Estimation of Transition Frequency during Continuous Translation Surface Perturbation
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Experimental Procedures
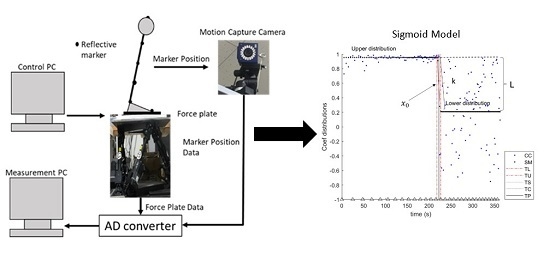
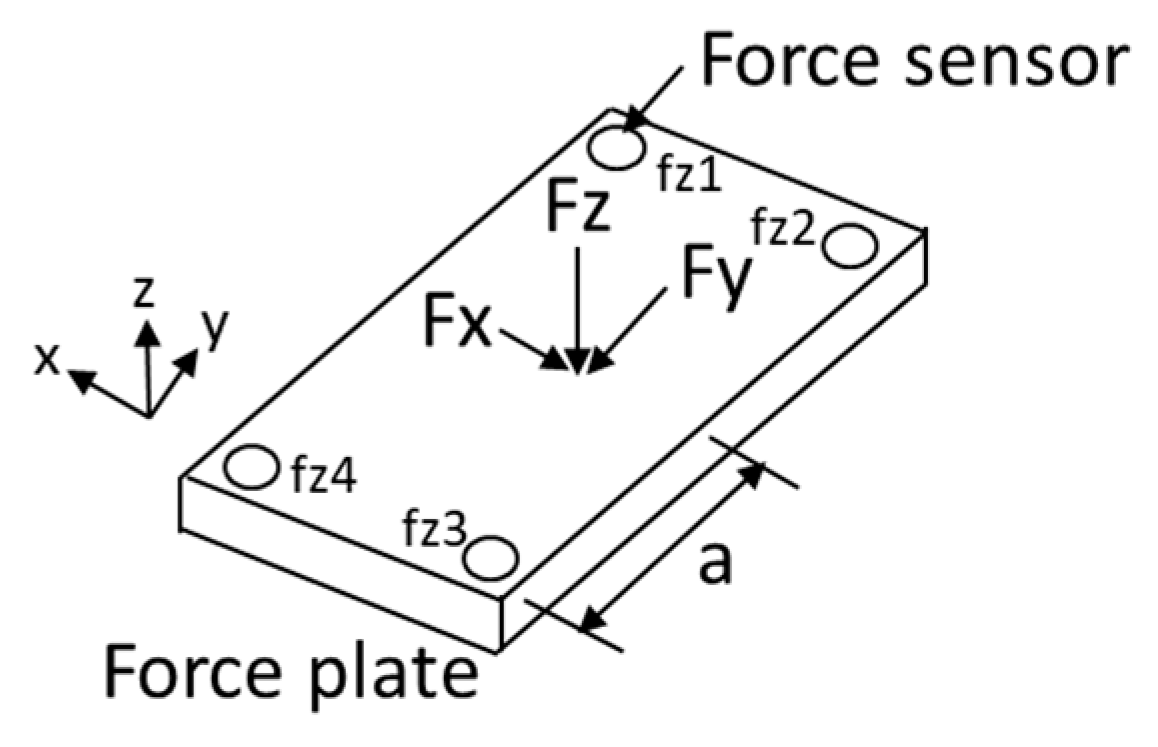
2.3. Experimental Apparatus
2.4. Data Collection
2.5. Data Analysis
2.5.1. Cross-Correlation Coefficient
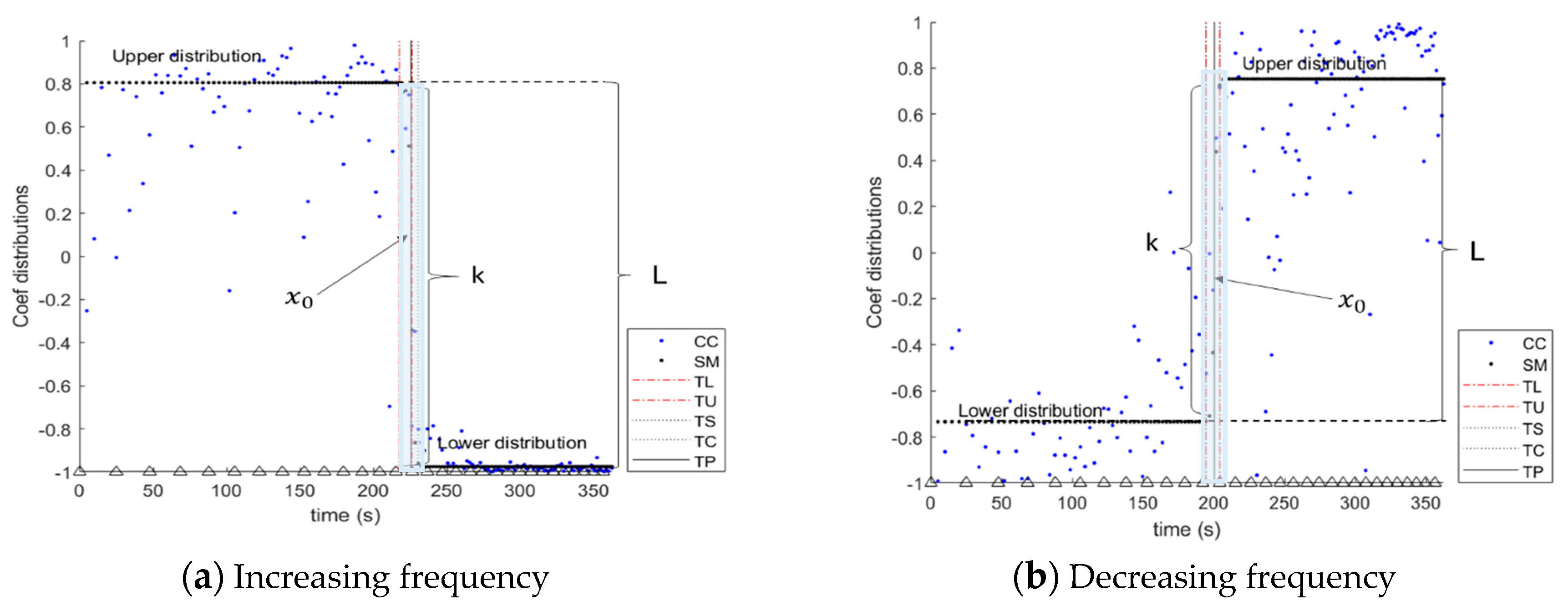
2.5.2. Logistic Function (Sigmoid Model)
2.6. Statistical Analysis
3. Results
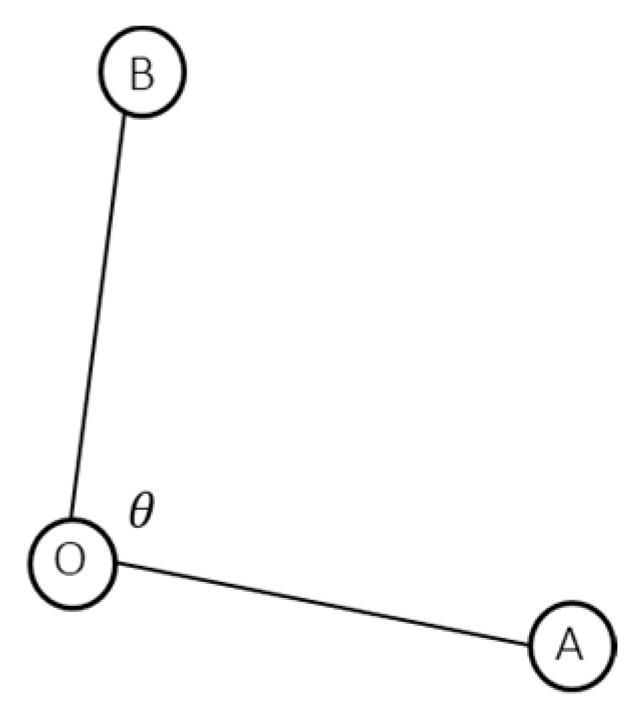
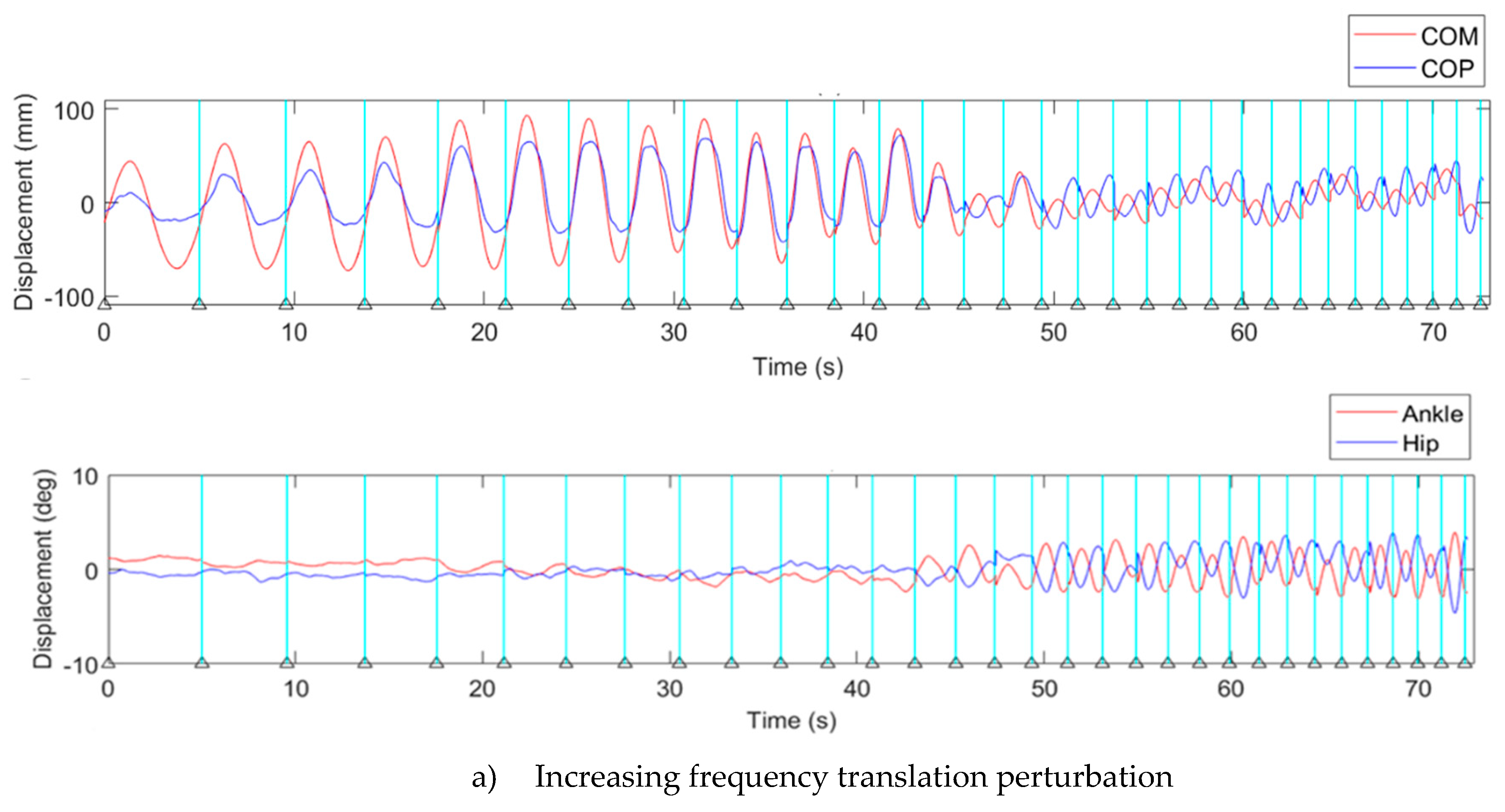
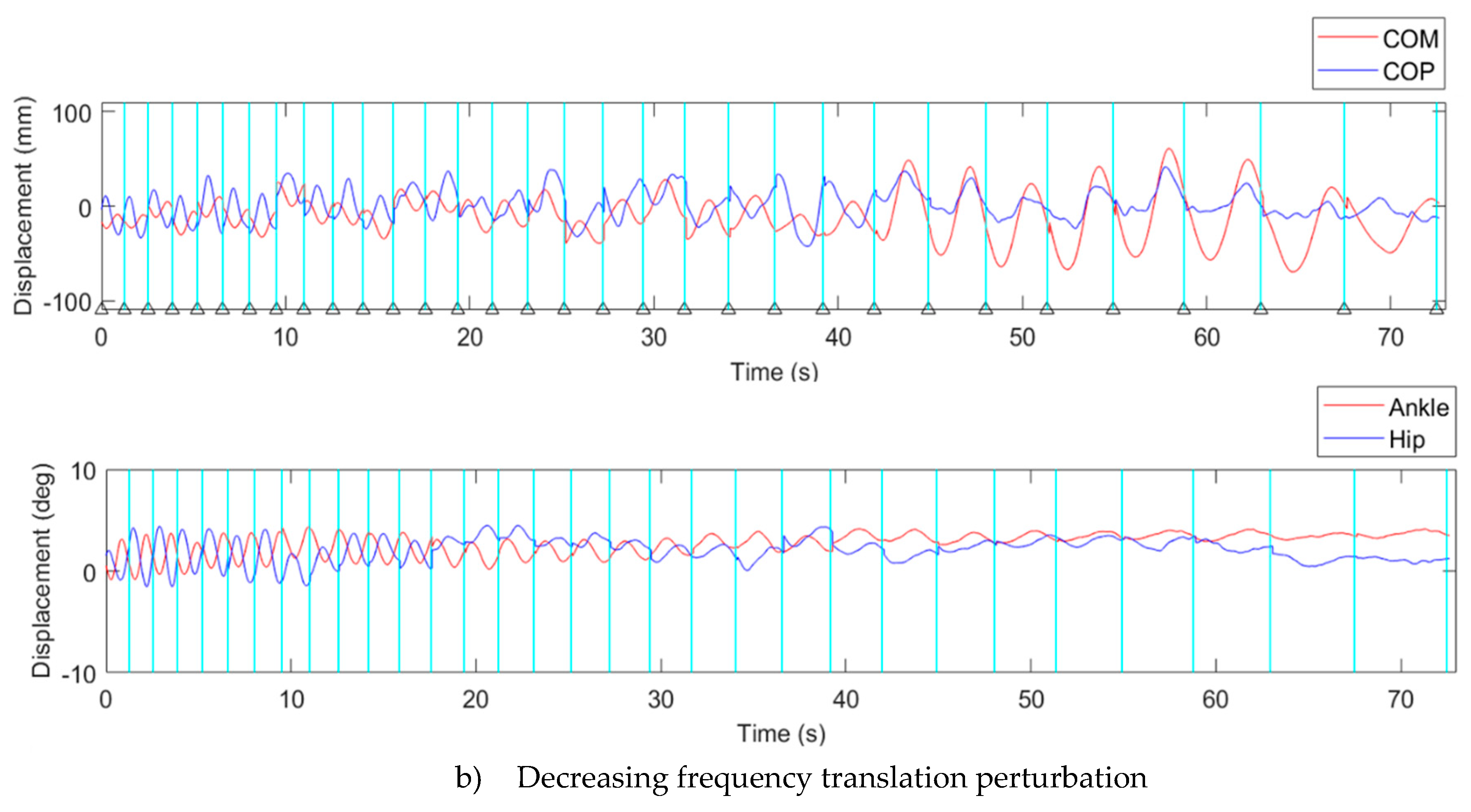
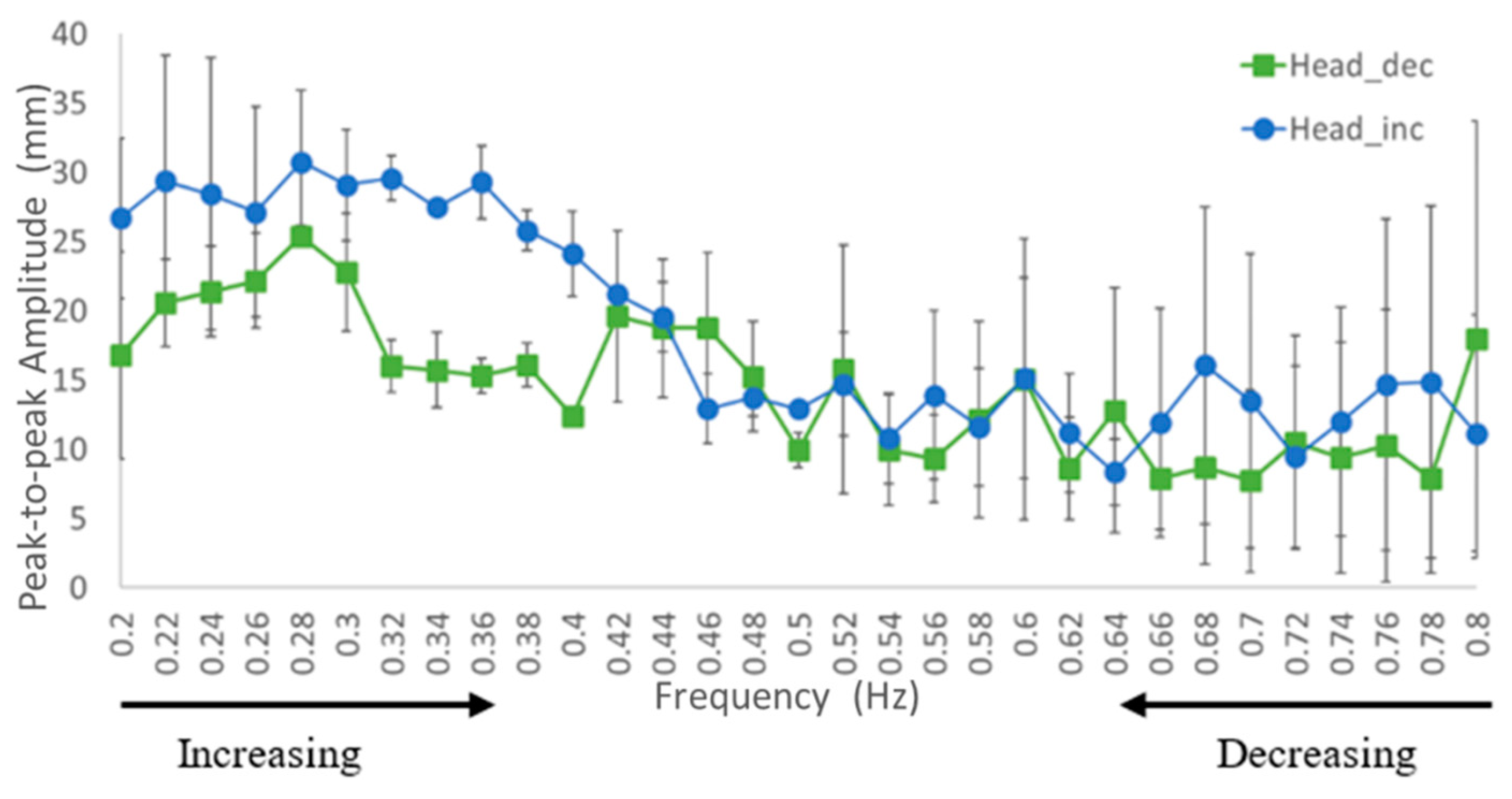
3.1. Displacement of Kinematics Parameters
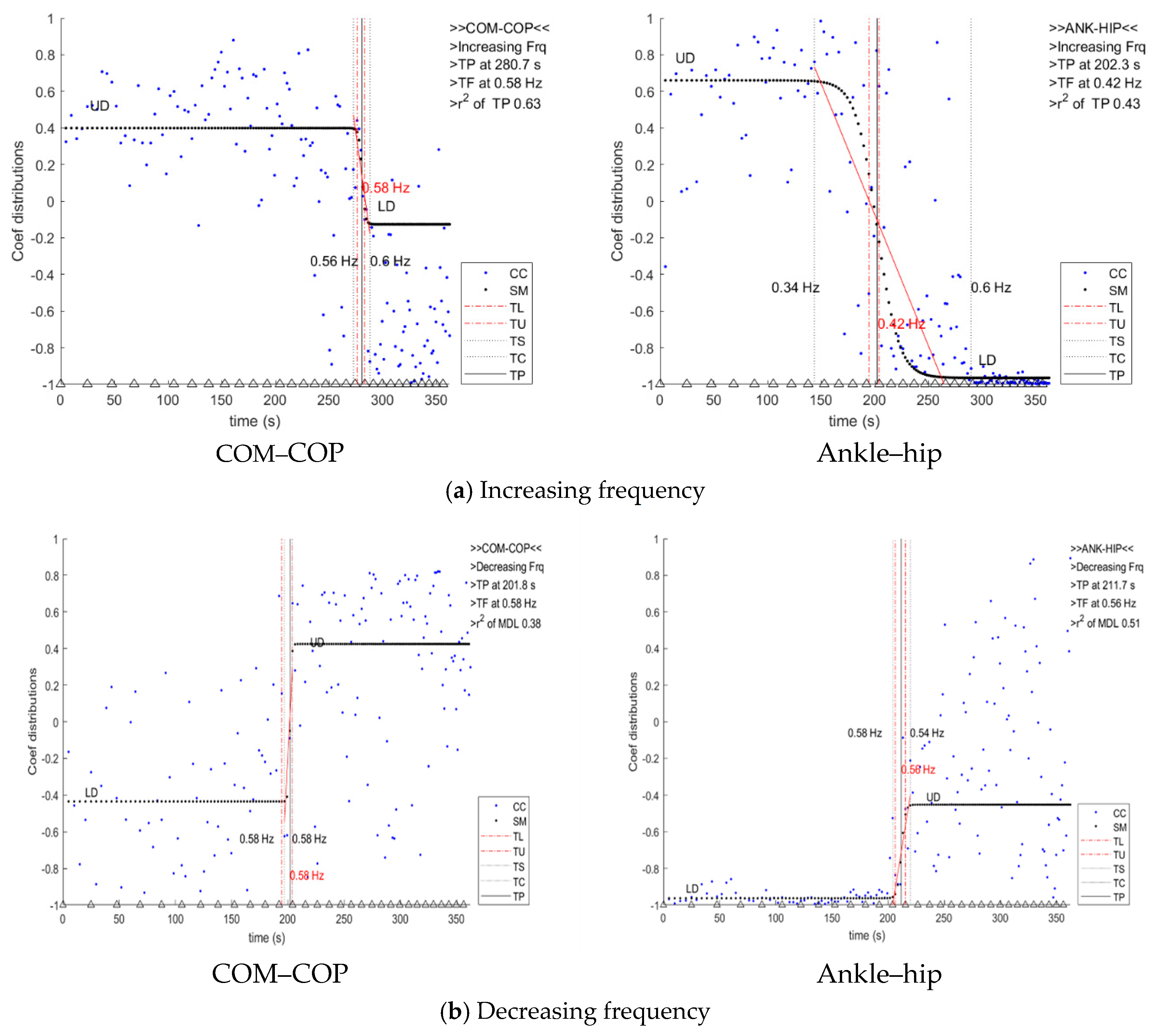
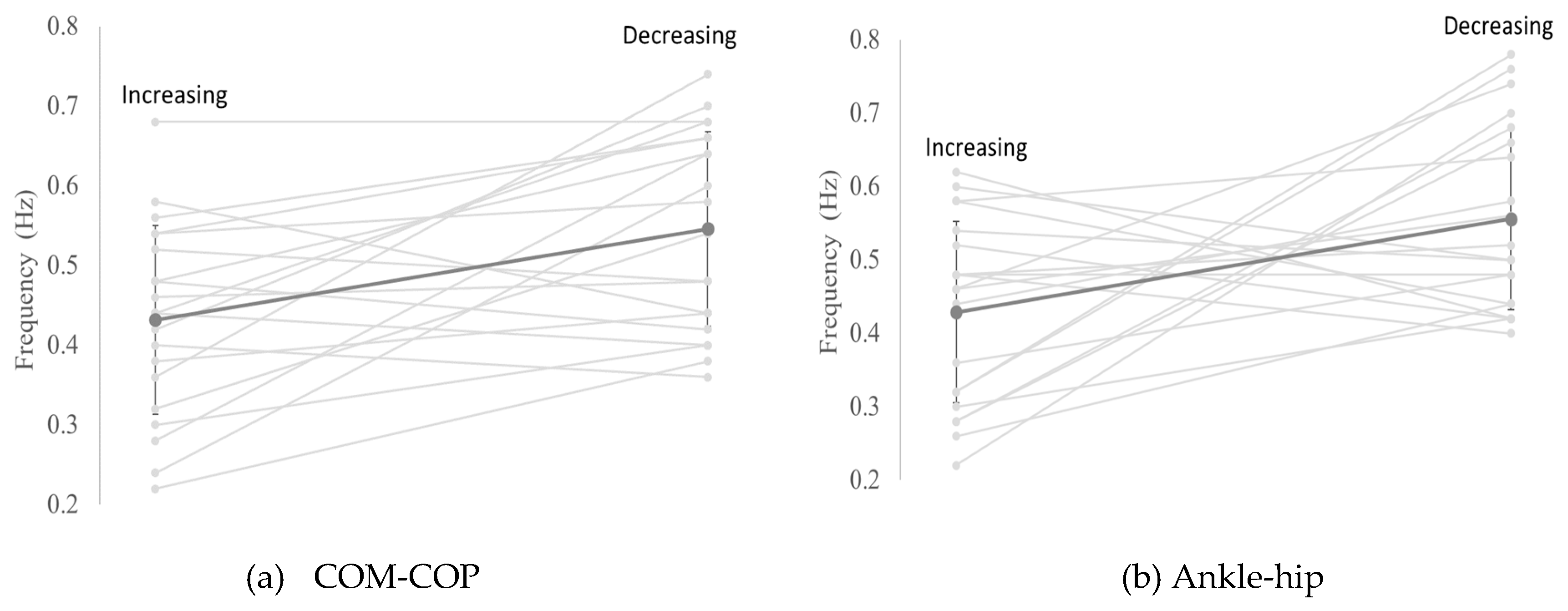
3.2. Transition Frequency
4. Discussion
4.1. Sigmoid Model Based on Cross-Correlation Coefficient Data for the Determination of Transition Frequency
4.2. Kinematic Characteristic of Continuous Translation Perturbation Frequencies
4.3. From a Single-Linked Model to Multi-Segmental Model
4.4. Study Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lord, S.R.; Clark, R.D.; Webster, I.W. Postural stability and associated physiological factors in a population of aged persons. J. Gerontol. 1991, 46, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Winter, D.A. Human balance and posture control during standing and walking. Gait Posture 1995, 3, 193–214. [Google Scholar] [CrossRef]
- Santos, M.J.; Kanekar, N.; Aruin, A.S. The role of anticipatory adjustment in compensatory control of posture: 2. Biomechanical analysis. J. Electromyogr. Kinesiol. 2013, 20, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Horak, F.B.; Nasher, L.M. Central programming of postural movement: Adaptation to altered support-surface configuration. J. Neurophysiol. 1986, 55, 1369–1381. [Google Scholar] [CrossRef]
- Nasher, L.M.; McCollum, G. The organization of human postural movement: A formal basis and experimental synthesis. Behav. Brain Sci. 1985, 8, 135–172. [Google Scholar] [CrossRef]
- Buchanan, J.J.; Horak, F.B. Emergence of postural patterns as a function of vision and translation frequency. J. Neurophysiol. 1999, 81, 2325–2339. [Google Scholar] [CrossRef]
- Horak, F.B.; Macpherson, J.M. Postural Orientation and Equilibrium; Rowell, L.B., Shepard, J.T., Eds.; Handbook of Physiology: Section 12, Exercise Regulation and Integration of Multiple Systems; Oxford University Press: New York, NY, USA, 1996; pp. 255–292. [Google Scholar]
- Bardy, B.G.; Oullier, O.; Bootsma, R.J.; Stoffregen, T.A. Dynamics of human postural transitions. J. Exp. Psychol. Hum. Percept. Perform. 2002, 28, 499–514. [Google Scholar] [CrossRef]
- Kelso, S. Phase transitions and critical behavior in human bimanual coordination. Am. J. Physiol. 1984, 246, R1000–R1004. [Google Scholar] [CrossRef]
- Hamill, J.; Bates, B.T.; Holt, K.G. Timing of lower extremity joint actions during treadmill running. Med. Sci. Sports Exerc. 1992, 24, 807–813. [Google Scholar] [CrossRef]
- Dutt-Mazumder, A.; Newell, K. Transition of postural coordination as a function of frequency of the moving support platform. Hum. Mov. Sci. 2017, 52, 24–35. [Google Scholar] [CrossRef]
- Sfar, A.R.; Challal, Y.; Moyal, P.; Natalizio, E. A Game Theoretic Approach for Privacy Preserving Model in IoT-Based Transportation. IEEE Trans. Intell. Transp. Syst. 2019. [Google Scholar] [CrossRef]
- Wu, G.; Say, B.; Sanner, S. Scalable Nonlinear Planning with Deep Neural Network Learned Transition Models. arXiv 2019, arXiv:1904.02873. [Google Scholar]
- Klimstra, M.; Zehr, E.P. A sigmoid function is the best fit for the ascending limb of the Hoffmann reflex recruitment curve. Exp. Brain Res. 2008, 186, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.-J.; Schultz, A.B.; Shepard, N.T.; Alexander, N.B. Postural control in young and elderly adults when stance is perturbed: Dynamics. J. Biomech. 1996, 29, 319–329. [Google Scholar] [CrossRef]
- Hughes, M.; Schenkman, M.; Chandler, J.; Studenski, S. Postural responses to platform perturbation: kinematics and electromyography. Clin. Biomech. 1995, 10, 318–322. [Google Scholar] [CrossRef]
- Keshner, E.A.; Woollacott, M.H.; Debû, B. Neck, trunk and limb muscle responses during postural perturbations in humans. Exp. Brain Res. 1988, 71, 455–466. [Google Scholar] [CrossRef]
- Woollacott, M.H.; Von Hosten, C.; Rösblad, B. Relation between muscle response onset and body segmental movements during postural perturbations in humans. Exp. Brain Res. 1988, 72, 593–604. [Google Scholar] [CrossRef]
- Alexander, N.B.; Shepard, N.; Gu, M.J.; Schultz, A. Postural Control in Young and Elderly Adults When Stance Is Perturbed: Kinematics. J. Gerontol. 1992, 47, 79–87. [Google Scholar] [CrossRef]
- Gage, W.H.; Winter, D.A.; Frank, J.S.; Adkin, A.L. Kinematic and Kinetic Validity of the Inverted Pendulum Model in Quiet Standing. Gait Posture 2004, 19, 124–132. [Google Scholar] [CrossRef]
- Ko, Y.-G.; Challis, J.H.; Newell, K.M. Postural coordination patterns as a function of dynamics of the support surface. Hum. Mov. Sci. 2001, 20, 737–764. [Google Scholar] [CrossRef]
- Winter, D.A. Biomechanics and Motor Control of Human Movement, 4th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; p. 86. ISBN 978-0-470-39818-0. [Google Scholar]
- Nelson-Wong, E.; Howarth, S.; Winter, D.A.; Callaghan, J.P. Application of Autocorrelation and Cross-correlation Analyses in Human Movement and Rehabilitation Research. J. Orthop. Sports Phys. Ther. 2009, 39, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.-H.; Challis, J.H.; Newell, K.M. Transition of COM–COP relative phase in a dynamic balance task. Hum. Mov. Sci. 2014, 38, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bardy, B.G.; Marin, L.; Stoffregen, T.A.; Bootsma, R.J. Postural coordination modes considered as emergent phenomena. J. Exp. Psychol. Hum. Percept. Perform. 1999, 25, 1284–1301. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Yamamoto, S.I.; Miyoshi, T.; Nakazawa, K.; Masani, K.; Nozaki, D. Anti-phase action between the angular accelerations of trunk and leg is reduced in the elderly. Gait Posture 2014, 40, 107–112. [Google Scholar] [CrossRef]
- Missenard, O.; Fernandez, L. Moving faster while preserving accuracy. Neuroscience 2011, 197, 233–241. [Google Scholar] [CrossRef]
- Aruin, A.S.; Forrest, W.R.; Latash, M.L. Anticipatory postural adjustments in conditions of postural instability. Electroencephalogr. Clin. Neurophysiol. 1998, 109, 350–359. [Google Scholar] [CrossRef]
- Azaman, A.; Yamamoto, S.I. Analysis of joint stiffness of human posture in response to balance ability and limited sensory input during dynamic perturbation. Int. J. Exp. Comput. Biomech. 2015, 3, 83–101. [Google Scholar] [CrossRef]
- Martin, L.; Cometti, G.; Pousson, M.; Morlon, B. Effect of electrical stimulation training on the contractile characteristics of the triceps surae muscle. Graefe’s Arch. Clin. Exp. Ophthalmol. 1993, 67, 457–461. [Google Scholar] [CrossRef]
- Martin, L.; Cahouet, V.; Ferry, M.; Fouque, F. Optimization model predictions for postural coordination modes. J. Biomech. 2006, 39, 170–176. [Google Scholar] [CrossRef]
- Runge, C.F.; Shupert, C.L.; Horak, F.B.; Zajac, F.E. Ankle and hip strategies defined by joint torques. Gait Posture 1999, 10, 161–170. [Google Scholar] [CrossRef]
- Aramaki, Y.; Nozaki, D.; Masani, K.; Sato, T.; Nakazawa, K.; Yano, H. Reciprocal angular acceleration of the ankle and hip joints during quiet standing in humans. Exp. Brain Res. 2001, 136, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kiemel, T.; Jeka, J. The influence of sensory information on two component coordination during quiet stance. Gait Posture 2007, 26, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Sasagawa, S.; Ushiyama, J.; Kouzaki, M.; Kanehisa, H. Effect of the hip motion on the body kinematics in the sagittal plane during human quiet standing. Neurosci. Lett. 2009, 450, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Creath, R.; Kiemel, T.; Horak, F.; Peterka, R.; Jeka, J. A unified view of quiet and perturbed stance: simultaneous co-existing excitable modes. Neurosci. Lett. 2005, 377, 75–80. [Google Scholar] [CrossRef]
- Kelso, J.S. Dynamic Patterns; MIT Press: Cambridge, MA, USA, 1995. [Google Scholar]
- van Emmerik, R.E.; van Wegen, E.E. On Variability and Stability in Human Movement. J. Appl. Biomech. 2000, 16, 394–406. [Google Scholar] [CrossRef]
- Bonnet, V.; Ramdani, S.; Fraisse, P.; Ramdani, N.; Lagarde, J.; Bardy, B.G. A structurally optimal control model for predicting and analyzing human postural coordination. J. Biomech. 2011, 44, 2123–2128. [Google Scholar] [CrossRef]
- Duncan, P.W.; Weiner, D.K.; Chandler, J.; Studenski, S. Functional reach: A new clinical measure of balance. J Gerontol. 1990, 45, M192–M197. [Google Scholar] [CrossRef]
- Pollock, A.S.; Durward, B.R.; Rowe, P.J.; Paul, J.P. What is balance? Clin. Rehabil. 2000, 14, 402–406. [Google Scholar] [CrossRef]
| Segment | Segment Weight/Total Body Weight |
|---|---|
| Head, arm, trunk (HAT) | 0.536 |
| Pelvis | 0.142 |
| Left/Right Thigh | 0.1 |
| Left/Right Leg | 0.0465 |
| Left/Right Foot | 0.0145 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohd Ramli, N.F.F.; Mat Dzahir, M.A.; Yamamoto, S.-I. Estimation of Transition Frequency during Continuous Translation Surface Perturbation. Appl. Sci. 2019, 9, 4891. https://doi.org/10.3390/app9224891
Mohd Ramli NFF, Mat Dzahir MA, Yamamoto S-I. Estimation of Transition Frequency during Continuous Translation Surface Perturbation. Applied Sciences. 2019; 9(22):4891. https://doi.org/10.3390/app9224891
Chicago/Turabian StyleMohd Ramli, Nur Fatin Fatina, Mohd Azuwan Mat Dzahir, and Shin-Ichiroh Yamamoto. 2019. "Estimation of Transition Frequency during Continuous Translation Surface Perturbation" Applied Sciences 9, no. 22: 4891. https://doi.org/10.3390/app9224891
APA StyleMohd Ramli, N. F. F., Mat Dzahir, M. A., & Yamamoto, S.-I. (2019). Estimation of Transition Frequency during Continuous Translation Surface Perturbation. Applied Sciences, 9(22), 4891. https://doi.org/10.3390/app9224891