The Effect of Whey Protein Supplementation on Myofibrillar Protein Synthesis and Performance Recovery in Resistance-Trained Men
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statement and Study Design
2.2. Participants
2.3. Pre-study Dietary Assessment, Control and Supplementation
2.4. Study Protocol
2.5. Resistance Training Protocol
2.6. Muscle Recovery Parameters
2.6.1. Isometric Squat Force
2.6.2. Countermovement Jump Height
2.6.3. Serum Creatine Kinase
2.6.4. Muscle Soreness
2.7. Muscle Analysis
2.7.1. Myofibrillar Protein Synthesis
2.7.2. Muscle RNA
2.8. Statistical Analysis
3. Results
3.1. Participants and Dietary Compliance
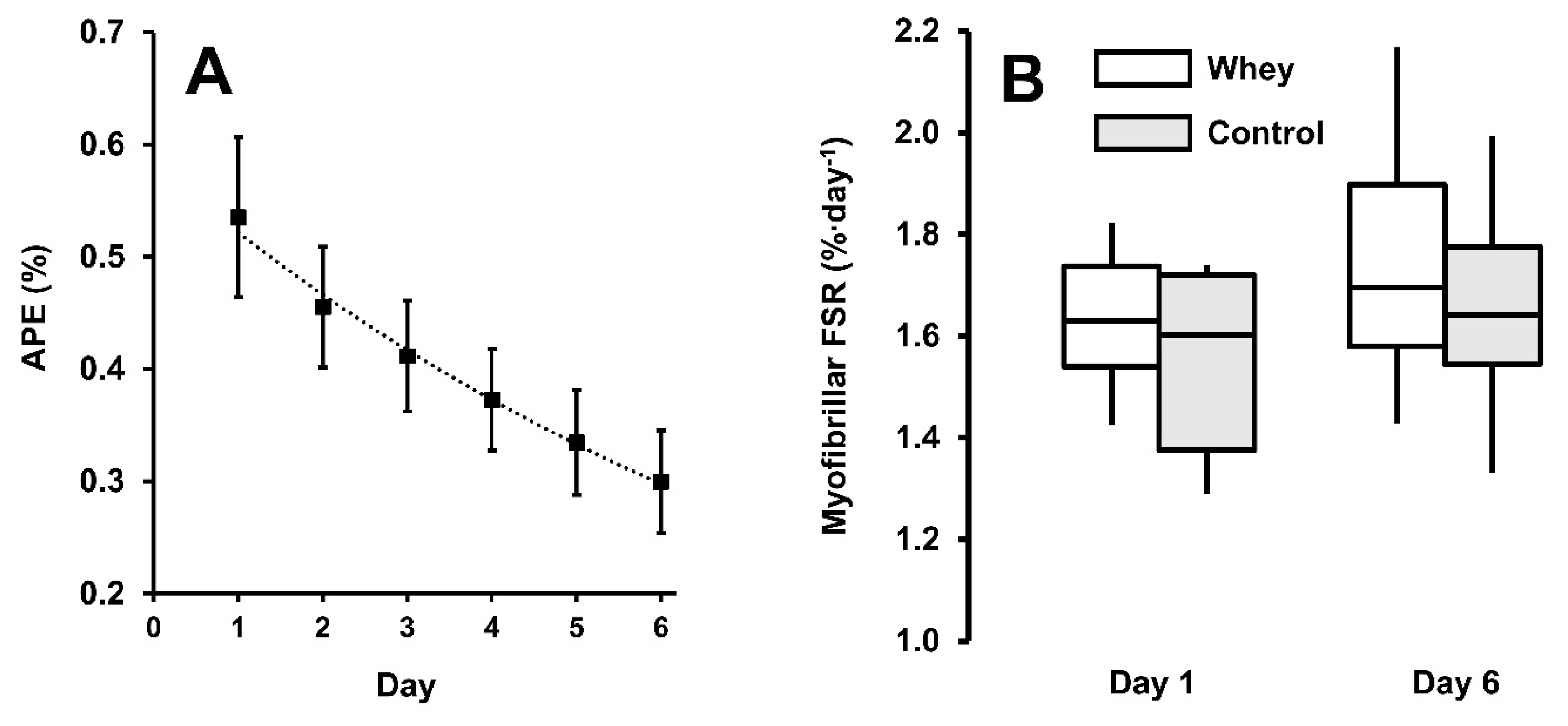
3.2. Myofibrillar Protein Synthesis
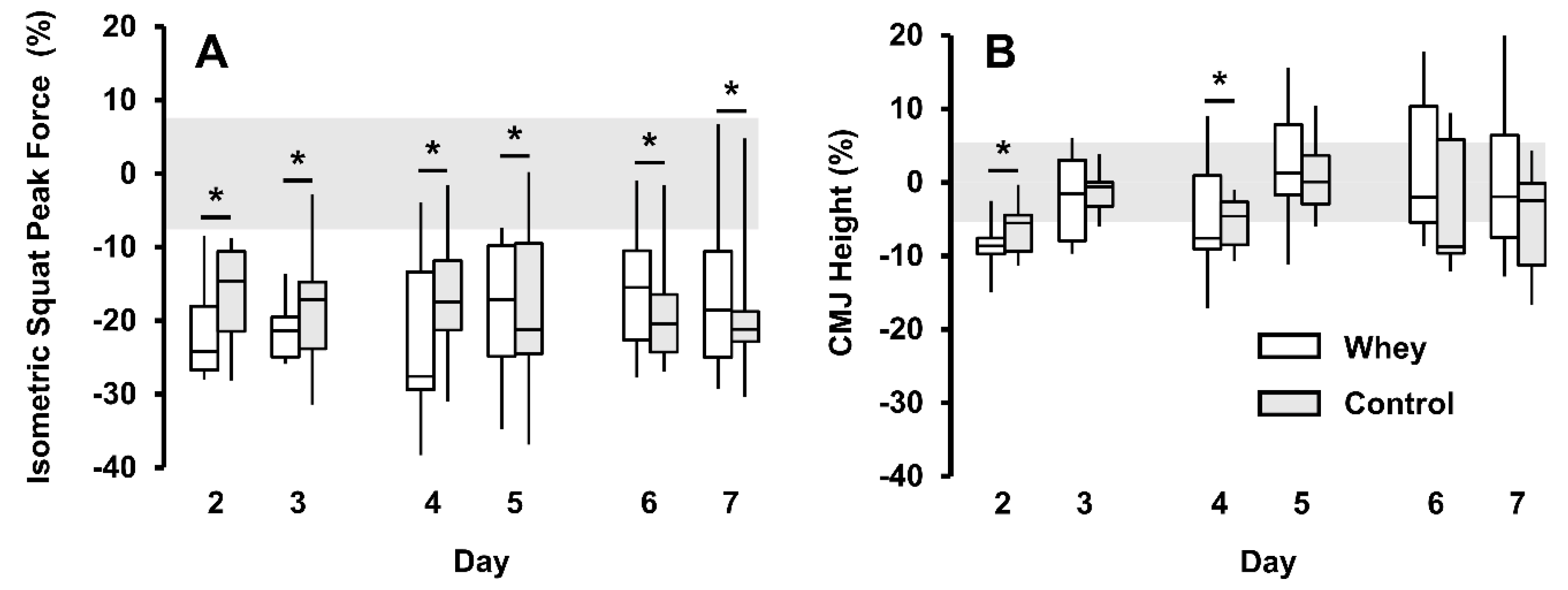
3.3. Muscle Recovery Measures and Resistance Training Performance
3.4. Muscle RNA
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Energy | Protein | Frequency | Timing | ||
|---|---|---|---|---|---|
| kJ·kg−1⸱d−1 | kJ·kg−1⸱EO−1 | g·kg−1⸱d−1 | g·kg−1⸱EO−1 | EO·d−1 | h·EO−1. |
| 154 (26) | 31 (6) | 1.9 (0.5) | 0.39 (0.1) | 5 (1) | 3.3 (0.6) |
Appendix B
| Energy (kJ·kg−1) | Protein (g·kg−1) | CHO (g·kg−1) | Fat (g·kg−1) | Frequency (d−1) | |
|---|---|---|---|---|---|
| Snacks | 16 | 0.34 | 0.43 | 0.07 | 2 |
| Meals | 38 | 0.34 | 1.22 | 0.29 | 3 |
| Daily Intake from Food | 146 | 1.7 | 4.5 | 1.0 | 5 |
Appendix C
| WHEY | CON | |||||
|---|---|---|---|---|---|---|
| mg·kg−1 | % | Dose (g) | mg·kg−1 | % | Dose (g) | |
| Alanine | 17 | 5 | 1.4 (0.2) | 33 | 10 | 2.6 (0.6) |
| Arginine | 8 | 2 | 0.7 (0.1) | 0 | 0 | 0 |
| Aspartic acid | 35 | 11 | 3.1 (0.4) | 41 | 12 | 3.2 (0.7) |
| Cysteine | 8 | 2 | 0.6 (0.1) | 0 | 0 | 0 |
| Glutamic acid | 56 | 17 | 4.9 (0.6) | 120 | 36 | 9.3 (2.0) |
| Glycine | 6 | 2 | 0.5 (0.1) | 13 | 4 | 1.0 (0.2) |
| Histidine | 6 | 2 | 0.5 (0.1) | 0 | 0 | 0 |
| Isoleucine | 20 | 6 | 1.8 (0.2) | 0 | 0 | 0 |
| Leucine | 34 | 10 | 3.0 (1.4) | 0 | 0 | 0 |
| Lysine | 30 | 9 | 2.7 (0.3) | 0 | 0 | 0 |
| Methionine | 7 | 2 | 0.6 (0.1) | 0 | 0 | 0 |
| Phenylalanine | 10 | 3 | 0.9 (0.1) | 0 | 0 | 0 |
| Proline | 19 | 6 | 1.7 (0.2) | 53 | 16 | 4.2 (0.9) |
| Serine | 17 | 5 | 1.4 (0.2) | 45 | 14 | 3.5 (0.8) |
| Threonine | 23 | 7 | 2.1 (0.3) | 0 | 0 | 0 |
| Tryptophan | 7 | 2 | 0.6 (0.1) | 0 | 0 | 0 |
| Tyrosine | 9 | 3 | 0.8 (0.1) | 25 | 8 | 2.0 (0.4) |
| Valine | 19 | 6 | 1.7 (0.2) | 0 | 0 | 0 |
| EAA | 160 | 48 | 13.7 (1.7) | 0 | 0 | 0 |
| NEAA | 170 | 52 | 15.2 (1.9) | 330 | 100 | 25.8 (5.6) |
| TAA | 330 | 100 | 28.9 (3.6) | 330 | 100 | 25.8 (5.6) |
Appendix D
| Group | Baseline | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | |
|---|---|---|---|---|---|---|---|---|
| ISq Force (N) | WHEY | 827 (195) | 670 (146) | 648 (149) | 643 (192) | 677 (194) | 672 (169) | 709 (173) |
| CON | 740 (235) | 609 (172) | 593 (162) | 608 (191) | 599 (212) | 591 (189) | 582 (148) | |
| Pooled | 776 (205) | 626 (154)* | 610 (154) * | 611 (172) * | 628 (183) * | 615 (163) * | 632 (162) * | |
| CMJ Height (cm) | WHEY | 38 (5) | 35 (4) | 37 (5) | 36 (4) | 38 (5) | 32 (14) | 39 (5) |
| CON | 37 (6) | 33 (6) | 35 (7) | 33 (6) | 35 (6) | 29 (13) | 33 (5) | |
| Pooled | 37 (6) | 34 (5) * | 36 (6) | 35 (5) * | 37 (6) | 32 (10) | 36 (6) | |
| VAS (cm) | WHEY | 0.4 (0.3) | 2.8 (1.4) | 3.0 (1.8) | 2.4 (1.4) | 1.9 (1.5) | 1.9 (1.8) | 1.8 (2.2) |
| CON | 0.3 (0.3) | 1.7 (1.1) | 1.5 (2.1) | 1.3 (1.7) | 1.5 (1.6) | 2.8 (2.0) | 1.5 (2.2) | |
| Pooled | 0.4 (0.3) | 2.3 (1.3)* | 2.3 (2.1) * | 1.8 (1.6) * | 1.7 (1.5) * | 2.4 (1.9) * | 1.6 (2.1) * | |
| CK (IU∙L−1) | WHEY | 157 (87) | 230 (88) | 166 (61) | 188 (78) | 153 (67) | 182 (82) | 158 (79) |
| CON | 130 (38) | 208 (42) | 176 (39) | 181 (36) | 157 (30) | 174 (54) | 152 (53) | |
| Pooled | 138 (87) | 215 (89) * | 168 (68) | 181 (78) | 153 (66) | 172 (93) | 149 (90) |
References
- Biolo, G.; Maggi, S.P.; Williams, B.D.; Tipton, K.D.; Wolfe, R.R. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am. J. Physiol. Endocrinol. Metab. 1995, 268, 514–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, S.M.; Tipton, K.D.; Aarsland, A.; Wolf, S.E.; Wolfe, R.R. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am. J. Physiol. Endocrinol. Metab. 1997, 273, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Atherton, P.J.; Smith, K. Muscle protein synthesis in response to nutrition and exercise. J. Physiol. 2012, 590, 1049–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, D.R.; Robinson, M.J.; Fry, J.L.; Tang, J.E.; Glover, E.I.; Wilkinson, S.B.; Prior, T.; Tarnopolsky, M.A.; Phillips, S.M. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am. J. Clin. Nutr. 2009, 89, 161–168. [Google Scholar] [CrossRef]
- Witard, O.C.; Jackman, S.R.; Breen, L.; Smith, K.; Selby, A.; Tipton, K.D. Myofibrillar muscle protein synthesis rates subsequent to a meal in response to increasing doses of whey protein at rest and after resistance exercise. Am. J. Clin. Nutr. 2013, 99, 86–95. [Google Scholar] [CrossRef] [Green Version]
- Churchward-Venne, T.A.; Breen, L.; Di Donato, D.M.; Hector, A.J.; Mitchell, C.J.; Moore, D.R.; Stellingwerff, T.; Breuille, D.; Offord, E.A.; Baker, S.K.; et al. Leucine supplementation of a low-protein mixed macronutrient beverage enhances myofibrillar protein synthesis in young men: A double-blind, randomized trial. Am. J. Clin. Nutr. 2014, 99, 276–286. [Google Scholar] [CrossRef] [Green Version]
- Davies, R.W.; Bass, J.J.; Carson, B.P.; Norton, C.; Kozior, M.; Amigo-Benavent, M.; Wilkinson, D.J.; Brook, M.S.; Atherton, P.J.; Smith, K.; et al. Differential Stimulation of Post-Exercise Myofibrillar Protein Synthesis in Humans Following Isonitrogenous, Isocaloric Pre-Exercise Feeding. Nutrients 2019, 11, E1657. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, C.J.; Churchward-Venne, T.A.; Parise, G.; Bellamy, L.; Baker, S.K.; Smith, K.; Atherton, P.J.; Phillips, S.M. Acute post-exercise myofibrillar protein synthesis is not correlated with resistance training-induced muscle hypertrophy in young men. PLoS ONE 2014, 9, e89431. [Google Scholar] [CrossRef]
- Wilkinson, D.J.; Franchi, M.V.; Brook, M.S.; Narici, M.V.; Williams, J.P.; Mitchell, W.K.; Szewczyk, N.J.; Greenhaff, P.L.; Atherton, P.J.; Smith, K. A validation of the application of D(2)O stable isotope tracer techniques for monitoring day-to-day changes in muscle protein subfraction synthesis in humans. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E571–E579. [Google Scholar] [CrossRef] [Green Version]
- Brook, M.S.; Wilkinson, D.J.; Mitchell, W.K.; Lund, J.N.; Phillips, B.E.; Szewczyk, N.J.; Greenhaff, P.L.; Smith, K.; Atherton, P.J. Synchronous deficits in cumulative muscle protein synthesis and ribosomal biogenesis underlie age-related anabolic resistance to exercise in humans. J. Physiol. 2016. [Google Scholar] [CrossRef] [Green Version]
- Damas, F.; Phillips, S.M.; Libardi, C.A.; Vechin, F.C.; Lixandrão, M.E.; Jannig, P.R.; Costa, L.A.R.; Bacurau, A.V.; Snijders, T.; Parise, G.; et al. Resistance training-induced changes in integrated myofibrillar protein synthesis are related to hypertrophy only after attenuation of muscle damage. J. Physiol. 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holwerda, A.M.; Paulussen, K.J.M.; Overkamp, M.; Smeets, J.S.J.; Gijsen, A.P.; Goessens, J.P.B.; Verdijk, L.B.; van Loon, L.J.C. Daily resistance-type exercise stimulates muscle protein synthesis in vivo in young men. J. Appl. Physiol. 2017, 124, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.R.; Ratamess, N.A.; Tranchina, C.P.; Rashti, S.L.; Kang, J.; Faigenbaum, A.D. Effect of a proprietary protein supplement on recovery indices following resistance exercise in strength/power athletes. Amino Acids 2010, 38, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Pasiakos, S.M.; Lieberman, H.R.; McLellan, T.M. Effects of protein supplements on muscle damage, soreness and recovery of muscle function and physical performance: A systematic review. Sports Med. 2014, 44, 655–670. [Google Scholar] [CrossRef]
- West, D.W.D.; Abou Sawan, S.; Mazzulla, M.; Williamson, E.; Moore, D.R. Whey Protein Supplementation Enhances Whole Body Protein Metabolism and Performance Recovery after Resistance Exercise: A Double-Blind Crossover Study. Nutrients 2017, 9, 735. [Google Scholar] [CrossRef]
- Davies, R.W.; Carson, B.P.; Jakeman, P.M. The Effect of Whey Protein Supplementation on the Temporal Recovery of Muscle Function Following Resistance Training: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 221. [Google Scholar] [CrossRef] [Green Version]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. Position of the Academy of Nutrition and Dietetics, Dietitians of Canada, and the American College of Sports Medicine: Nutrition and Athletic Performance. J. Acad. Nutr. Diet. 2016, 116, 501–528. [Google Scholar] [CrossRef]
- Maughan, R.J.; Burke, L.M.; Dvorak, J.; Larson-Meyer, D.E.; Peeling, P.; Phillips, S.M.; Rawson, E.S.; Walsh, N.P.; Garthe, I.; Geyer, H.; et al. IOC consensus statement: Dietary supplements and the high-performance athlete. Br. J. Sports Med. 2018, 52, 439–455. [Google Scholar] [CrossRef]
- Public Health England. Government Recommendations for Food Energy and Nutrients for Males and Females Aged 1–18 Years and 19+ Years; Public Health England: London, UK, 2016; pp. 9–11.
- Moore, D.R. Maximizing Post-exercise Anabolism: The Case for Relative Protein Intakes. Front. Nutr. 2019, 6. [Google Scholar] [CrossRef]
- Tipton, K.D.; Elliott, T.A.; Cree, M.G.; Aarsland, A.A.; Sanford, A.P.; Wolfe, R.R. Stimulation of net muscle protein synthesis by whey protein ingestion before and after exercise. Am. J. Physiol. Endocrinol. Metab. 2007, 292, 71–76. [Google Scholar] [CrossRef]
- Davies, R.W.; Carson, B.P.; Bass, J.J.; Holohan, S.; Jakeman, P.M. Acute reduction of lower-body contractile function following a microbiopsy of m. vastus lateralis. Scand. J. Med. Sci. Sports 2018, 28, 2638–2642. [Google Scholar] [CrossRef] [PubMed]
- Davies, R.W.; Carson, B.P.; Jakeman, P.M. Sex Differences in the Temporal Recovery of Neuromuscular Function Following Resistance Training in Resistance Trained Men and Women 18 to 35 Years. Front. Physiol. 2018, 9, 1480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komi, P.V.; Bosco, C. Utilization of stored elastic energy in leg extensor muscles by men and women. Med. Sci. Sports 1978, 10, 261–265. [Google Scholar] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front. Psychol. 2013, 4, 863. [Google Scholar] [CrossRef] [Green Version]
- Morton, R.W.; Murphy, K.T.; McKellar, S.R.; Schoenfeld, B.J.; Henselmans, M.; Helms, E.; Aragon, A.A.; Devries, M.C.; Banfield, L.; Krieger, J.W.; et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br. J. Sports Med. 2017, 52, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Nader, G.A.; Hornberger, T.A.; Esser, K.A. Translational control: Implications for skeletal muscle hypertrophy. Clin. Orthop. Relat. Res. 2002, 178–187. [Google Scholar] [CrossRef]
- Bickel, C.S.; Slade, J.; Mahoney, E.; Haddad, F.; Dudley, G.A.; Adams, G.R. Time course of molecular responses of human skeletal muscle to acute bouts of resistance exercise. J. Appl. Physiol. 2005, 98, 482–488. [Google Scholar] [CrossRef]
- West, D.W.; Baehr, L.M.; Marcotte, G.R.; Chason, C.M.; Tolento, L.; Gomes, A.V.; Bodine, S.C.; Baar, K. Acute resistance exercise activates rapamycin-sensitive and-insensitive mechanisms that control translational activity and capacity in skeletal muscle. J. Physiol. 2016, 594, 453–468. [Google Scholar] [CrossRef] [Green Version]
- Brook, M.S.; Wilkinson, D.J.; Smith, K.; Atherton, P.J. It’s not just about protein turnover: The role of ribosomal biogenesis and satellite cells in the regulation of skeletal muscle hypertrophy. Eur. J. Sport Sci. 2019, 19, 952–963. [Google Scholar] [CrossRef]
- Farup, J.; Rahbek, S.K.; Knudsen, I.S.; de Paoli, F.; Mackey, A.L.; Vissing, K. Whey protein supplementation accelerates satellite cell proliferation during recovery from eccentric exercise. Amino Acids 2014. [Google Scholar] [CrossRef] [PubMed]

| WHEY (n = 7) | CON (n = 8) | p-value | |
|---|---|---|---|
| Age (y) | 24 (4) | 23 (5) | 0.561 |
| Stature (m) | 1.81 (0.08) | 1.77 (0.04) | 0.276 |
| Body Mass (kg) | 81 (10) | 77 (17) | 0.649 |
| LBM (kg) | 63 (8) | 60 (10) | 0.488 |
| LBMI (kg·m−2) | 19 (2) | 19 (3) | 0.830 |
| Body fat (%) | 17 (6) | 18 (6) | 0.807 |
| 1RM (kg·kg−1) | 1.5 (0.3) | 1.5 (0.3) | 0.852 |
| RT-experience (y) | 2.4 (1.1) | 2.6 (1.5) | 0.783 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davies, R.W.; Bass, J.J.; Carson, B.P.; Norton, C.; Kozior, M.; Wilkinson, D.J.; Brook, M.S.; Atherton, P.J.; Smith, K.; Jakeman, P.M. The Effect of Whey Protein Supplementation on Myofibrillar Protein Synthesis and Performance Recovery in Resistance-Trained Men. Nutrients 2020, 12, 845. https://doi.org/10.3390/nu12030845
Davies RW, Bass JJ, Carson BP, Norton C, Kozior M, Wilkinson DJ, Brook MS, Atherton PJ, Smith K, Jakeman PM. The Effect of Whey Protein Supplementation on Myofibrillar Protein Synthesis and Performance Recovery in Resistance-Trained Men. Nutrients. 2020; 12(3):845. https://doi.org/10.3390/nu12030845
Chicago/Turabian StyleDavies, Robert W., Joseph J. Bass, Brian P. Carson, Catherine Norton, Marta Kozior, Daniel J. Wilkinson, Matthew S. Brook, Philip J. Atherton, Ken Smith, and Philip M. Jakeman. 2020. "The Effect of Whey Protein Supplementation on Myofibrillar Protein Synthesis and Performance Recovery in Resistance-Trained Men" Nutrients 12, no. 3: 845. https://doi.org/10.3390/nu12030845
APA StyleDavies, R. W., Bass, J. J., Carson, B. P., Norton, C., Kozior, M., Wilkinson, D. J., Brook, M. S., Atherton, P. J., Smith, K., & Jakeman, P. M. (2020). The Effect of Whey Protein Supplementation on Myofibrillar Protein Synthesis and Performance Recovery in Resistance-Trained Men. Nutrients, 12(3), 845. https://doi.org/10.3390/nu12030845









