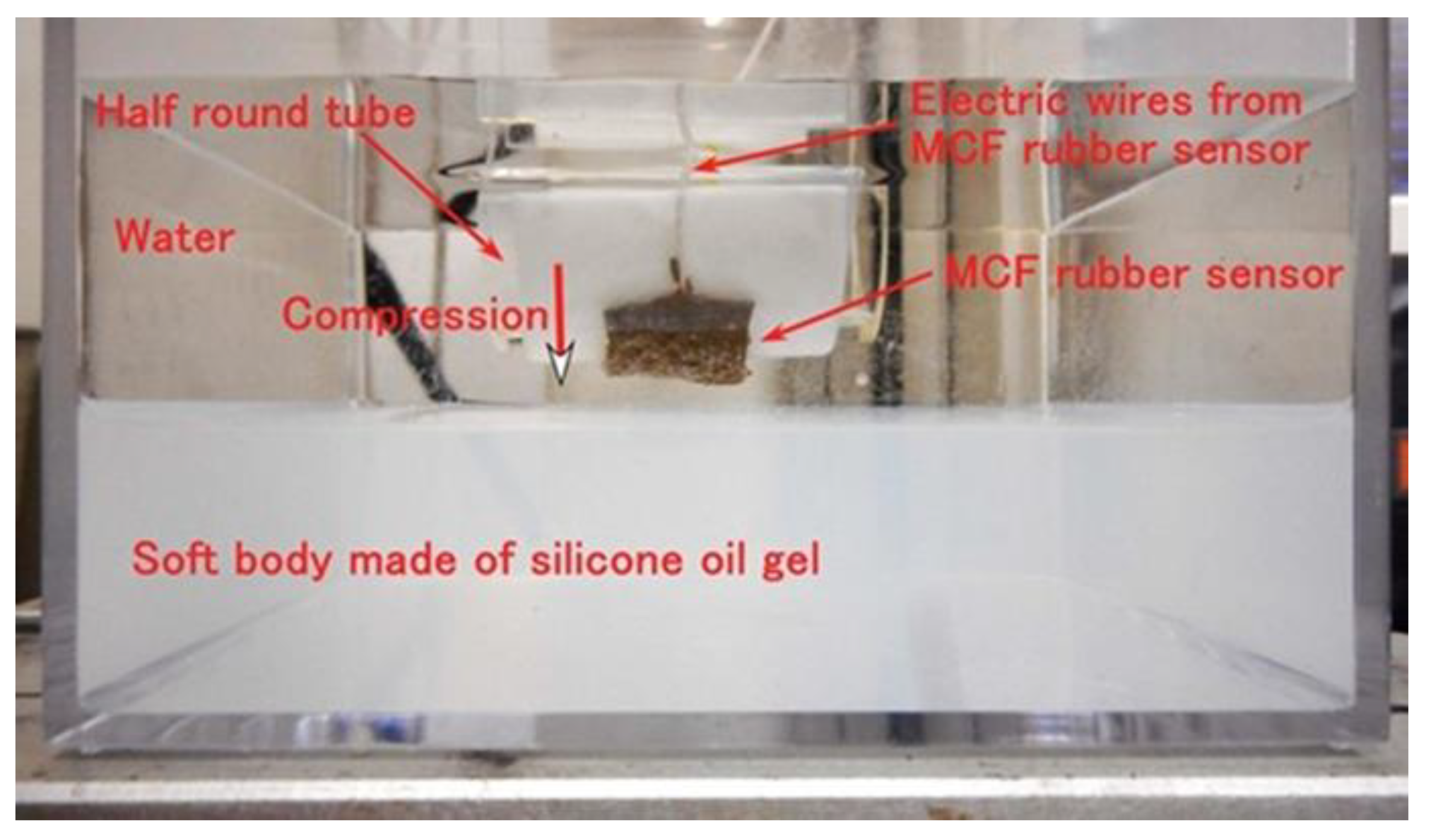
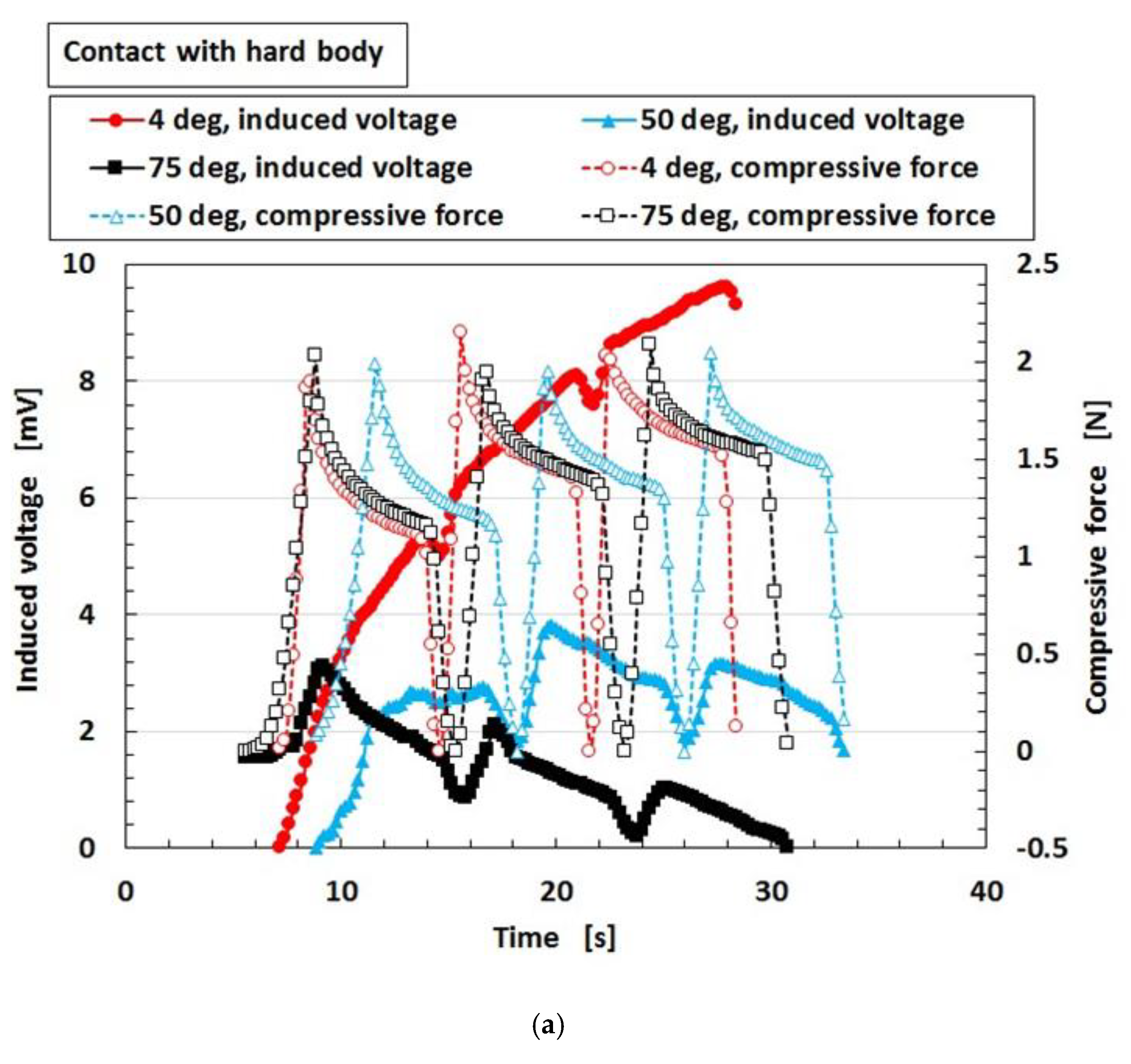
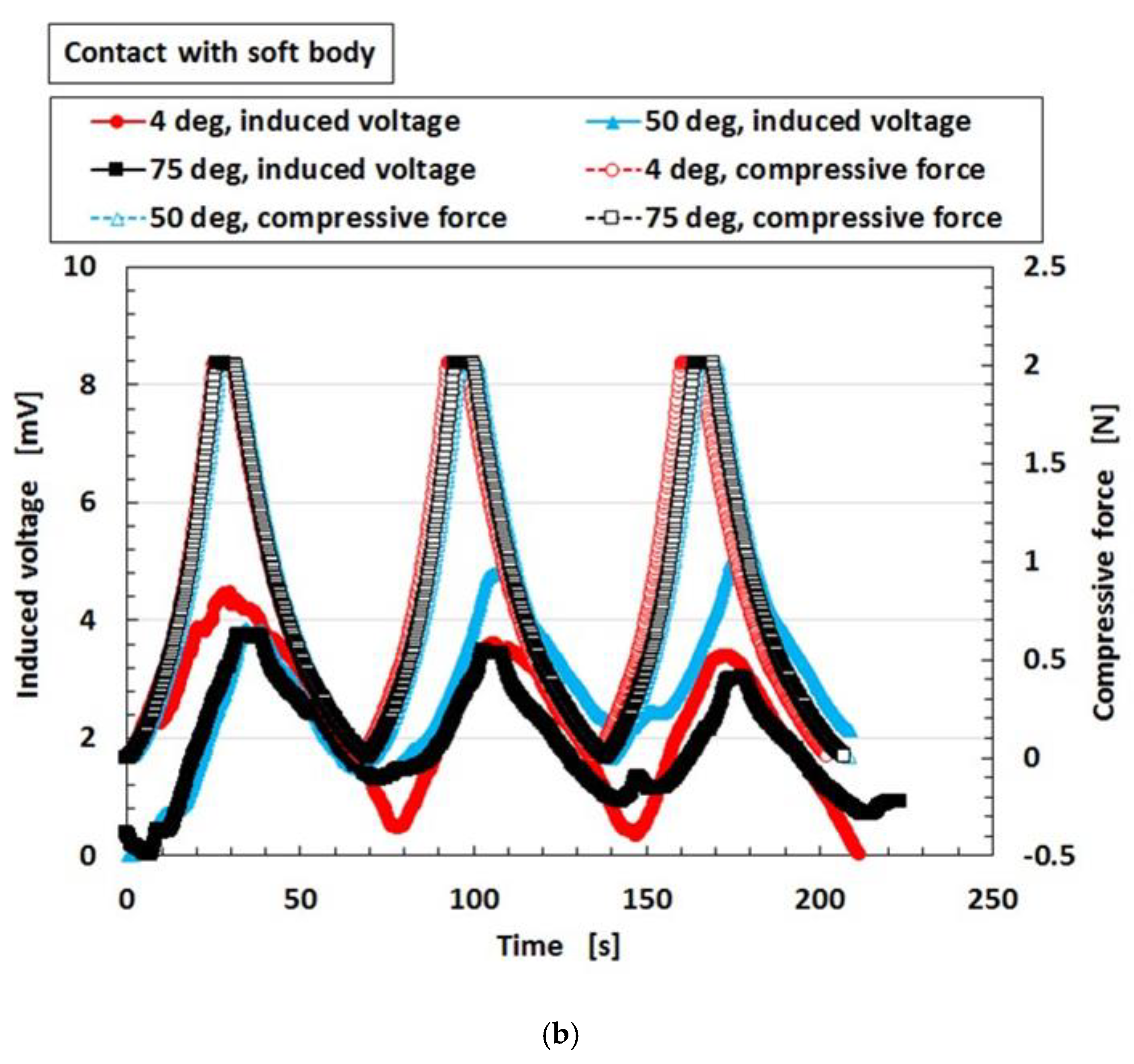
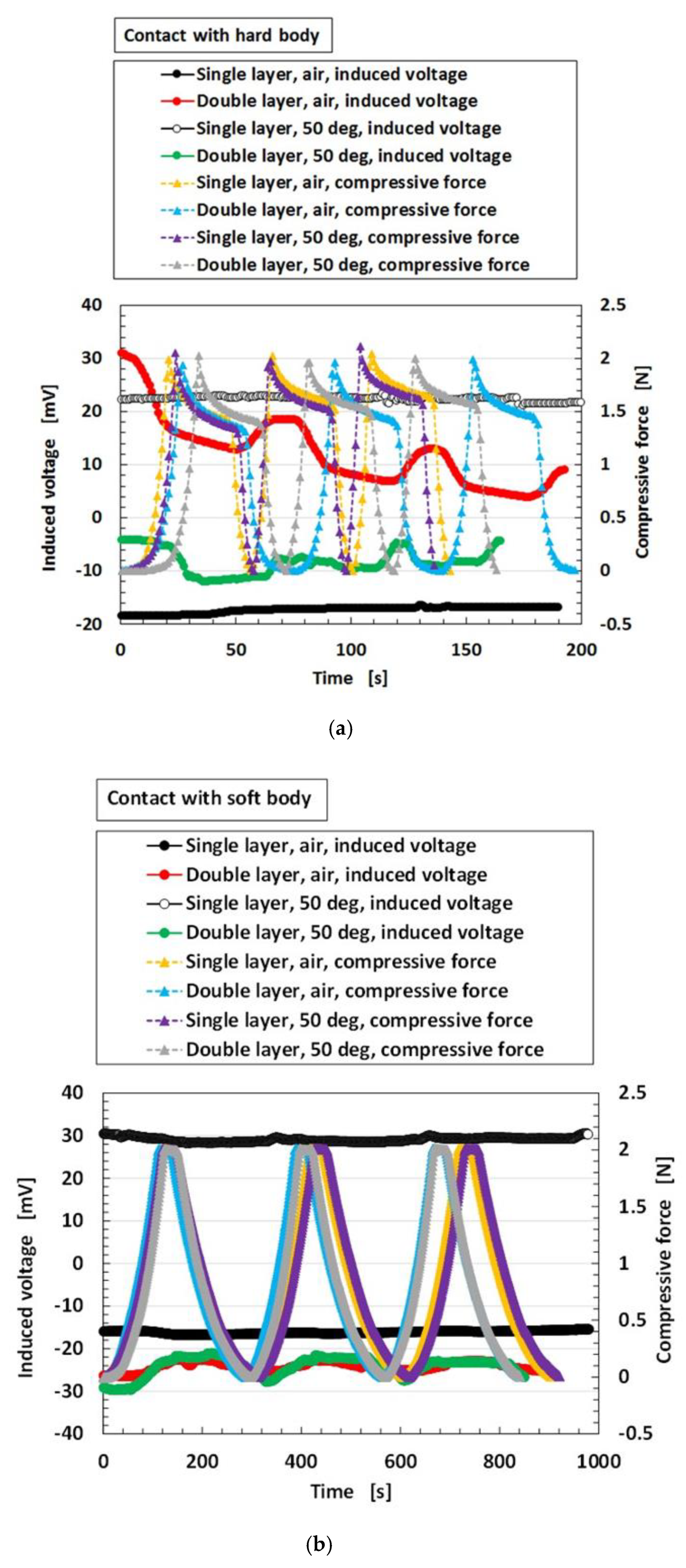
2.1. Factor That Influence Instability
Firstly, we will begin by discussing the factors that influence the instability of an MCF rubber sensor. In the previous study, we evaluated the secular stability of electrolytically polymerized MCF rubber sheets fabricated by sandwiching between opposing magnets [
1]. However, it is typically necessary to measure the electric properties during sandwiching of the MCF rubber between the opposing electrodes. This electric property has secular stability. However, when the electrodes are separated, the stability deteriorates with time. Instability can be divided into two phases: (a) temporary instability during sensing that fluctuates with time; (b) instability over a long span of time leading to aging degradation. However, MCF rubber sheets must be considered because they are fundamental components of MCF rubber sensors. From this perspective, examples of factors that influence instability include: (1) the evaporation of water from the MCF rubber because water is substantially involved in the fabrication process; (2) the vulcanization of the MCF rubber occurs during the electrolytic polymerization process with surpassing this point, in contrast to electrolytic polymerization in our previous studies; (3) the deformation of the MCF rubber by the Mullins effect. Factors (1–3) are related to (a) and (b).
In the case of (1), water is involved and is trapped in the inner MCF rubber, even if electrolytic polarization is performed, because the NR and CR latexes contain water. Therefore, due to the evaporation of water from the electrolytically polymerized MCF rubber, the MCF rubber sensor can exhibit a secular change of its electrical and mechanical properties. This will be discussed in the following section.
In the case of (2), the MCF rubber liquid should be taken into account because it is fundamental in the production of MCF rubber sensors. In the previous study, we experimentally investigated the influence of the aggregation of magnetic particles and rubber molecules of MCF rubber liquid on their electrical characteristics when a magnetic field was applied [
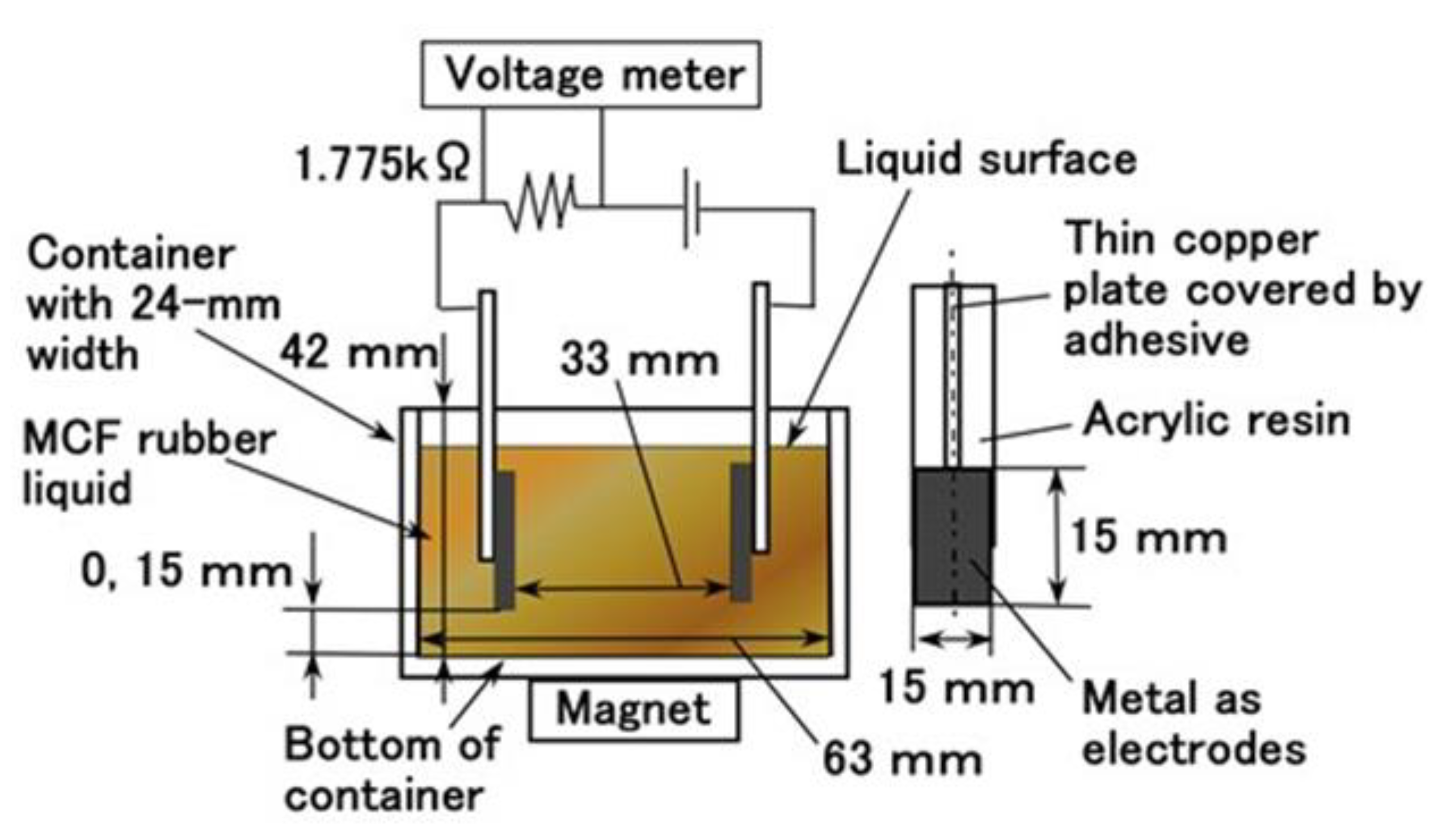
24]. The application of a magnetic field to a container immersed in the MCF rubber liquid resulted in the aggregation of particles and molecules to sediment. In the present report, we will introduce the result that the MCF rubber in the middle of vulcanization induces temporal instability by using an experimental apparatus with large electrodes gap as shown in
Figure A1 in the
Appendix A. The MCF rubber liquid consisted of 12 g Ni powder with particles on the order of microns and bumps on the surface (No. 123 by Yamaishi Co. Ltd., Noda, Japan), 3 g water-based MF with 50 wt% Fe
3O
4 (M-300, Sigma Hi-Chemical Co. Ltd., Tsutsujigasaki, Japan), 16 g NR-latex (Ulacol, Rejitex Co. Ltd., Atsugi, Japan) and 31 g of water. Using electrolytic polymerization, the MCF rubber is vulcanized as shown in
Figure A2 in the
Appendix A. The vulcanized MCF rubber grows from the anode surface towards the direction of the cathode as a crystal. The thickness of the vulcanized MCF rubber increases with time as shown in
Figure A3 in the
Appendix A. Isoprene molecules and magnetic clusters are aligned along the same direction as the electric and magnetic field lines, as explained in previous studies [
1,
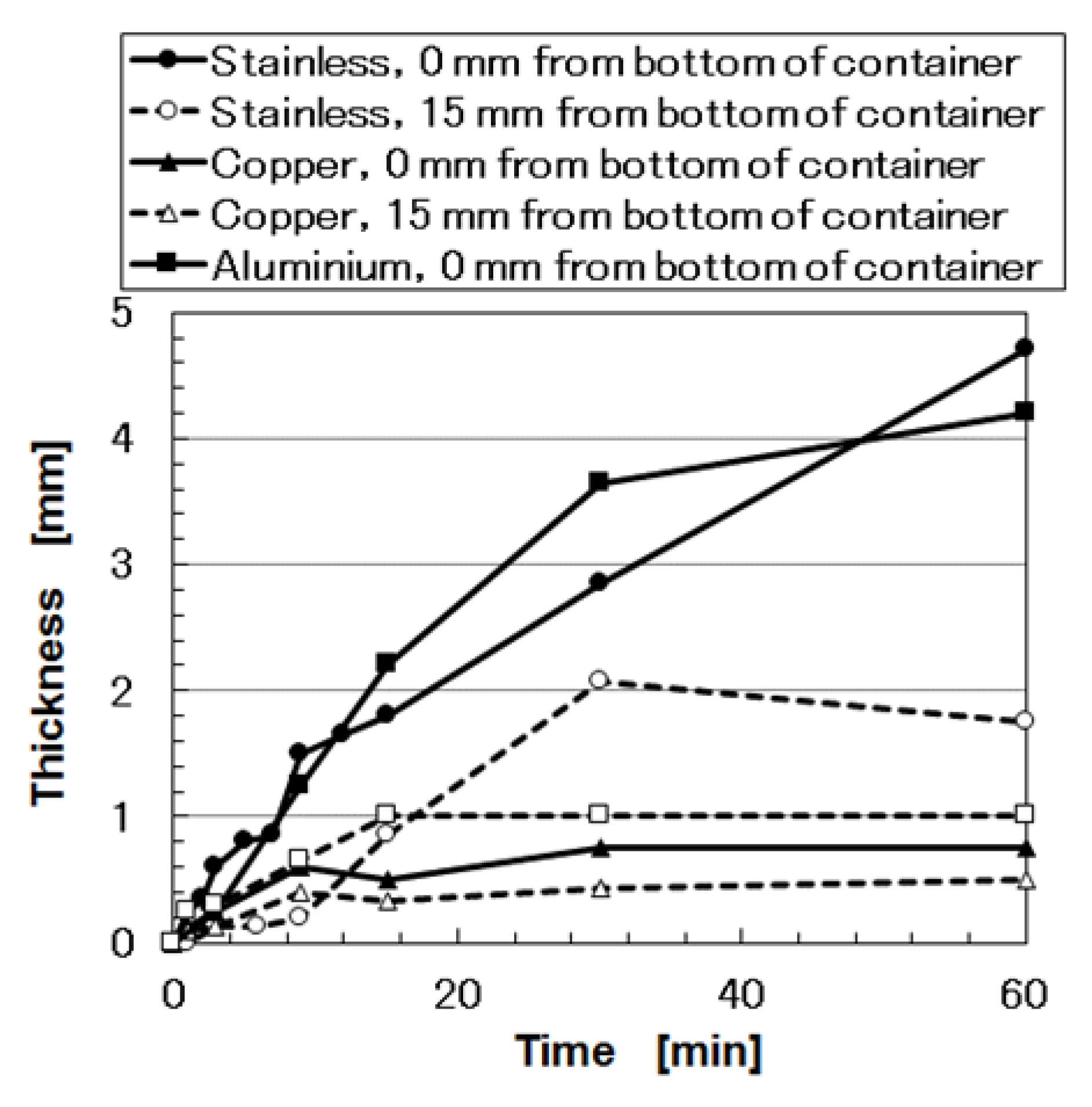
4]. The structure can be considered to be approximately quasi-regular crystalline, but not a crystalline lattice such as in hexagonal close-packed, body-centered cubic, and face-centered cubic structures. The density of the aggregation at 0 mm was larger than that at 15 mm such that the thickness of the vulcanize MCF rubber at 0 mm was larger than that at 15 mm. As shown in
Figure A4 in the
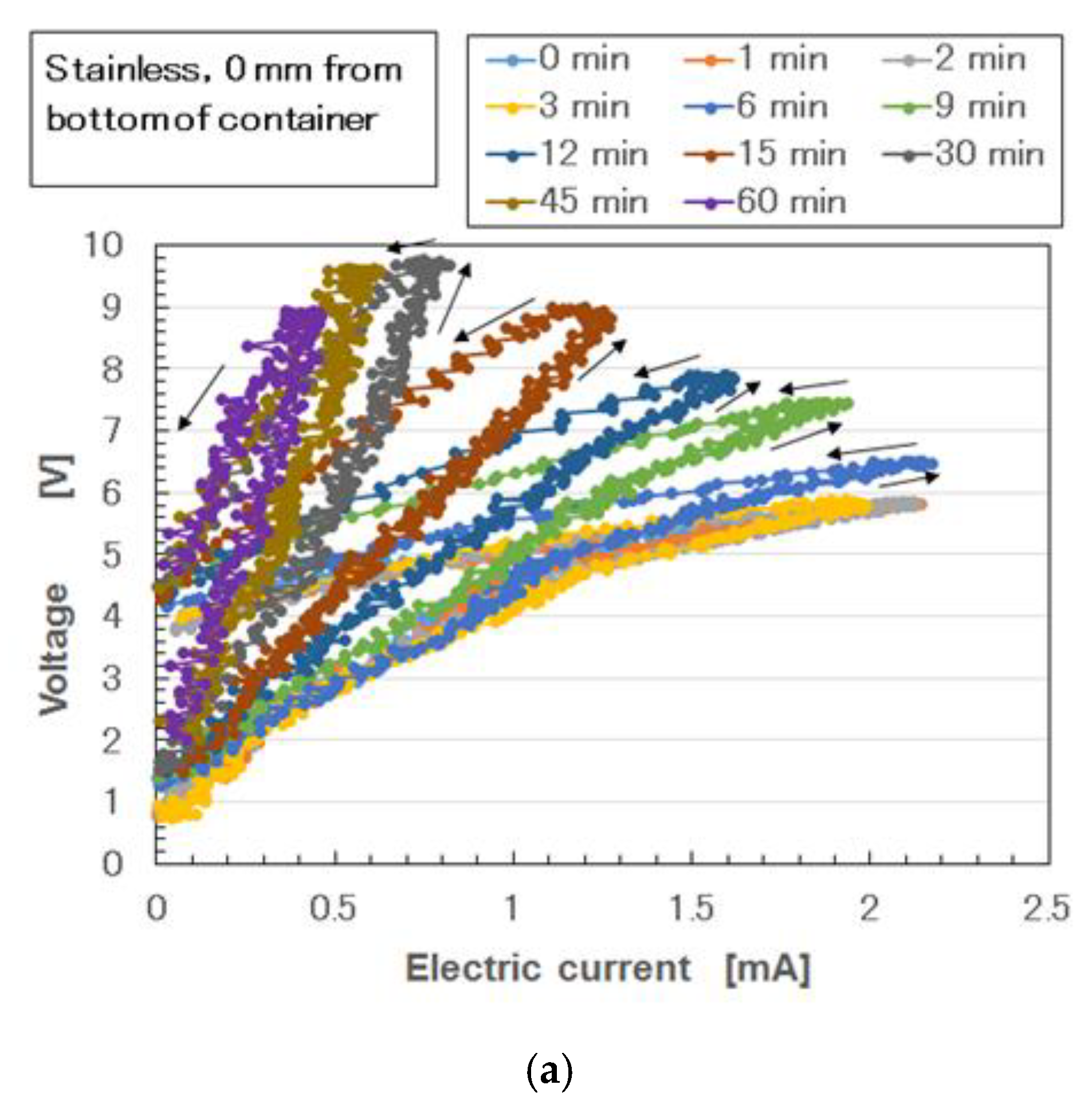
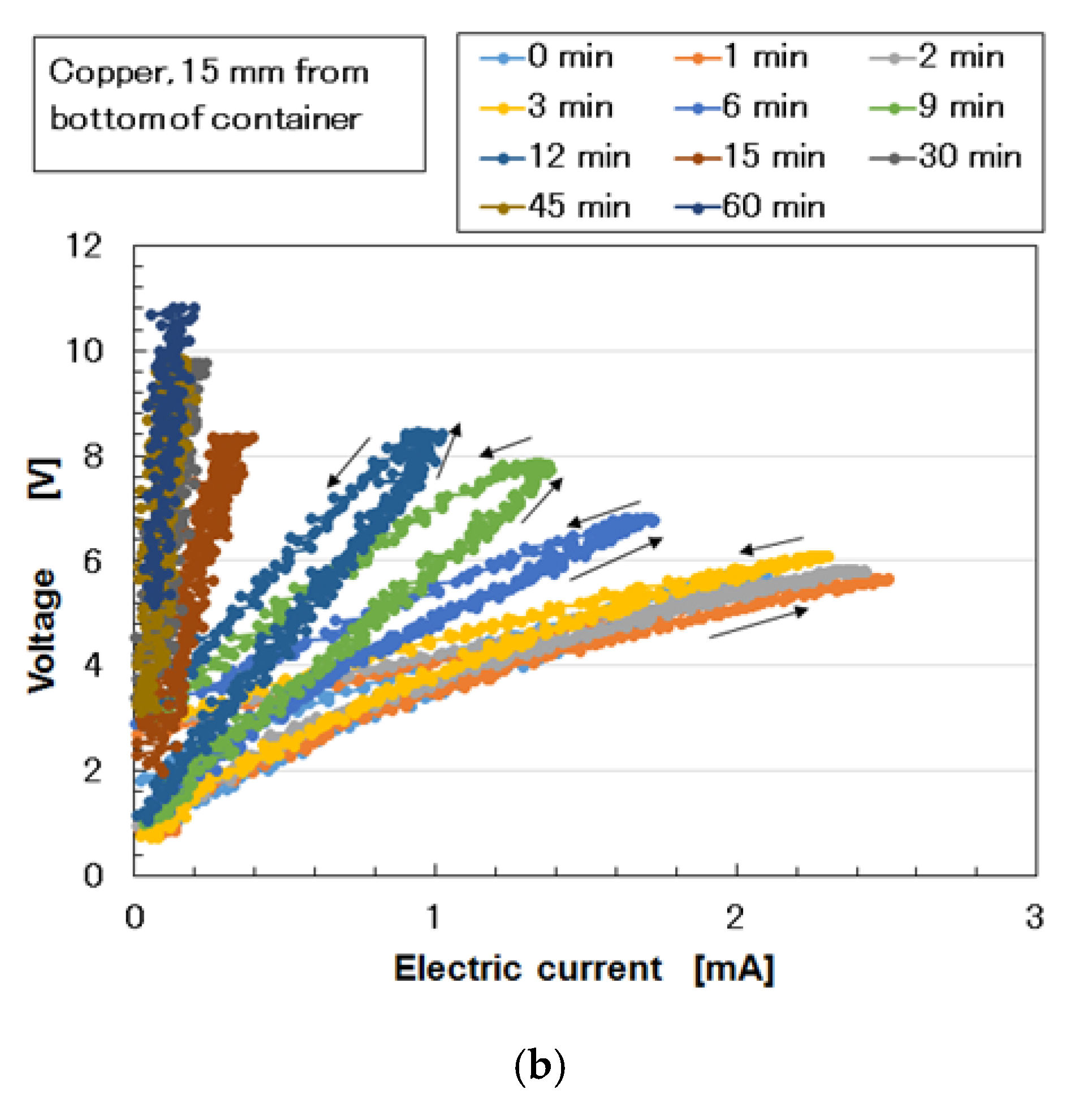
Appendix A, as the thickness of the vulcanized MCF rubber increases, the electrical conductivity decreases. Therefore, if the vulcanization of the MCF rubber occurs during the electrolytic polymerization process without surpassing this point, the electrical signal from the MCF rubber sensor becomes unstable when the piezoresistivity of MCF rubber sensor is examined. This refers to usage whereby a generated electric current is measured via the application of a voltage with any electric source.
In the case of (3), we must consider the change of the electric current flowing in the MCF rubber based on its deformation. The change of the electric current of the MCF rubber by compression has been elucidated in previous studies [
1,
2,
3]. In addition, this has been theoretically explained in other studies [
5,
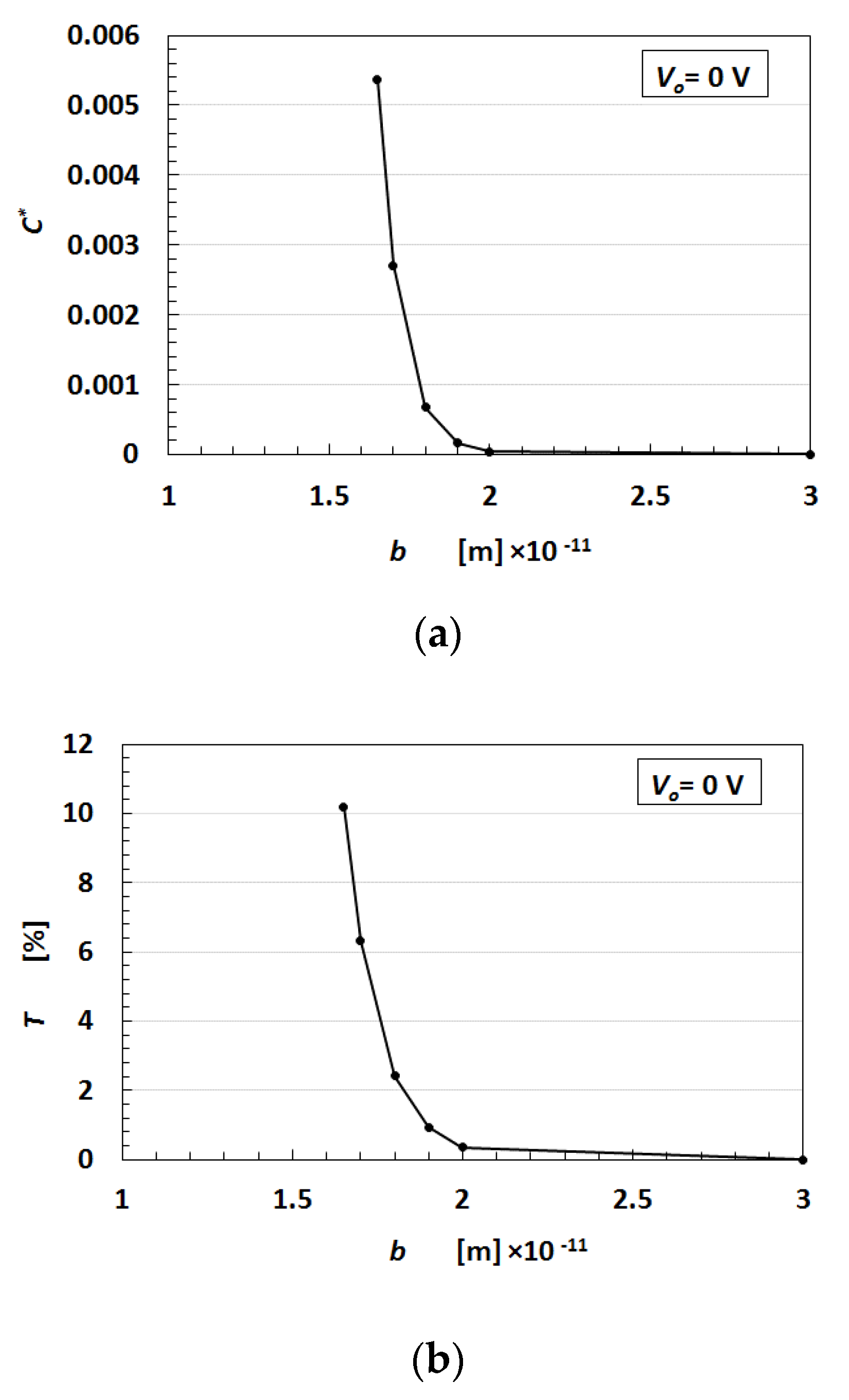
24]. The relationship between the transmitted probability
T, which represents the electric current flowing in the MCF rubber and the thickness
b, is shown in
Figure A5a in the
Appendix A. The relationship between the dimensionless capacitance
C* and the thickness
b is shown in
Figure A5b in the
Appendix.
T and
C* increase by enhancing
b.
T is considered to be re-expression of the electric current
I,
C* applies to the capacitance
C, and
b applies to the deformation quantity. The motion of electrons described by the tunnel theory is categorized as electron transfer in the field of complex chemistry. The mechanism of the electron transfer is divided into two types based on the distance among the particles of Fe
3O
4 and Ni, and the molecules of polyisoprene: outer-sphere electron transfer reaction (OSETR) and inter-sphere electron transfer reaction (ISETR), as shown in a previous study [
5]. Whether OSETR or ISETR is generated depends on the probability of the distance among the particles and molecules—OSETR takes place in the case of long distances and ISETR occurs for shorter distances. Decreasing
b represents a contraction of the MCF rubber. As
b becomes smaller, ISETR is dominant in the change of the electrical property of the MCF rubber due to the electric-chemical reaction. In contrast, as
b gets larger, OSETR is dominant. As the distance among the particles and molecules is reduced by deformation of the MCF rubber,
I and
C increase as shown in
Figure A5. The enhancement of
I and
C are presented as
ΔI and
ΔC, respectively. In the case of
ΔI and
ΔC, we can obtain the results as represented by Equations (1)–(3), together with Equation (A3) in the
Appendix. The notion is that
I represents the amount of electron transfer and that
C represents the amount of counter ion created by an anionic acceptor A
− and cationic donor D
+. A and D are generated by particles and molecules with semiconducting roles by mixing and electrolytic polymerization as shown in Equations (A4)–(A7) in the
Appendix, which has been presented in a previous study [
5]. However, the reaction occurs from the right-hand-side to the left-hand-side by irradiation of electromagnetic waves of γ-rays, microwave, light, etc. In the case of OSETR, which implies that the distance among the particles and molecules is large, the net reaction from the right-hand-side to the left-hand-side does not occur practically. In the case of ISETR, which implies that the distance among the particles and molecules is small, the net reaction from the right-hand-side to the left-hand-side occurs practically.
In the case of Equation (1), the voltage
V increases with time. The amount of A
− and D
+ is small because ISETR is dominant in the reaction as shown in Equations (A4)–(A7), due to the small distance among the particles and molecules. However, the amount of electron transfer is larger than that of the counter ion:
In the case of Equation (2), the voltage
V with time is constant. The amount of electron transfer is the same as that of counter ion:
In the case of Equation (3), the voltage
V with time decreases toward zero. The amount of A
− and D
+ is large because of OSETR is dominant in the reaction as shown in Equations (A4)–(A7) due to the large distance among the particles and molecules. However, the amount of electron transfer is smaller than that of the counter ion:
Mullins effect is induced by the inner deformation among the particles and molecules of the MCF rubber. MCF rubber was determined to exhibit the Mullins effect in previous studies [
1,
24]. Therefore, the instability related to the aforementioned (a) and (b) occurs, such as the alternation of increasing and decreasing voltage and electric current of the MCF rubber according to Equations (1)–(3). As a result, the instability based on the deformation of the MCF rubber by Mullins effect also occurs.
Moreover, the instability of the MCF rubber is significant when utilized as a sensor. To prevent the aforementioned instability: (1) a possible idea is to deposit some material on the MCF rubber sensor. However, for the MCF rubber or MCF rubber sensor, this is useless because of the attempt to prevent the aforementioned instability (2) as follows. At first, we can clarify that by comparing the electrical property between the covered and non-covered MCF rubber as shown in
Figure A6, the electrical sensitivity is reduced compared to the silicone oil rubber that covers the MCF rubber. Secondly, based on another experiment on coating using a liquid-rubber coating spray, the covering can be determined to be useless. In the case of the MCF rubber sensor produced using the procedure as shown in
Figure A1 in the previous report [
9], the MCF rubber sensor sprayed with liquid rubber-coating spray, which is an ordinary commercial liquid-rubber spray (LC-311SP, Jefcom, Co. Ltd., Osaka, Japan) exhibits the change that the 11.2 mV induced voltage at the production date becomes zero after 1 month. The MCF rubber sensor was consisted with the MCF rubber liquid with a hydrate as 1 g Ni, 0.75 g MF with 40 wt% Fe
3O
4 (W-40, Ichinen-Chemicals Co., Ltd., Shibaura, Japan), 3 g NR-latex (Ulacol), 3 g CR-latex (671A, Showa Denko Co. Ltd., Tokyo, Japan), 0.5 g TiO
2 (Anatase type, Fujifilm Wako Pure Chemical Co., Ltd., Osaka, Japan), and 0.5 g hydrates Na
2WO
4·2H
2O (Fujifilm Wako Pure Chemical Co., Ltd., Osaka, Japan), and MCF rubber without hydrate as 3 g Ni powder, 0.75 g MF (W-40), 3 g NR-latex (Ulacol), 3g CR-latex (671A), and 0.5 g TiO
2. Irrespective of the material used to coat the MCF rubber or MCF rubber sensor, the instability of the aforementioned (b) is still present in the MCF rubber so that the construction in the MCF rubber sensor must be improved.
Incidentally, the present MCF rubber sensor is sensitive to both normal and shear forces, which has been presented in detail in our previous study [
1,
2]: the electric current passed between electrodes is changed alternatingly according to the deformation of the rubber by the application of the forces, whose passing phenomena is created by tunnel effect as shown in
Figure A5a. For example, regarding the normal force, the electric resistance decreases abruptly by the application of minimal force as shown in
Figure A6. This change is different by kinds of dopant involved in the MCF rubber as shown in the reference [
2]. In addition, the electric resistivity of commercial pressure-sensitive electrically conductive rubbers (PSECRs) made of NR-latex (NR-latex), CR rubber (CR rubber) and silicon oil rubber has been presented, comparing with that of MCF rubber [
1].
2.2. Combination of NR and CR, and Q
To resolve the instability highlighted in the previous section, the resistance of the non-diene rubber to water, heat, etc. must be considered. Moreover, we attempt to combine the non-diene rubber to diene rubber. In the present report, we deal with Q as a non-diene rubber. It is structured as the basis of dimethylpolysiloxane (PDMS) as shown in Equation A8 in the
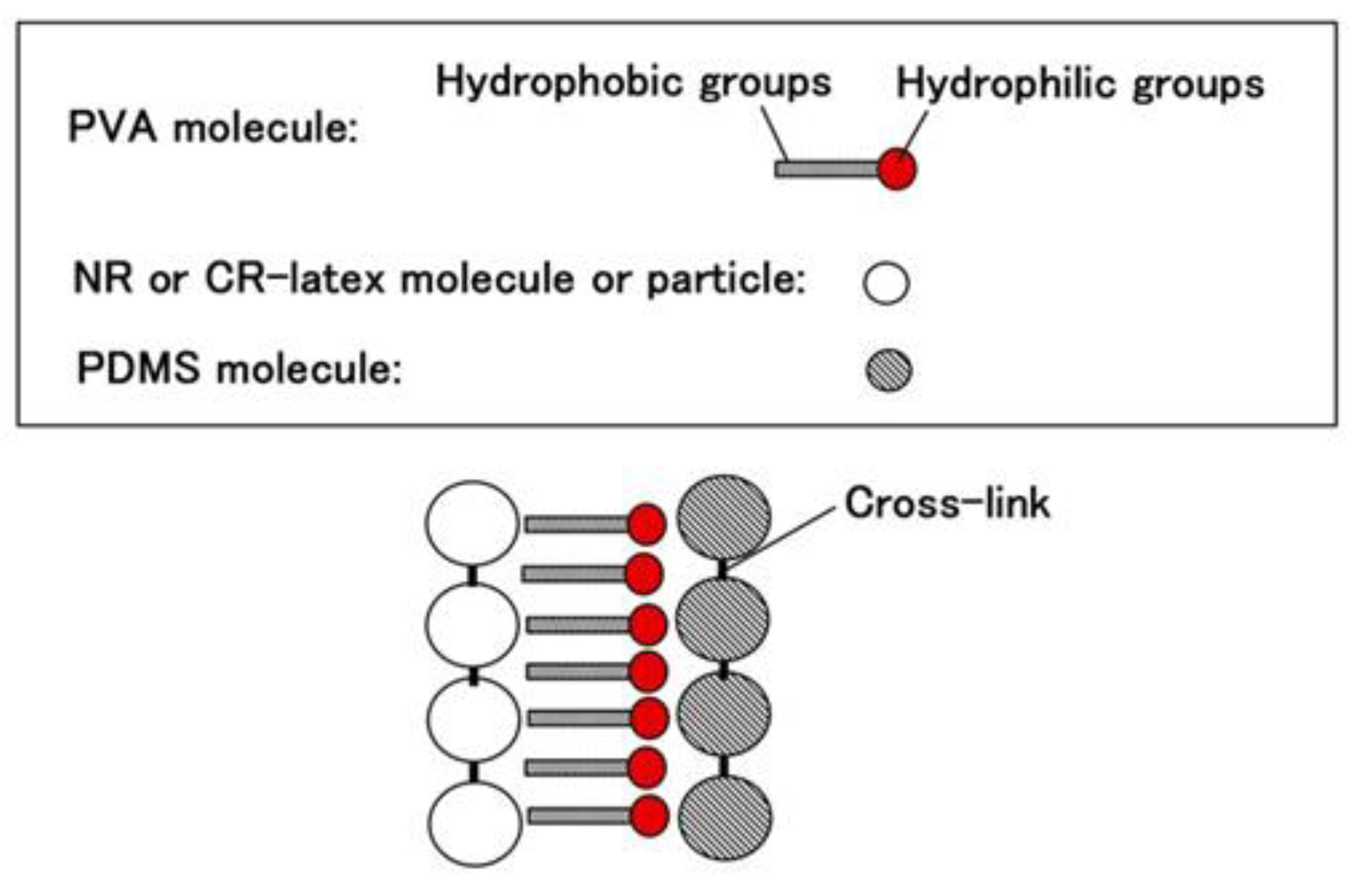
Appendix, which is similar to oil. On the contrary, NR and CR are similar to water. Therefore, when PDMS and NR or CR are combined, polyvinyl alcohol (PVA) is used, which is anionic as shown in Equation (4). PVA is an emulsifier, then NR-latex or CR-latex and PDMS can be combined by emulsion polymerization as shown in
Figure 1.
Q used in this report is KF96 (1 cSt, 50 cSt, 100 cSt, 1000 cSt) which is a silicone oil with the methyl group but not the rubber liquid, KE1400, and KE1300T that are hardened to be solid rubber using a curing agent. They are produced by Shin-Etsu Chemical Co. Ltd. (Tokyo, Japan). As for KE1400 and KE1300T, in general usage, they are solidified to be silicone rubber using a curing agent. KE1400 and KE1300T are improved because of solidification so that they are different from KF96; KE1400 and KE1300T are silicone oils with some silane, however, KF96 is a pure silicone oil without any silane. In the present study, we used only KE1400 and KE1300T without using the curing agent in the present combination. Therefore, the combination using KE1400 and KE1300T is different from that with KF96 from the perspective of the presence of silane. In the case of using PVA, we combined 3 g NR (Ulacol)- or CR (671A)- latex to the previously combined 3 g KF96, KE1400 or KE1300T with 3 g PVA.
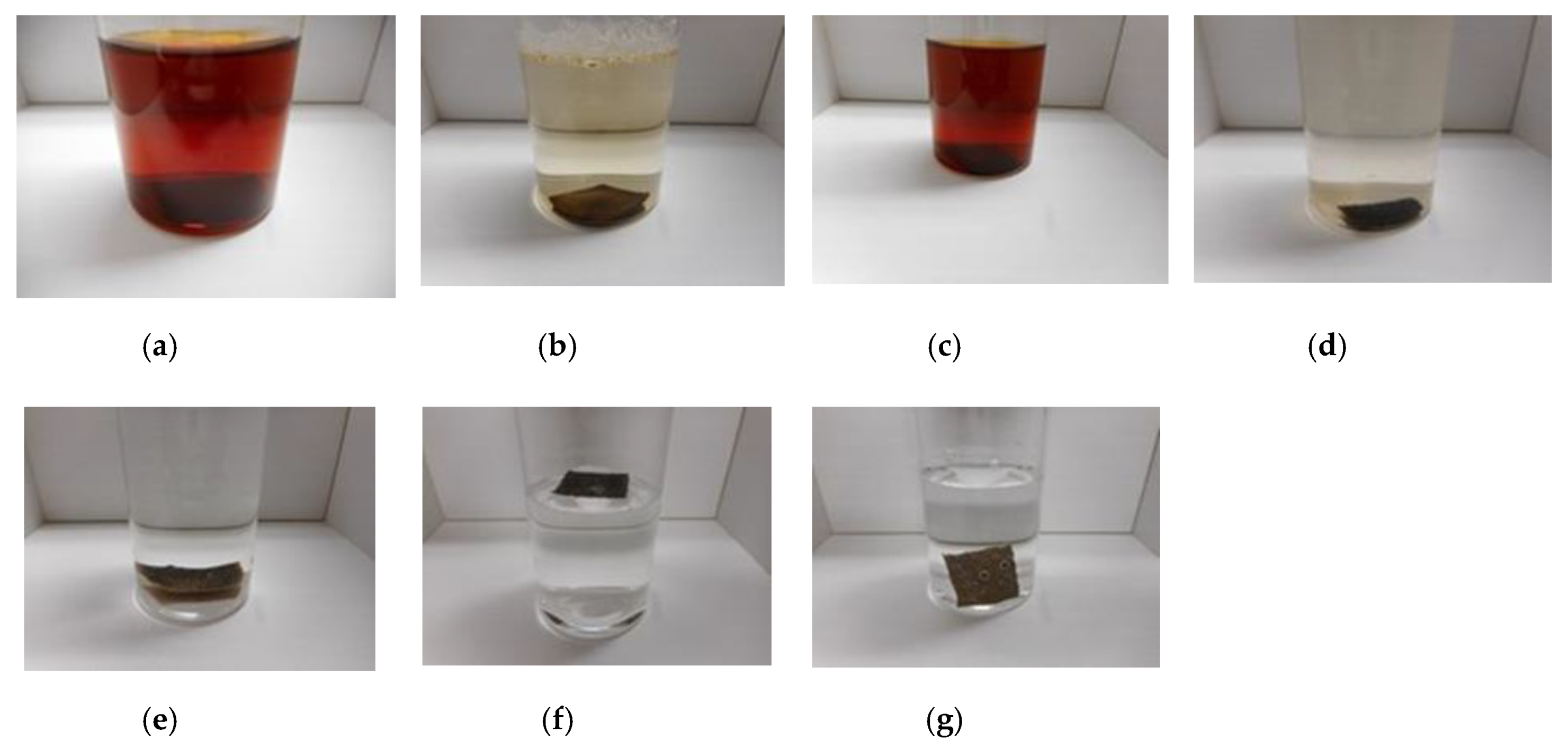
Figure 2 shows the appearance of the combination, which can achieve the same results by other combinations except for the figure.
As seen in
Figure 2a, there are many small granulated lumps that are non-combined ones of PDMS and NR- or CR-latex. By comparing
Figure 2a,b, PVA can be seen to have a role in the combination between diene and non-diene rubbers. In the case of
Figure 2d, after the combination of the KE1400 and CR-latex (671A) with PVA, MCF consisting of 3 g Ni and 0.75 g MF (W-40) was combined. The combination of diene and non-diene rubber latexes with MCF also results in a highly uniform dispersion.
Next, these combined liquids were electrolytically polymerized whereby a static magnetic field of 312 mT is applied to a pair of two stainless electrodes with a 1 mm gap using permanent magnets as paired opposites via the application of a constant electric field at 20 V, 2.7 A, and 5 min. The magnetic field strength can be determined by the production method such that has been presented in the previous studies [
1]. It can be measure with Gauss meter probe which is an ordinary instrument for measurement of magnetic field. From trying many experiments, 312 mT has been confirmed to be optimal magnetic field strength in case of our present production method with 1-mm electrodes gap using permanent magnets as paired opposites via the application of a constant electric field at 2.7 A during 6–30 V, and 5–30 min. Therefore, we used 312 mT during the present study. The mass of each component is the same as that of
Figure 2. In the case of using TiO
2, a liquid containing 0.5 g TiO
2 is combined beforehand to MF, combined with Ni, and then this liquid is combined to the combination of non-diene and diene rubber latexes with PVA. The combination of NR-latex and CR-latex is because the resistance to heat, ozone, etc. of CR-latex is superior to that of NR-latex. If we use just CR-latex as shown in
Figure 3e,g,i, the electrolytically polymerized MCF rubber has such a thin thickness that it is difficult to handle at the subsequent procedure of producing it as a sensor style, as demonstrated in another experiment.
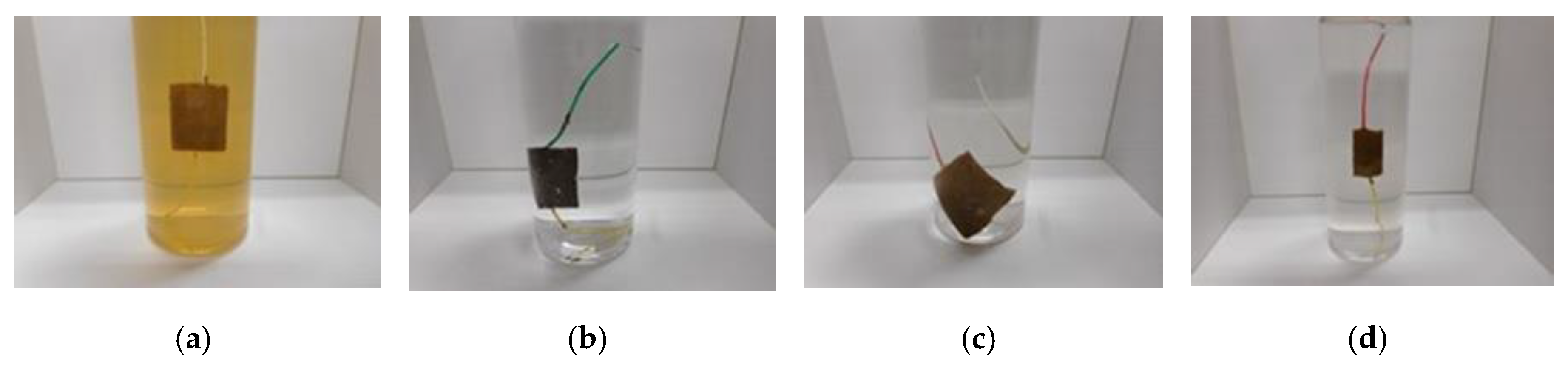
Figure 3 shows the surface of the MCF rubber on the cathode-side electrode after electrolytic polymerization. All surfaces are porous. The cause of creation of the porous by electrolytic polymerization in case of using PVA is due to the creation of hydrogen of water involved in the PVA, NR-latex and CR-latex. The electrolytic polymerization is correspondent to the electric degradation of water and the hydrogen is created on the cathode. In contrast, the surface of the anode-side electrode is non-porous because the vulcanization of the MCF rubber by electrolytic polymerization occurs on the anode. Using KE1300T or CR-latex, the porosity increases.
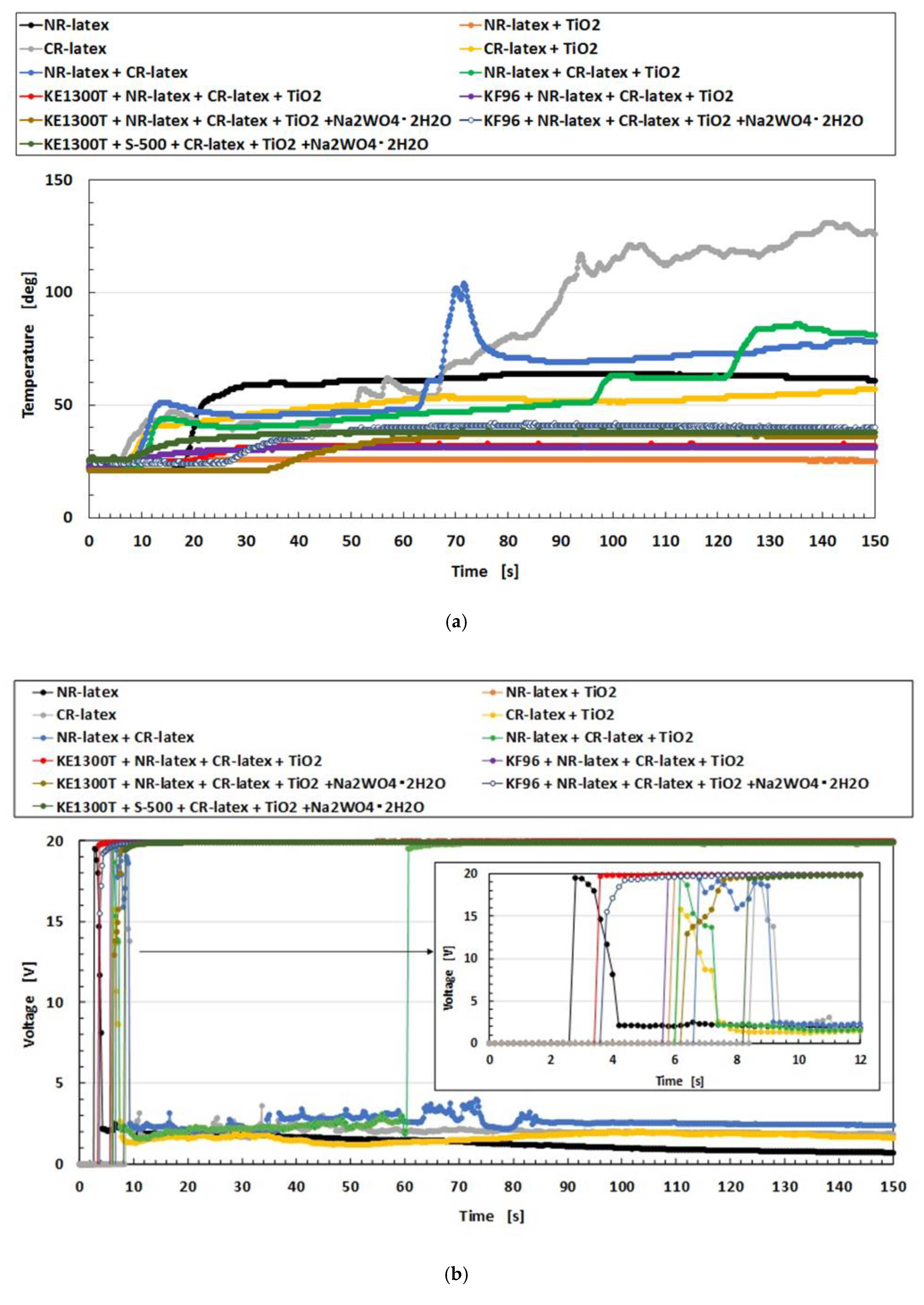
To investigate the microscopic mechanism of electrolytic polymerization of the MCF rubber by combining diene and non-diene rubber latexes, we will consider the changes in temperature, voltage, and electric current of the MCF rubber during electrolytic polymerization as shown in
Figure A7 in the
Appendix. In the case of using just NR-latex or CR-latex, the temperature due to electrolytic polymerization increases, the voltage decreases to a constant value, and electric current increases to be constant. However, for the combination of diene and non-diene rubber latexes, the temperature increase due to electrolytic polymerization is smaller compared to the case of only the NR-latex or CR-latex. The voltage is maintained at a constant at the applied voltage; the electric current increases temporarily and then decreases to zero. This result indicates that the electric current cannot easily flow inside the MCF rubber liquid because of the mixing with PDMS and PVA.
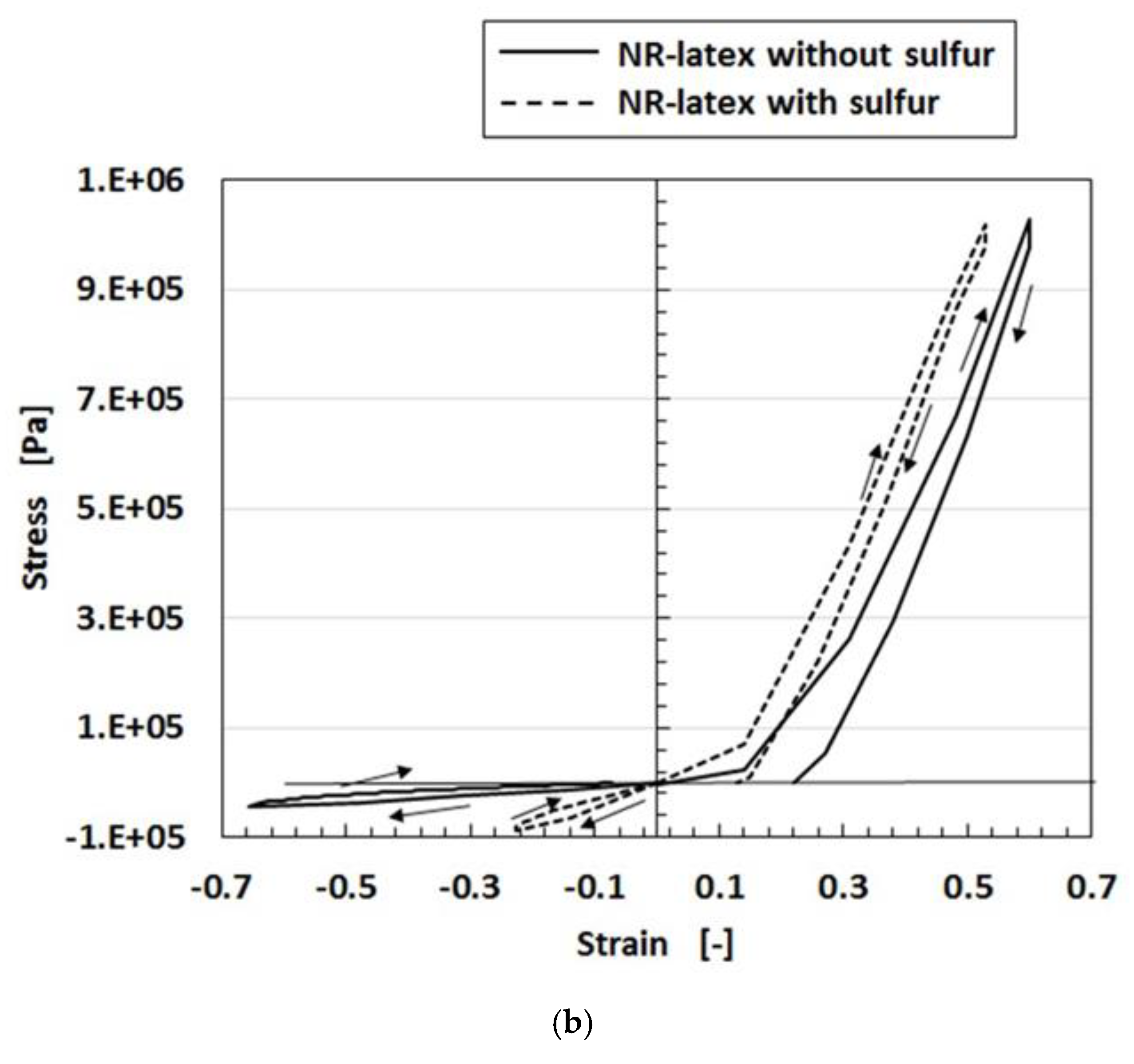
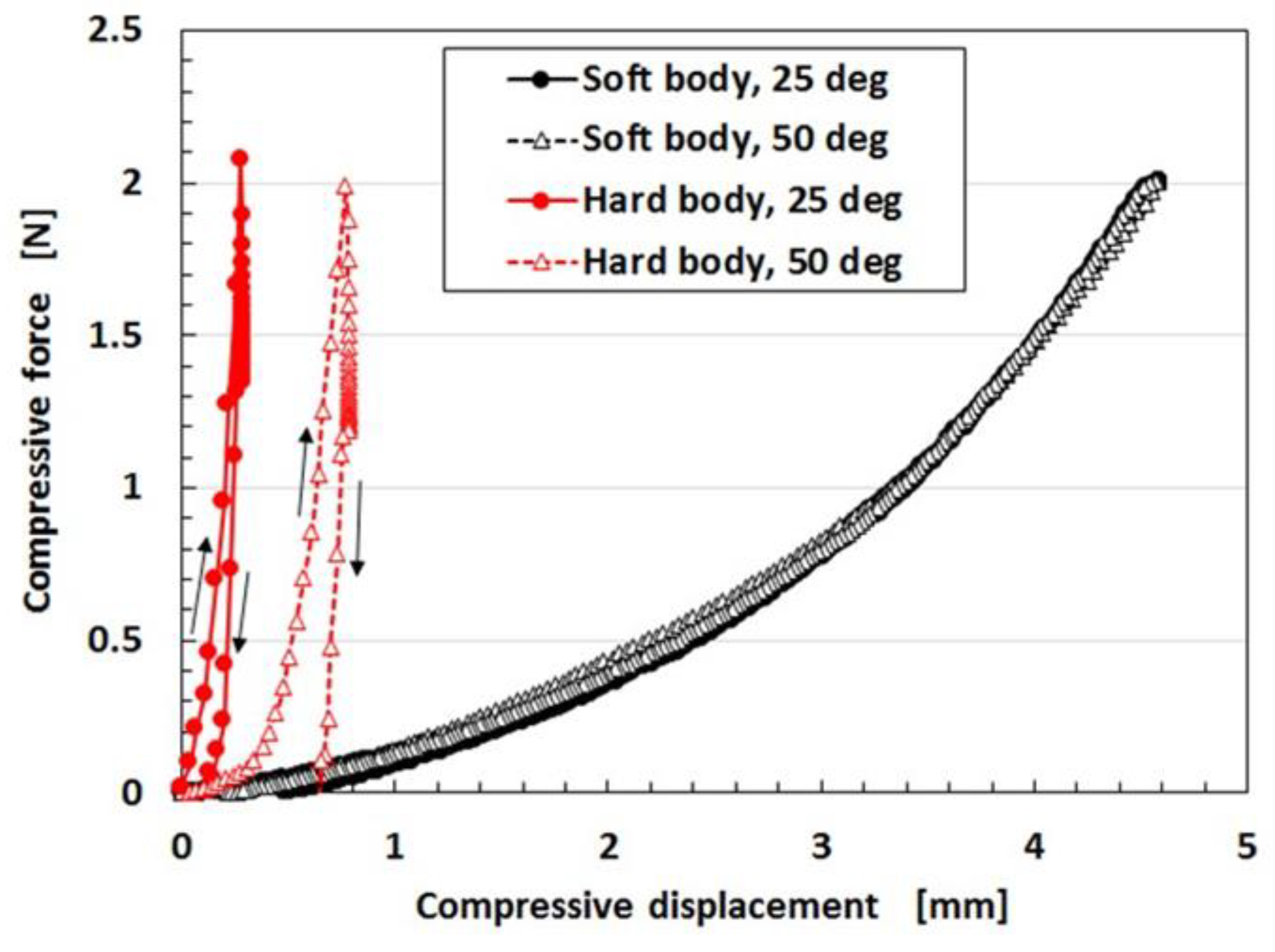
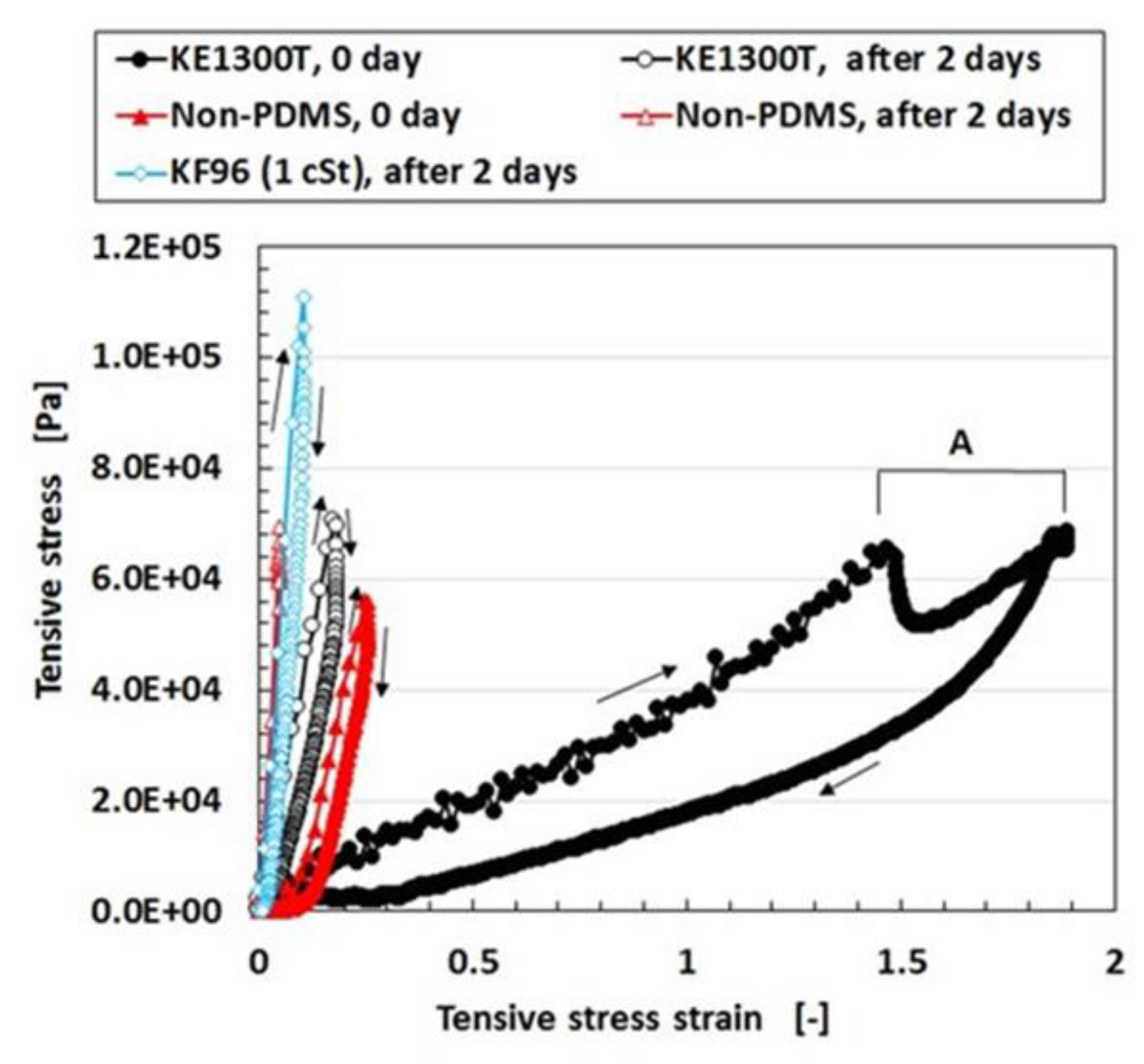
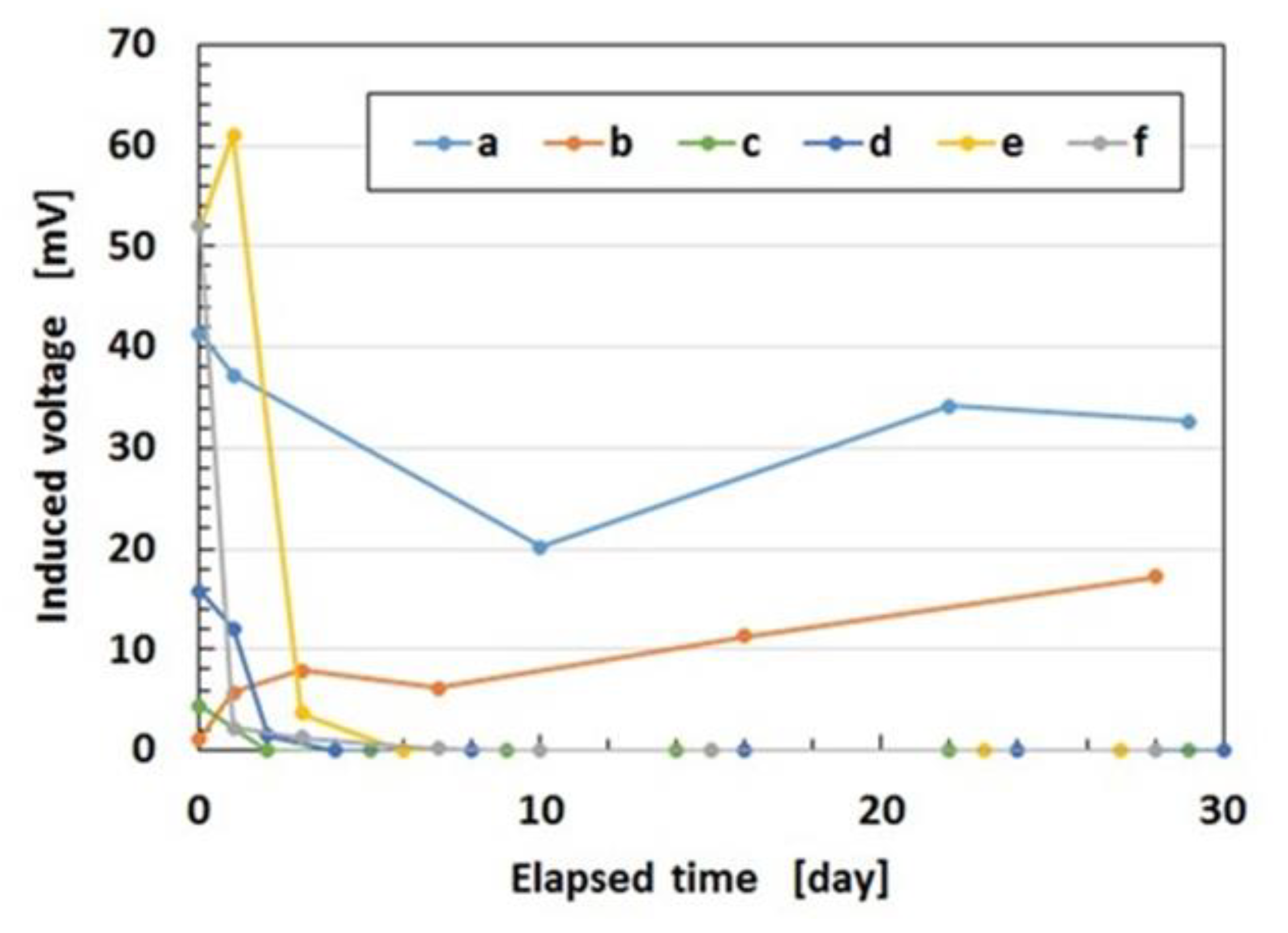
Regarding the instability of (1) as mentioned in the previous section, it was investigated using the experimental data on the mechanical property under tension as shown in
Figure 4 using a commercial, compact tensile testing machine (SL-6002, IMADA-SS Co. Ltd., Toyohashi, Japan). All test specimens with rectangular parallelepiped shapes were 1 mm thick 10 mm wide and 10 mm long in the initial stage before tension. The maximum tensile force was 0.5 N and the compression speed was 100 mm/min. The data is for the second tension when this parameter was repeated many times to eliminate the Mullins effect. The electrolytic polymerization conditions are the same as those of
Figure 3. The constituent of each MCF rubber is as follows: for KE1300T, 3 g KE1300T + 3 g NR-latex (Ulacol) + 3 g CR-latex (671A) + 3 g PVA + 0.75 g MF (W-40) + 3 g Ni; for KF96, 3 g KF96 (1 cSt) + 3 g NR-latex (Ulacol) + 3 g CR-latex (671A) + 3 g PVA + 0.75 g MF (W-40) + 3 g Ni; for Non-PDMS, 3 g NR-latex (Ulacol) + 3 g CR-latex (671A) + 0.75 g MF (W-40) + 3 g Ni. In the figure, the arrows indicate the cyclic tension of increasing and decreasing stress-strain. “0 day” indicates that the measurement was conducted on the same day as the production of the MCF rubber, and “after 2 days” indicates that the measurement was performed after being left in the air for 2 days from the production of the MCF rubber.
Table 1 shows the mass of each MCF rubber sheet and the ratio of water evaporation (RWE) from the MCF rubber, which can be obtained from the reduction of the mass.
KF96 (1 cSt) has kinematic viscosity at 1 cSt and KE1300T at 7810 cSt because the molecular weight of PDMS of KF96 (1 cSt) is smaller than that of KE1300T. The PDMS has the ability to contain the remaining water in the MCF rubber. From
Table 1, the RWE of KF96 (1 cSt) is larger than that of KE1300T. This is because the ability to contain the remaining water in the rubber deteriorates due to the smaller molecular weight of PDMS. In addition, KF96 (1 cSt) has more water in the rubber than KE1300T and the RWE of KF96 (1 cSt) is larger than that of KF96. Therefore, KF96 (1 cSt) easily hardens such that KE1300T is softer than KF96 (1 cSt) as shown in
Figure 4. As a result, when PDMS is used, a higher molecular weight is required for the production of MCF rubber sensors. However, the RWE of KE1300T is slightly smaller than that of the non-PDMS as shown in
Table 1. This is because PDMS has the ability to contain the remaining water in the rubber. Due to the water, KE1300T is softer than the non-PDMS as shown in
Figure 4. As a result of this softness, a temporary decreasing change is represented by “A” in
Figure 4. It depends on the deformation of the connection between PVA and the NR- or the CR-latex, and between the PVA and PDMS. With respect to the instability of (1), an argument will be presented after the following sensor production section.
2.3. Production of Sensor
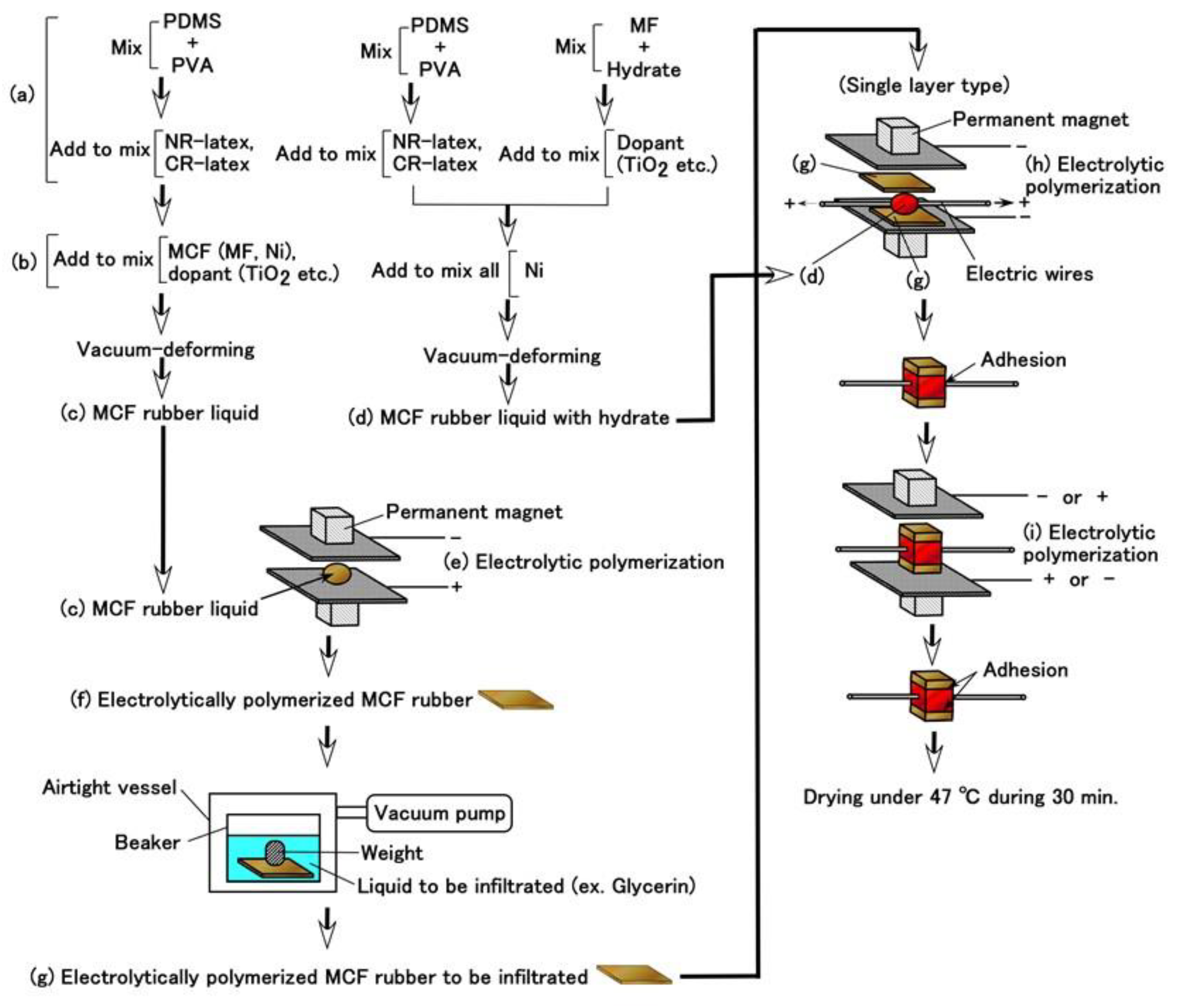
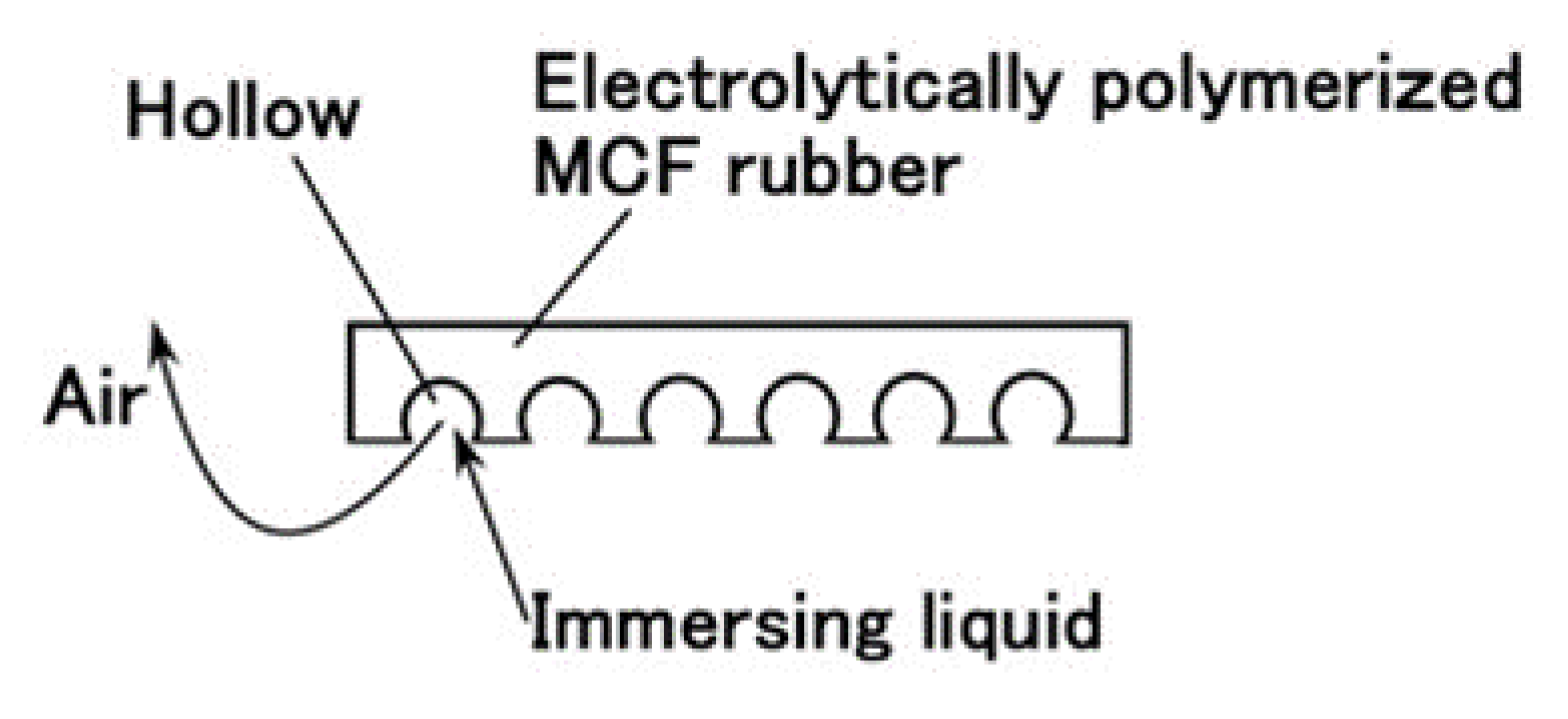
Given that we evaluated the effectiveness of utilizing PDMS in combination with NR- or CR- latex and Q, we attempt to produce a sensor using PDMS. It is evident from
Figure 3 that the MCF rubber that is electrolytically polymerized with PDMS is highly porous. Therefore, we infiltrated liquids using a vacuum as shown in (g) in
Figure 5 and
Figure 6Prior to the infiltration, we prepared the electrolytically polymerized MCF rubber with PDMS as shown in (f), which is the same as that shown in
Figure 3. The order of (a) and (b) in
Figure 5 is significant. As for (b) in
Figure 5, TiO
2 is appropriate for combining with MCF before mixing Ni. The electrolytic polymerization of (e) in
Figure 5 occurs for an applied electric field at 20 V, 2.7 A, and 5 min. The filtrated liquid can be anything, therefore, the MCF rubber can be fabricated using any intelligent liquid including battery acid, electrolyte, etc. Therefore, it is possible to produce a novel intelligent material using any intelligent fluid. In the present report, we used glycerin (99.5%, Kanto Chemical Co., Inc., Tokyo, Japan) because of the possibility of resolving instability (1). During filtration, the MCF rubber (f) is light enough to float upwards. Therefore, it must be held down by a weight in the infiltrating liquid.
In the case of instability (2), to abate the vulcanization of the MCF rubber from the time of electrolytic polymerization, which is not during the process but past the endpoint, the final procedure of drying the MCF rubber sensor shown in
Figure 5 is important. The vulcanization of the MCF rubber achieved via both electrolytic and thermal polymerization. The latter is for drying.
However, the MCF rubber liquid with hydrate must be prepared as shown in (d) in
Figure 5. The metal complex hydrate Na
2WO
4·2H
2O and Na
2MoO
4·2H
2O are suitable for the MCF rubber liquid combined with PDMS. Using the same MCF rubber sensor production process as in the previous study [
8], MCF rubber liquid with hydrate (d) inserted between the sandwiched MCF rubber (g) together with thin electrical wires is electrolytically polymerized as shown in (h) and (i) in
Figure 5. The electrolytic polymerization of (h) occurs at 30 V, 2.7 A, 5 min., and (i) 30 V, 2.7 A, 15 min in an air atmosphere. The constituent includes 3 g PDMS, 3 g PVA, 3 g NR-latex (Ulacol), 3 g CR-latex (671A), 0.5 g Na
2WO
4·2H
2O, 0.75 g MF (W-40), and 3 g Ni for (c) in
Figure 5, 1 g Ni for (d) in
Figure 5. At all electrolytic polymerizations (e), (h), (i), a static magnetic field intensity of 312 mT produced by permanent magnets as paired opposites is applied to a pair of two stainless electrodes with a 1 mm gap.
Incidentally, the hard adhesion of the MCF rubber with hydrate to metal could be confirmed. Most metals adhere including gold, silver, and platinum, except for Cr and Ti. In the case when the glass coated with TiO
2 is specific: such as when using KF96 and Na
2WO
4·2H
2O, or KE1300T and Na
2MoO
4·2H
2O, adhesion occurs. In the case when KF96 and Na
2MoO
4·2H
2O, or KE1300T and Na
2WO
4·2H
2O is used, there is no adhesion. The adhesion phenomenon is shown in
Figure 7 for an anode electrode in the case of electrolytic polymerization.
This is expected to induce the progression of metal coating to prevent corrosion and adhesive glue between the rubber and a metal as a novel adhesion technique. The present adhesion technique is superior to that proposed in the previous study [
8], which is applicable to stainless steel, iron, lead, gold, silver, platinum, and glass coated with TiO
2. Therefore, as shown
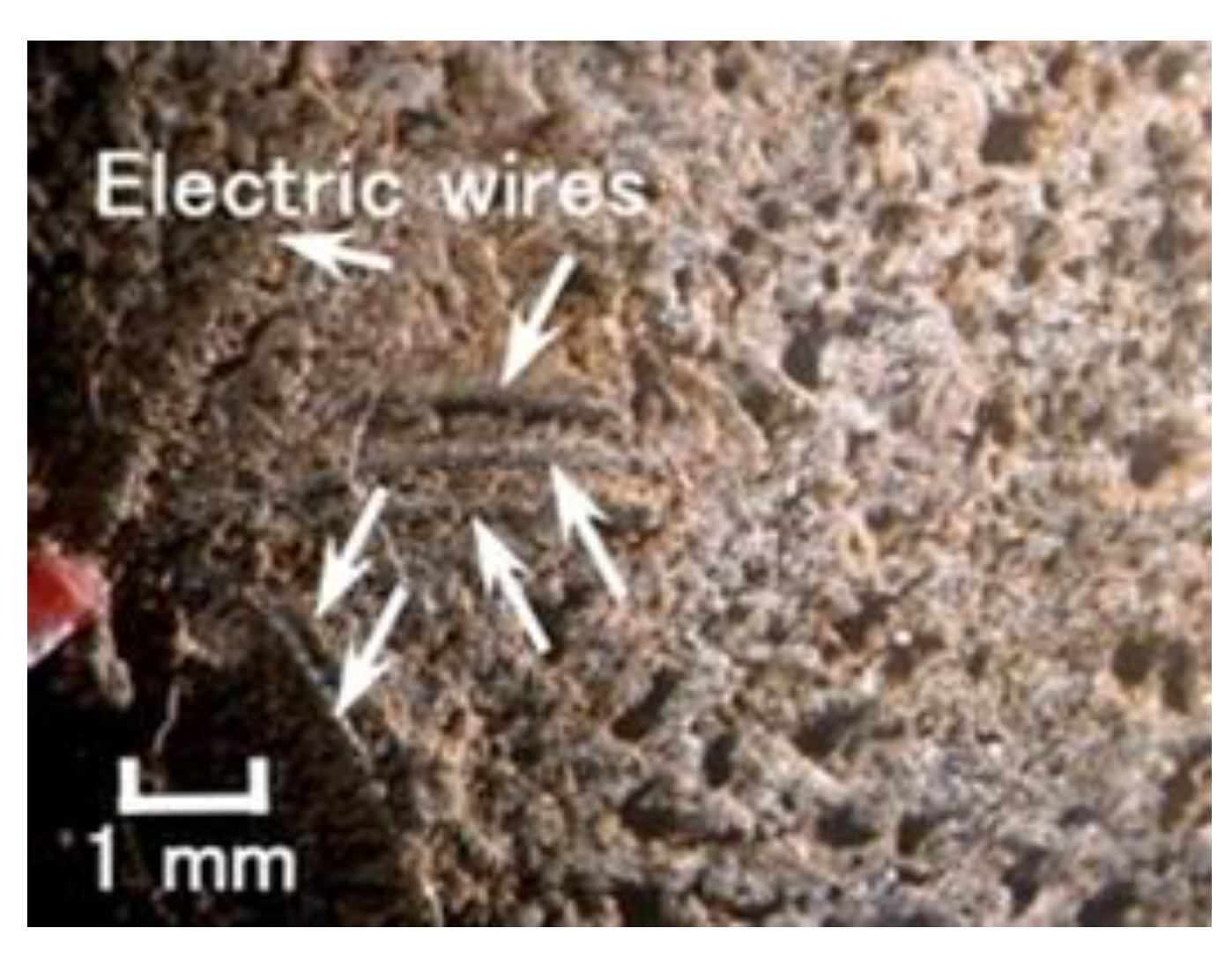
Figure 8, the thin electrical wires with an outer diameter of approximately φ 1.3 mm with seven thin silver-gilt electrical wires with a diameter of approximately φ 0.1 mm and a length of approximately 5 mm adhere in the MCF rubber. As presented in a previous study [
8], although the number of thin silver-gilt wires is a few and their diameter is very small, the wires cannot be detached from the electrolytically polymerized MCF rubber affixed around each thin wire.
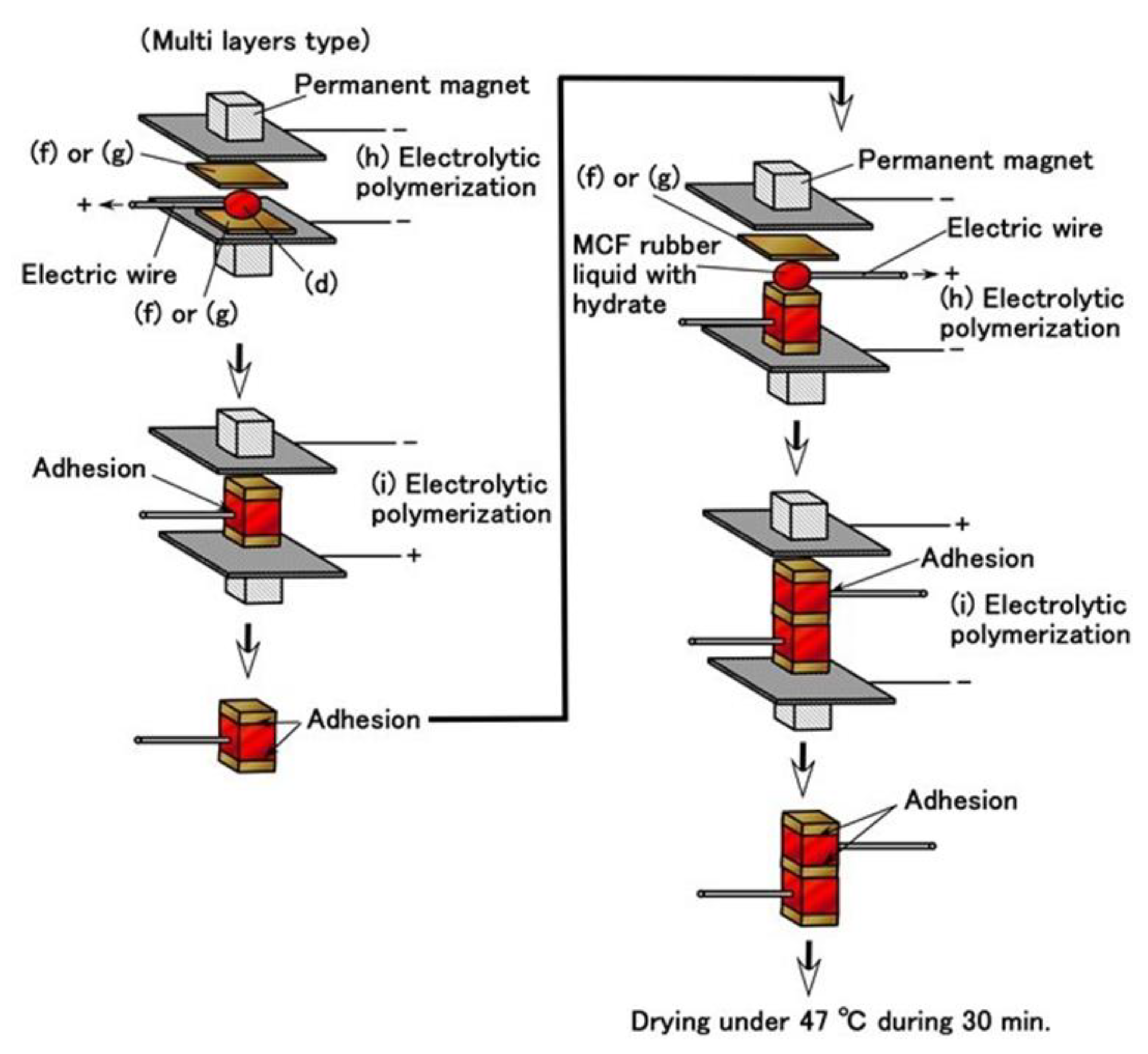
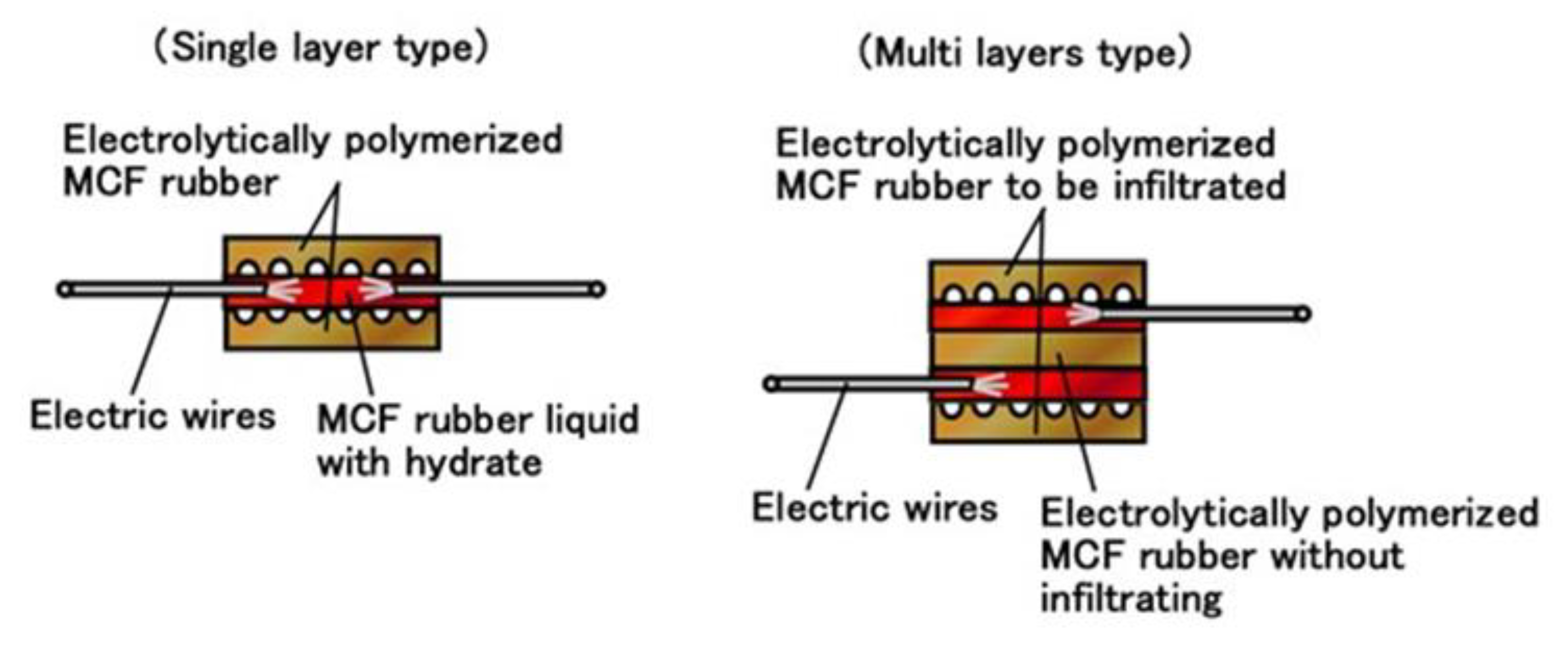
Moreover, we can produce many fabricated layers by repeating a single layer between the electrolytically polymerized rubbers as shown in (g) in
Figure 5. In the present report, we produced double layers via the production procedure shown in
Figure 9. The internal structure is shown in
Figure 10. For the electrolytically polymerized MCF rubbers such as (f) and (g) in
Figure 5, it does not matter whether they are infiltrated or not.