Differential Sympathetic Activation of Adipose Tissues by Brain-Derived Neurotrophic Factor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Cannula Implantation
2.3. Unilateral Denervation
2.4. Cannula Placement Verification and icv Injection Procedure
2.5. Body Weight, Body Composition, and Food Intake
2.6. Plasma Lipolytic Products, Corticosterone, and Catecholamines
2.7. Catecholamine Extraction and Measurement
2.8. Determination of NETO
2.9. Analysis of Gene Expression Using Real-Time Quantitative PCR
2.10. Analysis of Protein Expression Using Western Blot
2.11. Statistical Analysis
3. Results
3.1. Cohort 1: Effects of BDNF on Whole-Animal Lipid Mobilization
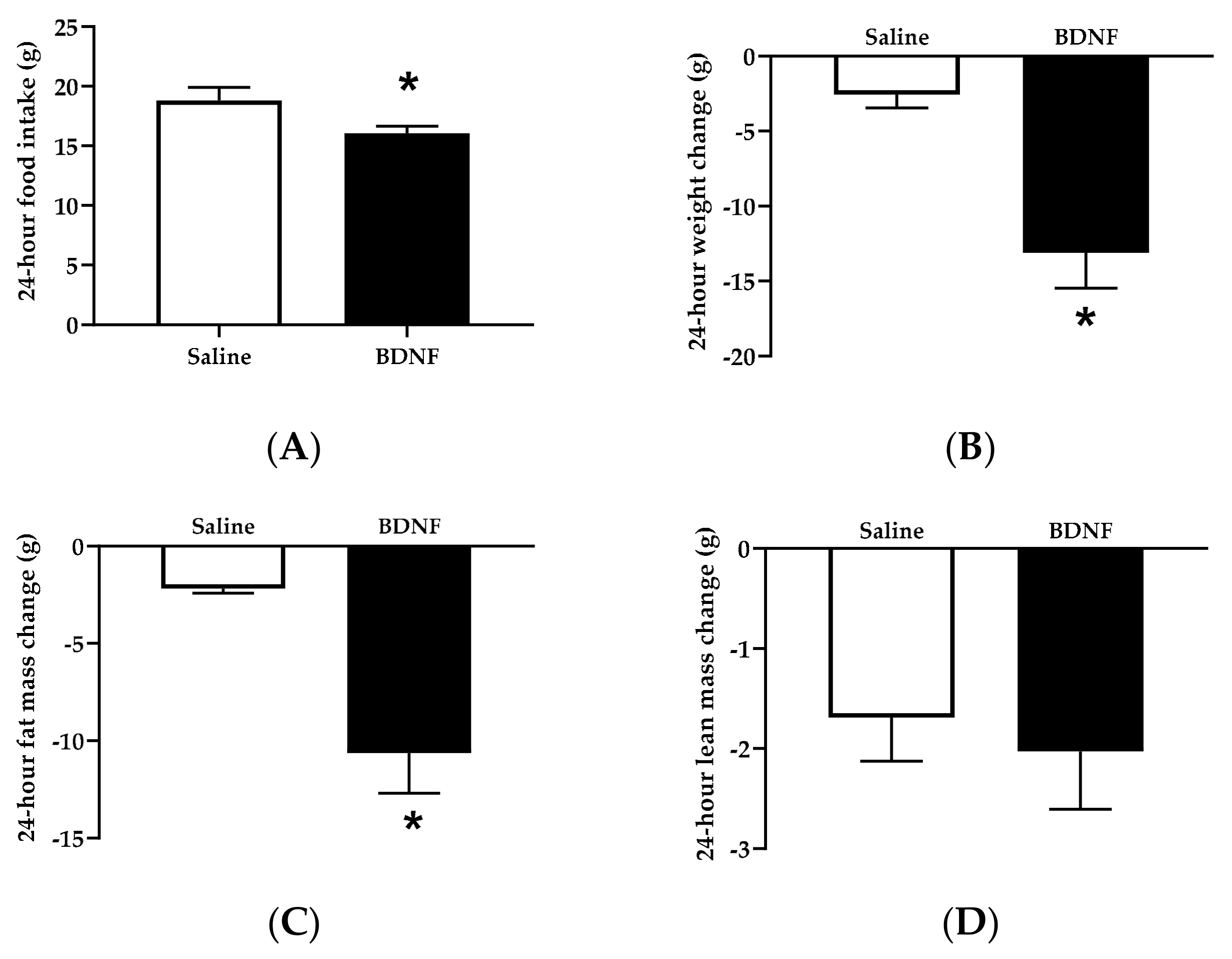
3.1.1. Food Intake, Body Weight, and Body Composition
3.1.2. Circulating Factors
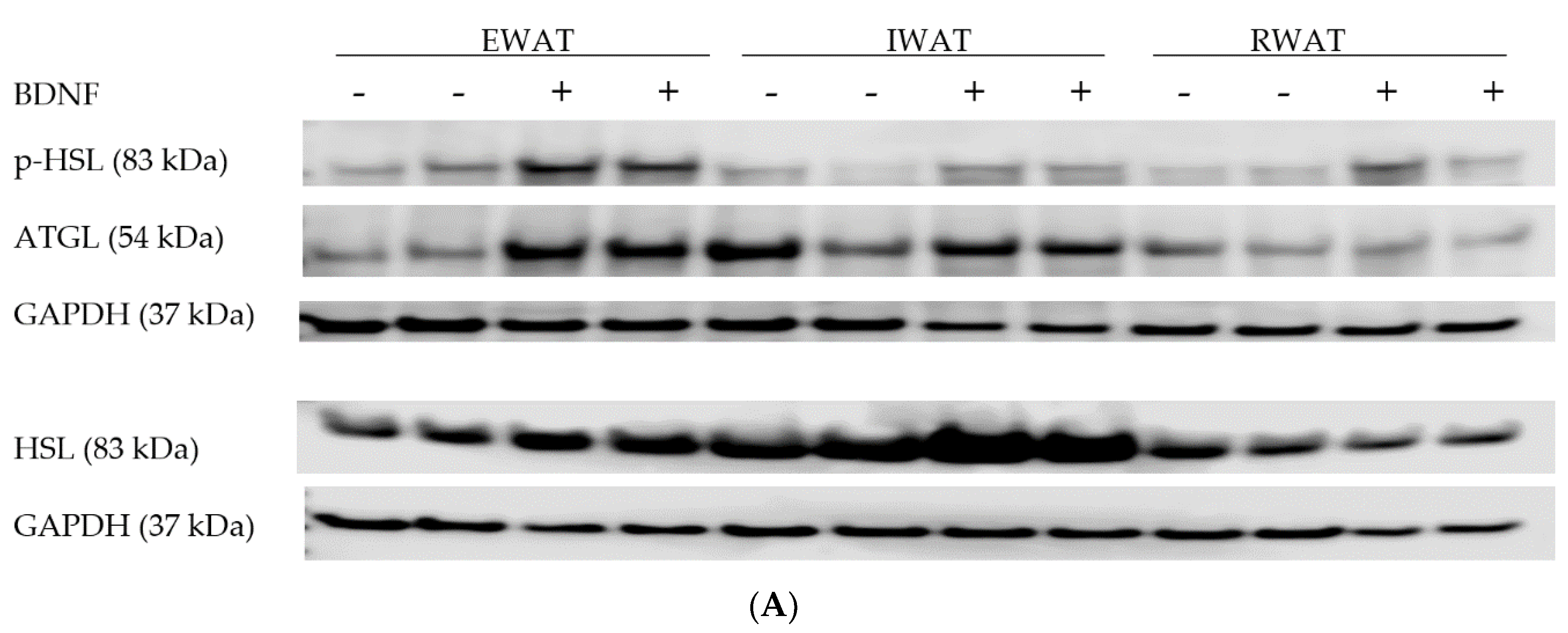
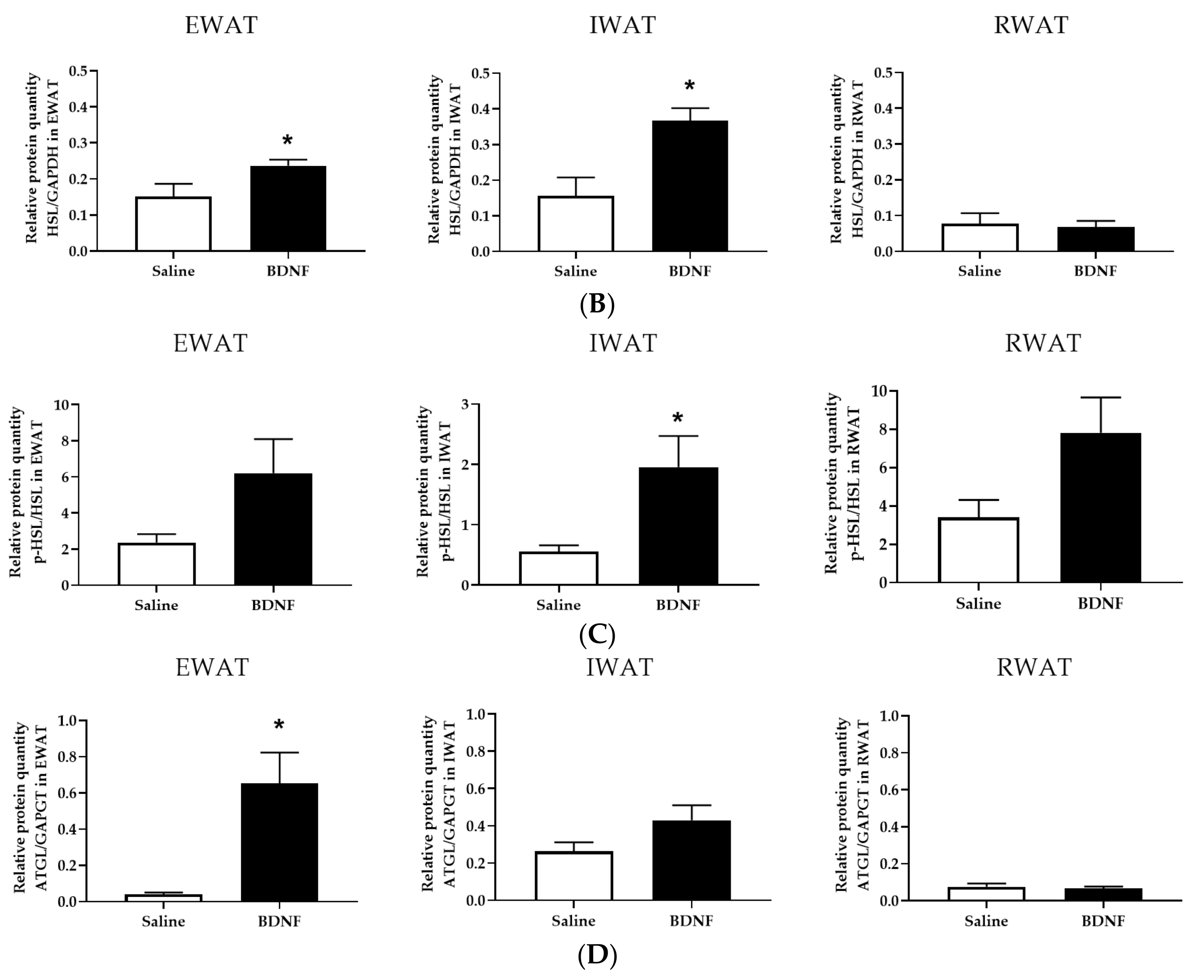
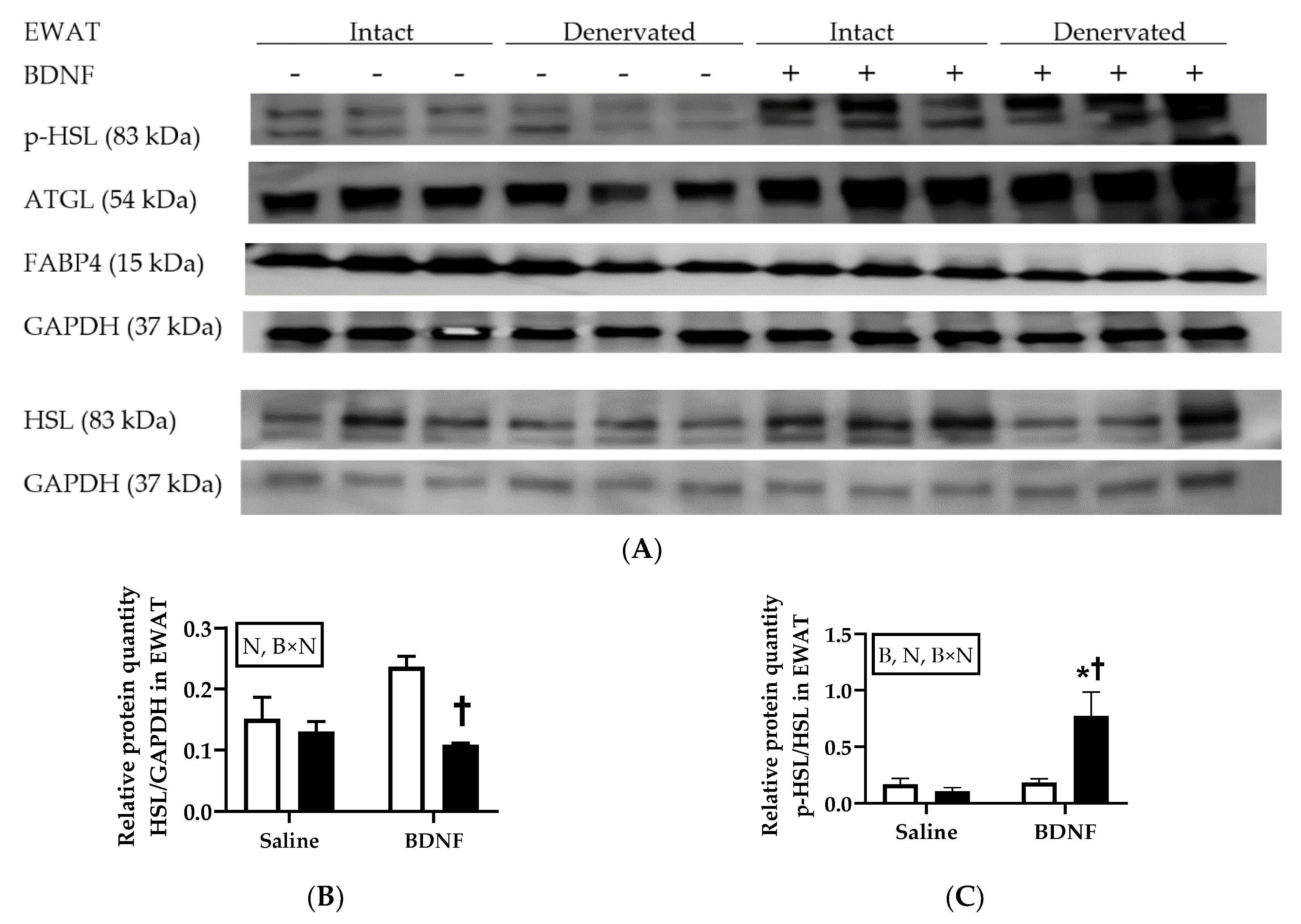
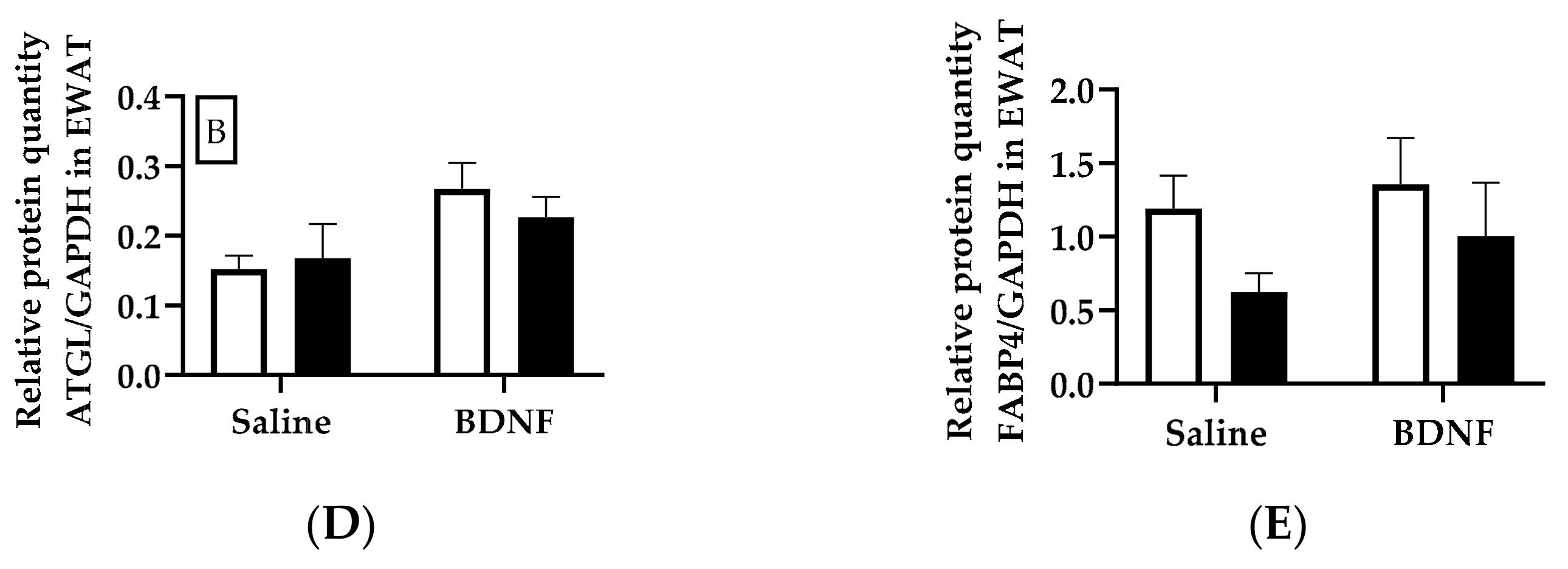
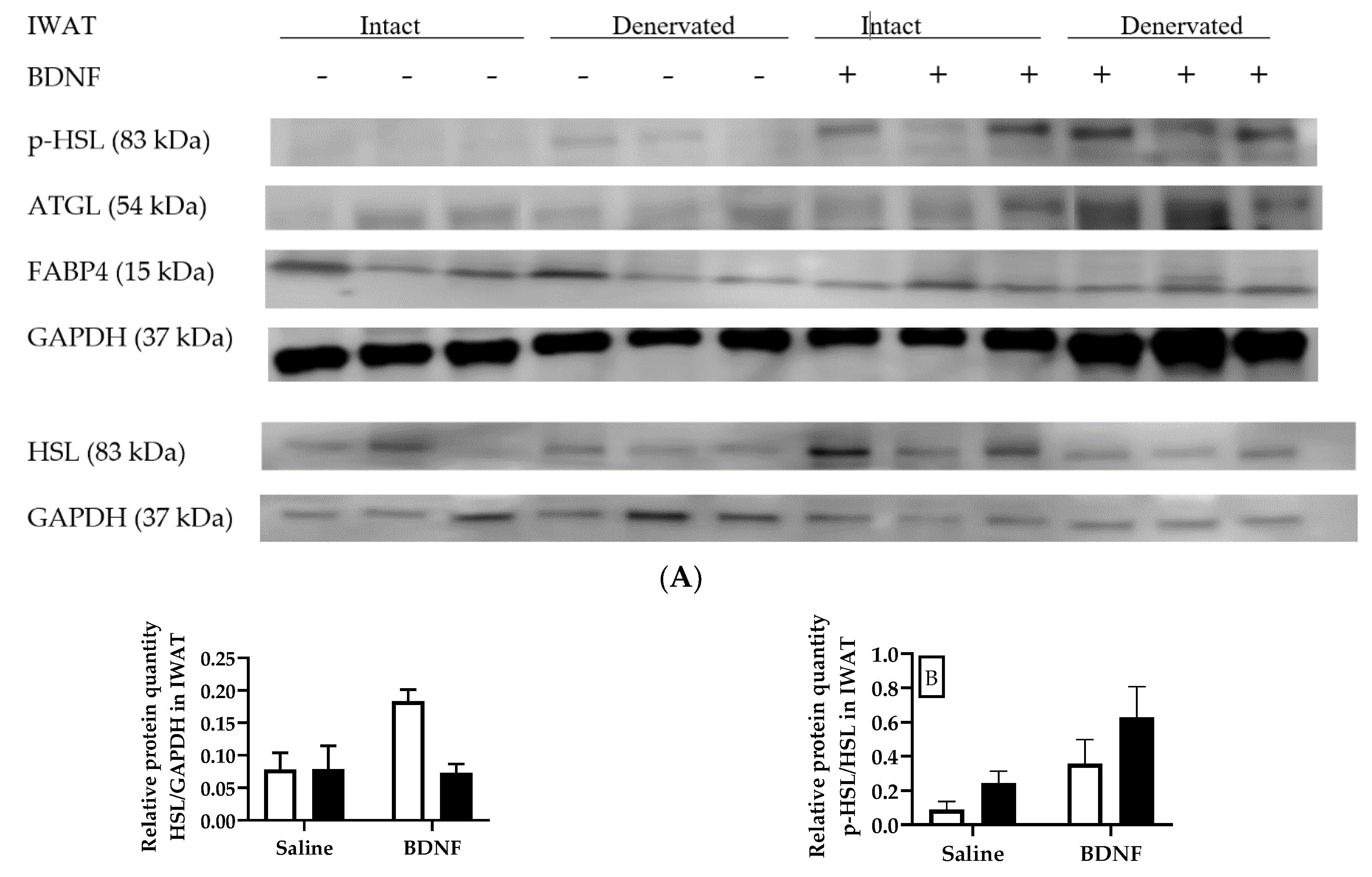
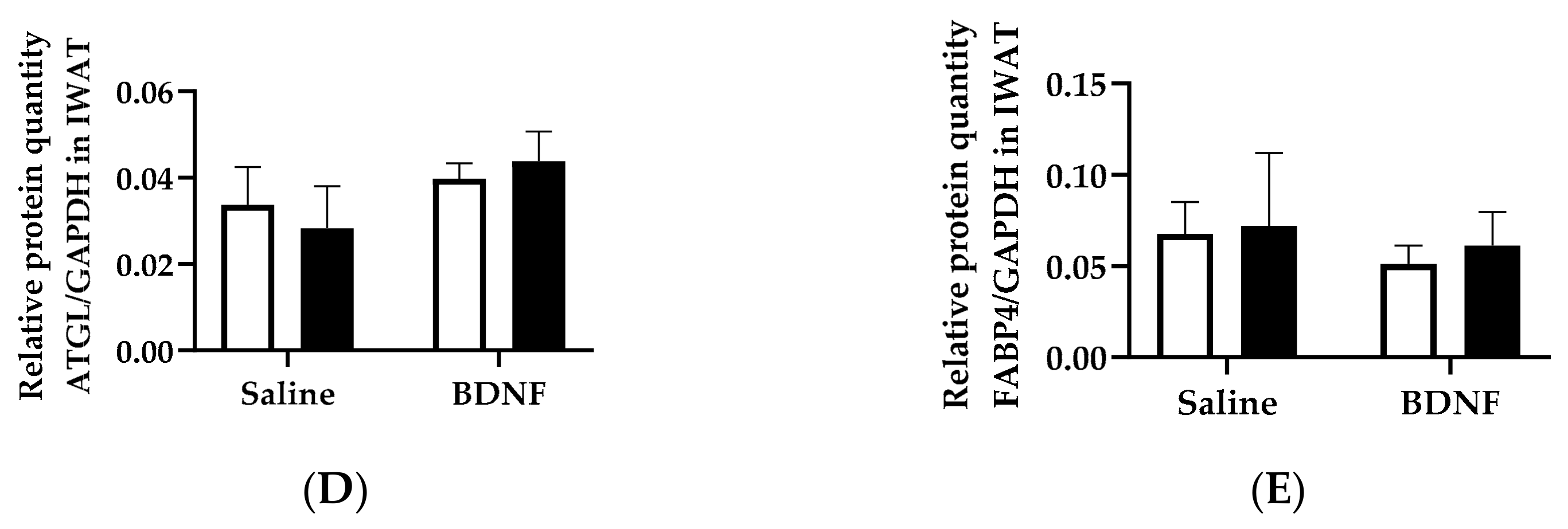
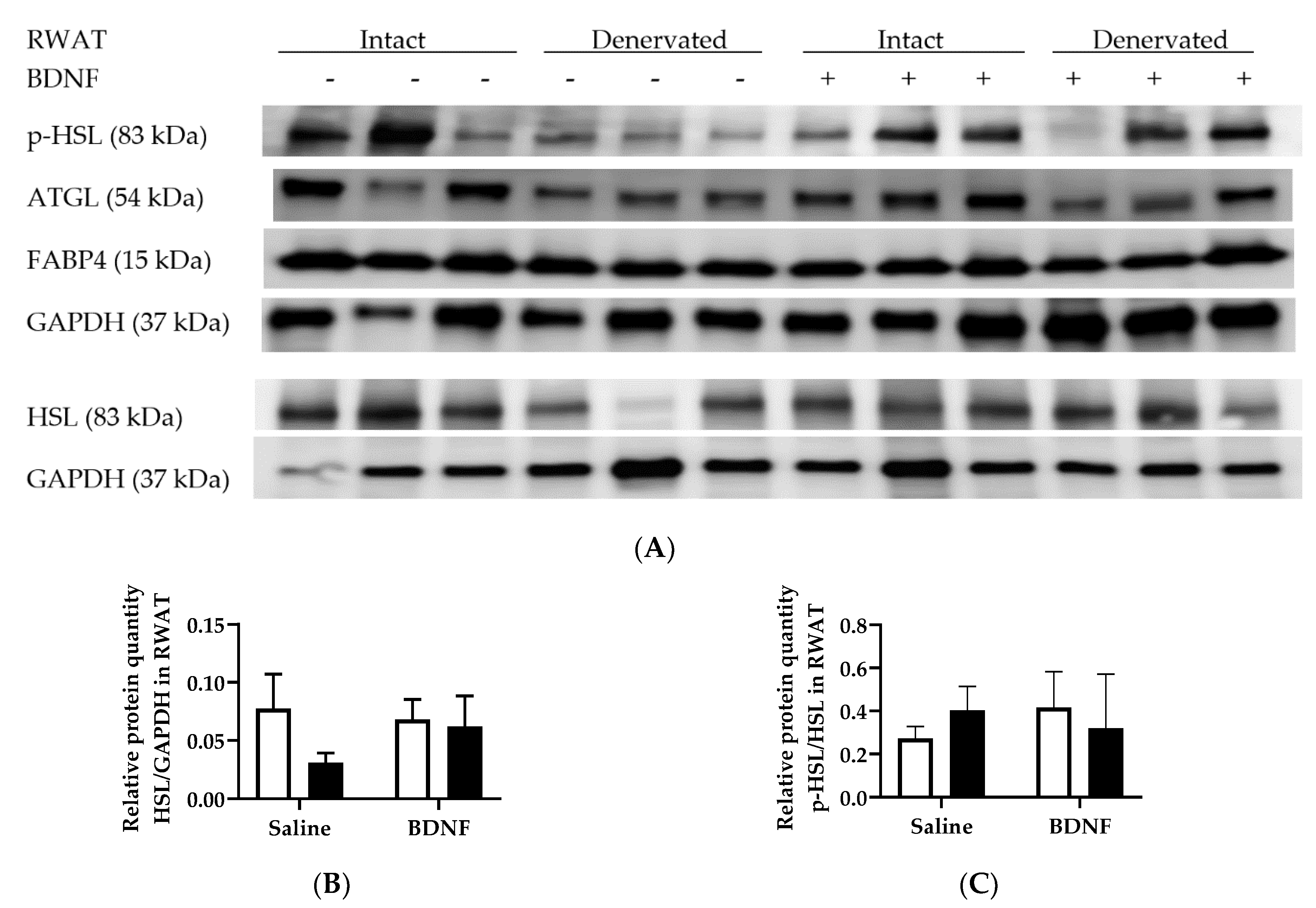
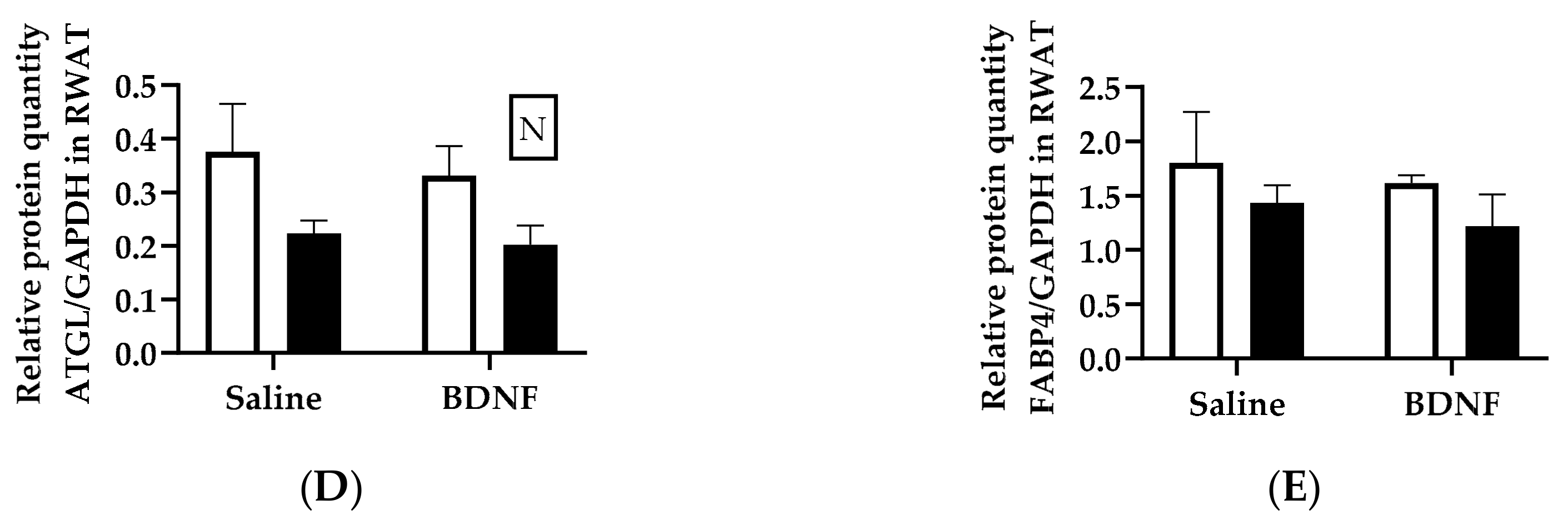
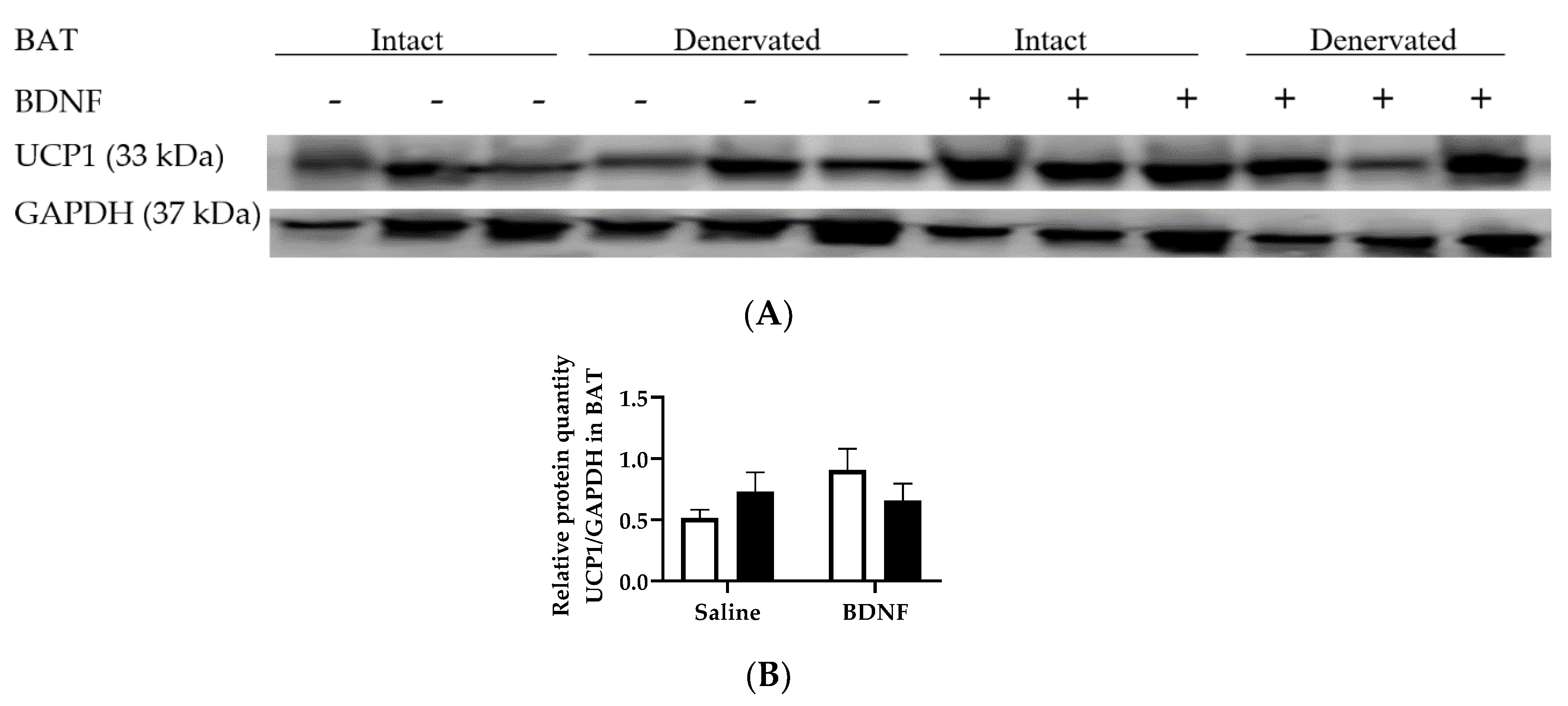
3.1.3. Expression of Lipolytic Proteins in Adipose Tissues
3.2. Cohort 2: Effects of BDNF on Adipose Lipolysis via Sympathetic Activation
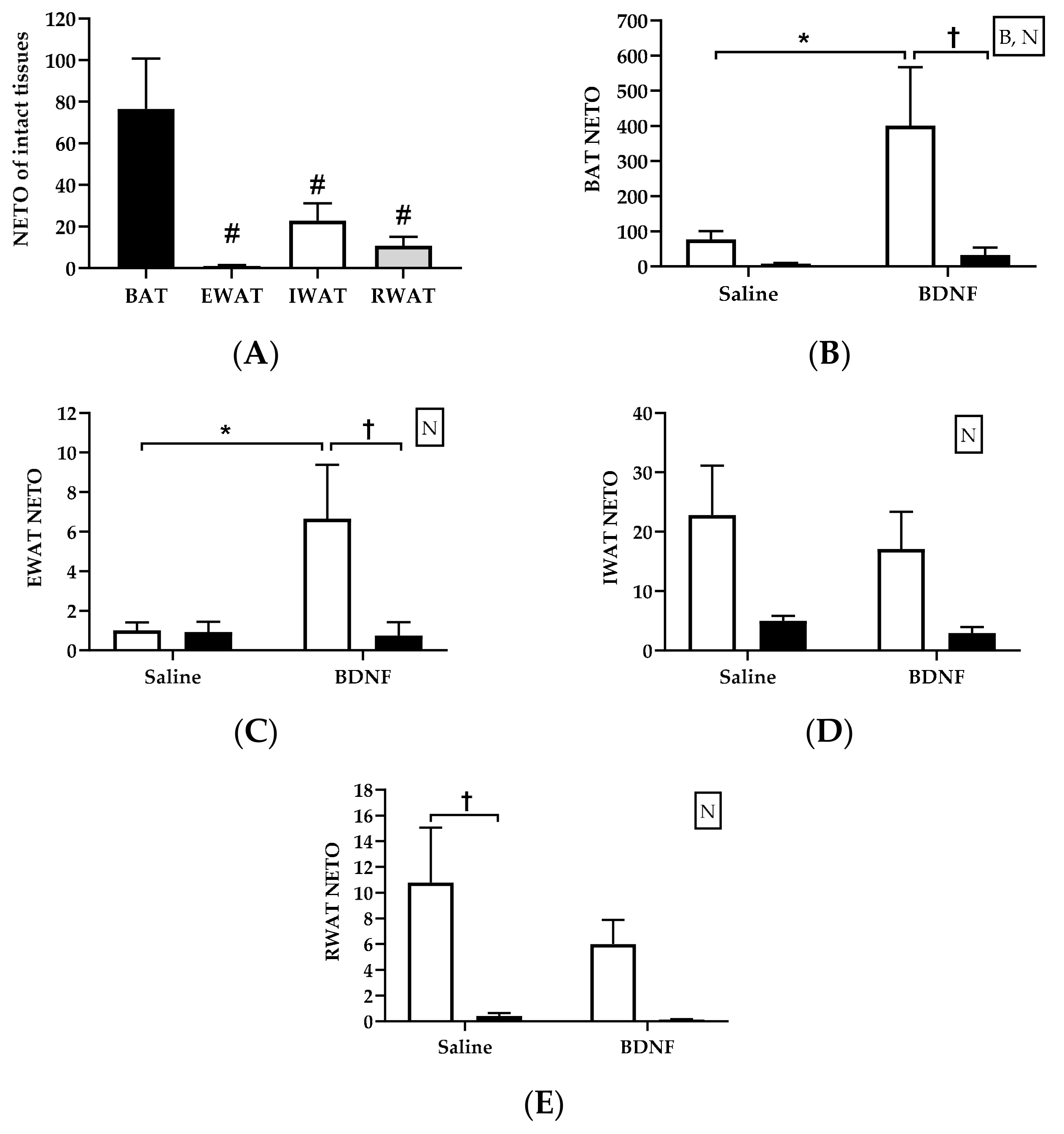
3.2.1. Adipose Tissue NE Concentration
3.2.2. Adipose Tissue NETO
3.2.3. Adipose Tissue Gene Expression
3.2.4. Adipose Tissue Protein Expression
4. Discussion
4.1. Summary of Findings
4.2. Sympathetic Activity Indicated by NETO
4.3. Expression of Lipolytic Genes and Proteins
4.4. Potential Mechanisms of BDNF Action via SNS
4.5. Potential Sites of BDNF Action
4.6. Future Studies
4.7. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhu, Q.; Glazier, B.J.; Hinkel, B.C.; Cao, J.; Liu, L.; Liang, C.; Shi, H. Neuroendocrine regulation of energy metabolism involving different types of adipose tissues. Int. J. Mol. Sci. 2019, 20, 2707. [Google Scholar] [CrossRef] [PubMed]
- Cannon, B.; Nedergaard, J. Brown adipose tissue: Function and physiological significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef] [PubMed]
- Kolditz, C.-I.; Langin, D. Adipose tissue lipolysis. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Noble, E.E.; Billington, C.J.; Kotz, C.M.; Wang, C. The lighter side of BDNF. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R1053–R1069. [Google Scholar] [CrossRef] [PubMed]
- Toriya, M.; Maekawa, F.; Maejima, Y.; Onaka, T.; Fujiwara, K.; Nakagawa, T.; Nakata, M.; Yada, T. Long-term infusion of brain-derived neurotrophic factor reduces food intake and body weight via a corticotrophin-releasing hormone pathway in the paraventricular nucleus of the hypothalamus. J. Neuroendocrinol. 2010, 22, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Bomberg, E.; Billington, C.; Levine, A.; Kotz, C.M. Brain-derived neurotrophic factor in the hypothalamic paraventricular nucleus increases energy expenditure by elevating metabolic rate. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R992–R1002. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Bomberg, E.; Billington, C.J.; Levine, A.S.; Kotz, C.M. Brain-derived neurotrophic factor (BDNF) in the hypothalamic ventromedial nucleus increases energy expenditure. Brain Res. 2010, 1336, 66–77. [Google Scholar] [CrossRef] [Green Version]
- Nonomura, T.; Tsuchida, A.; Ono-Kishino, M.; Nakagawa, T.; Taiji, M.; Noguchi, H. Brain-derived neurotrophic factor regulates energy expenditure through the central nervous system in obese diabetic mice. Int. J. Exp. Diabetes Res. 2001, 2, 201–209. [Google Scholar] [CrossRef]
- Kernie, S.G.; Liebl, D.J.; Parada, L.F. BDNF regulates eating behavior and locomotor activity in mice. EMBO J. 2000, 19, 1290–1300. [Google Scholar] [CrossRef] [Green Version]
- Rios, M.; Fan, G.; Fekete, C.; Kelly, J.; Bates, B.; Kuehn, R.; Lechan, R.M.; Jaenisch, R. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol. Endocrinol. 2001, 15, 1748–1757. [Google Scholar] [CrossRef]
- Xu, B.; Goulding, E.H.; Zang, K.; Cepoi, D.; Cone, R.D.; Jones, K.R.; Tecott, L.H.; Reichardt, L.F. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat. Neurosci. 2003, 6, 736–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuchida, A.; Nonomura, T.; Ono-Kishino, M.; Nakagawa, T.; Taiji, M.; Noguchi, H. Acute effects of brain-derived neurotrophic factor on energy expenditure in obese diabetic mice. Int. J. Obes. Relat. Metab. Disord. 2001, 25, 1286–1293. [Google Scholar] [CrossRef] [Green Version]
- An, J.J.; Liao, G.-Y.; Kinney, C.E.; Sahibzada, N.; Xu, B. Discrete BDNF neurons in the paraventricular hypothalamus control feeding and energy expenditure. Cell Metab. 2015, 22, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Brodie, B.B.; Costa, E.; Dlabac, A.; Neff, N.H.; Smookler, H.H. Applicaiton of steady state kinetics to the estimation of synthesis rate and turnover time of tissue catecholamines. J. Pharmacol. Exp. Ther. 1966, 154, 493–498. [Google Scholar] [PubMed]
- Vaughan, C.H.; Zarebidaki, E.; Ehlen, J.C.; Bartness, T.J. Analysis and measurement of the sympathetic and sensory innervation of white and brown adipose tissue. In Methods in Enzymology; Ormond, A.M., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 537, pp. 199–225. [Google Scholar]
- Zhu, Z.; Liu, X.; Senthil Kumar, S.P.D.; Zhang, J.; Shi, H. Central expression and anorectic effect of brain-derived neurotrophic factor are regulated by circulating estradiol levels. Horm. Behav. 2013, 63, 533–542. [Google Scholar] [CrossRef] [Green Version]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 7th ed.; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Shi, H.; Song, C.K.; Giordano, A.; Cinti, S.; Bartness, T.J. Sensory or sympathetic white adipose tissue denervation differentially affects depot growth and cellularity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R1028–R1037. [Google Scholar] [CrossRef] [PubMed]
- Bowers, R.R.; Festuccia, W.T.L.; Song, C.K.; Shi, H.; Migliorini, R.H.; Bartness, T.J. Sympathetic innervation of white adipose tissue and its regulation of fat cell number. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R1167–R1175. [Google Scholar] [CrossRef] [PubMed]
- Frontini, A.; Cinti, S. Distribution and development of brown adipocytes in the murine and human adipose organ. Cell Metab. 2010, 11, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Spicer, E.G.; Gavini, C.K.; Goudjo-Ako, A.J.; Novak, C.M.; Shi, H. Enhanced sympathetic activity in mice with brown adipose tissue transplantation (transBATation). Physiol. Behav. 2014, 125, 21–29. [Google Scholar] [CrossRef]
- Mefford, I.N.; Ward, M.M.; Miles, L.; Taylor, B.; Chesney, M.A.; Keegan, D.L.; Barchas, J.D. Determination of plasma catecholamines and free 3,4-dihydroxyphenylacetic acid in continuously collected human plasma by high performance liquid chromatography with electrochemical detection. Life Sci. 1981, 28, 477–483. [Google Scholar] [CrossRef]
- Shi, H.; Bowers, R.R.; Bartness, T.J. Norepinephrine turnover in brown and white adipose tissue after partial lipectomy. Physiol. Behav. 2004, 81, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Spector, S.; Sjoerdsma, A.; Udenfriend, S. Blockade of endogenous norepinephrine synthesis by alpha-methyl-tyrosine, an inhibitor of tyrosine hydroxylase. J. Pharmacol. Exp. Ther. 1965, 147, 86–95. [Google Scholar] [PubMed]
- Penn, D.M.; Jordan, L.C.; Kelso, E.W.; Davenport, J.E.; Harris, R.B.S. Effects of central or peripheral leptin administration on norepinephrine turnover in defined fat depots. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R1613–R1621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, J.; Zhu, Q.; Liu, L.; Glazier, B.J.; Hinkel, B.C.; Liang, C.; Shi, H. Global transcriptome analysis of brown adipose tissue of diet-induced obese mice. Int. J. Mol. Sci. 2018, 19, 1095. [Google Scholar] [CrossRef] [PubMed]
- Baron, D.M.; Clerte, M.; Brouckaert, P.; Raher, M.J.; Flynn, A.W.; Zhang, H.; Carter, E.A.; Picard, M.H.; Bloch, K.D.; Buys, E.S.; et al. In vivo noninvasive characterization of brown adipose tissue blood flow by contrast ultrasound in mice. Circ. Cardiovasc. Imaging 2012, 5, 652–659. [Google Scholar] [CrossRef]
- Migliorini, R.H.; Garofalo, M.A.; Kettelhut, I.C. Increased sympathetic activity in rat white adipose tissue during prolonged fasting. Am. J. Physiol. 1997, 272, R656–R661. [Google Scholar] [CrossRef]
- Brito, N.A.; Brito, M.N.; Bartness, T.J. Differential sympathetic drive to adipose tissues after food deprivation, cold exposure or glucoprivation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R1445–R1452. [Google Scholar] [CrossRef] [Green Version]
- McElroy, J.F.; Mason, P.W.; Hamilton, J.M.; Wade, G.N. Effects of diet and photoperiod on NE turnover and GDP binding in Siberian hamster brown adipose tissue. Am. J. Physiol. 1986, 250, R383–R388. [Google Scholar] [CrossRef]
- Gavini, C.K.; Mukherjee, S.; Shukla, C.; Britton, S.L.; Koch, L.G.; Shi, H.; Novak, C.M. Leanness and heightened nonresting energy expenditure: Role of skeletal muscle activity thermogenesis. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E635–E647. [Google Scholar] [CrossRef]
- Brito, M.N.; Brito, N.A.; Baro, D.J.; Song, C.K.; Bartness, T.J. Differential activation of the sympathetic innervation of adipose tissues by melanocortin receptor stimulation. Endocrinology 2007, 148, 5339–5347. [Google Scholar] [CrossRef]
- Jensen, M.D. Lipolysis: Contribution from regional fat. Annu. Rev. Nutr. 1997, 17, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Hartman, A.D.; Christ, D.W. Effect of cell size, age and anatomical location on the lipolytic response of adipocytes. Life Sci. 1978, 22, 1087–1096. [Google Scholar] [CrossRef]
- Fried, S.K.; Leibel, R.L.; Edens, N.K.; Kral, J.G. Lipolysis in intraabdominal adipose tissues of obese women and men. Obes. Res. 1993, 1, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.F. Differential control of sympathetic outflow. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 281, R683–R698. [Google Scholar] [CrossRef] [PubMed]
- Krotkiewski, M. The effects of estrogens on regional adipose tissue cellularity in the rat. Acta. Physiol. Scand. 1976, 96, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Young, J.B.; Daly, P.A.; Uemura, K.; Chaouloff, F. Effects of chronic lard feeding on sympathetic nervous system activity in the rat. Am. J. Physiol. 1994, 267, R1320–R1328. [Google Scholar] [CrossRef] [PubMed]
- Marcin, K.; Per, B. The effects of dexamethasone and starvation on body composition and regional adipose tissue cellularity in the rat. Acta. Endocrinol. (Cop.) 1975, 80, 667–675. [Google Scholar]
- Prentice, K.J.; Saksi, J.; Hotamisligil, G.S. Adipokine FABP4 integrates energy stores and counterregulatory metabolic responses. J. Lipid Res. 2019, 60, 734–740. [Google Scholar] [CrossRef] [Green Version]
- Youngstrom, T.G.; Bartness, T.J. Catecholaminergic innervation of white adipose tissue in Siberian hamsters. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1995, 268, R744–R751. [Google Scholar] [CrossRef]
- Kreier, F.; Kap, Y.S.; Mettenleiter, T.C.; van Heijningen, C.; van der Vliet, J.; Kalsbeek, A.; Sauerwein, H.P.; Fliers, E.; Romijn, J.A.; Buijs, R.M. Tracing from fat tissue, liver, and pancreas: A neuroanatomical framework for the role of the brain in type 2 diabetes. Endocrinology 2006, 147, 1140–1147. [Google Scholar] [CrossRef]
- Lafontan, M.; Berlan, M. Fat cell adrenergic receptors and the control of white and brown fat cell function. J. Lipid Res. 1993, 34, 1057–1091. [Google Scholar] [PubMed]
- Lafontan, M.; Bousquet-Melou, A.; Galitzky, J.; Barbe, P.; Carpéné, C.; Langin, D.; Berlan, M.; Valet, P.; Castan, I.; Bouloumié, A.; et al. Adrenergic receptors and fat cells: Differential recruitment by physiological amines and homologous regulation. Obes. Res. 1995, 3, 507S–514S. [Google Scholar] [CrossRef] [PubMed]
- Deveaud, C.; Beauvoit, B.; Salin, B.; Schaeffer, J.; Rigoulet, M.J.M.; Biochemistry, C. Regional differences in oxidative capacity of rat white adipose tissue are linked to the mitochondrial content of mature adipocytes. Mol. Cell. Biochem. 2004, 267, 157–166. [Google Scholar] [CrossRef] [PubMed]
- DiGirolamo, M.; Fine, J.B.; Tagra, K.; Rossmanith, R. Qualitative regional differences in adipose tissue growth and cellularity in male Wistar rats fed ad libitum. Am. J. Physiol. 1998, 274, R1460–R1467. [Google Scholar] [CrossRef] [PubMed]
- Egan, J.J.; Greenberg, A.S.; Chang, M.K.; Wek, S.A.; Moos, M.C.; Londos, C. Mechanism of hormone-stimulated lipolysis in adipocytes: Translocation of hormone-sensitive lipase to the lipid storage droplet. Proc. Natl. Acad. Sci. USA 1992, 89, 8537–8541. [Google Scholar] [CrossRef] [PubMed]
- Rydén, M.; Jocken, J.; Harmelen, V.; Dicker, A.; Hoffstedt, J.; Wirén, M.; Blomqvist, L.; Mairal, A.; Langin, D.; Blaak, E.; et al. Comparative studies of the role of hormone-sensitive lipase and adipose triglyceride lipase in human fat cell lipolysis. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E1847–E1855. [Google Scholar] [CrossRef] [Green Version]
- Schwanhäusser, B.; Busse, D.; Li, N.; Dittmar, G.; Schuchhardt, J.; Wolf, J.; Chen, W.; Selbach, M. Corrigendum: Global quantification of mammalian gene expression control. Nature 2013, 495, 126. [Google Scholar] [CrossRef] [PubMed]
- Altar, C.A.; Cai, N.; Bliven, T.; Juhasz, M.; Conner, J.M.; Acheson, A.L.; Lindsay, R.M.; Wiegand, S.J. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature 1997, 389, 856–860. [Google Scholar] [CrossRef]
- Johnson, F.; Hohmann, S.E.; DiStefano, P.S.; Bottjer, S.W. Neurotrophins Suppress Apoptosis Induced by Deafferentation of an Avian Motor-Cortical Region. J. Neurosci. 1997, 17, 2101–2111. [Google Scholar] [CrossRef] [Green Version]
- Pardridge, W.M.; Kang, Y.-S.; Buciak, J.L.J.P.R. Transport of human recombinant brain-derived neurotrophic factor (BDNF) through the rat blood-brain barrier in vivo using vector-mediated peptide drug delivery. Pharm. Res. 1994, 11, 738–746. [Google Scholar] [CrossRef]
- Soderquist, R.G.; Milligan, E.D.; Sloane, E.M.; Harrison, J.A.; Douvas, K.K.; Potter, J.M.; Hughes, T.S.; Chavez, R.A.; Johnson, K.; Watkins, L.R.; et al. PEGylation of brain-derived neurotrophic factor for preserved biological activity and enhanced spinal cord distribution. J. Biomed. Mater. Res. A 2009, 91, 719–729. [Google Scholar] [CrossRef]
- Pan, W.; Banks, W.A.; Fasold, M.B.; Bluth, J.; Kastin, A.J. Transport of brain-derived neurotrophic factor across the blood–brain barrier. Neuropharmacology 1998, 37, 1553–1561. [Google Scholar] [CrossRef]
- Khalin, I.; Alyautdin, R.; Wong, T.W.; Gnanou, J.; Kocherga, G.; Kreuter, J. Brain-derived neurotrophic factor delivered to the brain using poly (lactide-co-glycolide) nanoparticles improves neurological and cognitive outcome in mice with traumatic brain injury. Drug. Deliv. 2016, 23, 3520–3528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Young, L.R.; Schmelzer, C.H.; Burton, L.E. A common mechanism for recombinant human NGF, BDNF, NT-3, and murine NGF slow unfolding. Protein. Sci. 1999, 8, 2513–2518. [Google Scholar] [CrossRef] [PubMed]
- Nakagomi, A.; Okada, S.; Yokoyama, M.; Yoshida, Y.; Shimizu, I.; Miki, T.; Kobayashi, Y.; Minamino, T. Role of the central nervous system and adipose tissue BDNF/TrkB axes in metabolic regulation. NPJ Aging Mech. Dis. 2015, 1, 15009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelleymounter, M.A.; Cullen, M.J.; Wellman, C.L. Characteristics of BDNF-induced weight loss. Exp. Neurol. 1995, 131, 229–238. [Google Scholar] [CrossRef]
- Kovacev, V.P.; Scow, R.O. Effect of hormones on fatty acid release by rat adipose tissue in vivo. Am. J. Physiol. 1966, 210, 1199–1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Parameter | Saline | BDNF |
|---|---|---|
| Glycerol (mg/L) | 4.9184 ± 0.6983 | 8.4047 ± 1.3931 * |
| Free fatty acids (mmol/L) | 0.3381 ± 0.0197 | 0.4234 ± 0.0198 * |
| Corticosterone (ng/mL) | 496.6319 ± 99.2159 | 403.2675 ± 116.7263 |
| Norepinephrine (ng/mL) | 7.5759 ± 3.1380 | 9.2376 ± 2.3438 |
| Epinephrine (ng/mL) | 51.5562 ± 22.5392 | 37.9994 ± 9.0009 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Q.; Liu, X.; Glazier, B.J.; Krolick, K.N.; Yang, S.; He, J.; Lo, C.C.; Shi, H. Differential Sympathetic Activation of Adipose Tissues by Brain-Derived Neurotrophic Factor. Biomolecules 2019, 9, 452. https://doi.org/10.3390/biom9090452
Zhu Q, Liu X, Glazier BJ, Krolick KN, Yang S, He J, Lo CC, Shi H. Differential Sympathetic Activation of Adipose Tissues by Brain-Derived Neurotrophic Factor. Biomolecules. 2019; 9(9):452. https://doi.org/10.3390/biom9090452
Chicago/Turabian StyleZhu, Qi, Xian Liu, Bradley J. Glazier, Kristen N. Krolick, Shangyuwen Yang, Jingyan He, Chunmin C. Lo, and Haifei Shi. 2019. "Differential Sympathetic Activation of Adipose Tissues by Brain-Derived Neurotrophic Factor" Biomolecules 9, no. 9: 452. https://doi.org/10.3390/biom9090452






