Stability and Catalase-Like Activity of a Mononuclear Non-Heme Oxoiron(IV) Complex in Aqueous Solution
Abstract
:1. Introduction
2. Results and Discussion
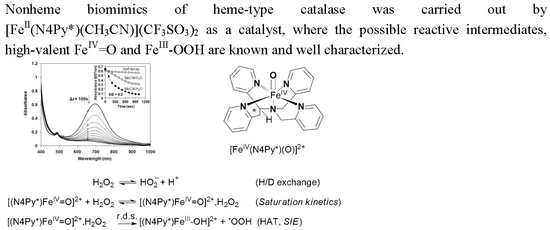
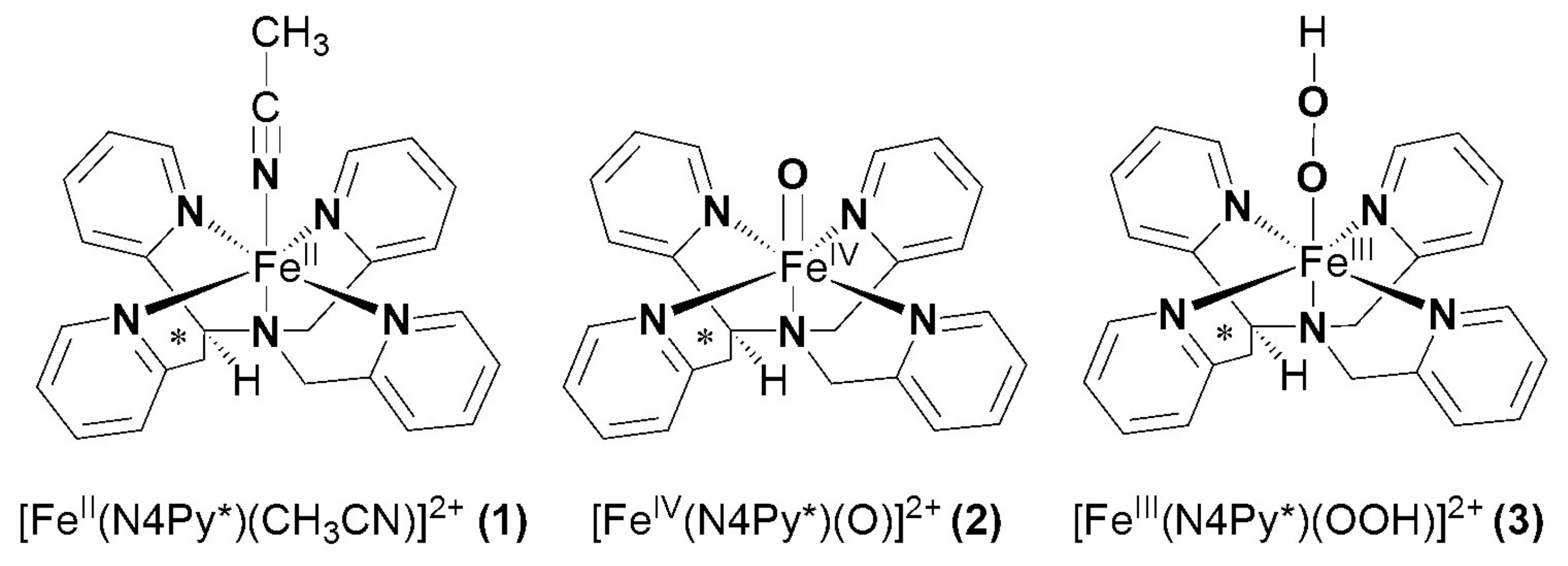
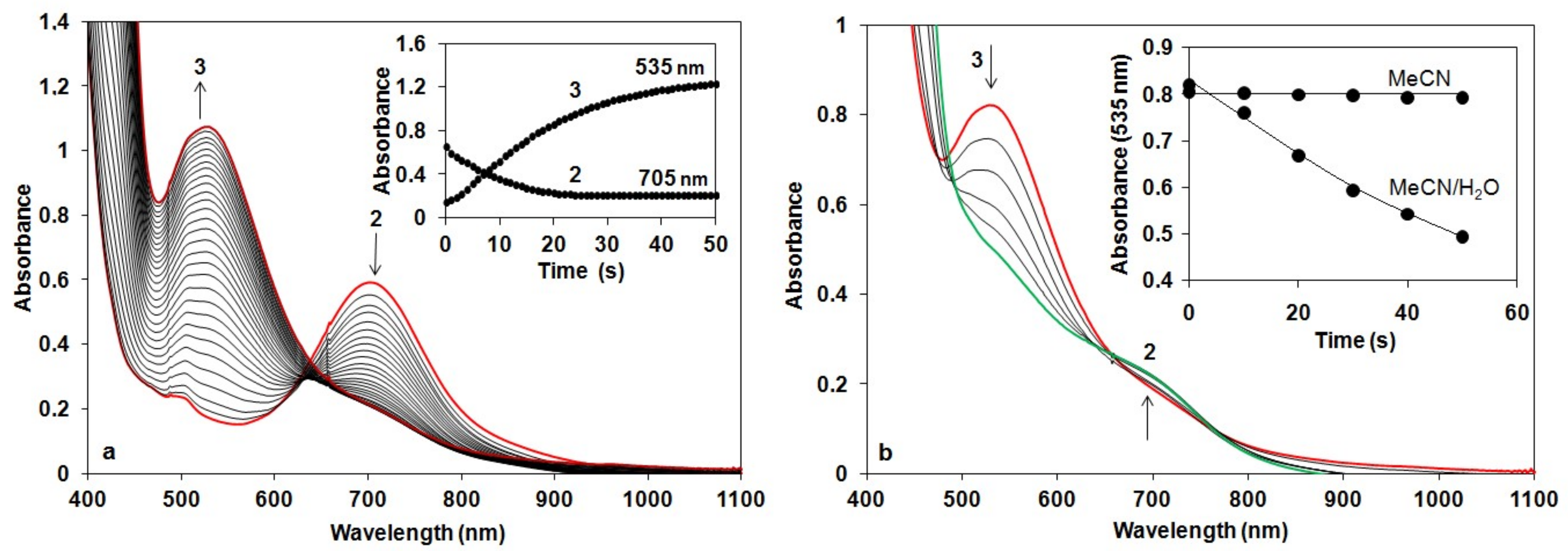
2.1. Catalase-Like Reactivity of [FeII(N4Py*)(CH3CN)](CF3SO3)2 in Aqueous Solution
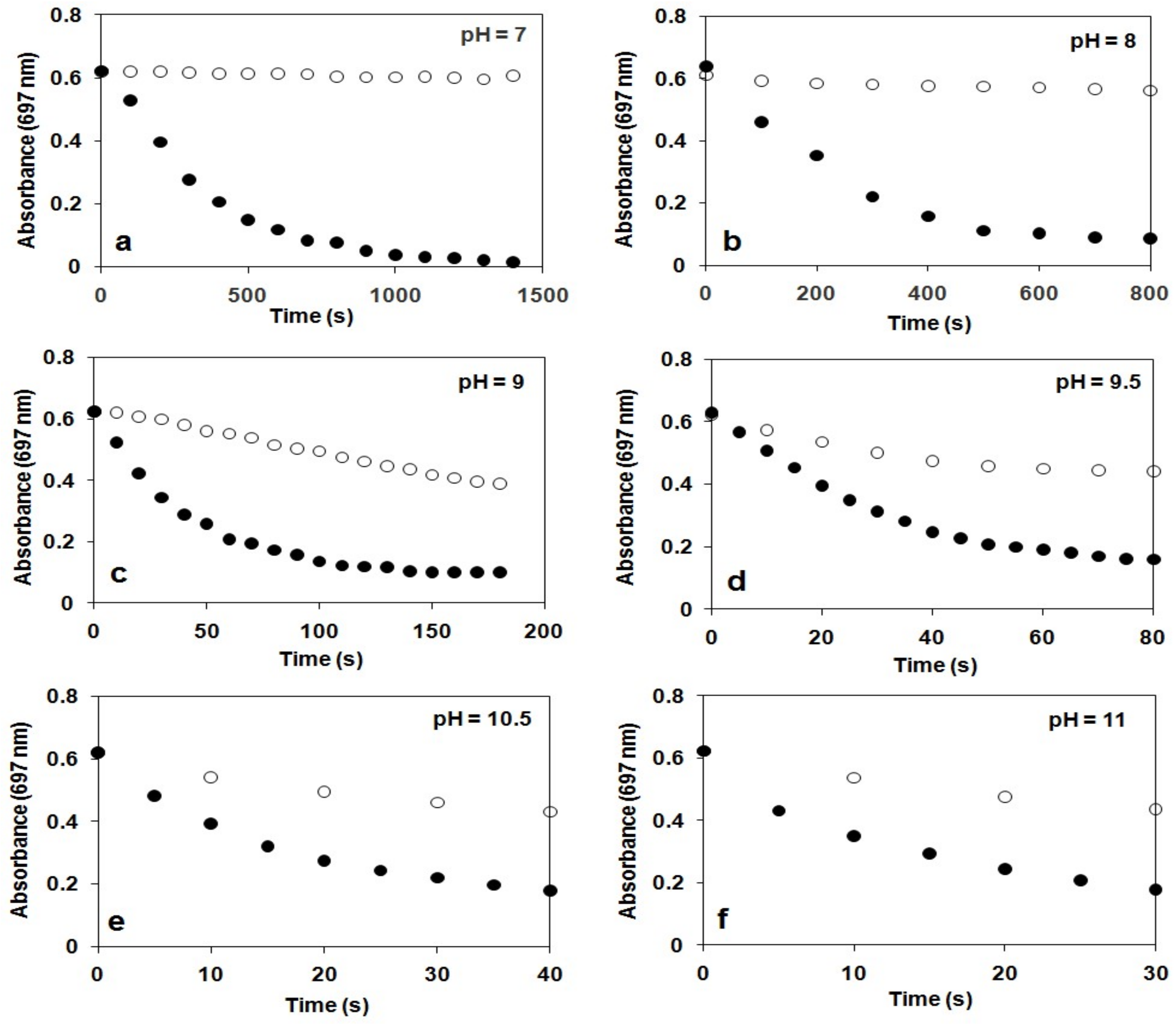
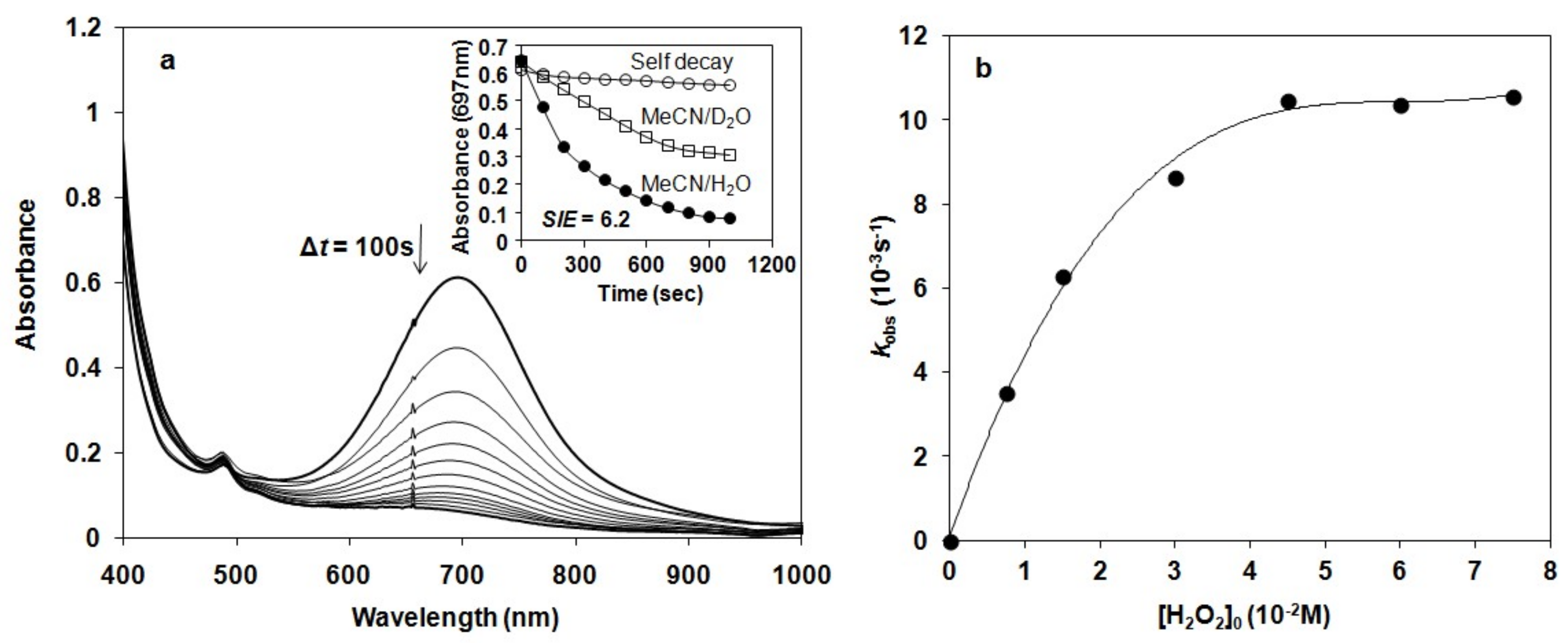
2.2. Catalase-Like Reactivity Mediated by [(N4Py*)FeIV=O](ClO4)2 in Aqueous Solution
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zamocky, M.; Furtmuller, P.G.; Obinger, C. Evolution of catalases from bacteria to humans. Antioxid. Redox Signal. 2008, 10, 1527–1548. [Google Scholar] [CrossRef]
- Kunsch, C.; Medford, R.M. Oxidative Stress as a Regulator of Gene Expression in the Vasculature. Circ. Res. 1999, 85, 753–766. [Google Scholar] [CrossRef]
- Balaban, R.S.; Nemoto, S.; Finkel, T. Mitochondria, Oxidants, and Aging. Cell 2005, 120, 483–495. [Google Scholar] [CrossRef] [Green Version]
- Giordano, F.J. Oxygen, oxidative stress, hypoxia, and heart failure. J. Clin. Invest. 2005, 115, 500–508. [Google Scholar] [CrossRef]
- Halliwell, B. Free radicals, antioxidants, and human disease: Curiosity, cause, or consequence? Lancet 1994, 344, 721–724. [Google Scholar] [CrossRef]
- Choua, S.; Pacheco, P.; Coquelet, C.; Bienvenüe, E. Catalase-like activity of a water-soluble complex of Ru(II). J. Inorg. Biochem. 1997, 65, 79–85. [Google Scholar] [CrossRef]
- Hempel, N.; Carrico, P.M.; Melendez, J.A. Manganese superoxide dismutase (Sod2) and redoxcontrol of signaling events that drive metastasis. Anticancer Agents Med. Chem. 2011, 11, 191–201. [Google Scholar] [CrossRef]
- Sampson, N.; Koziel, R.; Zenzmaier, C.; Bubendorf, L.; Plas, E.; Jansen-Durr, P.; Berger, P. ROS Signaling by NOX4 Drives Fibroblast-to-Myofibroblast Differentation in the Diseased Prostatic Stroma. Mol. Endocrinol. 2011, 25, 503–515. [Google Scholar] [CrossRef]
- Gao, M.C.; Jia, X.D.; Wu, Q.F.; Cheng, Y.; Chen, F.R.; Zhang, J. Silencing Prx1 and/or Prx5 sensitizes human esophageal cancer cells to ionizing radiation and increases apoptosis via intracellular ROS accumulation. Acta Pharmacol. Sin. 2011, 32, 528–536. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Y.; Su, Y. Peroxiredoxins, a novel target in cancer radiotherapy. Cancer Lett. 2009, 286, 154–160. [Google Scholar] [CrossRef]
- Holley, A.K.; Miao, L.; St Clair, D.K.; St Clair, W.H. Redox-Modulated Phenomena and Radiation Therapy: The Central Role of Superoxide Dismutases. Antioxid. Redox Signal. 2014, 20, 1567–1589. [Google Scholar] [CrossRef] [Green Version]
- Armogida, M.; Nistico, R.; Mercuri, N.B. Therapeutic potential of targeting hydrogen peroxide metabolism in the treatment of brain ischaemia. Br. J. Pharmacol. 2012, 166, 1211–1224. [Google Scholar] [CrossRef] [Green Version]
- Beyer, W.F.; Fridovich, I. Catalases-with and without heme. Basic Life Sci. 1988, 49, 651–661. [Google Scholar]
- Nicholls, P.; Fita, I.; Loewen, P.C. Enzymology and structure of catalases. Adv. Inorg. Chem. 2001, 51, 51–106. [Google Scholar]
- Ko, T.P.; Day, J.; Malkin, A.J.; McPherson, A. Structure of orthorhombic crystals of beef liver catalase. Acta Crystallogr. 1999, 55, 1383–1394. [Google Scholar] [CrossRef] [Green Version]
- Ivancich, A.; Jouve, H.M.; Sartor, B.; Gaillard, J. EPR investigation of compound I in Proteus mirabilis and bovin liver catalases: Formation of porphyrin and tyrosyl radical intermediates. Biochemistry 1997, 36, 9356–9364. [Google Scholar] [CrossRef]
- Kono, Y.; Fridovich, I. Isolation and characterization of the pseudocatalase of Lactobacillus plantarum. J. Biol. Chem. 1983, 258, 6015–6019. [Google Scholar]
- Barynin, V.V.; Whittaker, M.M.; Antonyuk, S.V.; Lamzin, V.S.; Harrison, P.M.; Artymiuk, P.J.; Whittaker, J.W. Crystal Structure of Manganese Catalase from Lactobacillus plantarum. Structure 2001, 9, 725–738. [Google Scholar] [CrossRef]
- Antonyuk, S.V.; Melik-Adman, V.R.; Popov, A.N.; Lamzin, V.S.; Hempstead, P.D.; Harrison, P.M.; Artymyuk, P.J.; Barynin, V.V. Three-dimensional structure of the enzyme dimanganese catalase from Thermus thermophilus at 1 Å resolution. Crystallogr. Rep. 2000, 45, 105–113. [Google Scholar] [CrossRef]
- Barynin, V.V.; Grebenko, A.I. T-catalase is nonheme catalase of the extremely thermophilic bacterium Thermus thermophilus HB8. Dokl. Akad. Nauk. USSR 1986, 286, 461–464. [Google Scholar]
- Allgood, G.S.; Perry, J.J. Characterization of a manganese-containing catalase from the obligate thermophile Thermoleophilum album. J. Bacteriol. 1986, 168, 563–567. [Google Scholar] [CrossRef]
- Amo, T.; Atomi, H.; Imanaka, T. Unique Presence of a Manganase Catalase in a Hyperthermophilic Archaeon, Pyrobaculum calidifontis VA1. J. Bacteriol. 2002, 184, 3305–3312. [Google Scholar] [CrossRef]
- Gao, J.; Martell, A.E.; Reibenspies, J.H. Novel dicopper(II) catalase-like model complexes: Synthesis, crystal structure, properties and kinetic studies. Inorg. Chim. Acta 2003, 346, 32–42. [Google Scholar] [CrossRef]
- Boelrijk, A.E.M.; Dismukes, G.C. Mechanism of Hydrogen Peroxide Dismutation by a Dimanganese Catalase Mimic: Dominant Role of an Intramolecular Base on Substrate Binding Affinity and Rate Acceleration. Inorg. Chem. 2000, 39, 3020. [Google Scholar] [CrossRef]
- Paschke, J.; Kirsch, M.; Korth, H.G.; Groot, H.; Sustmann, R. Catalase-Like Activity of a Non-Heme Dibenzotetraaza[14]annulene–Fe(III) Complex under Physiological Conditions. J. Am. Chem. Soc. 2001, 123, 11099–11100. [Google Scholar] [CrossRef]
- Okuno, T.; Ito, S.; Ohba, S.; Nishida, Y. µ-Oxo bridged diiron(III) complexes and hydrogen peroxide: Oxygenation and catalase-like activities. J. Chem. Soc. Dalton. Trans. 1997, 24, 3547–3551. [Google Scholar] [CrossRef]
- Mauerer, B.; Crane, J.; Schuler, J.; Wieghardt, K.; Nuber, B. A Hemerythrin Model Complex with Catalase Activity. Angew. Chem. Int. Ed. Engl. 1993, 32, 289–291. [Google Scholar] [CrossRef]
- Ménage, S.; Vincent, J.M.; Lambeaux, C.; Fontecave, M. µ-Oxo-bridged diiron(III) complexes and H2O2: Monooxygenase and catalase-like activities. J. Chem. Soc. Dalton Trans. 1994, 21, 2081–2084. [Google Scholar] [CrossRef]
- Sigel, H.; Wiss, K.; Fischer, B.E.; Prijs, B. Metal ions and hydrogen peroxide. Catalase-like activity of copper(2+) ion in aqueous solution and its promotion by the coordination of 2,2′-bipyridyl. Inorg. Chem. 1979, 18, 1354–1358. [Google Scholar] [CrossRef]
- Kaizer, J.; Csonka, R.; Speier, G.; Giorgi, M.; Réglier, M. Synthesis, structure and catalase-like activity of new dicopper(II) complexes with phenylglyoxylate and benzoate ligands. J. Mol. Catal. A Chem. 2005, 236, 12–17. [Google Scholar] [CrossRef]
- Kaizer, J.; Csay, T.; Speier, G.; Réglier, M.; Giorgi, M. Synthesis, structure and catalase-like activity of Cu(N-baa)(2)(phen) (phen=1, 10-phenanthroline, N-baaH = N-benzoylanthranilic acid). Inorg. Chem. Commun. 2006, 9, 1037–1039. [Google Scholar] [CrossRef]
- Pap, J.S.; Horvath, B.; Speier, G.; Kaizer, J. Synthesis and catalase-like activity of dimanganese complexes with phthalazine-based ligands. Transit. Met. Chem. 2011, 36, 603–609. [Google Scholar] [CrossRef]
- Pap, J.S.; Kripli, B.; Bors, I.; Bogáth, D.; Giorgi, M.; Kaizer, J.; Speier, G. Transition metal complexes bearing flexible N-3 or N3O donor ligands: Reactivity toward superoxide radical anion and hydrogen peroxide. J. Inorg. Biochem. 2012, 117, 60–70. [Google Scholar] [CrossRef]
- Kaizer, J.; Csay, T.; Kovari, P.; Speier, G.; Parkanyi, L. Catalase mimics of a manganese(II) complex: The effect of axial ligands and pH. J. Mol. Catal. A Chem. 2008, 280, 203–209. [Google Scholar] [CrossRef]
- Kaizer, J.; Kripli, B.; Speier, G.; Parkanyi, L. Synthesis, structure, and catalase-like activity of a novel manganese(II) complex: Dichloro[1,3-bis(2 ‘-benzimidazolylimino) isoindoline] manganese(II). Polyhedron 2009, 28, 933–936. [Google Scholar] [CrossRef]
- Horn, A., Jr.; Parrilha, G.I.; Melo, K.V.; Fernandes, C.; Horner, M.; Visentin, I.C.; Santos, J.A.S.; Santos, M.S.; Eleutherio, E.C.A.; Pereira, M.D. An iron-based cytosolic catalase and superoxide dismutase mimic complex. Inorg. Chem. 2010, 49, 1274–1276. [Google Scholar] [CrossRef]
- Carvalho, N.M.F.; Horn, A., Jr.; Faria, R.B.; Bortoluzzi, A.J.; Drago, V.; Antunes, O.A.C. Synthesis, characterization, X-ray molecular structure and catalase-like activity of a non-heme iron complex: Dichloro[N-propanoate-N,N-bis-(2-pyridylmethyl)amine] iron(III). Inorg. Chim. Acta 2006, 359, 4250–4258. [Google Scholar] [CrossRef]
- Dickman, M.H.; Pope, M.T. Peroxo and Superoxo Complexes of Chromium, Molybdenum, and Tungsten. Chem. Rev. 1994, 94, 569–584. [Google Scholar] [CrossRef]
- Wu, A.J.; Penner-Hahn, J.E.; Pecoraro, V.L. Structural, Spectroscopic, and Reactivity Models for the Manganese Catalases. Chem. Rev. 2004, 104, 903–938. [Google Scholar] [CrossRef]
- Gilbert, J.; Roecker, L.; Meyer, T.J. Hydrogen Atom Transfer in the Oxidation of Hydrogen Peroxide by [(bpy) 2(py)Ru IV=O] 2+ and by [(bpy) 2(py)Ru III-OH] 2+. Inorg. Chem. 1987, 26, 1126. [Google Scholar] [CrossRef]
- Crans, D.C.; Smee, J.J.; Gaidamauskas, E.; Yang, L. The Chemistry and Biochemistry of Vanadium and the Biological Activities Exerted by Vanadium Compounds. Chem. Rev. 2004, 104, 849–902. [Google Scholar] [CrossRef]
- Pires, B.M.; Silva, D.M.; Visentin, L.C.; Drago, V.; Carvalho, N.M.F.; Faria, R.B.; Antunes, O.A.C. Synthesis, characterization and catalase-like activity of the tetranuclear iron(III) complex involving a (μ-oxo)(μ-hydroxo)bis(μ-alkoxo)tetra(μ-carboxylato)tetrairon core. Inorg. Chim. Acta 2013, 407, 69–81. [Google Scholar] [CrossRef]
- Lakk-Bogáth, D.; Csonka, R.; Speier, G.; Reglier, M.; Simaan, A.J.; Naubron, J.V.; Giorgi, M.; Lazar, K.; Kaizer, J. Formation, Characterization, and Reactivity of Nonheme Iron(IV)-Oxo Complex Derived from the Chiral Pentadentate Ligand asN4Py. Inorg. Chem. 2016, 55, 10090. [Google Scholar] [CrossRef]
- Turcas, R.; Lakk-Bogáth, D.; Speier, G.; Kaizer, J. Steric Control and Mechanism of Benzaldehyde Oxidation by Polypyridyl Oxoiron(IV) Complexes: Aromatic versus Benzylic Hydroxylation of Aromatic Aldehydes. Dalton. Trans. 2018, 47, 3248. [Google Scholar] [CrossRef]
- Lakk-Bogáth, D.; Kripli, B.; Meena, B.I.; Speier, G.; Kaizer, J. Catalytic and stoichiometric oxidation of N,N-dimethylanilines mediated by nonheme oxoiron(IV) complex with tetrapyridyl ligand. Polyhedron 2019, 169, 169–175. [Google Scholar] [CrossRef]
- Lakk-Bogáth, D.; Kripli, B.; Meena, B.I.; Speier, G.; Kaizer, J. Catalytic and stoichiometric C-H oxidation of benzylalcohols and hydrocarbons mediated by nonheme oxoiron(IV) complex with chiral tetrapyridyl ligand. Inorg. Chem. Commun. 2019, 104, 165–170. [Google Scholar] [CrossRef]
- Kubota, R.; Imamura, S.; Shimizu, T.; Asayama, S.; Kawakami, H. Synthesis of Water-Soluble Dinuclear Mn-Porphyrin with Multiple Antioxidative Activities. ACS Med. Chem. Lett. 2014, 5, 639–643. [Google Scholar] [CrossRef] [Green Version]
- Jakopitsch, C.; Vlasits, J.; Wiseman, B.; Loewen, P.C.; Obinger, C. Redox Intermediates in the Catalase Cycle of Catalase-Peroxidases from Synechocystis PCC 6803, Burkholderia pseudomallei, and Mycobacterium tuberculosis. Biochemistry 2007, 46, 1183–1193. [Google Scholar] [CrossRef]
- Gelasco, A.; Bensiek, S.; Pecoraro, V.L. The [Mn2(2-OHsalpn)2]2-,1-,0 System: An Efficient Functional Model for the Reactivity and Inactivation of the Manganese Catalases. Inorg. Chem. 1998, 37, 3301–3309. [Google Scholar] [CrossRef]
- Larson, E.J.; Pecoraro, V.L. [Mn(III)(2-OHsalpn)]2 is an efficient functional model for the manganese catalases. J. Am. Chem. Soc. 1993, 115, 7928–7929. [Google Scholar]
- Kaizer, J.; Baráth, G.; Speier, G.; Réglier, M.; Giorgi, M. Synthesis, structure and catalase mimics of novel homoleptic manganese(II) complexes of 1,3-bis(2’-pyridylimino) isoindoline, Mn(4R-ind)2 (R= H., Me). Inorg. Chem. Commun. 2007, 10, 292–294. [Google Scholar] [CrossRef]
- Signorella, S.; Palopoli, C.; Ledesma, G. Rationally designed mimics of antioxidant manganoenzymes: Role of structural features in the quest for catalysts with catalaseand superoxide dismutase activity. Coord. Chem. Rev. 2018, 365, 75–102. [Google Scholar] [CrossRef]
- Chance, B.; Greenstein, D.S.; Roughton, F.J. The mechanism of catalase action. I. Steady-state analysis. Arch. Biochem. Biophys. 1952, 37, 301–321. [Google Scholar] [CrossRef]
- Braymer, J.J.; O’Neill, K.P.; Rohde, J.U.; Lim, M.H. The Reaction of a High-Valent Nonheme Oxoiron(IV) Intermediate with Hydrogen Peroxide. Angew. Chem. Int. Ed. 2012, 51, 1–6. [Google Scholar] [CrossRef]
- Chen, J.; Draksharapu, A.; Angelone, D.; Unjaroen, D.; Padamati, S.K.; Hage, R.; Swart, M.; Duboc, C.; Browne, W.R. H2O2 Oxidation by FeIII-OOH Intermediates and Its Effect on Catalytic Efficiency. ACS Catal. 2018, 8, 9665–9674. [Google Scholar] [CrossRef]
- Sastri, C.V.; Seo, M.S.; Park, M.J.; Kim, K.M.; Nam, W. Formation, stability, and reactivity of a mononuclear nonheme oxoiron(IV) complex in aqueous solution. Chem. Commun. 2005, 1405–1407. [Google Scholar] [CrossRef]
- Gilbert, J.A.; Gersten, S.W.; Meyer, T.J. H-D Kinetic Isotope Effects of 16 and 22 in the Oxidation of H2O2. J. Am. Chem. Soc. 1982, 104, 6872–6873. [Google Scholar] [CrossRef]


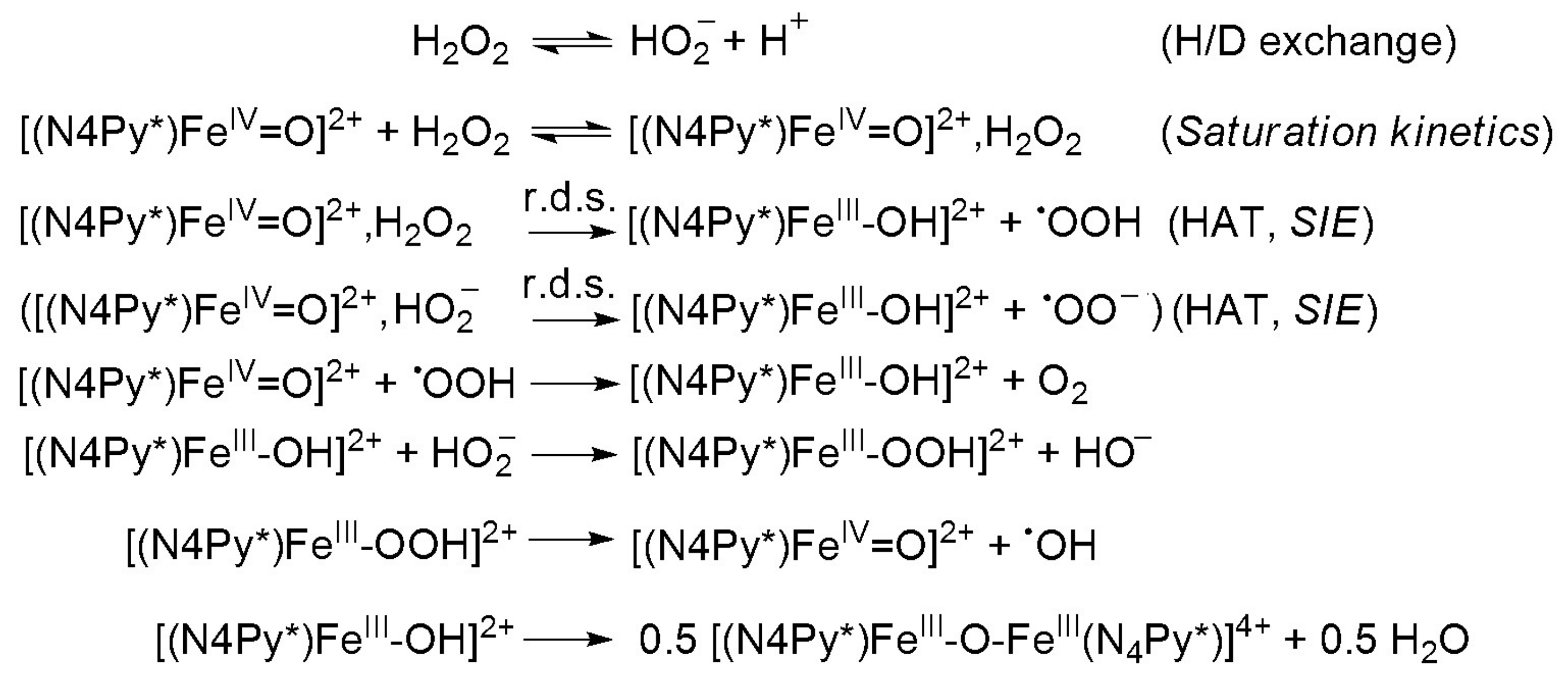
| Entry | Complex/Enzyme | KM (M) | kcat (s−1) | kcat/KM (s−1M−1) | Solvent | Refs. |
|---|---|---|---|---|---|---|
| 1 | SynKatG 1 | 0.0042 | H2O, pH 7 | [48] | ||
| 2 | BpKatG 2 | 0.0059 | H2O, pH 7 | [48] | ||
| 3 | MtbKatG 3 | 0.0025 | 1.2 × 103 | 5 × 108 | H2O, pH 7 | [48] |
| 4 | BLC 4 | 0.093 | 4.0 × 107 | H2O, pH 7 | [53] | |
| 5 | [FeII(N4Py*)(CH3CN)](ClO4)2 | 1.39 | 33.2 | 23.9 | H2O, pH 9.5 | this work. |
| 6 | [(N4Py*)FeIV=O](ClO4)2 | 0.018 | 0.014 | 0.754 | CH3CN/H2O, pH 8 | this work |
| 7 | [Fe4(μ -O) μ-OH)(μ-OAc)4(L2)]3+, 5 | 1.010 | 1.41 × 10−4 | 1.40 × 10−4 | H2O | [42] |
| 8 | [Fe4(μ-O) μ-OH)(μ-OAc)4(L2)]3+, 5 | 2.882 | 3.50 × 10−3 | 1.21 × 10−3 | H2O, pH 7.2 | [42] |
| 9 | [Fe4(μ-O) μ-OH)(μ-OAc)4(L2)]3+, 5 | 0.749 | 5.37 × 10−2 | 7.17 × 10−2 | CH3CN | [42] |
| 10 | T. thermophilus | 0.083 | 2.6 × 105 | 3.13 × 106 | H2O | [19,20] |
| 11 | T. album | 0.015 | 2.6 × 104 | 1.73 × 106 | H2O | [21] |
| 12 | L. plantarum | 0.35 | 2.0 × 105 | 0.57 × 106 | H2O | [17,18] |
| 13 | [Mn(indH)Cl2] 6 | 0.49 | 38.9 | 79.2 | H2O, pH 9.5 | [30] |
| 14 | [Mn(ind)2] 6 | 0.019 | 0.06 | 3.2 | DMF | [51] |
| 15 | [Mn(X-salpn)O]2 7 | 10–102 | 4.2–21.9 | 305–990 | CH3CN | [49,50] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kripli, B.; Sólyom, B.; Speier, G.; Kaizer, J. Stability and Catalase-Like Activity of a Mononuclear Non-Heme Oxoiron(IV) Complex in Aqueous Solution. Molecules 2019, 24, 3236. https://doi.org/10.3390/molecules24183236
Kripli B, Sólyom B, Speier G, Kaizer J. Stability and Catalase-Like Activity of a Mononuclear Non-Heme Oxoiron(IV) Complex in Aqueous Solution. Molecules. 2019; 24(18):3236. https://doi.org/10.3390/molecules24183236
Chicago/Turabian StyleKripli, Balázs, Bernadett Sólyom, Gábor Speier, and József Kaizer. 2019. "Stability and Catalase-Like Activity of a Mononuclear Non-Heme Oxoiron(IV) Complex in Aqueous Solution" Molecules 24, no. 18: 3236. https://doi.org/10.3390/molecules24183236