Glycation of Plant Proteins: Regulatory Roles and Interplay with Sugar Signalling?
Abstract
:1. Introduction
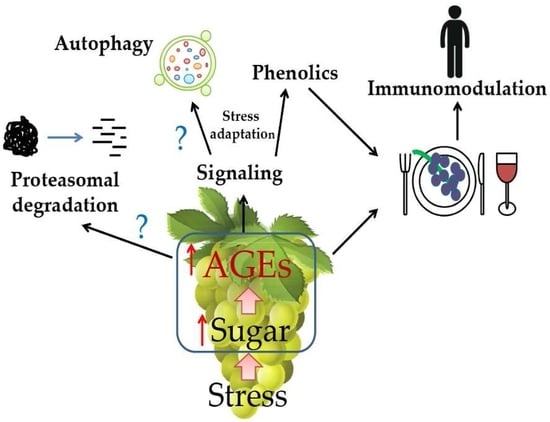
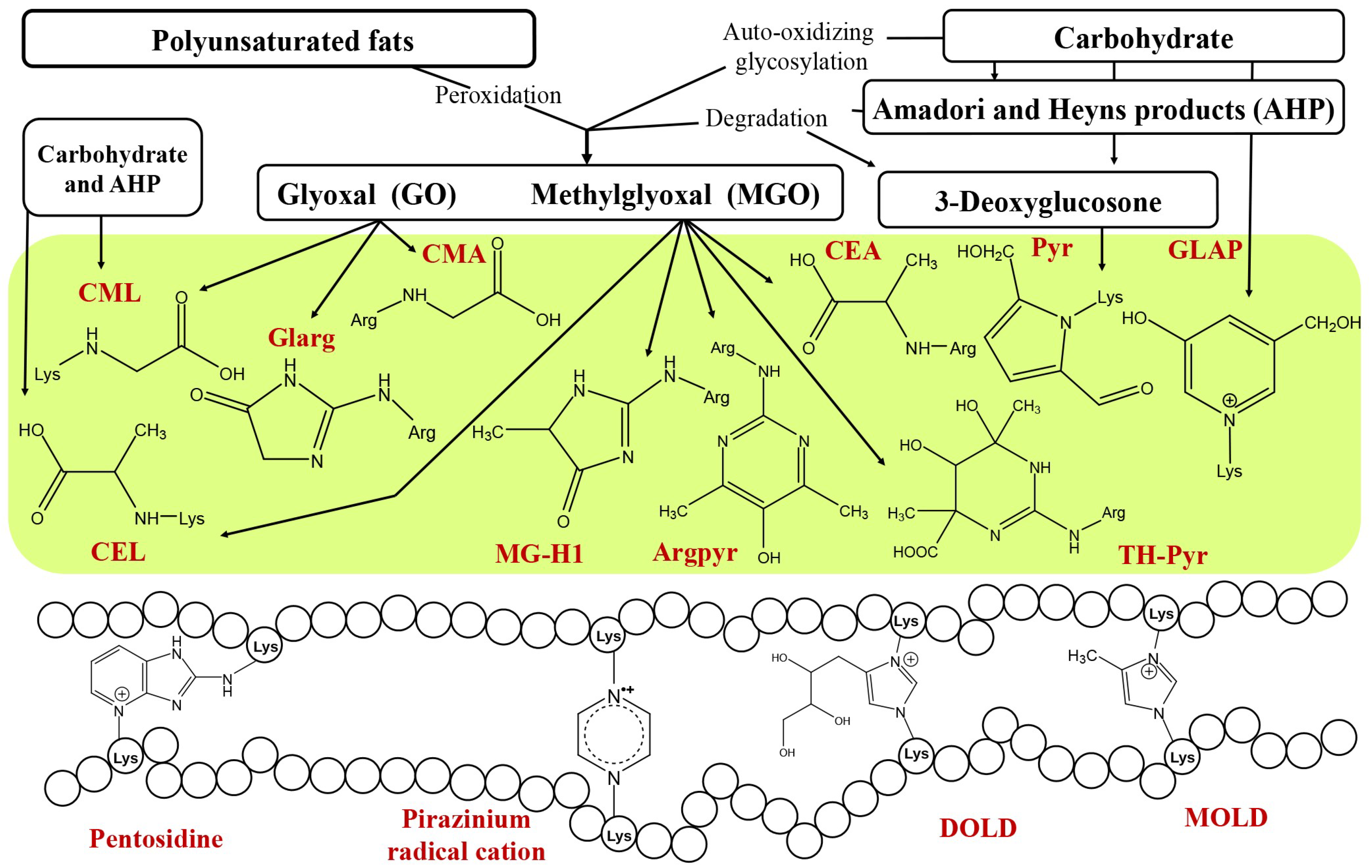
2. AGEs in Plant-Derived Foods
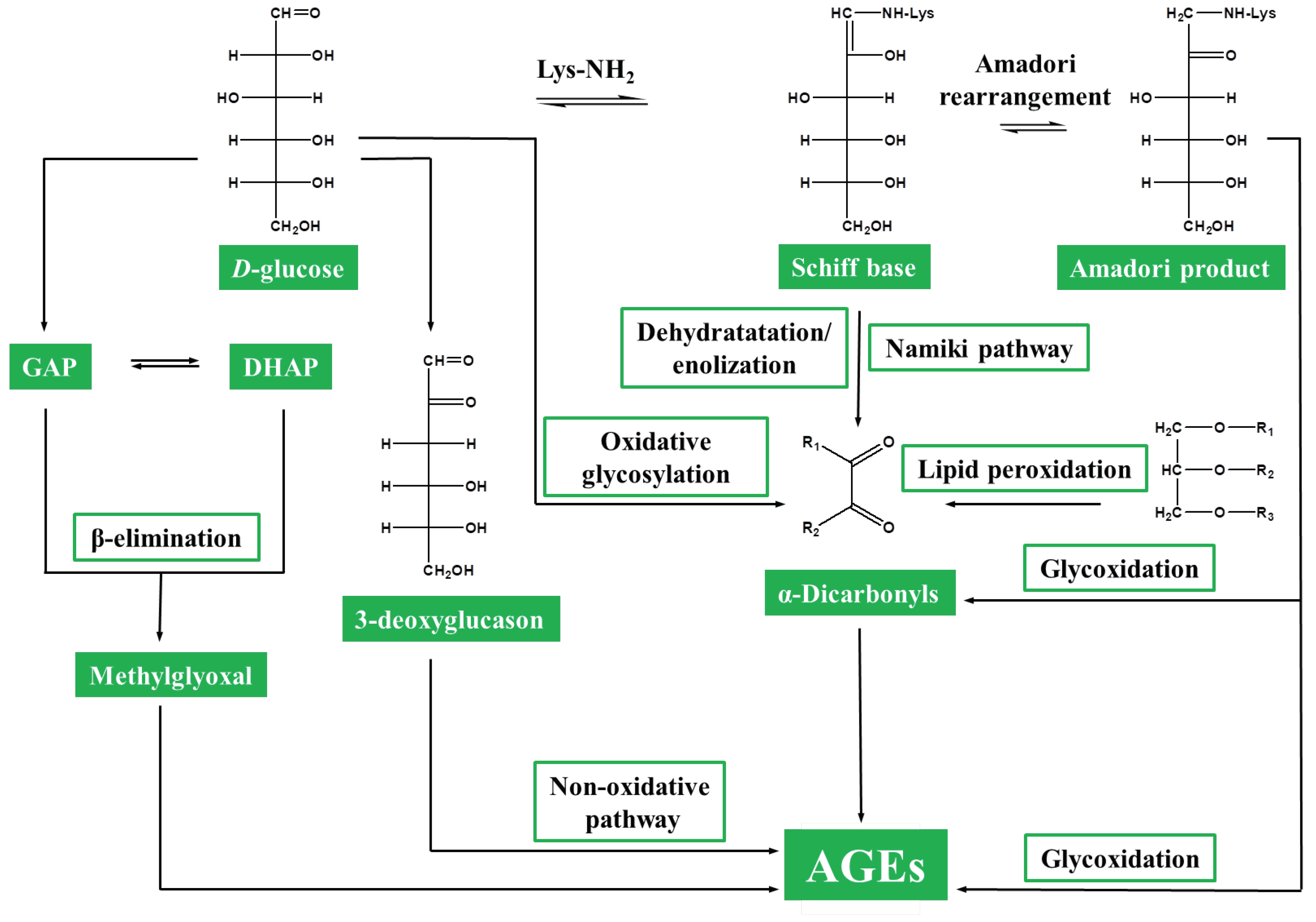
3. Protein Glycation in Plants: From Non-Specificity to Glycation Hotspots
4. Possible Role of Protein Glycation in Plant Physiology
4.1. Glycation as the Marker of Ageing, Senescence and Tag for Protein Degradation
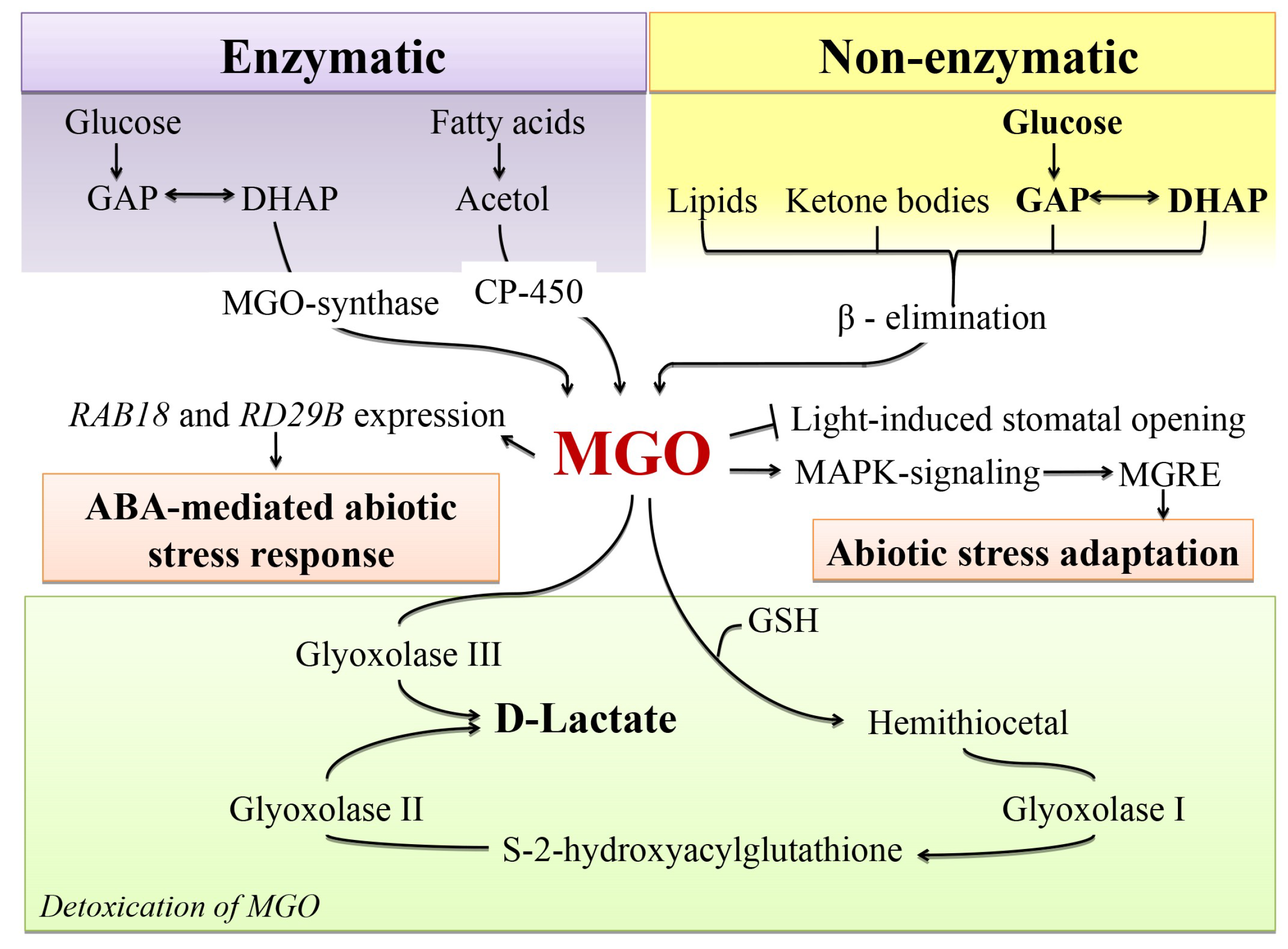
4.2. Glycation as a Possible Mechanism behind MGO Signalling
4.3. Glycation and Non-Enzymatic Antiglycative/Antioxidant Defense
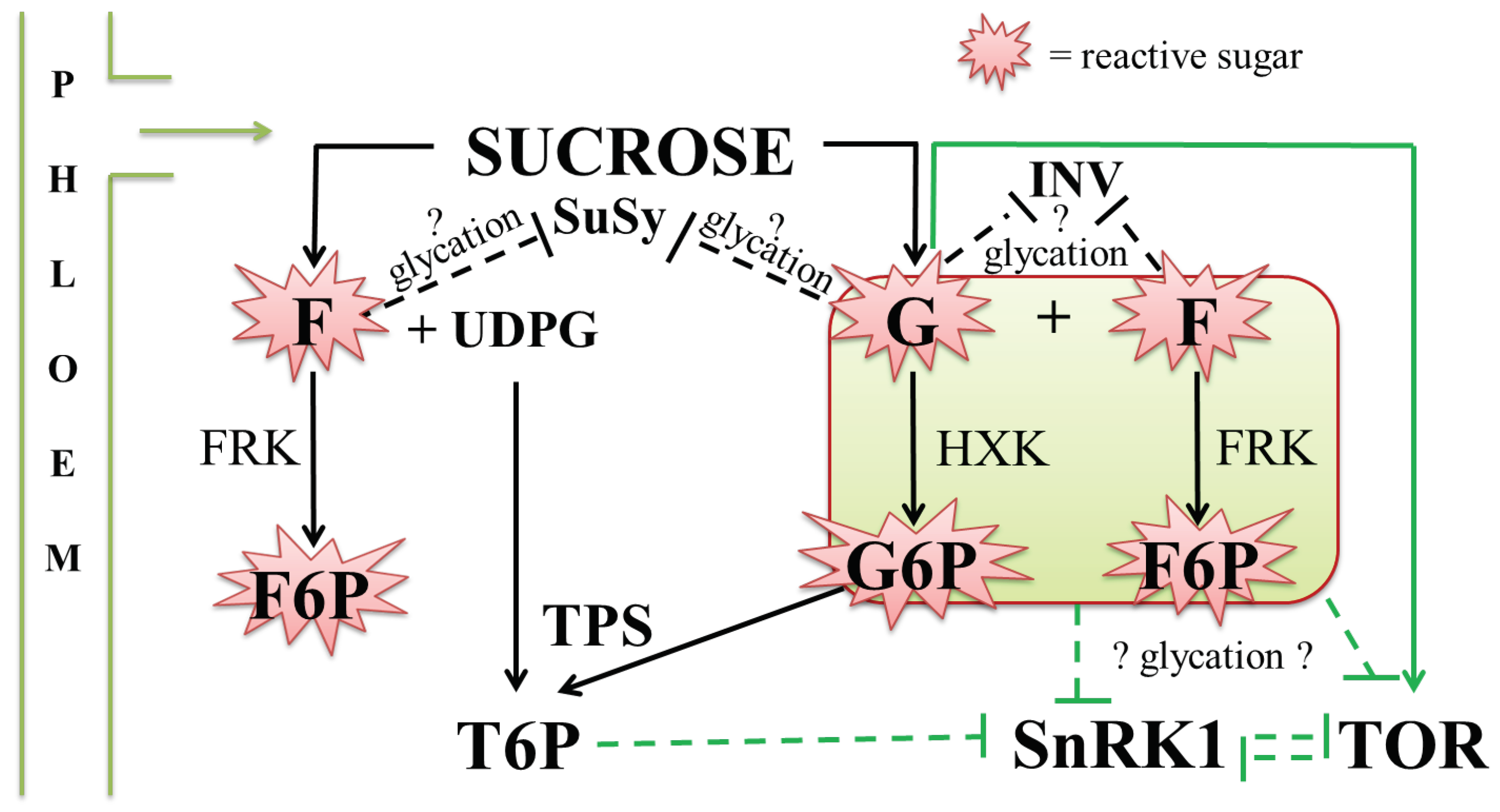
4.4. Possible Interplay between Glycation and Sugar Signalling
5. Conclusions
Author contributions
Funding
Conflicts of Interest
References
- Milkovska-Stamenova, S.; Schmidt, R.; Frolov, A.; Birkemeyer, C. GC-MS method for the quantitation of carbohydrate intermediates in glycation systems. J. Agric. Food Chem. 2015, 63, 5911–5919. [Google Scholar] [CrossRef]
- Ansari, N.A.; Moinuddin; Ali, R. Glycated lysine residues: A marker for non-enzymatic protein glycation in age-related diseases. Dis. Markers 2011, 30, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Greifenhagen, U.; Frolov, A.; Blüher, M.; Hoffmann, R. Site-specific analysis of advanced glycation end products in plasma proteins of type 2 diabetes mellitus patients. Anal. Bioanal. Chem. 2016, 408, 5557–5566. [Google Scholar] [CrossRef] [PubMed]
- Bennmann, D.; Horstkorte, R.; Hofmann, B.; Jacobs, K.; Navarrete-Santos, A.; Simm, A.; Bork, K.; Gnanapragassam, V.S. Advanced glycation end products interfere with adhesion and neurite outgrowth. PLoS ONE 2014, 9, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Leiva, G.E.; Naranjo, G.B.; Malec, L.S. A study of different indicators of Maillard reaction with whey proteins and different carbohydrates under adverse storage conditions. Food Chem. 2017, 215, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Filipov, N.M.; Guo, T.L. Dietary glycation products regulate immune homeostasis: Early glycation products promote prostate cancer cell proliferation through modulating macrophages. Mol. Nutr. Food Res. 2018, 62. [Google Scholar] [CrossRef] [PubMed]
- Hodge, J.E. The Amadori rearrangement. Adv. Carbohydr. Chem. 1955, 10, 169–205. [Google Scholar]
- Ulrich, P.; Cerami, A. Protein glycation, diabetes, and aging. Recent Prog. Horm. Res. 2001, 56, 1–21. [Google Scholar] [CrossRef]
- Heyns, K.; Noack, H. Die umsetzung von d-fructose mit l-lysine und l-arginin undderen beziehung zu nichtenzymatischenbräunungsreaktionen. Chem. Ber. 1962, 95, 720–727. [Google Scholar] [CrossRef]
- Pilková, L.; Pokornу, J.; Davídek, J. Browning reactions of Heyns rearrangement products. Food Nahr. 1990, 34, 759–764. [Google Scholar] [CrossRef]
- Treibmann, S.; Hellwig, A.; Hellwig, M.; Henle, T. Lysine-derived protein-bound Heyns compounds in bakery products. J. Agric. Food Chem. 2017, 65, 10562–10570. [Google Scholar] [CrossRef]
- Kislinger, T.; Humeny, A.; Pischetsrieder, M. Analysis of protein glycation products by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Curr. Med. Chem. 2004, 11, 2185–2193. [Google Scholar] [CrossRef]
- Sanders, K.A.; Delker, D.A.; Huecksteadt, T.; Beck, E.; Wuren, T.; Chen, Y.; Zhang, Y.; Hazel, M.W.; Hoidal, J.R. RAGE is a critical mediator of pulmonary oxidative stress, alveolar macrophage activation and emphysema in response to cigarette smoke. Sci. Rep. 2019, 9, 231. [Google Scholar] [CrossRef]
- Soboleva, A.; Modzel, M.; Didio, A.; Płóciennik, H.; Kijewska, M.; Grischina, T.; Karonova, T.; Bilova, T.; Stefanov, V.; Stefanowicz, P.; et al. Quantification of prospective type 2 diabetes mellitus biomarkers by stable isotope dilution with bi-labeled standard glycated peptides. Anal. Methods 2017, 9, 409–418. [Google Scholar] [CrossRef]
- Arancio, O.; Zhang, H.P.; Chen, X.; Lin, C.; Trinchese, F.; Puzzo, D.; Liu, S.; Hegde, A.; Yan, S.F.; Stern, A.; et al. RAGE potentiates abeta-induced perturbation of neuronal function in transgenic mice. EMBO J. 2004, 23, 4096–4105. [Google Scholar] [CrossRef]
- Abate, G.; Marziano, M.; Rungratanawanich, W.; Memo, M.; Uberti, D. Nutrition and AGE-ing: Focusing on Alzheimer’s disease. Oxid. Med. Cell. Longev. 2017, 2017, 7039816. [Google Scholar] [CrossRef]
- Achouiti, A.; Föll, D.; Vogl, T.; van Till, J.W.O.; Laterre, P.-F.; Dugernier, T.; Wittebole, X.; Boermeester, M.A.; Roth, J.; van der Poll, T.; et al. S100A12 and soluble receptor for advanced glycation end products levels during human severe sepsis. Shock Augusta Ga 2013, 40, 188–194. [Google Scholar] [CrossRef]
- Saremi, A.; Howell, S.; Schwenke, D.C.; Bahn, G.; Beisswenger, P.J.; Reaven, P.D. VADT investigators advanced glycation end products, oxidation products, and the extent of atherosclerosis during the VA diabetes trial and follow-up study. Diabetes Care 2017, 40, 591–598. [Google Scholar] [CrossRef]
- Stinghen, A.E.M.; Massy, Z.A.; Vlassara, H.; Striker, G.E.; Boullier, A. Uremic toxicity of advanced glycation end products in CKD. J. Am. Soc. Nephrol. JASN 2016, 27, 354–370. [Google Scholar] [CrossRef]
- Sugimoto, K.; Yasujima, M.; Yagihashi, S. Role of advanced glycation end products in diabetic neuropathy. Curr. Pharm. Des. 2008, 14, 953–961. [Google Scholar] [CrossRef]
- Goldin, A.; Beckman, J.A.; Schmidt, A.M.; Creager, M.A. Advanced glycation end products: Sparking the development of diabetic vascular injury. Circulation 2006, 114, 597–605. [Google Scholar] [CrossRef]
- Vlassara, H.; Uribarri, J. Advanced glycation end products (AGE) and diabetes: Cause, effect, or both? Curr. Diab. Rep. 2014, 14, 453. [Google Scholar] [CrossRef]
- Aldini, G.; Vistoli, G.; Stefek, M.; Chondrogianni, N.; Grune, T.; Sereikaite, J.; Sadowska-Bartosz, I.; Bartosz, G. Molecular strategies to prevent, inhibit, and degrade advanced glycoxidation and advanced lipoxidation end products. Free Radic. Res. 2013, 47 (Suppl. 1), 93–137. [Google Scholar] [CrossRef] [Green Version]
- Cohen, M.P. Intervention strategies to prevent pathogenetic effects of glycated albumin. Arch. Biochem. Biophys. 2003, 419, 25–30. [Google Scholar] [CrossRef]
- Greifenhagen, U.; Frolov, A.; Hoffmann, R. Oxidative degradation of Nε-fructosylamine-substituted peptides in heated aqueous systems. Amino Acids 2015, 47, 1065–1076. [Google Scholar] [CrossRef]
- Hodge, J.E. Dehydrated foods, chemistry of browning reactions in model systems. J. Agric. Food Chem. 1953, 1, 928–943. [Google Scholar] [CrossRef]
- Wolff, S.P.; Dean, R.T. Glucose autoxidation and protein modification. The potential role of “autoxidative glycosylation” in diabetes. Biochem. J. 1987, 245, 243–250. [Google Scholar] [CrossRef]
- Hunt, J.V.; Dean, R.T.; Wolff, S.P. Hydroxyl radical production and autoxidative glycosylation. Glucose utilization throughout protein damage in the experimental glycation model of diabetes mellitus and ageing. Biochem. J. 1988, 256, 205–212. [Google Scholar] [CrossRef]
- Niwa, T.; Chromatogr, J. 3-Deoxyglucosone: Metabolism, analysis, biological activity, and clinical implication. B. Biomed. Sci. Appl. 1999, 731, 23–36. [Google Scholar] [CrossRef]
- Semchyshyn, H.M. Fructation in vivo: Detrimental and protective effects of fructose. BioMed Res. Int. 2013, 2013, 343914. [Google Scholar] [CrossRef]
- Thornalley, P.J.; Langborg, A.; Minhas, H.S. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem. J. 1999, 344 Pt 1, 109–116. [Google Scholar] [CrossRef]
- Toyosaki, T. Effects of medium-chain triacylglycerols on Maillard reaction in bread baking. J. Sci. Food Agric. 2018, 98, 3169–3174. [Google Scholar] [CrossRef]
- Anderson, M.M.; Requena, J.R.; Crowley, J.R.; Thorpe, S.R.; Heinecke, J.W. The myeloperoxidase system of human phagocytes generates nepsilon-(carboxymethyl)lysine on proteins: A mechanism for producing advanced glycation end products at sites of inflammation. J. Clin. Investig. 1999, 104, 103–113. [Google Scholar] [CrossRef]
- Maessen, D.E.M.; Stehouwer, C.D.A.; Schalkwijk, C.G. The role of methylglyoxal and the glyoxalase system in diabetes and other age-related diseases. Clin. Sci. Lond. Engl. 2015, 128, 839–861. [Google Scholar] [CrossRef]
- Collier, T.A.; Nash, A.; Birch, H.L.; de Leeuw, N.H. Effect on the mechanical properties of type I collagen of intra-molecular lysine-arginine derived advanced glycation end-product cross-linking. J. Biomech. 2018, 67, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.; Tiwari, S. Therapeutic interventions for advanced glycation end products and its receptor-mediated cardiovascular disease. Curr. Pharm. Des. 2017, 23, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Nass, N.; Bartling, B.; Navarrete Santos, A.; Scheubel, R.J.; Börgermann, J.; Silber, R.E.; Simm, A. Advanced glycation end products, diabetes and ageing. Z. Gerontol. Geriatr. 2007, 40, 349–356. [Google Scholar] [CrossRef]
- Bakker, S.F.; Tushuizen, M.E.; Gözütok, E.; Çiftci, A.; Gelderman, K.A.; Mulder, C.J.; Simsek, S. Advanced glycation end products (AGEs) and the soluble receptor for AGE (sRAGE) in patients with type 1 diabetes and coeliac disease. Nutr. Metab. Cardiovasc. Dis. NMCD 2015, 25, 230–235. [Google Scholar] [CrossRef]
- Ott, C.; Jacobs, K.; Haucke, E.; Navarrete Santos, A.; Grune, T.; Simm, A. Role of advanced glycation end products in cellular signalling. Redox Biol. 2014, 2, 411–429. [Google Scholar] [CrossRef]
- Neeper, M.; Schmidt, A.M.; Brett, J.; Yan, S.D.; Wang, F.; Pan, Y.C.; Elliston, K.; Stern, D.; Shaw, A. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J. Biol. Chem. 1992, 267, 14998–15004. [Google Scholar]
- Xie, J.; Méndez, J.D.; Méndez-Valenzuela, V.; Aguilar-Hernández, M.M. Cellular signalling of the receptor for advanced glycation end products (RAGE). Cell. Signal. 2013, 25, 2185–2197. [Google Scholar] [CrossRef]
- Wendt, T.M.; Tanji, N.; Guo, J.; Kislinger, T.R.; Qu, W.; Lu, Y.; Bucciarelli, L.G.; Rong, L.L.; Moser, B.; Markowitz, G.S.; et al. RAGE drives the development of glomerulosclerosis and implicates podocyte activation in the pathogenesis of diabetic nephropathy. Am. J. Pathol. 2003, 162, 1123–1137. [Google Scholar] [CrossRef]
- Miyata, T.; van Ypersele de Strihou, C.; Ueda, Y.; Ichimori, K.; Inagi, R.; Onogi, H.; Ishikawa, N.; Nangaku, M.; Kurokawa, K. Angiotensin II receptor antagonists and angiotensin-converting enzyme inhibitors lower in vitro the formation of advanced glycation end products: Biochemical mechanisms. J. Am. Soc. Nephrol. JASN 2002, 13, 2478–2487. [Google Scholar] [CrossRef]
- Nakamura, S.; Makita, Z.; Ishikawa, S.; Yasumura, K.; Fujii, W.; Yanagisawa, K.; Kawata, T.; Koike, T. Progression of nephropathy in spontaneous diabetic rats is prevented by OPB-9195, a novel inhibitor of advanced glycation. Diabetes 1997, 46, 895–899. [Google Scholar] [CrossRef]
- Semchyshyn, H.M.; Lozinska, L.M.; Miedzobrodzki, J.; Lushchak, V.I. Fructose and glucose differentially affect aging and carbonyl/oxidative stress parameters in Saccharomyces cerevisiae cells. Carbohydr. Res. 2011, 346, 933–938. [Google Scholar] [CrossRef]
- Bolouri Moghaddam, M.R.; Van den Ende, W. Sugars, the clock and transition to flowering. Front. Plant Sci. 2013, 4, 22. [Google Scholar] [CrossRef]
- Szwergold, B.S. Maillard reactions in hyperthermophilic archaea: Implications for better understanding of non-enzymatic glycation in biology. Rejuvenation Res. 2013, 16, 259–272. [Google Scholar] [CrossRef]
- Poulsen, M.W.; Hedegaard, R.V.; Andersen, J.M.; de Courten, B.; Bügel, S.; Nielsen, J.; Skibsted, L.H.; Dragsted, L.O. Advanced glycation endproducts in food and their effects on health. Food Chem. Toxicol. 2013, 60, 10–37. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Lund, M.N.; Li, B.; Hu, Y.; Zhang, X. Kinetic investigation of the trapping of Nε-(carboxymethyl)lysine by 4-methylbenzoquinone: A new mechanism to control Nε-(carboxymethyl)lysine levels in foods. Food Chem. 2018, 244, 25–28. [Google Scholar] [CrossRef]
- Stitt, A.W.; Moore, J.E.; Sharkey, J.A.; Murphy, G.; Simpson, D.A.; Bucala, R.; Vlassara, H.; Archer, D.B. Advanced glycation end products in vitreous: Structural and functional implications for diabetic vitreopathy. Investig. Ophthalmol. Vis. Sci. 1998, 39, 2517–2523. [Google Scholar]
- Ahmed, N.; Thornalley, P.J. Quantitative screening of protein biomarkers of early glycation, advanced glycation, oxidation and nitrosation in cellular and extracellular proteins by tandem mass spectrometry multiple reaction monitoring. Biochem. Soc. Trans. 2003, 31, 1417–1422. [Google Scholar] [CrossRef]
- Soboleva, A.; Vikhnina, M.; Grishina, T.; Frolov, A. Probing protein glycation by chromatography and mass spectrometry: Analysis of glycation adducts. Int. J. Mol. Sci. 2017, 18, 2557. [Google Scholar] [CrossRef]
- Hull, G.L.J.; Woodside, J.V.; Ames, J.M.; Cuskelly, G.J. Nε-(carboxymethyl)lysine content of foods commonly consumed in a Western style diet. Food Chem. 2012, 131, 170–174. [Google Scholar] [CrossRef]
- Goldberg, T.; Cai, W.; Peppa, M.; Dardaine, V.; Baliga, B.S.; Uribarri, J.; Vlassara, H. Advanced glycoxidation end products in commonly consumed foods. J. Am. Diet. Assoc. 2004, 104, 1287–1291. [Google Scholar] [CrossRef]
- Vlassara, H.; Cai, W.; Crandall, J.; Goldberg, T.; Oberstein, R.; Dardaine, V.; Peppa, M.; Rayfield, E.J. Inflammatory mediators are induced by dietary glycotoxins, a major risk factor for diabetic angiopathy. Proc. Natl. Acad. Sci. USA 2002, 99, 15596–15601. [Google Scholar] [CrossRef] [Green Version]
- Uribarri, J.; Woodruff, S.; Goodman, S.; Cai, W.; Chen, X.; Pyzik, R.; Yong, A.; Striker, G.E.; Vlassara, H. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J. Am. Diet. Assoc. 2010, 110, 911–916. [Google Scholar] [CrossRef]
- Henle, T. Maillard reaction of proteins and advanced glycation end products (AGEs) in food. In Process-Induced Food Toxicants; Park, J.H., Penning, T.M., Stadler, R.H., Lineback, D.R., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2008; pp. 215–242. ISBN 978-0-470-43010-1. [Google Scholar]
- Assar, S.; Moloney, C.; Lima, M.; Magee, R.; Ames, J. Determination of Nɛ-(carboxymethyl)lysine in food systems by ultra performance liquid chromatography-mass spectrometry. Amino Acids 2009, 36, 317–326. [Google Scholar] [CrossRef]
- Krajcovicova-Kudlackova, M.; Sebekova, K.; Schinzel, R.; Klvanová, J. Advanced glycation end products and nutrition. Physiol. Res. 2002, 51, 313–316. [Google Scholar]
- Turner, D.P. Advanced Glycation End-Products: A biological consequence of lifestyle contributing to cancer disparity. Cancer Res. 2015, 75, 1925–1929. [Google Scholar] [CrossRef]
- Bechtold, U.; Rabbani, N.; Mullineaux, P.M.; Thornalley, P.J. Quantitative measurement of specific biomarkers for protein oxidation, nitration and glycation in Arabidopsis leaves. Plant J. Cell Mol. Biol. 2009, 59, 661–671. [Google Scholar] [CrossRef]
- Thornalley, P.J.; Rabbani, N. Detection of oxidized and glycated proteins in clinical samples using mass spectrometry—a user’s perspective. Biochim. Biophys. Acta 2014, 1840, 818–829. [Google Scholar] [CrossRef]
- Greifenhagen, U.; Frolov, A.; Blüher, M.; Hoffmann, R. Plasma proteins modified by advanced glycation end products (AGEs) reveal site-specific susceptibilities to glycemic control in patients with type 2 diabetes. J. Biol. Chem. 2016, 291, 9610–9616. [Google Scholar] [CrossRef]
- Ahmed, U.; Anwar, A.; Savage, R.S.; Thornalley, P.J.; Rabbani, N. Protein oxidation, nitration and glycation biomarkers for early-stage diagnosis of osteoarthritis of the knee and typing and progression of arthritic disease. Arthritis Res. Ther. 2016, 18, 250. [Google Scholar] [CrossRef]
- Smuda, M.; Henning, C.; Raghavan, C.T.; Johar, K.; Vasavada, A.R.; Nagaraj, R.H.; Glomb, M.A. Comprehensive analysis of maillard protein modifications in human lenses: Effect of age and cataract. Biochemistry 2015, 54, 2500–2507. [Google Scholar] [CrossRef]
- Frolov, A.; Bilova, T.; Paudel, G.; Berger, R.; Balcke, G.U.; Birkemeyer, C.; Wessjohann, L.A. Early responses of mature Arabidopsis thaliana plants to reduced water potential in the agar-based polyethylene glycol infusion drought model. J. Plant Physiol. 2017, 208, 70–83. [Google Scholar] [CrossRef]
- Paudel, G.; Bilova, T.; Schmidt, R.; Greifenhagen, U.; Berger, R.; Tarakhovskaya, E.; Stöckhardt, S.; Balcke, G.U.; Humbeck, K.; Brandt, W.; et al. Osmotic stress is accompanied by protein glycation in Arabidopsis thaliana. J. Exp. Bot. 2016, 67, 6283–6295. [Google Scholar] [CrossRef]
- Bilova, T.; Lukasheva, E.; Brauch, D.; Greifenhagen, U.; Paudel, G.; Tarakhovskaya, E.; Frolova, N.; Mittasch, J.; Balcke, G.U.; Tissier, A.; et al. A snapshot of the plant glycated proteome: Structural, functional and mechanistic aspects. J. Biol. Chem. 2016, 291, 7621–7636. [Google Scholar] [CrossRef]
- Zhang, Q.; Monroe, M.E.; Schepmoes, A.A.; Clauss, T.R.W.; Gritsenko, M.A.; Meng, D.; Petyuk, V.A.; Smith, R.D.; Metz, T.O. Comprehensive identification of glycated peptides and their glycation motifs in plasma and erythrocytes of control and diabetic subjects. J. Proteome Res. 2011, 10, 3076–3088. [Google Scholar] [CrossRef]
- Zhang, Q.; Petyuk, V.A.; Schepmoes, A.A.; Orton, D.J.; Monroe, M.E.; Yang, F.; Smith, R.D.; Metz, T.O. Analysis of non-enzymatically glycated peptides: Neutral-loss-triggered MS3 versus multi-stage activation tandem mass spectrometry. Rapid Commun. Mass Spectrom. RCM 2008, 22, 3027–3034. [Google Scholar] [CrossRef]
- Schmidt, R.; Böhme, D.; Singer, D.; Frolov, A. Specific tandem mass spectrometric detection of AGE-modified arginine residues in peptides. J. Mass Spectrom. JMS 2015, 50, 613–624. [Google Scholar] [CrossRef]
- Greifenhagen, U.; Nguyen, V.D.; Moschner, J.; Giannis, A.; Frolov, A.; Hoffmann, R. Sensitive and site-specific identification of carboxymethylated and carboxyethylated peptides in tryptic digests of proteins and human plasma. J. Proteome Res. 2015, 14, 768–777. [Google Scholar] [CrossRef]
- Chaplin, A.K.; Chernukhin, I.; Bechtold, U. Profiling of advanced glycation end products uncovers abiotic stress-specific target proteins in Arabidopsis. J. Exp. Bot. 2019, 70, 653–670. [Google Scholar] [CrossRef]
- Mustafa, G.; Komatsu, S. Quantitative proteomics reveals the effect of protein glycosylation in soybean root under flooding stress. Front. Plant Sci. 2014, 5. [Google Scholar] [CrossRef]
- Dubey, R.S.; Singh, A.K. Salinity induces accumulation of soluble sugars and alters the activity of sugar metabolising enzymes in rice plants. Biol. Plant. 1999, 42, 233–239. [Google Scholar] [CrossRef]
- McLoughlin, F.; Arisz, S.A.; Dekker, H.L.; Kramer, G.; de Koster, C.G.; Haring, M.A.; Munnik, T.; Testerink, C. Identification of novel candidate phosphatidic acid-binding proteins involved in the salt-stress response of Arabidopsis thaliana roots. Biochem. J. 2013, 450, 573–581. [Google Scholar] [CrossRef]
- Demidchik, V.; Cuin, T.A.; Svistunenko, D.; Smith, S.J.; Miller, A.J.; Shabala, S.; Sokolik, A.; Yurin, V. Arabidopsis root K+-efflux conductance activated by hydroxyl radicals: Single-channel properties, genetic basis and involvement in stress-induced cell death. J. Cell Sci. 2010, 123, 1468–1479. [Google Scholar] [CrossRef]
- Makavitskaya, M.; Svistunenko, D.; Navaselsky, I.; Hryvusevich, P.; Mackievic, V.; Rabadanova, C.; Tyutereva, E.; Samokhina, V.; Straltsova, D.; Sokolik, A.; et al. Novel roles of ascorbate in plants: Induction of cytosolic Ca2+ signals and efflux from cells via anion channels. J. Exp. Bot. 2018, 69, 3477–3489. [Google Scholar] [CrossRef]
- Mayta, M.L.; Lodeyro, A.F.; Guiamet, J.J.; Tognetti, V.B.; Melzer, M.; Hajirezaei, M.R.; Carrillo, N. Expression of a plastid-targeted flavodoxin decreases chloroplast reactive oxygen species accumulation and delays senescence in aging tobacco leaves. Front. Plant Sci. 2018, 9, 1039. [Google Scholar] [CrossRef]
- Gkogkolou, P.; Böhm, M. Advanced glycation end products. Dermatoendocrinology 2012, 4, 259–270. [Google Scholar] [CrossRef] [Green Version]
- Bilova, T.; Paudel, G.; Shilyaev, N.; Schmidt, R.; Brauch, D.; Tarakhovskaya, E.; Milrud, S.; Smolikova, G.; Tissier, A.; Vogt, T.; et al. Global proteomic analysis of advanced glycation end products in the Arabidopsis proteome provides evidence for age-related glycation hot spots. J. Biol. Chem. 2017, 292, 15758–15776. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, Y.; Yuk, I.; Pai, R.; McKay, P.; Eigenbrot, C.; Dennis, M.; Katta, V.; Francissen, K.C. Unveiling a glycation hot spot in a recombinant humanized monoclonal antibody. Anal. Chem. 2008, 80, 2379–2390. [Google Scholar] [CrossRef] [PubMed]
- Matamoros, M.A.; Kim, A.; Peñuelas, M.; Ihling, C.; Griesser, E.; Hoffmann, R.; Fedorova, M.; Frolov, A.; Becana, M. Protein carbonylation and glycation in legume nodules. Plant Physiol. 2018, 177, 1510–1528. [Google Scholar] [CrossRef]
- Wolff, S.P.; Bascal, Z.A.; Hunt, J.V. “Autoxidative glycosylation”: Free radicals and glycation theory. Prog. Clin. Biol. Res. 1989, 304, 259–275. [Google Scholar]
- Wolff, S.P.; Jiang, Z.Y.; Hunt, J.V. Protein glycation and oxidative stress in diabetes mellitus and ageing. Free Radic. Biol. Med. 1991, 10, 339–352. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox sensing and signalling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiol. Plant. 2003, 119, 355–364. [Google Scholar] [CrossRef]
- Sekiguchi, Y.; Mitsuhashi, N.; Inoue, Y.; Yagisawa, H.; Mimura, T. Analysis of sugar phosphates in plants by ion chromatography on a titanium dioxide column with pulsed amperometric detection. J. Chromatogr. 2004, 1039, 71–76. [Google Scholar] [CrossRef]
- Han, J.; Tschernutter, V.; Yang, J.; Eckle, T.; Borchers, C.H. Analysis of selected sugars and sugar phosphates in mouse heart tissue by reductive amination and liquid chromatography-electrospray ionization mass spectrometry. Anal. Chem. 2013, 85, 5965–5973. [Google Scholar] [CrossRef] [PubMed]
- Soboleva, A.; Schmidt, R.; Vikhnina, M.; Grishina, T.; Frolov, A. Maillard proteomics: Opening new pages. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Bassham, J.A.; Benson, A.A.; Calvin, M. The path of carbon in photosynthesis. J. Biol. Chem. 1950, 185, 781–787. [Google Scholar] [PubMed]
- Szwergold, B.S.; Howell, S.; Beisswenger, P.J. Human fructosamine-3-kinase: Purification, sequencing, substrate specificity, and evidence of activity in vivo. Diabetes 2001, 50, 2139–2147. [Google Scholar] [CrossRef]
- Collard, F.; Wiame, E.; Bergans, N.; Fortpied, J.; Vertommen, D.; Vanstapel, F.; Delpierre, G.; Van Schaftingen, E. Fructosamine 3-kinase-related protein and deglycation in human erythrocytes. Biochem. J. 2004, 382, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Fortpied, J.; Gemayel, R.; Stroobant, V.; van Schaftingen, E. Plant ribulosamine/erythrulosamine 3-kinase, a putative protein-repair enzyme. Biochem. J. 2005, 388, 795–802. [Google Scholar] [CrossRef] [Green Version]
- Ravichandran, G.; Lakshmanan, D.K.; Raju, K.; Elangovan, A.; Nambirajan, G.; Devanesan, A.A.; Thilagar, S. Food advanced glycation end products as potential endocrine disruptors: An emerging threat to contemporary and future generation. Environ. Int. 2019, 123, 486–500. [Google Scholar] [CrossRef]
- Demidchik, V. Mechanisms of oxidative stress in plants: From classical chemistry to cell biology. Environ. Exp. Bot. 2015, 109, 212–228. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Ejiri, Y.; Tanaka, K. Glycation by ascorbic acid causes loss of activity of ribulose-1,5-bisphosphate carboxylase/oxygenase and its increased susceptibility to proteases. Plant Cell Physiol. 2002, 43, 1334–1341. [Google Scholar] [CrossRef]
- Kato, Y.; Yamamoto, Y.; Murakami, S.; Sato, F. Post-translational regulation of CND41 protease activity in senescent tobacco leaves. Planta 2005, 222, 643–651. [Google Scholar] [CrossRef]
- Vistoli, G.; De Maddis, D.; Cipak, A.; Zarkovic, N.; Carini, M.; Aldini, G. Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): An overview of their mechanisms of formation. Free Radic. Res. 2013, 47 (Suppl. 1), 3–27. [Google Scholar] [CrossRef]
- Havé, M.; Leitao, L.; Bagard, M.; Castell, J.-F.; Repellin, A. Protein carbonylation during natural leaf senescence in winter wheat, as probed by fluorescein-5-thiosemicarbazide. Plant Biol. Stuttg. Ger. 2015, 17, 973–979. [Google Scholar] [CrossRef]
- Murthy, U.M.N.; Kumar, P.P.; Sun, W.Q. Mechanisms of seed ageing under different storage conditions for Vigna radiata (L.) wilczek: Lipid peroxidation, sugar hydrolysis, Maillard reactions and their relationship to glass state transition. J. Exp. Bot. 2003, 54, 1057–1067. [Google Scholar] [CrossRef]
- Mamontova, T.; Lukasheva, E.; Mavropolo-Stolyarenko, G.; Proksch, C.; Bilova, T.; Kim, A.; Babakov, V.; Grishina, T.; Hoehenwarter, W.; Medvedev, S.; et al. Proteome map of pea (Pisum sativum L.) embryos containing different amounts of residual chlorophylls. Int. J. Mol. Sci. 2018, 19, 4066. [Google Scholar] [CrossRef]
- Korovila, I.; Hugo, M.; Castro, J.P.; Weber, D.; Höhn, A.; Grune, T.; Jung, T. Proteostasis, oxidative stress and aging. Redox Biol. 2017, 13, 550–567. [Google Scholar] [CrossRef] [PubMed]
- Janse van Rensburg, H.C.; Van den Ende, W.; Signorelli, S. Autophagy in plants: Both a puppet and a puppet master of sugars. Front. Plant Sci. 2019, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Queisser, M.A.; Yao, D.; Geisler, S.; Hammes, H.-P.; Lochnit, G.; Schleicher, E.D.; Brownlee, M.; Preissner, K.T. Hyperglycemia impairs proteasome function by methylglyoxal. Diabetes 2010, 59, 670–678. [Google Scholar] [CrossRef]
- Yoshida, H.; Kisugi, R. Mechanisms of LDL oxidation. Clin. Chim. Acta Int. J. Clin. Chem. 2010, 411, 1875–1882. [Google Scholar] [CrossRef]
- Herrmann, J.; Soares, S.M.; Lerman, L.O.; Lerman, A. Potential role of the ubiquitin-proteasome system in atherosclerosis: Aspects of a protein quality disease. J. Am. Coll. Cardiol. 2008, 51, 2003–2010. [Google Scholar] [CrossRef]
- Borysiuk, K.; Ostaszewska-Bugajska, M.; Vaultier, M.-N.; Hasenfratz-Sauder, M.-P.; Szal, B. Enhanced formation of methylglyoxal-derived advanced glycation end products in Arabidopsis under ammonium nutrition. Front. Plant Sci. 2018, 9, 667. [Google Scholar] [CrossRef]
- Takagi, D.; Inoue, H.; Odawara, M.; Shimakawa, G.; Miyake, C. The Calvin cycle inevitably produces sugar-derived reactive carbonyl methylglyoxal during photosynthesis: A potential cause of plant diabetes. Plant Cell Physiol. 2014, 55, 333–340. [Google Scholar] [CrossRef]
- Thornalley, P.J. Population genetics of human glyoxalases. Heredity 1991, 67, 139–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welchen, E.; Schmitz, J.; Fuchs, P.; García, L.; Wagner, S.; Wienstroer, J.; Schertl, P.; Braun, H.-P.; Schwarzländer, M.; Gonzalez, D.H.; et al. d-Lactate dehydrogenase links methylglyoxal degradation and electron transport through cytochrome c. Plant Physiol. 2016, 172, 901–912. [Google Scholar] [CrossRef]
- Hoque, T.S.; Uraji, M.; Hoque, M.A.; Nakamura, Y.; Murata, Y. Methylglyoxal induces inhibition of growth, accumulation of anthocyanin, and activation of glyoxalase I and II in Arabidopsis thaliana. J. Biochem. Mol. Toxicol. 2017, 31, e21901. [Google Scholar] [CrossRef] [PubMed]
- Kaur, C.; Kushwaha, H.R.; Mustafiz, A.; Pareek, A.; Sopory, S.K.; Singla-Pareek, S.L. Analysis of global gene expression profile of rice in response to methylglyoxal indicates its possible role as a stress signal molecule. Front. Plant Sci. 2015, 6, 682. [Google Scholar] [CrossRef]
- Kaur, C.; Sharma, S.; Singla-Pareek, S.L.; Sopory, S.K. Methylglyoxal detoxification in plants: Role of glyoxalase pathway. Indian J. Plant Physiol. 2016, 21, 377–390. [Google Scholar] [CrossRef]
- Shin, S.-M.; Song, S.-H.; Lee, J.-W.; Kwak, M.-K.; Kang, S.-O. Methylglyoxal synthase regulates cell elongation via alterations of cellular methylglyoxal and spermidine content in Bacillus subtilis. Int. J. Biochem. Cell Biol. 2017, 91, 14–28. [Google Scholar] [CrossRef] [PubMed]
- Kaur, C.; Singla-Pareek, S.L.; Sopory, S.K. Glyoxalase and methylglyoxal as biomarkers for plant stress tolerance. Crit. Rev. Plant Sci. 2014, 33, 429–456. [Google Scholar] [CrossRef]
- Yadav, S.K.; Singla-Pareek, S.L.; Ray, M.; Reddy, M.K.; Sopory, S.K. Methylglyoxal levels in plants under salinity stress are dependent on glyoxalase I and glutathione. Biochem. Biophys. Res. Commun. 2005, 337, 61–67. [Google Scholar] [CrossRef]
- Ghosh, A.; Pareek, A.; Sopory, S.K.; Singla-Pareek, S.L. A glutathione responsive rice glyoxalase II, OsGLYII-2, functions in salinity adaptation by maintaining better photosynthesis efficiency and anti-oxidant pool. Plant J. Cell Mol. Biol. 2014, 80, 93–105. [Google Scholar] [CrossRef] [Green Version]
- Gupta, B.K.; Sahoo, K.K.; Ghosh, A.; Tripathi, A.K.; Anwar, K.; Das, P.; Singh, A.K.; Pareek, A.; Sopory, S.K.; Singla-Pareek, S.L. Manipulation of glyoxalase pathway confers tolerance to multiple stresses in rice. Plant Cell Environ. 2018, 41, 1186–1200. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, J.A.; Hasanuzzaman, M.; Nahar, K.; Rahman, A.; Hossain, M.S.; Fujita, M. γ-aminobutyric acid (GABA) confers chromium stress tolerance in Brassica juncea L. by modulating the antioxidant defense and glyoxalase systems. Ecotoxicol. Lond. Engl. 2017, 26, 675–690. [Google Scholar] [CrossRef]
- Chetyrkin, S.; Mathis, M.; Pedchenko, V.; Sanchez, O.A.; McDonald, W.H.; Hachey, D.L.; Madu, H.; Stec, D.; Hudson, B.; Voziyan, P. Glucose autoxidation induces functional damage to proteins via modification of critical arginine residues. Biochemistry 2011, 50, 6102–6112. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Hardas, S.S.; Bader Lange, M.L. Oxidatively modified glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and Alzheimer disease: Many pathways to neurodegeneration. J. Alzheimers Dis. JAD 2010, 20, 369–393. [Google Scholar] [CrossRef]
- Hoque, T.S.; Uraji, M.; Tuya, A.; Nakamura, Y.; Murata, Y. Methylglyoxal inhibits seed germination and root elongation and up-regulates transcription of stress-responsive genes in ABA-dependent pathway in Arabidopsis. Plant Biol. Stuttg. Ger. 2012, 14, 854–858. [Google Scholar] [CrossRef] [PubMed]
- Hoque, T.S.; Okuma, E.; Uraji, M.; Furuichi, T.; Sasaki, T.; Hoque, M.A.; Nakamura, Y.; Murata, Y. Inhibitory effects of methylglyoxal on light-induced stomatal opening and inward K+ channel activity in Arabidopsis. Biosci. Biotechnol. Biochem. 2012, 76, 617–619. [Google Scholar] [CrossRef] [PubMed]
- Kalapos, M.P. Methylglyoxal and glucose metabolism: A historical perspective and future avenues for research. Drug Metabol. Drug Interact. 2008, 23, 69–91. [Google Scholar] [CrossRef] [PubMed]
- Moller, I.M. Plant mitochondria and oxidative stress: Electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 561–591. [Google Scholar] [CrossRef] [PubMed]
- Prestes, A.d.S.; dos Santos, M.M.; Ecker, A.; Zanini, D.; Schetinger, M.R.C.; Rosemberg, D.B.; da Rocha, J.B.T.; Barbosa, N.V. Evaluation of methylglyoxal toxicity in human erythrocytes, leukocytes and platelets. Toxicol. Mech. Methods 2017, 27, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Atlante, A.; de Bari, L.; Valenti, D.; Pizzuto, R.; Paventi, G.; Passarella, S. Transport and metabolism of d-lactate in Jerusalem artichoke mitochondria. Biochim. Biophys. Acta 2005, 1708, 13–22. [Google Scholar] [CrossRef]
- Kwon, K.; Choi, D.; Hyun, J.K.; Jung, H.S.; Baek, K.; Park, C. Novel glyoxalases from Arabidopsis thaliana. FEBS J. 2013, 280, 3328–3339. [Google Scholar] [CrossRef]
- Thornalley, P.J. Glyoxalase I—structure, function and a critical role in the enzymatic defence against glycation. Biochem. Soc. Trans. 2003, 31, 1343–1348. [Google Scholar] [CrossRef]
- Vander Jagt, D.L. Glyoxalase II: Molecular characteristics, kinetics and mechanism. Biochem. Soc. Trans. 1993, 21, 522–527. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, A.; Kushwaha, H.R.; Hasan, M.R.; Pareek, A.; Sopory, S.K.; Singla-Pareek, S.L. Presence of unique glyoxalase III proteins in plants indicates the existence of shorter route for methylglyoxal detoxification. Sci. Rep. 2016, 6, 18358. [Google Scholar] [CrossRef]
- Demidchik, V.; Tyutereva, E.V.; Voitsekhovskaja, O.V. The role of ion disequilibrium in induction of root cell death and autophagy by environmental stresses. Funct. Plant Biol. 2018, 45, 28–46. [Google Scholar] [CrossRef]
- Pompella, A.; Visvikis, A.; Paolicchi, A.; De Tata, V.; Casini, A.F. The changing faces of glutathione, a cellular protagonist. Biochem. Pharmacol. 2003, 66, 1499–1503. [Google Scholar] [CrossRef]
- Berdanier, A.B.; Clark, J.S. Multiyear drought-induced morbidity preceding tree death in southeastern U.S. forests. Ecol. Appl. Publ. Ecol. Soc. Am. 2016, 26, 17–23. [Google Scholar] [CrossRef]
- Hassan, W.; Noreen, H.; Rehman, S.; Gul, S.; Kamal, M.A.; Kamdem, J.P.; Zaman, B.; da Rocha, J.B.T. Oxidative stress and antioxidant potential of one hundred medicinal plants. Curr. Top. Med. Chem. 2017, 17, 1336–1370. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. PPB 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Tuteja, N.; Gill, S.S. (Eds.) Crop Improvement Under Adverse Conditions; Springer-Verlag: New York, NY, USA, 2013; ISBN 978-1-4614-4632-3. [Google Scholar]
- Wang, L.; Liang, W.; Xing, J.; Tan, F.; Chen, Y.; Huang, L.; Cheng, C.-L.; Chen, W. Dynamics of chloroplast proteome in salt-stressed mangrove Kandelia candel (L.) Druce. J. Proteome Res. 2013, 12, 5124–5136. [Google Scholar] [CrossRef]
- Mhamdi, A.; Queval, G.; Chaouch, S.; Vanderauwera, S.; Van Breusegem, F.; Noctor, G. Catalase function in plants: A focus on Arabidopsis mutants as stress-mimic models. J. Exp. Bot. 2010, 61, 4197–4220. [Google Scholar] [CrossRef]
- Griffith, O.W.; Meister, A. Translocation of intracellular glutathione to membrane-bound gamma-glutamyl transpeptidase as a discrete step in the gamma-glutamyl cycle: Glutathionuria after inhibition of transpeptidase. Proc. Natl. Acad. Sci. USA 1979, 76, 268–272. [Google Scholar] [CrossRef]
- Meister, A.; Anderson, M.E. Glutathione. Annu. Rev. Biochem. 1983, 52, 711–760. [Google Scholar] [CrossRef]
- Bela, K.; Horváth, E.; Gallé, Á.; Szabados, L.; Tari, I.; Csiszár, J. Plant glutathione peroxidases: Emerging role of the antioxidant enzymes in plant development and stress responses. J. Plant Physiol. 2015, 176, 192–201. [Google Scholar] [CrossRef]
- Hayes, J.D.; Flanagan, J.U.; Jowsey, I.R. Glutathione transferases. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 51–88. [Google Scholar] [CrossRef]
- Couto, N.; Wood, J.; Barber, J. The role of glutathione reductase and related enzymes on cellular redox homoeostasis network. Free Radic. Biol. Med. 2016, 95, 27–42. [Google Scholar] [CrossRef]
- Asadi Karam, E.; Maresca, V.; Sorbo, S.; Keramat, B.; Basile, A. Effects of triacontanol on ascorbate-glutathione cycle in Brassica napus L. exposed to cadmium-induced oxidative stress. Ecotoxicol. Environ. Saf. 2017, 144, 268–274. [Google Scholar] [CrossRef]
- Kagan, V.E.; Shvedova, A.; Serbinova, E.; Khan, S.; Swanson, C.; Powell, R.; Packer, L. Dihydrolipoic acid-a universal antioxidant both in the membrane and in the aqueous phase. Reduction of peroxyl, ascorbyl and chromanoxyl radicals. Biochem. Pharmacol. 1992, 44, 1637–1649. [Google Scholar] [CrossRef]
- Jiang, Q. Natural forms of vitamin E: Metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic. Biol. Med. 2014, 72, 76–90. [Google Scholar] [CrossRef]
- Jomova, K.; Valko, M. Health protective effects of carotenoids and their interactions with other biological antioxidants. Eur. J. Med. Chem. 2013, 70, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Packer, L.; Witt, E.H.; Tritschler, H.J. Alpha-lipoic acid as a biological antioxidant. Free Radic. Biol. Med. 1995, 19, 227–250. [Google Scholar] [CrossRef]
- Glasauer, A.; Chandel, N.S. Targeting antioxidants for cancer therapy. Biochem. Pharmacol. 2014, 92, 90–101. [Google Scholar] [CrossRef]
- Smuda, M.; Glomb, M.A. Maillard degradation pathways of vitamin C. Angew. Chem. Int. Ed. 2013, 52, 4887–4891. [Google Scholar] [CrossRef] [PubMed]
- Henning, C.; Liehr, K.; Girndt, M.; Ulrich, C.; Glomb, M.A. Extending the spectrum of α-dicarbonyl compounds in vivo. J. Biol. Chem. 2014, 289, 28676–28688. [Google Scholar] [CrossRef]
- Li, X.; Makavitskaya, M.; Samokhina, V.; Mackievic, V.; Navaselsky, I.; Hryvusevich, P.; Smolikova, G.; Medvedev, S.; Shabala, S.; Yu, M.; et al. Effects of exogenously-applied l-ascorbic acid on root expansive growth and viability of the border-like cells. Plant Signal. Behav. 2018, 13, 1514895. [Google Scholar] [CrossRef] [PubMed]
- Ortwerth, B.J.; Speaker, J.A.; Prabhakaram, M.; Lopez, M.G.; Li, E.Y.; Feather, M.S. Ascorbic acid glycation: The reactions of l-threose in lens tissue. Exp. Eye Res. 1994, 58, 665–674. [Google Scholar] [CrossRef]
- Selão, T.T.; Zhang, L.; Knoppovaá, J.; Komenda, J.; Norling, B. Photosystem II assembly steps take place in the thylakoid membrane of the cyanobacterium Synechocystis sp. PCC6803. Plant Cell Physiol. 2016, 57, 878. [Google Scholar] [CrossRef]
- Pischetsrieder, M.; Larisch, B.; Severin, T. The Maillard reaction of ascorbic acid with amino acids and proteins - identification of products. Mail. React. Foods Med. 2005, 107–112. [Google Scholar] [CrossRef]
- Moore, B.; Zhou, L.; Rolland, F.; Hall, Q.; Cheng, W.-H.; Liu, Y.-X.; Hwang, I.; Jones, T.; Sheen, J. Role of the Arabidopsis glucose sensor HXK1 in nutrient, light and hormonal signalling. Science 2003, 300, 332–336. [Google Scholar] [CrossRef]
- Hellmann, H.A.; Smeekens, S. Sugar sensing and signalling in plants. Front. Plant Sci. 2014, 5, 113. [Google Scholar] [CrossRef]
- Sheen, J. Master regulators in plant glucose signalling networks. J. Plant Biol. 2014, 57, 67–79. [Google Scholar] [CrossRef] [PubMed]
- LeClere, S.; Schmelz, E.A.; Chourey, P.S. Sugar levels regulate tryptophan-dependent auxin biosynthesis in developing maize kernels. Plant Physiol. 2010, 153, 306–318. [Google Scholar] [CrossRef]
- Sakr, S.; Wang, M.; Dedaldechamp, F.; Perez-Garcia, M.-D.; Oge, L.; Hamama, L.; Atanassova, R. The Sugar-Signaling Hub: Overview of regulators and interaction with the hormonal and metabolic network. Int. J. Mol. Sci. 2018, 19, 2506. [Google Scholar] [CrossRef]
- Mason, M.G.; Ross, J.J.; Babst, B.A.; Wienclaw, B.N.; Beveridge, C.A. Sugar demand, not auxin, is the initial regulator of apical dominance. Proc. Natl. Acad. Sci. USA 2014, 111, 6092–6097. [Google Scholar] [CrossRef] [Green Version]
- Rolland, F.; Baena-Gonzalez, E.; Sheen, J. Sugar sensing and signalling in plants: Conserved and novel mechanisms. Annu. Rev. Plant Biol. 2006, 57, 675–709. [Google Scholar] [CrossRef]
- Granot, D.; Kelly, G.; Stein, O.; David-Schwartz, R. Substantial roles of hexokinase and fructokinase in the effects of sugars on plant physiology and development. J. Exp. Bot. 2014, 65, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.-L.; Jin, Y.; Yang, Y.-J.; Li, G.-J.; Boyer, J.S. Sugar input, metabolism, and signalling mediated by invertase: Roles in development, yield potential, and response to drought and heat. Mol. Plant 2010, 3, 942–955. [Google Scholar] [CrossRef]
- Kunz, S.; Pesquet, E.; Kleczkowski, L.A. Functional dissection of sugar signals affecting gene expression in Arabidopsis thaliana. PLoS ONE 2014, 9, 100312. [Google Scholar] [CrossRef]
- Li, P.; Wind, J.J.; Shi, X.; Zhang, H.; Hanson, J.; Smeekens, S.C.; Teng, S. Fructose sensitivity is suppressed in Arabidopsis by the transcription factor ANAC089 lacking the membrane-bound domain. Proc. Natl. Acad. Sci. USA 2011, 108, 3436–3441. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-H.; Yoo, S.-D. Signalling role of fructose mediated by FINS1/FBP in Arabidopsis thaliana. PLOS Genet. 2011, 7, 1001263. [Google Scholar] [CrossRef]
- Lastdrager, J.; Hanson, J.; Smeekens, S. Sugar signals and the control of plant growth and development. J. Exp. Bot. 2014, 65, 799–807. [Google Scholar] [CrossRef] [Green Version]
- Wingler, A. Transitioning to the next phase: The role of sugar signaling throughout the plant life cycle. Plant Physiol. 2018, 176, 1075–1084. [Google Scholar] [CrossRef]
- Peshev, D.; Van den Ende, W. Fructans: Prebiotics and immunomodulators. J. Funct. Foods 2014, 8, 348–357. [Google Scholar] [CrossRef]
- Van den Ende, W.; El-Esawe, S. Sucrose signaling pathways leading to fructan and anthocyanin accumulation: A dual function in abiotic and biotic stress responses? Environ. Exp. Bot. 2013, 108, 4–13. [Google Scholar] [CrossRef]
- Payyavula, R.S.; Singh, R.K.; Navarre, D.A. Transcription factors, sucrose, and sucrose metabolic genes interact to regulate potato phenylpropanoid metabolism. J. Exp. Bot. 2013, 64, 5115–5131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morkunas, I.; Narożna, D.; Nowak, W.; Samardakiewicz, S.; Remlein-Starosta, D. Cross-talk interactions of sucrose and Fusarium oxysporum in the phenylpropanoid pathway and the accumulation and localization of flavonoids in embryo axes of yellow lupine. J. Plant Physiol. 2011, 168, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Delatte, T.L.; Sedijani, P.; Kondou, Y.; Matsui, M.; de Jong, G.J.; Somsen, G.W.; Wiese-Klinkenberg, A.; Primavesi, L.F.; Paul, M.J.; Schluepmann, H. Growth arrest by trehalose-6-phosphate: An astonishing case of primary metabolite control over growth by way of the SnRK1 signalling pathway. Plant Physiol. 2011, 157, 160–174. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, Y.; Li, Z.; Hsu, C.-C.; Liu, X.; Fu, L.; Hou, Y.-J.; Du, Y.; Xie, S.; Zhang, C.; et al. Reciprocal regulation of the TOR kinase and ABA receptor balances plant growth and stress response. Mol. Cell 2018, 69, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Dobrenel, T.; Marchive, C.; Sormani, R.; Moreau, M.; Mozzo, M.; Montané, M.-H.; Menand, B.; Robaglia, C.; Meyer, C. Regulation of plant growth and metabolism by the TOR kinase. Biochem. Soc. Trans. 2011, 39, 477–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.-L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef] [Green Version]
- Yadav, U.P.; Ivakov, A.; Feil, R.; Duan, G.Y.; Walther, D.; Giavalisco, P.; Piques, M.; Carillo, P.; Hubberten, H.-M.; Stitt, M.; et al. The sucrose–trehalose 6-phosphate (Tre6P) nexus: Specificity and mechanisms of sucrose signalling by Tre6P. J. Exp. Bot. 2014, 65, 1051–1068. [Google Scholar] [CrossRef]
- Janse van Rensburg, H.C.; Van den Ende, W. UDP-glucose: A potential signalling molecule in plants? Front. Plant Sci. 2018, 8, 2230. [Google Scholar] [CrossRef]
- Zhai, Z.; Liu, H.; Shanklin, J. Phosphorylation of WRINKLED1 by KIN10 results in its proteasomal degradation, providing a link between energy homeostasis and lipid biosynthesis. Plant Cell 2017, 29, 871–889. [Google Scholar] [CrossRef]
- Nunes, C.; Primavesi, L.F.; Patel, M.K.; Martinez-Barajas, E.; Powers, S.J.; Sagar, R.; Fevereiro, P.S.; Davis, B.G.; Paul, M.J. Inhibition of SnRK1 by metabolites: Tissue-dependent effects and cooperative inhibition by glucose 1-phosphate in combination with trehalose 6-phosphate. Plant Physiol. Biochem. 2013, 63, 89–98. [Google Scholar] [CrossRef]
- Xiong, Y.; Mc Cormack, M.; Li, L.; Hall, Q.; Xiang, C.; Sheen, J. Glucose–TOR signalling reprograms the transcriptome and activates meristems. Nature 2013, 496, 181–186. [Google Scholar] [CrossRef]
- Moellering, R.E.; Cravatt, B.F. Functional lysine modification by an intrinsically reactive primary glycolytic metabolite. Science 2013, 341, 549–553. [Google Scholar] [CrossRef] [Green Version]
- Demidchik, V.; Straltsova, D.; Medvedev, S.S.; Pozhvanov, G.A.; Sokolik, A.; Yurin, V. Stress-induced electrolyte leakage: The role of K+-permeable channels and involvement in programmed cell death and metabolic adjustment. J. Exp. Bot. 2014, 65, 1259–1270. [Google Scholar] [CrossRef]
- Fedorova, M.; Frolov, A.; Hoffmann, R. Fragmentation behavior of Amadori-peptides obtained by non-enzymatic glycosylation of lysine residues with ADP-ribose in tandem mass spectrometry. J. Mass Spectrom. 2010, 45, 664–669. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shumilina, J.; Kusnetsova, A.; Tsarev, A.; Janse van Rensburg, H.C.; Medvedev, S.; Demidchik, V.; Van den Ende, W.; Frolov, A. Glycation of Plant Proteins: Regulatory Roles and Interplay with Sugar Signalling? Int. J. Mol. Sci. 2019, 20, 2366. https://doi.org/10.3390/ijms20092366
Shumilina J, Kusnetsova A, Tsarev A, Janse van Rensburg HC, Medvedev S, Demidchik V, Van den Ende W, Frolov A. Glycation of Plant Proteins: Regulatory Roles and Interplay with Sugar Signalling? International Journal of Molecular Sciences. 2019; 20(9):2366. https://doi.org/10.3390/ijms20092366
Chicago/Turabian StyleShumilina, Julia, Alena Kusnetsova, Alexander Tsarev, Henry C. Janse van Rensburg, Sergei Medvedev, Vadim Demidchik, Wim Van den Ende, and Andrej Frolov. 2019. "Glycation of Plant Proteins: Regulatory Roles and Interplay with Sugar Signalling?" International Journal of Molecular Sciences 20, no. 9: 2366. https://doi.org/10.3390/ijms20092366