Water Cooking Stability of Dried Noodles Enriched with Different Particle Size and Concentration Green Tea Powders
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Particle Size Analysis and Color Measurement of GTP
2.3. Dried Green Tea Noodle Production
2.4. Cooking Properties of DGTN
2.5. Measurement of Free Phenolic Content (FPC), Total Chlorophyll, Chlorophyll a and Chlorophyll b Contents
2.6. Antioxidant Activity
2.6.1. Extraction
2.6.2. 1-Diphenyl-2-Picrylhydrazyl (DPPH) Scavenging Activity
2.6.3. ABTS•+ Scavenging Activity
2.6.4. Ferric Reducing Antioxidant Power (FRAP)
2.7. Texture Analysis of DGTN
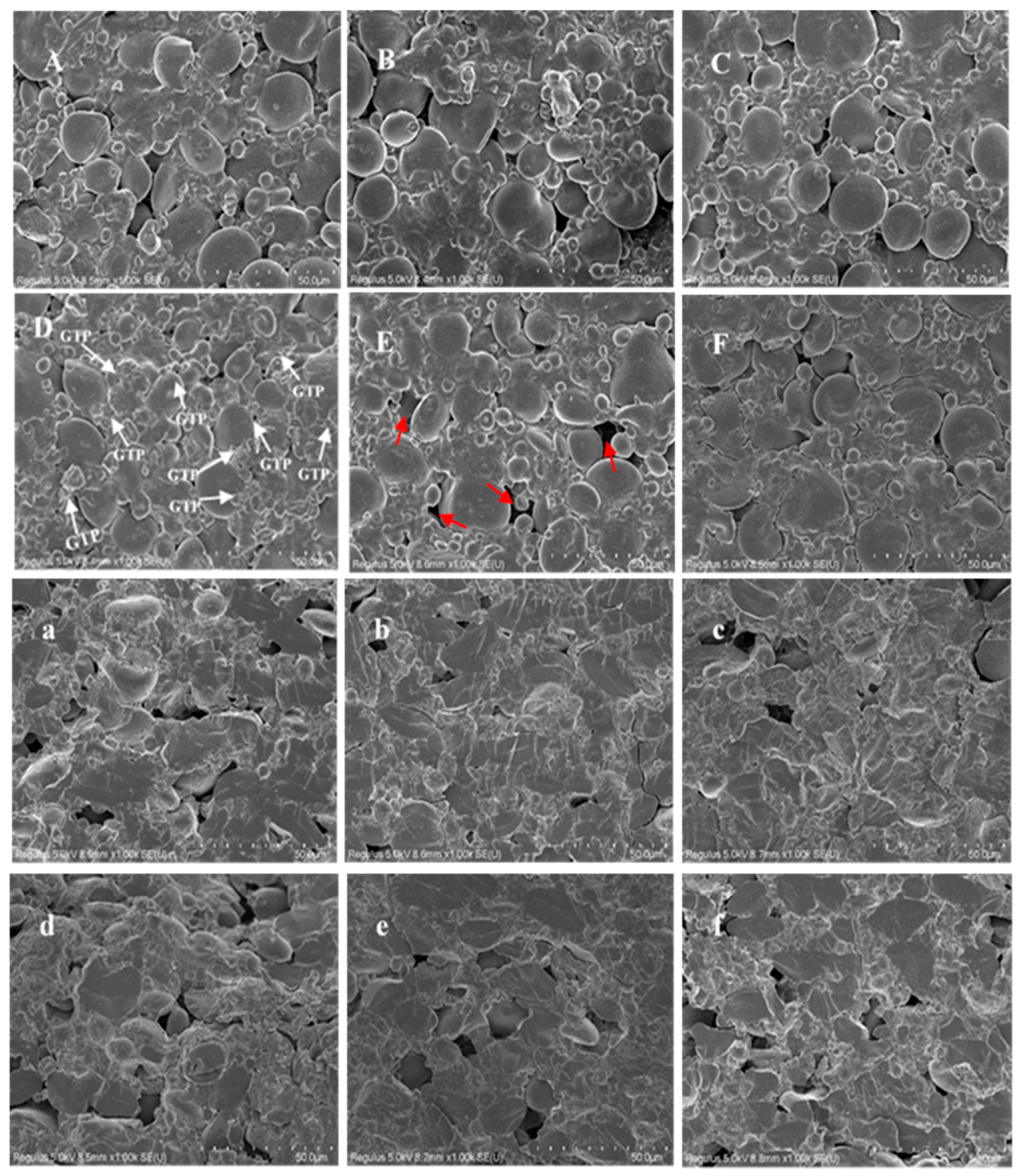
2.8. Scanning Electron Microscope (SEM) of GTP and DGTN
2.9. Statistical Analysis
3. Results and Discussion
3.1. Composition, Color, Particle size, and Microstructure of GTP
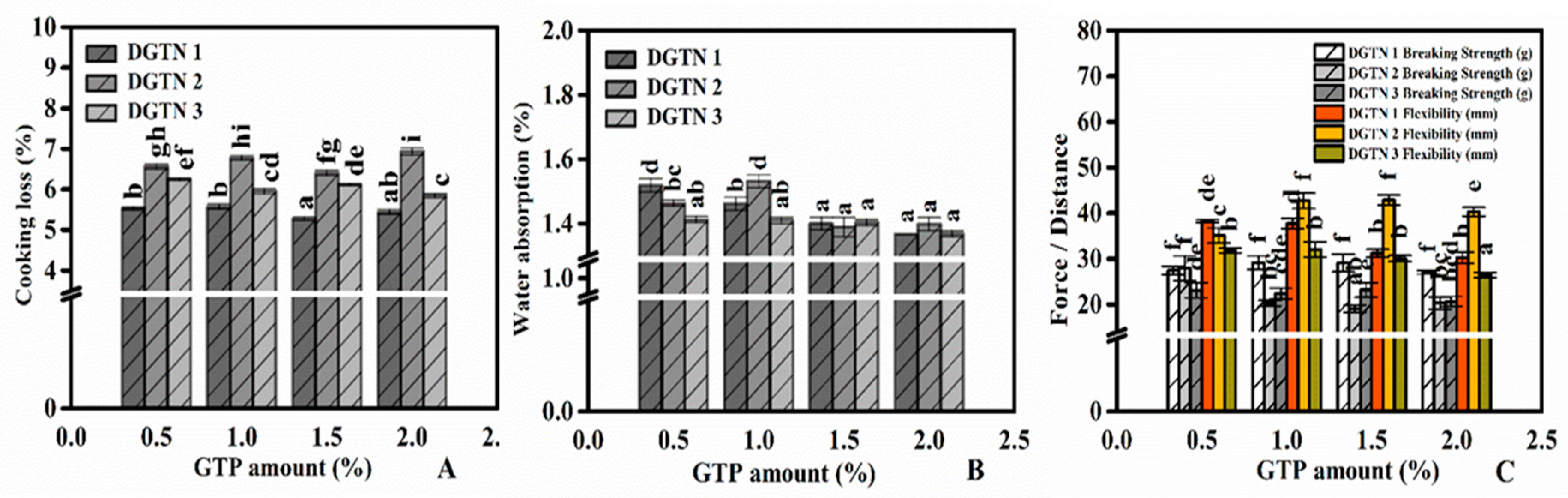
3.2. Water Cooking Stability of DGTN
3.2.1. Cooking Loss and Water Absorption
3.2.2. Functional Component Leakages
3.2.3. Antioxidant Activity Changes
3.3. Texture Properties of DGTN
3.4. Microstructures of DGTN
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yu, P.; Low, M.Y.; Zhou, W. Development of a partial least squares-artificial neural network (PLS-ANN) hybrid model for the prediction of consumer liking scores of ready-to-drink green tea beverages. Food Res. Int. 2018, 103, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.F.; Park, S.C.; Chung, J.O.; Baik, J.H.; Park, S.K. The compounds contributing to the greenness of green tea. J. Food Sci. 2004, 69, S301–S305. [Google Scholar] [CrossRef]
- Wang, K.B.; Ruan, J.-Y. Analysis of chemical components in green tea in relation with perceived quality, a case study with Longjing teas. Int. J. Food Sci. Technol. 2009, 44, 2476–2484. [Google Scholar] [CrossRef]
- Sakurai, Y.; Mise, R.; Kimura, S.; Noguchi, S.; Iwao, Y.; Itai, S. Novel method for improving the water dispersibility and flowability of fine green tea powder using a fluidized bed granulator. J. Food Eng. 2017, 206, 118–124. [Google Scholar] [CrossRef]
- Kurauchi, Y.; Devkota, H.P.; Hori, K.; Nishihara, Y.; Hisatsune, A.; Katsuki, T.S.H. Anxiolytic activities of Matcha tea powder, extracts, and fractions in mice: Contribution of dopamine D1 receptor- and serotonin 5-HT 1A receptor-mediated mechanisms. J. Funct. Foods 2019, 59, 301–308. [Google Scholar] [CrossRef]
- Li, M.; Zhang, J.H.; Zhu, K.X.; Peng, W.; Zhang, S.K.; Wang, B.; Zhu, Y.J.; Zhou, H.M. Effect of superfine green tea powder on the thermodynamic, rheological and fresh noodle making properties of wheat flour. LWT Food Sci. Technol. 2012, 46, 23–28. [Google Scholar] [CrossRef]
- Sharma, A.; Zhou, W.B. A stability study of green tea catechins during the biscuit making process. Food Chem. 2011, 126, 568–573. [Google Scholar] [CrossRef]
- Wang, R.; Zhou, W.B.; Jiang, X.H. Mathematical modeling of the stability of green tea catechin epigallocatechin gallate (EGCG) during bread baking. J. Food Eng. 2008, 87, 505–513. [Google Scholar] [CrossRef]
- Verardo, V.; Roman, D.A.; Carretero, A.S.; Marconi, E.; Gutierrez, A.F.; Caboni, M.F. Determination of Free and Bound Phenolic Compounds in Buckwheat Spaghetti by RP-HPLC-ESI-TOF-MS: Effect of Thermal Processing from Farm to Fork. J. Agric. Food Chem. 2011, 59, 7700–7707. [Google Scholar] [CrossRef]
- Paula, R.D.; Rabalski, I.; Messia, M.C.; Abdel-Aal, E.M.; Marconi, E. Effect of processing on phenolic acids composition and radical scavenging capacity of barley pasta. Food Res. Int. 2017, 102, 136–143. [Google Scholar] [CrossRef]
- Fares, C.; Menga, V.; Martina, A.; Pellegrini, N.; Scazzina, F.; Torriani, S. Nutritional profile and cooking quality of a new functional pasta naturally enriched in phenolic acids, added with b -glucan and Bacillus coagulans GBI-30, 6086. J. Cereal Sci. 2015, 65, 260–266. [Google Scholar] [CrossRef]
- Rocchetti, G.; Lucini, L.; Chiodelli, G.; Giuberti, G.; Montesano, D.; Masoero, F.; Trevisan, M. Impact of boiling on free and bound phenolic profile and antioxidant activity of commercial gluten-free pasta. Food Res. Int. 2017, 100, 69–77. [Google Scholar] [CrossRef] [PubMed]
- AACC. Method 46-12.01. -Crude Protein-Kjeldahl Method, Boric Acid Modification. In American Association of Cereal Chemists, 10th ed.; AACC International: St Paul, MN, USA, 2000. [Google Scholar]
- Yu, K.; Zhou, H.M.; Zhu, K.X.; Guo, X.N.; Peng, W. Increasing the physicochemical stability of stored green tea noodles: Analysis of the quality and chemical components. Food Chem. 2019, 278, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Inglett, G.E.; Peterson, S.C.; Carriere, C.J.; Maneepun, S. Rheological, textural, and sensory properties of Asian noodles containing an oat cereal hydrocolloid. Food Chem. 2005, 90, 1–8. [Google Scholar] [CrossRef]
- Savlak, N.; Turker, B.; Yesilkanat, N. Effects of particle size distribution on some physical, chemical and functional properties of unripe banana flour. Food Chem. 2016, 213, 180–186. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, L.; Yang, T.; Liu, Q.; Chen, X.; Zhang, Y.; Shu, G.; Li, J. Production of superfine green tea powder from processing wastes: Characterization of chemical composition and exploration of antimicrobial potential against Ralstonia solanacearum. LWT Food Sci. Technol. 2019, 104, 142–147. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, W.; Cao, Y.; Ji, G.; Gao, C.; Han, L. The effect of ultrafine and coarse grinding on the suspending and precipitating properties of black tea powder particles. J. Food Eng. 2018, 223, 124–131. [Google Scholar] [CrossRef]
- Chen, J.S.; Fei, M.J.; Shi, C.L.; Tian, J.C.; Sun, C.L.; Zhang, H.; Ma, Z.; Dong, H.X. Effect of particle size and addition level of wheat bran on quality of dry white Chinese noodles. J. Cereal Sci. 2011, 53, 217–224. [Google Scholar] [CrossRef]
- Abreu, J.; Quintino, I.; Pascoal, G.; Postingher, B.; Cadena, R.; Teodoro, A. Antioxidant capacity, phenolic compound content and sensory properties of cookies produced from organic grape peel (Vitis labrusca) flour. Int. J. Food Sci. Technol. 2019, 54, 1215–1224. [Google Scholar] [CrossRef]
- Xu, L.; Pan, H.; Lei, Q.; Xiao, W.; Peng, Y.; Xiao, P. Insect tea, a wonderful work in the Chinese tea culture. Food Res. Int. 2013, 53, 629–635. [Google Scholar] [CrossRef]
- Rocchetti, G.; Giuberti, G.; Gallo, A.; Bernardi, J.; Marocco, A.; Lucini, L. Effect of dietary polyphenols on the in vitro starch digestibility of pigmented maize varieties under cooking conditions. Food Res. Int. 2018, 108, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Sakulnak, R.; Wang, S. Effect of black tea on antioxidant, textural, and sensory properties of Chinese steamed bread. Food Chem. 2016, 194, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Cao, H.; Hou, M.; Nirasawa, S.; Tatsumi, E.; Foster, T.J.; Cheng, Y. Effect of konjac glucomannan on physical and sensory properties of noodles made from low-protein wheat flour. Food Res. Int. 2013, 51, 879–885. [Google Scholar] [CrossRef]
- Ma, X.; Ryu, G. Effects of green tea contents on the quality and antioxidant properties of textured vegetable protein by extrusion-cooking. Food Sci. Biotechnol. 2019, 28, 67–74. [Google Scholar] [CrossRef]
- Pongpichaiudom, A.; Songsermpong, S. Evaluation of microstructure and quality characteristics of microwave-dried instant noodles enriched with chicken meat, egg yolk, and seaweed. J. Food Meas. Charact. 2017, 12, 22–34. [Google Scholar] [CrossRef]
- Yang, Z.; Yu, W.; Xu, D.; Guo, L.; Wu, F.; Xu, X. Impact of frozen storage on whole wheat starch and its A-Type and B-Type granules isolated from frozen dough. Carbohydr. Polym. 2019, 223, 115142. [Google Scholar] [CrossRef]

| - | GTP 1 | GTP 2 | GTP 3 |
|---|---|---|---|
| Average volume particle diameter (μm) | 6.60 ± 1.91 a | 15.53 ± 2.62 b | 9.78 ± 2.01 ab |
| Protein (%) | 24.43 ± 0.16 c | 22.62 ± 0.13 b | 20.32 ± 0.10 a |
| Moisture (%) | 4.19 ± 0.00 a | 5.88 ± 0.01 c | 5.08 ± 0.02 b |
| Free phenolic (mg/g) | 163.59 ± 0.21 c | 139.75 ± 0.11 a | 147.11 ± 0.09 b |
| Chlorophyll a (Ca/mg/g) | 3.64 ± 0.03 c | 3.18 ± 0.02 b | 2.74 ± 0.06 a |
| Chlorophyll b (Cb/mg/g) | 1.97 ± 0.01 b | 1.83 ± 0.01 a | 1.82 ± 0.04 a |
| Total chlorophyll (TC/mg/g) | 5.61 ± 0.05 c | 5.00 ± 0.08 b | 4.56 ± 0.10 a |
| Color | - | - | - |
| L* | 60.41 ± 0.00 b | 55.23 ± 0.20 a | 55.82 ± 0.41 a |
| a* | -12.33 ± 0.01 a | -9.01 ± 0.31 b | -7.43 ± 0.03 c |
| b* | 27.07 ± 0.22 c | 25.08 ± 0.30 b | 24.12 ± 0.31 a |
| Addition Amount | DGTN 1 | DGTN 2 | DGTN 3 | |||
|---|---|---|---|---|---|---|
| 0.5% | 2% | 0.5% | 2% | 0.5% | 2% | |
| Chlorophyll a | - | - | - | - | - | - |
| Uncooked (μg/g) | 17.37 ± 0.04 b | 60.73 ± 1.60 e | 13.76 ± 0.37 a | 52.36 ± 0.23 d | 13.62 ± 0.05 a | 45.84 ± 0.06 c |
| Cooked (μg/g) | 14.50 ± 0.10 a | 46.74 ± 1.09 c | 13.67 ± 0.11 a | 44.90 ± 2.40 c | 13.06 ± 0.06 a | 40.64 ± 0.66 b |
| Retention rate (%) | 83.48 ± 0.73 ab | 76.96 ± 0.23 a | 99.47 ± 3.52 c | 85.77 ± 4.94 b | 95.87 ± 0.84 c | 88.65 ± 1.32 b |
| Chlorophyll b | - | - | - | - | ||
| Uncooked (μg/g) | 13.85 ± 0.05 b | 32.46 ± 0.73 e | 11.83 ± 0.09 a | 28.74 ± 0.29 d | 11.40 ± 0.15 a | 24.99 ± 0.21 c |
| Cooked (μg/g) | 12.32 ± 0.15 b | 24.83 ± 0.40 e | 11.15 ± 0.03 a | 22.87 ± 0.85 d | 10.72 ± 0.00 a | 20.64 ± 0.31 c |
| Retention rate (%) | 88.95 ± 0.71 c | 76.50 ± 0.49 a | 94.25 ± 0.44 d | 79.62 ± 3.78 ab | 94.10 ± 1.28 d | 82.59 ± 1.94 b |
| Total chlorophyll | - | - | - | - | ||
| Uncooked (μg/g) | 31.22 ± 0.09 b | 93.20 ± 2.33 e | 25.58 ± 0.46 a | 81.10 ± 0.52 d | 25.02 ± 0.21 a | 70.83 ± 0.15 c |
| Cooked (μg/g) | 26.82 ± 0.05 a | 71.56 ± 1.49 c | 24.82 ± 0.08 a | 67.76 ± 3.25 c | 23.78 ± 0.06 a | 61.28 ± 0.97 b |
| Retention rate (%) | 85.91 ± 0.01 b | 76.80 ± 0.32 a | 97.04 ± 2.07 c | 83.59 ± 4.54 b | 95.07 ± 1.04 c | 86.51 ± 1.55 b |
| Free phenolic content | - | - | - | - | - | - |
| Uncooked (mg/g) | 3.18 ± 0.02 b | 5.42 ± 0.00 d | 2.46 ± 0.03 a | 4.92 ± 0.18 c | 2.76 ± 0.05 a | 4.70 ± 0.22 c |
| Cooked (mg/g) | 1.71 ± 0.06 a | 3.41 ± 0.09 c | 1.57 ± 0.01 a | 3.34 ± 0.01 bc | 1.64 ± 0.01 a | 3.19 ± 0.10 b |
| Retention rate (%) | 53.76 ± 2.31 a | 62.86 ± 1.54 b | 63.96 ± 0.43 bc | 67.92 ± 2.74 c | 59.37 ± 0.69 b | 67.82 ± 1.08 c |
| Sample | DPPH/IC50 (mg/mL) | ABTS/mmol/g | FRAP/mmol/g | |||
|---|---|---|---|---|---|---|
| GTP 1 | 0.71 ± 0.02 a | 2.24 ± 0.17 b | 2.28 ± 0.06 c | |||
| GTP 2 | 0.84 ± 0.00 b | 1.50 ± 0.08 a | 1.55 ± 0.10 b | |||
| GTP 3 | 0.87 ± 0.00 c | 1.51 ± 0.10 a | 1.22 ± 0.01 a | |||
| - | DPPH/IC50 (mg/mL) | ABTS/mmol/kg | FRAP/mmol/kg | |||
| - | Uncooked | Cooked | Uncooked | Cooked | Uncooked | Cooked |
| 0.5% DGTN 1 | 188.5 ± 7.7 a | 295.8 ± 3.3 a | 7.76 ± 0.00 c | 5.22 ± 0.29 a | 4.05 ± 0.28 a | 3.16 ± 0.02 a |
| 0.5% DGTN 2 | 199.7 ± 7.6 a | 315.6 ± 44.1 a | 6.82 ± 0.05 a | 6.07 ± 0.16 a | 3.69 ± 0.18 a | 2.91 ± 0.54 a |
| 0.5% DGTN 3 | 224.3 ± 4.1 b | 288.5 ± 17.1 a | 7.14 ± 0.13 b | 5.65 ± 0.49 a | 3.77 ± 0.11 a | 3.43 ± 0.07 a |
| - | - | - | - | - | - | - |
| 2% DGTN 1 | 55.7 ± 1.1 a | 79.7 ± 6.9 a | 24.48 ± 1.03 b | 16.64 ± 1.69 a | 18.63 ± 1.29 a | 12.44 ± 0.06 a |
| 2% DGTN 2 | 67.7 ± 1.5 b | 91.5 ± 1.0 b | 22.32 ± 0.68 a | 16.06 ± 0.15 a | 17.28 ± 0.88 a | 11.87 ± 0.85 a |
| 2% DGTN 3 | 66.0 ± 0.6 b | 89.1 ± 2.0 ab | 21.05 ± 0.31 a | 16.06 ± 0.12 a | 17.88 ± 0.28 a | 11.85 ± 1.48 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, K.; Zhou, H.-M.; Zhu, K.-X.; Guo, X.-N.; Peng, W. Water Cooking Stability of Dried Noodles Enriched with Different Particle Size and Concentration Green Tea Powders. Foods 2020, 9, 298. https://doi.org/10.3390/foods9030298
Yu K, Zhou H-M, Zhu K-X, Guo X-N, Peng W. Water Cooking Stability of Dried Noodles Enriched with Different Particle Size and Concentration Green Tea Powders. Foods. 2020; 9(3):298. https://doi.org/10.3390/foods9030298
Chicago/Turabian StyleYu, Kun, Hui-Ming Zhou, Ke-Xue Zhu, Xiao-Na Guo, and Wei Peng. 2020. "Water Cooking Stability of Dried Noodles Enriched with Different Particle Size and Concentration Green Tea Powders" Foods 9, no. 3: 298. https://doi.org/10.3390/foods9030298
APA StyleYu, K., Zhou, H.-M., Zhu, K.-X., Guo, X.-N., & Peng, W. (2020). Water Cooking Stability of Dried Noodles Enriched with Different Particle Size and Concentration Green Tea Powders. Foods, 9(3), 298. https://doi.org/10.3390/foods9030298






