1 h Postload Glycemia Is Associated with Low Endogenous Secretory Receptor for Advanced Glycation End Product Levels and Early Markers of Cardiovascular Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Biochemical Analyses
2.3. Carotid Ultrasound Examination
2.4. Arterial Stiffness Evaluation
2.4.1. Pulse Wave Velocity
2.4.2. Pulse Wave Analysis
2.5. Statistical Analyses
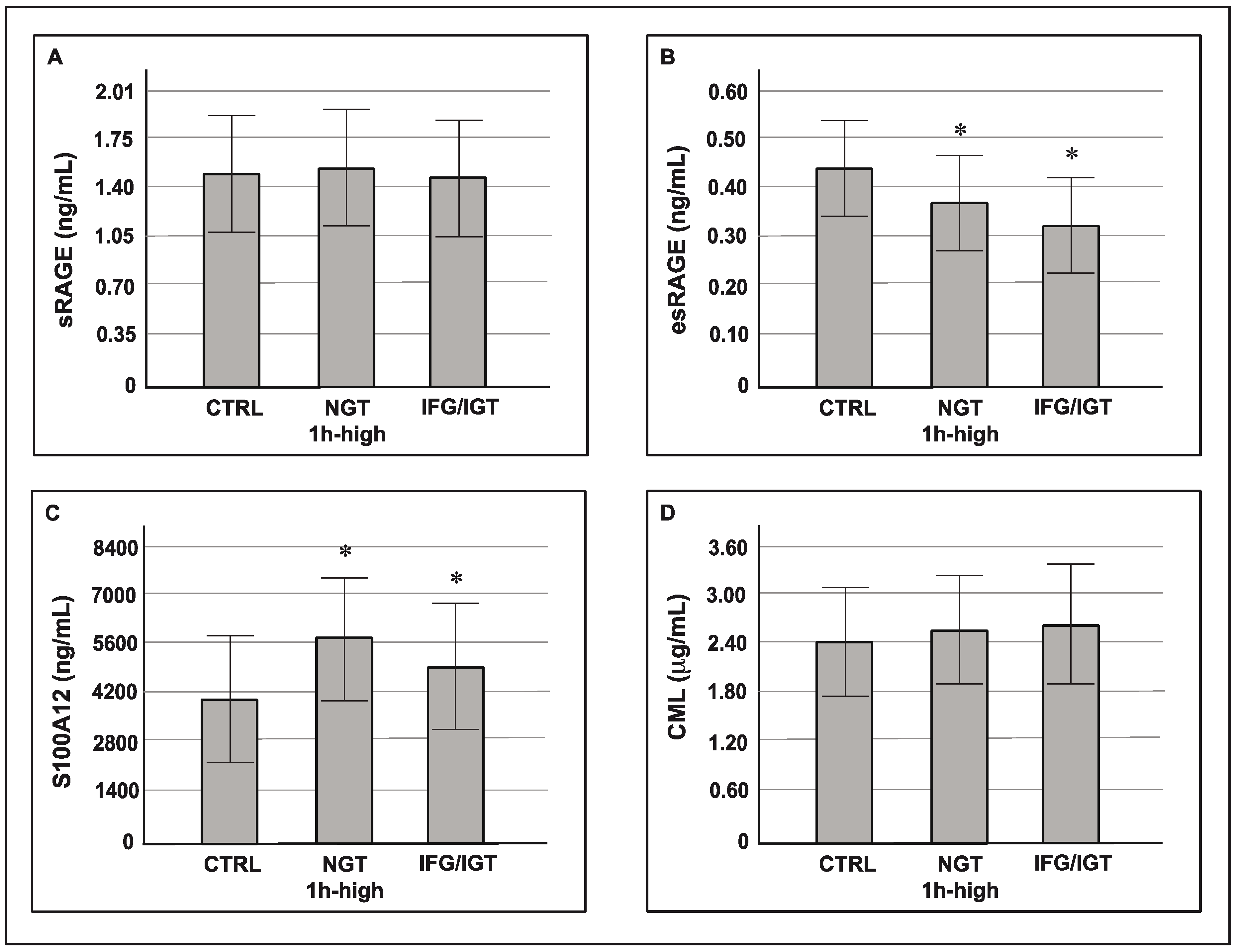
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Unwin, N.; Shaw, J.; Zimmet, P.; Alberti, K.G.M.M. Impaired glucose tolerance and impaired fasting glycaemia: The current status on definition and intervention. Diabet. Med. 2002, 19, 708–723. [Google Scholar] [PubMed]
- Di Pino, A.; DeFronzo, R.A. Insulin Resistance and Atherosclerosis: Implications for Insulin Sensitizing Agents. Endocr. Rev. 2019. [Google Scholar] [CrossRef] [PubMed]
- Matthew, C.; Riddle, M. Standards of Medical Care in Diabetes—2019. Available online: https://www.passeidireto.com/arquivo/62387919/standards-of-medical-care-in-diabetes-2019 (accessed on 25 January 2019).
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M. Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [PubMed]
- Li, G.; Zhang, P.; Wang, J.; An, Y.; Gong, Q.; Gregg, E.W.; Yang, W.; Zhang, B.; Shuai, Y.; Hong, J.; et al. Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: A 23-year follow-up study. Lancet Diabetes Endocrinol. 2014, 2, 474–480. [Google Scholar] [CrossRef]
- Uusitupa, M.; Peltonen, M.; Lindstrom, J.; Aunola, S.; Ilanne-Parikka, P.; Keinanen-Kiukaanniemi, S.; Valle, T.T.; Eriksson, J.G.; Tuomilehto, J. Ten-year mortality and cardiovascular morbidity in the Finnish Diabetes Prevention Study—Secondary analysis of the randomized trial. PLoS ONE 2009, 4, e5656. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Ghani, M.A.; Abdul-Ghani, T.; Ali, N.; Defronzo, R.A. One-hour plasma glucose concentration and the metabolic syndrome identify subjects at high risk for future type 2 diabetes. Diabetes Care 2008, 31, 1650–1655. [Google Scholar] [CrossRef]
- Fiorentino, T.V.; Marini, M.A.; Andreozzi, F.; Arturi, F.; Succurro, E.; Perticone, M.; Sciacqua, A.; Hribal, M.L.; Perticone, F.; Sesti, G. One-hour postload hyperglycemia is a stronger predictor of type 2 diabetes than impaired fasting glucose. J. Clin. Endocrinol. Metab. 2015, 100, 3744–3751. [Google Scholar] [CrossRef]
- Priya, M.; Anjana, R.M.; Chiwanga, F.S.; Gokulakrishnan, K.; Deepa, M.; Mohan, V. 1-Hour Venous Plasma Glucose and Incident Prediabetes and Diabetes in Asian Indians. Diabetes Technol. Ther. 2013, 15, 497–502. [Google Scholar] [CrossRef]
- Alyass, A.; Almgren, P.; Akerlund, M.; Dushoff, J.; Isomaa, B.; Nilsson, P.; Tuomi, T.; Lyssenko, V.; Groop, L.; Meyre, D. Modelling of OGTT curve identifies 1 h plasma glucose level as a strong predictor of incident type 2 diabetes: Results from two prospective cohorts. Diabetologia 2015, 58, 87–97. [Google Scholar] [CrossRef]
- Succurro, E.; Marini, M.A.; Arturi, F.; Grembiale, A.; Lugarà, M.; Andreozzi, F.; Sciacqua, A.; Lauro, R.; Hribal, M.L.; Perticone, F.; et al. Elevated one-hour post-load plasma glucose levels identifies subjects with normal glucose tolerance but early carotid atherosclerosis. Atherosclerosis 2009, 207, 245–249. [Google Scholar] [CrossRef]
- Marcovecchio, M.L.; Bagordo, M.; Marisi, E.; de Giorgis, T.; Chiavaroli, V.; Chiarelli, F.; Mohn, A. One-hour post-load plasma glucose levels associated with decreased insulin sensitivity and secretion and early makers of cardiometabolic risk. J. Endocrinol. Investig. 2017, 40, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Andreozzi, F.; Mannino, G.C.; Perticone, M.; Perticone, F.; Sesti, G. Elevated 1-h post-load plasma glucose levels in subjects with normal glucose tolerance are associated with a pro-atherogenic lipid profile. Atherosclerosis 2017, 256, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Basta, G. Receptor for advanced glycation endproducts and atherosclerosis: From basic mechanisms to clinical implications. Atherosclerosis 2008, 196, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Yonekura, H.; Yamamoto, Y.; Sakurai, S.; Petrova, R.G.; Abedin, M.J.; Li, H.; Yasui, K.; Takeuchi, M.; Makita, Z.; Takasawa, S.; et al. Novel splice variants of the receptor for advanced glycation end-products expressed in human vascular endothelial cells and pericytes, and their putative roles in diabetes-induced vascular injury. Biochem. J. 2003, 370, 1097–1109. [Google Scholar] [CrossRef] [PubMed]
- Selvin, E.; Steffes, M.W.; Zhu, H.; Matsushita, K.; Wagenknecht, L.; Pankow, J.; Coresh, J.; Brancati, F.L. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N. Engl. J. Med. 2010, 362, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Malmstedt, J.; Kärvestedt, L.; Swedenborg, J.; Brismar, K. The receptor for advanced glycation end products and risk of peripheral arterial disease, amputation or death in type 2 diabetes: A population-based cohort study. Cardiovasc. Diabetol. 2015, 14. [Google Scholar] [CrossRef] [PubMed]
- Di Pino, A.; Urbano, F.; Zagami, R.M.; Filippello, A.; Di Mauro, S.; Piro, S.; Purrello, F.; Rabuazzo, A.M. Low Endogenous Secretory Receptor for Advanced Glycation End-Products Levels Are Associated With Inflammation and Carotid Atherosclerosis in Prediabetes. J. Clin. Endocrinol. Metab. 2016, 101, 1701–1709. [Google Scholar] [CrossRef] [Green Version]
- Calanna, S.; Urbano, F.; Piro, S.; Zagami, R.M.; Di Pino, A.; Spadaro, L.; Purrello, F.; Rabuazzo, A.M. Elevated plasma glucose-dependent insulinotropic polypeptide associates with hyperinsulinemia in metabolic syndrome. Eur. J. Endocrinol. 2012, 166, 917–922. [Google Scholar] [CrossRef] [Green Version]
- Calanna, S.; Scicali, R.; Di Pino, A.; Knop, F.K.; Piro, S.; Rabuazzo, A.M.; Purrello, F. Alpha- and beta-cell abnormalities in haemoglobin A1c-defined prediabetes and type 2 diabetes. Acta Diabetol. 2014, 51, 567–575. [Google Scholar] [CrossRef]
- Di Pino, A.; Currenti, W.; Urbano, F.; Scicali, R.; Piro, S.; Purrello, F.; Rabuazzo, A.M. High intake of dietary advanced glycation end-products is associated with increased arterial stiffness and inflammation in subjects with type 2 diabetes. Nutr. Metab. Cardiovasc. Dis. 2017, 11, 978–984. [Google Scholar] [CrossRef]
- Mosca, A.; Goodall, I.; Hoshino, T.; Jeppsson, J.O.; John, W.G.; Little, R.R.; Miedema, K.; Myers, G.L.; Reinauer, H.; Sacks, D.B.; et al. Global standardization of glycated hemoglobin measurement: The position of the IFCC Working Group. Clin. Chem. Lab. Med. 2007, 45, 1077–1080. [Google Scholar] [CrossRef]
- Zagami, R.M.; Di Pino, A.; Urbano, F.; Piro, S.; Purrello, F.; Rabuazzo, A.M. Low circulating Vitamin D levels are associated with increased arterial stiffness in prediabetic subjects identified according to HbA1c. Atherosclerosis 2015, 243, 395–401. [Google Scholar] [CrossRef]
- Di Pino, A.; Alagona, C.; Piro, S.; Calanna, S.; Spadaro, L.; Palermo, F.; Urbano, F.; Purrello, F.; Rabuazzo, A.M. Separate impact of metabolic syndrome and altered glucose tolerance on early markers of vascular injuries. Atherosclerosis 2012, 223, 458–462. [Google Scholar] [CrossRef]
- Marini, M.A.; Fiorentino, T.V.; Andreozzi, F.; Mannino, G.C.; Perticone, M.; Sciacqua, A.; Perticone, F.; Sesti, G. Elevated 1-h post-challenge plasma glucose levels in subjects with normal glucose tolerance or impaired glucose tolerance are associated with whole blood viscosity. Acta Diabetol. 2017, 54, 775–784. [Google Scholar] [CrossRef] [Green Version]
- Succurro, E.; Arturi, F.; Grembiale, A.; Iorio, F.; Fiorentino, T.V.; Andreozzi, F.; Sciacqua, A.; Hribal, M.L.; Perticone, F.; Sesti, G. One-hour post-load plasma glucose levels are associated with elevated liver enzymes. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 713–718. [Google Scholar] [CrossRef]
- Perticone, F.; Sciacqua, A.; Perticone, M.; Arturi, F.; Scarpino, P.E.; Quero, M.; Sesti, G. Serum uric acid and 1-h postload glucose in essential hypertension. Diabetes Care 2012, 35, 153–157. [Google Scholar] [CrossRef]
- Sciacqua, A.; Perticone, M.; Grillo, N.; Falbo, T.; Bencardino, G.; Angotti, E.; Arturi, F.; Parlato, G.; Sesti, G.; Perticone, F. Vitamin D and 1-h post-load plasma glucose in hypertensive patients. Cardiovasc. Diabetol. 2014, 13, 1–8. [Google Scholar] [CrossRef]
- Sesti, G.; Fiorentino, T.V.; Succurro, E.; Perticone, M.; Arturi, F.; Sciacqua, A.; Perticone, F. Elevated 1-h post-load plasma glucose levels in subjects with normal glucose tolerance are associated with unfavorable inflammatory profile. Acta Diabetol. 2014, 51, 927–932. [Google Scholar] [CrossRef]
- Di Pino, A.; Mangiafico, S.; Urbano, F.; Scicali, R.; Scandura, S.; D’Agate, V.; Piro, S.; Tamburino, C.; Purrello, F.; Rabuazzo, A.M. HbA 1c identifies subjects with prediabetes and subclinical left ventricular diastolic dysfunction. J. Clin. Endocrinol. Metab. 2017, 102, 3756–3764. [Google Scholar] [CrossRef]
- Katakami, N.; Matsuhisa, M.; Kaneto, H.; Matsuoka, T.-A.; Sakamoto, K.; Yasuda, T.; Yamasaki, Y. Endogenous secretory RAGE but not soluble RAGE is associated with carotid atherosclerosis in type 1 diabetes patients. Diabetes Vasc. Dis. Res. 2008, 5, 190–197. [Google Scholar] [CrossRef] [Green Version]
- Koyama, H.; Shoji, T.; Yokoyama, H.; Motoyama, K.; Mori, K.; Fukumoto, S.; Emoto, M.; Shoji, T.; Tamei, H.; Matsuki, H.; et al. Plasma level of endogenous secretory RAGE is associated with components of the metabolic syndrome and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2587–2593. [Google Scholar] [CrossRef]
- Sciacqua, A.; Miceli, S.; Greco, L.; Arturi, F.; Naccarato, P.; Mazzaferro, D.; Tassone, E.J.; Turano, L.; Martino, F.; Sesti, G.; et al. One-hour postload plasma glucose levels and diastolic function in hypertensive patients. Diabetes Care 2011, 34, 2291–2296. [Google Scholar] [CrossRef]
- Fiorentino, T.V.; Andreozzi, F.; Mannino, G.C.; Pedace, E.; Perticone, M.; Sciacqua, A.; Perticone, F.; Sesti, G. One-hour postload hyperglycemia confers higher risk of hepatic steatosis to HbA1c-defined prediabetic subjects. J. Clin. Endocrinol. Metab. 2016, 101, 4030–4038. [Google Scholar] [CrossRef]
| NGT 1 h <155 mg/dL (n = 123) | NGT 1 h ≥155 mg/dL (n = 84) | IFG/IGT (n = 75) | |
|---|---|---|---|
| Age (year) | 45 ± 10.2 | 47.5 ± 10 * | 49.5 ± 7.8 *# |
| BMI (Kg/m2) | 29.5 ± 5 | 29.2 ± 4 | 29.8 ± 6.1 |
| Waist circumference (cm) | 99.9 ± 11.7 | 100 ± 10.8 | 98.3 ± 9.6 |
| Fasting glucose (mg/dL) | 86.4 ± 8.6 | 90.5 ± 8.6 * | 99.4 ± 14 *# |
| 1 h postload glucose (mg/dL) | 121.3 ± 21.1 | 176 ± 22.4 * | 187.6 ± 35.4 |
| 2 h postload glucose (mg/dL) | 105.3 ± 22 | 119 ± 21.4 * | 168.4 ± 22.4 |
| HbA1c (%) | 5.6 ± 0.3 | 5.8 ± 0.38 * | 6.0 ± 0.31 |
| Fasting insulin (μU/mL) | 7.2 ± 3.6 | 9 ± 6.5 | 10.8 ± 5.6 |
| Total cholesterol (mg/dL) | 192.2 ± 36.7 | 197.4 ± 42.1 | 199.4 ± 40.4 |
| HDL cholesterol (mg/dL) | 49.1 ± 11.4 | 44 ± 12.3 * | 43.1 ± 11.8 * |
| Triglycerides (mg/dL) | 86 (66–122) | 100 (76–137) | 125.5 (85–173) |
| LDL cholesterol (mg/dL) | 124.1 ± 32.4 | 128.4 ± 41.1 * | 125.2 ± 33.5 |
| Systolic BP (mmHg) | 118.2 ± 15.6 | 121 ± 13.5 | 123.9 ± 14.2 * |
| Diastolic BP (mmHg) | 73.2 ± 11 | 74.4 ± 10.6 | 75.1 ± 10.4 |
| HOMA-IR | 1.55 ± 0.84 | 2.09 ± 1.55 * | 2.3 ± 1.5 *# |
| Hypertension | 16% | 26% | 31% |
| ACE inhibitors/ARB | 50% | 50% | 52% |
| Calcium channel blockers | 20% | 22% | 22% |
| Beta blockers | 5% | 9% | 8% |
| Thiazide diuretics | 15% | 5% | 8% |
| No therapy | 10% | 14% | 12% |
| Statin therapy | 18% | 20% | 22% |
| Active smokers | 30% | 26% | 34% |
| Sex (M/F) | 41/82 | 41/42 | 35/40 |
| NGT 1 h <155 mg/dL (n = 123) | NGT 1 h ≥155 mg/dL (n = 84) | IFG/IGT (n = 75) | |
|---|---|---|---|
| IMT (mm) | 0.69 (0.56–0.71) | 0.78 (0.68–0.83) * | 0.83 (0.71–0.92) *# |
| PWV (cm/sec) | 7.22 ± 1.6 | 7.7 ± 1.4 * | 8.2 ± 1.6 *# |
| Aug P (mmHg) | 9.2 ± 6.5 | 11.6 ± 6.1 * | 12.7 ± 6.1 * |
| Aug I (%) | 25.2 ± 12 | 28.6 ± 11.7 * | 30.6 ± 11.7 * |
| SEVR (%) | 162 ± 27.7 | 158 ± 30.8 | 158.2 ± 32.3 |
| Coefficient β | p-Value | |
|---|---|---|
| esRAGE | ||
| Model 1 * | ||
| Systolic BP | 0.29 | 0.03 |
| Model 2 ** | ||
| HbA1c | −0.18 | 0.01 |
| Model 3 *** | ||
| HbA1c | −0.27 | 0.05 |
| hs-CRP | −0.35 | 0.04 |
| IMT | ||
| Model 1 * | ||
| age | 0.52 | 0.001 |
| Model 2 ** | ||
| age | 0.51 | 0.001 |
| HbA1c | 0.2 | 0.05 |
| Model 3 *** | ||
| age | 0.51 | 0.001 |
| HbA1c | 0.35 | 0.04 |
| esRAGE | −0.21 | 0.005 |
| PWV | ||
| Model 1 * | ||
| age | 0.25 | 0.006 |
| Systolic BP | 2.4 | 0.01 |
| HDL | −2.5 | 0.01 |
| Model 2 ** | ||
| age | 0.16 | 0.04 |
| Systolic BP | 0.31 | 0.001 |
| Model 3 *** | ||
| S100A12 | 0.31 | 0.04 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Pino, A.; Urbano, F.; Scicali, R.; Di Mauro, S.; Filippello, A.; Scamporrino, A.; Piro, S.; Purrello, F.; Rabuazzo, A.M. 1 h Postload Glycemia Is Associated with Low Endogenous Secretory Receptor for Advanced Glycation End Product Levels and Early Markers of Cardiovascular Disease. Cells 2019, 8, 910. https://doi.org/10.3390/cells8080910
Di Pino A, Urbano F, Scicali R, Di Mauro S, Filippello A, Scamporrino A, Piro S, Purrello F, Rabuazzo AM. 1 h Postload Glycemia Is Associated with Low Endogenous Secretory Receptor for Advanced Glycation End Product Levels and Early Markers of Cardiovascular Disease. Cells. 2019; 8(8):910. https://doi.org/10.3390/cells8080910
Chicago/Turabian StyleDi Pino, Antonino, Francesca Urbano, Roberto Scicali, Stefania Di Mauro, Agnese Filippello, Alessandra Scamporrino, Salvatore Piro, Francesco Purrello, and Agata Maria Rabuazzo. 2019. "1 h Postload Glycemia Is Associated with Low Endogenous Secretory Receptor for Advanced Glycation End Product Levels and Early Markers of Cardiovascular Disease" Cells 8, no. 8: 910. https://doi.org/10.3390/cells8080910






