Bixa orellana L. (Bixaceae) and Dysphania ambrosioides (L.) Mosyakin & Clemants (Amaranthaceae) Essential Oils Formulated in Nanocochleates against Leishmania amazonensis
Abstract
1. Introduction
2. Results
2.1. Antileishmanial Activity and Cytotoxicity
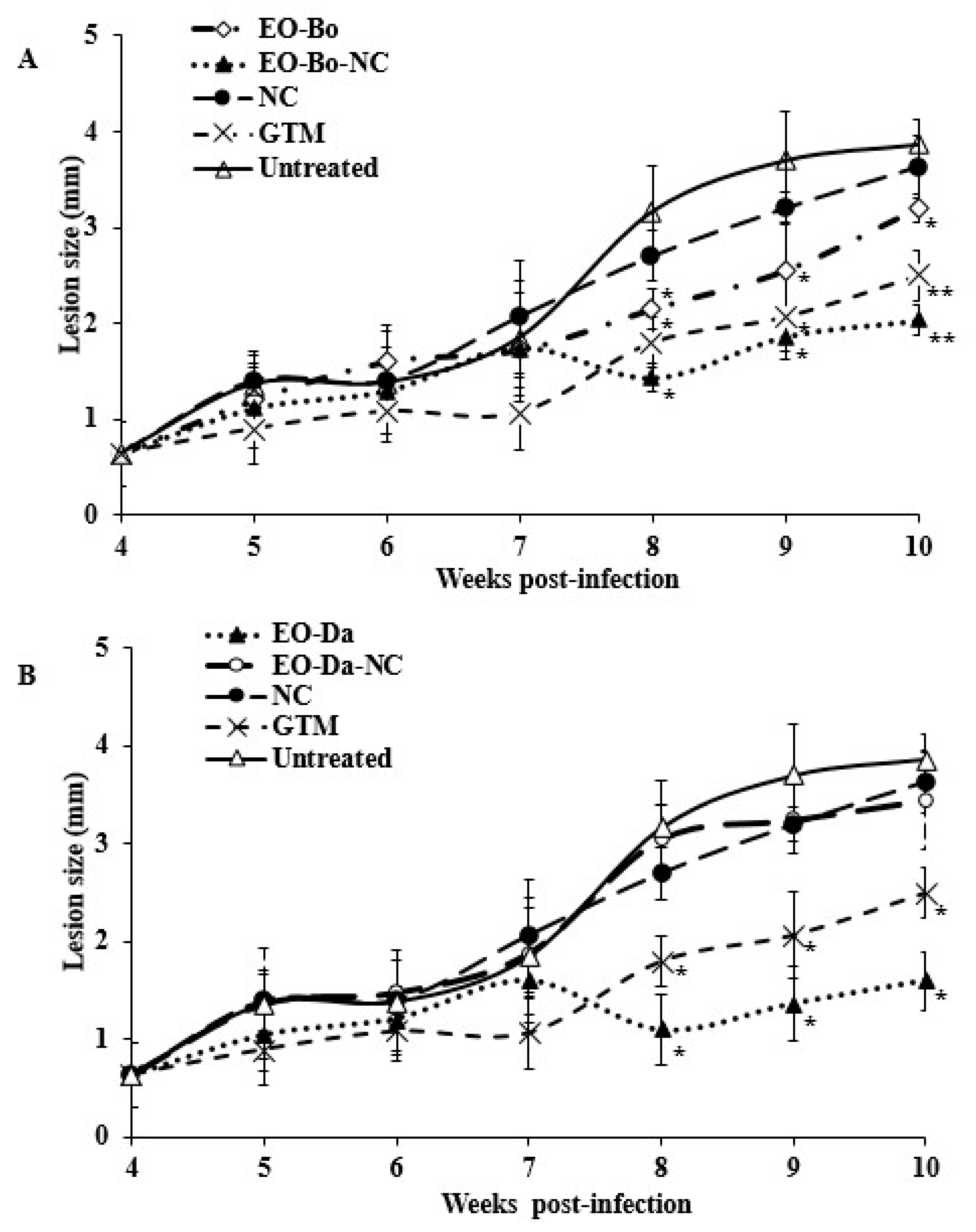
2.2. In Vivo Effect on Experimental Cutaneous Leishmaniasis
3. Discussion
4. Materials and Methods
4.1. Essential Oils from B. orellana and D. ambrosioides
4.2. Reference Drug
4.3. Nanocochleate Formulations
4.4. Parasites
4.5. Animals
4.6. Antiamastigote Assay
4.7. Cytotoxicity Assay
4.8. In Vivo Studies
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Steverding, D. The history of leishmaniasis. Parasit. Vectors 2017, 10, 82. [Google Scholar] [CrossRef] [PubMed]
- Kevric, I.; Cappel, M.A.; Keeling, J.H. New world and old world leishmania infections: A practical review. Dermatol. Clin. 2015, 33, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Torres-Guerrero, E.; Quintanilla-Cedillo, M.R.; Ruiz-Esmenjaud, J.; Arenas, R. Leishmaniasis: A review. F1000Research 2017, 6, 750. [Google Scholar] [CrossRef] [PubMed]
- Monzote, L. Antileishmanial patents antileishmanial current drugs and relevant patents. Recent Pat. Antiinfect. Drug Discov. 2011, 6, 1–26. [Google Scholar] [CrossRef]
- Andrews, K.T.; Fisher, G.; Skinner-Adams, T.S. Drug repurposing and human parasitic protozoan diseases. Int. J. Parasitol. Drugs Drug Resist. 2014, 4, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Lehnhardt Pires, C.; Rodrigues, S.D.; Bristot, D.; Gaeta, H.H.; de Oliveira Toyama, D.; Farias, W.R.L.; Toyama, M.H. Evaluation of macroalgae sulfated polysaccharides on the Leishmania (L.) amazonensis promastigote. Mar. Drugs 2013, 11, 934–943. [Google Scholar] [CrossRef]
- Armeli Minicante, S.; Michelet, S.; Bruno, F.; Castelli, G.; Vitale, F.; Sfriso, A.; Morabito, M.; Genovese, G. Bioactivity of phycocolloids against the mediterranean protozoan Leishmania infantum: An inceptive study. Sustainability 2016, 8, 1131. [Google Scholar] [CrossRef]
- Ogungbe, I.V.; Singh, M.; Setzer, W.N. Antileishmanial natural products from plants. Stud. Nat. Prod. Chem. 2012, 36, 331–381. [Google Scholar]
- Rodrigues, I.A.; Mazotto, A.M.; Cardoso, V.; Alves, R.L.; Amaral, A.C.F.; de Andrade Silva, J.R.; Pinheiro, A.S.; Vermelho, A.B. Natural products: Insights into Leishmaniasis inflammatory response. Mediat. Inflamm. 2015, 2015, 1–12. [Google Scholar] [CrossRef]
- Monzote, L.; Herrera, I.; Satyal, P.; Setzer, W.N. In-Vitro evaluation of 52 commercially-available essential oils against Leishmania amazonensis. Molecules 2019, 24, 1248. [Google Scholar] [CrossRef]
- Monzote, L.; Garcia, M.; Scull, R.; Cuellar, A.; Setzer, W.N. Antileishmanial activity of the essential oil from Bixa orellana. Phyther. Res. 2014, 28, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Monzote, L.; García, M.; Pastor, J.; Gil, L.; Scull, R.; Maes, L.; Cos, P.; Gille, L. Essential oil from Chenopodium ambrosioides and main components: Activity against Leishmania, their mitochondria and other microorganisms. Exp. Parasitol. 2014, 136, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Monzote, L.; García, M.; Montalvo, A.M.; Linares, R.; Scull, R. Effect of oral treatment with the essential oil from Chenopodium ambrosioides against cutaneous leishmaniasis in BALB/c mice, caused by Leishmania amazonensis. Complement. Med. Res. 2009, 16, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Monzote, L.; Pastor, J.; Scull, R.; Gille, L. Antileishmanial activity of essential oil from Chenopodium ambrosioides and its main components against experimental cutaneous leishmaniasis in BALB/c mice. Phytomedicine 2014, 21, 1048–1052. [Google Scholar] [CrossRef]
- Turek, C.; Stintzing, F.C. Stability of essential oils: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Martinho, N.; Damgé, C.; Reis, C.P. Recent advances in drug delivery systems. J. Biomater. Nanobiotechnol. 2011, 2, 510–526. [Google Scholar] [CrossRef]
- Papahadjopoulos, D.; Vail, W.J.; Jacobson, K.; Poste, G. Cochleate lipid cylinders: Formation by fusion of unilamellar lipid vesicles. Biochim. Biophys. Acta (BBA)-Biomembr. 1975, 394, 483–491. [Google Scholar] [CrossRef]
- Delmarre, D.; Lu, R.; Tatton, N.; Krause-Elsmore, S.; Gould-Fogerite, S.; Mannino, R.J. Formulation of hydrophobic drugs into cochleate delivery vehicles: A simplified protocol & formulation kit. Drug Deliv. Technol. 2004, 4, 64–69. [Google Scholar]
- Rawat, M.; Singh, D.; Saraf, S.; Saraf, S. Lipid carriers: A versatile delivery vehicle for proteins and peptides. Yakugaku Zasshi 2008, 128, 269–280. [Google Scholar] [CrossRef]
- Romero, E.L.; Morilla, M.J. Drug delivery systems against leishmaniasis? Still an open question. Expert Opin. Drug Deliv. 2008, 5, 805–823. [Google Scholar] [CrossRef]
- Smith, D.M.; Simon, J.K.; Baker, J.R. Applications of nanotechnology for immunology. Nat. Rev. Immunol. 2013, 13, 592–605. [Google Scholar] [CrossRef] [PubMed]
- Iborra, S.; Solana, J.C.; Requena, J.M.; Soto, M. Vaccine candidates against Leishmania under current research. Expert Rev. Vaccines 2018, 17, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A. Plant-derived compounds in treatment of leishmaniasis. Iran. J. Vet. Res. 2015, 16, 1–19. [Google Scholar] [PubMed]
- Bilia, A.R.; Guccione, C.; Isacchi, B.; Righeschi, C.; Firenzuoli, F.; Bergonzi, M.C. Essential oils loaded in nanosystems: A developing strategy for a successful therapeutic approach. Evidence-Based Complement. Altern. Med. 2014, 2014, 651593. [Google Scholar] [CrossRef]
- Islamuddin, M.; Chouhan, G.; Want, M.Y.; Tyagi, M.; Abdin, M.Z.; Sahal, D.; Afrin, F. Leishmanicidal activities of Artemisia annua leaf essential oil against visceral Leishmaniasis. Front. Microbiol. 2014, 5, 626. [Google Scholar] [CrossRef]
- Kayser, O.; Olbrich, C.; Croft, S.L.; Kiderlen, A.F. Formulation and biopharmaceutical issues in the development of drug delivery systems for antiparasitic drugs. Parasitol. Res. 2003, 90, S63–S70. [Google Scholar] [CrossRef]
- Maurin, M.; Raoult, D. Bartonella (Rochalimaea) quintana infections. Clin. Microbiol. Rev. 1996, 9, 273–292. [Google Scholar] [CrossRef]
- Santangelo, R.; Paderu, P.; Delmas, G.; Chen, Z.W.; Mannino, R.; Zarif, L.; Perlin, D.S. Efficacy of oral cochleate-amphotericin B in a mouse model of systemic candidiasis. Antimicrob. Agents Chemother. 2000, 44, 2356–2360. [Google Scholar] [CrossRef]
- Thornton, S.J.; Wasan, K.M. The reformulation of amphotericin B for oral administration to treat systemic fungal infections and visceral leishmaniasis. Expert Opin. Drug Deliv. 2009, 6, 271–284. [Google Scholar] [CrossRef]
- Sesana, A.M.; Monti-Rocha, R.; Vinhas, S.A.; Morais, C.G.; Dietze, R.; Lemos, E.M. In vitro activity of amphotericin B cochleates against Leishmania chagasi. Mem. Inst. Oswaldo Cruz 2011, 106, 251–253. [Google Scholar] [CrossRef]
- Pérez, O.; Bracho, G.; Lastre, M.; Mora, N.; Del Campo, J.; Gil, D.; Zayas, C.; Acevedo, R.; González, D.; López, J.A.; et al. Novel adjuvant based on a proteoliposome-derived cochleate structure containing native lipopolysaccharide as a pathogen-associated molecular pattern. Immunol. Cell Biol. 2004, 82, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Tamargo, B.; Monzote, L.; Piñón, A.; Machín, L.; García, M.; Scull, R.; Setzer, W.N. In vitro and in vivo evaluation of essential oil from Artemisia absinthium L. formulated in nanocochleates against cutaneous leishmaniasis. Medicines 2017, 4, 38. [Google Scholar] [CrossRef] [PubMed]
- Syed, U.M.; Woo, A.F.; Plakogiannis, F.; Jin, T.; Zhu, H. Cochleates bridged by drug molecules. Int. J. Pharm. 2008, 363, 118–125. [Google Scholar] [CrossRef]
- Soppimath, K.S.; Aminabhavi, T.M.; Kulkarni, A.R.; Rudzinski, W.E. Biodegradable polymeric microparticles as drug delivery devices. J. Control. Release 2015, 49, 187–190. [Google Scholar]
- Gomes, C.; Moreira, R.G.; Castell-Perez, E. Poly (DL-lactide-co-glycolide) (PLGA) nanoparticles with entrapped trans-cinnamaldehyde and eugenol for antimicrobial delivery applications. J. Food Sci. 2011, 76, N16–N24. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, R.R.; Ghodake, P.P.; Mane, A.N.; Ghadge, A.A. Nanocochleates: A novel carrier for drug transfer. J. Sci. Innov. Res. 2013, 2, 964–969. [Google Scholar]
- Soong, L.; Henard, C.A.; Melby, P.C. Immunopathogenesis of non-healing American cutaneous leishmaniasis and progressive visceral leishmaniasis. Semin. Immunopathol. 2012, 34, 735–751. [Google Scholar] [CrossRef]
- Torres-Santos, E.C.; Moreira, D.L.; Kaplan, M.A.C.; Meirelles, M.N.; Rossi-Bergmann, B. Selective effect of 2′,6′-dihydroxy-4′-methoxychalcone isolated from Piper aduncum on Leishmania amazonensis. Antimicrob. Agents Chemother. 1999, 43, 1234–1241. [Google Scholar] [CrossRef]
- Sladowski, D.; Steer, S.J.; Clothier, R.H.; Balls, M. An improved MTT assay. J. Immunol. Methods 1993, 157, 203–207. [Google Scholar] [CrossRef]
- Buffet, P.A.; Sulahian, A.; Garin, Y.J.F.; Nassar, N.; Derouin, F. Culture microtitration: A sensitive method for quantifying Leishmania infantum in tissues of infected mice. Antimicrob. Agents Chemother. 1995, 39, 2167–2168. [Google Scholar] [CrossRef]
Sample Availability: Samples are no longer available. |
| Products | IC50a ± SDb (μg/mL) | CC50c ± SDb (μg/mL) | Selectivity Index |
|---|---|---|---|
| EO-Bo | 8.5 ± 0.8 | 61.8 ± 5.9 | 7 |
| EO-Bo-NC | 15.4 ± 1.3* | 94.6 ± 2.2* | 6 |
| EO-Da | 4.9 ± 1.1 | 57.9 ± 3.7 | 12 |
| EO-Da-NC | >60* | 46.9 ± 4.4* | - |
| GTM | 11.0 ± 3.4 | >1500 | >136 |
| NC | ~25% of infection at maximum volume tested. | ~70% of mortality at maximum volume tested. | - |
| Group of Animals (Number of Animals) | Average of | Variation of Body Weight (%)a | |||||
|---|---|---|---|---|---|---|---|
| Initial Weight (g) | 5 w.p.i. | 6 w.p.i. | 7 w.p.i. | 8 w.p.i. | 9 w.p.i. | 10 w.p.i. | |
| EO-Bo (8) | 19.2 | +8.1 | +9.4 | +10.7 | +12.7 | +13.2 | +16.4 |
| EO-Bo-NC (8) | 19.0 | +3.5 | +4.6 | +7.9 | +8.9 | +9.3 | +9.5 |
| EO-Da (8) | 19.3 | +3.1 | +3.7 | +3.5 | +7.3 | +5.9 | +11.8 |
| EO-Da-NC (8) | 19.5 | +0.3 | +3.1 | +3.3 | +10.7 | +12.2 | +11.2 |
| NC (8) | 19.7 | −2.1 | +0.3 | +4.6 | +8.0 | +8.6 | +8.6 |
| GTM (8) | 19.2 | +2.9 | +6.3 | +5.5 | +8.6 | +8.4 | +9.5 |
| Untreated (8) | 19.3 | +4.0 | +3.7 | +5.8 | +6.4 | +6.3 | +8.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machín, L.; Tamargo, B.; Piñón, A.; Atíes, R.C.; Scull, R.; Setzer, W.N.; Monzote, L. Bixa orellana L. (Bixaceae) and Dysphania ambrosioides (L.) Mosyakin & Clemants (Amaranthaceae) Essential Oils Formulated in Nanocochleates against Leishmania amazonensis. Molecules 2019, 24, 4222. https://doi.org/10.3390/molecules24234222
Machín L, Tamargo B, Piñón A, Atíes RC, Scull R, Setzer WN, Monzote L. Bixa orellana L. (Bixaceae) and Dysphania ambrosioides (L.) Mosyakin & Clemants (Amaranthaceae) Essential Oils Formulated in Nanocochleates against Leishmania amazonensis. Molecules. 2019; 24(23):4222. https://doi.org/10.3390/molecules24234222
Chicago/Turabian StyleMachín, Laura, Beatriz Tamargo, Abel Piñón, Regla C. Atíes, Ramón Scull, William N. Setzer, and Lianet Monzote. 2019. "Bixa orellana L. (Bixaceae) and Dysphania ambrosioides (L.) Mosyakin & Clemants (Amaranthaceae) Essential Oils Formulated in Nanocochleates against Leishmania amazonensis" Molecules 24, no. 23: 4222. https://doi.org/10.3390/molecules24234222
APA StyleMachín, L., Tamargo, B., Piñón, A., Atíes, R. C., Scull, R., Setzer, W. N., & Monzote, L. (2019). Bixa orellana L. (Bixaceae) and Dysphania ambrosioides (L.) Mosyakin & Clemants (Amaranthaceae) Essential Oils Formulated in Nanocochleates against Leishmania amazonensis. Molecules, 24(23), 4222. https://doi.org/10.3390/molecules24234222








