Acute Effect of the Timing of Resistance Exercise and Nutrient Intake on Muscle Protein Breakdown
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
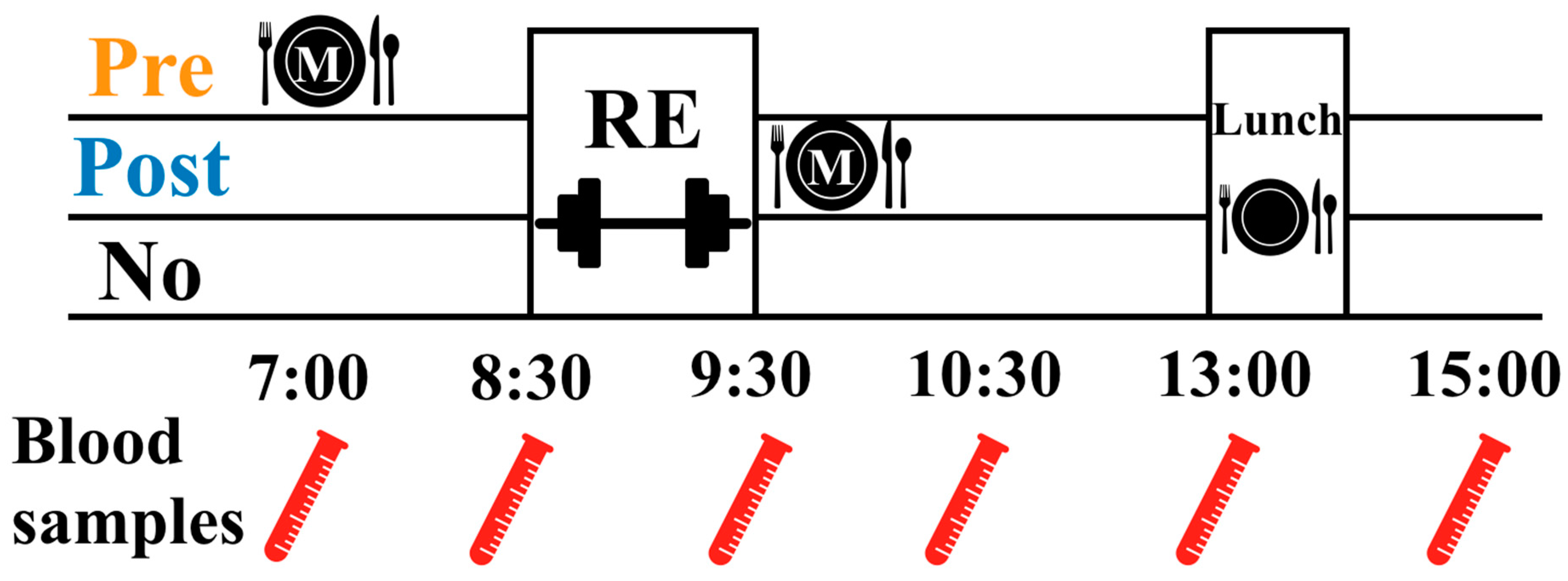
2.2. Experimental Design
2.3. Pre-Measurement
2.4. Meal Control
2.5. Meal Contents
2.6. Resistance Exercise
2.7. Blood Sampling
2.8. Statistical Analysis
3. Results
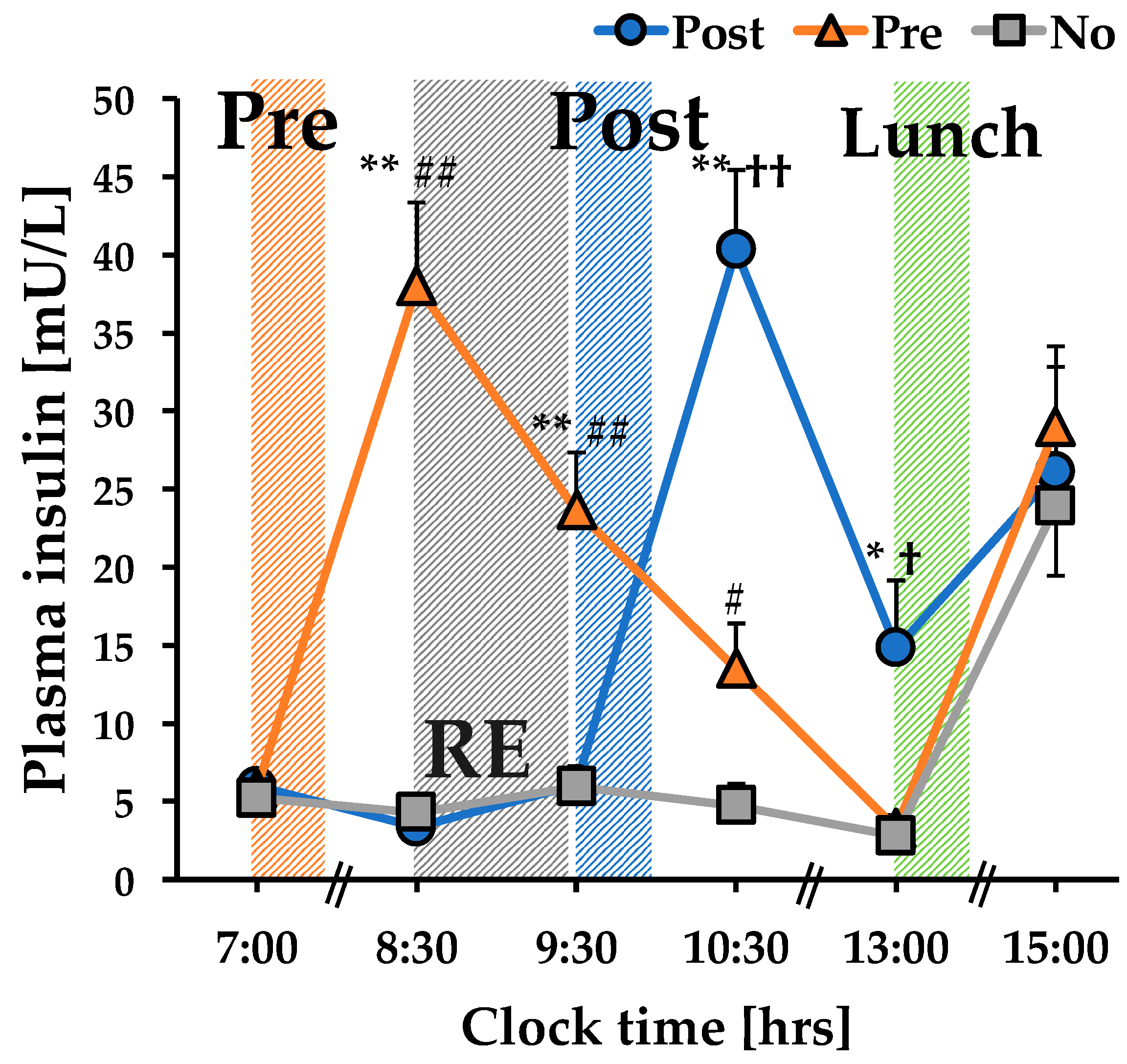
3.1. The Concentration of Plasma Insulin
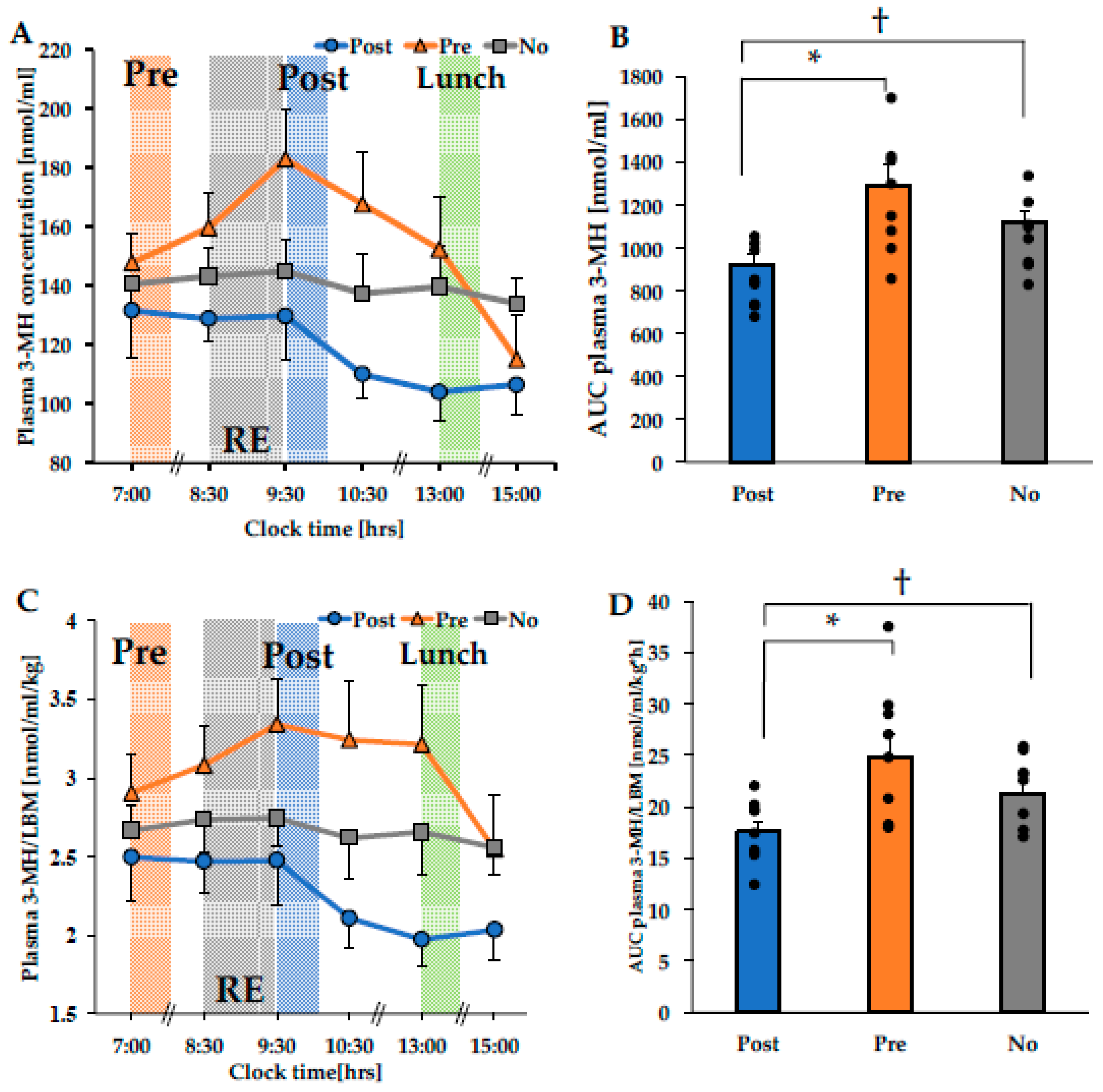
3.2. The Concentration of Plasma 3-MH
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Biolo, G.; Maggi, S.P.; Williams, B.D.; Tipton, K.D.; Wolfe, R.R. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am. J. Physiol. 1995, 268, E514–E520. [Google Scholar] [CrossRef]
- Tipton, K.D.; Hamilton, D.L.; Gallagher, I.J. Assessing the role of muscle protein breakdown in response to nutrition and exercise in humans. Sport Med. 2018, 48 (Suppl. 1), S53–S64. [Google Scholar] [CrossRef]
- Bird, S.P.; Tarpenning, K.M.; Marino, F.K. Independent and combined effects of liquid carbohydrate/essential amino acid ingestion on hormonal and muscular adaptations following resistance training in untrained men. Eur. J. Appl. Physiol. 2006, 97, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Biolo, G.; Tipton, K.D.; Wolfe, R.R. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am. J. Physiol. 1997, 273, E122–E129. [Google Scholar] [CrossRef] [PubMed]
- Biolo, G.; Williams, B.D.; Fleming, R.Y.; Wolfe, R.R. Insulin action on muscle protein kinetics and amino acid transport during recovery after resistance exercise. Diabetes 1999, 48, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Tipton, K.D.; Wolfe, R.R. Exercise-induced change in protein metabolism. Acta Physiol. Scand. 1998, 162, 377–387. [Google Scholar] [CrossRef]
- Tipton, K.D.; Wolfe, R.R. Protein and amino acids for athletes. J. Sports Sci. 2004, 22, 65–79. [Google Scholar] [CrossRef]
- Rasmussen, B.B.; Tipton, K.D.; Miller, S.L.; Wolf, S.E.; Wolfe, R.R. An oral essential amino acid-carbohydrate supplement enhances muscle protein anabolism after resistance exercise. J. Appl. Physiol. 2000, 88, 386–392. [Google Scholar] [CrossRef]
- Aragon, A.A.; Schoenfeld, B.J. Nutrient revisited: There a post-exercise anabolic window? J. Int. Soc. Sports Nutr. 2013, 10, 5. [Google Scholar] [CrossRef]
- Tipton, K.D.; Rasmussen, B.B.; Miller, S.L.; Wolf, S.E.; Owens-Stovall, S.K.; Petrini, B.E.; Wolfe, R.R. Timing of amino acid-carbohydrate ingestion alters anabolic response of muscle to resistance exercise. Am. J. Physiol. Endocrinol. Metab. 2001, 281, E197–E206. [Google Scholar] [CrossRef]
- Fujita, S.; Dreyer, H.C.; Drummond, M.J.; Glynn, E.L.; Volpi, E.; Rasmussen, B.B. Essential amino acid and carbohydrate ingestion before resistance exercise does not enhance postexercise muscle protein synthesis. J. Appl. Physiol. 2009, 106, 1730–1739. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, H.C.; Fujita, S.; Glynn, E.L.; Drummond, M.J.; Volpi, E.; Rasmussen, B.B. Resistance exercise increase leg muscle protein synthesis and mTOR signaling independent of sex. Acta Physiol. (Oxf.) 2010, 199, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Nabuco, H.C.G.; Tomeleri, C.M.; Sugihara Junior, P.; Fernandes, R.R.; Cavalcante, E.F.; Antunes, M.; Ribeiro, A.S.; Teixeira, D.C.; Silva, A.M.; Sardinha, L.B.; et al. Effects of whey protein supplementation Pre- or Post- resistance training on muscle mass, muscular strength, and functional capacity in Pre-conditioned older women: A randomized clinical trial. Nutrients 2018, 10, 563. [Google Scholar] [CrossRef] [PubMed]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Alhazzani, W.; Calder, P.C.; Casaer, M.P.; Hiesmayr, M.; Mayer, K.; Montejo, J.C.; Pichael, C.; et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin. Nutr. 2019, 38, 48–79. [Google Scholar] [CrossRef]
- Van Vliet, S.; Shy, E.L.; Sawan, S.A.; Beals, J.W.; West, D.W.; Skinner, S.K.; Ulanov, A.V.; Li, Z.; Paliska, S.A.; Parsons, C.M.; et al. Consumption of Whole egg promotes greater stimulation of postexercise muscle protein synthesis than consumption of isonitrogenous amounts of egg whites in young men. Am. J. Clin. Nutr. 2017, 106, 1401–1412. [Google Scholar] [CrossRef]
- Schoenfeld, B.J.; Aragon, A.A.; Krieger, J.W. The effect of protein timing on muscle strength and hypertrophy: A meta-analysis. J. Int. Soc. Sports Nutr. 2013, 10, 53. [Google Scholar] [CrossRef]
- Morton, R.R.; Murphy, K.T.; Mckellar, S.R.; Schoenfeld, B.J.; Henselmans, M.; Helms, E.; Aragon, A.A.; Devris Banfield, L.; Krieger, J.W.; Phillips, S.M. A systematic review, meta-analysis and meta-regession of the effect of protein supplementation on resistance training–induced gain in muscle mass and strength in healthy adults. Br. J. Sports Med. 2018, 52, 376–384. [Google Scholar]
- O’Neil, C.E.; Byrd-Bredbenner, C.; Hayes, D.; Jana, L.; Klinger, S.E.; Stephenson-Martin, S. The role of breakfast in health: Definition and criteria for a quality breakfast. J. Acad. Nutr. Diet. 2014, 114, 8–26. [Google Scholar] [CrossRef]
- Tolfrey, K.; Zakrzewski, J.K. Breakfast, glycemic index and health in young people. J. Sport Health Sci. 2012, 1, 149–159. [Google Scholar] [CrossRef]
- Japan Ministry of health, Labour and Welfare. National Health and Nutrition Survey 2016. 2016. Available online: https://www.mhlw.go.jp/stf/houdou/0000177189.html (accessed on 3 March 2020).
- Phillips, S.M. Protein requirements and supplement in strength sports. Nutrition 2004, 20, 689–695. [Google Scholar] [CrossRef]
- Yasuda, J.; Asako, M.; Arimitsu, T.; Fujita, S. Association of protein intake in three meals with muscle mass in healthy young subjects: A cross-sectional study. Nutrients 2019, 11, 612. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, J.; Tomita, T.; Arimitsu, T.; Fujita, S. Evenly distributed protein intake over three meals augments resistance exercise-induced muscle hypertrophy in healthy young men. J. Nutr. 2020, in press. [Google Scholar]
- Scott, D.; Park, M.S.; Kim, T.N.; Ryu, J.Y.; Hong, H.C.; Yoo, H.J.; Baik, S.H.; Jones, G.; Choi, K.M. Associations of low muscle mass and the metabolic syndrome in Caucasian and Asian middle-aged and older Adults. J. Nutr. Health Aging 2016, 20, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Son, J.W.; Lee, S.S.; Kim, S.R.; Yoo, S.J.; Cha, B.Y.; Son, H.Y.; Cho, N.H. Low muscle mass and risk of type 2 diabetes in middle-aged and older adults: Findings from the KoGES. Diabetologia 2017, 60, 865–872. [Google Scholar] [CrossRef]
- Shimokata, H.; Ando, F.; Yuki, A.; Otsuka, R. Age-related changes in skeletal muscle mass among community-dwelling Japanese: A 12-year longitudinal study. Geriatr. Gerontol. Int. 2014, 14 (Suppl. 1), 85–92. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederrholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Glynn, E.L.; Fry, C.S.; Drummond, M.J.; Dreyer, H.C.; Dhanani, S.; Volpi, E.; Rasmussen, B.B. Muscle protein has a minor role in the protein anabolic response to essential amino acid and carbohydrate intake following resistance exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R533–R540. [Google Scholar] [CrossRef]
- Lukaski, H.C.; Mendez, J.; Buskirk, E.R.; Cohn, S.H. Relationship between endogenous 3-methylhistidine excretion and body composition. Am. J. Physiol. 1981, 240, E302–E307. [Google Scholar] [CrossRef]
- Moon, J.S.; Lee, J.E.; Yoon, J.S. Variation in serum creatinine level is correlated to risk of type 2 diabetes. Endocrinol. Metab. (Seoul) 2013, 28, 207–213. [Google Scholar] [CrossRef]
- Greenhaff, P.L.; Karagounis, L.G.; Peirce, N.; Simpson, E.J.; Hazell, M.; Layfield, R.; Wackerhage, H.; Smith, K.; Atherton, P.; Selby, A.; et al. Disassociation between the effects of amino acids and insulin on signaling, ubiquitin ligases, and protein turnover in human muscle. Am. J. physiol. Endocrinol. Metab. 2008, 295, E595–E604. [Google Scholar] [CrossRef]
- Staples, A.W.; Burd, N.A.; West, W.D.; Currie, K.D.; Atherton, P.J.; Moore, D.R.; Rennie, M.J.; Macdonald, M.J.; Baker, S.K.; Phillips, S.M. Carbohydrate dose not augment exercise-induced protein accretion versus protein alone. Med. Sci. Sports Exerc. 2011, 43, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Trappe, S.; Gallagher, P.; Harber, M.; Carrithers, J.; Fluckey, J.; Trappe, T. Single muscle fibre contractile properties in young and old men and women. J. Physiol. 2003, 552, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.; Trappe, T.; Crameri, R.M.; Qvortrup, K.; Kjaer, M.; Langberg, H. Myofibrillar proteolysis in response to voluntary electrically stimulated muscle contractions in humans. Scand. J. Med. Sci. Sports 2009, 19, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.W. Griffith’s Instructions for Patients, 8th ed.; Elsevier: Amsterdam, The Netherlands, 2010; p. 219. [Google Scholar]
- Moore, D.R.; Churchward-Vanne, T.A.; Witard, O.; Breen, L.; Burd, N.A.; Tipton, K.D.; Phillips, S.M. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J. Gerintol. A Biol. Sci. Med. Sci. 2015, 70, 57–62. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kume, W.; Yasuda, J.; Hashimoto, T. Acute Effect of the Timing of Resistance Exercise and Nutrient Intake on Muscle Protein Breakdown. Nutrients 2020, 12, 1177. https://doi.org/10.3390/nu12041177
Kume W, Yasuda J, Hashimoto T. Acute Effect of the Timing of Resistance Exercise and Nutrient Intake on Muscle Protein Breakdown. Nutrients. 2020; 12(4):1177. https://doi.org/10.3390/nu12041177
Chicago/Turabian StyleKume, Wataru, Jun Yasuda, and Takeshi Hashimoto. 2020. "Acute Effect of the Timing of Resistance Exercise and Nutrient Intake on Muscle Protein Breakdown" Nutrients 12, no. 4: 1177. https://doi.org/10.3390/nu12041177
APA StyleKume, W., Yasuda, J., & Hashimoto, T. (2020). Acute Effect of the Timing of Resistance Exercise and Nutrient Intake on Muscle Protein Breakdown. Nutrients, 12(4), 1177. https://doi.org/10.3390/nu12041177



