Jasmonate and Ethylene-Regulated Ethylene Response Factor 22 Promotes Lanolin-Induced Anthocyanin Biosynthesis in ‘Zaosu’ Pear (Pyrus bretschneideri Rehd.) Fruit
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
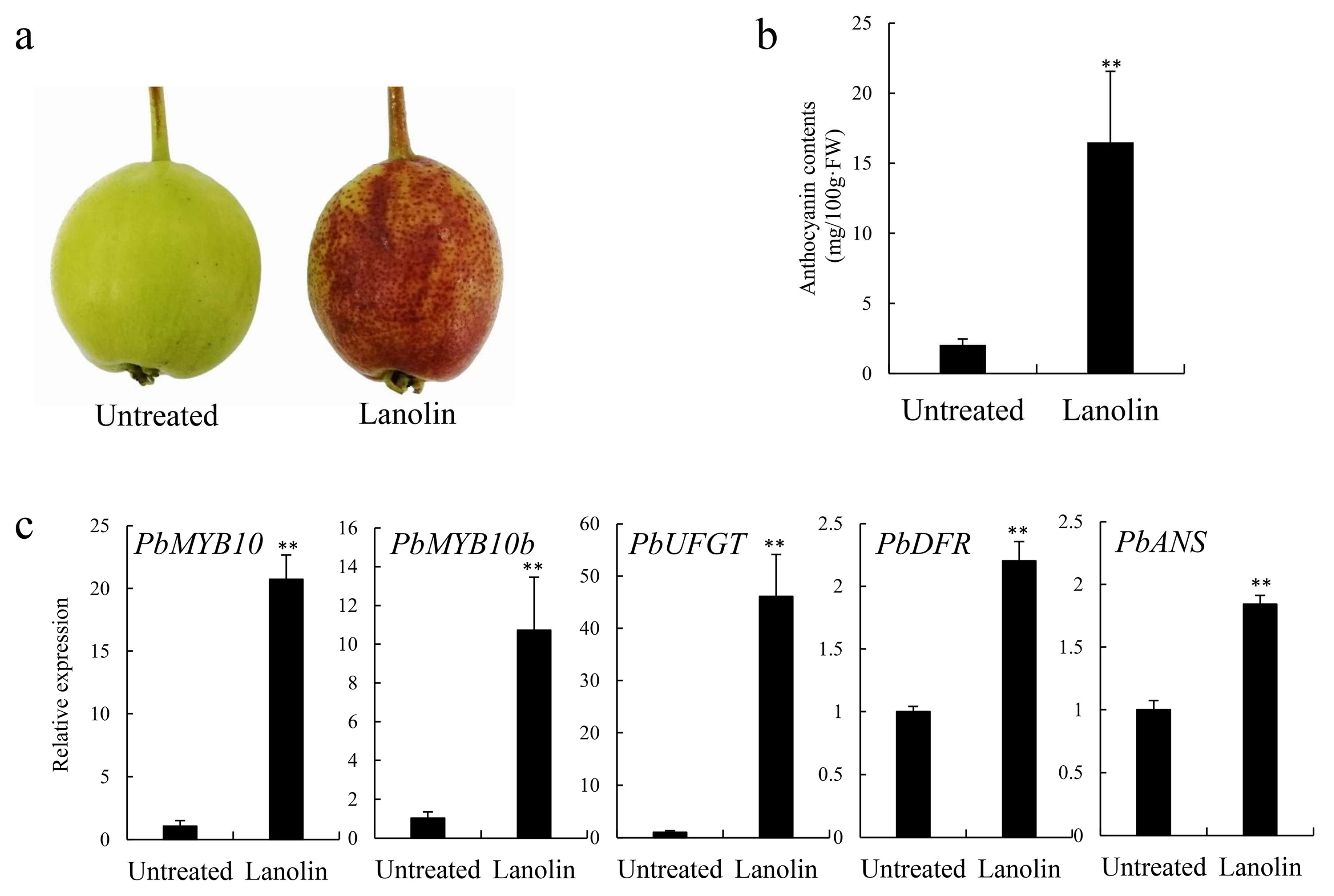
2.2. Measurement of the Anthocyanin Contents
2.3. RNA Extraction and cDNA Synthesis
2.4. Library Construction and Transcriptome Analysis
2.5. Quantitative Real-Time PCR Validation
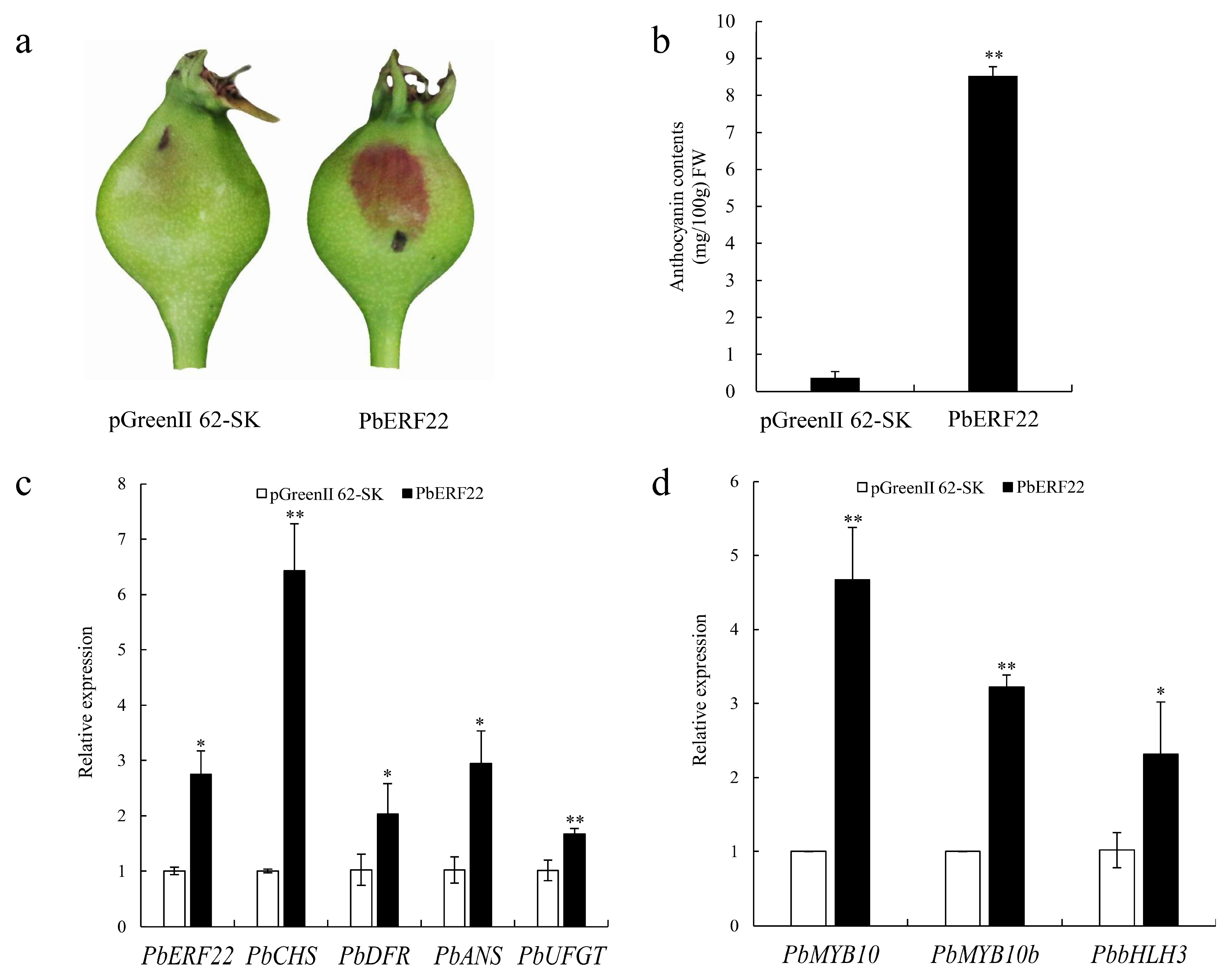
2.6. Transient Overexpression Experiments
2.7. Dual Luciferase Assay
2.8. Statistical Analyses
3. Results
3.1. Changes in Coloration and Expression Levels of Anthocyanin Biosynthesis-Related Genes in Lanolin-Treated ‘Zaosu’ Pear
3.2. Transcriptome Data Analysis
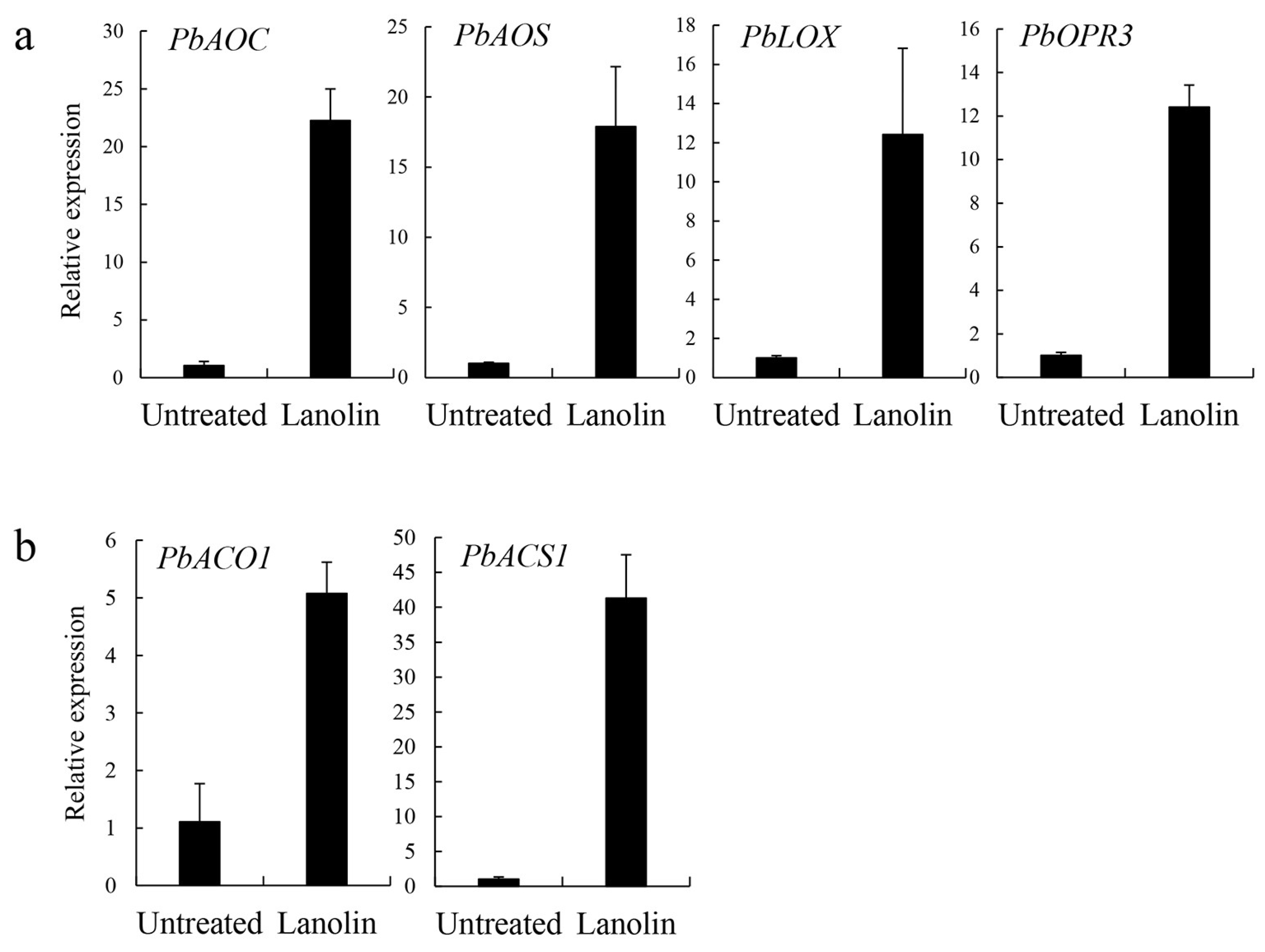
3.3. Lanolin-Induced Expression Levels of Jasmonate and Ethylene Synthesis-Related Genes
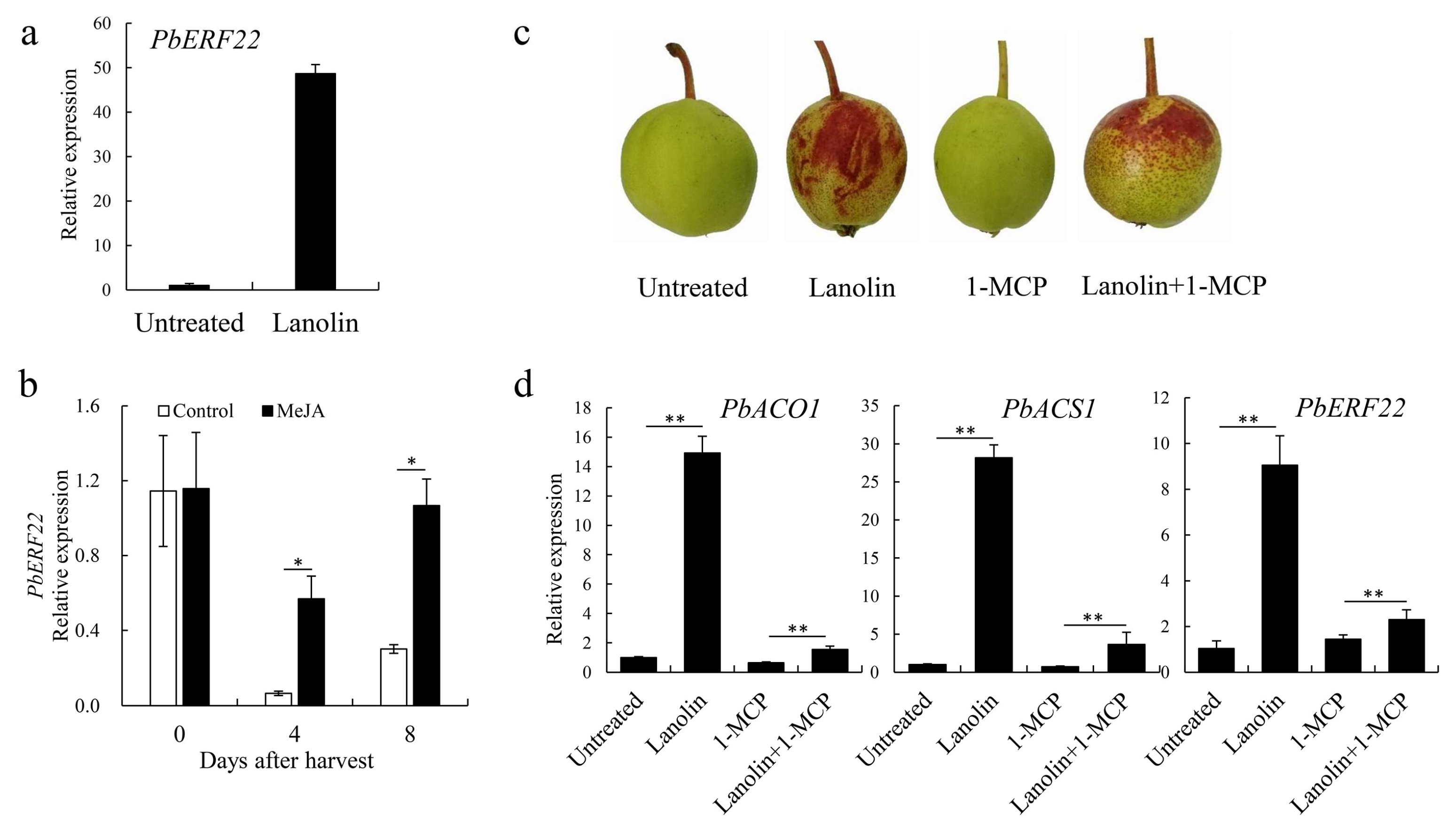
3.4. Identification of PbERF22 As a Candidate Gene Involved in Anthocyanin Biosynthesis
3.5. Transient Overexpression of PbERF22 in ‘Zaosu’ Pear
3.6. Effects of PbERF22 on PbUFGT Promoter Activity
4. Discussion
4.1. Lanolin Promotes Anthocyanin Biosynthesis in ‘Zaosu’ Pear Fruit
4.2. Lanolin Affects the Levels of Several Hormones in ‘Zaosu’ Pear Fruit
4.3. PbERF22 Responses to Jasmonate and Ethylene and is Correlated with Anthocyanin Biosynthesis
4.4. Functional Validation of PbERF22 in Anthocyanin Biosynthesis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jia, Z.; Ma, P.; Bian, X.; Yang, Q.; Guo, X.; Xie, Y. Biosynthesis Metabolic pathway and molecular regulation of plants anthocyanin. Acta Bot. Bor-Occid. Sin. 2014, 34, 1496–1506. [Google Scholar]
- Tsurunaga, Y.; Takahashi, T.; Katsube, T.; Kudo, A.; Kuramitsu, O.; Ishiwata, M.; Matsumoto, S. Effects of UV-B irradiation on the levels of anthocyanin, rutin and radical scavenging activity of buckwheat sprouts. Food Chem. 2013, 141, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhou, T.; Wei, C.; Lan, W.; Zhao, Y.; Pan, Y.; Wu, V.C. Antibacterial effect and mechanism of anthocyanin rich Chinese wild blueberry extract on various foodborne pathogens. Food Control 2018, 94, 155–161. [Google Scholar] [CrossRef]
- Reyes, L.F.; Cisneros, Z.L. Wounding stress increases the phenolic content and antioxidant capacity of purple-flesh potatoes (Solanum tuberosum L.). J. Agric. Food Chem. 2003, 51, 5296–5300. [Google Scholar] [CrossRef]
- Cui, Z.; Bi, W.; Hao, X.; Li, P.; Duan, Y.; Walker, M.A.; Xu, Y.; Wang, Q. Drought stress enhances up-regulation of anthocyanin biosynthesis in grapevine leafroll-associated virus 3-Infected in vitro grapevine (Vitis vinifera) leaves. Plant Dis. 2017, 101, 1606–1615. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Yao, G.; Yue, W.; Zhang, S.; Wu, J. Transcriptome profiling reveals differential gene expression in proanthocyanidin biosynthesis associated with red/green skin color mutant of pear (Pyrus communis L.). Front. Plant Sci. 2015, 6, 795. [Google Scholar] [CrossRef] [Green Version]
- Zhai, R.; Liu, X.; Feng, W.; Chen, S.; Xu, L.; Wang, Z.; Zhang, J.; Li, P.; Ma, F.; Liu, J. Different biosynthesis patterns among flavonoid 3-glycosides with distinct effects on accumulation of other flavonoid metabolites in pears (Pyrus bretschneideri Rehd.). PLoS ONE 2014, 9, e91945. [Google Scholar] [CrossRef]
- Feng, S.; Sun, S.; Chen, X.; Wu, S.; Wang, D.; Chen, X.; Zhang, P. PyMYB10 and PyMYB10.1 interact with bHLH to enhance anthocyanin accumulation in pears. PLoS ONE 2015, 10, e0142112. [Google Scholar] [CrossRef]
- Takos, A.M.; Jaffe, F.W.; Jacob, S.R.; Bogs, J.; Robinson, S.P.; Walker, A.R. Light-induced expression of a MYB gene regulates anthocyanin biosynthesis in red apples. Plant Physiol. 2006, 142, 1216–1232. [Google Scholar] [CrossRef] [Green Version]
- Espley, R.V.; Brendolise, C.; Chagne, D.; Kutty, A.S.; Green, S.; Volz, R.; Putterill, J.; Schouten, H.J.; Gardiner, S.E.; Hellens, R.P. Multiple repeats of a promoter segment causes transcription factor autoregulation in red apples. Plant Cell 2009, 21, 168–183. [Google Scholar] [CrossRef] [Green Version]
- Rahim, M.A.; Busatto, N.; Trainotti, L. Regulation of anthocyanin biosynthesis in peach fruits. Planta 2014, 240, 913–929. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zhang, S.; Lin, D.; Wei, Y.; Yan, Y.; Liu, G.; Reiter, R.; Chan, Z. Zinc finger of Arabidopsis thaliana 6 is involved in melatonin-mediated auxin signaling through interacting INDETERMINATE DOMAIN15 and INDOLE-3-ACETIC ACID 17. J. Pineal Res. 2018, 65, e12494. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Zhang, X.; You, C.; Bi, S.; Wang, X.; Hao, Y. MdWRKY40 promotes wounding-induced anthocyanin biosynthesis in association with MdMYB1 and undergoes MdBT2-mediated degradation. New Phytol. 2019, 224, 380–395. [Google Scholar] [CrossRef] [PubMed]
- Tao, R.; Bai, S.; Ni, J.; Yang, Q.; Zhao, Y.; Teng, Y. The blue light signal transduction pathway is involved in anthocyanin accumulation in ‘Red Zaosu’ pear. Planta 2018, 248, 37–48. [Google Scholar] [CrossRef]
- Gu, C.; Guo, Z.; Hao, P.; Wang, G.; Jin, Z.; Zhang, S. Multiple regulatory roles of AP2/ERF transcription factor in angiosperm. Bot. Stud. 2017, 58, 6. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Xu, Y.; Zhang, L.; Ji, Y.; Tan, D.; Yuan, H.; Wang, A. The jasmonate-activated transcription factor MdMYC2 regulates ethylene response factor and ethylene biosynthetic genes to promote ethylene biosynthesis during apple fruit ripening. Plant Cell 2017, 29, 1316–1334. [Google Scholar] [CrossRef] [Green Version]
- Lorenzo, O.; Piqueras, R.; Sánchez-serrano, J.J.; Solano, R. ETHYLENE RESPONSE FACTOR1 integrates signals from ethylene and jasmonate pathways in plant defense. Plant Cell 2003, 15, 165–178. [Google Scholar] [CrossRef] [Green Version]
- Maruyama, Y.; Yamoto, N.; Suzuki, Y.; Chiba, Y.; Yamazaki, K.; Sato, T.; Yamaguchi, J. The Arabidopsis transcriptional repressor ERF9 participates in resistance against necrotrophic fungi. Plant Sci. 2013, 213, 79–87. [Google Scholar] [CrossRef]
- An, J.; Zhang, X.; Bi, S.; You, C.; Wang, X.; Hao, Y. The ERF transcription factor MdERF38 promotes drought stress-induced anthocyanin biosynthesis in apple. Plant J. 2019, 101, 573–589. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, H.; Wang, N.; Jiang, S.; Fang, H.; Zhang, Z.; Yang, G.; Wang, Y.; Su, M.; Xu, L. The ethylene response factor MdERF1B regulates anthocyanin and proanthocyanidin biosynthesis in apple. Plant Mol. Biol. 2018, 98, 205–218. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, P.; Zhao, Q.; Tang, Y.; Chen, Y.; Li, M.; Jiang, H.; Wu, G. Overexpression of a phosphate starvation response AP2/ERF gene from physic nut in Arabidopsis alters root morphological traits and phosphate starvation-induced anthocyanin accumulation. Front. Plant Sci. 2018, 9, 1186. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.; Ming, M.; Allan, A.C.; Gu, C.; Li, L.; Wu, X.; Wang, R.; Chang, Y.; Qi, K.; Zhang, S.; et al. Map-based cloning of the pear gene MYB114 identifies an interaction with other transcription factors to coordinately regulate fruit anthocyanin biosynthesis. Plant J. 2017, 92, 437–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, J.; Bai, S.; Zhao, Y.; Qian, M.; Tao, R.; Yin, L.; Gao, L.; Teng, Y. Ethylene response factors Pp4ERF24 and Pp12ERF96 regulate blue light-induced anthocyanin biosynthesis in ‘Red Zaosu’ pear fruits by interacting with MYB114. Plant Mol. Biol. 2018, 99, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Schlossman, M.; McCarthy, J. Lanolin and its derivatives. JAOCS 1978, 55, 447–450. [Google Scholar] [CrossRef]
- Fischnich, O. Über den einfluss von β-Indolylessigsäure auf die blattbewegungen und die adventivwurzelbildung von Coleus. Planta 1935, 24, 552–583. [Google Scholar] [CrossRef]
- Castillo, F.; Daniel, H.; Gabriel, G.; Mendez, M.; Rodriguez, R.; Reyes, A.; Aguilar, C.N. In vitro antifungal activity of plant extracts obtained with alternative organic solvents against Rhizoctonia solani Kühn. Ind. Crop. Prod. 2010, 32, 324–328. [Google Scholar] [CrossRef]
- Laibach, F.; Kornmann, P. Zur Frage des Wuchsstofftransportes in der Haferkoleoptile. Planta 1933, 21, 396–418. [Google Scholar] [CrossRef]
- Laibach, F.; Mai, G.; Müller, A. Über ein Zellteilungshormon. Naturwissenschaften 1934, 22, 288. [Google Scholar] [CrossRef]
- Hikosaka, S.; Sugiyama, N. Effects of exogenous plant growth regulators on yield, fruit growth, and concentration of endogenous hormones in gynoecious parthenocarpic cucumber (Cucumis sativus L.). Horticult. J. 2015, 84, 342–349. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.X.; Whiting, M.D. Improving ‘Bing’ sweet cherry fruit quality with plant growth regulators. Sci. Hortic. 2011, 127, 341–346. [Google Scholar] [CrossRef]
- Cooper, W.C. Hormones in relation to root formation on stem cuttings. Plant Physiol. 1935, 10, 789–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDavid, C.R.; Sagar, G.R.; Marshall, C. The effect of auxin from shoot on root development in Pisum Sativum L. New Phytol. 1972, 71, 1027–1032. [Google Scholar] [CrossRef]
- Hao, B.Z.; Wu, J.L. Laticifer differentiation in Hevea brasiliensis: Induction by exogenous jasmonic acid and linolenic acid. Ann. Bot. 2000, 85, 37–43. [Google Scholar] [CrossRef] [Green Version]
- Kost, C.; Heil, M. Herbivore-induced plant volatiles induce an indirect defence in neighbouring plants. J. Ecol. 2006, 94, 619–628. [Google Scholar] [CrossRef]
- Zuniga-Mayo, V.M.; Banos-Bayardo, C.R.; Diaz-Ramirez, D.; Marsch-Martinez, N.; de Folter, S. Conserved and novel responses to cytokinin treatments during flower and fruit development in Brassica napus and Arabidopsis thaliana. Sci Rep. 2018, 8. [Google Scholar] [CrossRef]
- Gerchikov, N.; Keren-Keiserman, A.; Perl-Treves, R.; Ginzberg, I. Wounding of melon fruits as a model system to study rind netting. Sci. Hortic. 2008, 117, 115–122. [Google Scholar] [CrossRef]
- Henzell, R.F.; Briscoe, M.R.; Lauren, D.R. Evaluation of two plant growth regulators as chemical pruning agents for kiwifruit vines in summer. N. Z. J. Exp. Agr. 1986, 14, 199–203. [Google Scholar] [CrossRef]
- Freiman, Z.E.; Rosianskey, Y.; Dasmohapatra, R.; Kamara, I.; Flaishman, M.A. The ambiguous ripening nature of the fig (Ficus carica L.) fruit: A gene-expression study of potential ripening regulators and ethylene-related genes. J. Exp. Bot. 2015, 66, 3309–3324. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Shu, S.; Bai, S.; Tao, R.; Qian, M.; Teng, Y. Genome-wide survey and analysis of the TIFY gene family and its potential role in anthocyanin synthesis in Chinese sand pear (Pyrus pyrifolia). Tree Genet. Genomes. 2018, 14, 25. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and measurement of anthocyanins by UV-visible spectroscopy. In Current Protocols in Food Analytical Chemistry; Wrolstad, R.E., Acree, T.E., An, H., Decker, E.A., Penner, M.H., Reid, D.S., Schwartz, S.J., Shoemaker, C.F., et al., Eds.; John Wiley & Sons: New York, NY, USA, 2001; pp. F1.2.1–F1.2.13. [Google Scholar]
- Wang, Z.; Du, H.; Zhai, R.; Song, L.; Ma, F.; Xu, L. Transcriptome analysis reveals candidate genes related to color fading of ‘Red Bartlett’ (Pyrus communis L.). Front. Plant Sci. 2017, 8, 455. [Google Scholar] [CrossRef] [Green Version]
- Zhai, R.; Zhao, Y.; Wu, M.; Yang, J.; Li, X.; Liu, H.; Wu, T.; Liang, F.; Yang, C.; Wang, Z.; et al. The MYB transcription factor PbMYB12b positively regulates flavonol biosynthesis in pear fruit. BMC Plant Biol. 2019, 19, 85. [Google Scholar] [CrossRef] [PubMed]
- Zhai, R.; Wang, Z.; Zhang, S.; Meng, G.; Xu, L. Two MYB transcription factors regulate flavonoid biosynthesis in pear fruit (Pyrus bretschneideri Rehd.). J. Exp. Bot. 2015, 67, 1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, J.; Yao, J.; Xu, R.; You, C.; Wang, X.; Hao, Y. An apple NAC transcription factor enhances salt stress tolerance by modulating the ethylene response. Physiol. Plant. 2018, 164, 279–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, L.; Zhang, Y.; Yang, X.; Xiao, J.; Zhang, H.; Zhang, Z.; Wang, Y.; Jiang, G. Colored light-quality selective plastic films affect anthocyanin content, enzyme activities, and the expression of flavonoid genes in strawberry (Fragaria × ananassa) fruit. Food Chem. 2016, 207, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Varasteh, F.; Arzani, K.; Barzegar, M.; Zamani, Z. Changes in anthocyanins in arils of chitosan-coated pomegranate (Punica granatum L. cv. Rabbab-e-Neyriz) fruit during cold storage. Food Chem. 2012, 130, 267–272. [Google Scholar] [CrossRef]
- Qian, M.; Yu, B.; Li, X.; Sun, Y.; Zhang, D.; Teng, Y. Isolation and expression analysis of anthocyanin biosynthesis genes from the red Chinese sand pear, Pyrus pyrifolia Nakai cv. Mantianhong, in response to methyl jasmonate treatment and UV-B/VIS conditions. Plant. Mol. Biol. Rep. 2014, 32, 428–437. [Google Scholar] [CrossRef]
- Zieslin, N.; Starkman, F.; Zamski, E. Growth of rose flower peduncles and effects of applied plant growth regulators. Plant. Growth Regul. 1989, 8, 65–76. [Google Scholar]
- Nogueira, M.R.; Ferraz, M.V.; Bezerra, A.K.; Mazziniguedes, R.B.; Costa, C.R.X.; De Almeida, L.C.; Pereira, S.T.; Pivetta, K.F. Indolbutyric acid and time of the year influence on rooting of chrysanthemum cuttings. Am. J. Plant. Sci. 2018, 09, 507–516. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Duan, G.; Li, C.; Liu, L.; Han, G.; Zhang, Y.; Wang, C. The crosstalks between jasmonic acid and other plant hormone signaling highlight the involvement of jasmonic acid as a core component in plant response to biotic and abiotic stresses. Front. Plant. Sci. 2019, 10, 1349. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, N. Hormone signaling pathways under stress combinations. Plant. Signal. Behav. 2016, 11, e1247139. [Google Scholar] [CrossRef] [Green Version]
- Loreti, E.; Povero, G.; Novi, G.; Solfanelli, C.; Alpi, A.; Perata, P. Gibberellins, jasmonate and abscisic acid modulate the sucrose-induced expression of anthocyanin biosynthetic genes in Arabidopsis. New Phytol. 2008, 179, 1004–1016. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Mao, J.; Yang, S.; Guo, Z.; Ma, Z.; Dawuda, M.M.; Zuo, C.; Chu, M.; Chen, B. Anthocyanin accumulation correlates with hormones in the fruit skin of ‘Red Delicious’ and its four generation bud sport mutants. BMC Plant. Biol. 2018, 18, 363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, S.T.; Goto-Yamamoto, N.; Kobayashi, S.; Esaka, M. Effects of plant hormones and shading on the accumulation of anthocyanins and the expression of anthocyanin biosynthetic genes in grape berry skins. Plant. Sci. 2004, 167, 247–252. [Google Scholar] [CrossRef]
- Delker, C.; Stenzel, I.; Hause, B.; Miersch, O.; Feussner, I.; Wasternack, C. Jasmonate biosynthesis in arabidopsis thaliana-enzymes, products, regulation. Plant. Biol. 2006, 8, 297–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Paepe, A.; Van Der Straeten, D. Ethylene biosynthesis and signaling: An overview. Vitam. Horm. 2005, 72, 399–430. [Google Scholar] [PubMed]
- Müller, M.; Munné-bosch, S. Ethylene response factors: A key regulatory hub in hormone and stress signaling. Plant. Physiol. 2015, 169, 32–41. [Google Scholar] [CrossRef] [Green Version]
- Stepanova, L.A.; Alonso, W.J. Ethylene signaling and response: Where different regulatory modules meet. Curr. Opin. Plant. Biol. 2009, 12, 548–555. [Google Scholar] [CrossRef]
- Ouaked, F. A MAPK pathway mediates ethylene signaling in plants. EMBO J. 2003, 22, 1282–1288. [Google Scholar] [CrossRef] [Green Version]
- McGrath, K.C.; Dombrecht, B.; Manners, J.M.; Schenk, P.M.; Edgar, C.I.; Maclean, D.J.; Wolf-Rüdiger, S.; Udvardi, M.K.; Kazan, K. Repressor- and activator-type ethylene response factors functioning in jasmonate signaling and disease resistance identified via a genome-wide screen of arabidopsis transcription factor gene expression. Plant. Physiol. 2005, 139, 949–959. [Google Scholar] [CrossRef] [Green Version]
- Tan, X.; Fan, Z.; Shan, W.; Yin, X.; Kuang, J.; Lu, W.; Chen, J. Association of BrERF72 with methyl jasmonate-induced leaf senescence of Chinese flowering cabbage through activating JA biosynthesis-related genes. Hortic. Res. 2018, 5, 22. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Chen, M.; Li, L.; Xu, Z.; Chen, X.; Guo, J.; Ma, Y. Overexpression of the soybean GmERF3 gene, an AP2/ERF type transcription factor for increased tolerances to salt, drought, and diseases in transgenic tobacco. J. Exp. Bot. 2009, 60, 3781–3796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Huang, Z.; Xie, B.; Chen, Q.; Tian, X.; Zhang, X.; Zhang, H.; Lu, X.; Huang, D.; Huang, R. The ethylene-, jasmonate-, abscisic acid- and NaCl-responsive tomato transcription factor JERF1 modulates expression of GCC box-containing genes and salt tolerance in tobacco. Planta 2004, 220, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Rouster, J.; Leah, R.; Mundy, J.; Cameron-Mills, V. Identification of a methyl jasmonate-responsive region in the promoter of a lipoxygenase 1 gene expressed in barley grain. Plant. J. 1997, 11, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Koyama, T.; Sato, F. The function of ethylene response factor genes in the light-induced anthocyanin production of Arabidopsis thaliana leaves. Plant. Biotechnol. 2018, 35, 87–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Meng, D.; Wang, A.; Li, T.; Jiang, S.; Cong, P.; Li, T. The Methylation of the PcMYB10 promoter is associated with green-skinned sport in Max Red Bartlett pear. Plant. Physiol. 2013, 162, 885–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, S.; Wang, Y.; Yang, S.; Xu, Y.; Chen, X. Anthocyanin biosynthesis in pears is regulated by a R2R3-MYB transcription factor PyMYB10. Planta 2010, 232, 245–255. [Google Scholar] [CrossRef]


| Correlation of Factor | PbERF22 | PbMYB10 | PbMYB10b | PbUFGT | PbDFR | PbANS |
|---|---|---|---|---|---|---|
| PbERF22 | 1 | 0.734 ** | 0.565 | 0.800 ** | 0.456 | 0.121 |
| PbMYB10 | 1 | 0.469 | 0.749 ** | 0.399 | 0.581 * | |
| PbMYB10b | 1 | 0.769 ** | 0.693 * | −0.159 | ||
| PbUFGT | 1 | 0.689 * | −0.066 | |||
| PbDFR | 1 | −0.114 | ||||
| PbANS | 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, T.; Liu, H.-T.; Zhao, G.-P.; Song, J.-X.; Wang, X.-L.; Yang, C.-Q.; Zhai, R.; Wang, Z.-G.; Ma, F.-W.; Xu, L.-F. Jasmonate and Ethylene-Regulated Ethylene Response Factor 22 Promotes Lanolin-Induced Anthocyanin Biosynthesis in ‘Zaosu’ Pear (Pyrus bretschneideri Rehd.) Fruit. Biomolecules 2020, 10, 278. https://doi.org/10.3390/biom10020278
Wu T, Liu H-T, Zhao G-P, Song J-X, Wang X-L, Yang C-Q, Zhai R, Wang Z-G, Ma F-W, Xu L-F. Jasmonate and Ethylene-Regulated Ethylene Response Factor 22 Promotes Lanolin-Induced Anthocyanin Biosynthesis in ‘Zaosu’ Pear (Pyrus bretschneideri Rehd.) Fruit. Biomolecules. 2020; 10(2):278. https://doi.org/10.3390/biom10020278
Chicago/Turabian StyleWu, Ting, Han-Ting Liu, Guang-Ping Zhao, Jun-Xing Song, Xiao-Li Wang, Cheng-Quan Yang, Rui Zhai, Zhi-Gang Wang, Feng-Wang Ma, and Ling-Fei Xu. 2020. "Jasmonate and Ethylene-Regulated Ethylene Response Factor 22 Promotes Lanolin-Induced Anthocyanin Biosynthesis in ‘Zaosu’ Pear (Pyrus bretschneideri Rehd.) Fruit" Biomolecules 10, no. 2: 278. https://doi.org/10.3390/biom10020278
APA StyleWu, T., Liu, H.-T., Zhao, G.-P., Song, J.-X., Wang, X.-L., Yang, C.-Q., Zhai, R., Wang, Z.-G., Ma, F.-W., & Xu, L.-F. (2020). Jasmonate and Ethylene-Regulated Ethylene Response Factor 22 Promotes Lanolin-Induced Anthocyanin Biosynthesis in ‘Zaosu’ Pear (Pyrus bretschneideri Rehd.) Fruit. Biomolecules, 10(2), 278. https://doi.org/10.3390/biom10020278




