Human Balance in Response to Continuous, Predictable Translations of the Support Base: Integration of Sensory Information, Adaptation to Perturbations, and the Effect of Age, Neuropathy and Parkinson’s Disease
Abstract
:Featured Application
Abstract
1. Introduction
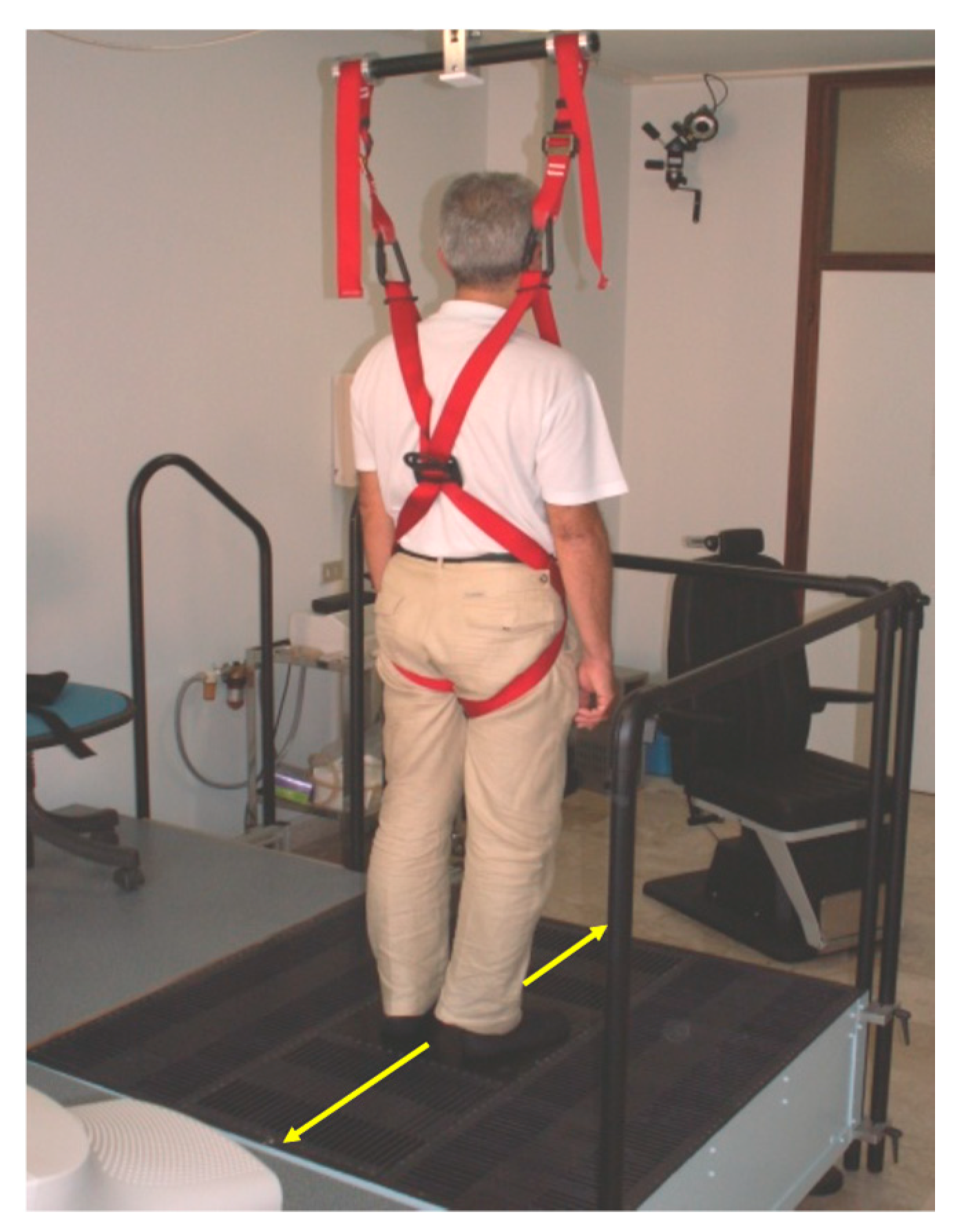
2. The Continuous Predictable Balance Perturbations Administered by the Antero-Posterior Translation of the Platform
3. Muscle Activities
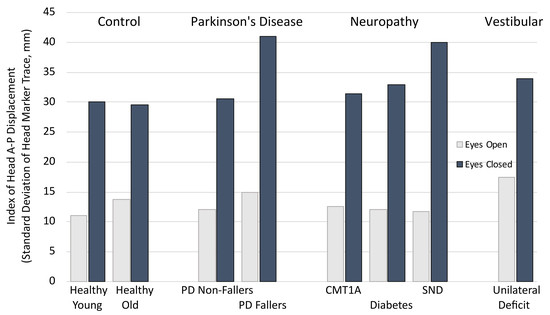
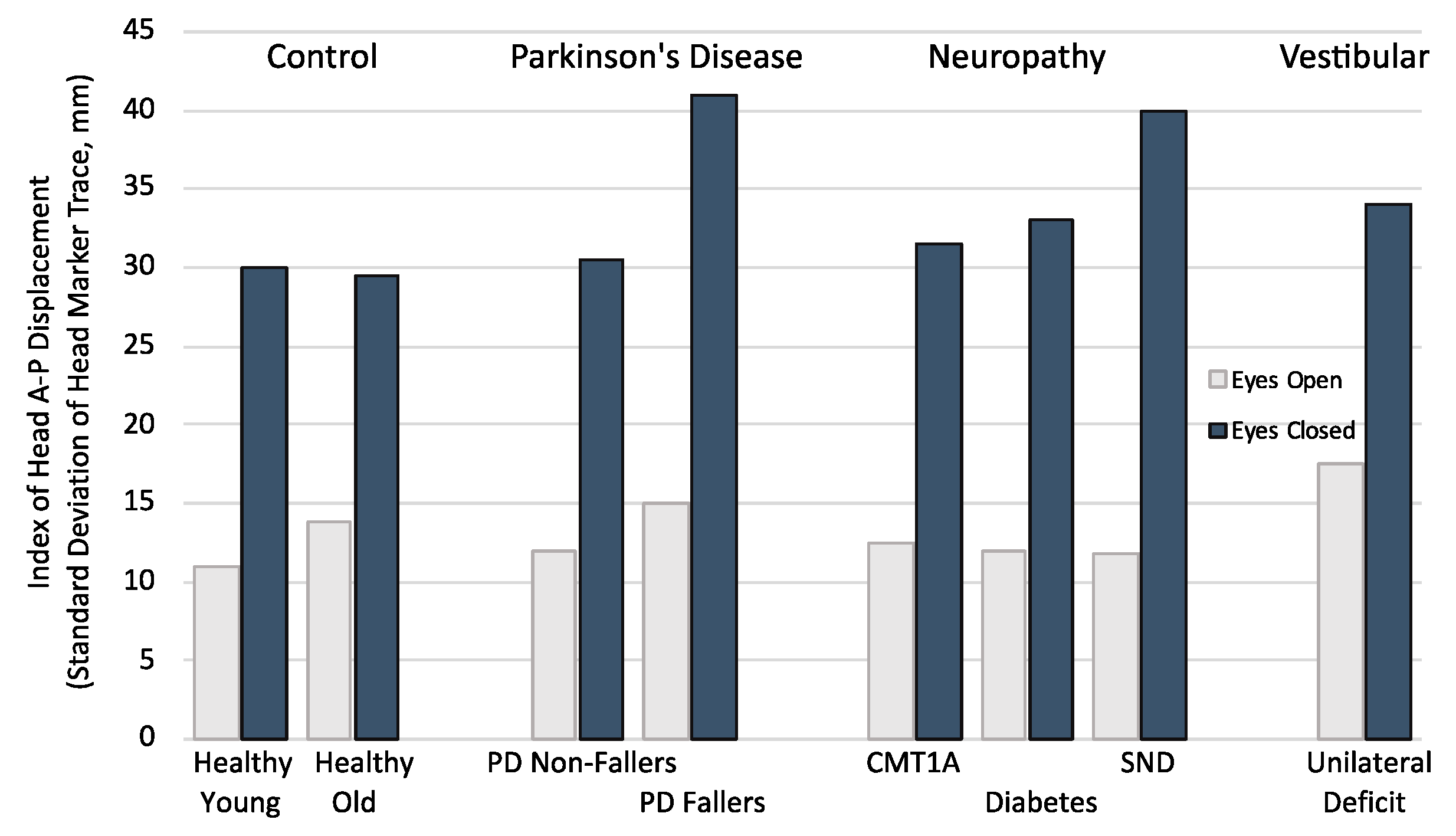
4. Vision
5. Playing with Vision
6. Aging
7. Neuropathy
8. Parkinson’s Disease
9. Adaptation to the Repeated Perturbation Cycles
10. Rehabilitation
11. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Collins Dictionary. Available online: https://www.collinsdictionary.com/dictionary/english/balance (accessed on 4 October 2019).
- Rietdyk, S.; Patla, A.E.; Winter, D.A.; Ishac, M.G.; Little, C.E. Balance recovery from medio-lateral perturbations of the upper body during standing. J. Biomech. 1999, 32, 1149–1158. [Google Scholar] [CrossRef]
- Balasubramaniam, R.; Wing, A.M. The dynamics of standing balance. Trends Cogn. Sci. 2002, 6, 531–536. [Google Scholar] [CrossRef]
- Guglielmetti, S.; Nardone, A.; De Nunzio, A.M.; Godi, M.; Schieppati, M. Walking along circular trajectories in Parkinson’s disease. Mov. Disord. 2009, 24, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Godi, M.; Giardini, M.; Schieppati, M. Walking along curved trajectories. Changes with age and Parkinson’s Disease. Hints to rehabilitation. Front. Neurol. 2019, 10, 532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Fabio, R.P.; Badke, M.B.; Duncan, P.W. Adapting human postural reflexes following localized cerebrovascular lesion: Analysis of bilateral long latency responses. Brain Res. 1986, 363, 257–264. [Google Scholar] [CrossRef]
- Bove, M.; Nardone, A.; Schieppati, M. Effects of leg muscle tendon vibration on group Ia and group II reflex responses to stance perturbation in humans. J. Physiol. 2003, 550, 617–630. [Google Scholar] [CrossRef]
- Schieppati, M.; Nardone, A. Free and supported stance in Parkinson’s disease. The effect of posture and ‘postural set’ on leg muscle responses to perturbation, and its relation to the severity of the disease. Brain 1991, 114, 1227–1244. [Google Scholar] [CrossRef]
- Rogers, M.W.; Mille, M.L. Balance perturbations. Handb. Clin. Neurol. 2018, 159, 85–105. [Google Scholar] [CrossRef]
- Allum, J.H.; Honegger, F. Synergies and strategies underlying normal and vestibulary deficient control of balance: Implication for neuroprosthetic control. Prog. Brain Res. 1993, 97, 331–348. [Google Scholar] [CrossRef]
- Schieppati, M.; Nardone, A. Group II spindle afferent fibers in humans: Their possible role in the reflex control of stance. Prog. Brain Res. 1999, 123, 461–472. [Google Scholar] [CrossRef]
- Meyer, P.F.; Oddsson, L.I.; De Luca, C.J. The role of plantar cutaneous sensation in unperturbed stance. Exp. Brain Res. 2004, 156, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, O.W.; Polskaia, N.; Tokuno, C.D. The effects of foot cooling on postural muscle responses to an unexpected loss of balance. Hum. Mov. Sci. 2017, 54, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Abbruzzese, M.; Rubino, V.; Schieppati, M. Task-dependent effects evoked by foot muscle afferents on leg muscle activity in humans. Electroencephalogr. Clin. Neurophysiol. 1996, 101, 339–348. [Google Scholar] [CrossRef]
- Horak, F.B.; Buchanan, J.; Creath, R.; Jeka, J. Vestibulospinal control of posture. Adv. Exp. Med. Biol. 2002, 508, 139–345. [Google Scholar] [CrossRef]
- Goodworth, A.D.; Peterka, R.J. Influence of bilateral vestibular loss on spinal stabilization in humans. J. Neurophysiol. 2010, 103, 1978–1987. [Google Scholar] [CrossRef] [Green Version]
- Corna, S.; Nardone, A.; Prestinari, A.; Galante, M.; Grasso, M.; Schieppati, M. Comparison of Cawthorne-Cooksey exercises and sinusoidal support surface translations to improve balance in patients with unilateral vestibular deficit. Arch. Phys. Med. Rehabil. 2003, 84, 1173–1184. [Google Scholar] [CrossRef]
- Miall, R.C.; Wolpert, D.M. Forward models for physiological motor control. Neural Netw. 1996, 9, 1265–1279. [Google Scholar] [CrossRef]
- Schieppati, M.; Giordano, A.; Nardone, A. Variability in a dynamic postural task attests ample flexibility in balance control mechanisms. Exp. Brain Res. 2002, 144, 200–210. [Google Scholar] [CrossRef]
- Buchanan, J.J.; Horak, F.B. Emergence of postural patterns as a function of vision and translation frequency. J. Neurophysiol. 1999, 81, 2325–2339. [Google Scholar] [CrossRef] [Green Version]
- Corna, S.; Tarantola, J.; Nardone, A.; Giordano, A.; Schieppati, M. Standing on continuously moving platform: Is body inertia counteracted or exploited? Exp. Brain Res. 1999, 124, 331–341. [Google Scholar] [CrossRef]
- Schieppati, M.; Nardone, A.; Siliotto, R.; Grasso, M. Early and late stretch responses of human foot muscles induced by perturbation of stance. Exp. Brain Res. 1995, 105, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Kelly, L.A.; Kuitunen, S.; Racinais, S.; Cresswell, A.G. Recruitment of the plantar intrinsic foot muscles with increasing postural demand. Clin. Biomech. 2012, 27, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Nardone, A.; Giordano, A.; Corrà, T.; Schieppati, M. Responses of leg muscles in humans displaced while standing. Effects of types of perturbation and of postural set. Brain 1990, 113, 65–84. [Google Scholar] [CrossRef]
- Carpenter, M.G.; Allum, J.H.; Honegger, F. Directional sensitivity of stretch reflexes and balance corrections for normal subjects in the roll and pitch planes. Exp. Brain Res. 1999, 129, 93–113. [Google Scholar] [CrossRef]
- Granata, K.P.; Slota, G.P.; Bennett, B.C. Paraspinal muscle reflex dynamics. J. Biomech. 2004, 37, 241–247. [Google Scholar] [CrossRef]
- Boudreau, S.A.; Falla, D. Chronic neck pain alters muscle activation patterns to sudden movements. Exp. Brain Res. 2014, 232, 2011–2020. [Google Scholar] [CrossRef]
- Van Drunen, P.; Koumans, Y.; van der Helm, F.C.; van Dieën, J.H.; Happee, R. Modulation of intrinsic and reflexive contributions to low-back stabilization due to vision, task instruction, and perturbation bandwidth. Exp. Brain Res. 2015, 233, 735–749. [Google Scholar] [CrossRef] [Green Version]
- Massion, J. Movement, posture and equilibrium: Interaction and coordination. Prog. Neurobiol. 1992, 38, 35–56. [Google Scholar] [CrossRef]
- Aruin, A.S.; Forrest, W.R.; Latash, M.L. Anticipatory postural adjustments in conditions of postural instability. Electroencephalogr. Clin. Neurophysiol. 1998, 109, 350–359. [Google Scholar] [CrossRef]
- Bouisset, S.; Do, M.C. Posture, dynamic stability, and voluntary movement. Neurophysiol. Clin. 2008, 38, 345–362. [Google Scholar] [CrossRef]
- Aruin, A.S. Enhancing Anticipatory Postural Adjustments: A Novel Approach to Balance Rehabilitation. J. Nov. Physiother. 2016, 6, e144. [Google Scholar] [CrossRef] [PubMed]
- Sozzi, S.; Nardone, A.; Schieppati, M. Calibration of the Leg Muscle Responses Elicited by Predictable Perturbations of Stance and the Effect of Vision. Front. Hum. Neurosci. 2016, 10, 419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khasnis, A.; Gokula, R.M. Romberg’s test. J. Postgrad. Med. 2003, 49, 169. [Google Scholar] [PubMed]
- Redfern, M.S.; Yardley, L.; Bronstein, A.M. Visual influences on balance. J. Anxiety Disord. 2001, 15, 81–94. [Google Scholar] [CrossRef]
- Paulus, W.; Straube, A.; Brandt, T.H. Visual postural performance after loss of somatosensory and vestibular function. J. Neurol. Neurosurg. Psychiatry 1987, 50, 1542–1545. [Google Scholar] [CrossRef] [PubMed]
- Nardone, A.; Schieppati, M. Balance control under static and dynamic conditions in patients with peripheral neuropathy. G Ital. Med. Lav. Ergon. 2007, 29, 101–104. [Google Scholar] [CrossRef]
- Nardone, A.; Schieppati, M. The role of instrumental assessment of balance in clinical decision making. Eur. J. Phys. Rehabil. Med. 2010, 46, 221–237. [Google Scholar]
- Assländer, L.; Hettich, G.; Mergner, T. Visual contribution to human standing balance during support surface tilts. Hum. Mov. Sci. 2015, 41, 147–164. [Google Scholar] [CrossRef] [Green Version]
- Ghai, S.; Ghai, I.; Effenberg, A.O. Effects of dual tasks and dual-task training on postural stability: A systematic review and meta-analysis. Clin. Interv. Aging 2017, 12, 557. [Google Scholar] [CrossRef] [Green Version]
- Schmid, M.; Bottaro, A.; Sozzi, S.; Schieppati, M. Adaptation to continuous perturbation of balance: Progressive reduction of postural muscle activity with invariant or increasing oscillations of the center of mass depending on perturbation frequency and vision conditions. Hum. Mov. Sci. 2011, 30, 262–278. [Google Scholar] [CrossRef] [Green Version]
- Schmid, M.; Casabianca, L.; Bottaro, A.; Schieppati, M. Graded changes in balancing behavior as a function of visual acuity. Neuroscience 2008, 153, 1079–1091. [Google Scholar] [CrossRef] [PubMed]
- Schmid, M.; Nardone, A.; De Nunzio, A.M.; Schmid, M.; Schieppati, M. Equilibrium during static and dynamic tasks in blind subjects: No evidence of cross-modal plasticity. Brain 2007, 130, 2097–2107. [Google Scholar] [CrossRef] [PubMed]
- Sozzi, S.; Nardone, A.; Schieppati, M. Vision Does Not Necessarily Stabilize the Head in Space During Continuous Postural Perturbations. Front. Neurol. 2019, 10, 748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Nunzio, A.M.; Schieppati, M. Time to reconfigure balancing behaviour in man: Changing visual condition while riding a continuously moving platform. Exp. Brain Res. 2007, 178, 18–36. [Google Scholar] [CrossRef]
- Sozzi, S.; Monti, A.; De Nunzio, A.M.; Do, M.C.; Schieppati, M. Sensori-motor integration during stance: Time adaptation of control mechanisms on adding or removing vision. Hum. Mov. Sci. 2011, 30, 172–189. [Google Scholar] [CrossRef]
- Honeine, J.L.; Crisafulli, O.; Sozzi, S.; Schieppati, M. Processing time of addition or withdrawal of single or combined balance-stabilizing haptic and visual information. J. Neurophysiol. 2015, 114, 3097–3110. [Google Scholar] [CrossRef] [Green Version]
- McCrum, C.; Gerards, M.H.G.; Karamanidis, K.; Zijlstra, W.; Meijer, K. A systematic review of gait perturbation paradigms for improving reactive stepping responses and falls risk among healthy older adults. Eur. Rev. Aging Phys. Act. 2017, 14, 3. [Google Scholar] [CrossRef] [Green Version]
- Tsai, Y.C.; Hsieh, L.F.; Yang, S. Age-related changes in posture response under a continuous and unexpected perturbation. J. Biomech. 2014, 47, 482–490. [Google Scholar] [CrossRef]
- Alcock, L.; O’Brien, T.D.; Vanicek, N. Association between somatosensory, visual and vestibular contributions to postural control, reactive balance capacity and healthy ageing in older women. Health Care Women Int. 2018, 39, 1366–1380. [Google Scholar] [CrossRef]
- Schieppati, M.; Grasso, M.; Siliotto, R.; Nardone, A. Effect of age, chronic diseases and parkinsonism on postural control. In Sensorimotor Impairment in the Elderly; Stelmach, G.E., Hömberg, V., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1993; pp. 355–373. [Google Scholar]
- Schieppati, M.; Hugon, M.; Grasso, M.; Nardone, A.; Galante, M. The limits of equilibrium in young and elderly normal subjects and in parkinsonians. Electroencephalogr. Clin. Neurophysiol. 1994, 93, 286–298. [Google Scholar] [CrossRef]
- Nardone, A.; Siliotto, R.; Grasso, M.; Schieppati, M. Influence of aging on leg muscle reflex responses to stance perturbation. Arch. Phys. Med. Rehabil. 1995, 76, 158–165. [Google Scholar] [CrossRef]
- Studenski, S.; Duncan, P.W.; Chandler, J. Postural responses and effector factors in persons with unexplained falls: Results and methodologic issues. J. Am. Geriatr. Soc. 1991, 39, 229–234. [Google Scholar] [CrossRef]
- Woollacott, M.H.; Shumway-Cook, A.; Nashner, L.M. Aging and posture control: Changes in sensory organization and muscular coordination. Int. J. Aging Human Dev. 1986, 23, 97–114. [Google Scholar] [CrossRef] [PubMed]
- Wiesmeier, I.K.; Dalin, D.; Maurer, C. Elderly use proprioception rather than visual and vestibular cues for postural motor control. Front. Aging Neurosci. 2015, 7, 97. [Google Scholar] [CrossRef] [PubMed]
- Nardone, A.; Grasso, M.; Tarantola, J.; Corna, S.; Schieppati, M. Postural coordination in elderly subjects standing on a periodically moving platform. Arch. Phys. Med. Rehabil. 2000, 81, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Bugnariu, N.; Sveistrup, H. Age-related changes in postural responses to externally- and self-triggered continuous perturbations. Arch. Gerontol. Geriatr. 2006, 42, 73–89. [Google Scholar] [CrossRef]
- Van Ooteghem, K.; Frank, J.S.; Horak, F.B. Practice-related improvements in posture control differ between young and older adults exposed to continuous, variable amplitude oscillations of the support surface. Exp. Brain Res. 2009, 199, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Castro, P.; Kaski, D.; Schieppati, M.; Furman, M.; Arshad, Q.; Bronstein, A. Subjective stability perception is related to postural anxiety in older subjects. Gait Posture 2019, 68, 538–544. [Google Scholar] [CrossRef]
- Trivedi, S.; Pandit, A.; Ganguly, G.; Das, S.K. Epidemiology of Peripheral Neuropathy: An Indian Perspective. Ann. Indian Acad. Neurol. 2017, 20, 173–184. [Google Scholar] [CrossRef]
- Brisset, M.; Nicolas, G. Peripheral neuropathies and aging. Geriatr. Psychol. Neuropsychiatr. Vieil 2018, 16, 409–413. [Google Scholar] [CrossRef]
- Feldman, E.L.; Callaghan, B.C.; Pop-Busui, R.; Zochodne, D.W.; Wright, D.E.; Bennett, D.L.; Bril, V.; Russell, J.W.; Viswanathan, V. Diabetic neuropathy. Nat. Rev. Dis. Primers 2019, 5, 41. [Google Scholar] [CrossRef]
- Vinik, A.I. Diabetic Sensory and Motor Neuropathy. N. Engl. J. Med. 2016, 374, 1455–1464. [Google Scholar] [CrossRef] [PubMed]
- Schieppati, M.; Tacchini, E.; Nardone, A.; Tarantola, J.; Corna, S. Subjective perception of body sway. J. Neurol. Neurosurg. Psychiatry 1999, 66, 313–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lencioni, T.; Rabuffetti, M.; Piscosquito, G.; Pareyson, D.; Aiello, A.; Di Sipio, E.; Padua, L.; Stra, F.; Ferrarin, M. Postural stabilization and balance assessment in Charcot-Marie-Tooth 1A subjects. Gait Posture 2014, 40, 481–486. [Google Scholar] [CrossRef]
- Anson, E.; Bigelow, R.T.; Swenor, B.; Deshpande, N.; Studenski, S.; Jeka, J.J.; Agrawal, Y. Loss of Peripheral Sensory Function Explains Much of the Increase in Postural Sway in Healthy Older Adults. Front. Aging Neurosci. 2017, 9, 202. [Google Scholar] [CrossRef] [Green Version]
- Nardone, A.; Grasso, M.; Schieppati, M. Balance control in peripheral neuropathy: Are patients equally unstable under static and dynamic conditions? Gait Posture 2006, 23, 364–373. [Google Scholar] [CrossRef]
- Nardone, A.; Tarantola, J.; Miscio, G.; Pisano, F.; Schenone, A.; Schieppati, M. Loss of large-diameter spindle afferent fibres is not detrimental to the control of body sway during upright stance: Evidence from neuropathy. Exp. Brain Res. 2000, 135, 155–162. [Google Scholar] [CrossRef]
- Kars, H.J.; Hijmans, J.M.; Geertzen, J.H.; Zijlstra, W. The effect of reduced somatosensation on standing balance: A systematic review. J. Diabetes Sci. Technol. 2009, 3, 931–943. [Google Scholar] [CrossRef] [Green Version]
- Nardone, A.; Galante, M.; Pareyson, D.; Schieppati, M. Balance control in Sensory Neuron Disease. Clin. Neurophysiol. 2007, 118, 538–550. [Google Scholar] [CrossRef]
- Whitlock, J.R. Posterior parietal cortex. Curr. Biol. 2017, 27, R691–R695. [Google Scholar] [CrossRef] [Green Version]
- Burke, D.; Hagbarth, K.E.; Löfstedt, L.; Wallin, B.G. The responses of human muscle spindle endings to vibration during isometric contraction. J. Physiol. 1976, 261, 695–711. [Google Scholar] [CrossRef]
- Burke, D.; Hagbarth, K.E.; Löfstedt, L.; Wallin, B.G. The responses of human muscle spindle endings to vibration of non-contracting muscles. J. Physiol. 1976, 261, 673–693. [Google Scholar] [CrossRef]
- De Nunzio, A.M.; Nardone, A.; Schieppati, M. Head stabilization on a continuously oscillating platform: The effect of a proprioceptive disturbance on the balancing strategy. Exp. Brain Res. 2005, 165, 261–272. [Google Scholar] [CrossRef]
- Staines, W.R.; McIlroy, W.E.; Brooke, J.D. Cortical representation of whole-body movement is modulated by proprioceptive discharge in humans. Exp. Brain Res. 2001, 138, 235–242. [Google Scholar] [CrossRef]
- Konczak, J.; Corcos, D.M.; Horak, F.; Poizner, H.; Shapiro, M.; Tuite, P.; Volkmann, J.; Maschke, M. Proprioception and motor control in Parkinson’s disease. J. Mot. Behav. 2009, 41, 543–552. [Google Scholar] [CrossRef]
- Schoneburg, B.; Mancini, M.; Horak, F.; Nutt, J.G. Framework for understanding balance dysfunction in Parkinson’s disease. Mov. Disord. 2013, 28, 1474–1482. [Google Scholar] [CrossRef] [Green Version]
- Hwang, S.; Agada, P.; Grill, S.; Kiemel, T.; Jeka, J.J. A central processing sensory deficit with Parkinson’s disease. Exp. Brain Res. 2016, 234, 2369–2379. [Google Scholar] [CrossRef] [Green Version]
- Ghai, S.; Ghai, I.; Schmitz, G.; Effenberg, A.O. Effect of rhythmic auditory cueing on parkinsonian gait: A systematic review and meta-analysis. Sci. Rep. 2018, 8, 506. [Google Scholar] [CrossRef]
- Boonstra, T.A.; van Vugt, J.P.; van der Kooij, H.; Bloem, B.R. Balance asymmetry in Parkinson’s disease and its contribution to freezing of gait. PLoS ONE 2014, 9, e102493. [Google Scholar] [CrossRef]
- Weissblueth, E.; Schwartz, M.; Hocherman, S. Adaptation to cyclic stance perturbations in Parkinson’s disease depends on postural demands. Parkinsonism Relat. Disord. 2008, 14, 489–494. [Google Scholar] [CrossRef]
- Van Ooteghem, K.; Frank, J.S.; Horak, F.B. Postural motor learning in Parkinson’s disease: The effect of practice on continuous compensatory postural regulation. Gait Posture 2017, 57, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Nardone, A.; Schieppati, M. Balance in Parkinson’s disease under static and dynamic conditions. Mov. Disord. 2006, 21, 1515–1520. [Google Scholar] [CrossRef] [PubMed]
- De Nunzio, A.M.; Nardone, A.; Schieppati, M. The control of equilibrium in Parkinson’s disease patients: Delayed adaptation of balancing strategy to shifts in sensory set during a dynamic task. Brain Res. Bull. 2007, 74, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.V.; Horak, F.B. Abnormal proprioceptive-motor integration contributes to hypometric postural responses of subjects with Parkinson’s disease. Neuroscience 2006, 141, 999–1009. [Google Scholar] [CrossRef]
- Blouin, J.S.; Descarreaux, M.; Bélanger-Gravel, A.; Simoneau, M.; Teasdale, N. Attenuation of human neck muscle activity following repeated imposed trunk-forward linear acceleration. Exp. Brain Res. 2003, 150, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Nardone, A.; Pasetti, C.; Schieppati, M. Spinal and supraspinal stretch responses of postural muscles in early Parkinsonian patients. Exp. Neurol. 2012, 237, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Keshner, E.A.; Allum, J.H.; Pfaltz, C.R. Postural coactivation and adaptation in the sway stabilizing responses of normals and patients with bilateral vestibular deficit. Exp. Brain Res. 1987, 69, 77–92. [Google Scholar] [CrossRef]
- Fujiwara, K.; Maeda, K.; Irei, M.; Mammadova, A.; Kiyota, N. Changes in event-related potentials associated with postural adaptation during floor oscillation. Neuroscience 2012, 213, 122–132. [Google Scholar] [CrossRef]
- Siegmund, G.P.; Blouin, J.S.; Inglis, J.T. Does startle explain the exaggerated first response to a transient perturbation? Exerc. Sport Sci. Rev. 2008, 36, 76–82. [Google Scholar] [CrossRef]
- Allum, J.H.; Tang, K.S.; Carpenter, M.G.; Oude Nijhuis, L.B.; Bloem, B.R. Review of first trial responses in balance control: Influence of vestibular loss and Parkinson’s disease. Hum. Mov. Sci. 2011, 30, 279–295. [Google Scholar] [CrossRef]
- Schmid, M.; Sozzi, S. Temporal features of postural adaptation strategy to prolonged and repeatable balance perturbation. Neurosci. Lett. 2016, 628, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.; Bugnariu, N.; Guevel, A.; Sveistrup, H. Adaptation of the feedforward postural response to repeated continuous postural perturbations. Neurosci. Med. 2013, 4, 45–49. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, A.; Guevel, A.; Sveistrup, H. Impact of ankle muscle fatigue and recovery on the anticipatory postural adjustments to externally initiated perturbations in dynamic postural control. Exp. Brain Res. 2012, 223, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Joseph Jilk, D.; Safavynia, S.A.; Ting, L.H. Contribution of vision to postural behaviors during continuous support-surface translations. Exp. Brain Res. 2014, 232, 169–180. [Google Scholar] [CrossRef] [Green Version]
- Welch, T.D.; Ting, L.H. Mechanisms of motor adaptation in reactive balance control. PLoS ONE 2014, 9, e96440. [Google Scholar] [CrossRef] [Green Version]
- Nardone, A.; Godi, M.; Artuso, A.; Schieppati, M. Balance rehabilitation by moving platform and exercises in patients with neuropathy or vestibular deficit. Arch. Phys. Med. Rehabil. 2010, 91, 1869–1877. [Google Scholar] [CrossRef]
- Buchanan, J.J.; Horak, F.B. Vestibular loss disrupts control of head and trunk on a sinusoidally moving platform. J. Vestib. Res. 2002, 11, 371–389. [Google Scholar]
- Nardone, A.; Turcato, A.M.; Schieppati, M. Effects of balance and gait rehabilitation in cerebellar disease of vascular or degenerative origin. Restor. Neurol. Neurosci. 2014, 32, 233–245. [Google Scholar] [CrossRef]
- Missaoui, B.; Thoumie, P. Balance training in ataxic neuropathies. Effects on balance and gait parameters. Gait Posture 2013, 38, 471–476. [Google Scholar] [CrossRef]
- Nanhoe-Mahabier, W.; Allum, J.H.; Overeem, S.; Borm, G.F.; Oude Nijhuis, L.B.; Bloem, B.R. First trial reactions and habituation rates over successive balance perturbations in Parkinson’s disease. Neuroscience 2012, 217, 123–129. [Google Scholar] [CrossRef]
- Giardini, M.; Nardone, A.; Godi, M.; Guglielmetti, S.; Arcolin, I.; Pisano, F.; Schieppati, M. Instrumental or Physical-Exercise Rehabilitation of Balance Improves Both Balance and Gait in Parkinson’s Disease. Neural Plast. 2018, 7, 5614242. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghai, S.; Nardone, A.; Schieppati, M. Human Balance in Response to Continuous, Predictable Translations of the Support Base: Integration of Sensory Information, Adaptation to Perturbations, and the Effect of Age, Neuropathy and Parkinson’s Disease. Appl. Sci. 2019, 9, 5310. https://doi.org/10.3390/app9245310
Ghai S, Nardone A, Schieppati M. Human Balance in Response to Continuous, Predictable Translations of the Support Base: Integration of Sensory Information, Adaptation to Perturbations, and the Effect of Age, Neuropathy and Parkinson’s Disease. Applied Sciences. 2019; 9(24):5310. https://doi.org/10.3390/app9245310
Chicago/Turabian StyleGhai, Shashank, Antonio Nardone, and Marco Schieppati. 2019. "Human Balance in Response to Continuous, Predictable Translations of the Support Base: Integration of Sensory Information, Adaptation to Perturbations, and the Effect of Age, Neuropathy and Parkinson’s Disease" Applied Sciences 9, no. 24: 5310. https://doi.org/10.3390/app9245310