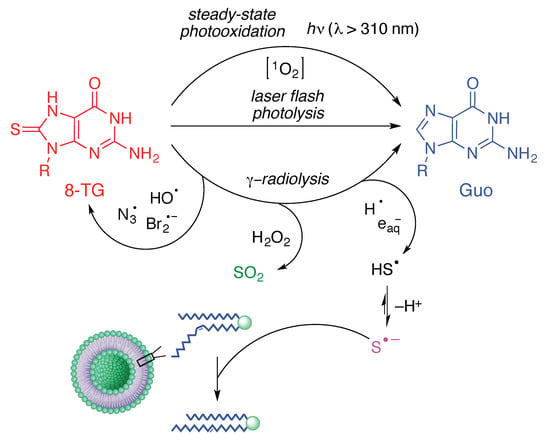
Converging Fate of the Oxidation and Reduction of 8-Thioguanosine
Abstract
1. Introduction
2. Results and Discussion
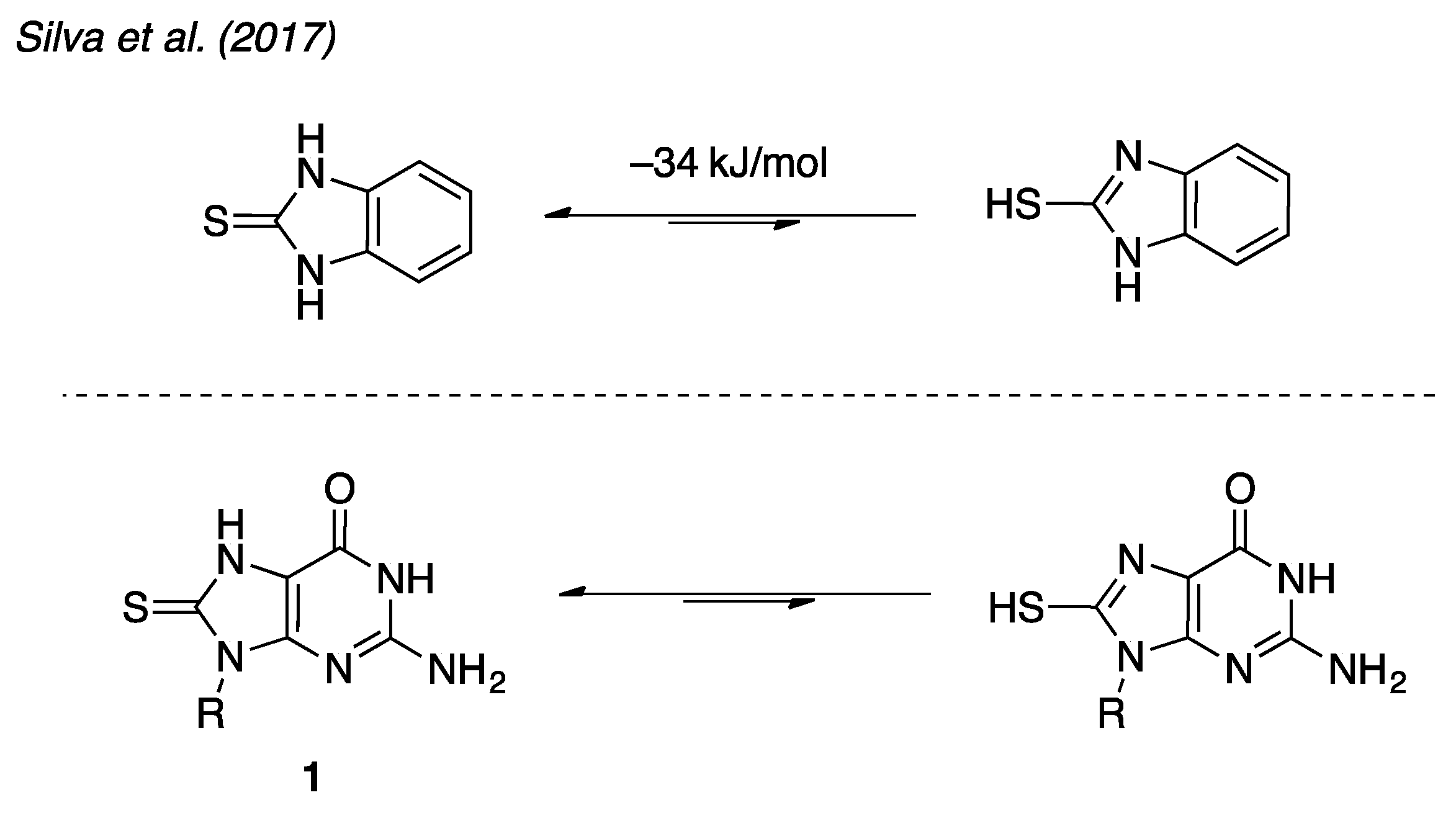
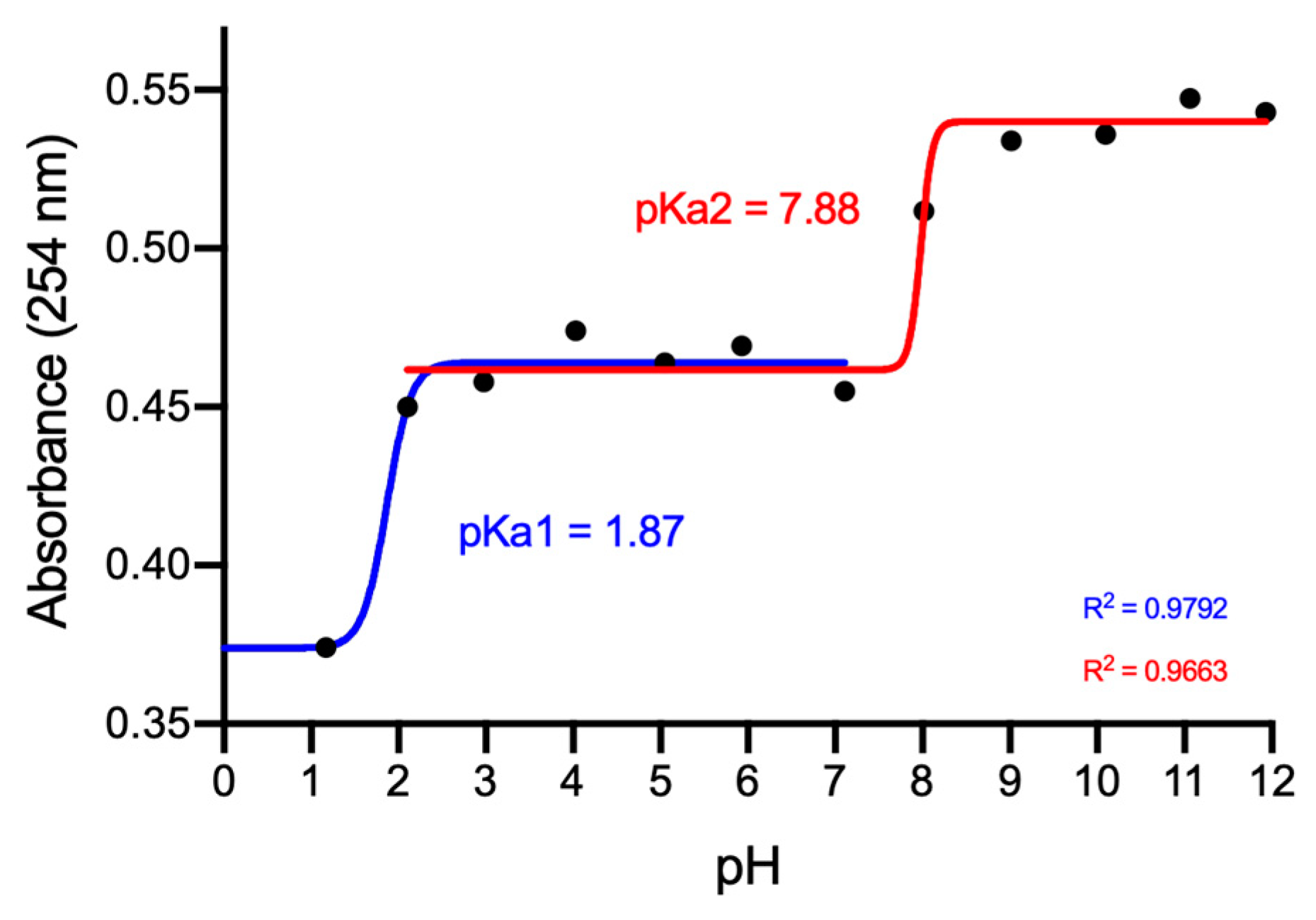
2.1. Structural Characterization of 8-Thioguanosine (8-TG)
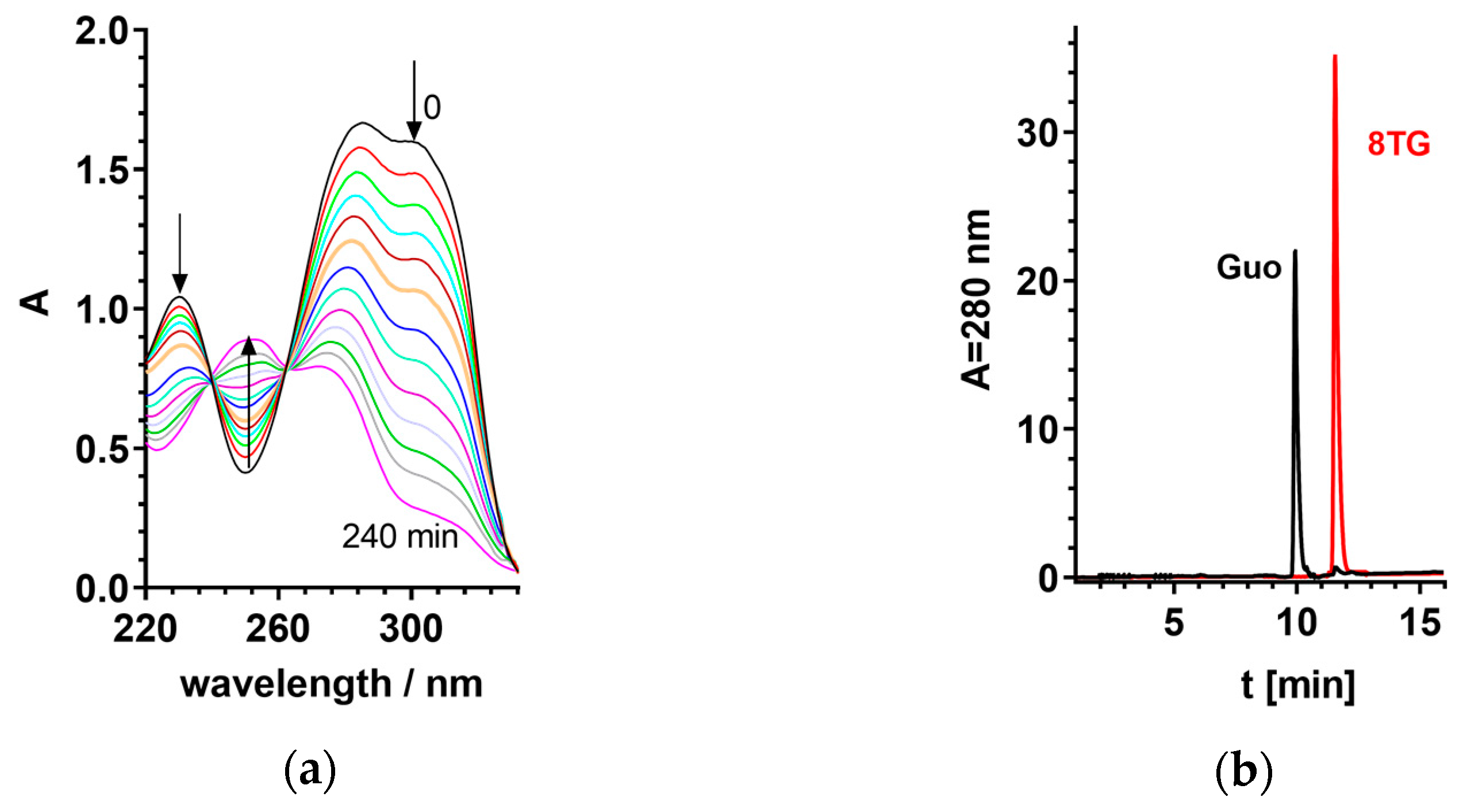
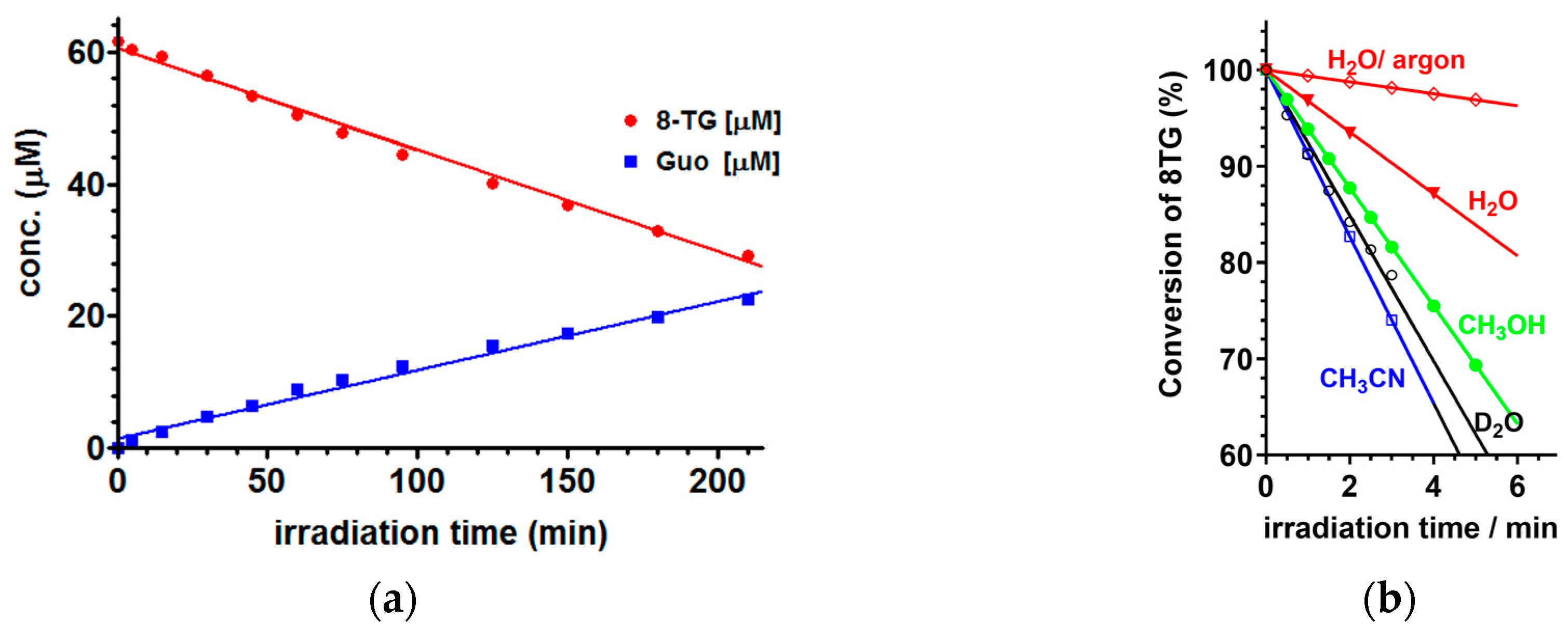
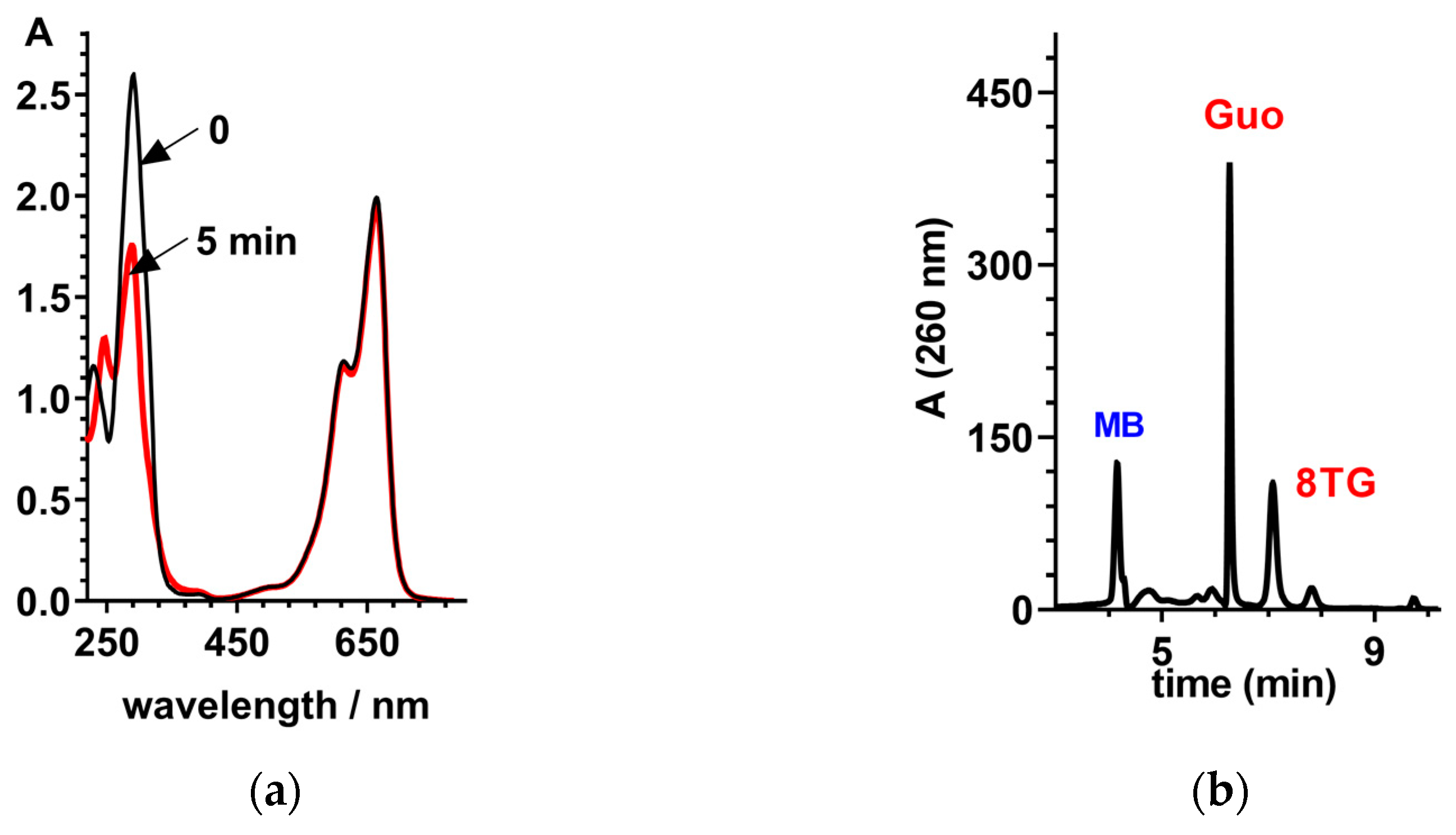
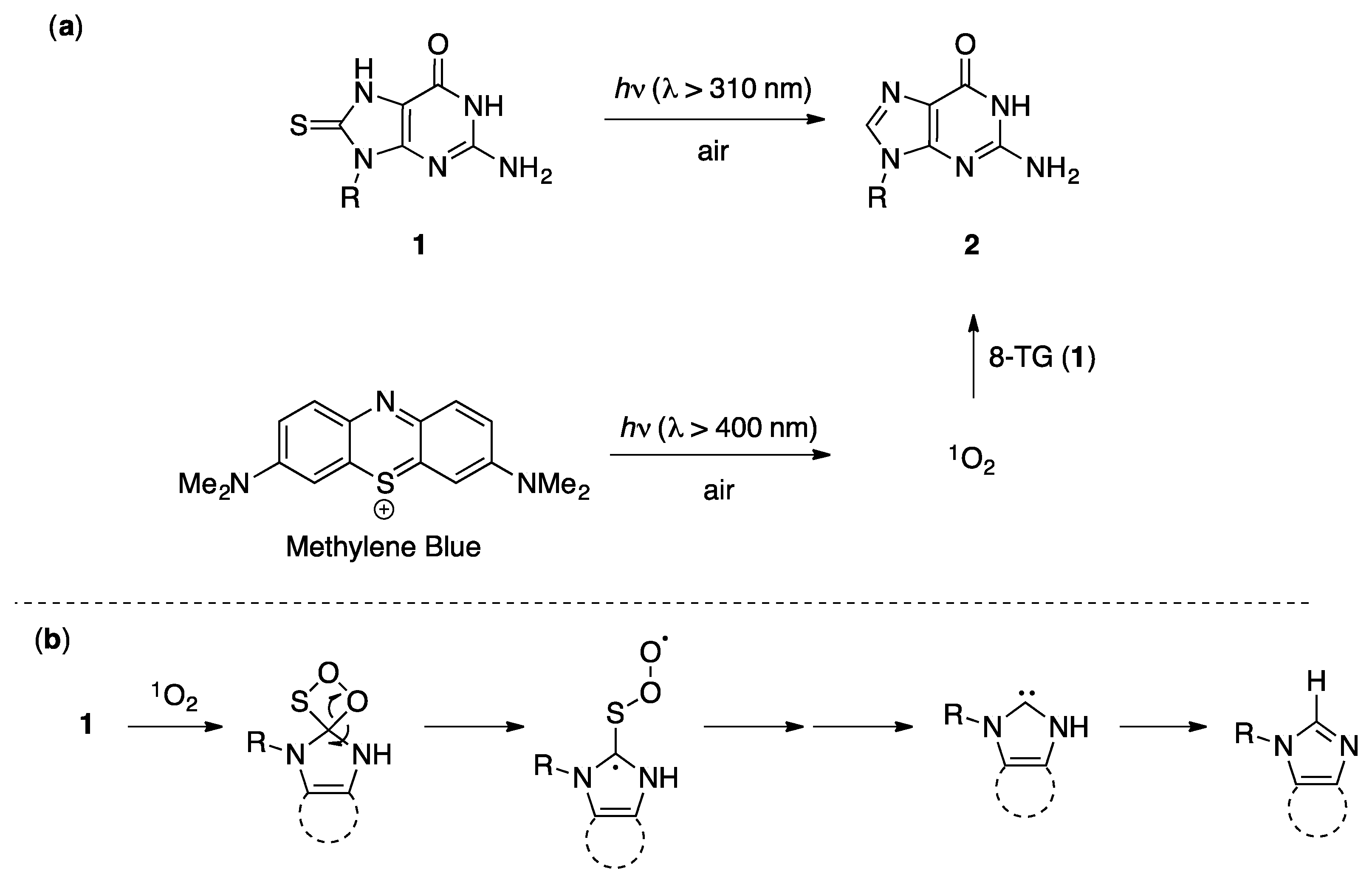
2.2. Steady-State Photochemistry
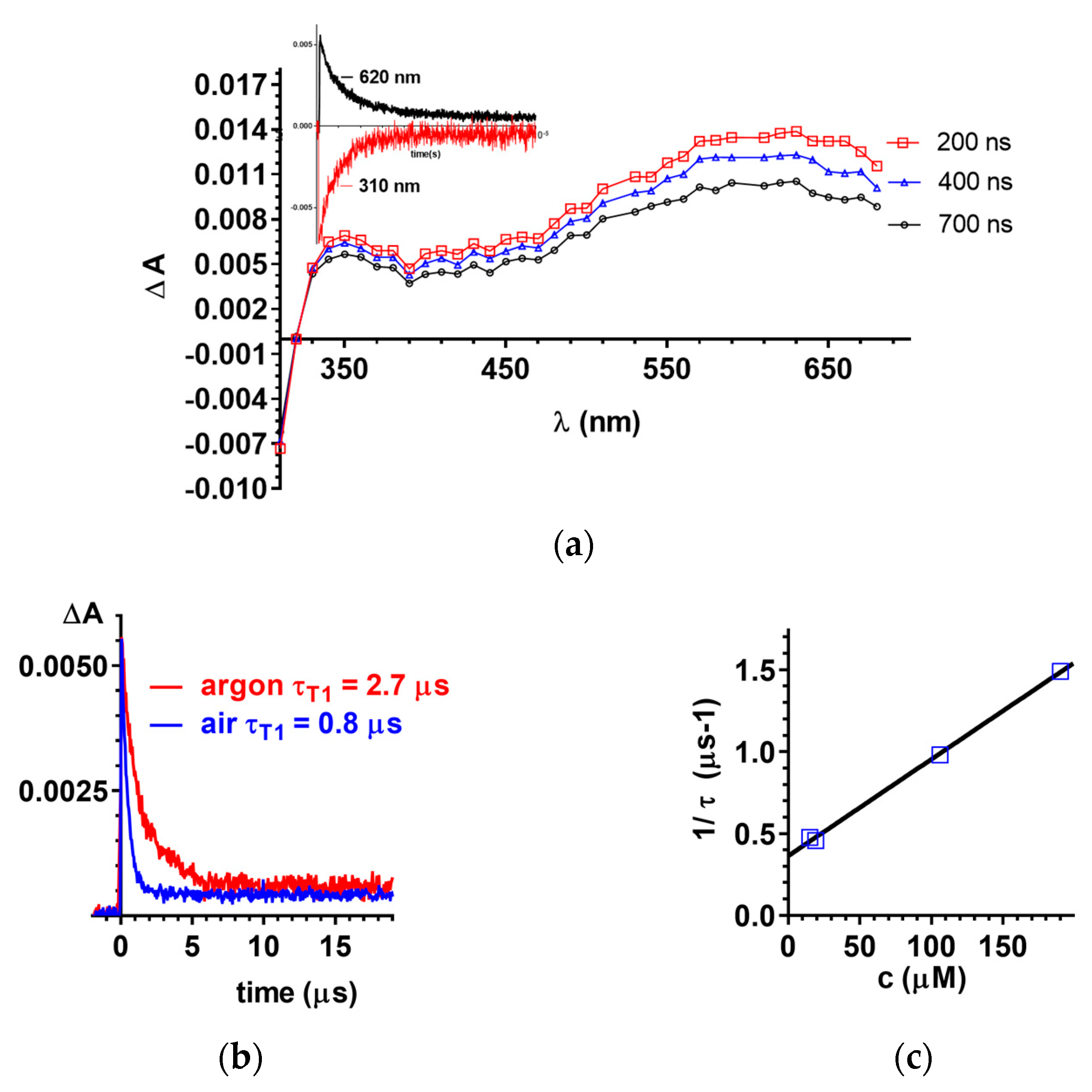
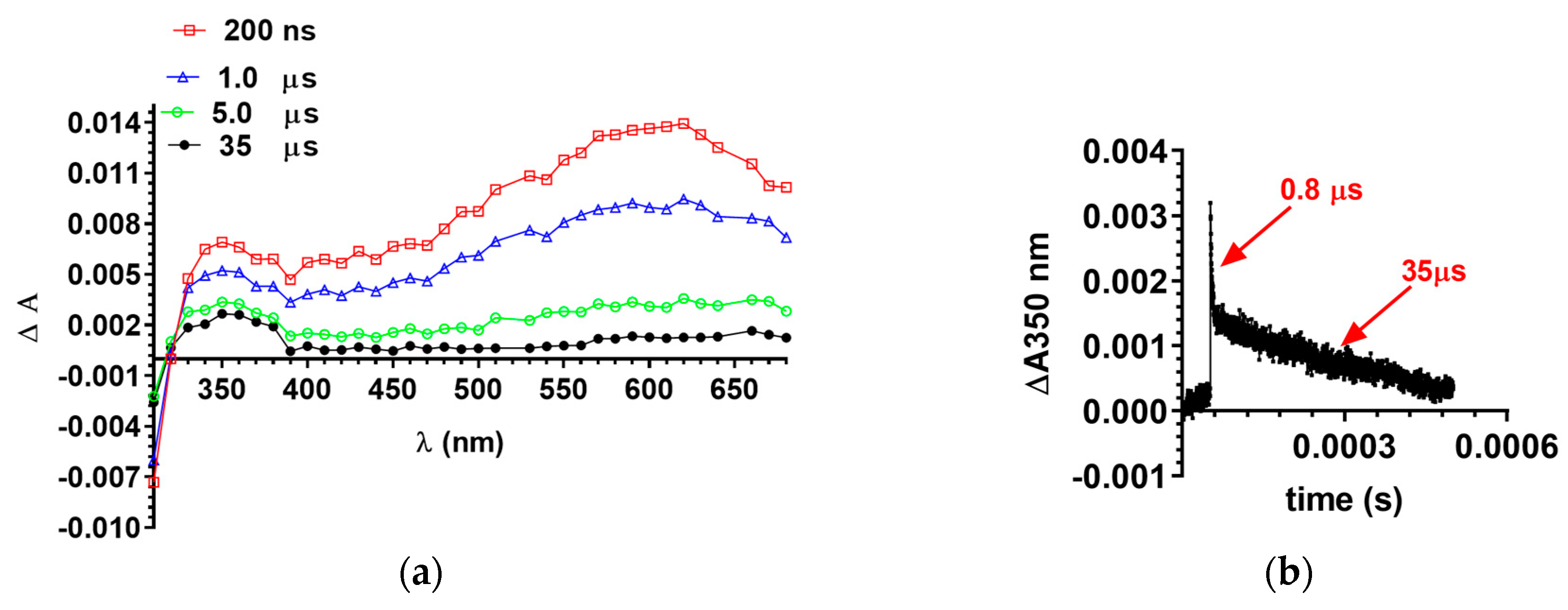
2.3. Laser Flash Photolysis
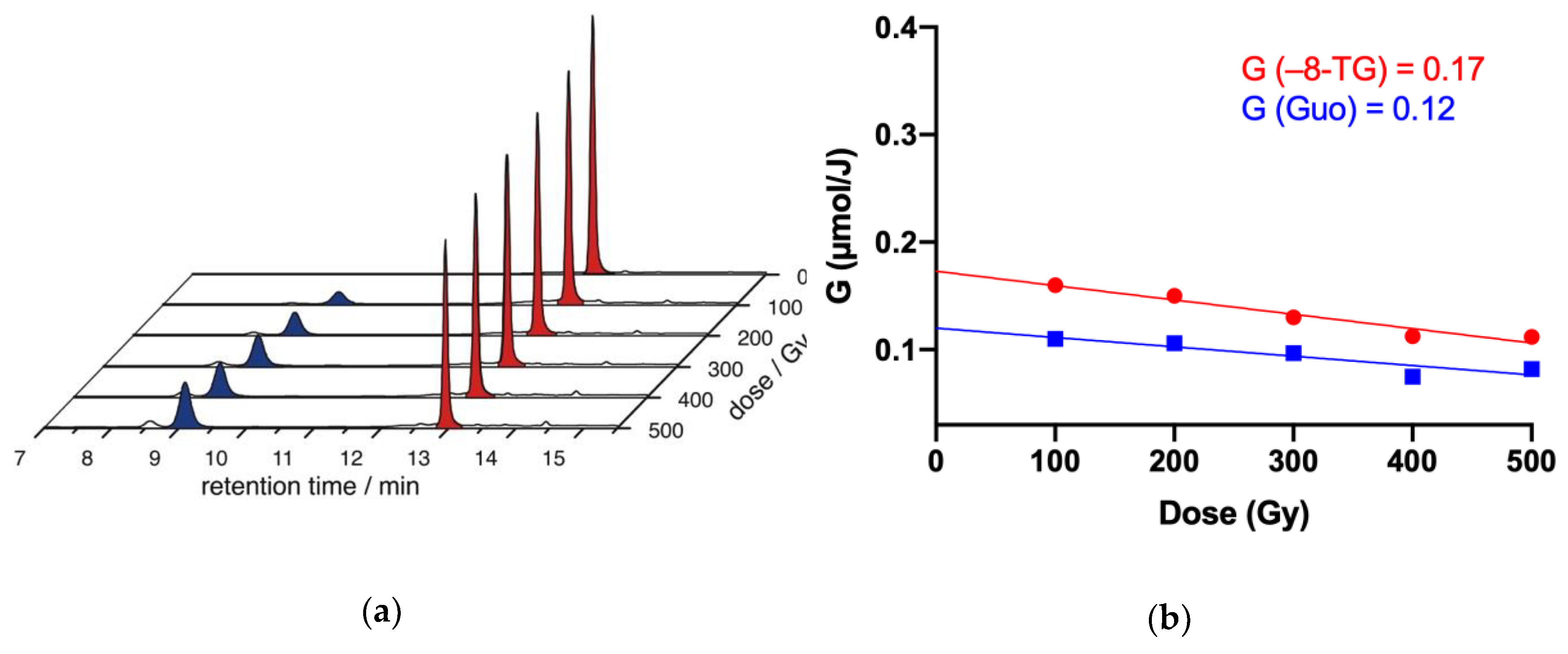
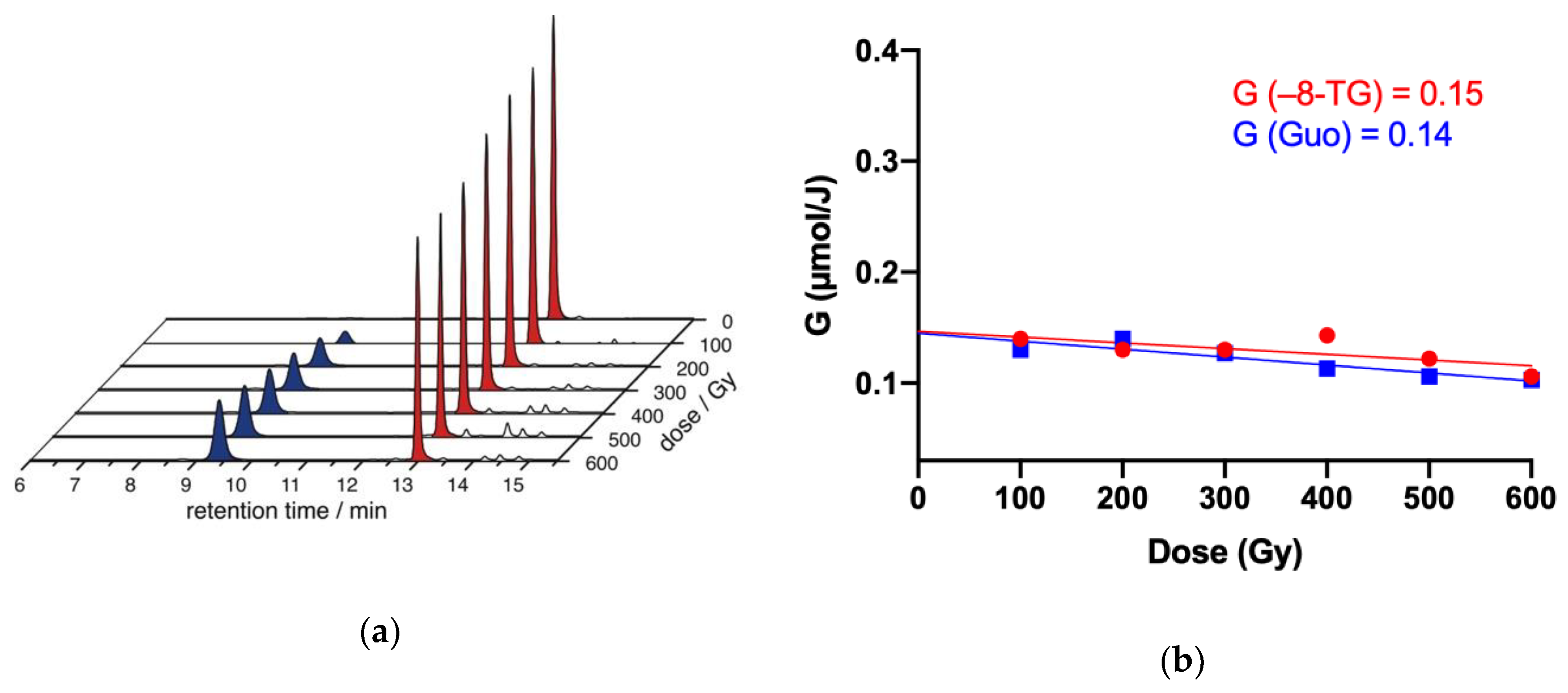
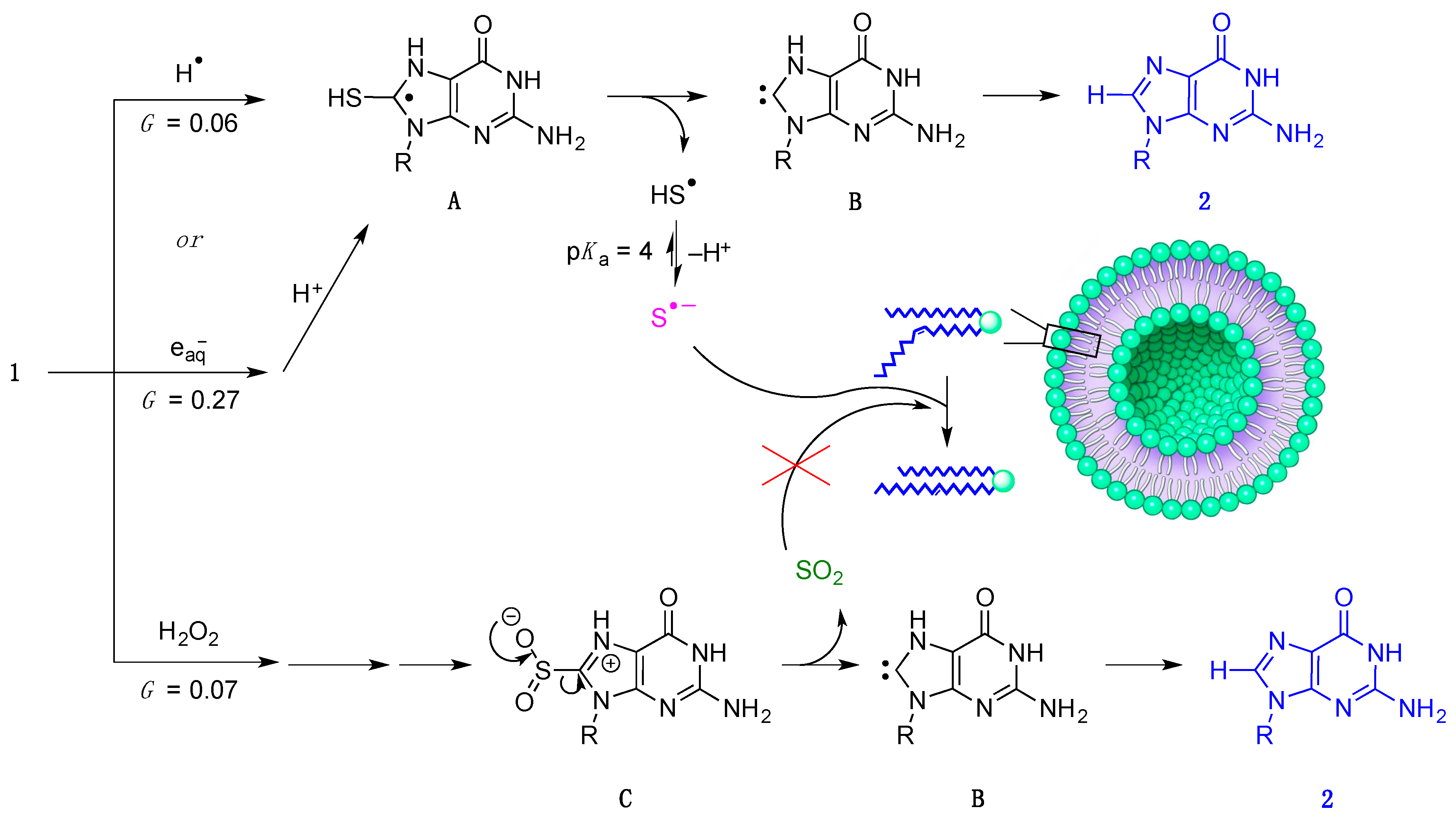
2.4. γ-Radiolysis of 8-TG in Aqueous Solutions
2.4.1. γ-radiolysis of 8-TG in Aqueous Solutions under Reductive Conditions
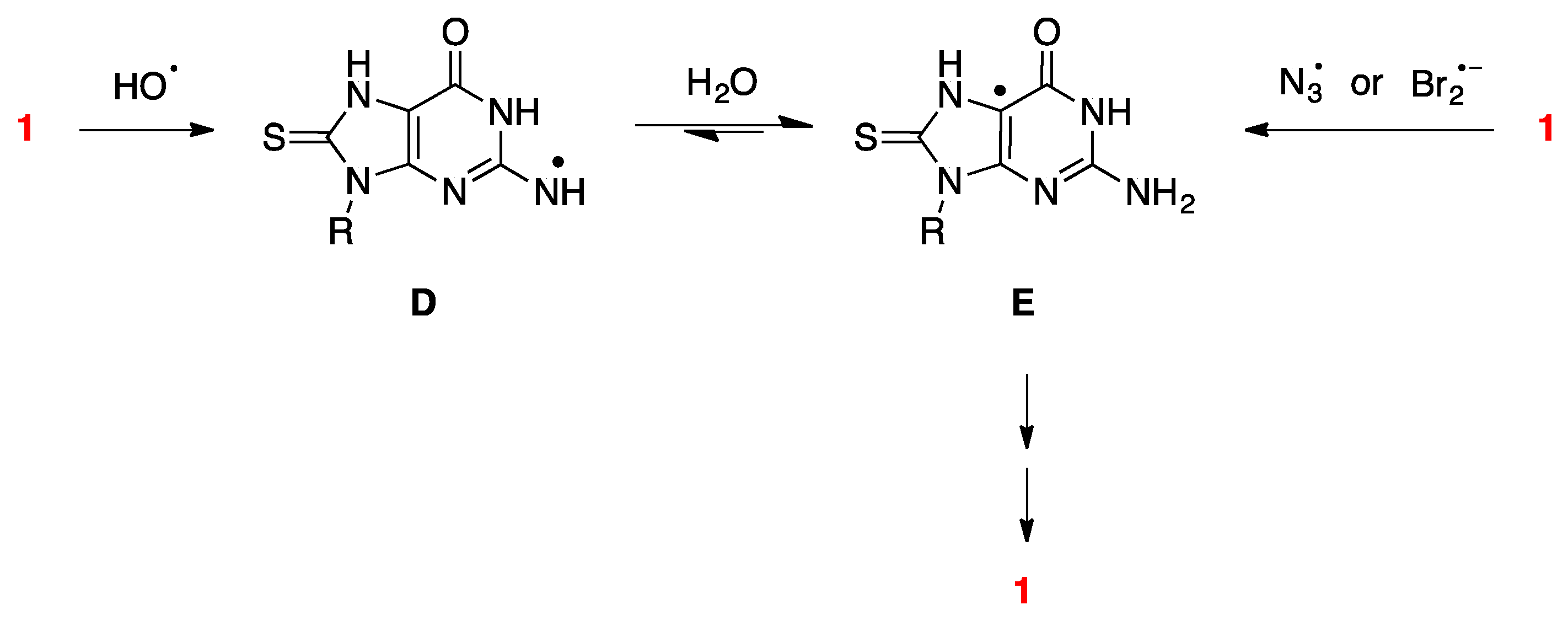
2.4.2. The Role of HO• Radicals and Other Oxidants
3. Materials and Methods
3.1. Materials
3.2. UV-Vis Absorption Spectra
3.3. HPLC-MS Analysis
3.4. Steady-State Irradiation
3.5. Laser Flash Photolysis (LFP) Experiments
3.6. Experiments in Gammacell
3.7. Preparation of 1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) Liposomes
3.8. GC Analysis of Fatty Acid Methyl Esters
3.9. NMR Analysis of 8-TG
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 8-TG | 8-thioguanosine |
| 6-TG | 6-thioguanosine |
| ROS | reactive oxygen species |
| Guo/G | guanosine |
| FAME | fatty acid methyl ester |
| HPLC-MS | high performance liquid chromatography mass spectrometry |
| GC | gas chromatography |
| POPC | 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine |
| MB | Methylene Blue |
| RB | Rose Bengal |
| 8-oxo-G | 8-oxo-guanosine |
References
- De Clercq, E. Antiviral drug discovery: Ten more compounds, and ten more stories (part B). Med. Res. Rev. 2009, 29, 571–610. [Google Scholar] [CrossRef] [PubMed]
- Cantara, W.A.; Crain, P.F.; Rozenski, J.; McCloskey, J.A.; Harris, K.A.; Zhang, X.; Vendeix, F.A.P.; Fabris, D.; Agris, P.F. The RNA modification database, RNAMDB: 2011 update. Nucleic Acids Res. 2010, 39, D195–D201. [Google Scholar] [CrossRef] [PubMed]
- Elion, G.B. The purine path to chemotherapy. Vitro Cell. Dev. Biol. 1989, 25, 321–330. [Google Scholar] [CrossRef]
- Dervieux, T.; Blanco, J.G.; Krynetski, E.Y.; Vanin, E.F.; Roussel, M.F.; Relling, M.V. Differing contribution of thiopurine methyltransferase to mercaptopurine versus thioguanine effects in human leukemic cells. Cancer Res. 2001, 61, 5810. [Google Scholar] [PubMed]
- Karran, P.; Attard, N. Thiopurines in current medical practice: Molecular mechanisms and contributions to therapy-related cancer. Nat. Rev. Cancer 2008, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Massey, A.; Xu, Y.-Z.; Karran, P. Photoactivation of DNA thiobases as a potential novel therapeutic option. Curr. Biol. 2001, 11, 1142–1146. [Google Scholar] [CrossRef]
- Attard, N.R.; Karran, P. UVA photosensitization of thiopurines and skin cancer in organ transplant recipients. Photochem. Photobiol. Sci. 2012, 11, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Ashwood, B.; Pollum, M.; Crespo-Hernández, C.E. Photochemical and photodynamical properties of sulfur-substituted nucleic acid bases. Photochem. Photobiol. 2019, 95, 33–58. [Google Scholar] [CrossRef] [PubMed]
- Favre, A.; Saintomé, C.; Fourrey, J.L.; Clivio, P.; Laugâa, P. Thionucleobases as intrinsic photoaffinity probes of nucleic acid structure and nucleic acid-protein interactions. J. Photochem. Photobiol. B Biol. 1998, 42, 109–124. [Google Scholar] [CrossRef]
- Brem, R.; Daehn, I.; Karran, P. Efficient DNA interstrand crosslinking by 6-thioguanine and UVA radiation. DNA Repair 2011, 10, 869–876. [Google Scholar] [CrossRef]
- Brem, R.; Karran, P. Reactive oxygen-mediated damage to a human DNA replication and repair protein. Cancer Res. 2012, 77, 4787–4797. [Google Scholar] [CrossRef] [PubMed]
- Pollum, M.; Ortiz-Rodrıguez, L.A.; Jockusch, S.; Crespo-Hernández, C.E. The triplet state of 6-thio-2′-deoxyguanosine: Intrinsic properties and reactivity toward molecular oxygen. Photochem. Photobiol. 2016, 92, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Brem, R.; Guven, M.; Karran, P. Oxidatively-generated damage to DNA and proteins mediated by photosensitized UVA. Free Radic. Biol. Med. 2017, 107, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Goodman, M.G. Mechanism of synergy between T cell signals and C8-substituted guanine nucleosides in humoral immunity: B lymphotropic cytokines induce responsiveness to 8-mercaptoguanosine. J. Immunol. 1986, 136, 3335. [Google Scholar] [PubMed]
- Silva, A.L.R.; Ribeiro da Silva, M.D.M.C. Energetic, structural and tautomeric analysis of 2-mercaptobenzimidazole. J. Therm. Anal. Calorim. 2017, 129, 1679–1688. [Google Scholar] [CrossRef]
- Sahu, S.; Rani Sahoo, P.; Patel, S.; Mishra, B.K. Oxidation of thiourea and substituted thioureas: A review. J. Sulfur Chem. 2011, 32, 171–197. [Google Scholar] [CrossRef]
- Shakeel, A.; Altaf, A.A.; Qureshi, A.M.; Badshah, A. Thiourea derivatives in drug design and medicinal chemistry: A short review. J. Drug Des. Med. Chem. 2016, 2, 10. [Google Scholar] [CrossRef]
- Pandey, M.; Srivastava, A.K.; D’Souza, S.F.; Penna, S. Thiourea, a ROS scavenger, regulates source-to-sink relationship to enhance crop yield and oil content in Brassica juncea (L.). PLoS ONE 2013, 8, e73921. [Google Scholar] [CrossRef]
- Lou, E.K.; Cathro, P.; Marino, V.; Damiani, F.; Heithersay, G.S. Evaluation of hydroxyl radical diffusion and acidified thiourea as a scavenger during intracoronal bleaching. J. Endod. 2016, 42, 1126–1130. [Google Scholar] [CrossRef]
- Grivas, S.; Ronne, E. Facile desulfurization of cyclic thioureas by hydrogen peroxide in acetic acid. Acta Chem. Scand. 1995, 49, 225–229. [Google Scholar] [CrossRef][Green Version]
- Kuhn, N.; Kratz, T. Synthesis of imidazol-2-ylidenes by reduction of imidazole-2(3H)-thiones. Synthesis 1993, 561–562. [Google Scholar] [CrossRef]
- Azarifar, D.; Golbaghi, M. Selective and facile oxidative desulfurization of thioureas and thiobarbituric acids with singlet molecular oxygen generated from trans-3,5-dihydroperoxy-3,5-dimethyl-1,2-dioxolane. J. Sulfur Chem. 2016, 37, 1–13. [Google Scholar] [CrossRef]
- Dey, G.R.; Naik, D.B.; Kishore, K.; Moorthy, P.N. Nature of the transient species formed in the pulse radiolysis of some thiourea derivatives. J. Chem. Soc. Perkin Trans. 2 1994, 1625–1629. [Google Scholar] [CrossRef]
- Dey, G.R.; Naik, D.B.; Kishore, K.; Moorthy, P.N. Kinetic and spectral characteristics of transients formed in the pulse radiolysis of phenylthiourea in aqueous solution. Radiat. Phys. Chem. 1994, 43, 365–369. [Google Scholar] [CrossRef]
- Dey, G.R.; Naik, D.B.; Kishore, K.; Moorthy, P.N. Kinetic and spectral properties of the intermediates formed in the pulse radiolysis of 2-mercaptobenzimidazole. Res. Chem. Intermed. 1995, 21, 47–58. [Google Scholar] [CrossRef]
- Wang, W.-F.; Schuchmann, M.N.; Schuchmann, H.-P.; Knolle, W.; von Sonntag, J.; von Sonntag, C. Radical cations in the OH-radical-induced oxidation of thiourea and tetramethylthiourea in aqueous solution. J. Am. Chem. Soc. 1999, 121, 238–245. [Google Scholar] [CrossRef]
- Dey, G. A comparative study of radical cations of thiourea, thiosemicarbazide, and diethylthiourea in aqueous sulphuric acid media employing pulse radiolysis technique. J. Phys. Org. Chem. 2013, 26, 927–932. [Google Scholar] [CrossRef]
- Serobatse, K.R.N.; Kabanda, M.M. An appraisal of the hydrogen atom transfer mechanism for the reaction between thiourea derivatives and OH radical: A case-study of dimethylthiourea and diethylthiourea. Comput. Theor. Chem. 2017, 1101, 83–95. [Google Scholar] [CrossRef]
- Gain, S.; Das, R.S.; Banerjee, R.; Mukhopadhyay, S. Kinetics and mechanism of oxidation of thiourea by a bridging superoxide in the presence of Ellman’s reagent. J. Coord. Chem. 2016, 69, 2136–2147. [Google Scholar] [CrossRef]
- Miyata, S.; Hoshino, M.; Isozaki, T.; Yamada, T.; Sugimura, H.; Xu, Y.-Z.; Suzuki, T. Acid dissociation equlibrium and singlet molecular oxygen quantum yield of acetylated 6,8-dithioguanosine in aqueous buffer solution. J. Phys. Chem. B 2018, 122, 2912–2921. [Google Scholar] [CrossRef]
- Ioele, M.; Bazzanini, R.; Chatgilialoglu, C.; Mulazzani, Q.G. Chemical radiation studies of 8-bromoguanosine in aqueous solutions. J. Am. Chem. Soc. 2000, 122, 1900–1907. [Google Scholar] [CrossRef]
- Baptista, M.S.; Cadet, J.; Di Mascio, P.; Ghogare, A.A.; Greer, A.; Hamblin, M.R.; Lorente, C.; Nunez, S.C.; Ribeiro, M.S.; Thomas, A.H.; et al. Type I and type II photosensitized oxidation reactions: Guidelines and mechanistic pathways. Photochem. Photobiol. 2017, 93, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.J.; Muthuramu, K.; Ramamurthy, V. Oxidations of thio ketones by singlet and triplet oxygen. J. Org. Chem. 1982, 47, 127–131. [Google Scholar] [CrossRef]
- Wenska, G.; Koput, J.; Burdziński, G.; Taras-Goslinska, K.; Maciejewski, A. Photophysical and photochemical properties of the T1 excited state of thioinosine. J. Photochem. Photobiol. A Chem. 2009, 206, 93–101. [Google Scholar] [CrossRef]
- Hemmens, V.J.; Moore, D.E. Photo-oxidation of 6-mercaptopurine in aqueous solution. J. Chem. Soc. Perkin Trans. 2 1984, 209–211. [Google Scholar] [CrossRef]
- Ren, X.; Li, F.; Jeffs, G.; Zhang, X.; Xu, Y.-Z.; Karran, P. Guanine sulphinate is a major stable product of photochemical oxidation of DNA 6-thioguanine by UVA irradiation. Nucleic Acids Res. 2009, 38, 1832–1840. [Google Scholar] [CrossRef]
- Zou, X.; Zhao, H.; Yu, Y.; Su, H. Formation of guanine-6-sulfonate from 6-thioguanine and singlet oxygen: A combined theoretical and experimental study. J. Am. Chem. Soc. 2013, 135, 4509–4515. [Google Scholar] [CrossRef] [PubMed]
- Miyata, S.; Yamada, T.; Isozaki, T.; Sugimura, H.; Xu, Y.Z.; Suzuki, T. Absorption characteristics and quantum yields of singlet oxygen generation of thioguanosine derivatives. Photochem. Photobiol. 2018, 94, 677–684. [Google Scholar] [CrossRef]
- Miyata, S.; Tanabe, S.; Isozaki, T.; Xu, Y.-Z.; Suzuki, T. Characteristics of the excited triplet states of thiolated guanosine derivatives and singlet oxygen generation. Photochem. Photobiol. Sci. 2018, 17, 1469–1476. [Google Scholar] [CrossRef]
- Bensasson, R.V.; Land, E.J.; Truscott, T.G. Excited States and Free Fadicals in Biology and Medicine; Oxford University Press Inc.: New York, NY, USA, 1993; p. 150. [Google Scholar]
- Pedzinski, T.; Markiewicz, A.; Marciniak, B. Photosensitized oxidation of methionine derivatives. Laser flash photolysis studies. Res. Chem. Intermed. 2009, 35, 497–506. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals ((•OH)/•O−) in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Spinks, J.W.T.; Woods, R.J. An Introduction to Radiation Chemistry, 3rd ed.; John-Wiley and Sons, Inc.: New York, NY, USA, 1990; p. 100. [Google Scholar]
- Lykakis, I.N.; Ferreri, C.; Chatgilialoglu, C. The sulfhydryl radical (HS•/S•−): A contender for the isomerization of double bonds in membrane lipids. Angew. Chem. Int. Ed. 2007, 46, 1914–1916. [Google Scholar] [CrossRef] [PubMed]
- Chatgilialoglu, C.; Ferreri, C.; Melchiorre, M.; Sansone, A.; Torreggiani, A. Lipid geometrical isomerism: From chemistry to biology and diagnostics. Chem. Rev. 2014, 114, 255–284. [Google Scholar] [CrossRef] [PubMed]
- Chatgilialoglu, C.; Ferreri, C.; Torreggiani, A.; Salzano, A.M.; Renzone, G.; Scaloni, A. Radiation-induced reductive modifications of sulfur-containing amino acids within peptides and proteins. J. Proteom. 2011, 74, 2264–2273. [Google Scholar] [CrossRef]
- Chatgilialoglu, C.; Caminal, C.; Altieri, A.; Vougioukalakis, G.C.; Mulazzani, Q.G.; Gimisis, T.; Guerra, M. Tautomerism in the guanyl radical. J. Am. Chem. Soc. 2006, 128, 13796–13805. [Google Scholar] [CrossRef]
- Chatgilialoglu, C.; Caminal, C.; Guerra, M.; Mulazzani, Q.G. Tautomers of one-electron-oxidized guanosine. Angew. Chem. Int. Ed. 2005, 44, 6030–6032. [Google Scholar] [CrossRef] [PubMed]
- Chatgilialoglu, C.; D’Angelantonio, M.; Guerra, M.; Kaloudis, P.; Mulazzani, Q.G. A reevaluation of the ambident reactivity of the guanine moiety towards hydroxyl radicals. Angew. Chem. Int. Ed. 2009, 48, 2214–2217. [Google Scholar] [CrossRef]
- Chatgilialoglu, C.; D’Angelantonio, M.; Kciuk, G.; Bobrowski, K. New insights into the reaction paths of hydroxyl radicals with 2′-deoxyguanosine. Chem. Res. Toxicol. 2011, 24, 2200–2206. [Google Scholar] [CrossRef]
- D’Angelantonio, M.; Russo, M.; Kaloudis, P.; Mulazzani, Q.G.; Wardman, P.; Guerra, M.; Chatgilialoglu, C. Reaction of hydrated electrons with guanine derivatives: Tautomerism of intermediate species. J. Phys. Chem. B 2009, 113, 2170–2176. [Google Scholar] [CrossRef]
- Kaloudis, P.; D’Angelantonio, M.; Guerra, M.; Spadafora, M.; Cismaş, C.; Gimisis, T.; Mulazzani, Q.G.; Chatgilialoglu, C. Comparison of isoelectronic 8-HO-G and 8-NH2-G derivatives in redox processes. J. Am. Chem. Soc. 2009, 131, 15895–15902. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Olson, F.; Hunt, C.A.; Szoka, F.C.; Vail, W.J.; Papahadjopoulos, D. Preparation of liposomes of defined size distribution by extrusion through polycarbonate membranes. Biochim. Biophys. Acta Biomembr. 1979, 557, 9–23. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
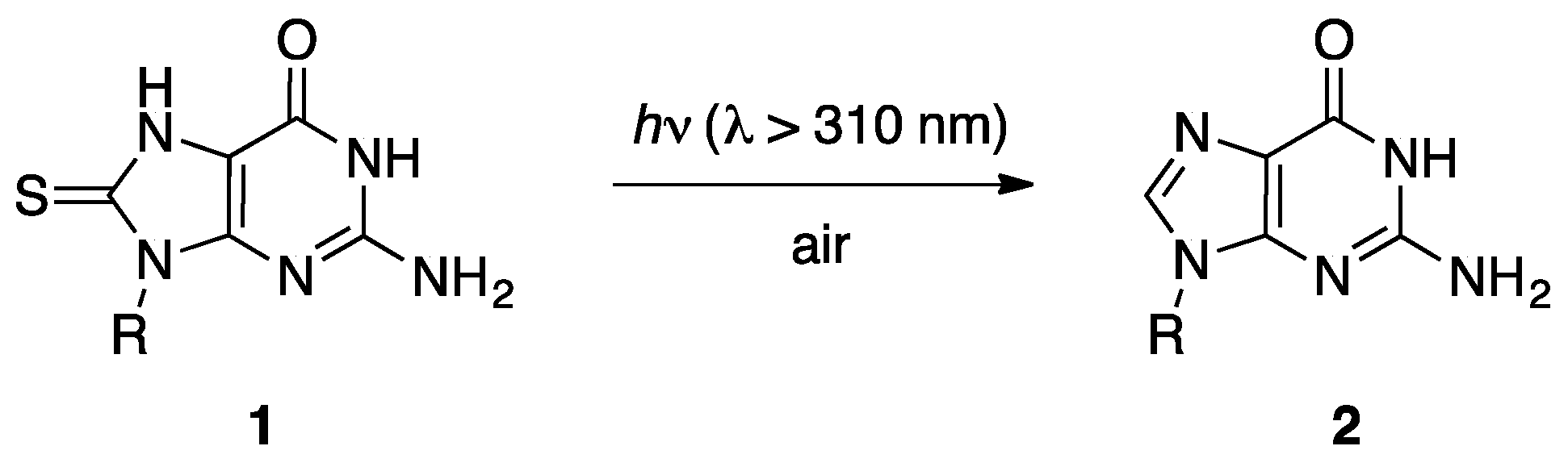

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taras-Goslinska, K.; Vetica, F.; Barata-Vallejo, S.; Triantakostanti, V.; Marciniak, B.; Chatgilialoglu, C. Converging Fate of the Oxidation and Reduction of 8-Thioguanosine. Molecules 2019, 24, 3143. https://doi.org/10.3390/molecules24173143
Taras-Goslinska K, Vetica F, Barata-Vallejo S, Triantakostanti V, Marciniak B, Chatgilialoglu C. Converging Fate of the Oxidation and Reduction of 8-Thioguanosine. Molecules. 2019; 24(17):3143. https://doi.org/10.3390/molecules24173143
Chicago/Turabian StyleTaras-Goslinska, Katarzyna, Fabrizio Vetica, Sebastián Barata-Vallejo, Virginia Triantakostanti, Bronisław Marciniak, and Chryssostomos Chatgilialoglu. 2019. "Converging Fate of the Oxidation and Reduction of 8-Thioguanosine" Molecules 24, no. 17: 3143. https://doi.org/10.3390/molecules24173143
APA StyleTaras-Goslinska, K., Vetica, F., Barata-Vallejo, S., Triantakostanti, V., Marciniak, B., & Chatgilialoglu, C. (2019). Converging Fate of the Oxidation and Reduction of 8-Thioguanosine. Molecules, 24(17), 3143. https://doi.org/10.3390/molecules24173143