In Vivo Metabolic Response upon Exposure to Gold Nanorod Core/Silver Shell Nanostructures: Modulation of Inflammation and Upregulation of Dopamine
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterizations of Au@Ag NRs
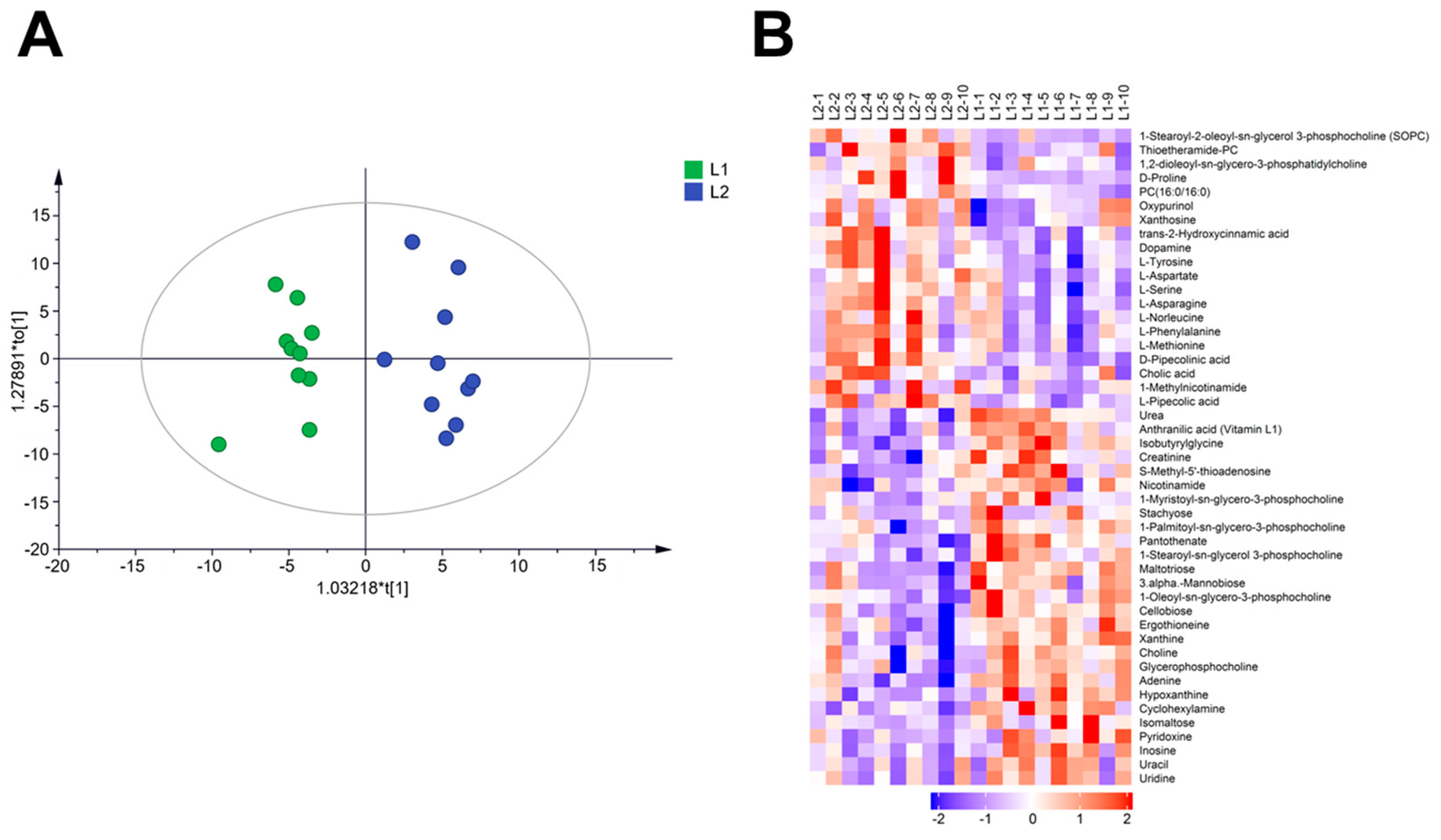
2.2. Effect of Au@Ag NRs on Metabolomics in Mouse Liver
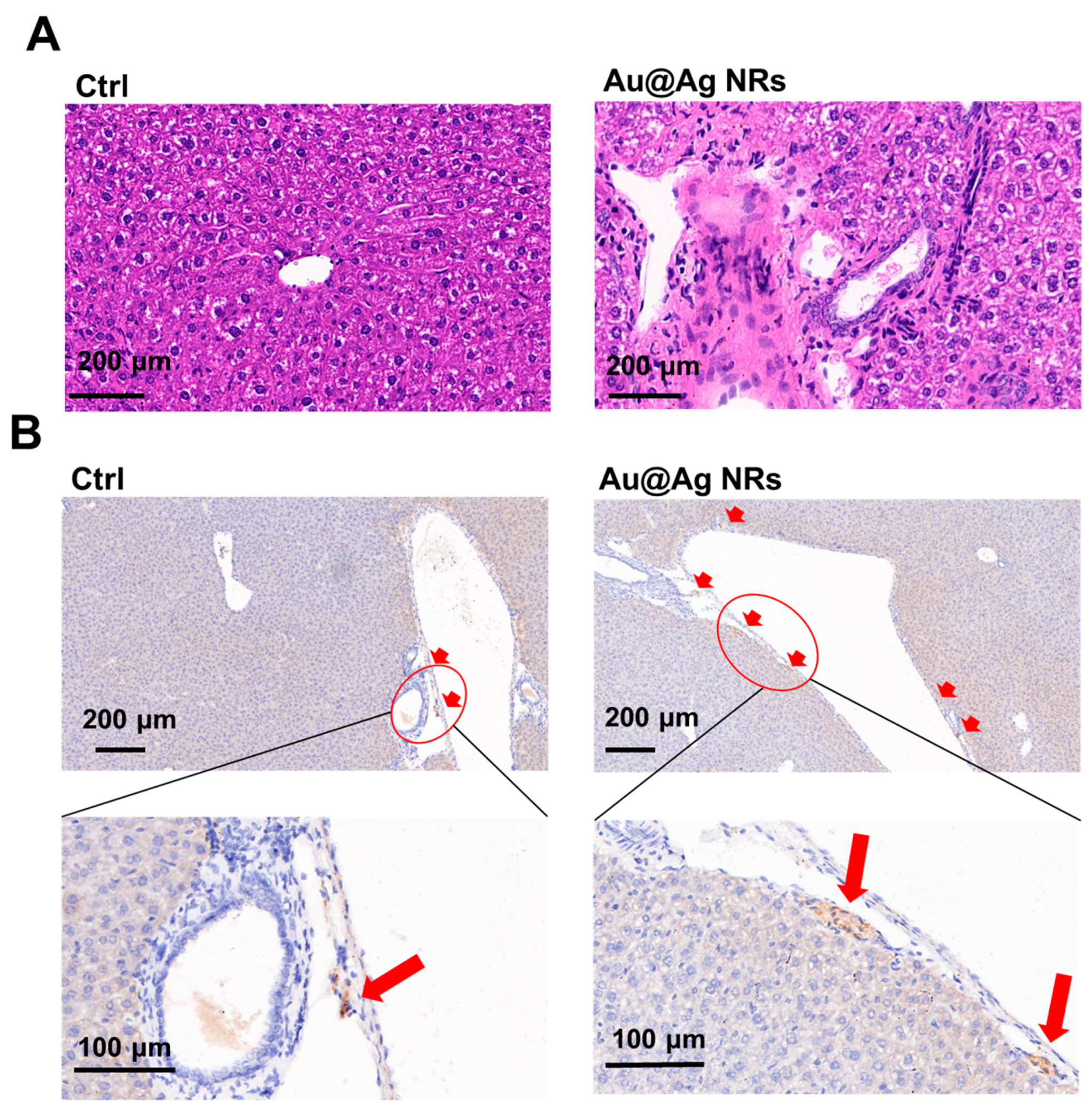
2.3. Effect of Au@Ag NRs on Inflammation and Dopamine Synthesis in Mouse Liver
2.4. Cell Viability and Redox Balance Affected by Au@Ag NRs in Raw 264.7 Cells
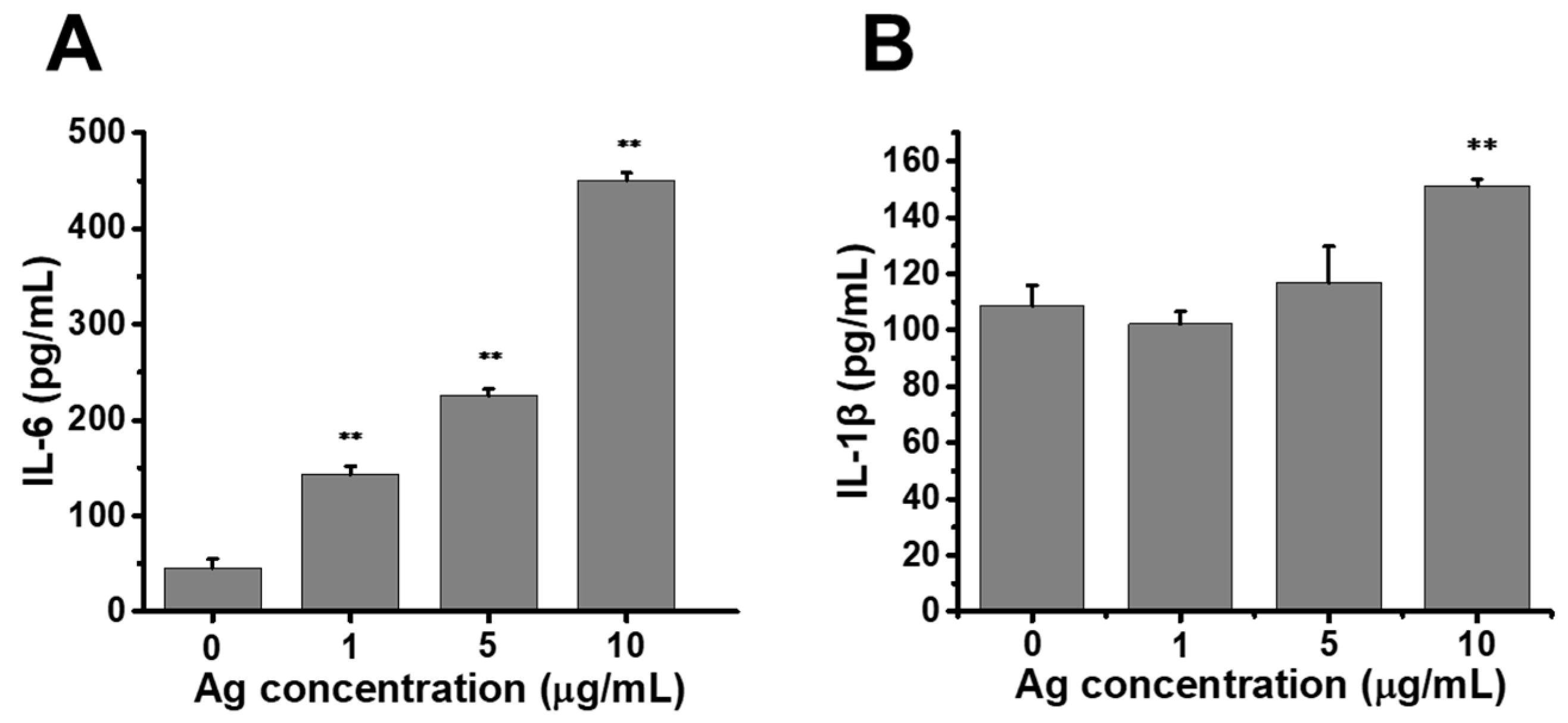
2.5. Release of Proinflammatory Cytokines Enhanced by Au@Ag NRs in Raw264.7 Cells
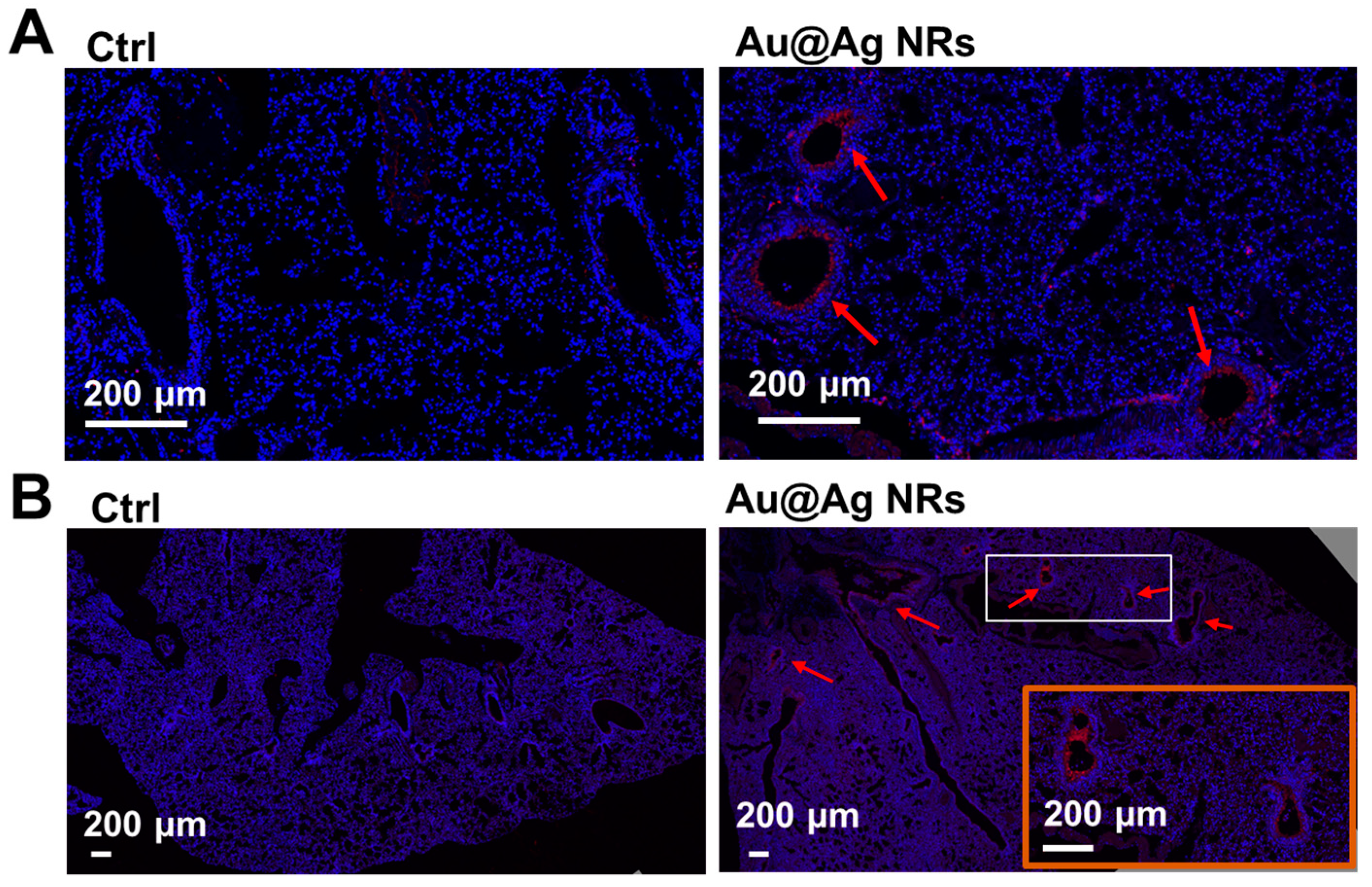
2.6. Inflammation and Dopamine Synthesis in Lungs
3. Materials and Methods
3.1. Chemicals Materials and Lab Animals
3.2. Preparation and Characterization of Gold Nanorod Core/Silver Shell Nanostructures (Au@Ag NRs)
3.3. Intravenous Administration of Au@Ag NRs in Mice
3.4. Metabolomics Analysis
3.5. Determination of Silver Content in Liver and Lung Tissues by ICP-MS
3.6. Histopathological Evaluation and Immunohistochemical Test
3.7. Treg Cells Isolation and Dopamine Assay
3.8. Cell Culture and Cell Viability Assay
3.9. Two-Photon Luminescence (TPL) Imaging of Au@Ag NRs in Raw264.7 Cells
3.10. Intracellular Reactive Oxygen Species (ROS) Detection
3.11. Enzyme-Linked Immunosorbent Assay (ELISA)
3.12. Western Blotting
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, Z.; Xia, T.; Liu, S.J. Mechanisms of nanosilver-induced toxicological effects: More attention should be paid to its sublethal effects. Nanoscale 2015, 7, 7470–7481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, C.; Li, Y.; Tjong, S.C. Bactericidal and Cytotoxic Properties of Silver Nanoparticles. Int. J. Mol. Sci. 2019, 20, 449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Yan, L.; Liu, J.; Chen, C.; Zhao, Y. Quantification of Nanomaterial/Nanomedicine Trafficking in Vivo. Anal. Chem. 2018, 90, 589–614. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Zhang, W.; Ji, Y.; Meng, J.; Guo, H.; Liu, J.; Wu, X.; Xu, H. Revealing silver cytotoxicity using Au nanorods/Ag shell nanostructures: Disrupting cell membrane and causing apoptosis through oxidative damage. Rsc Adv. 2013, 3, 2296. [Google Scholar] [CrossRef]
- Jiang, X.M.; Wang, L.M.; Ji, Y.L.; Tang, J.L.; Tian, X.; Cao, M.J.; Li, J.X.; Bi, S.Y.; Wu, X.C.; Chen, C.Y.; et al. Interference of Steroidogenesis by Gold Nanorod Core/Silver Shell Nanostructures: Implications for Reproductive Toxicity of Silver Nanomaterials. Small 2017, 13. [Google Scholar] [CrossRef]
- Onodera, A.; Nishiumi, F.; Kakiguchi, K.; Tanaka, A.; Tanabe, N.; Honma, A.; Yayama, K.; Yoshioka, Y.; Nakahira, K.; Yonemura, S.; et al. Short-term changes in intracellular ROS localisation after the silver nanoparticles exposure depending on particle size. Toxicol. Rep. 2015, 2, 574–579. [Google Scholar] [CrossRef] [Green Version]
- De Matteis, V.; Cascione, M.; Toma, C.C.; Leporatti, S. Morphomechanical and organelle perturbation induced by silver nanoparticle exposure. J. Nanopart. Res. 2018, 20. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, J.; Boudreau, M.; Meng, J.; Yin, J.J.; Liu, J.; Xu, H. Intravenous administration of silver nanoparticles causes organ toxicity through intracellular ROS-related loss of inter-endothelial junction. Part. Fibre Toxicol. 2016, 13, 21. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.J.; Rahman, M.M.; Lee, S.M.; Kim, J.M.; Park, K.; Kang, J.H.; Seo, Y.R. Assessment of in vivo genotoxicity of citrated-coated silver nanoparticles via transcriptomic analysis of rabbit liver tissue. Int. J. Nanomed. 2019, 14, 393–405. [Google Scholar] [CrossRef] [Green Version]
- Clarke, C.J.; Haselden, J.N. Metabolic profiling as a tool for understanding mechanisms of toxicity. Toxicol. Pathol. 2008, 36, 140–147. [Google Scholar] [CrossRef]
- Cui, L.; Wang, X.; Sun, B.; Xia, T.; Hu, S. Predictive Metabolomic Signatures for Safety Assessment of Metal Oxide Nanoparticles. ACS Nano 2019, 13, 13065–13082. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.L.; Ng, C.T.; Zou, L.; Lu, Y.; Chen, J.; Bay, B.H.; Shen, H.M.; Ong, C.N. Targeted metabolomics reveals differential biological effects of nanoplastics and nanoZnO in human lung cells. Nanotoxicology 2019, 13, 1117–1132. [Google Scholar] [CrossRef] [PubMed]
- Williams, N.C.; O’Neill, L.A.J. A Role for the Krebs Cycle Intermediate Citrate in Metabolic Reprogramming in Innate Immunity and Inflammation. Front. Immunol. 2018, 9, 141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lodhi, I.J.; Wei, X.; Yin, L.; Feng, C.; Adak, S.; Abou-Ezzi, G.; Hsu, F.F.; Link, D.C.; Semenkovich, C.F. Peroxisomal lipid synthesis regulates inflammation by sustaining neutrophil membrane phospholipid composition and viability. Cell Metab. 2015, 21, 51–64. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Lopez, E.; Zhong, Z.; Stubelius, A.; Sweeney, S.R.; Booshehri, L.M.; Antonucci, L.; Liu-Bryan, R.; Lodi, A.; Terkeltaub, R.; Lacal, J.C.; et al. Choline Uptake and Metabolism Modulate Macrophage IL-1beta and IL-18 Production. Cell Metab. 2019, 29, 1350–1362 e7. [Google Scholar] [CrossRef]
- Yan, Y.; Jiang, W.; Liu, L.; Wang, X.; Ding, C.; Tian, Z.; Zhou, R. Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell 2015, 160, 62–73. [Google Scholar] [CrossRef] [Green Version]
- Carrola, J.; Bastos, V.; Ferreira de Oliveira, J.M.; Oliveira, H.; Santos, C.; Gil, A.M.; Duarte, I.F. Insights into the impact of silver nanoparticles on human keratinocytes metabolism through NMR metabolomics. Arch. Biochem. Biophys. 2016, 589, 53–61. [Google Scholar] [CrossRef]
- Yu, Y.; Ren, W.; Ren, B. Nanosize titanium dioxide cause neuronal apoptosis: A potential linkage between nanoparticle exposure and neural disorder. Neurol. Res. 2008, 30, 1115–1120. [Google Scholar] [CrossRef]
- Kwon, H.J.; Kim, D.; Seo, K.; Kim, Y.G.; Han, S.I.; Kang, T.; Soh, M.; Hyeon, T. Ceria Nanoparticle Systems for Selective Scavenging of Mitochondrial, Intracellular, and Extracellular Reactive Oxygen Species in Parkinson’s Disease. Angew. Chem. Int. Ed. Engl. 2018, 57, 9408–9412. [Google Scholar] [CrossRef]
- Fatemi Tabatabaie, S.R.; Mehdiabadi, B.; Moori Bakhtiari, N.; Tabandeh, M.R. Silver nanoparticle exposure in pregnant rats increases gene expression of tyrosine hydroxylase and monoamine oxidase in offspring brain. Drug Chem. Toxicol. 2017, 40, 440–447. [Google Scholar] [CrossRef]
- Dichtl, S.; Haschka, D.; Nairz, M.; Seifert, M.; Volani, C.; Lutz, O.; Weiss, G. Dopamine promotes cellular iron accumulation and oxidative stress responses in macrophages. Biochem. Pharmacol. 2018, 148, 193–201. [Google Scholar] [CrossRef]
- Wu, M.; Chen, L.; Li, R.; Dan, M.; Liu, H.; Wang, X.; Wu, X.; Liu, Y.; Xu, L.; Xie, L. Bio-distribution and bio-availability of silver and gold in rat tissues with silver/gold nanorod administration. Rsc Adv. 2018, 8, 12260–12268. [Google Scholar] [CrossRef] [Green Version]
- Becker, J.; Zins, I.; Jakab, A.; Khalavka, Y.; Schubert, O.; Sonnichsen, C. Plasmonic focusing reduces ensemble linewidth of silver-coated gold nanorods. Nano Lett. 2008, 8, 1719–1723. [Google Scholar] [CrossRef]
- Mishra, A.R.; Zheng, J.; Tang, X.; Goering, P.L. Silver Nanoparticle-Induced Autophagic-Lysosomal Disruption and NLRP3-Inflammasome Activation in HepG2 Cells Is Size-Dependent. Toxicol. Sci. 2016, 150, 473–487. [Google Scholar] [CrossRef]
- Ramadi, K.B.; Mohamed, Y.A.; Al-Sbiei, A.; Almarzooqi, S.; Bashir, G.; Al Dhanhani, A.; Sarawathiamma, D.; Qadri, S.; Yasin, J.; Nemmar, A.; et al. Acute systemic exposure to silver-based nanoparticles induces hepatotoxicity and NLRP3-dependent inflammation. Nanotoxicology 2016, 10, 1061–1074. [Google Scholar] [CrossRef]
- Sun, B.; Wang, X.; Ji, Z.; Li, R.; Xia, T. NLRP3 inflammasome activation induced by engineered nanomaterials. Small 2013, 9, 1595–1607. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Sun, X.; Chen, H.; Xi, G.; Hou, Y.; Wu, J.; Liu, D.; Wang, H.; Hou, Y.; Yu, J. The protective effect of dopamine on ventilator-induced lung injury via the inhibition of NLRP3 inflammasome. Int. Immunopharmacol. 2017, 45, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Daubner, S.C.; Le, T.; Wang, S. Tyrosine hydroxylase and regulation of dopamine synthesis. Arch. Biochem. Biophys. 2011, 508, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rangasamy, S.B.; Dasarathi, S.; Pahan, P.; Jana, M.; Pahan, K. Low-Dose Aspirin Upregulates Tyrosine Hydroxylase and Increases Dopamine Production in Dopaminergic Neurons: Implications for Parkinson’s Disease. J. Neuroimmune Pharmacol. 2019, 14, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, M.; Fietta, A.M.; Ferrari, M.; Rasini, E.; Bombelli, R.; Carcano, E.; Saporiti, F.; Meloni, F.; Marino, F.; Lecchini, S. Human CD4+CD25+ regulatory T cells selectively express tyrosine hydroxylase and contain endogenous catecholamines subserving an autocrine/paracrine inhibitory functional loop. Blood 2007, 109, 632–642. [Google Scholar] [CrossRef] [Green Version]
- Sandberg, W.J.; Låg, M.; Holme, J.A.; Friede, B.; Gualtieri, M.; Kruszewski, M.; Schwarze, P.E.; Skuland, T.; Refsnes, M. Comparison of non-crystalline silica nanoparticles in IL-1β release from macrophages. Part. Fibre Toxicol. 2012, 9, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loeschner, K.; Hadrup, N.; Qvortrup, K.; Larsen, A.; Gao, X.Y.; Vogel, U.; Mortensen, A.; Lam, H.R.; Larsen, E.H. Distribution of silver in rats following 28 days of repeated oral exposure to silver nanoparticles or silver acetate. Part. Fibre Toxicol. 2011, 8, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Zande, M.; Vandebriel, R.J.; Van Doren, E.; Kramer, E.; Rivera, Z.H.; Serrano-Rojero, C.S.; Gremmer, E.R.; Mast, J.; Peters, R.J.B.; Hollman, P.C.H.; et al. Distribution, Elimination, and Toxicity of Silver Nanoparticles and Silver Ions in Rats after 28-Day Oral Exposure. ACS Nano 2012, 6, 7427–7442. [Google Scholar] [CrossRef] [PubMed]
- Haak, A.J.; Kostallari, E.; Sicard, D.; Ligresti, G.; Choi, K.M.; Caporarello, N.; Jones, D.L.; Tan, Q.; Meridew, J.; Espinosa, A.M.D.; et al. Selective YAP/TAZ inhibition in fibroblasts via dopamine receptor D1 agonism reverses fibrosis. Sci. Transl. Med. 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Taura, P.; Fuster, J.; Mercadal, J.; Martinez-Palli, G.; Fondevila, C.; Blasi, A.; Balust, J.; Garcia-Valdecasas, J.C. The use of beta-adrenergic drugs improves hepatic oxygen metabolism in cirrhotic patients undergoing liver resection. J. Hepatol. 2010, 52, 340–347. [Google Scholar] [CrossRef]



| Organs | Ag Content (μg/g) |
|---|---|
| liver | 49.31 ± 11.65 |
| lung | 9.61 ± 3.08 |
| Mode | Metabolites | Trend | VIP | p-Value | Pathway |
|---|---|---|---|---|---|
| negative | Alpha-D-Glucose | down | 18.5136 | 0.014065 | Energy metabolism |
| negative | L-Malic acid | down | 3.14375 | 0.002261 | |
| negative | DL-lactate | down | 3.10708 | 0.006937 | |
| negative | Glycerol | down | 1.83996 | 0.073435 | |
| positive | PC(16:0/16:0) | up | 4.06947 | 0.019537 | Membrane metabolism and choline metabolism |
| positive | Thioetheramide-PC | up | 5.29022 | 0.012911 | |
| positive | 1-Stearoyl-sn-glycerol-3-phosphocholine | down | 7.59854 | 0.002209 | |
| positive | 1,2-dioleoyl-sn-glycero-3-phosphatidylcholine | up | 2.33406 | 0.067463 | |
| positive | 1-Myristoyl-sn-glycero-3-phosphocholine | down | 1.90661 | 0.07697 | |
| positive | 1-Oleoyl-sn-glycero-3-phosphocholine | down | 7.27013 | 0.005697 | |
| positive | 1-Palmitoyl-sn-glycero-3-phosphocholine | down | 6.09609 | 0.011949 | |
| positive | 1-Stearoyl-2-oleoyl-sn-glycerol 3-phosphocholine (SOPC) | up | 3.54098 | 0.003904 | |
| positive | Glycerophosphocholine | down | 6.89478 | 0.022855 | |
| positive | Phosphorylcholine | down | 1.79248 | 0.003768 | |
| positive | Choline | down | 1.0697 | 0.020519 | |
| positive | L-Phenylalanine | up | 5.14322 | 0.078525 | Dopamine synthesis |
| positive | L-Tyrosine | up | 2.80009 | 0.006615 | |
| positive | Dopamine | up | 1.19994 | 0.003964 | |
| positive | Cholic acid | up | 5.21803 | 0.049867 | cholic acid synthesis |
| negative | Chenodeoxycholate | up | 2.19638 | 0.097443 | |
| positive | Hypoxanthine | down | 2.56096 | 0.005989 | Purine metabolism |
| positive | Xanthine | down | 3.20653 | 0.000404 | |
| positive | Adenine | down | 2.30405 | 0.000537 | |
| positive | Inosine | down | 4.63986 | 0.003509 | |
| negative | Adenosine 3′-monophosphate | up | 1.01864 | 0.078576 | |
| positive | Nicotinamide | down | 6.21993 | 0.0432 | Redox metabolism |
| negative | Nicotinate | down | 1.06332 | 0.003276 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Wen, T.; Wang, T.; Ji, Y.; Shen, Y.; Chen, J.; Xu, H.; Wu, X. In Vivo Metabolic Response upon Exposure to Gold Nanorod Core/Silver Shell Nanostructures: Modulation of Inflammation and Upregulation of Dopamine. Int. J. Mol. Sci. 2020, 21, 384. https://doi.org/10.3390/ijms21020384
Li H, Wen T, Wang T, Ji Y, Shen Y, Chen J, Xu H, Wu X. In Vivo Metabolic Response upon Exposure to Gold Nanorod Core/Silver Shell Nanostructures: Modulation of Inflammation and Upregulation of Dopamine. International Journal of Molecular Sciences. 2020; 21(2):384. https://doi.org/10.3390/ijms21020384
Chicago/Turabian StyleLi, Haiyun, Tao Wen, Tao Wang, Yinglu Ji, Yaoyi Shen, Jiaqi Chen, Haiyan Xu, and Xiaochun Wu. 2020. "In Vivo Metabolic Response upon Exposure to Gold Nanorod Core/Silver Shell Nanostructures: Modulation of Inflammation and Upregulation of Dopamine" International Journal of Molecular Sciences 21, no. 2: 384. https://doi.org/10.3390/ijms21020384
APA StyleLi, H., Wen, T., Wang, T., Ji, Y., Shen, Y., Chen, J., Xu, H., & Wu, X. (2020). In Vivo Metabolic Response upon Exposure to Gold Nanorod Core/Silver Shell Nanostructures: Modulation of Inflammation and Upregulation of Dopamine. International Journal of Molecular Sciences, 21(2), 384. https://doi.org/10.3390/ijms21020384





