Chromium (VI) Adsorption on Modified Activated Carbons
Abstract
1. Introduction
2. Materials and Methods
- -
- under static conditions from C0 = 1 ÷ 500 mg/dm3.
- -
- under dynamic conditions C0 = 1 mg/dm3.
2.1. Materials
- (a)
- Activated carbon WD—extra—which in the further part of the paper will be called WDA
- (b)
- Activated carbon WD—extra modified with hydrochloric acid (HCl), called WD(HCl)
- (c)
- Activated carbon WD—extra modified with nitric acid HNO3- marked as—WD(HNO3).
2.1.1. Modification of Activated Carbon with Hydrochloric Acid HCl
2.1.2. Modification of Activated Carbon with Nitric Acid HNO3
2.2. Tests under Static Conditions
- (a)
- determination of the solution pH and carbon modification effect on chromium sorption (pH 2–10), for C0 = 10 mg/dm3, carbon dose 1 g/dm3, adsorption time 12 h.
- (b)
- determination of the chromate ions sorption kinetics (for adsorption times from 0.25–12 h).
- (c)
- preparation of Freundlich adsorption isotherms for two selected carbons at three different temperatures 288.15 K (15 °C), 313.15 K (40 °C) and 338.15 K (65 °C) (for C0 = 1–500 mg/dm3 and carbon dose 1 g/dm3).
2.3. Tests under Flow-Through Conditions
- P—sorption capacity of the deposit (mg/g)
- V—volume of treated water (dm3)
- C0, Ck—initial and final concentration of chromium compounds (mg/dm3)
- M—mass of the deposit (g)
3. Results and Discussion
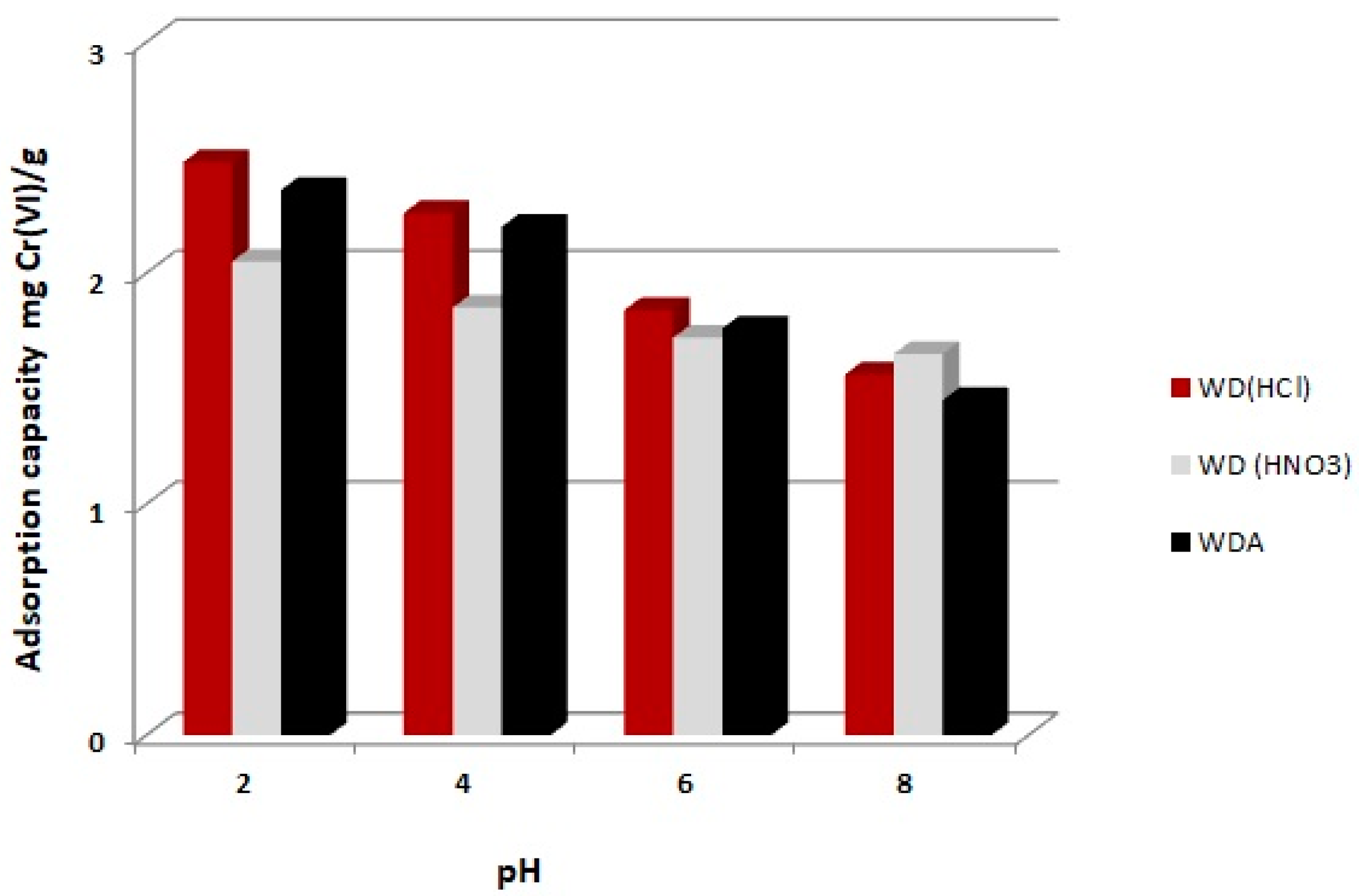
3.1. Effect of pH
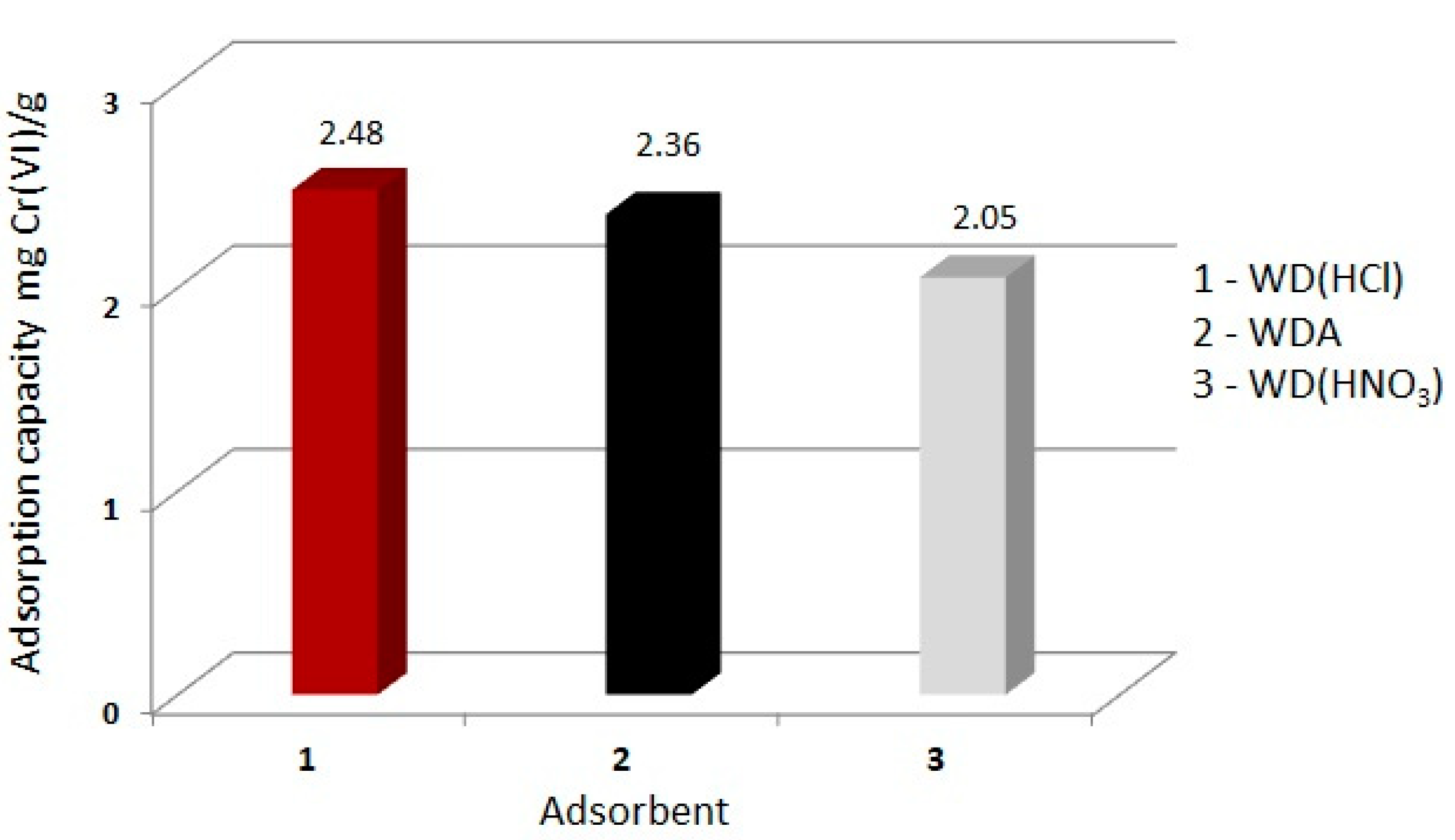
3.2. Effect of Modification on Adsorption
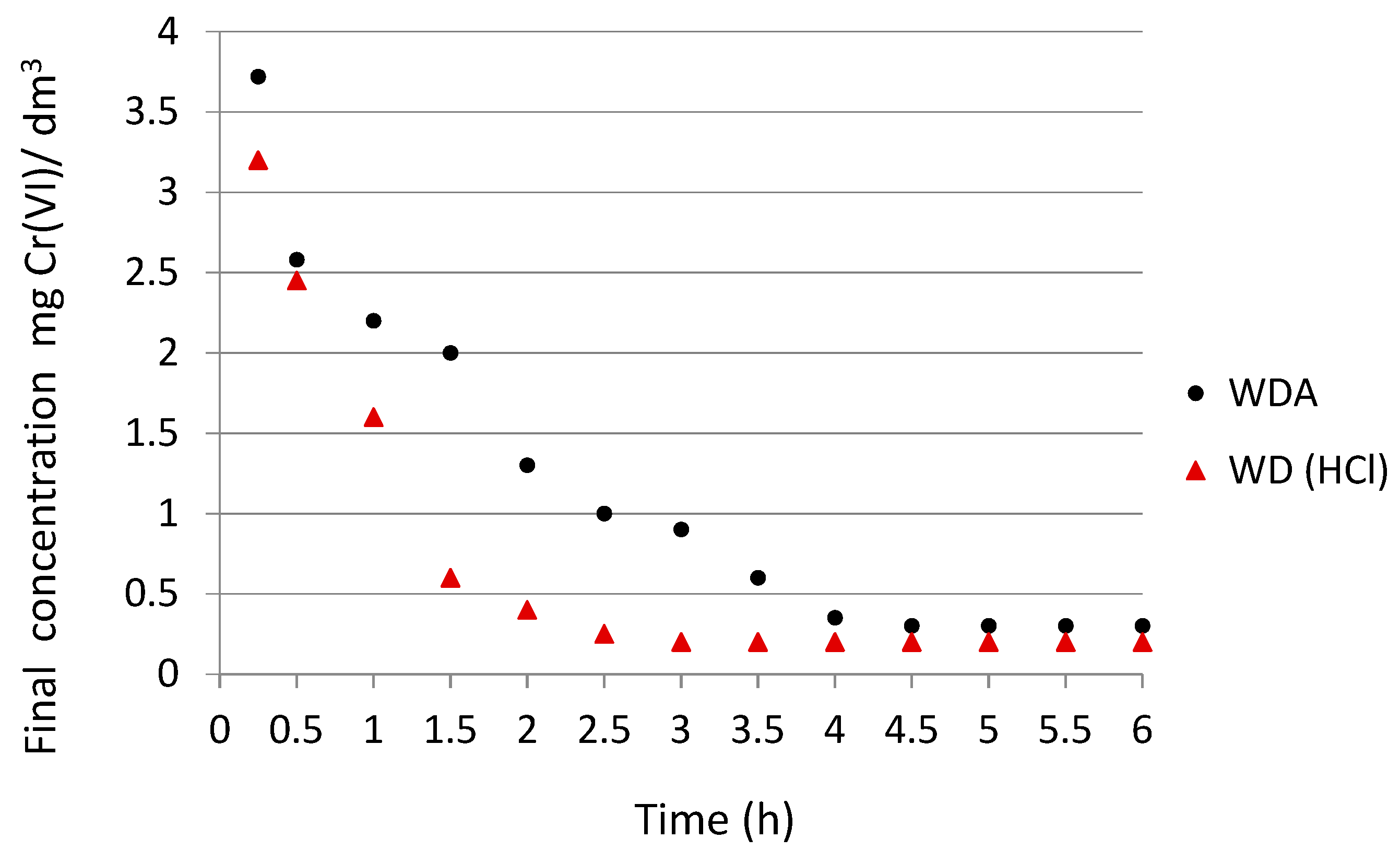
3.3. Adsorption Kinetics
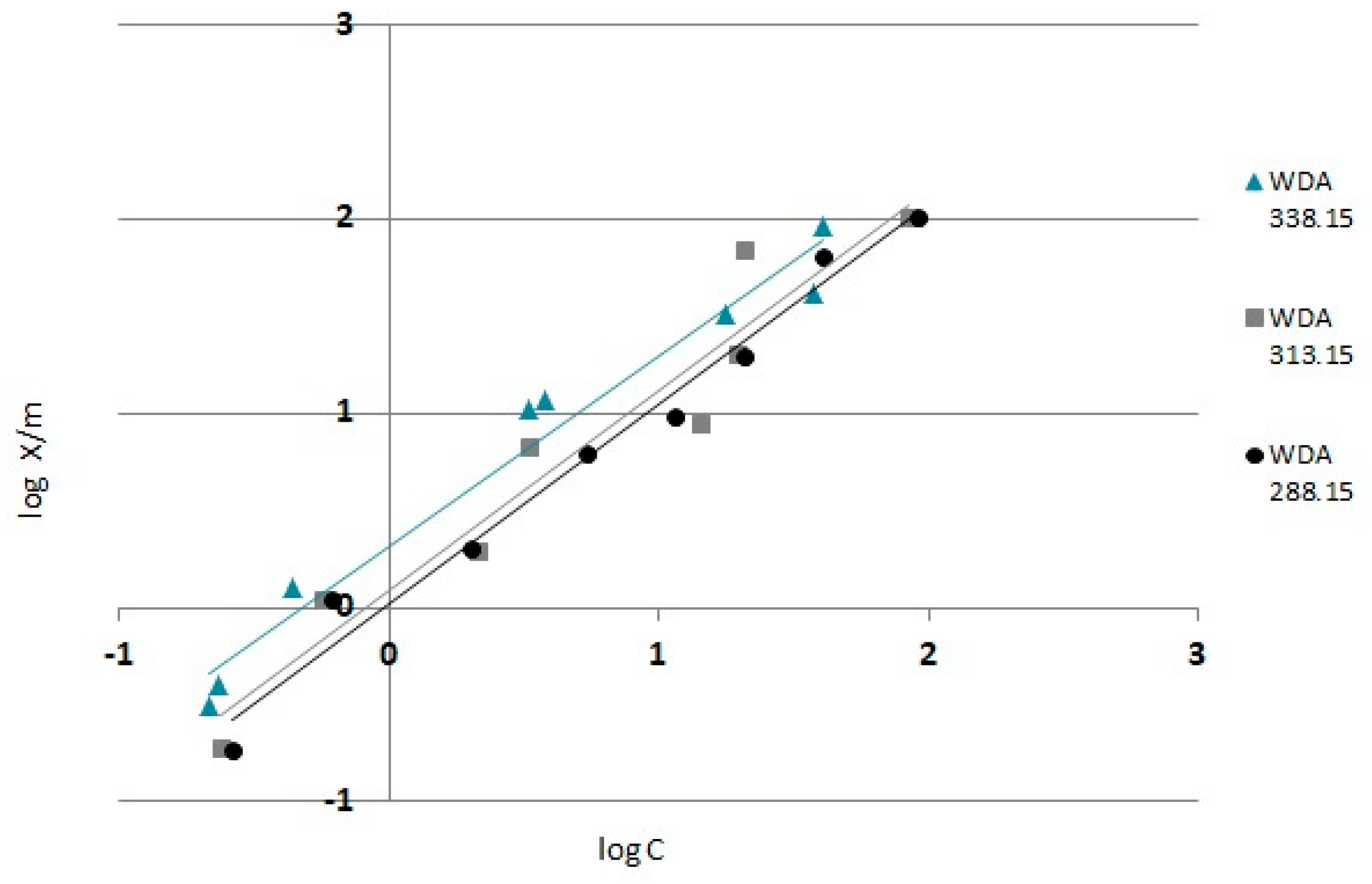
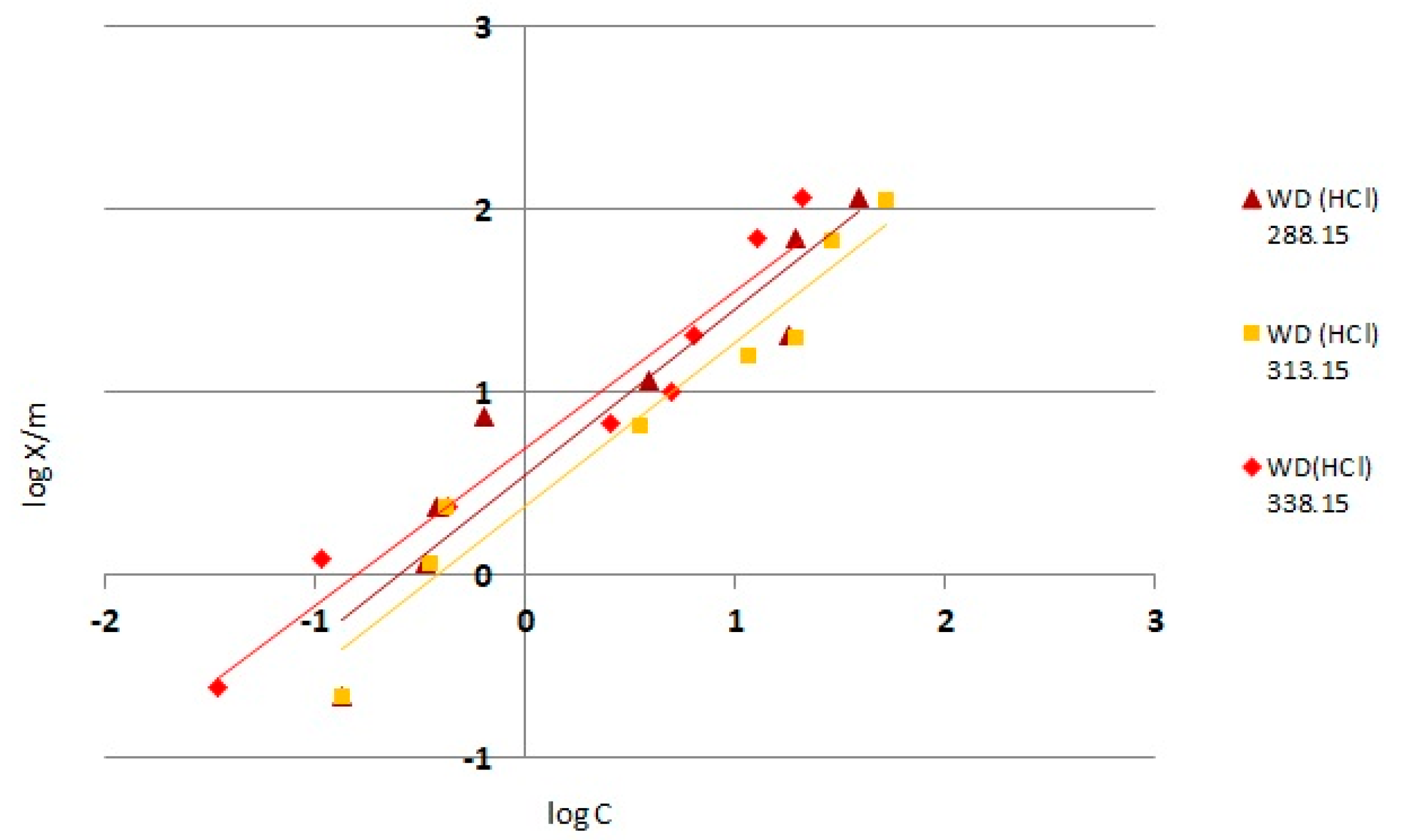
3.4. Adsorption Isotherms
- →
- X—amount of adsorbed substance [mg]
- →
- M—adsorbent mass [g]
- →
- C—equilibrium concentration [mg/dm3]
- →
- K, n—isotherm constants
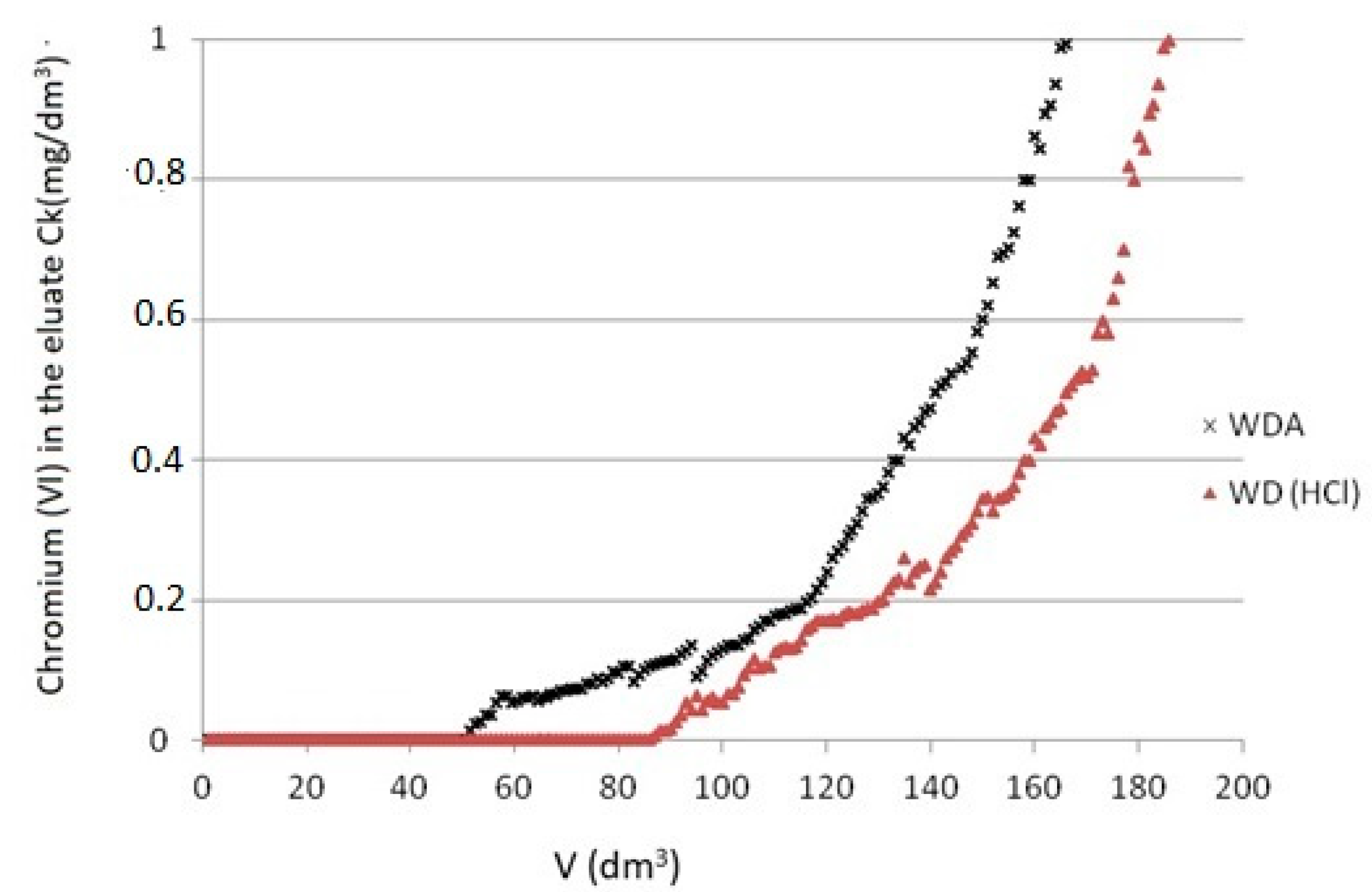
3.5. Adsorption Dynamics
4. Conclusions
- The solution pH significantly affected the adsorption capacity of the tested activated carbons. For the WD carbon (HNO3) the adsorption decreased slightly with increasing the solution pH. For carbons WDA and WD (HCl), the effect of the reaction on the chromium adsorption capacity was greater and definitely more beneficial at a lower pH.
- The action of inorganic acids (chemical modification) on activated commercial carbon WD-extra caused a change in its sorption properties. The use of hydrochloric acid contributed to the increase in the adsorption capacity of chromium (VI), by increasing the specific surface area of carbon as a result of the mesoporous ash removal.
- Modification with nitric acid (oxidizing) caused a decrease in the adsorption capacity, most likely associated with a change in the chemical nature of the carbon surface and partial destruction of the pore structure due to carbon oxidation.
- The adsorption time had a significant effect on the efficiency of chromium (VI) removal by the specific coal. For carbon WD(HCl) the adsorption equilibrium occurred after 2.5 h. The maximum reduction of chromate ions with respect to WDA occurred after 4.5 h.
- Analyzing the determined isotherms, it can be concluded that as the temperature increased, the adsorbents showed better adsorption properties, whereas the highest adsorption capacity of chromium (VI) was demonstrated by WD(HCl) modified carbon.
- Adsorption under flow through conditions showed that a modified WD(HCl) carbon bed worked much more efficiently than WDA. The filtration cycle to the bed breakthrough point lasted twice as long, and the chromium compounds were removed almost entirely. Adsorption capacities were obtained PbWD(HCl) = 2.25 mg/g (breakthrough point) and PeWD (HCl) = 4.35 mg/g (after exhaustion).
- In the light of the carried-out research, the modified WD (HCl) carbon effectively removed chromium (VI) compounds from water, which makes it possible to use it in water treatment systems.
Author Contributions
Funding
Conflicts of Interest
References
- Barabasz, W.; Chmiel, M.; Gałus, A.; Paśmionka, I. Ekotoksykologia chrome. Chem. Inżynieria Ekol. 1998, 8–9, 665–674. [Google Scholar]
- Altundogan, H.S. Cr(VI) removal from aqueous solution by iron (III) hyroxide-loaded sugar beet pulp. Process Biochem. 2005, 40, 1443–1452. [Google Scholar] [CrossRef]
- Ozkan, A.; Yekeler, M. Coagulation and flocculation characteristics of celesite with different inorganic salts and polymers. Chem. Eng. Process. 2004, 43, 873–879. [Google Scholar] [CrossRef]
- Dubey, P.S. Adsorption of chromium (VI) on low cost adsorbents derived from agricultural waste material: A comparative study. J. Hazard. Mater. 2007, 145, 465–470. [Google Scholar] [CrossRef]
- Puszkarewicz, A. Analiza adsorpcji fenolu na surowych i modyfikowanych diatomitach karpackich. Chem. Dydakt. Ekol. Metrol. 2010, 15, 189–192. [Google Scholar]
- Chang, Y.; Li, C.-W.; Benjamin, M.M. Iron oxide-coated media for NOM sorption and particulate filtration. J. Am. Water Work. Assoc. 1997, 89, 100–113. [Google Scholar] [CrossRef]
- Alther, G. Cleaning wastewater: Removing oil from water with organoclays. Filtr. Sep. 2008, 45, 22–24. [Google Scholar] [CrossRef]
- Dantas, T.D.C.; Neto, A.; Moura, M.D.A. Removal of chromium from aqueous solutions by diatomite treated with microemulsion. Water Res. 2001, 35, 2219–2224. [Google Scholar] [CrossRef]
- Ma, Y.; Gao, N.; Chu, W.; Li, C. Removal of phenol by powdered activated car-bon adsorption. Front. Environ. Sci. Eng. 2013, 7, 158–165. [Google Scholar] [CrossRef]
- Lalvani, S.B.; Wiltowski, T.; Hübner, A.; Weston, A.; Mandich, N. Removal of hexavalent chromium and metal cations by a selective and novel carbon adsorbent. Carbon 1998, 36, 1567–1571. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U., Jr. Activated carbons and low cost adsorbents for remediation of tri- and hexavalent chromium from water. J. Hazard. Mater. 2006, 137, 762–811. [Google Scholar] [CrossRef] [PubMed]
- Sajjad, M.; Khan, S.; Baig, S.A.; Munir, S.; Naz, A.; Ahmad, S.S.; Khan, A. Removal of potentially toxic elements from aqueous solutions and industrial wastewater using activated carbon. Water Sci. Technol. 2017, 75, 2571–2579. [Google Scholar] [CrossRef] [PubMed]
- Grabas, K. Sferyczne węgle aktywne do usuwania zanieczyszczeń organicznych z wody. Inżynieria Ochr. Środowiska 2000, 3–4, 435–441. [Google Scholar]
- Fazlzadeh, M.; Khosravi, R.; Zarei, A. Green synthesis of zinc oxide nanoparticles using Peganum harmala seed extract, and loaded on Peganum harmala seed powdered activated carbon as new adsorbent for removal of Cr(VI) from aqueous solution. Ecol. Eng. 2017, 103, 180–190. [Google Scholar] [CrossRef]
- Moralı, U.; Demiral, H.; Şensöz, S. Optimization of activated carbon production from sunflower seed extracted meal: Taguchi design of experiment approach and analysis of variance. J. Clean. Prod. 2018, 189, 602–611. [Google Scholar] [CrossRef]
- Zubrik, A.; Matik, M.; Hredzák, S.; Lovás, M.; Danková, Z.; Kováčová, M.; Briančin, J. Preparation of chemically activated carbon from waste biomass by single-stage and two-stage pyrolysis. J. Clean. Prod. 2017, 143, 643–653. [Google Scholar] [CrossRef]
- Choma, J.; Kloske, M. Otrzymywanie i właściwości impregnowanych węgli aktywnych. Ochr. Środowiska 1999, 21, 3–17. [Google Scholar]
- Sun, Y.; Yue, Q.; Gao, B.; Gao, Y.; Li, Q.; Wang, Y. Adsorption of hexavalent chromium on Arundo donax Linn activated carbon amine-crosslinked copolymer. Chem. Eng. J. 2013, 217, 240–247. [Google Scholar] [CrossRef]
- Yao, S.; Zhang, J.; Shen, D.; Xiao, R.; Gu, S.; Zhao, M.; Liang, J. Removal of Pb(II) from water by the activated carbon modified by nitric acid under microwave heating. J. Colloid Interface Sci. 2016, 463, 118–127. [Google Scholar] [CrossRef]
- Lach, J. Lach Adsorption of Chloramphenicol on Commercial and Modified Activated Carbons. Water 2019, 11, 1141. [Google Scholar] [CrossRef]
- Yorgun, S.; Vural, N.; Demiral, H. Preparation of high-surface area activated carbons from Paulownia wood by ZnCl2 activation. Microporous Mesoporous Mater. 2009, 122, 189–194. [Google Scholar] [CrossRef]
- Selomulya, C.; Meeyoo, V.; Amal, R. Mechanisms of Cr(VI) removal from water by various types of activated carbons. J. Chem. Technol. Biotech. 1999, 74, 111–122. [Google Scholar] [CrossRef]
- Karnjanakom, S.; Maneechakr, P. Adsorption behaviors and capacities of Cr(VI) onto environmentally activated carbon by cationic (HDTMA and DDAB) surfacants. J. Mol. Struct. 2019, 1186, 80–90. [Google Scholar] [CrossRef]
- Jain, M.; Yadaw, M.; Kohout, T. Development of iron oxide/ activated carbon nanoparticle composite for removal of Cr(VI), Cu(II) and Cd(II) ions from agueous solution. Water Resour. Ind. 2018, 20, 54–74. [Google Scholar] [CrossRef]
- Janusz, W. The electrical double layer parameters for the group 4 metal oxide/electrolyte system. Adsorpt. Sci. Technol. 2000, 18, 117–134. [Google Scholar] [CrossRef]
- Sorbak, Z. Wykorzystanie sorbentów w procesach oczyszczania gruntu. Chem. Dydakt. Ekol. Metrol. 2010, 15, 77–92. [Google Scholar]
- Lach, J. Chromium Adsorption from Waters of Different Chemical Composition. Environ. Prot. Eng. 2016, 19, 353–362. [Google Scholar] [CrossRef]
- Repelewicz, M.; Jedynak, K.; Choma, J. Struktura porowata i chemia powierzchni węgli aktywnych modyfikowanych kwasami nieorganicznymi. Ochrona Śr. 2009, 31, 45–50. [Google Scholar]
- Rai, M.; Shahi, G.; Meena, V.; Meena, R.; Chakraborty, S.; Singh, R.; Rai, B. Removal of hexavalent chromium Cr (VI) using activated carbon prepared from mango kernel activated with H3PO4. Resour. Technol. 2016, 2, S63–S70. [Google Scholar] [CrossRef]
- Yüksel, S.; Orhan, R. The Removal of Cr(VI) from Aqueous Solution by Activated Carbon Prepared from Apricot, Peach Stone and Almond Shell Mixture in a Fixed-Bed Column. Arab. J. Sci. Eng. 2019, 44, 5345–5357. [Google Scholar] [CrossRef]
- An, F.; Gao, B.; Feng, X. Adsorption mechanism and property of novel composite material PMAA/SiO2 towards phenol. Chem. Eng. J. 2009, 153, 108–113. [Google Scholar] [CrossRef]
| Indicator | Volume |
|---|---|
| Density bulk density, [g/L] | 390 ÷ 415 |
| Granulation, [mm] | 1 ÷ 1.5 |
| Specific surface, [m2/g] | 950 ÷ 1050 |
| Aggregate volume of pores, [cm3/g] | 0.85 ÷ 0.95 |
| Adsorption of iodine, [mg/g] | 900 ÷ 1000 |
| Dechloration capacity, [cm] | 4 ÷ 5 |
| Mechanical durability [%] | 90 |
| Temperature | N | K | Coefficients R2 |
|---|---|---|---|
| 288.15 K | 0.972 | 1.035 | 0.97 |
| 313.15 K | 0.973 | 1.238 | 0.93 |
| 338.15 K | 1.028 | 2.032 | 0.97 |
| Temperature | N | K | Coefficients R2 |
|---|---|---|---|
| 288.15 K | 1.179 | 2.454 | 0.94 |
| 313.15 K | 1.106 | 3.589 | 0.90 |
| 338.15 K | 1.157 | 5.073 | 0.95 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puszkarewicz, A.; Kaleta, J. Chromium (VI) Adsorption on Modified Activated Carbons. Appl. Sci. 2019, 9, 3549. https://doi.org/10.3390/app9173549
Puszkarewicz A, Kaleta J. Chromium (VI) Adsorption on Modified Activated Carbons. Applied Sciences. 2019; 9(17):3549. https://doi.org/10.3390/app9173549
Chicago/Turabian StylePuszkarewicz, Alicja, and Jadwiga Kaleta. 2019. "Chromium (VI) Adsorption on Modified Activated Carbons" Applied Sciences 9, no. 17: 3549. https://doi.org/10.3390/app9173549
APA StylePuszkarewicz, A., & Kaleta, J. (2019). Chromium (VI) Adsorption on Modified Activated Carbons. Applied Sciences, 9(17), 3549. https://doi.org/10.3390/app9173549





