 |
|---|---|---|---|
| 7a | 1.43 | - | 85.1 |
| 7b | 2.25 | 2.41 | 50.1 |
| 7c | 13.3 | 30.7 | 12.5 |
| 7d | 10.2 | - | 53.8 |
| 11a | 62.0 | - | 34.2 |
| 11b | 1.46 | 0.66 | 3.67 |
| 11c | 1.77 | 1.13 | 16.6 |
| 11d | 13.7 | 8.98 | 5.89 |
| 15a | 8.50 | 11.0 | 15.0 |
| 15b | 4.22 | 2.70 | 136 |
| 19 | 9.45 | 20.6 | 7.15 |
| Comp | LC50 Values of Compounds for Cell Line, µM (M ± SD) | ||||||
|---|---|---|---|---|---|---|---|
| Jurkat | MCF-7 | HCT-116 | HEK293 | HL-60 | HL-60/adr | HL-60/vinc | |
| 11b | 10.15 ± 1.43 | 15.66 ± 2.54 | 4.22 ± 0.46 | 23.33 ± 4.38 | 7.67 ± 1.17 | 16.23 ± 1.68 | 16.79 ± 3.34 |
| 11c | 9.47 ± 1.10 | 9.97 ± 0.99 | 1.90 ± 0.24 | 13.61 ± 0.80 | 6.59 ± 0.96 | 10.63 ± 1.53 | 12.06 ± 3.27 |
| 11d | 5.30 ± 0.75 | 9.02 ± 0.90 | 3.10 ± 0.37 | 8.41 ± 1.26 | 4.69 ± 0.54 | 8.4 ± 1.21 | 2.32 ± 1.06 |
| 7c | 16.54 ± 2.33 | 27.75 ± 6.06 | 16.87 ± 3.90 | 42.71 ± 8.65 | 24.02 ± 8.66 | 18.58 ± 2.09 | 49.46 ± 9.47 |
| 15a | 43.58 ± 16.73 | 13.66 ± 2.67 | 8.67 ± 1.02 | 33.73 ± 7.52 | 34.42 ± 7.89 | 32.37 ± 3.44 | 36.55 ± 4.74 |
| 19 | 24.86 ± 3.80 | 13.55 ± 0.78 | 11.3 ± 1.01 | 33.36 ± 11.76 | 46.49 ± 17.31 | 32.31 ± 5.32 | 21.73 ± 3.39 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez, R.; Di Geronimo, B.; Pastor, M.; Zapico, J.M.; Coderch, C.; Panchuk, R.; Skorokhyd, N.; Maslyk, M.; Ramos, A.; de Pascual-Teresa, B. Multitarget Anticancer Agents Based on Histone Deacetylase and Protein Kinase CK2 Inhibitors. Molecules 2020, 25, 1497. https://doi.org/10.3390/molecules25071497
Martínez R, Di Geronimo B, Pastor M, Zapico JM, Coderch C, Panchuk R, Skorokhyd N, Maslyk M, Ramos A, de Pascual-Teresa B. Multitarget Anticancer Agents Based on Histone Deacetylase and Protein Kinase CK2 Inhibitors. Molecules. 2020; 25(7):1497. https://doi.org/10.3390/molecules25071497
Chicago/Turabian StyleMartínez, Regina, Bruno Di Geronimo, Miryam Pastor, José María Zapico, Claire Coderch, Rostyslav Panchuk, Nadia Skorokhyd, Maciej Maslyk, Ana Ramos, and Beatriz de Pascual-Teresa. 2020. "Multitarget Anticancer Agents Based on Histone Deacetylase and Protein Kinase CK2 Inhibitors" Molecules 25, no. 7: 1497. https://doi.org/10.3390/molecules25071497
APA StyleMartínez, R., Di Geronimo, B., Pastor, M., Zapico, J. M., Coderch, C., Panchuk, R., Skorokhyd, N., Maslyk, M., Ramos, A., & de Pascual-Teresa, B. (2020). Multitarget Anticancer Agents Based on Histone Deacetylase and Protein Kinase CK2 Inhibitors. Molecules, 25(7), 1497. https://doi.org/10.3390/molecules25071497