Application of Non-Thermal Plasma on Biofilm: A Review
Abstract
:1. Introduction
2. Literature Review Strategy
3. Biofilm and Its Formation
4. Non-Thermal Plasma
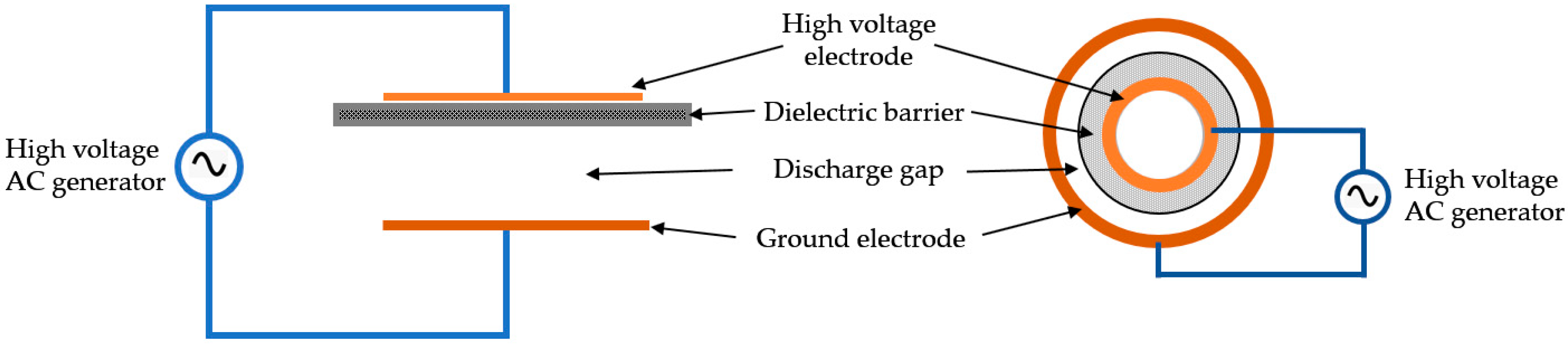
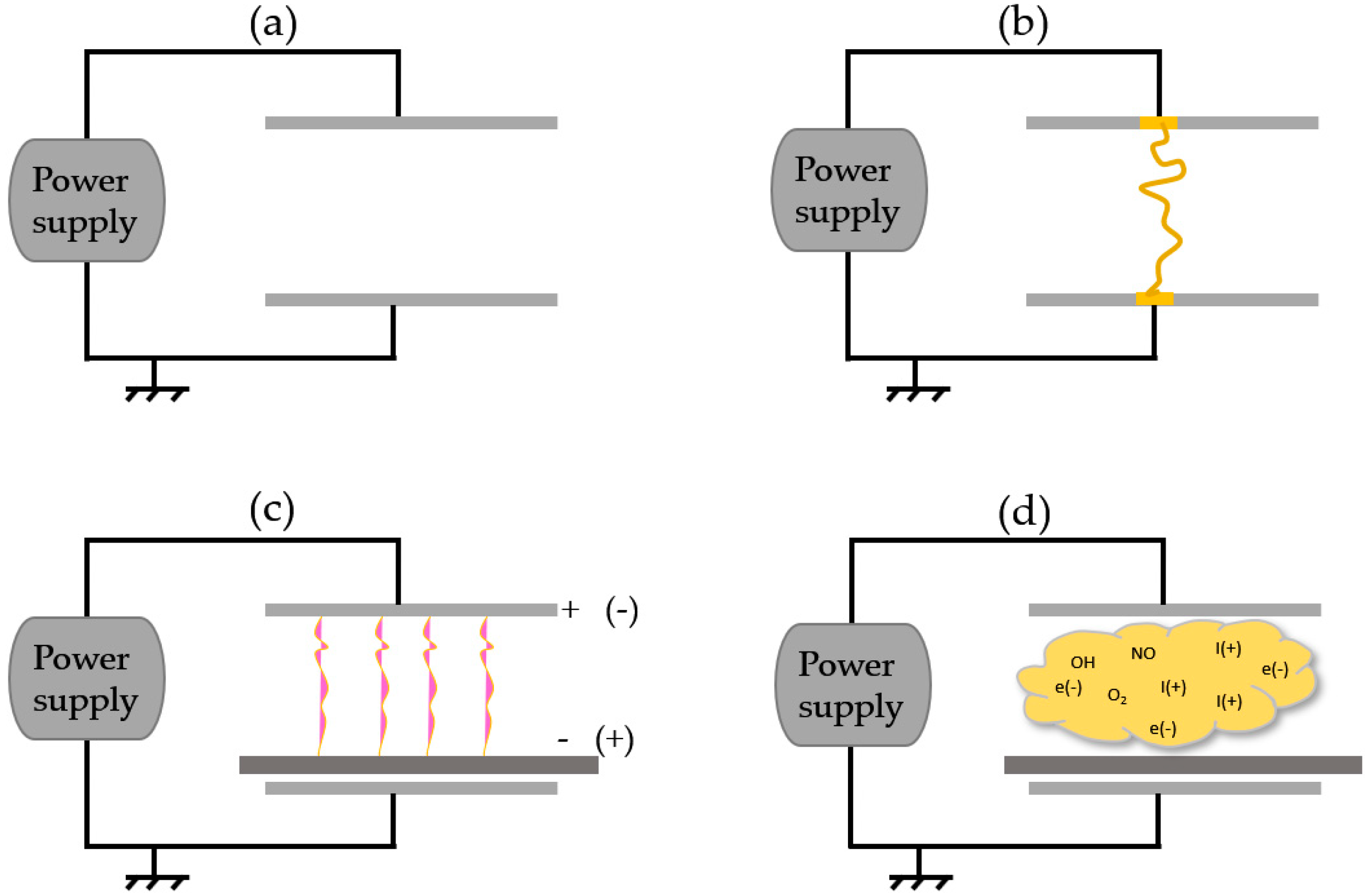
4.1. Plasma Generation and Treatment
4.2. Active Plasma Agents
5. Factors Influencing the Antimicrobial Efficacy of NTP
5.1. Plasma Treatment Time and the Distance between the Plasma Source and the Sample
5.2. Frequency and Electrical Input Power (Voltage)
5.3. Role of the Gas or the Gas Mixture with its Flow Rate
6. Biomedical Applications of NTP on Biofilm
7. Other Medical Applications
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smith, A.W. Biofilms and antibiotic therapy: Is there a role for combating bacterial resistance by the use of novel drug delivery systems? Adv. Drug Deliv. Rev. 2005, 57, 1539–1550. [Google Scholar] [CrossRef] [PubMed]
- Puligundla, P.; Mok, C. Potential applications of nonthermal plasmas against biofilm-associated micro-organisms in vitro. J. Appl. Microbiol. 2017, 122, 1134–1148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkawareek, M.Y.; Algwari, Q.T.; Laverty, G.; Gorman, S.P.; Graham, W.G.; O’Connell, D.; Gilmore, B.F. Eradication of Pseudomonas aeruginosa biofilms by atmospheric pressure non-thermal plasma. PLoS ONE 2012, 7, e44289. [Google Scholar] [CrossRef] [PubMed]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.; Moser, C.; Wang, H.-Z.; Høiby, N.; Song, Z.-J. Strategies for combating bacterial biofilm infections. Int. J. Oral Sci. 2015, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Cornell, R.S.; Hood, C.R. What You Should Know About Biofilm and Implants. Podiatry Today 2015, 28, 38–46. Available online: https://www.podiatrytoday.com/what-you-should-know-about-biofilm-and-implants (accessed on 15 August 2019).
- Wolcott, R.; Dowd, S. The role of biofilms: Are we hitting the right target? Plast. Reconstr. Surg. 2011, 127, 28S–35S. [Google Scholar] [CrossRef]
- Abreu, A.C.; Tavares, R.R.; Borges, A.; Mergulhão, F.; Simões, M. Current and emergent strategies for disinfection of hospital environments. J. Antimicrob. Chemother. 2013, 68, 2718–2732. [Google Scholar] [CrossRef]
- Khardori, N.; Yassien, M. Biofilms in device-related infections. J. Ind. Microbiol. Biotechnol. 1995, 15, 141–147. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms and device-associated infections. Emerg. Infect. Dis. 2001, 7, 277. [Google Scholar] [CrossRef]
- Murakami, M.; Nishi, Y.; Seto, K.; Kamashita, Y.; Nagaoka, E. Dry mouth and denture plaque microflora in complete denture and palatal obturator prosthesis wearers. Gerodontology 2015, 32, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Borgwardt, L.; Høiby, N.; Wu, H.; Sørensen, T.S.; Borgwardt, A. Prosthesis infections after orthopedic joint replacement: The possible role of bacterial biofilms. Orthop. Rev. 2013, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.L.; Lowry, N.; Campbell, T.; Reid, T.W.; Webster, D.R.; Tobin, E.; Aslani, A.; Mosley, T.; Dertien, J.; Colmer-Hamood, J.A. An organoselenium compound inhibits Staphylococcus aureus biofilms on hemodialysis catheters in vivo. Antimicrob. Agents Chemother. 2012, 56, 972–978. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.P.A.; Watanabe, E.; Andrade, D. de Biofilm on artificial pacemaker: Fiction or reality? Arq. Bras. Cardiol. 2011, 97, e113–e120. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, N.; Cahill, O.; Daniels, S.; Galvin, S.; Humphreys, H. Cold atmospheric pressure plasma and decontamination. Can it contribute to preventing hospital-acquired infections? J. Hosp. Infect. 2014, 88, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.A.; Ahmad, I.; Sajid, M.; Cameotra, S.S. Current and emergent control strategies for medical biofilms. In Antibiofilm Agents; Springer: Berlin/Heidelberg, Germany, 2014; pp. 117–159. [Google Scholar]
- Rutala, W.A.; Weber, D.J. Guideline for Disinfection and Sterilization in Healthcare Facilities. 2008. Available online: http://www.cdc.gov/hicpac/pdf/guidelines/Disinfection_Nov_2008.pdf (accessed on 15 August 2019).
- Weinstein, R.A.; Hota, B. Contamination, disinfection, and cross-colonization: Are hospital surfaces reservoirs for nosocomial infection? Clin. Infect. Dis. 2004, 39, 1182–1189. [Google Scholar] [CrossRef] [PubMed]
- Cahill, O.J.; Claro, T.; O’Connor, N.; Cafolla, A.A.; Stevens, N.T.; Daniels, S.; Humphreys, H. Cold air plasma to decontaminate inanimate surfaces of the hospital environment. Appl. Environ. Microbiol. 2014, 80, 2004–2010. [Google Scholar] [CrossRef]
- Lindsay, D.; Von Holy, A. Bacterial biofilms within the clinical setting: What healthcare professionals should know. J. Hosp. Infect. 2006, 64, 313–325. [Google Scholar] [CrossRef]
- Bose, S.; Ghosh, A.K. Fungal Biofilm & Medical Device Associated Infection: It’s Formation, Diagnosis & Future Trends: A Review. Ann. Pathol. Lab. Med. 2016, 3, R1–R9. [Google Scholar]
- Brelles-Mariño, G. Challenges in biofilm inactivation: The use of cold plasma as a new approach. J. Bioprocess. Biotech. 2012, 2, e107. [Google Scholar]
- Donlan, R.M. Biofilm formation: A clinically relevant microbiological process. Clin. Infect. Dis. 2001, 33, 1387–1392. [Google Scholar] [CrossRef] [PubMed]
- McConoughey, S.J.; Howlin, R.; Granger, J.F.; Manring, M.M.; Calhoun, J.H.; Shirtliff, M.; Kathju, S.; Stoodley, P. Biofilms in periprosthetic orthopedic infections. Future Microbiol. 2014, 9, 987–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall-Stoodley, L.; Stoodley, P. Developmental regulation of microbial biofilms. Curr. Opin. Biotechnol. 2002, 13, 228–233. [Google Scholar] [CrossRef]
- Izano, E.A.; Amarante, M.A.; Kher, W.B.; Kaplan, J.B. Differential roles of poly-N-acetylglucosamine surface polysaccharide and extracellular DNA in Staphylococcus aureus and Staphylococcus epidermidis biofilms. Appl. Environ. Microbiol. 2008, 74, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Høiby, N.; Ciofu, O.; Johansen, H.K.; Song, Z.; Moser, C.; Jensen, P.Ø.; Molin, S.; Givskov, M.; Tolker-Nielsen, T.; Bjarnsholt, T. The clinical impact of bacterial biofilms. Int. J. Oral Sci. 2011, 3, 55. [Google Scholar] [CrossRef]
- Mah, T.-F.C.; O’Toole, G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001, 9, 34–39. [Google Scholar] [CrossRef]
- Stewart, P.S.; Costerton, J.W. Antibiotic resistance of bacteria in biofilms. Lancet 2001, 358, 135–138. [Google Scholar] [CrossRef]
- Rupf, S.; Lehmann, A.; Hannig, M.; Schäfer, B.; Schubert, A.; Feldmann, U.; Schindler, A. Killing of adherent oral microbes by a non-thermal atmospheric plasma jet. J. Med. Microbiol. 2010, 59, 206–212. [Google Scholar] [CrossRef] [Green Version]
- Koban, I.; Holtfreter, B.; Hübner, N.O.; Matthes, R.; Sietmann, R.; Kindel, E.; Weltmann, K.D.; Welk, A.; Kramer, A.; Kocher, T. Antimicrobial efficacy of non-thermal plasma in comparison to chlorhexidine against dental biofilms on titanium discs in vitro—Proof of principle experiment. J. Clin. Periodontol. 2011, 38, 956–965. [Google Scholar] [CrossRef]
- Gupta, T.T.; Ayan, H. Non-Thermal Plasma in Conjunction with Chlorhexidine (Chx) Digluconate Sterilize The Biofilm Contaminated Titanium Surface. In Proceedings of the 2017 IEEE International Conference on Plasma Science (ICOPS), Atlantic City, NJ, USA, 21–25 May 2017; p. 1. [Google Scholar]
- Gupta, T.T. Characterization and Optimization of Non-Thermal Plasma for Biofilm Sterilization. Ph.D. Thesis, University of Toledo, Toledo, OH, USA, 2018. [Google Scholar]
- Jha, N.; Ryu, J.J.; Choi, E.H.; Kaushik, N.K. Generation and role of reactive oxygen and nitrogen species induced by plasma, lasers, chemical agents, and other systems in dentistry. Oxid. Med. Cell. Longev. 2017, 2017, 7542540. [Google Scholar] [CrossRef]
- Gaunt, L.F.; Beggs, C.B.; Georghiou, G.E. Bactericidal action of the reactive species produced by gas-discharge nonthermal plasma at atmospheric pressure: A review. IEEE Trans. Plasma Sci. 2006, 34, 1257–1269. [Google Scholar] [CrossRef]
- Graves, D.B. The emerging role of reactive oxygen and nitrogen species in redox biology and some implications for plasma applications to medicine and biology. J. Phys. D Appl. Phys. 2012, 45, 263001. [Google Scholar] [CrossRef]
- Moreau, M.; Orange, N.; Feuilloley, M.G.J. Non-thermal plasma technologies: New tools for bio-decontamination. Biotechnol. Adv. 2008, 26, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, C.; Berganza, C.; Zhang, J. Cold Atmospheric Plasma: Methods of production and application in dentistry and oncology. Med. Gas Res. 2013, 3, 21. [Google Scholar] [CrossRef] [PubMed]
- Fridman, G.; Friedman, G.; Gutsol, A.; Shekhter, A.B.; Vasilets, V.N.; Fridman, A. Applied plasma medicine. Plasma Process. Polym. 2008, 5, 503–533. [Google Scholar] [CrossRef]
- Keidar, M.; Shashurin, A.; Volotskova, O.; Ann Stepp, M.; Srinivasan, P.; Sandler, A.; Trink, B. Cold atmospheric plasma in cancer therapy. Phys. Plasmas 2013, 20, 57101. [Google Scholar] [CrossRef]
- Ayan, H. Uniform Dielectric Barrier Discharge with Nanosecond Pulse Excitation for Biomedical Applications. Ph.D. Thesis, Drexel University, Philadelphia, PA, USA, 2009. [Google Scholar]
- Xu, L.; Tu, Y.; Yu, Y.; Tan, M.; Li, J.; Chen, H. Augmented survival of Neisseria gonorrhoeae within biofilms: Exposure to atmospheric pressure non-thermal plasmas. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Sun, K.; Liang, Y.; Sun, P.; Yang, X.; Wang, J.; Zhang, J.; Zhu, W.; Fang, J.; Becker, K.H. Cold plasma therapy of a tooth root canal infected with Enterococcus faecalis biofilms in vitro. J. Endod. 2013, 39, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Molnar, I.; Papp, J.; Simon, A.; Anghel, S.D. Deactivation of Streptococcus mutans biofilms on a tooth surface using he dielectric barrier discharge at atmospheric pressure. Plasma Sci. Technol. 2013, 15, 535. [Google Scholar] [CrossRef]
- Marchal, F.; Robert, H.; Merbahi, N.; Fontagne-Faucher, C.; Yousfi, M.; Romain, C.E.; Eichwald, O.; Rondel, C.; Gabriel, B. Inactivation of Gram-positive biofilms by low-temperature plasma jet at atmospheric pressure. J. Phys. D Appl. Phys. 2012, 45, 345202. [Google Scholar] [CrossRef]
- Xiong, Z.; Du, T.; Lu, X.; Cao, Y.; Pan, Y. How deep can plasma penetrate into a biofilm? Appl. Phys. Lett. 2011, 98, 221503. [Google Scholar] [CrossRef]
- Joshi, S.G.; Paff, M.; Friedman, G.; Fridman, G.; Fridman, A.; Brooks, A.D. Control of methicillin-resistant Staphylococcus aureus in planktonic form and biofilms: A biocidal efficacy study of nonthermal dielectric-barrier discharge plasma. Am. J. Infect. Control 2010, 38, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Ziuzina, D.; Patil, S.; Cullen, P.J.; Boehm, D.; Bourke, P. Dielectric barrier discharge atmospheric cold plasma for inactivation of Pseudomonas aeruginosa biofilms. Plasma Med. 2014, 4, 137–152. [Google Scholar] [CrossRef]
- Xu, Z.; Shen, J.; Zhang, Z.; Ma, J.; Ma, R.; Zhao, Y.; Sun, Q.; Qian, S.; Zhang, H.; Ding, L. Inactivation Effects of Non-Thermal Atmospheric-Pressure Helium Plasma Jet on Staphylococcus aureus Biofilms. Plasma Process. Polym. 2015, 12, 827–835. [Google Scholar] [CrossRef]
- Traba, C.; Chen, L.; Liang, J.F. Low power gas discharge plasma mediated inactivation and removal of biofilms formed on biomaterials. Curr. Appl. Phys. 2013, 13, S12–S18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ermolaeva, S.A.; Varfolomeev, A.F.; Chernukha, M.Y.; Yurov, D.S.; Vasiliev, M.M.; Kaminskaya, A.A.; Moisenovich, M.M.; Romanova, J.M.; Murashev, A.N.; Selezneva, I.I. Bactericidal effects of non-thermal argon plasma in vitro, in biofilms and in the animal model of infected wounds. J. Med. Microbiol. 2011, 60, 75–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taghizadeh, L.; Brackman, G.; Nikiforov, A.; van der Mullen, J.; Leys, C.; Coenye, T. Inactivation of biofilms using a low power atmospheric pressure argon plasma jet; the role of entrained nitrogen. Plasma Process. Polym. 2015, 12, 75–81. [Google Scholar] [CrossRef]
- Matthes, R.; Bender, C.; Schlüter, R.; Koban, I.; Bussiahn, R.; Reuter, S.; Lademann, J.; Weltmann, K.D.; Kramer, A. Antimicrobial Efficacy of Two Surface Barrier Discharges with Air Plasma against In Vitro Biofilms. PLoS ONE 2013, 8, e70462. [Google Scholar] [CrossRef]
- Koban, I.; Matthes, R.; Hübner, N.-O.; Welk, A.; Meisel, P.; Holtfreter, B.; Sietmann, R.; Kindel, E.; Weltmann, K.-D.; Kramer, A. Treatment of Candida albicans biofilms with low-temperature plasma induced by dielectric barrier discharge and atmospheric pressure plasma jet. New J. Phys. 2010, 12, 73039. [Google Scholar] [CrossRef]
- Maisch, T.; Shimizu, T.; Isbary, G.; Heinlin, J.; Karrer, S.; Klämpfl, T.G.; Li, Y.-F.; Morfill, G.; Zimmermann, J.L. Contact-free inactivation of Candida albicans biofilms by cold atmospheric air plasma. Appl. Environ. Microbiol. 2012, 78, 4242–4247. [Google Scholar] [CrossRef]
- Vandervoort, K.G.; Brelles-Mariño, G. Plasma-mediated inactivation of Pseudomonas aeruginosa biofilms grown on borosilicate surfaces under continuous culture system. PLoS ONE 2014, 9, e108512. [Google Scholar] [CrossRef] [PubMed]
- Matthes, R.; Koban, I.; Bender, C.; Masur, K.; Kindel, E.; Weltmann, K.; Kocher, T.; Kramer, A.; Hübner, N. Antimicrobial efficacy of an atmospheric pressure plasma jet against biofilms of Pseudomonas aeruginosa and Staphylococcus epidermidis. Plasma Process. Polym. 2013, 10, 161–166. [Google Scholar] [CrossRef]
- Jiang, C.; Schaudinn, C.; Jaramillo, D.E.; Webster, P.; Costerton, J.W. In Vitro Antimicrobial Effect of a Cold Plasma Jet against Enterococcus faecalis Biofilms. ISRN Dent. 2012, 2012, 295736. [Google Scholar] [CrossRef] [PubMed]
- Alkawareek, M.Y.; Algwari, Q.T.; Gorman, S.P.; Graham, W.G.; O’Connell, D.; Gilmore, B.F. Application of atmospheric pressure nonthermal plasma for the in vitro eradication of bacterial biofilms. FEMS Immunol. Med. Microbiol. 2012, 65, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Kovalóvá, Z.; Zahoran, M.; Zahoranová, A.; Machala, Z. Streptococci biofilm decontamination on teeth by low-temperature air plasma of dc corona discharges. J. Phys. D Appl. Phys. 2014, 47, 224014. [Google Scholar] [CrossRef]
- Fricke, K.; Koban, I.; Tresp, H.; Jablonowski, L.; Schröder, K.; Kramer, A.; Weltmann, K.D.; von Woedtke, T.; Kocher, T. Atmospheric pressure plasma: A high-performance tool for the efficient removal of biofilms. PLoS ONE 2012, 7, e42539. [Google Scholar] [CrossRef] [PubMed]
- Zelaya, A.J.; Stough, G.; Rad, N.; Vandervoort, K.; Brelles-Mariño, G. Pseudomonas aeruginosa biofilm inactivation: Decreased cell culturability, adhesiveness to surfaces, and biofilm thickness upon high-pressure nonthermal plasma treatment. IEEE Trans. Plasma Sci. 2010, 38, 3398–3403. [Google Scholar] [CrossRef]
- Weltmann, K.; Kindel, E.; Brandenburg, R.; Meyer, C.; Bussiahn, R.; Wilke, C.; Von Woedtke, T. Atmospheric pressure plasma jet for medical therapy: Plasma parameters and risk estimation. Contrib. Plasma Phys. 2009, 49, 631–640. [Google Scholar] [CrossRef]
- Bekeschus, S.; Schmidt, A.; Weltmann, K.-D.; von Woedtke, T. The plasma jet kINPen—A powerful tool for wound healing. Clin. Plasma Med. 2016, 4, 19–28. [Google Scholar] [CrossRef]
- Winter, J.; Wende, K.; Masur, K.; Iseni, S.; Dünnbier, M.; Hammer, M.U.; Tresp, H.; Weltmann, K.D.; Reuter, S. Feed gas humidity: A vital parameter affecting a cold atmospheric-pressure plasma jet and plasma-treated human skin cells. J. Phys. D Appl. Phys. 2013, 46, 295401. [Google Scholar] [CrossRef]
- Heinlin, J.; Zimmermann, J.L.; Zeman, F.; Bunk, W.; Isbary, G.; Landthaler, M.; Maisch, T.; Monetti, R.; Morfill, G.; Shimizu, T. Randomized placebo-controlled human pilot study of cold atmospheric argon plasma on skin graft donor sites. Wound Repair Regen. 2013, 21, 800–807. [Google Scholar] [CrossRef]
- Brehmer, F.; Haenssle, H.A.; Daeschlein, G.; Ahmed, R.; Pfeiffer, S.; Görlitz, A.; Simon, D.; Schön, M.P.; Wandke, D.; Emmert, S. Alleviation of chronic venous leg ulcers with a hand-held dielectric barrier discharge plasma generator (PlasmaDerm® VU-2010): Results of a monocentric, two-armed, open, prospective, randomized and controlled trial (NCT01415622). J. Eur. Acad. Dermatol. Venereol. 2015, 29, 148–155. [Google Scholar] [CrossRef]
- Isbary, G.; Heinlin, J.; Shimizu, T.; Zimmermann, J.L.; Morfill, G.; Schmidt, H.; Monetti, R.; Steffes, B.; Bunk, W.; Li, Y. Successful and safe use of 2 min cold atmospheric argon plasma in chronic wounds: Results of a randomized controlled trial. Br. J. Dermatol. 2012, 167, 404–410. [Google Scholar] [CrossRef]
- Gupta, T.T.; Karki, S.B.; Matson, J.S.; Gehling, D.J.; Ayan, H. Sterilization of Biofilm on a Titanium Surface Using a Combination of Nonthermal Plasma and Chlorhexidine Digluconate. BioMed Res. Int. 2017, 2017, 6085741. [Google Scholar] [CrossRef]
- Gupta, T.T.; Matson, J.S.; Ayan, H. Antimicrobial Effectiveness of Regular Dielectric-Barrier Discharge (DBD) and Jet DBD on the Viability of Pseudomonas aeruginosa. IEEE Trans. Radiat. Plasma Med. Sci. 2018, 2, 68–76. [Google Scholar] [CrossRef]
- Ayan, H.; Staack, D.; Fridman, G.; Gutsol, A.; Mukhin, Y.; Starikovskii, A.; Fridman, A.; Friedman, G. Application of nanosecond-pulsed dielectric barrier discharge for biomedical treatment of topographically non-uniform surfaces. J. Phys. D Appl. Phys. 2009, 42, 125202. [Google Scholar] [CrossRef]
- Fridman, G.; Peddinghaus, M.; Balasubramanian, M.; Ayan, H.; Fridman, A.; Gutsol, A.; Brooks, A. Blood coagulation and living tissue sterilization by floating-electrode dielectric barrier discharge in air. Plasma Chem. Plasma Process. 2006, 26, 425–442. [Google Scholar] [CrossRef]
- Kong, M.G.; Kroesen, G.; Morfill, G.; Nosenko, T.; Shimizu, T.; Van Dijk, J.; Zimmermann, J.L. Plasma medicine: An introductory review. New J. Phys. 2009, 11, 115012. [Google Scholar] [CrossRef]
- Laroussi, M. Low temperature plasma-based sterilization: Overview and state-of-the-art. Plasma Process. Polym. 2005, 2, 391–400. [Google Scholar] [CrossRef]
- Isbary, G.; Stolz, W.; Shimizu, T.; Monetti, R.; Bunk, W.; Schmidt, H.-U.; Morfill, G.E.; Klämpfl, T.G.; Steffes, B.; Thomas, H.M. Cold atmospheric argon plasma treatment may accelerate wound healing in chronic wounds: Results of an open retrospective randomized controlled study in vivo. Clin. Plasma Med. 2013, 1, 25–30. [Google Scholar] [CrossRef]
- Ayan, H.; Fridman, G.; Gutsol, A.F.; Vasilets, V.N.; Fridman, A.; Friedman, G. Nanosecond-pulsed uniform dielectric-barrier discharge. IEEE Trans. Plasma Sci. 2008, 36, 504–508. [Google Scholar] [CrossRef]
- Sanaei, N.; Ayan, H. Bactericidal efficacy of dielectric barrier discharge plasma on methicillin-resistant staphylococcus aureus and Escherichia coli in planktonic phase and colonies in vitro. Plasma Med. 2015, 5, 1–16. [Google Scholar] [CrossRef]
- Stoffels, E.; Kieft, I.E.; Sladek, R.E.J.; Van den Bedem, L.J.M.; Van der Laan, E.P.; Steinbuch, M. Plasma needle for in vivo medical treatment: Recent developments and perspectives. Plasma Sources Sci. Technol. 2006, 15, S169. [Google Scholar] [CrossRef]
- Sladek, R.E.J.; Stoffels, E.; Walraven, R.; Tielbeek, P.J.A.; Koolhoven, R.A. Plasma treatment of dental cavities: A feasibility study. IEEE Trans. Plasma Sci. 2004, 32, 1540–1543. [Google Scholar] [CrossRef]
- Fridman, G.; Shereshevsky, A.; Jost, M.M.; Brooks, A.D.; Fridman, A.; Gutsol, A.; Vasilets, V.; Friedman, G. Floating electrode dielectric barrier discharge plasma in air promoting apoptotic behavior in melanoma skin cancer cell lines. Plasma Chem. Plasma Process. 2007, 27, 163–176. [Google Scholar] [CrossRef]
- Karki, S.B.; Gupta, T.T.; Yildirim-Ayan, E.; Eisenmann, K.M.; Ayan, H. Investigation of non-thermal plasma effects on lung cancer cells within 3D collagen matrices. J. Phys. D Appl. Phys. 2017, 50, 315401. [Google Scholar] [CrossRef]
- Karki, S.B.; Yildirim-Ayan, E.; Eisenmann, K.M.; Ayan, H. Miniature Dielectric Barrier Discharge Nonthermal Plasma Induces Apoptosis in Lung Cancer Cells and Inhibits Cell Migration. BioMed Res. Int. 2017, 2017, 8058307. [Google Scholar] [CrossRef]
- Kamath, U.; Rajasekhara, S.; Nazar, N.; Sathar, A. Cold Atmospheric Plasma; Break Through in Dentistry—A Review. Paripex-Indian J. Res. 2016, 5. [Google Scholar]
- Singh, S.; Chandra, R.; Tripathi, S.; Rahman, H.; Tripathi, P.; Jain, A.; Gupta, P. The bright future of dentistry with cold plasma-review. J. Dent. Med. Sci. 2014, 13, 6–13. [Google Scholar] [CrossRef]
- Cheruthazhekatt, S.; Černák, M.; Slavíček, P.; Havel, J. Gas plasmas and plasma modified materials in medicine. J. Appl. Biomed. 2010, 8, 55–66. [Google Scholar] [CrossRef] [Green Version]
- Scholtz, V.; Pazlarova, J.; Souskova, H.; Khun, J.; Julak, J. Nonthermal plasma—A tool for decontamination and disinfection. Biotechnol. Adv. 2015, 33, 1108–1119. [Google Scholar] [CrossRef]
- Von Woedtke, T.; Reuter, S.; Masur, K.; Weltmann, K.-D. Plasmas for medicine. Phys. Rep. 2013, 530, 291–320. [Google Scholar] [CrossRef]
- Kogelschatz, U. Dielectric-barrier discharges: Their history, discharge physics, and industrial applications. Plasma Chem. Plasma Process. 2003, 23, 1–46. [Google Scholar] [CrossRef]
- Ehlbeck, J.; Schnabel, U.; Polak, M.; Winter, J.; Von Woedtke, T.; Brandenburg, R.; Von dem Hagen, T.; Weltmann, K.D. Low temperature atmospheric pressure plasma sources for microbial decontamination. J. Phys. D Appl. Phys. 2010, 44, 13002. [Google Scholar] [CrossRef]
- Laimer, J.; Störi, H. Recent Advances in the Research on Non-Equilibrium Atmospheric Pressure Plasma Jets. Plasma Process. Polym. 2007, 4, 266–274. [Google Scholar] [CrossRef]
- Kogelschatz, U. Atmospheric-pressure plasma technology. Plasma Phys. Control. Fusion 2004, 46, B63. [Google Scholar] [CrossRef]
- Neuber, J.U. Non-Thermal Atmospheric-Pressure Plasma for Sterilization of Surfaces and Biofilms. Master’s Thesis, Old Dominion University, Norfolk, VA, USA, 2016. [Google Scholar]
- Weltmann, K.D.; von Woedtke, T. Plasma medicine—Current state of research and medical application. Plasma Phys. Control. Fusion 2016, 59, 14031. [Google Scholar] [CrossRef]
- Winter, J.; Brandenburg, R.; Weltmann, K.D. Atmospheric pressure plasma jets: An overview of devices and new directions. Plasma Sources Sci. Technol. 2015, 24, 64001. [Google Scholar] [CrossRef]
- Isbary, G.; Shimizu, T.; Li, Y.-F.; Stolz, W.; Thomas, H.M.; Morfill, G.E.; Zimmermann, J.L. Cold atmospheric plasma devices for medical issues. Expert Rev. Med. Devices 2013, 10, 367–377. [Google Scholar] [CrossRef]
- Gupta, T.; Karki, S.; Fournier, R.; Ayan, H. Mathematical Modelling of the Effects of Plasma Treatment on the Diffusivity of Biofilm. Appl. Sci. 2018, 8, 1729. [Google Scholar] [CrossRef]
- Kieft, I.E. Plasma Needle: Exploring Biomedical Applications of Non-Thermal Plasmas; Technische Universiteit Eindhoven: Eindhoven, The Netherlands, 2005; ISBN 9038627378. [Google Scholar]
- Liao, X.; Liu, D.; Xiang, Q.; Ahn, J.; Chen, S.; Ye, X.; Ding, T. Inactivation mechanisms of non-thermal plasma on microbes: A review. Food Control 2017, 75, 83–91. [Google Scholar] [CrossRef]
- Joshi, S.G.; Cooper, M.; Yost, A.; Paff, M.; Ercan, U.K.; Fridman, G.; Friedman, G.; Fridman, A.; Brooks, A.D. Nonthermal dielectric-barrier discharge plasma-induced inactivation involves oxidative DNA damage and membrane lipid peroxidation in Escherichia coli. Antimicrob. Agents Chemother. 2011, 55, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Kalghatgi, S.U. Mechanisms of Interaction of Non-Thermal Plasma with Living Cells; Drexel University: Philadelphia, PA, USA, 2010. [Google Scholar]
- Alkawareek, M.Y.; Gorman, S.P.; Graham, W.G.; Gilmore, B.F. Potential cellular targets and antibacterial efficacy of atmospheric pressure non-thermal plasma. Int. J. Antimicrob. Agents 2014, 43, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Flynn, P.B.; Gilmore, B.F. Understanding plasma biofilm interactions for controlling infection and virulence. J. Phys. D Appl. Phys. 2018, 51, 263001. [Google Scholar] [CrossRef]
- Kvam, E.; Davis, B.; Mondello, F.; Garner, A.L. Nonthermal atmospheric plasma rapidly disinfects multidrug-resistant microbes by inducing cell surface damage. Antimicrob. Agents Chemother. 2012, 56, 2028–2036. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Ghimire, B.; Li, Y.; Adhikari, M.; Veerana, M.; Kaushik, N.; Jha, N.; Adhikari, B.; Lee, S.-J.; Masur, K. Biological and medical applications of plasma-activated media, water and solutions. Biol. Chem. 2018, 400, 39–62. [Google Scholar] [CrossRef] [PubMed]
- Anzai, K.; Aoki, T.; Koshimizu, S.; Takaya, R.; Tsuchida, K.; Takajo, T. Formation of reactive oxygen species by irradiation of cold atmospheric pressure plasma jet to water depends on the irradiation distance. J. Clin. Biochem. Nutr. 2019, 64, 187–193. [Google Scholar] [CrossRef] [Green Version]
- Barekzi, N.; Laroussi, M. Dose-dependent killing of leukemia cells by low-temperature plasma. J. Phys. D Appl. Phys. 2012, 45, 422002. [Google Scholar] [CrossRef]
- Lackmann, J.-W.; Bandow, J.E. Inactivation of microbes and macromolecules by atmospheric-pressure plasma jets. Appl. Microbiol. Biotechnol. 2014, 98, 6205–6213. [Google Scholar] [CrossRef]
- Halfmann, H.; Bibinov, N.; Wunderlich, J.; Awakowicz, P. A double inductively coupled plasma for sterilization of medical devices. J. Phys. D Appl. Phys. 2007, 40, 4145. [Google Scholar] [CrossRef]
- Halfmann, H.; Denis, B.; Bibinov, N.; Wunderlich, J.; Awakowicz, P. Identification of the most efficient VUV/UV radiation for plasma based inactivation of Bacillus atrophaeus spores. J. Phys. D Appl. Phys. 2007, 40, 5907. [Google Scholar] [CrossRef]
- Lu, H.; Patil, S.; Keener, K.M.; Cullen, P.J.; Bourke, P. Bacterial inactivation by high-voltage atmospheric cold plasma: Influence of process parameters and effects on cell leakage and DNA. J. Appl. Microbiol. 2014, 116, 784–794. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Wang, C.; Qiu, Y. Aging of surface properties of ultra high modulus polyethylene fibers treated with He/O2 atmospheric pressure plasma jet. Surf. Coat. Technol. 2008, 202, 2670–2676. [Google Scholar] [CrossRef]
- Park, J.; Henins, I.; Herrmann, H.W.; Selwyn, G.S.; Jeong, J.Y.; Hicks, R.F.; Shim, D.; Chang, C.S. An atmospheric pressure plasma source. Appl. Phys. Lett. 2000, 76, 288–290. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.J.; Chung, T.H.; Bae, S.H.; Leem, S.H. Bacterial inactivation using atmospheric pressure single pin electrode microplasma jet with a ground ring. Appl. Phys. Lett. 2009, 94, 141502. [Google Scholar] [CrossRef]
- Laroussi, M.; Leipold, F. Evaluation of the roles of reactive species, heat, and UV radiation in the inactivation of bacterial cells by air plasmas at atmospheric pressure. Int. J. Mass Spectrom. 2004, 233, 81–86. [Google Scholar] [CrossRef]
- Thiyagarajan, M.; Sarani, A.; Nicula, C. Optical emission spectroscopic diagnostics of a non-thermal atmospheric pressure helium-oxygen plasma jet for biomedical applications. J. Appl. Phys. 2013, 113, 233302. [Google Scholar] [CrossRef]
- Xu, Z.; Shen, J.; Cheng, C.; Hu, S.; Lan, Y.; Chu, P.K. In vitro antimicrobial effects and mechanism of atmospheric-pressure He/O2 plasma jet on Staphylococcus aureus biofilm. J. Phys. D Appl. Phys. 2017, 50, 105201. [Google Scholar] [CrossRef]
- Herrmann, H.W.; Henins, I.; Park, J.; Selwyn, G.S. Decontamination of chemical and biological warfare (CBW) agents using an atmospheric pressure plasma jet (APPJ). Phys. Plasmas 1999, 6, 2284–2289. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.-Y.; Park, B.J.; Lee, D.H.; Lee, I.-S.; Hyun, S.O.; Chung, K.-H.; Park, J.-C. Sterilization of Escherichia coli and MRSA using microwave-induced argon plasma at atmospheric pressure. Surf. Coat. Technol. 2005, 193, 35–38. [Google Scholar] [CrossRef]
- Park, B.J.; Lee, D.H.; Park, J.-C.; Lee, I.-S.; Lee, K.-Y.; Hyun, S.O.; Chun, M.-S.; Chung, K.-H. Sterilization using a microwave-induced argon plasma system at atmospheric pressure. Phys. Plasmas 2003, 10, 4539–4544. [Google Scholar] [CrossRef]
- Lee, M.H.; Park, B.J.; Jin, S.C.; Kim, D.; Han, I.; Kim, J.; Hyun, S.O.; Chung, K.-H.; Park, J.-C. Removal and sterilization of biofilms and planktonic bacteria by microwave-induced argon plasma at atmospheric pressure. New J. Phys. 2009, 11, 115022. [Google Scholar] [CrossRef]
- Nishime, T.M.C.; Borges, A.C.; Koga-Ito, C.Y.; Machida, M.; Hein, L.R.O.; Kostov, K.G. Non-thermal atmospheric pressure plasma jet applied to inactivation of different microorganisms. Surf. Coat. Technol. 2017, 312, 19–24. [Google Scholar] [CrossRef] [Green Version]
- Goree, J.; Liu, B.; Drake, D. Gas flow dependence for plasma-needle disinfection of S. mutans bacteria. J. Phys. D Appl. Phys. 2006, 39, 3479. [Google Scholar] [CrossRef]
- Sladek, R.E.J.; Filoche, S.K.; Sissons, C.H.; Stoffels, E. Treatment of Streptococcus mutans biofilms with a nonthermal atmospheric plasma. Lett. Appl. Microbiol. 2007, 45, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wu, L.; Babu, J.P.; Hottel, T.L.; Garcia-Godoy, F.; Hong, L. Effects of atmospheric non-thermal argon/oxygen plasma on biofilm viability and hydrophobicity of oral bacteria. Am. J. Dent. 2017, 30, 52–56. [Google Scholar]
- Bhatt, S.; Mehta, P.; Chen, C.; Schneider, C.L.; White, L.N.; Chen, H.-L.; Kong, M.G. Efficacy of low-temperature plasma-activated gas disinfection against biofilm on contaminated GI endoscope channels. Gastrointest. Endosc. 2019, 89, 105–114. [Google Scholar] [CrossRef]
- Patenall, B.L.; Hathaway, H.; Sedgwick, A.C.; Thet, N.T.; Williams, G.T.; Young, A.E.; Allinson, S.L.; Short, R.D.; Jenkins, A.T.A. Limiting Pseudomonas aeruginosa Biofilm Formation Using Cold Atmospheric Pressure Plasma. Plasma Med. 2018, 8, 269–277. [Google Scholar] [CrossRef]
- Lu, P.; Ziuzina, D.; Cullen, P.J.; Bourke, P. Inner surface biofilm inactivation by atmospheric pressure helium porous plasma jet. Plasma Process. Polym. 2018, 15, e1800055. [Google Scholar] [CrossRef]
- Kalghatgi, S.U.; Fridman, G.; Cooper, M.; Nagaraj, G.; Peddinghaus, M.; Balasubramanian, M.; Vasilets, V.N.; Gutsol, A.F.; Fridman, A.; Friedman, G. Mechanism of blood coagulation by nonthermal atmospheric pressure dielectric barrier discharge plasma. IEEE Trans. Plasma Sci. 2007, 35, 1559–1566. [Google Scholar] [CrossRef]
- Lloyd, G.; Friedman, G.; Jafri, S.; Schultz, G.; Fridman, A.; Harding, K. Gas plasma: Medical uses and developments in wound care. Plasma Process. Polym. 2010, 7, 194–211. [Google Scholar] [CrossRef]
- Heinlin, J.; Morfill, G.; Landthaler, M.; Stolz, W.; Isbary, G.; Zimmermann, J.L.; Shimizu, T.; Karrer, S. Plasma medicine: Possible applications in dermatology. JDDG 2010, 8, 968–976. [Google Scholar] [CrossRef] [PubMed]



| Indwelling Medical Device | Organisms |
|---|---|
| Central venous catheter | Coagulase-negative staphylococci, Staphylococcus aureus, Enterococcus faecalis, Klebsiella pneumoniae, Pseudomonas aeruginosa, Candida albicans |
| Prosthetic heart valve | Viridans Streptococcus, coagulase-negative staphylococci, enterococci, Staphylococcus aureus |
| Urinary catheter | Staphylococcus epidermidis, Escherichia coli, Klebsiella pneumoniae, Enterococcus faecalis, Proteus mirabilis |
| Artificial hip prosthesis | Coagulase-negative staphylococci, b-hemolytic streptococci, enterococci, Proteus mirabilis, Bacterioides species, Staphylococcus aureus, viridans Streptococcus, Escherichia coli, Pseudomonas aeruginosa |
| Artificial voice prosthesis | Candida albicans, Streptococcus mitis, Streptococcus salivarius, Rothia dentrocariosa, Candida tropicalis, Streptococcus sobrinus, Staphylococcus epidermidis, Stomatococcus mucilaginous |
| Intrauterine device | Staphylococcus epidermidis, Corynebacterium species, Staphylococcus aureus, Micrococcus species, Lactobacillus plantarum, Group B streptococci, Enterococcus species, Candida albicans |
| Bacterial Strain | Plasma Type and Parameters (Voltage, Frequency, Power, Working Gas, and Flow Rate) | Inactivation Yield | Substrate for Biofilm Formation with Time for Decontamination or Sterilization | Ref. |
|---|---|---|---|---|
| Neisseria gonorrhoeae | Jet: 10 kV and 10 kHz, He at 2 L/min | 7 log reduction | Coverslips (20 min) | [42] |
| Enterococcus faecalis | Jet: 18 kV and 10 kHz, Ar/O2 (2%) at 5 L/min | No CFU detected | Root canal (10 min) | [43] |
| Streptococcus mutants | DBD: 580 kHz and 2 W/cm3 power density, He at 2 L/min | 98% killed | Tooth slices (30 s) | [44] |
| Weissella confusa | Jet: 20 kHz | 2.63 and 2.16 log reduction with and without sucrose | Cellulose ester membrane (20 min) | [45] |
| Porphyromonas gingivalis | Jet: 8 kV and 8 kHz, He/O2 (1%) at 1 L/min | All cells killed in 15 µm biofilm | Cover slip (5 min) | [46] |
| Staphylococcus aureus | FE-DBD: 120 V and 0.13 W/cm2 power | All biofilms were sterilized | Cover slip and 96 well plate (<2 min) | [47] |
| Pseudomonas aeruginosa | Jet: 6 kV with 20 and 40 kHz, He/O2 (0.5%) at 2 L/min | 4 log reduction at 20 kHz and complete eradication at 40 kHz | Peg lid of Calgary biofilm device and polycarbonate coupon (4 min) | [3] |
| Pseudomonas aeruginosa | DBD: 120 kV and 50 Hz | Biofilm reduced to undetectable level | 96 well plate and coverslips (5 min) | [48] |
| Staphylococcus aureus | Jet: 20 kV and 38 kHz, He at 6.7 L/min | 3.06 log reduction | Borosilicate slices (10 min) | [49] |
| Staphylococcus aureus, epidermidis and Escherichia coli | Low power gas discharge: 60 W power, oxygen, argon, and nitrogen at flow rate of 2.4 ft3 h−1 | All biofilms killed | Polyethylene terephthalate (PET) films, silicon wafers and cover-glass chambers (25–30 min) | [50] |
| Burkholderia cenocepacia and Pseudomonas aeruginosa | MicroPlaSter B device plasma with argon gas | 0.005% and 2% bacteria survived | Coverglass (10 min) | [51] |
| Staphylococcus aureus, Pseudomonas aeruginosa and Candida albicans | Jet: 5 kV and 61 kHz, 2.5–3.5 W power, Ar+O2 | 27%, 39%, and 35% cells survived of S. aureus, P. aeruginosa, and C. albicans | 96 well plate (3 min) | [52] |
| Streptococcus mutans and saliva multispecies | Jet (KINPen 09): Ar (5 slm) and Ar+1%(O2) at 0.05 slm, HDBD (hollow DBD): 8.4 kV and 37.6 kHz, Ar and Ar+O2 at 1 and 0.01 slm, and VDBD (volume DBD):10 kV and 40 kHz, Ar at 0.05 slm | 5.38 for S. mutans and 5.67 for saliva biofilm | Titanium disc (10 min) | [31] |
| Pseudomonas aeruginosa and Staphylococcus epidermidis | Surface dielectric barrier discharge (SBD)-Structured electrode planar SBDA: (13 kV and 20 kHz) and a wire electrode SBD-B: (8 kV and 30 kHz with compressed air at 0.5 slm) | 7.1 and 3.8 log reduction by SBD-A in P. aeruginosa and SBD-B and 3.4 and 2.7 log reduction by SBD-A and SBD-B in S. epidermidis | Polycarbonate disc (10 min) | [53] |
| Candida albicans | Jet(KINPen09): 220 V and 50/60 Hz, 8 W power, Ar at 5 slm and Ar+(1%)O2 at 0.05 slm, HDBD:9 kV and 37.6 kHz, 9W power, Ar at 6 slm and Ar+(1%)O2 at 0.06 slm and VDBD: 10 kV and 40 kHz, 16 W power | 5 log reduction | Titanium disc (10 min) | [54] |
| Candida albicans | Surface microdischarge plasma technology (SMD): 9 kV and 1 kHz | 6 log reduction | 6 well plate (8 min) | [55] |
| Pseudomonas aeruginosa | Atomflo 300 reactor plasma Jet: 13.56 MHz and 100 W R, He at 20.4 L/min and N2 at 0.135 L/min at 35 W | Complete biofilm inactivation | Borosilicate coupon (30 min) | [56] |
| Pseudomonas aeruginosa and Staphylococcus epidermidis | Jet (Kinpen09): 2–6 kVpp and 1.1 MHz, 3.5 W power, Ar and Ar+(1%)O2 at 5 slm | 5.41 and 5.10 log reduction in Ar and Ar + O2 plasma for P. aeruginosa and 3.14 and 2.21 log reduction in Ar and Ar + O2 plasma for S. epidermidis | Microtiter plate (5 min) | [57] |
| Enterococcus faecalis | Plasma dental probe:6 kV and 1 kHz, 0.7 W power, He/(1%)O2 at 1 slm | 93.1% biofilm killing | Hydroxyapatite discs (5 min) | [58] |
| Bacillus cereus, Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa | Jet:6 kV and 20 kHz, He/(0.5%)O2 at 2 slm | Complete biofilm eradication | Peg lid of Calgary Biofilm Device (<4 min and 10 min) | [59] |
| Streptococci | Atmospheric pressure air plasma of corona discharge: Positive corona (PC) −8 kV and 20 kHz, negative corona (NC) −7 kV and 0.25 to 2 MHz | 3 log reduction | Tooth surfaces (10 min) | [60] |
| Candida albicans | Jet (KINPen 08): 2–6 kVpp and 1.7 MHz, Ar at 5 slm and Ar/[1%]O2 at 0.05 slm | Complete biofilm removal with Ar+O2 | Polystyrene (PS) wafers (5 min) | [61] |
| Pseudomonas aeruginosa | Atomflo 300 reactor plasma Jet: 13.56 MHz and 100 W RF, He at 20.4 L/min and N2 at 0.135 L/min at 35 W | 100% ianctivation | CDC biofilm reactor on borosilicate coupons (5 min) | [62] |
| Molecular Marker Involved in Plasma Treatment | Significance |
|---|---|
| 8-hydroxydeoxyguanosine (8-OHdG) and Ƴ-H2AX | Ubiquitous marker of oxidative stress and a by-product of oxidative DNA damage [99,100] |
| 3-nitrotyrosine | Protein oxidative damage marker causes chemical fragmentation, inactivation, and proteolytic degradation [100] |
| Proteinase K | Plasma exposure reduces the catalytic activity of proteinase K by damaging the protein [101] |
| Malondialdehyde (MDA) and 4-hydroxynonenal (4-HNE) | A marker for oxidative stress that measures lipid peroxidation. It damages DNA and proteins through the formation of covalent adducts [99,102] |
| Polyunsaturated fatty acids (PUFA) | Causes lipid peroxidation of bacterial cell membrane by extracting H atom from PUFA by plasma Reactive oxygen species (ROS) [98] |
| Intracellular ATP | Poly(ADP-ribose) polymerase-1 (PARP-1) results in decreased ATP level which signifies cell surface damage caused by leaking cellular proteins/nucleic acids [103,104] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, T.T.; Ayan, H. Application of Non-Thermal Plasma on Biofilm: A Review. Appl. Sci. 2019, 9, 3548. https://doi.org/10.3390/app9173548
Gupta TT, Ayan H. Application of Non-Thermal Plasma on Biofilm: A Review. Applied Sciences. 2019; 9(17):3548. https://doi.org/10.3390/app9173548
Chicago/Turabian StyleGupta, Tripti Thapa, and Halim Ayan. 2019. "Application of Non-Thermal Plasma on Biofilm: A Review" Applied Sciences 9, no. 17: 3548. https://doi.org/10.3390/app9173548
APA StyleGupta, T. T., & Ayan, H. (2019). Application of Non-Thermal Plasma on Biofilm: A Review. Applied Sciences, 9(17), 3548. https://doi.org/10.3390/app9173548





