Nutraceutical Boom in Cancer: Inside the Labyrinth of Reactive Oxygen Species
Abstract
1. Introduction
1.1. Nutraceuticals
1.2. Nutraceuticals and Cancer
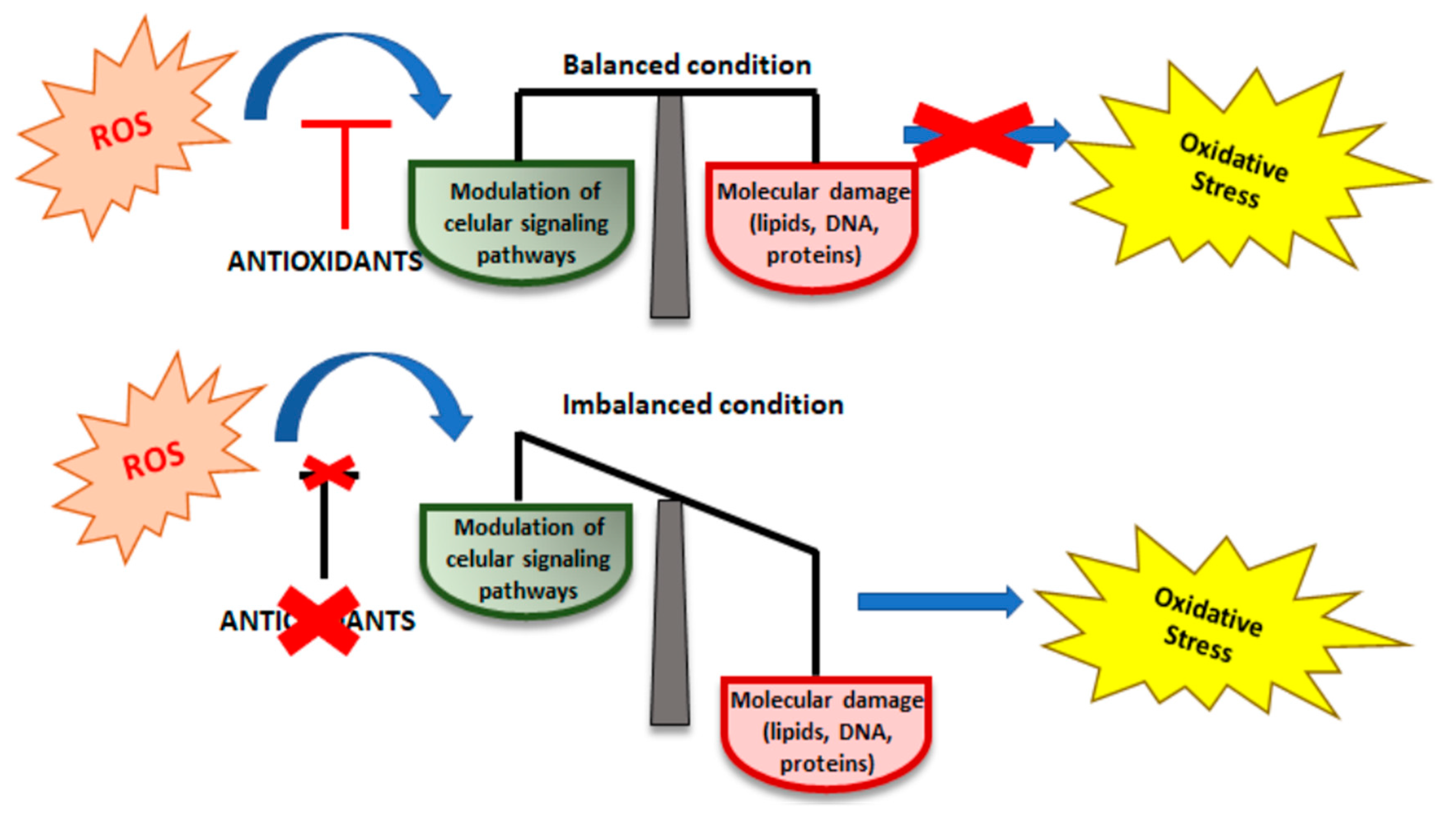
2. Oxidative Stress (OS)
2.1. Antioxidant Activity of Nutraceuticals
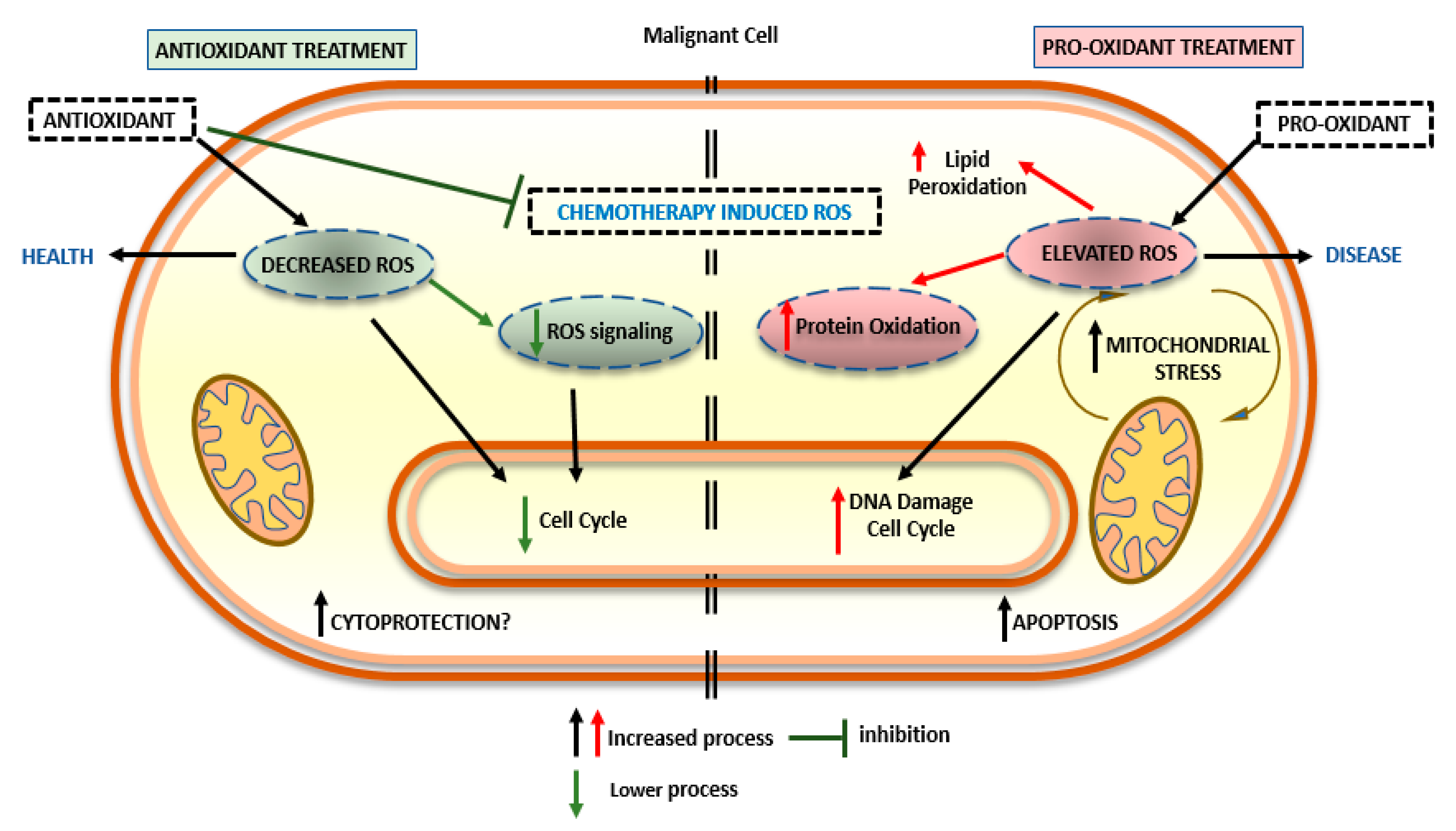
2.2. Pro-Oxidant Activity of Nutraceuticals
2.3. Combination of Nutraceuticals and Antioxidant Substances
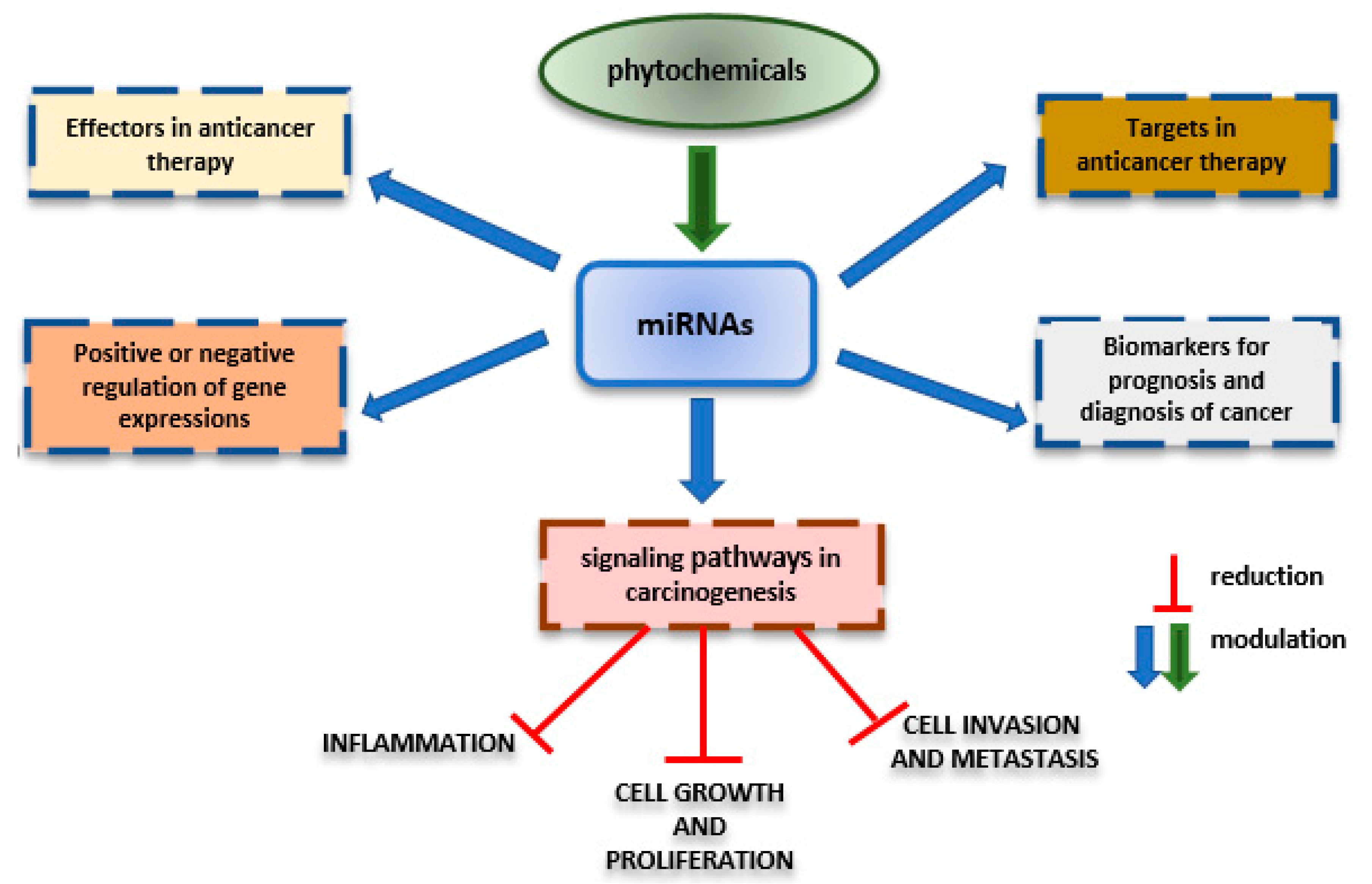
3. Effects of Nutraceuticals on MicroRNAs
Nutraceuticals’ Impact on Epigenetic Phenomena in Cancer
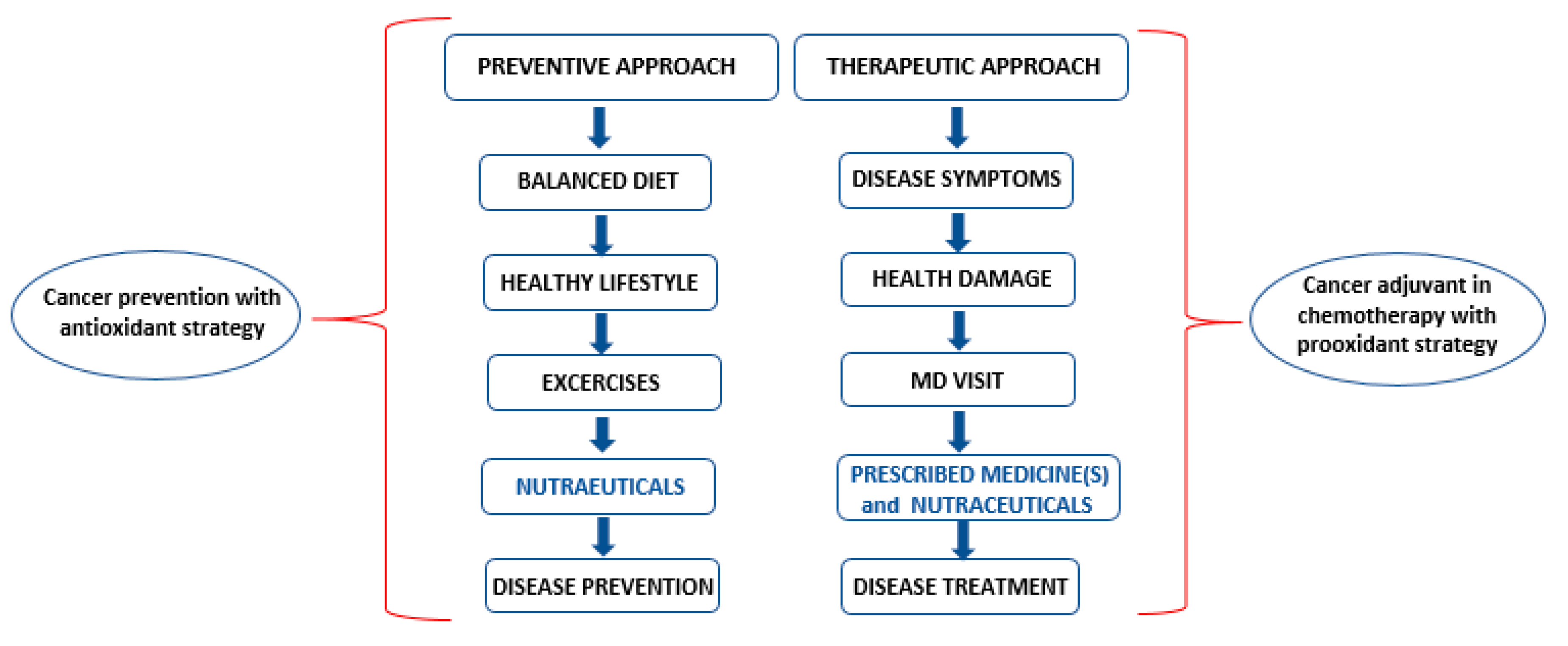
4. Nutraceuticals: Prevention, Cure and Chemotherapy
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AC | Caffeic acid |
| AMPK | Adenosine monophosphate protein kinase |
| BBR | berberine |
| BC | breast cancer |
| Bcl-2 | b-cell lymphoma-2 |
| CAT | catalase |
| COX-1 | Cyclooxygenase 1 |
| COX-2 | Cyclooxigenase 2 |
| CYP | Cytochrome P450 |
| DNMT | DNA methylation |
| EGC | epicatechin gallate |
| GPx | glutathione peroxidase |
| HDAC | DNA histone deacetylases |
| IL-2 | interleukin 2 |
| IL-6 | interleukin 6 |
| IL-17 | interleukin 17 |
| MAPK | mitogen-activated protein kinase |
| Met | metformin |
| miRNA | microRNA |
| MMP-9 | metallo-proteinases-9 |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NK | natural killer |
| NOS | nitric oxide synthase |
| NRF2 | nuclear factor (erythroid-derived 2)-like 2 |
| OS | oxidative stress |
| RES | resveratrol |
| ROS | reactive oxygen species |
| RONS | reactive oxygen nitrogen species |
| RNS | reactive nitrogen species |
| SOD | superoxide dismutase |
| Trx | thioredoxin |
| TNF-α | tumor necrosis factor-alpha |
| PDGF | platelet-derived growth factor |
| VEGF | vascular endothelial growth factor |
References
- DeFelice, S.L. The Nutraceutical Revolution: Fueling a Powerful, New International Market; The Foundation for Innovation in Medicine: Mountside, NJ, USA, 1989. [Google Scholar]
- Bergamin, A.; Mantzioris, E.; Cross, G.; Deo, P.; Garg, S.; Hill, A.M. Nutraceuticals: Reviewing their role in chronic disease prevention and management. Pharm. Med. 2019, 33, 291–309. [Google Scholar] [CrossRef] [PubMed]
- Santini, A.; Tenore, G.C.; Novellino, E. Nutraceuticals: A paradigm of proactive medicine. Eur. J. Pharm. Sci. 2017, 96, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Lachance, P.A.; Das, Y.T. Nutraceuticals. In Comprehensive Medicinal Chemistry II; Triggle, D.J., Taylor, J.B., Eds.; Elsevier: Oxford, UK, 2007; pp. 449–461. [Google Scholar]
- Hardy, G. Nutraceuticals and functional foods: Introduction and meaning. Nutrition 2000, 16, 688–697. [Google Scholar] [CrossRef]
- Jain, N.; Ramawat, K.G. Nutraceuticals and Antioxidants in Prevention of Diseases. In Natural Products; Springer: Berlin, Germany, 2013; pp. 2559–2580. [Google Scholar]
- Prankash, D.; Gupta, C.; Sharma, G. Importance of phytochemicals in nutraceuticals. JCMRD 2012, 1, 70–78. [Google Scholar]
- Nivya, M.A.; Kaliyappan, R.; Vel, K.; Sasidharan, S.; Seethpathy, G.S. Role of nutraceutical in cancer. Int. J. Pharm. Pharm. Sci. 2012, 4, l4. [Google Scholar]
- Benzie, I.F.F. Evolution of antioxidant defence mechanisms. Eur. J. Nutr. 2000, 39, 53–61. [Google Scholar] [CrossRef]
- Yamini, B.T.; Pratibha, T.; Arjmandi, B.H. Nutraceuticals and cancer management. Front. Biosci. 2005, 10, 1607–1618. [Google Scholar]
- Zheng, J.; Zhou, Y.; Li, Y.; Xu, D.P.; Li, S.; Li, H.B. Spices for prevention and treatment of cancers. Nutrients 2016, 8, 495. [Google Scholar] [CrossRef]
- Hiramatsu, M.; Yoshikava, T.; Packer, L. Molecular Interventions in Lifestyle-Related Diseases; CRC Taylor and Francis: London, UK, 2005. [Google Scholar]
- Aggarwal, B.B.; Sundaram, C.; Prasad, S.; Kannappan, R. Tocotrienols, the vitamin E of the 21st century: Its potential against cancer and other chronic diseases. Biochem. Pharmacol. 2010, 80, 1613–1631. [Google Scholar] [CrossRef]
- McCubrey, J.A.; Lertpiriyapong, K.; Steelman, L.S.; Abrams, S.L.; Yang, L.V. Effects of resveratrol, curcumin, berberine and other nutraceuticals on aging, cancer development, cancer stem cells and microRNAs. Aging (Albany) 2017, 9, 1477–1536. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Sailo, B.L.; Banik, K.; Harsha, C.; Prasad, S.; Gupta, S.C.; Bharti, A.C.; Aggarwal, B.B. Chronic diseases, inflammation, and spices: How are they linked? J. Transl. Med. 2018, 16, 14. [Google Scholar] [CrossRef]
- Diaz-Gerevini, G.T.; Repossi, G.; Dain, A.; Tarres, M.C.; Das, U.N.; Eynard, A.R. Beneficial action of resveratrol: How and why? Nutrition 2016, 32, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Xia, J.; Gao, J.; Inagaki, Y.; Tang, W.; Kokudo, N. Anti-tumor effects and cellular mechanisms of resveratrol. Drug Discov. Ther. 2015, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cen, L.; Hutzen, B.; Ball, S.; DeAngelis, S.; Chen, C.L.; Fuchs, J.R.; Li, C.; Li, P.K.; Lin, J. New structural analogues of curcumin exhibit potent growth suppressive activity in human colorectal carcinoma cells. BMC Cancer 2009, 9, 99. [Google Scholar]
- Das, L.; Vinayak, M. Long term effect of curcumin in restoration of tumour suppressor p53 and phase-II antioxidant enzymes via activation of NRF2 signalling and modulation of inflammation in prevention of cancer. PLoS ONE 2015, 10, e0124000. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, S.; Meng, X.; Gan, R.Y.; Zhang, J.J.; Li, H.B. Dietary natural products for prevention and treatment of breast cancer. Nutrients 2017, 9, 728. [Google Scholar]
- Ferrucci, L.M.; McCorkel, R.; Smith, T.; Stein, K.D.; Cartmel, B. Factors related to the use of dietary supplements by cancer survivors. J. Altern. Complement Med. 2009, 15, 673–680. [Google Scholar] [CrossRef]
- Reedy, J.; Haines, P.S.; Steckler, A.; Campbell, M.K. Qualitative comparison of dietary choices and dietary supplement use among older adults with and without a history of colorectal cancer. J. Nutr. Educ. Behav. 2005, 37, 252–258. [Google Scholar] [CrossRef]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative stress, prooxidants, and antioxidants: The interplay. Biomed. Res. Int. 2014, 2014, 761264. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Cornelli, U. Antioxidant use in Nutraceuticals. Clin. Dermatol. 2009, 27, 175–194. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, P.; Haber, E.P.; Hirabara, S.M.; Rebelato, E.L.; Procopio, J.; Morgan, D.; Oliveira-Emilio, H.C.; Carpinelli, A.R.; Curi, R. Diabetes associated cell stress and dysfunction: Role of mitochondrial and non-mitochondrial ROS production and activity. J. Physiol. 2007, 583, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Panieri, E.; Santoro, M.M. ROS homeostasis and metabolism: A dangerous liaison in cancer cells. Cell Death Dis. 2017, 7, e2253. [Google Scholar] [CrossRef]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, M.V. Role of ROS and RNS sources in physiological and pathological conditions. Oxid. Med. Cell. Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-mediated cellular signaling. Oxid. Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef]
- Liou, G.Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef]
- Lee, M.T.; Lin, W.C.; Yu, B.; Lee, T.T. Antioxidant capacity of phytochemicals and their potential effects on oxidative status in animals—A review. Asian Australas J. Anim. Sci. 2017, 30, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Mendoza, N.; Morales-González, A.; Madrigal-Santillán, E.O.; Madrigal-Bujaidar, E.; Álvarez-González, I.; Garcia-Melo, R.F.; Anguiano-Robledo, L.; Fregoso-Aguilar, T.; Morales-Gonzalez, J.A. Antioxidant and adaptative response mediated by Nrf2 during physical exercise. Antioxidants 2019, 8, 196. [Google Scholar] [CrossRef] [PubMed]
- Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes; National Academy Press: Washington, DC, USA, 2002. [Google Scholar]
- Zhang, Y.J.; Gan, R.Y.; Li, S.; Zhou, Y.; Li, A.N.; Xu, D.P.; Li, H.B. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef]
- Ratnam, D.V.; Ankola, D.D.; Bhardwaj, V.; Sahana, D.K.; Kumar, M.N. Role of antioxidants in prophylaxis and therapy: A pharmaceutical perspective. J. Control. Release 2006, 113, 189–207. [Google Scholar] [CrossRef]
- Wallace, T.C.; Slavin, M.; Frankelfeld, C.L. Systematic review of anthocyanins and markers of cardiovascular disease. Nutrients 2016, 8, 32. [Google Scholar] [CrossRef] [PubMed]
- Na, H.K.; Surh, Y.J. Modulation of Nrf2-mediated antioxidant and detoxifying enzyme induction by the green tea polyphenol EGCG. Food Chem. Toxicol. 2008, 46, 1271–1278. [Google Scholar] [CrossRef]
- Saw, C.L.; Yang, A.Y.; Cheng, D.C.; Boyanapalli, S.S.; Su, Z.Y.; Khor, T.O.; Gao, S.; Wang, J.; Jiang, Z.H.; Kong, A.N. Pharmacodynamics of ginsenosides: Antioxidant activities, activation of Nrf2, and potential synergistic effects of combinations. Chem. Res. Toxicol. 2012, 25, 1574–1580. [Google Scholar] [CrossRef]
- Costa, A.; Garcia-Diaz, D.F.; Jimenez, P.; Silva, P.I. Bioactive compounds and health benefits of exotic tropical red-black berries. J. Funct. Food. 2013, 5, 539–549. [Google Scholar] [CrossRef]
- Dahlen, E.M.; Tengblad, A.; Lanne, T.; Clinchy, B.; Ernerudh, J.; Nystrom, S.H.; Ostgren, C.J. Abdominal obesity and low-grade systemic inflammation as markers of subclinical organ damage in type 2 diabetes. Diabetes Metab. 2014, 40, 76–81. [Google Scholar] [CrossRef]
- Prakash, D.; Kumar, N. Cost effective natural antioxidants. In Nutrients, Dietary Supplements and Nutriceuticals; Watson, R.R., Gerald, J.K., Preedy, V.R., Eds.; Humana Press: Totowa, NJ, USA, 2011; pp. 163–188. [Google Scholar]
- McCullough, M.L.; Peterson, J.J.; Patel, R.; Jacques, P.F.; Shah, R.; Dwyer, J.T. Flavonoid intake and cardiovascular disease mortality in a prospective cohort of US adults. Am. J. Clin. Nutr. 2012, 95, 454–464. [Google Scholar] [CrossRef]
- Cieślik, E.; Greda, A.; Adamus, W. Contents of polyphenols in fruits and vegetables. Food Chem. 2006, 94, 135–142. [Google Scholar] [CrossRef]
- Ferrucci, F.; Boffa, I.; De Masi, G.; Zollo, M. Natural compounds for pediatric cancer treatment. Naunyn Schmiedebergs Arch. Pharmacol. 2006, 389, 131–149. [Google Scholar] [CrossRef] [PubMed]
- Bernatoniene, J.; Kopustinskiene, D.M. The role of catechins in cellular responses to oxidative stress. Molecules 2018, 23, 965. [Google Scholar] [CrossRef] [PubMed]
- Sahin, K.; Orhan, C.; Tuzcu, M.; Ali, S.; Sahin, N.; Hayirli, A. Epigallocatechin-3-gallate prevents lipid peroxidation and enhances antioxidant defense system via modulating hepatic nuclear transcription factors in heat-stressed quails. Poult. Sci. 2010, 89, 2251–2258. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Ma, T.; Gu, Y.; Zhang, X.; Qiu, X.; Zhang, L.; Xu, R.; Yu, Y. Berbamine derivatives: A novel class of compounds for anti-leukemia activity. Eur. J. Med. Chem. 2009, 44, 3293–3298. [Google Scholar] [CrossRef]
- Ortiz, L.; Lombardi, P.; Tillhon, M.; Scovassi, A.L. Berberine, an epiphany against cancer. Molecules 2014, 19, 12349–12367. [Google Scholar] [CrossRef]
- Gupta, S.B.; Kim, J.H.; Prasad, S.; Aggarwal, B.B. Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer Met. Rev. 2010, 29, 405–434. [Google Scholar] [CrossRef]
- Toti, E.; Chen Oliver, C.-Y.; Palmery, M.; Viilano Valencia, D.; Peluso, I. Bob-Provitamin A and Provitamin A Carotenoids as Immunomodulators: Recommended Dietary Allowance, Therapeutic Index, or Personalized Nutrition? Oxid. Med. Cell. Longev. 2018, 2018, 4637861. [Google Scholar] [CrossRef]
- Linnewiel, K.; Ernst, H.; Caris-Veyrat, C.; Ben-Dor, A.; Kampf, A.; Salman, H.; Danilenko, M.; Levy, J.; Sharoni, Y. Structure activity relationship of carotenoid derivatives in activation of the electrophile/antioxidant response element transcription system. Free Radic. Biol. Med. 2009, 47, 659–667. [Google Scholar] [CrossRef]
- Wang, X.D. Enzymatic metabolites of lycopene induce Nrf2-mediated expression of phase II detoxifying/antioxidant enzymes in human bronchial epithelial cells. Int. J. Cancer 2008, 123, 1262–1268. [Google Scholar]
- Po-Min, Y.; Huang-Zhi, C.; Yu-Ting, H.; Chia-Wen, H.; Being-Sun, W. Lycopene inhibits NF-κB activation and adhesion molecule expression through Nrf2-mediated heme oxygenase-1 in endothelial cells. Int. J. Mol. Med. 2017, 39, 1533–1540. [Google Scholar]
- Takeshima, M.; Ono, M.; Higuchi, T.; Chen, C.; Hara, T.; Nakano, S. Anti-Proliferative and apoptosis-inducing activity of lycopene against three subtypes of human breast cancer cell lines. Cancer Sci. 2014, 105, 252–257. [Google Scholar] [CrossRef] [PubMed]
- He, H.J.; Wang, G.Y.; Gao, Y.; Ling, W.H.; Yu, Z.W.; Jin, T.R. Curcumin attenuates Nrf2 signaling defect, oxidative stress in muscle and glucose intolerance in high fat diet-fed mice. World J. Diabetes 2012, 3, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B. Targeting inflammation-induced obesity and metabolic diseases by curcumin and other nutraceuticals. Annu. Rev. Nutr. 2010, 30, 173–199. [Google Scholar] [CrossRef] [PubMed]
- Tseng, S.H.; Lin, S.M.; Chen, J.C.; Su, Y.H.; Huang, H.Y.; Chen, C.K.; Lin, P.Y.; Chen, Y. Resveratrol suppresses the angiogenesis and tumor growth of gliomas in rats. Clin. Cancer Res. 2004, 10, 2190–2202. [Google Scholar] [CrossRef] [PubMed]
- Khanduja, K.L.; Bhardwaj, A.; Kaushik, G. Resveratrol inhibits N-nitrosodiethylamine-induced ornithine decarboxylase and cyclooxygenase in mice. J. Nutr. Sci. Vitaminol. 2004, 50, 61–65. [Google Scholar] [CrossRef]
- Frémont, L. Biological effects of resveratrol. Life Sci. 2000, 66, 663–673. [Google Scholar] [CrossRef]
- Boudet, A.M. Evolution and current status of research in phenolic compounds. Phytochemistry 2007, 68, 2722–2735. [Google Scholar] [CrossRef]
- Bezerra, D.P.; Nascimento Soares, A.K.; Pergentino de Sousa, D. Overview of the role of vanillin on redox status and cancer development. Oxid. Med. Cell. Longev. 2016, 2016, 9734816. [Google Scholar] [CrossRef]
- Pedroso, L.S.; Fávero, G.M.; De Camargo, L.E.A.; Mainardes, R.M.; Khalil, N.M. Effect of the o-methyl catechols apocynin, curcumin and vanillin on the cytotoxicity activity of tamoxifen. J. Enzym. Inhib. Med. Chem. 2013, 28, 734–740. [Google Scholar] [CrossRef]
- Banerjee, K.; Mandal, M. Oxidative stress triggered by naturally occurring flavone apigenin results in senescence and chemotherapeutic effect in human colorectal cancer cells. Redox Biol. 2015, 5, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Mahomoodally, M.F.; Aumeeruddy, M.Z.; Rengasamy, K.R.R.; Roshan, S.; Hammad, S.; Pandohee, J.; Hu, X.; Zengin, G. Ginger and its active compounds in cancer therapy: From folk uses to nano-therapeutic applications. Semin. Cancer Biol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.J.; Nam, H.H.; Chung, M.S. Conversion of 6-gingerol to 6-shogaol in ginger (Zingiber officinale) pulp and peel during subcritical water extraction. Food Chem. 2019, 270, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, D.P.; Militao Gadelha, C.G.; Castro de Morais, M.; de Sousa, P. The dual antioxidant/prooxidant effect of eugenol and its action in cancer development and treatment. Nutrients 2017, 9, 1367. [Google Scholar] [CrossRef] [PubMed]
- Jordán, M.J.; Tandon, K.; Shaw, P.E.; Goodner, K.L. Aromatic profile of aqueous banana essence and banana fruit by gas chromatography-mass spectrometry (GC-MS) and gas chromatography-olfactometry (GC-O). J. Agric. Food Chem. 2001, 49, 4813–4817. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.G.; Shibamoto, T. Antioxidant properties of aroma compounds isolated from soybeans and mung beans. J. Agric. Food Chem. 2000, 48, 4290–4293. [Google Scholar] [CrossRef]
- Kühnau, J. The flavonoids: A class of semi-essential food components: Their role in human nutrition. World Rev. Nutr. Diet. 1976, 24, 117–191. [Google Scholar]
- Sutherland, B.A.; Rahman, R.M.A.; Appleton, I. Mechanisms of action of green tea catechins, with a focus on ischemia-induced neurodegeneration. J. Nutr. Biochem. 2006, 17, 291–306. [Google Scholar] [CrossRef]
- Gardner, E.J.; Ruxton, C.H.S.; Leeds, A.R. Black tea—Helpful or harmful? A review of the evidence. Eur. J. Clin. Nutr. 2007, 61, 3–18. [Google Scholar] [CrossRef]
- Nardini, M.; Cirillo, E.; Natella, F.; Scaccini, C. Absorption of phenolic acids in humans after coffee consumption. J. Agric. Food Chem. 2002, 50, 5735–5741. [Google Scholar] [CrossRef]
- Vinson, J.A.; Su, X.; Zubik, L.; Bose, P. Phenol antioxidant quantity and quality in foods: Fruits. J. Agric. Food Chem. 2001, 49, 5315–5321. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Aviram, M.; Zhang, Y.; Henning, S.M.; Feng, L.; Dreher, M.; Heber, D. Comparison of antioxidant potency of commonly consumed polyphenol-rich beverages in the United States. J. Agric. Food Chem. 2008, 56, 1415–1422. [Google Scholar] [CrossRef]
- Vinson, J.A.; Hao, Y.; Su, X.; Zubik, L. Phenol antioxidant quantity and quality in foods: Vegetables. J. Agric. Food Chem. 1998, 46, 3630–3634. [Google Scholar] [CrossRef]
- Lodovici, M.; Guglielmi, F.; Casalini, C.; Meoni, M.; Cheynier, V.; Dolara, P. Antioxidant and radical scavenging properties in vitro of polyphenolic extracts from red wine. Eur. J. Nutr. 2001, 40, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Vinson, J.A.; Proch, J.; Zubik, L. Phenol antioxidant quantity and quality in foods: Cocoa, dark chocolate, and milk chocolate. J. Agric. Food Chem. 1999, 47, 4821–4824. [Google Scholar] [CrossRef] [PubMed]
- Nitta, Y.; Kikuzaki, H.; Ueno, H. Food components inhibiting recombinant human histidine decarboxylase activity. J. Agric. Food Chem. 2007, 55, 299–304. [Google Scholar] [CrossRef]
- Macheix, J.J.; Fleuriet, A.; Billot, J. Fruit Phenolics; CRC Press Inc.: Boca Raton, FL, USA, 1990; pp. 272–273. [Google Scholar]
- Romero, C.; Medina, E.; Vargas, J.; Brenes, M.; De Castro, A. In vitro activity of olive oil polyphenols against Helicobacter pylori. J. Agric. Food Chem. 2007, 55, 680–686. [Google Scholar] [CrossRef]
- Song, J.M.; Lee, K.H.; Seong, B.L. Antiviral effect of catechins in green tea on influenza virus. Antivir. Res. 2005, 68, 66–74. [Google Scholar] [CrossRef]
- Perron, N.R.; Brumaghim, J.L. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef]
- Rohenkohl, C.C.; Carniel, A.P.; Colpo, E. Antioxidants Consumption during Chemotherapy Treatment; ABCD Arquivos Brasileiros De Cirurgia Digestiva: Sao Paulo, Brazil, 2011. [Google Scholar]
- Buettner, G.R.; Jurkiewicz, B.A. Catalytic metals, ascorbate and free radicals: Combinations to avoid. Radiat. Res. 1996, 145, 532–541. [Google Scholar] [CrossRef]
- Buettner, G.R. The pecking order of free radicals and antioxidants: Lipid peroxidation, α-tocopherol, and ascorbate. Arch. Biochem. Biophys. 1993, 300, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Van Heeswijk, R.P.; Cooper, C.L.; Foster, B.C.; Chauhan, B.M.; Shirazi, F.; Seguin, I.; Phillips, E.J.; Mills, E. Effect of high-dose vitamin C on hepatic cytochrome P450 3A4 activity. Pharmacotherapy 2005, 25, 1725–1728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.-F.; Zheng, Y.-F.; Zhu, H.-J.; Shen, X.-Y.; Zhu, X.-Q. Effects of kaempferol and quercetin on cytochrome 450 activities in primarily cultured rat hepatocytes. Zhejiang Da Xue Xue Bao Yi Xue Ban 2006, 35, 18–22. [Google Scholar] [PubMed]
- Villanueva, C.; Kross, R.D. Antioxidant-induced stress. Int. J. Mol. Sci. 2012, 13, 2091–2109. [Google Scholar] [CrossRef] [PubMed]
- De Araujo, Q.R.; Gattward, J.N.; Almoosawi, S.; Silva, M.D.; Dantas, P.A.; De Araujo Junior, Q.R. Cocoa and human health: From head to foot—A review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.K.; Siddiqui, I.A.; El-Abd, S.; Mukhtar, H.; Ahmad, N. Combination chemoprevention with grape antioxidants. Mol. Nutr. Food Res. 2016, 60, 1406–1415. [Google Scholar] [CrossRef]
- Hegazy, A.M.; El-Sayed, E.M.; Ibrahim, K.S.; Abdel-Azeem, A.S. Dietary antioxidant for disease prevention corroborated by the Nrf2 pathway. J. Comp. Int. Med. 2019, 16. [Google Scholar] [CrossRef]
- Liu, R.H. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am. J. Clin. Nutr. 2003, 78, 517S–520S. [Google Scholar] [CrossRef]
- Liu, R.H. Potential synergy of phytochemicals in cancer prevention: Mechanism of action. J. Nutr. 2004, 134, 3479S–3485S. [Google Scholar] [CrossRef]
- Van Breda, S.G.J.; de Kok, T.M.C.M. Smart combinations of bioactive compounds in fruits and vegetables may guide new strategies for personalized prevention of chronic diseases. Mol. Nutr. Food Res. 2018, 62, 1700597. [Google Scholar] [CrossRef]
- Sun, J.; Chu, Y.-F.; Wu, X.; Liu, R.H. Antioxidant and antiproliferative activities of fruits. J. Agric. Food Chem. 2002, 50, 7449–7454. [Google Scholar] [CrossRef] [PubMed]
- Linnewiel-Hermoni, K.; Khanin, M.; Danilenko, M.; Zango, G.; Amosi, Y.; Levy, J.; Sharoni, Y. The anti-cancer effects of carotenoids and other phytonutrients resides in their combined activity. Arch. Biochem. Biophys. 2015, 572, 28. [Google Scholar] [CrossRef] [PubMed]
- Holcombe, R.F.; Martinez, M.; Planutis, K.; Planutiene, M. Effects of a grape-supplemented diet on proliferation and Wnt signaling in the colonic mucosa are greatest for those over age 50 and with high arginine consumption. Nutr. J. 2015, 14, 62. [Google Scholar] [CrossRef] [PubMed]
- Fuchs-Tarlovsky, V. Role of antioxidants in cancer therapy. Nutrition 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Niedzwiecki, A.; Roomi, M.W.; Kalinovsky, T.; Rath, M. Anticancer efficacy of polyphenols and their combinations. Nutrients 2016, 8, 552. [Google Scholar] [CrossRef]
- Piao, L.; Mukherjee, S.; Chang, Q.; Xie, X.; Li, H.; Castellanos, M.R.; Banerjee, P.; Iqbal, H.; Ivancic, R.; Wang, X.; et al. TriCurin, a novel formulation of curcumin, epicatechin gallate and resveratrol, inhibits the tumorigenicity of human papillomavirus-positive head and neck squamous cell carcinoma. Oncotarget 2017, 8, 60025–60035. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Singh, M.; Srivastava, A.K.; Bhui, K.; Roy, P.; Chaturvedi, P.K.; Shukla, Y. Resveratrol and black tea polyphenol combination synergistically suppress mouse skin tumors growth by inhibition of activated MAPKS and p53. PLoS ONE 2011, 6, 1–12. [Google Scholar] [CrossRef]
- Khurana, R.K.; Jain, A.; Jain, A.; Sharma, T.; Singh, B.; Kesharwani, P. Administration of antioxidants in cancer: Debate of the decade. Drug Disc. Tod. 2018, 23, 763–770. [Google Scholar] [CrossRef]
- FDA. Ingredients Declared as Evaporated Cane Juice: Guidance for Industry; U.S. Department of Health and Human Services Food and Drug Administration, Center for Food Safety and Applied Nutrition: College Park, MD, USA, 2016. [Google Scholar]
- FDA. Guidance for Industry: Food Labeling; Nutrient Content Claims; Definition for “High Potency” and Definition for “Antioxidant” for Use in Nutrient Content Claims for Dietary Supplements and Conventional Foods; Small Entity Compliance Guide; U.S. Department of Health and Human Services Food and Drug Administration, Center for Food Safety and Applied Nutrition: College Park, MD, USA, 2008. [Google Scholar]
- Pokorný, J. Are natural antioxidants better and safer than synthetic antioxidants? Eur. J. Lipid Sci. Technol. 2017, 109, 629–664. [Google Scholar] [CrossRef]
- Nosrati, N.; Bakovic, M.; Paliyath, G. Molecular mechanisms and pathways as targets for cancer prevention and progression with dietary compounds. Int. J. Mol. Sci. 2017, 18, 2050. [Google Scholar] [CrossRef]
- Kapinova, A.; Kubatka, P.; Golubnitschaja, O.; Kello, M.; Zubor, P.; Solar, P.; Pec, M. Dietary phytochemicals in breast cancer research: Anticancer effects and potential utility for effective chemoprevention. Environ. Health Prev. Med. 2018, 23, 36. [Google Scholar] [CrossRef] [PubMed]
- Bosutti, A.; Zanconati, F.; Grassi, G.; Dapas, B.; Passamonti, S.; Scaggiante, B. Epigenetic and miRNAs dysregulation in prostate cancer: The role of nutraceuticals. Anticancer Agents Med. Chem. 2016, 16, 1385–1402. [Google Scholar] [CrossRef] [PubMed]
- Lichner, Z.; Fendler, A.; Saleh, C.; Nasser, A.N.; Boles, D.; Al-Haddad, S.; Kupchak, P.; Dharsee, M.; Nuin, P.S.; Evans, K.R.; et al. MicroRNA signature helps distinguish early from late biochemical failure in prostate cancer. Clin. Chem. 2013, 59, 1595–1603. [Google Scholar] [CrossRef] [PubMed]
- Allegra, A.; Alonci, A.; Campo, S.; Penna, G.; Petrungaro, A.; Gerace, D.; Musolino, C. Circulating microRNAs: New biomarkers in diagnosis, prognosis and treatment of cancer (review). Int. J. Oncol. 2012, 41, 1897–1912. [Google Scholar] [CrossRef]
- Celano, M.; Rosignolo, F.; Maggisano, V.; Pecce, V.; Iannone, M.; Russo, D.; Bulotta, S. MicroRNAs as biomarkers in thyroid carcinoma. Int. J. Genom. 2017, 2017, 1–11. [Google Scholar] [CrossRef]
- Allegri, L.; Rosignolo, F.; Mio, C.; Filetti, S.; Baldan, F.; Damante, G. Effects of nutraceuticals on anaplastic thyroid cancer cells. J. Cancer Res. Clin. Onc. 2018, 144, 285–294. [Google Scholar] [CrossRef]
- Lelli, D.; Pedone, C.; Sahebkar, A. Curcumin and treatment of melanoma: The potential role of microRNAs. Biomed. Pharm. 2017, 88, 832–834. [Google Scholar] [CrossRef]
- Vardi, A.; Bosviel, R.; Rabiau, N.; Adjakly, M.; Satih, S.; Dechelotte, P.; Boiteux, J.P.; Fontana, L.; Bignon, Y.J.; Guy, L.; et al. Soy phytoestrogens modify DNA methylation of GSTP1, RASSF1A, EPH2 and BRCA1 promoter in prostate cancer cells. Vivo 2010, 24, 393–400. [Google Scholar]
- Phuah, N.H.; Nagoor, N.H. Regulation of microRNAs by natural agents: New strategies in cancer therapies. Biomed. Res. Int. 2014, 2014, 804510. [Google Scholar] [CrossRef]
- Dhar, S.; Hicks, C.; Levenson, A.S. Resveratrol and prostate cancer: Promising role for microRNAs. Mol. Nutr. Food Res. 2011, 55, 1219–1229. [Google Scholar] [CrossRef]
- Hagiwara, K.; Kosaka, N.; Yoshioka, Y.; Takahashi, R.U.; Takeshita, F.; Ochiya, T. Stilbene derivatives promote Ago2-dependent tumour-suppressive microRNA activity. Sci. Rep. 2012, 2, 314. [Google Scholar] [CrossRef] [PubMed]
- Khor, T.O.; Huang, Y.; Wu, T.Y.; Shu, L.; Lee, J.; Kong, A.N. Pharmacodynamics of curcumin as DNA hypomethylation agent in restoring the expression of Nrf2 via promoter CpGs demethylation. Biochem. Pharmacol. 2011, 82, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Hardy, T.M.; Tollefsbol, T.O. Epigenetic diet: Impact on the epigenome and cancer. Epigenomics 2011, 3, 503–518. [Google Scholar] [CrossRef] [PubMed]
- Carlos-Reyes, A.; López-González, J.S.; Meneses-Flores, M.; Gallardo-Rincón, D.; Ruíz-García, E.; Marchant, L.A.; Astudillo-de la Vega, H.; Hernandez de la Cruz, O.N.; Lopez-Camarillo, C. Dietary compounds as epigenetic modulating agents in cancer. Front Genet. 2019, 10, 79. [Google Scholar] [CrossRef] [PubMed]
- Vanhees, K.; Coort, S.; Ruijters, E.J.; Godschalk, R.W.; van Schooten, F.J.; Barjesteh van Waalwijk van Doorn-Khosrovani, S. Epigenetics: Prenatal exposure to genistein leaves a permanent signature on the hematopoietic lineage. FASEB J. 2011, 25, 797–807. [Google Scholar] [CrossRef]
- Patil, V.S.; Zhou, R.; Rana, T.M. Gene regulation by non-coding RNAs. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 16–32. [Google Scholar] [CrossRef]
- Krakowsky, R.H.E.; Tollefsbol, T.O. Impact of nutrition on non-coding RNA epigenetics in breast and gynecological cancer. Front. Nutr. 2015, 2, 16. [Google Scholar] [CrossRef]
- Yuanyuan, L.; Sabita, N.S.; Tollefsbol, T.O. Impact of epigenetic dietary compounds on transgenerational prevention of human diseases. Rev. Artic. AAPS J. 2014, 16, 27–36. [Google Scholar]
- Strogantsev, R.; Ferguson-Smith, A.C. Proteins involved in establishment and maintenance of imprinted methylation marks. Brief. Funct. Genom. 2012, 11, 227–239. [Google Scholar] [CrossRef]
- Luczak, M.W.; Jagodziński, P.P. The role of DNA methylation in cancer development. Folia. Histochem. Cytobiol. 2006, 44, 143–154. [Google Scholar]
- Sundaram, M.K.; Ansari, M.Z.; Mutery, A.A.; Ashraf, M.; Nasab, R.; Rai, S.; Hussain, A. Genistein induces alterations of epigenetic modulatory signatures in human cervical cancer cells. Anticancer Agents Med. Chem. 2017, 18, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Shankar, E.; Kanwal, R.; Candamo, M.; Gupta, S. Dietary phytochemicals as epigenetic modifiers in cancer: Promise and challenges. In Seminars in Cancer Biology Vol. 40; Academic Press: Cambridge, MA, USA, 2016; pp. 82–99. [Google Scholar]
- Khan, M.A.; Hussain, A.; Sundaram, M.K.; Alalami, U.; Gunasekera, D.; Ramesh, L.; Hamza, A.; Quraishi, U. (−)-Epigallocatechin-3-gallate reverses the expression of various tumor-suppressor genes by inhibiting DNA methyltransferases and histone deacetylases in human cervical cancer cells. Oncol. Rep. 2015, 33, 1976–1984. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Kwak, J.; Choi, H.K.; Choi, K.C.; Kim, S.; Lee, J.; Jun, W.; Park, H.J.; Yoon, H.G. EGCG suppresses prostate cancer cell growth modulating acetylation of androgen receptor by anti-histone acetyltransferase activity. Int. J. Mol. Med. 2012, 30, 69–74. [Google Scholar] [PubMed]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A review of its’ effects on human health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Kumar, U.; Sharma, U.; Rathi, G. Reversal of hypermethylation and reactivation of glutathione S-transferase pi 1 gene by curcumin in breast cancer cell line. Tumor Biol. 2017, 39. [Google Scholar] [CrossRef]
- Du, L.; Xie, Z.; Wu, L.C.; Chiu, M.; Lin, J.; Chan, K.K.; Liu, S.; Liu, Z. Reactivation of RASSF1A in breast cancer cells by curcumin. Nutr. Cancer 2012, 64, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- Jiang, A.; Wang, X.; Shan, X.; Li, Y.; Wang, P.; Jiang, P.; Feng, Q. Curcumin reactivates silenced tumor suppressor gene RARβ by reducing DNA methylation. Phytother. Res. 2015, 29, 1237–1245. [Google Scholar] [CrossRef]
- Wu, B.; Yao, X.; Nie, X.; Xu, R. Epigenetic reactivation of RANK in glioblastoma cells by curcumin: Involvement of STAT3 inhibition. DNA Cell Biol. 2013, 32, 292–297. [Google Scholar] [CrossRef]
- Yu, J.; Peng, Y.; Wu, L.C.; Xie, Z.; Deng, Y.; He, S.; Mo, X.; Chiu, M.; Wang, Q.E.; He, X.; et al. Curcumin down-regulates DNA methyltransferase 1 and plays an anti-leukemic role in acute myeloid leukemia. PLoS ONE 2013, 8, e55934. [Google Scholar] [CrossRef]
- Zhao, W.; Zhou, X.; Qi, G.; Guo, Y. Curcumin suppressed the prostate cancer by inhibiting JNK pathways via epigenetic regulation. J. Biochem. Mol. Toxicol. 2018, 32, e22049. [Google Scholar] [CrossRef]
- Borriello, A. Resveratrol in cancer prevention and treatment: Focusing on molecular targets and mechanism of action. Multidiscip. Dig. Publish. Inst. Proc. 2017, 1, 97. [Google Scholar] [CrossRef]
- Dhar, S.; Kumar, A.; Li, K.; Tzivion, G.; Levenson, A.S. Resveratrol regulates PTEN/Akt pathway through inhibition of MTA1/HDAC unit of the NuRD complex in prostate cancer. Biochim. Biophys. Acta Mol. Cell Res. 2015, 1853, 265–275. [Google Scholar] [CrossRef]
- Jefferson Myrna Brind Center of Integrative Medicine. Antioxidant Supplementation in Cancer: Potential Interactions with Conventional Chemotherapy and Radiation Therapy; Thomas Jefferson University: Philadelphia, PA, USA, 2006. [Google Scholar]
- Drisko, J.A.; Chapman, J.; Hunter, V.J. The use of antioxidants with first-line chemotherapy in two cases of ovarian cancer. J. Am. Coll. Nutr. 2003, 22, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhu, H.; Huang, J.; Zhu, Y.; Hong, M.; Zhu, H.; Zhang, J.; Li, S.; Yang, L.; Wang, S.; et al. The synergy of Vitamin C with decitabine activates TET2 in leukemic cells and significantly improves overall survival in elderly patients with acute myeloid leukemia. Leuk. Res. 2018, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zeichner, S.B.; Koru-Sengul, T.; Shah, N. Improved clinical outcomes associated with vitamin D supplementation during adjuvant chemotherapy in patients with HER2+ nonmetastatic breast cancer. Clin. Breast Cancer 2015, 15, e1–e11. [Google Scholar] [CrossRef]
- Soodabeh, S.; Abdollahi, M. Antioxidants: Friends or foe in prevention or treatment of cancer: The debate of the century. Toxic. Appl. Pharm. 2013, 271, 49–63. [Google Scholar]
- Pascual-Teresa, S.D. Molecular mechanisms involved in the cardiovascular and neuroprotective effects of Anthocyanins. Arch. Biochem. Biophys. 2014, 559, 68–74. [Google Scholar] [CrossRef]
- Moss, R.W. Do antioxidants interfere with radiation therapy for cancer? Integr. Cancer Ther. 2007, 6, 281–292. [Google Scholar] [CrossRef]
- Young, J.S. NF-kB and Nrf2 as potential chemopreventive targets of some anti-inflammatory and antioxidative phytonutrients with anti-inflammatory and anti-oxidative activities. Asia Pac. J. Clin. Nutr. 2008, 17, 269–272. [Google Scholar]
- Doraii, T.; Aggarwal, B.B. Role of chemopreventive agents in cancer therapy. Cancer Lett. 2004, 215, 129–140. [Google Scholar] [CrossRef]
- Wargovich, M.J.; Morris, J.; Brown, V.; Ellis, J.; Logothetis, B.; Weber, R. Nutraceutical use in late-stage cancer. Cancer Metastasis Rev. 2010, 29, 503–510. [Google Scholar] [CrossRef]
- Gwilt, P.R.; Lear, C.L.; Tempero, M.A.; Birt, D.D.; Grandjean, A.C.; Ruddon, R.W.; Nagel, D.L. The effect of garlic extract on human metabolism of acetaminophen. Cancer Epidemiol. Biomark. Prev. 1994, 3, 155–160. [Google Scholar]
- Hu, Z.; Yang, X.; Ho, P.C.; Chan, S.Y.; Heng, P.W.; Chan, E.; Duan, W.; Koh, H.L.; Zhou, S. Herb–drug interactions: A literature review. Drugs 2005, 65, 1239–1282. [Google Scholar] [CrossRef] [PubMed]
- Vaes, L.P.; Chyka, P.A. Interactions of warfarin with garlic, ginger, ginkgo, or ginseng: Nature of the evidence. Ann. Pharmacother. 2000, 34, 1478–1482. [Google Scholar] [CrossRef] [PubMed]
- Conklin, K.A. Chemotherapy associated oxidative stress: Impact on chemotherapeutic effectiveness. Integr. Cancer Ther. 2004, 3, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Perumal, S.S.; Shanthi, P.; Sachdanandam, P. Combined efficacy of tamoxifen and coenzyme Q10 on the status of lipid peroxidation and antioxidant in DMBA induced breast cancer. Mol. Cell. Biochem. 2005, 273, 151–160. [Google Scholar] [CrossRef]
- Perumal, S.S.; Shanthi, P.; Sachdanandam, P. Augmented efficacy of tamoxifen in rat breast tumorigenesis when gavaged along with riboflavin, niacin, and CoQ10: Effects on lipid peroxidation and antioxidants in mitochondria. Chem. Biol. Interact. 2005, 152, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Bhori, M.; Kasu, Y.A.; Bhat, G.; Marar, T. Antioxidants as precision weapons in war against cancer chemotherapy induced toxicity—Exploring the armoury of obscurity. Saudi Pharm. J. 2018, 26, 177–190. [Google Scholar] [CrossRef]
- Prasad, K.N.; Colea, W.C.; Kumara, B.; Prasad, C.K. Pros and cons of antioxidant use during radiation therapy. Cancer Treat. Rev. 2002, 28, 79–91. [Google Scholar] [CrossRef]
- Simone, C.B., II; Simone, N.L.; Simone, V.; Simone, C.B. Antioxidants and other nutrients do not interfere with chemotherapy or radiation therapy and can increase kill and increase survival, part II. Altern. Ther. Health Med. 2007, 13, 40–47. [Google Scholar]
- Simone, C.B., II; Simone, N.L.; Simone, V.; Simone, C.B. Antioxidants and other nutrients do not interfere with chemotherapy or radiation therapy and can increase kill and increase survival, part 1. Altern. Ther. Health Med. 2007, 13, 22–28. [Google Scholar] [PubMed]
- Tosetti, F.; Ferrari, N.; Flora, S.D.; Albini, A. ‘Angioprevention:’ angiogenesis is a common and key target for cancer chemopreventive agents. FASEB J. 2002, 16, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Oak, M.H.; Bedoui, J.E.L.; Valerie, B.; Kerth, S. Antiangiogenic properties of natural polyphenols from red wine and green tea. J. Nutr. Biochem. 2005, 16, 1–8. [Google Scholar] [CrossRef]
- Lamy, S.; Blanchette, M.; Michaud-Levesque, J.; Lafleur, R.; Durocher, Y.; Moghrabi, A.; Barrerre, S.; Gingras, D.; Beliveau, R. Delphinidin, a dietary anthocyanidin, inhibits vascular endothelial growth factor receptor-2 phosphorylation. Carcinogenesis 2006, 27, 989–996. [Google Scholar] [CrossRef]
- Labrecque, L.; Lamy, S.; Chapus, A.; Mihoubi, S.; Durocher, Y.; Cass, B.; Bojanowski, M.W.; Gingras, D.; Beliveau, R. Combined inhibition of PDGF and VEGF receptors by ellagic acid, a dietary derived phenolic compound. Carcinogenesis 2005, 26, 821–826. [Google Scholar] [CrossRef]
- Chen, X.; Hu, P.Z.; Yang, X.X.; Huang, M.; Gao, Y.; Tang, W.; Chan, S.Y.; Dai, X.; Je, J.; Ho, P.C.; et al. Monitoring of immune responses to a herbal immuno-modulator in patients with advanced colorectal cancer. Int. Immunopharmacol. 2006, 6, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Dijsselbloem, N.; Berghe, W.V.; Naeyer, A.D.; Haegeman, G. Soy isoflavonephyto-pharmaceuticals in interleukin-6 affections multi-purpose nutraceuticals at the crossroad of hormone replacement, anti-cancer and anti-inflammatory therapy. Biochem. Pharmacol. 2004, 68, 1171–1785. [Google Scholar] [CrossRef]
- Surh, Y.J. Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer 2003, 3, 768–780. [Google Scholar] [CrossRef] [PubMed]
- Greenlee, H.; Kwan, M.L.; Kushi, L.H. Antioxidant supplement use after breast cancer diagnosis and mortality in the LACE cohort. Cancer 2012, 118, 2048–2058. [Google Scholar] [CrossRef] [PubMed]
- Greenlee, H.; Shaw, J.; Ingar Lau, Y.; Naini, A.; Maurer, M. Lack of effect of coenzyme Q10 on doxorubicin cytotoxicity in breast cancer cell cultures. Integr. Cancer Ther. 2012, 11, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Nechuta, S.; Lu, W.; Chen, Z.; Zheng, Y.; Gu, K.; Cai, H.; Zheng, W.; Shu, X.O. Vitamin supplement use during breast cancer treatment and survival: A prospective cohort study. Cancer Epidemiol. Biomark. Prev. 2011, 20, 262. [Google Scholar] [CrossRef] [PubMed]
- Lamson, D.W.; Brignall, M.S. Antioxidant in cancer therapy: Their actions and interactions with oncologic therapies. Altern. Med. Rev. 1999, 4, 304–329. [Google Scholar] [PubMed]
- Kline, K.; Yu, W.; Sanders, B.G. Vitamin E and breast cancer. J. Nutr. 2004, 134, 3458–3462. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Jeong, J.H.; Lee, I.H.; Lee, J.; Jung, J.H.; Park, H.Y.; Lee, D.H.; Chae, Y.S. Effect of high-dose vitamin C combined with anti-cancer treatment on breast cancer cells. Anticancer Res. 2019, 39, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhang, F.; Zhao, Y.; Shao, D.; Zheng, X.; Chen, Y.; He, K.; Li, J.; Chen, L. Berberine Enhances Chemosensitivity and Induces Apoptosis Through Dose-orchestrated AMPK Signaling in Breast Cancer. J. Cancer. 2017, 8, 1679–1689. [Google Scholar] [CrossRef]
- Puri, T.; Julka, P.K.; Goyal, S.; Nair, O.; Sharma, D.N.; Rath, G.K. Role of natural lycopene and phytonutrients along with radiotherapy and chemotherapy in high-grade gliomas. J. Clin. Oncol. 2005, 16, 1561. [Google Scholar] [CrossRef]
- Gloria, N.F.; Soares, N.; Brand, C.; Oliveira, F.L.; Borojevic, R.; Teodoro, A.J. Lycopene and beta-carotene induce cell-cycle arrest and apoptosis in human breast cancer cell lines. Anticancer Res. 2014, 34, 1377–1386. [Google Scholar]
- Sur, S.; Panda, C.K. Molecular aspects of cancer chemopreventive and therapeutic efficacies of tea and tea polyphenols. Nutrition 2017, 43–44, 8–15. [Google Scholar] [CrossRef]
- Wang, J.; Sun, P.; Wang, Q.; Zhang, P.; Wang, Y.; Zi, C.; Wang, X.; Sheng, J. (−)-Epigallocatechin-3-gallate derivatives combined with cisplatin exhibit synergistic inhibitory effects on non-small-cell lung cancer cells. Cancer Cell Int. 2019, 19, 266. [Google Scholar] [CrossRef]
- Banerjee, S.; Singh, S.K.; Chowdhury, I.; Lillard, J.W., Jr.; Singh, R. Combinatorial effect of curcumin with docetaxel modulates apoptotic and cell survival molecules in prostate cancer. Front. Biosci. 2017, 9, 235–245. [Google Scholar]
- Al-Sharif, I.; Remmal, A.; Aboussekhra, A. Eugenol triggers apoptosis in breast cancer cells through E2F1/survivin down-regulation. BMC Cancer 2013, 13. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.S.; Al-Sharif, I.; Sultan, A.; Al-Mazrou, A.; Remmal, A.; Aboussekhra, A. Eugenol potentiates cisplatin anti-cancer activity through inhibition of ALDH-positive breast cancer stem cells and the NF-κB signaling pathway. Mol. Carcinog. 2018, 57, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Pons, D.G.; Nadal-Serrano, M.; Torrens-Mas, M.; Oliver, J.; Roca, P. The phytoestrogen genistein affects breast cancer cells treatment depending on the ER alpha/ER beta ratio. J. Cell. Biochem. 2016, 117, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.H.; Hong, S.S.; Cho, Y.R.; Seo, D.W. 10-Gingerol inhibits proliferation and invasion of MDA-MB-231 breast cancer cells through suppression of Akt and p38(MAPK) activity. Oncol. Rep. 2016, 35, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zha, L.; Luo, L.; Chen, X.; Zhang, Q.; Gao, C.; Zhuang, X.; Yuan, S.; Qiao, T. [6]-Gingerol enhances the cisplatin sensitivity of gastric cancer cells through inhibition of proliferation and invasion via PI3K/AKT signaling pathway. Phytother. Res. 2019, 33, 1353–1362. [Google Scholar] [CrossRef]
- Sun, S.; Gong, F.; Liu, P.; Miao, Q. Metformin combined with quercetin synergistically repressed prostate cancer cells via inhibition of VEGF/PI3K/Akt signaling pathway. Gene 2018, 664, 50–57. [Google Scholar] [CrossRef]
- Lee, A.M.; Shandala, T.; Soo, P.P.; Su, P.P.; Su, Y.W.; King, T.J.; Chen, K.M.; Howe, P.R.; Xian, C.J. Effects of resveratrol supplementation on methotrexate chemotherapy-induced bone loss. Nutrients 2017, 9, 255. [Google Scholar] [CrossRef]
| Nutraceuticals | Doses | Model | Effects of Nutraceutical Alone | Combined Effect with Chemotherapy | Ref. |
|---|---|---|---|---|---|
| Ascorbic acid (vitamin C) | Vitamin C (0–20 mM) + various doses of chemotherapic drugs | In vitro | increased ROS and anti-tumorigenic effect with high doses of vit C in cancer cells without meaningful toxicities to normal cells | high-doses vitamin C inhibited cancer cells proliferation, increased apoptosis in BC cells; additional inhibitory effect on cells growth | [176] |
| Berberine | BBR from 2.5∼320 µM doxorubicin different concentrations | In vivo and in vitro | AMPK activator, activated caspase; PARP-1 cleavage; Cytochrome c release; cell cycle arrest | BBR sensitized drug-resistant BC to chemotherapy; directly induced apoptosis through the dose-orchestrated AMPK signaling pathway | [177] |
| Carotenoids, lycopene | 0.5–10 μM | In vitro | cell proliferation inhibition, cell cycle arrest, increased apoptosis of cancer cells | Protective effects against cisplatin-induced nephrotoxicity and doxorubicin-induced cardiotoxicity | [178,179] |
| Epigallocatechin-3-gallate (EGCG) derivatives | ECGC derivatives + cisplatin (2mg/kg per 2 days) | In vivo (mouse) and in vitro | cell-cycle arrest, inducted apoptosis and ROS, inhibited NF-ĸB, HER-2/neu, (IGF-1)-mediated and EGF-mediated signaling pathways, inhibited proteasome activity, iNOS, MMPs, VEGF, AP-1, MAPKs and COX-2 expression | EGCG derivatives inhibited cell viability and colony formation, caused cell cycle redistribution, induced apoptosis. Co-treatment enhanced apoptosis rate; reduced tumor growth. | [180,181] |
| Curcumin | 20 μM curcumin10nM docetaxel | In vitro | cell proliferation inhibition, anti-invasive activity, angiogenesis inhibition; Nrf2 enzymes activation; promoted tumor suppressor p53 and TGF-β and COX-2 reduction | curcumin enhanced efficacy of docetaxel, inducted apoptosis, inhibited proliferation, down-regulated NF-κB, COX-2, RTKs, and kinases PI3K and phospho-AKT by combined treatment | [182] |
| Eugenol | 1 μM eugenol + 30 μM cisplatin (vitro) 2 mg/kg cisplatin + 50 mg/kg eugenol (in vivo) | In vitro and in vivo (mouse) | growth and proliferation inhibition, induced apoptosis through targeting the E2F1/surviving pathway | Co-treatment significantly increased cytotoxic and pro-apoptotic effects, eugenol potentiated cisplatin inhibition of the NF-κB signaling pathway. Down-regulation of the IL-6 and IL-8 cytokines; inhibited epithelial-to-mesenchymal transition and stemness markers in tumor xenografts. | [183,184] |
| Genistein | 1 μM genistein, 10 μM cisplatin, 10 nM paclitaxel, 10 μM tamoxifen | In vitro | cell cycle arrest, improved mitochondrial functionality, regulated OS, uncoupling proteins, antioxidant enzymes and sirtuin, enhanced effects of anticancer drugs | Co-treatment increased cell viability and antioxidant protein levels, decreased ROS, decreased autophagy and apoptosis | [185] |
| Gingerol | 10 μM gingerol or 300 μM gingerol+ 2 μg/mL cisplatin | In vitro | inhibited proliferation and metastasis, cell cycle arrest through inactivation of Akt and p38MAPK activity, suppressed epidermal growth factor receptor expression | Co-treatment inhibited cell viability, enhanced cell cycle arrest at G1 phase; inhibited cell migration and invasion ability; decreased cyclin D1, cyclin A2, MMP-9, p-PI3K, AKT, and p-AKT protein expressions and increased P21 and P27 mRNA levels. | [186,187] |
| Quercetin | 20 μM quercitin + 40 μM metformin | In vivo (nude mice) in vitro | induced apoptosis, induced ER stress, activated pSTAT3/Bcl2 axis, induced protective autophagy | Co-treatment synergistically inhibited growth, migration and invasion of cancer cells, strongly inhibited the VEGF/Akt/PI3K pathway; the increased apoptosis was caspase-dependent and by down-regulation of Bcl-2 family members | [188] |
| Resveratrol | Differential concentrations | In vivo (rats) | Inhibited CYPA1 drug metabolism and COX activity. Suppressed TNF-α and IL-17. Influenced fatty acids oxidation, mitochondrial biogenesis, respiration, gluconeogenesis | RES prevented bone loss from MTX chemotherapy–induced and bone marrow adiposity; bone-protective properties (pro-osteogenic, antiresorptive, and antiadipogenic) | [17,189] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvani, M.; Pasha, A.; Favre, C. Nutraceutical Boom in Cancer: Inside the Labyrinth of Reactive Oxygen Species. Int. J. Mol. Sci. 2020, 21, 1936. https://doi.org/10.3390/ijms21061936
Calvani M, Pasha A, Favre C. Nutraceutical Boom in Cancer: Inside the Labyrinth of Reactive Oxygen Species. International Journal of Molecular Sciences. 2020; 21(6):1936. https://doi.org/10.3390/ijms21061936
Chicago/Turabian StyleCalvani, Maura, Amada Pasha, and Claudio Favre. 2020. "Nutraceutical Boom in Cancer: Inside the Labyrinth of Reactive Oxygen Species" International Journal of Molecular Sciences 21, no. 6: 1936. https://doi.org/10.3390/ijms21061936
APA StyleCalvani, M., Pasha, A., & Favre, C. (2020). Nutraceutical Boom in Cancer: Inside the Labyrinth of Reactive Oxygen Species. International Journal of Molecular Sciences, 21(6), 1936. https://doi.org/10.3390/ijms21061936





