1. Introduction
Glycation of proteins by the Maillard reaction is a promising food-grade method to modify food proteins [
1,
2,
3,
4,
5,
6]. Glycated proteins have improved functional properties in foods such as superior solubility, heat stability, emulsification, foaming, and gelation properties [
3,
7]. Glycation of proteins may help people who suffer from food protein allergies by lowering IgE-binding capacity [
8]. Glycation uses the first step in the Maillard reaction to create a reversible Schiff-base linkage between the free amino moiety in the protein and the carbonyl moiety in the polysaccharide [
9,
10,
11,
12,
13]. The Schiff-base linkage can be created via the dry-heating method [
9,
14] or the wet-heating method [
15,
16]. In the dry-heating method, an aqueous mixture of the protein and polysaccharide is first dried and then heated for a certain time (2 h to 9 days) at a fixed temperature (60 to 130 °C) and relative humidity (60 to 80%). In the wet-heating method, the aqueous solution is heated for a specific time (2 h to 2 days) at a fixed temperature (60 to 95 °C).
Schiff base formation is a reversible condensation reaction that generates water as a by-product. By Le Chatelier’s principle [
17], the presence of water in the reaction mixture drives the reaction in reverse, lowering yield. For example, the yield of glycation using the wet method is low (<5%) [
15,
16]. Subsequently yield was increased to 18% using a reaction time of 24 h at 60 °C [
18]. The dry-heating method uses a desiccator to remove water generated by the condensation reaction, shifting the chemical equilibrium towards product formation, which increases yield. For example, whey protein-maltodextrin powders heated for 2 h at 80 °C in a desiccator at 79% relative humidity formed substantial amounts of glycates with little un-glycated protein remaining in the reaction mixture [
14].
The purpose of the present study was to measure the time course and equilibrium yield of the glycation reaction between dextran and whey protein isolate (WPI) or glycomacropeptide (GMP) using the dry-heating method. The reaction between polysaccharides and proteins via the Maillard reaction is different from the reaction between simple sugars and proteins. In either case, the Maillard reaction pathway starts with the formation of a Schiff base between the free amino moiety in the protein and the carbonyl moiety in the carbohydrate [
19]. For simple sugars the Schiff base undergoes spontaneous rearrangement to either an Amadori or Heyn’s compound [
20]. Protein-polysaccharide glycation products are not subject to post-Amadori–Maillard reaction steps [
4]. The Maillard reaction stops at Schiff base formation [
15]. The protein-polysaccharide product is colorless and has no odor [
4]. It was not the purpose of the present work to explore the sequence of elementary chemical reactions that make up the Maillard reaction pathway by using the tools of chemical kinetics. Rather, the present work was aimed at measuring the time course and equilibrium yield of the glycate formation reaction and extracting apparent rate constants using a simple mathematical model and a fitting procedure.
In order to determine the time course and equilibrium yield, a quantitative method was required to measure the concentration of reactants (un-glycated protein and dextran) and products (glycated protein) versus time. We used a new method for the chromatographic analysis of whey protein-dextran glycation products and a complementary fluorescence laser densitometry method for this purpose. The present work is important because producing glycates quickly and in high yield is essential for delivering on the potential benefits of this new food-grade method for protein modification.
2. Materials and Methods
Whey protein isolate (WPI) and glycomacropeptide (GMP) were from Davisco Foods International (Le Sueur, MN, USA). WPI contained 92.7% protein, 2.0% ash, 5.0% moisture, 0.0% lactose, and 0.3% lipids. GMP contained 86% protein, 6.5% ash, 6.0% moisture, 1.0% lactose, and 0.5% lipids. Dialysis for removal of the 1% lactose in GMP was deemed unnecessary based on past research [
4]. Dextran T10 was from Pharmacosmos Company (Holbaek, Denmark) and had an average molecular mass of 5.2 kDa. Precast gels (Tris-Glycine, 4–20% linear gradient, 18 wells), pre-stained molecular mass standards, Tris/glycine/SDS premixed buffer, Laemmli sample buffer, and Coomassie Blue G-250 stain were from Bio-Rad Laboratories (Hercules, CA, USA). Pierce GelCode glycoprotein staining kit was from Thermo Fisher Scientific (Waltham, MA, USA). SYPRO Red Protein Gel Stain was from Lonza Rockland (Rockland, ME, USA). Other chemicals were from Fisher Scientific (Pittsburgh, PA, USA). Buffers were prepared at 22 °C. Centrifugal filter units (Amicon Ultra-15, 3 kDa) were from MilliporeSigma (Burlington, MA, USA).
2.1. Synthesis of Glycated Proteins
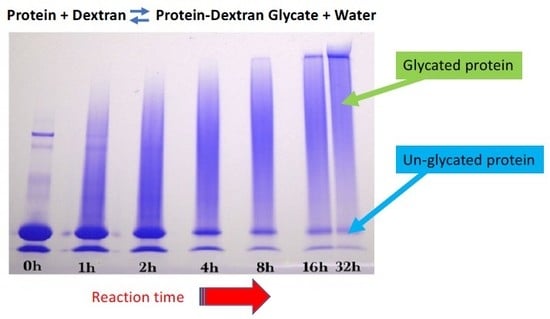
Glycation was conducted using the dry-heating method. Dextran and WPI or GMP were dissolved in 10 mM sodium phosphate buffer (pH 6.5) at a 3:1 mass ratio, resulting in a dextran-WPI molar ratio of 10:1. The liquid reaction mixture was frozen, lyophilized and ground into a powder using a mortar and pestle to form particles of about 0.1–3 mm diameter, and then dispensed into seven 20 mL glass scintillation vials. All seven vials without caps were equilibrated to 85% relative humidity at 22 °C for 24 h in a desiccator containing saturated potassium chloride solution. One vial was taken before the start of the reaction and capped. The remaining six un-capped vials were placed into a pre-heated desiccator at 70 °C that contained saturated potassium chloride solution giving a relative humidity of 80%. At time points of 1, 2, 4, 8, 16, and 32 h, one vial was taken from the desiccator and capped. The seven vials were stored at −20 °C prior to analysis.
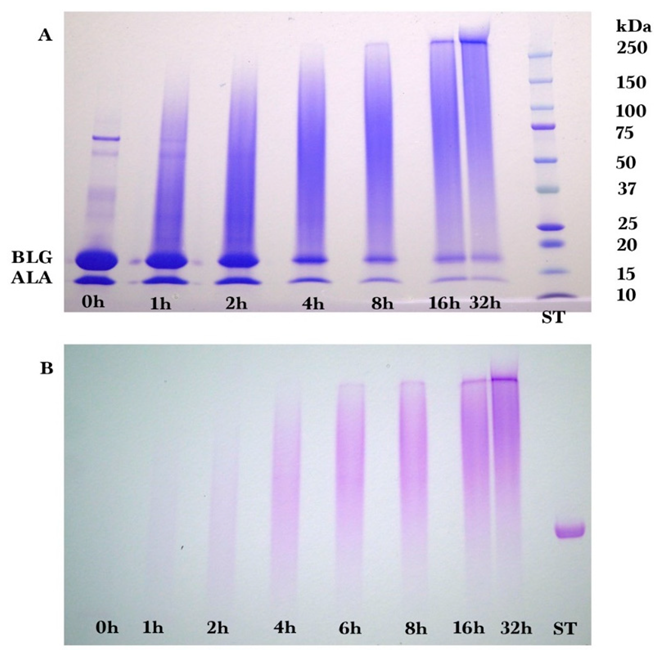
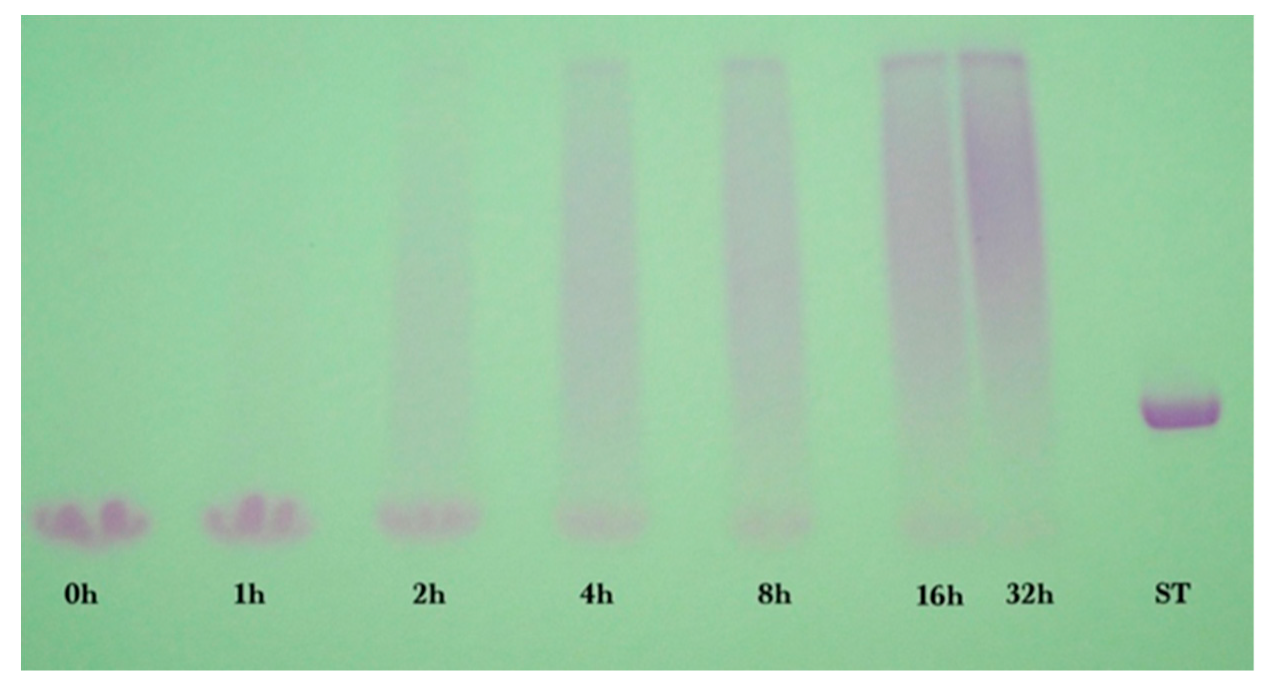
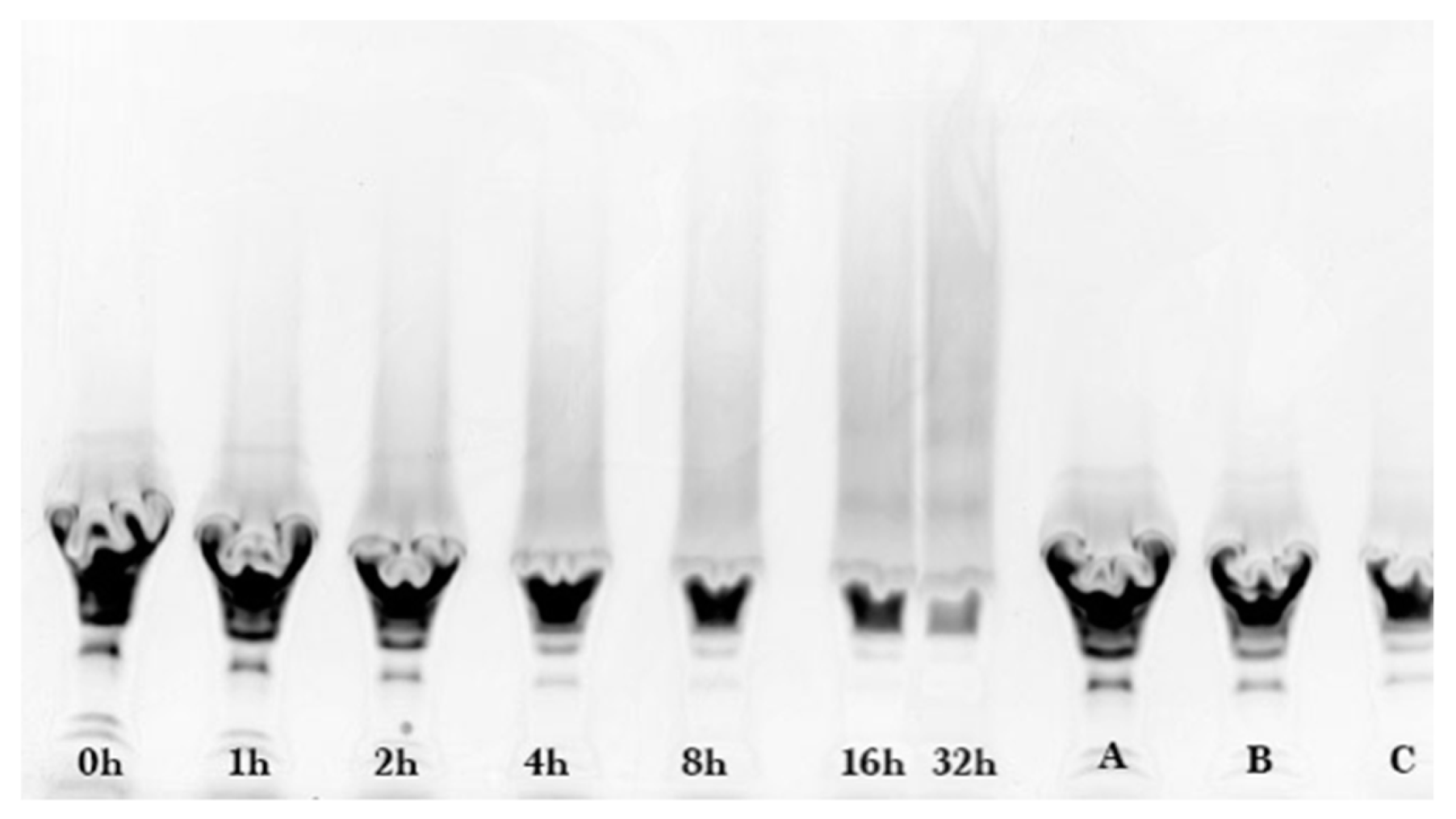
2.2. Gel Electrophoresis
Sodium dodecyl sulfate-polyacrylamide (SDS-PAGE) gel electrophoresis was performed to measure the progress of the glycation reaction, following the protocol of Bund et al. [
18]. Dried reaction products were fully dissolved in water, subjected to the SDS sample preparation procedure, loaded into the gel, and visualized by Coomassie Blue staining. Precision Plus Protein Kaleidoscope Pre-stained Protein Standards (ST) were also applied on the Coomassie gel as molecular mass makers. The ST mixture contained ten recombinant proteins of molecular mass 10 to 250 kDa. A Pierce GelCode glycoprotein staining kit was used for the detection of glycoproteins in the glycoprotein stained gel.
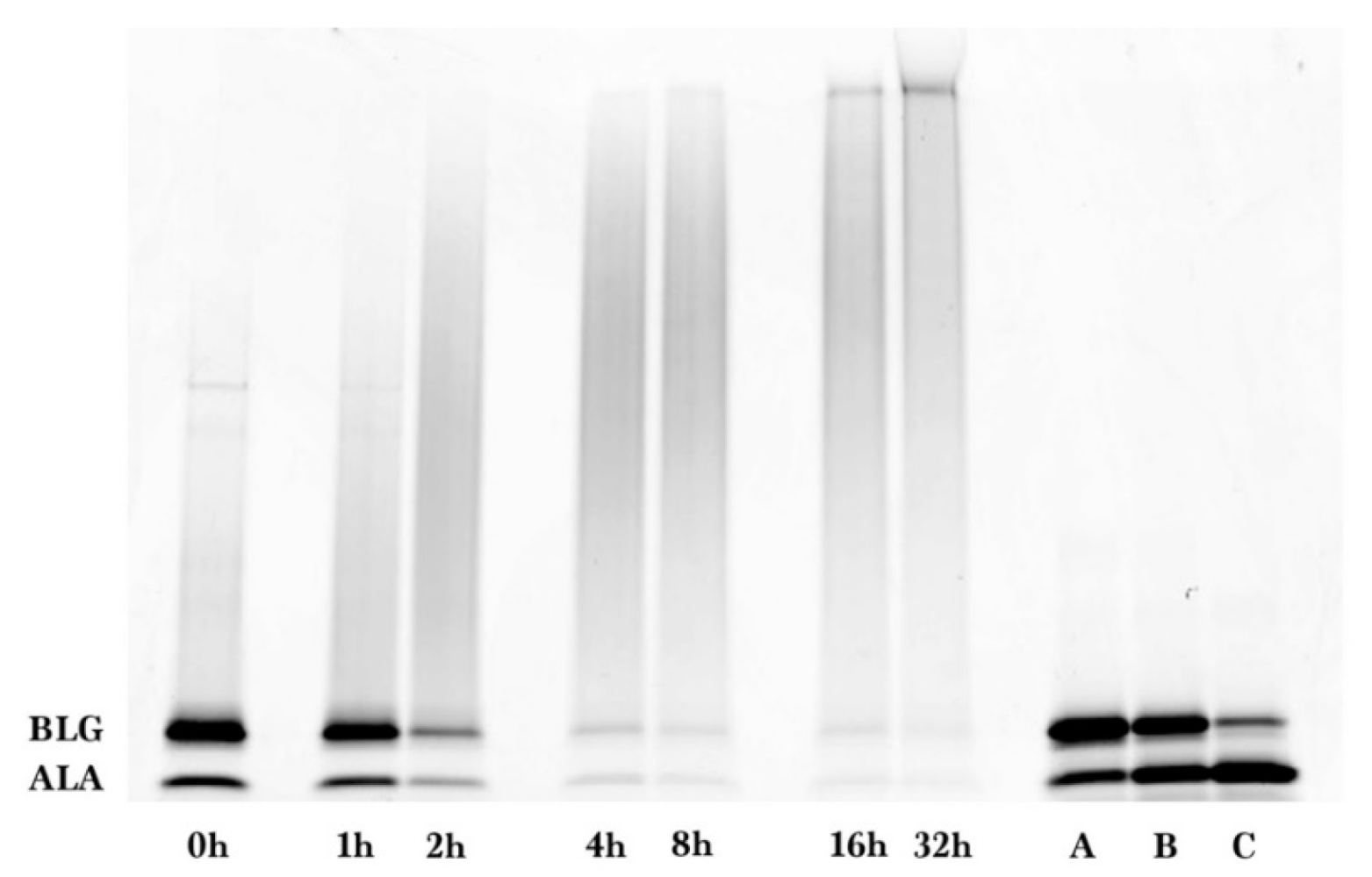
Coomassie and glycoprotein stained gels provided a qualitative result, but more quantitative data were needed for the kinetic analysis. Therefore, fluorescence laser densitometry after staining by SYPRO Red was used for protein quantification. Gels were scanned on TYHOON FLA 9000 laser densitometer (GE Healthcare, Piscataway, NJ, USA) in fluorescence mode using an excitation at 532 nm and emission at 610 nm. Bands were quantified using ImageQuantTL software (GE healthcare). Calibration of band volume to protein concentration was accomplished by applying internal standards to three lanes of the gel (lanes A, B, C). The internal standards consisted of three liquids each containing known concentrations of ALA and BLG of 0.1 to 0.3 mg/mL such that the total was 0.4 mg/mL.
2.3. Chromatographic Analysis
Glycated samples of WPI were analyzed by cation exchange chromatography using a 5 mL HiTrap MacroCap SP column from GE healthcare (Marlborough, MA, USA) connected to an ÄKTA Explorer 100 HPLC system (GE Healthcare). Glycated protein samples were reconstituted in 50 mM sodium lactate, pH 4.0 (Buffer A) and syringe filtered using a 0.22 µm PVDF filter (MilliporeSigma, Burlington, MA, USA). The chromatographic method consisted of 4 steps: (1) column equilibration using 5 column volumes (CV) of buffer A; (2) loading the sample into the column using a 2 mL sample injection loop to bind the glycated and un-glycated proteins to the column; (3) elution of the glycated protein using 12 CV of 40% buffer B (1 M NaCl in Buffer A); and (4) elution of unreacted un-glycated protein using 2.5 CV of 100% Buffer B. The column was then re-equilibrated using a 5 CV of buffer A and cleaned using 0.1 M NaOH. Glycated protein eluted in the “low salt” peak (40% buffer B) and unreacted un-glycated protein eluted in the “high salt” peak (100% buffer B). Unicorn 5.0 software was used to set up the chromatographic method and calculate peak area at 280 nm (PA280). Protein (µg) ≈ 25 × PA280, where 25 is the flow rate (5 mL/min) times the path length correction (10 mm/2 mm) for the detector flow cell.
2.4. Kinetic Model of the Glycation Reaction
In Schiff base formation, free amino acids on the protein react reversibly with carbonyls on the polysaccharide to produce glycated protein as shown in Equation (1) [
4,
9,
21]:
where P is un-glycated protein, D is dextran, PD is protein-dextran glycate, and W is water. Dextran and water were in ten-fold molar excess or more in the present work and can be considered to be constant. The kinetic equations are then shown in Equations (2) and (3) [
22]:
where k is the apparent rate constant and [P] and [PD] are the concentrations of un-glycated protein and protein-dextran glycate, respectively, at time t. The value of [P] at time zero is [P]
0. The values of [P] and [PD] at equilibrium (t → ∞) are [P]
eq and [PD]
eq, respectively. In the present work, measured values of [P] and [PD] versus time were used to obtain the fitted parameter values [P]
eq, [PD]
eq, and k.
2.5. Statistical Analysis
Equations (2) and (3) were fitted to the experimental data for the time course of the reaction by nonlinear regression using the JMP Pro software, version 11 (SAS Institute, Gary, NC, USA) to obtain the fitted parameter values [P]eq, [PD]eq, and k. Results were expressed as mean ± standard error. Point-by-point comparisons between the data and the fitted equations were made by t-test using SAS studio 3.5 (SAS Institute, Gary, NC, USA). The p-value was reported.
4. Discussion
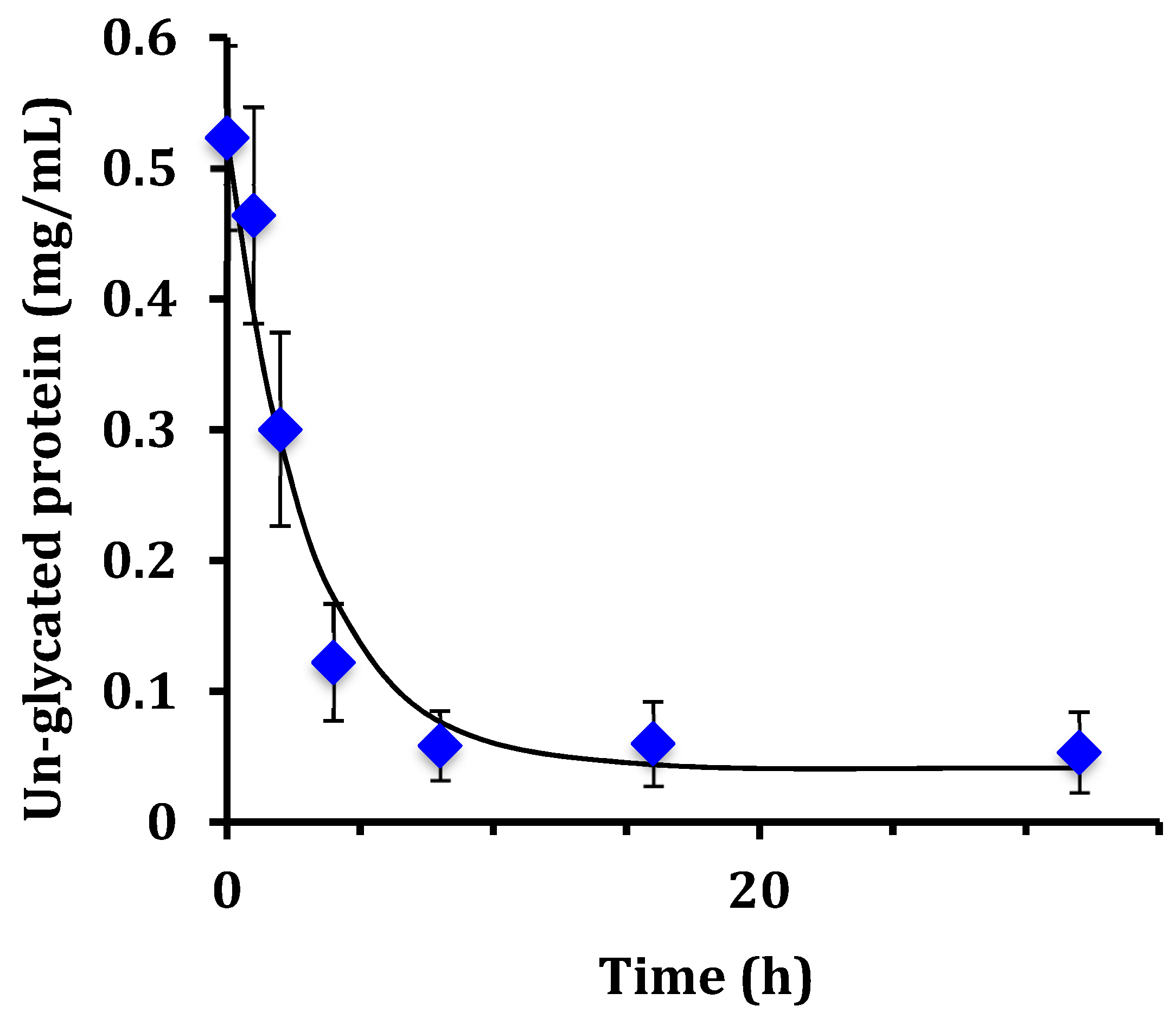
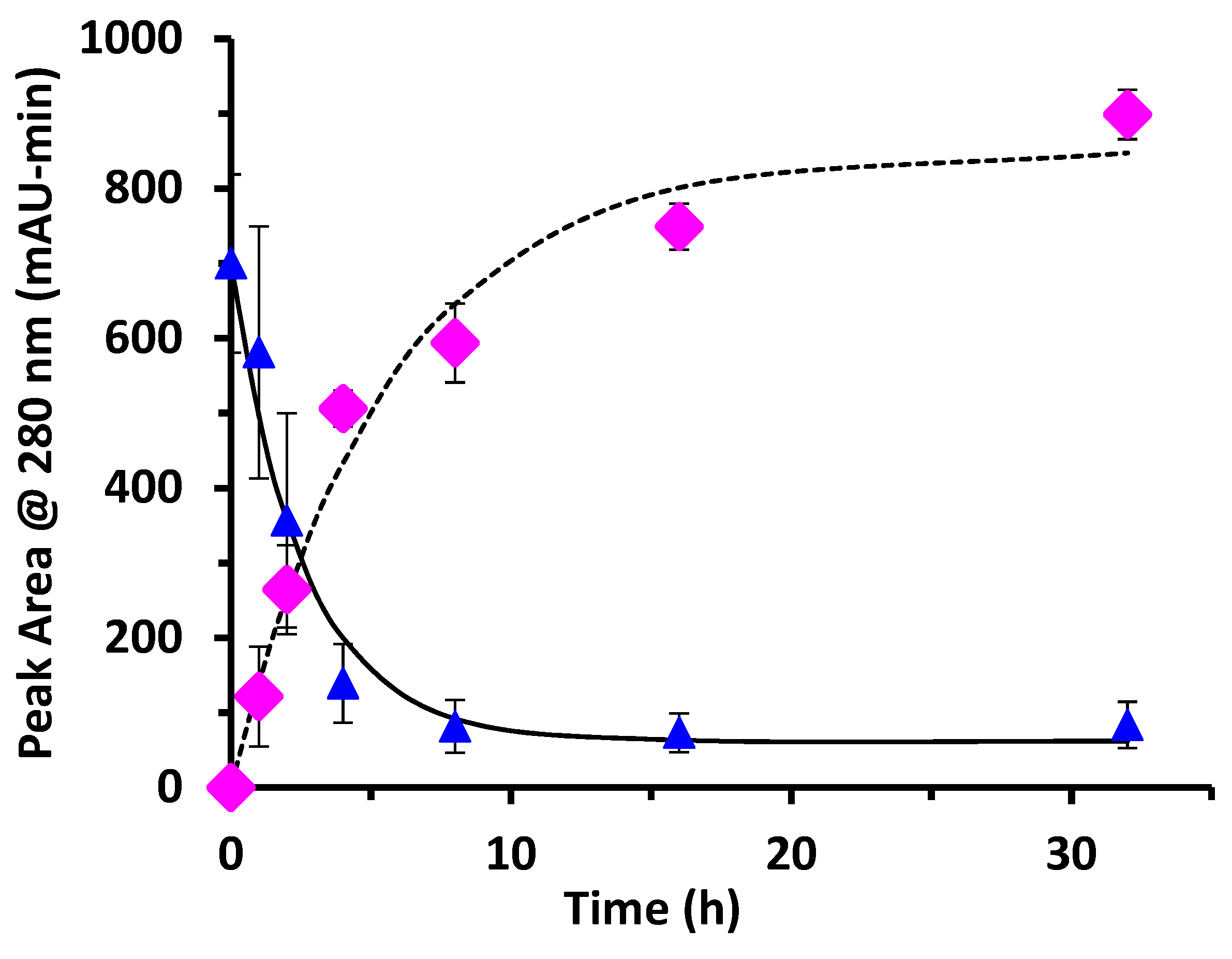
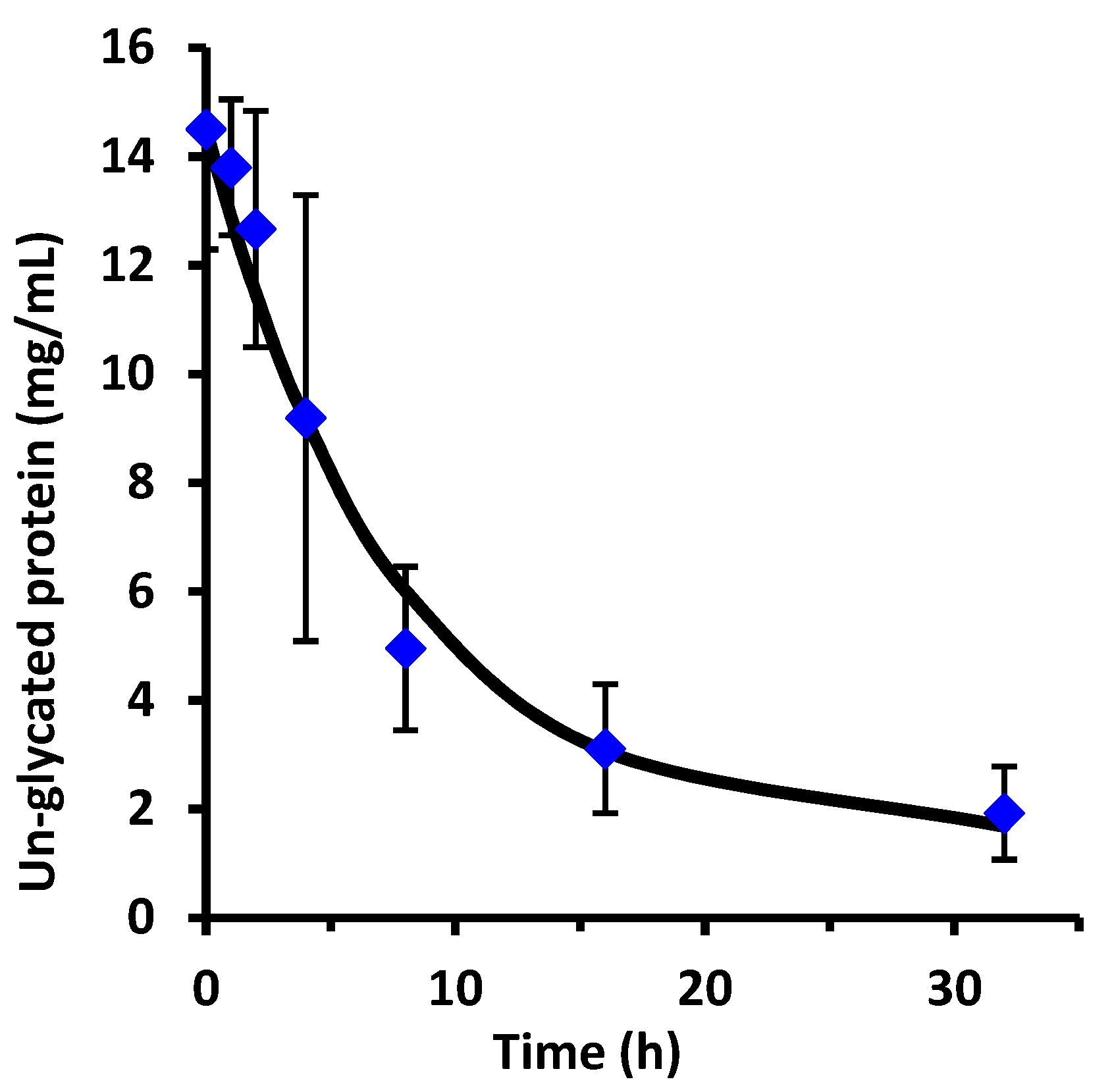
This work examined the kinetics and yield of whey protein glycation using dextran and the dry-heating method. Previous researchers have centered focus on the food properties of the un-purified reaction mixture after heating, but the kinetics of the protein-polysaccharide glycation reaction has been largely unstudied. The appropriate reaction time at 70 °C can be assessed by the reaction half-life (t
1/2). From the disappearance of un-glycated protein, glycation of WPI had t
1/2 = 1.8 h for WPI as measured by the chromatographic method, and t
1/2 = 2.1 h for WPI as measured by the SDS-PAGE method. These times are not greatly different than the work of Akhtar and Dickinson [
14] where a heating time of 2 h at 80 °C was used for the dry-heating method to glycate WPI using maltodextrin.
Glycation of GMP had t
1/2 = 5.3 h at 70 °C as measured by the SDS-PAGE method. Conversion at 32 h was 87% for GMP. Glycation of GMP by dextran was substantially (61%) slower than glycation of WPI, although yield was similar to WPI. GMP when pure has an unusual amino acid composition compared to WPI. GMP is deficient in Asp, Leu, and Lys, compared to WPI, and is missing six amino acids altogether: Cys, Tyr, Phe, Trp, His, and Arg [
25]. Furthermore, GMP contains double the amount of the following five amino acids compared to WPI: Thr, Ile, Pro, Ser, Val. Considering that Schiff base formation involves primarily Lys, and to a lesser extent His and Trp [
9], and that GMP has half the Lys content of WPI, and no His and Trp, then it is logical that glycation of GMP was slower than for WPI.
The WPI-dextran and the GMP-dextran glycation reactions were well described by the kinetic model. The reason the error bars were larger for
Figure 7 than
Figure 4 was the greater uncertainty in calculating the band area for the irregular king’s crown shape of
Figure 6 versus the regular rectangular shape of
Figure 2. For WPI-dextran, there were no statistical differences between the data points and the fit using the kinetic model (
p > 0.05) for 20 out of 21 time points. For GMP-dextran, there were no statistical differences between the data points and the fit using the kinetic model for seven out of seven time points (
p > 0.05). These observations support the hypothesis that protein-dextran glycation using the dry-heating method is an apparent first-order reversible reaction.
For the WPI-dextran reaction, the rate constants measured for the disappearance of un-glycated protein by SDS-PAGE (k = 0.33 h
−1) and by chromatographic analysis (k = 0.38 h
−1) were not statistically different (
p > 0.05). The rate constant for creation of glycated protein by chromatographic analysis (k = 0.18 h
−1) was about half the expected value. This discrepancy in the rate constant for creation of glycated protein was because of the last two data points of
Figure 4 (16 and 32 h) where the peak area for glycated protein [PD] continued to rise, while the peak area for un-glycated protein [P] did not continue to fall. It is possible that the protein extinction coefficient increases with glycation causing the peak area at 280 nm for un-glycated and glycated protein to be different, especially at long times where the extent of glycation is highest.
Yield was not previously measured for the dry-heating method. In the present work, yield for the glycation of WPI by dextran at 8 h was 88% for un-glycated protein based on chromatographic analysis, and 89% based on SDS-PAGE analysis. For comparison, the wet-heating method for dextran and WPI had at best a yield of 18% using a reaction time of 24 h at 60 °C [
18]. In summary, the dry-heating method increased yield from 18% to nearly 90% and cut the reaction time from 24 h to 8 h for dextran and WPI glycation. These results are not surprising if one considers that the glycation reaction is a reversible condensation reaction. Condensation reactions eliminate water and should be run in a dry state to achieve high yield.
5. Conclusions
This work examined the kinetics of the dry-heating method for the glycation of WPI and GMP by dextran. The dry-heating method had a reaction half-life of about 2 h at 70 °C and about 90% yield. The dry-heating method had a 5× higher yield than the wet-heating method. Glycation using the dry-heating method was well described using a reversible first-order kinetic model. As the reaction time increased, the amount of un-glycated protein fell exponentially, and the amount of glycated protein rose exponentially. Glycation of GMP was slower than glycation of WPI. The half-life for GMP glycation was about 5 h compared to 2 h for WPI glycation. This was attributed to the lower Lys and other reactive amino acid content of GMP compared to WPI. The glycation reaction starts with Schiff base formation which is a reversible condensation reaction that makes water. By Le Chatelier’s principle, the presence of water in the reaction mixture drives the reaction in reverse, lowering yield. This may explain why the dry-heating method of protein glycation by dextran was superior to the wet-heating method in terms of speed and yield. It is important to note that reversible condensation reactions can be driven in reverse (towards reactants in Equation (1)) by adding water. The reverse reaction of Equation (1) happens when protein-dextran glycates in powder form are added to wet foods such as beverages, emulsions, gels, and foams. In this case the glycates may fall apart by hydrolysis and return to the form of the reactants: free un-glycated protein and dextran. Glycation of proteins by dextran via the Maillard reaction is a food-grade method to improve the physical properties of proteins for expanded use of proteins in foods. The present work is useful to the food industry to expand the use of glycated proteins in creating new food products.