Short-Term Protocols to Obtain Insulin-Producing Cells from Rat Adipose Tissue: Signaling Pathways and In Vivo Effect
Abstract
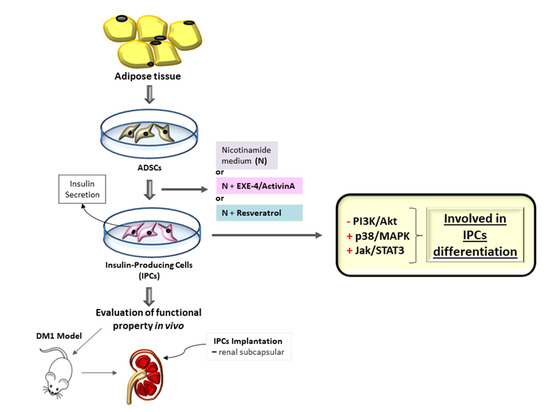
1. Introduction
2. Results
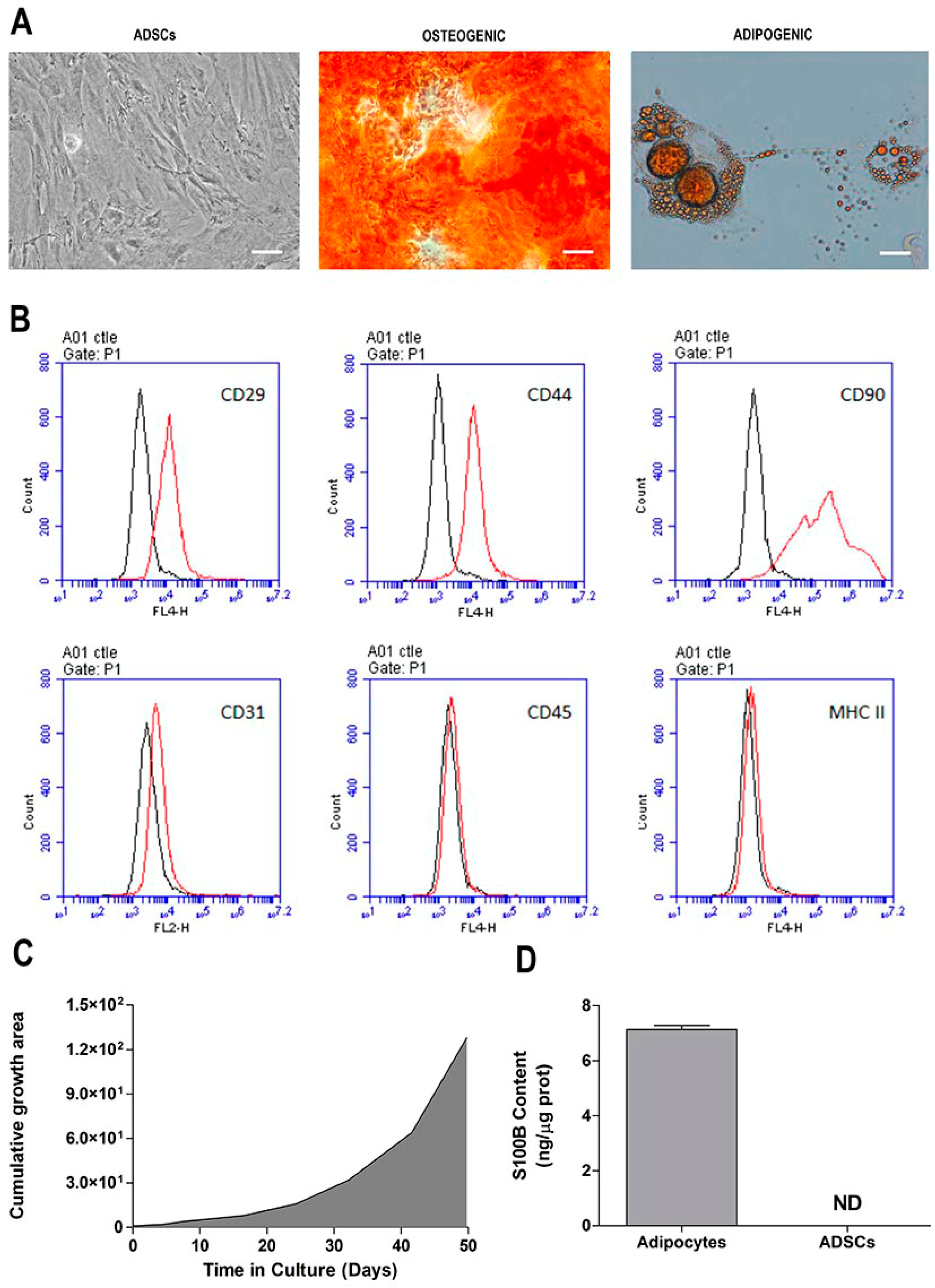
2.1. Morphological and Phenotypic Characterization of ADSC Culture
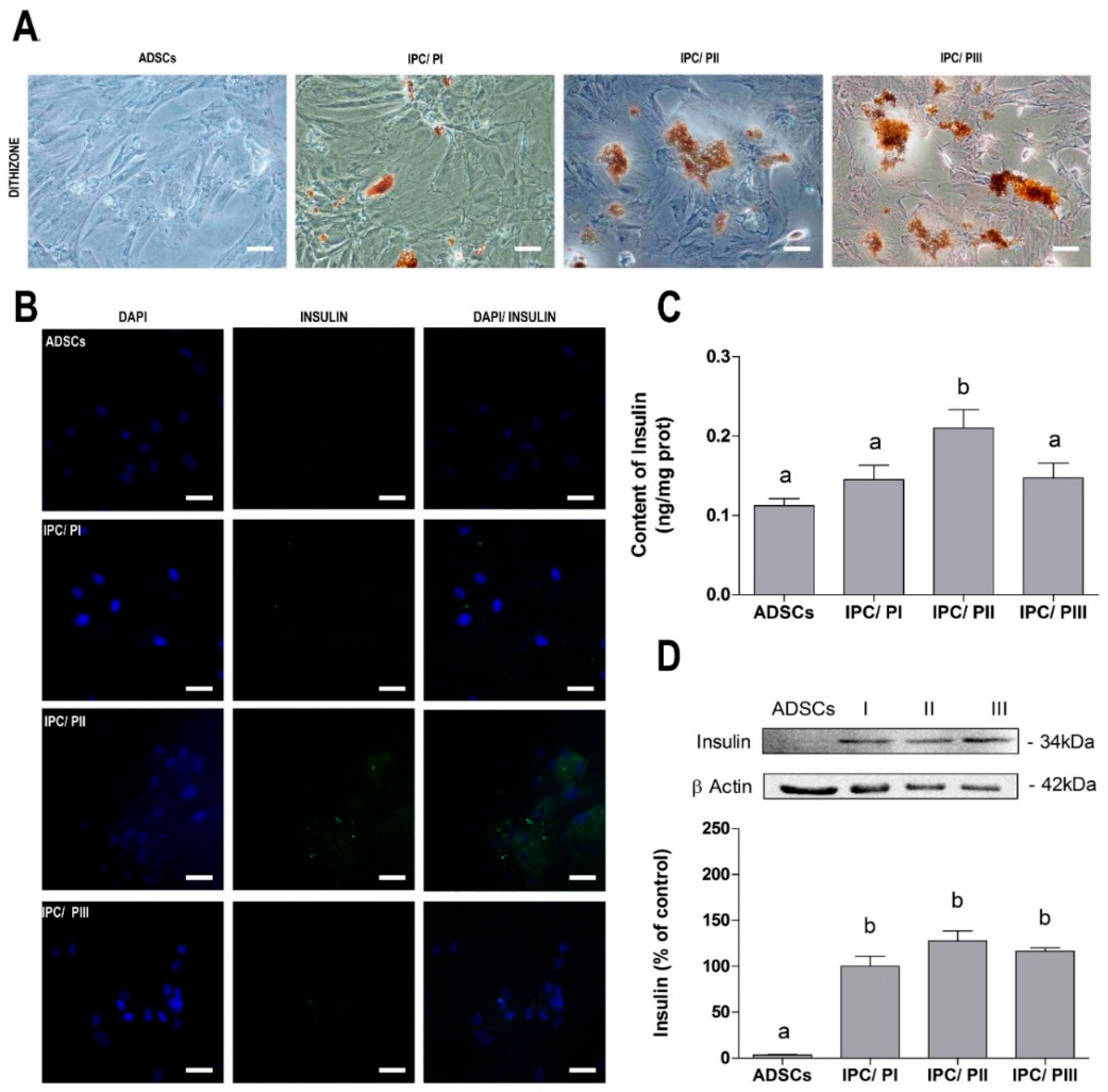
2.2. Differentiation of ADSC into Insulin-Producing Cells (IPCs)
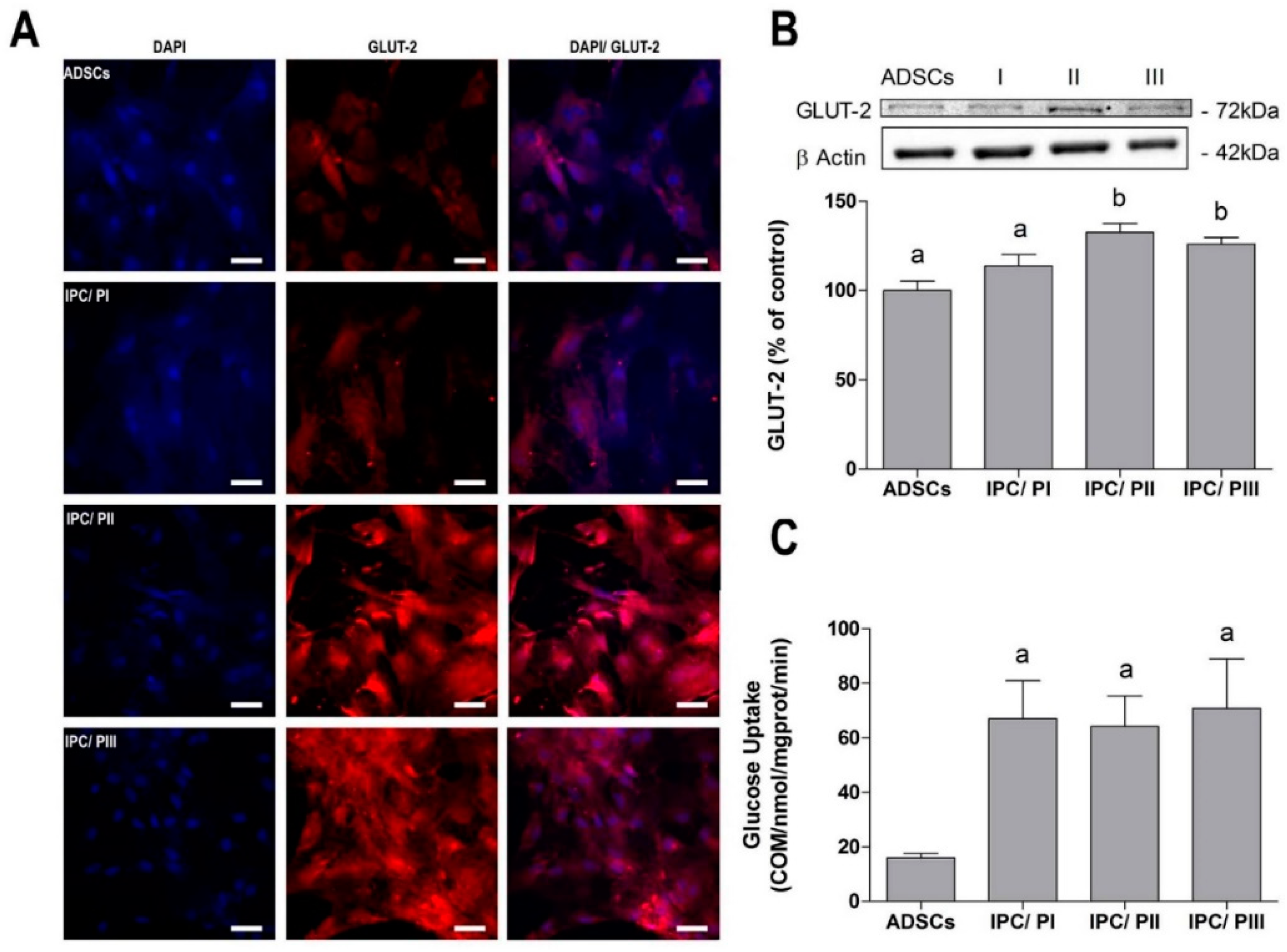
2.3. Transport of Glucose in IPCs
2.4. Secretion of Insulin in Response to Glucose Stimulation in IPCs
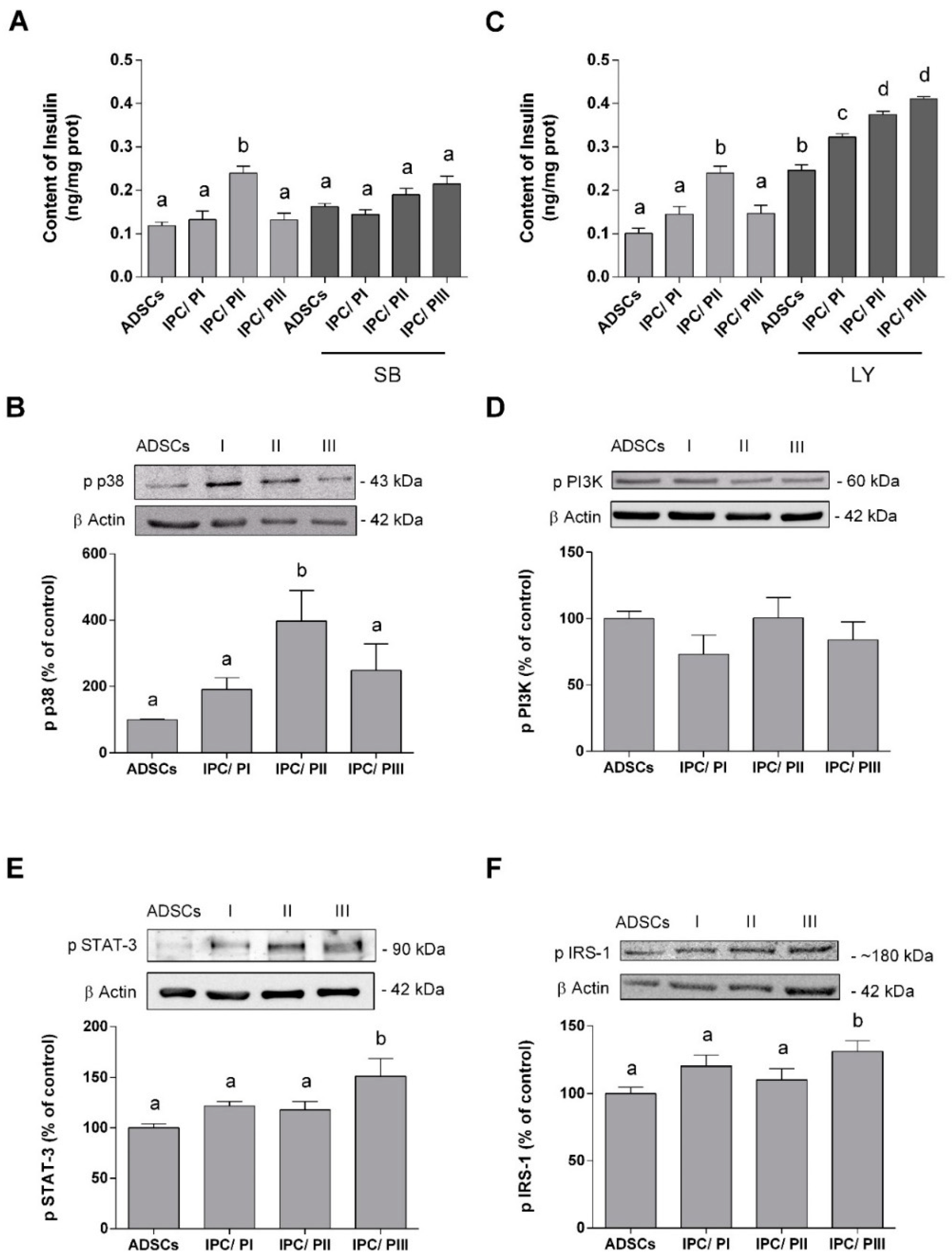
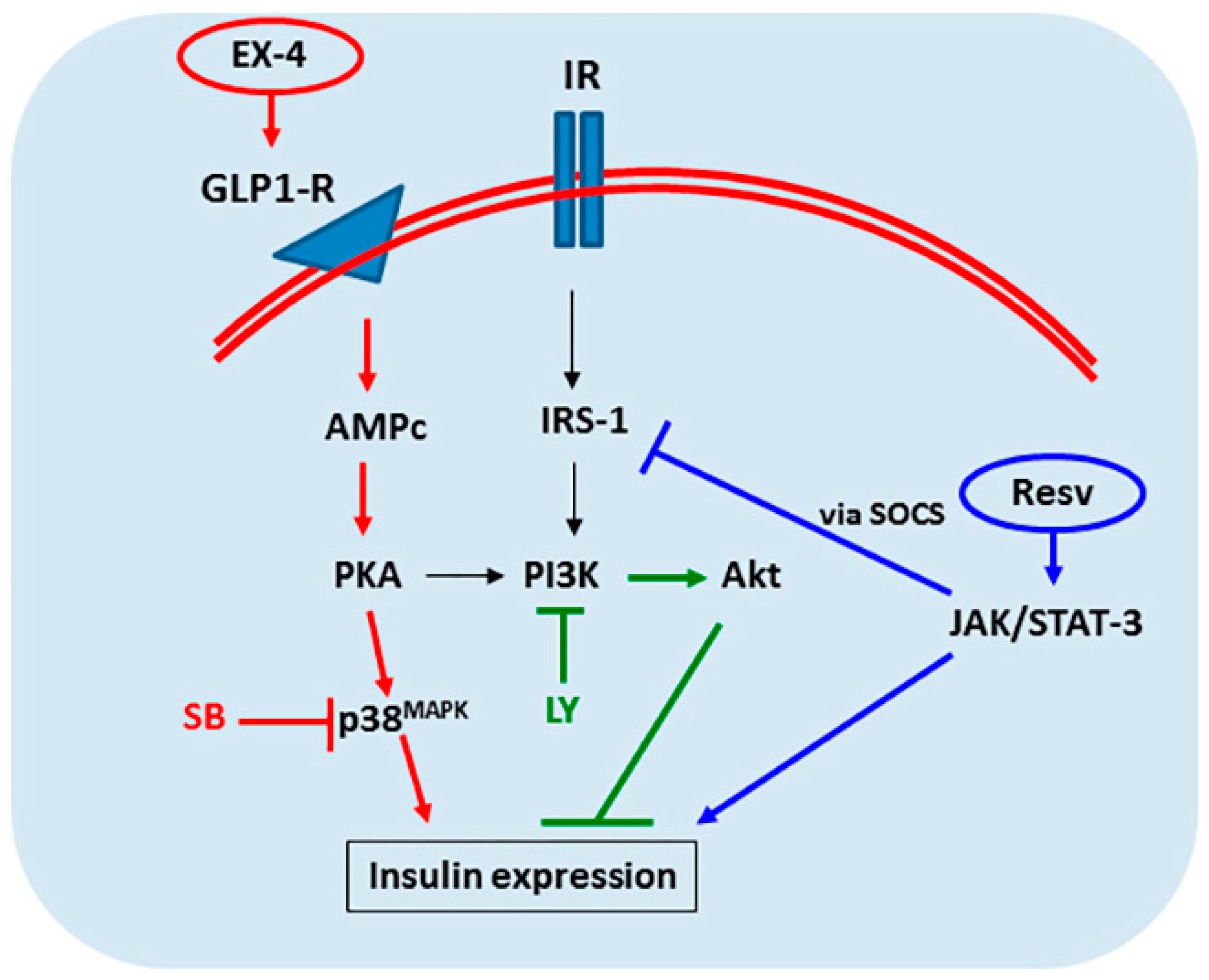
2.5. IPC Signaling Involved in Insulin Expression
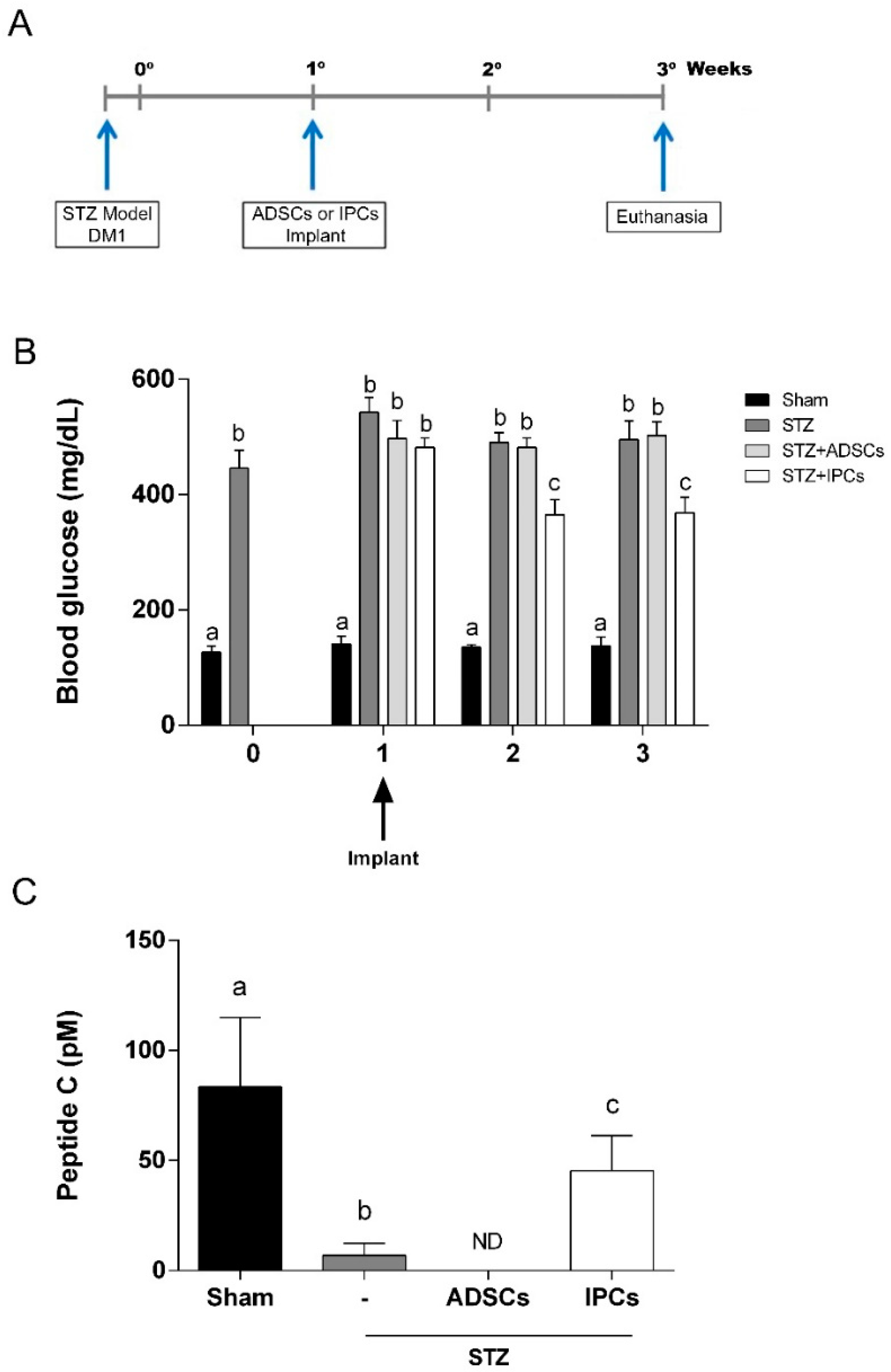
2.6. Implant of IPCs from ADSCs in Diabetic Rats
3. Discussion
4. Material and Methods
4.1. Animals
4.2. Chemicals
4.3. Isolation and Expansion of Rat Adipose Tissue-Derived Stromal Cells (ADSCs)
4.4. Characterization of ADSCs
4.5. ELISA for S100B
4.6. Protocol for Differentiation of Mesenchymal Stromal Cells into Insulin-Producing Cells (IPCs)
4.7. Staining with Dithizone
4.8. Immunofluorescence
4.9. Insulin Content
4.10. Evaluation of Glucose-Stimulated Insulin Secretion
4.11. Glucose Uptake Assay
4.12. Western Blot Analysis
4.13. DM1 Model and Surgical Procedure–IPCs Implant
4.14. Protein Determination
4.15. Statistical Analysis
5. Conclusions
Data Availability
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van Belle, T.L.; Coppieters, K.T.; von Herrath, M.G. Type 1 diabetes: Etiology, immunology, and therapeutic strategies. Physiol. Rev. 2011, 91, 79–118. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, P.; Brayman, K.L. Stem cell therapy to cure type 1 diabetes: From hype to hope. Stem Cells Transl. Med. 2013, 2, 328–336. [Google Scholar] [CrossRef]
- Aguayo-Mazzucato, C.; Bonner-Weir, S. Pancreatic β Cell Regeneration as a Possible Therapy for Diabetes. Cell Metab. 2018, 27, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Daley, G.Q. The promise and perils of stem cell therapeutics. Cell Stem Cell 2012, 10, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-B.; Jiang, X.-B.; Yang, L. Differentiation of rat marrow mesenchymal stem cells into pancreatic islet beta-cells. World J. Gastroenterol. 2004, 10, 3016–3020. [Google Scholar] [CrossRef]
- Karnieli, O.; Izhar-Prato, Y.; Bulvik, S.; Efrat, S. Generation of insulin-producing cells from human bone marrow mesenchymal stem cells by genetic manipulation. Stem Cells Dayt. Ohio 2007, 25, 2837–2844. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.-H.; Muzzonigro, T.M.; Bae, S.-H.; LaPlante, J.M.; Hatch, H.M.; Petersen, B.E. Adult bone marrow-derived cells trans-differentiating into insulin-producing cells for the treatment of type I diabetes. Lab. Investig. J. Tech. Methods Pathol. 2004, 84, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Paz, A.H.; Salton, G.D.; Ayala-Lugo, A.; Gomes, C.; Terraciano, P.; Scalco, R.; Laurino, C.C.F.C.; Passos, E.P.; Schneider, M.R.; Meurer, L.; et al. Betacellulin overexpression in mesenchymal stem cells induces insulin secretion in vitro and ameliorates streptozotocin-induced hyperglycemia in rats. Stem Cells Dev. 2011, 20, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Chandra, V.; Swetha, G.; Muthyala, S.; Jaiswal, A.K.; Bellare, J.R.; Nair, P.D.; Bhonde, R.R. Islet-like cell aggregates generated from human adipose tissue derived stem cells ameliorate experimental diabetes in mice. PLoS ONE 2011, 6, e20615. [Google Scholar] [CrossRef]
- Francese, R.; Fiorina, P. Immunological and regenerative properties of cord blood stem cells. Clin. Immunol. Orlando Fla 2010, 136, 309–322. [Google Scholar] [CrossRef]
- Domínguez-Bendala, J.; Lanzoni, G.; Inverardi, L.; Ricordi, C. Concise Review: Mesenchymal Stem Cells for Diabetes. STEM CELLS Transl. Med. 2012, 1, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Yoon, B.S.; Moon, J.H.; Kim, J.; Jun, E.K.; Lee, J.H.; Kim, J.S.; Baik, C.S.; Kim, A.; Whang, K.Y.; et al. Differentiation of human labia minora dermis-derived fibroblasts into insulin-producing cells. Exp. Mol. Med. 2012, 44, 26–35. [Google Scholar] [CrossRef]
- Khorsandi, L.; Saremy, S.; Khodadadi, A.; Dehbashi, F. Effects of Exendine-4 on The Differentiation of Insulin Producing Cells from Rat Adipose-Derived Mesenchymal Stem Cells. Cell J. 2016, 17, 720–729. [Google Scholar] [PubMed]
- Nejad-Dehbashi, F.; Hashemitabar, M.; Orazizadeh, M.; Bahramzadeh, S.; Shahhosseini Pourshoushtary, E.; Khorsandi, L. The effects of exendine-4 on insulin producing cell differentiation from rat bone marrow-derived mesenchymal stem cells. Cell J. 2014, 16, 187–194. [Google Scholar] [PubMed]
- Xin, Y.; Jiang, X.; Wang, Y.; Su, X.; Sun, M.; Zhang, L.; Tan, Y.; Wintergerst, K.A.; Li, Y.; Li, Y. Insulin-Producing Cells Differentiated from Human Bone Marrow Mesenchymal Stem Cells In Vitro Ameliorate Streptozotocin-Induced Diabetic Hyperglycemia. PLoS ONE 2016, 11, e0145838. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Stoffers, D.A.; Habener, J.F.; Bonner-Weir, S. Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes 1999, 48, 2270–2276. [Google Scholar] [CrossRef] [PubMed]
- Greig, N.H.; Holloway, H.W.; De Ore, K.A.; Jani, D.; Wang, Y.; Zhou, J.; Garant, M.J.; Egan, J.M. Once daily injection of exendin-4 to diabetic mice achieves long-term beneficial effects on blood glucose concentrations. Diabetologia 1999, 42, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Nardin, P.; Zanotto, C.; Hansen, F.; Batassini, C.; Gasparin, M.S.; Sesterheim, P.; Gonçalves, C.-A. Peripheral Levels of AGEs and Astrocyte Alterations in the Hippocampus of STZ-Diabetic Rats. Neurochem. Res. 2016, 41, 2006–2016. [Google Scholar] [CrossRef]
- Das, S.; Das, D.K. Anti-inflammatory responses of resveratrol. Inflamm. Allergy Drug Targets 2007, 6, 168–173. [Google Scholar] [CrossRef]
- Kao, C.-L.; Tai, L.-K.; Chiou, S.-H.; Chen, Y.-J.; Lee, K.-H.; Chou, S.-J.; Chang, Y.-L.; Chang, C.-M.; Chen, S.-J.; Ku, H.-H.; et al. Resveratrol promotes osteogenic differentiation and protects against dexamethasone damage in murine induced pluripotent stem cells. Stem Cells Dev. 2010, 19, 247–258. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, S.; Zhao, L.; Zhang, Y.; Li, Q.; Chai, X.; Zhang, Y. Resveratrol Enhances Cardiomyocyte Differentiation of Human Induced Pluripotent Stem Cells through Inhibiting Canonical WNT Signal Pathway and Enhancing Serum Response Factor-miR-1 Axis. Stem Cells Int. 2016, 2016, 2524092. [Google Scholar] [CrossRef]
- Dai, Z.; Li, Y.; Quarles, L.D.; Song, T.; Pan, W.; Zhou, H.; Xiao, Z. Resveratrol enhances proliferation and osteoblastic differentiation in human mesenchymal stem cells via ER-dependent ERK1/2 activation. Phytomedicine Int. J. Phytother. Phytopharm. 2007, 14, 806–814. [Google Scholar] [CrossRef]
- Ding, H.; Xu, X.; Qin, X.; Yang, C.; Feng, Q. Resveratrol promotes differentiation of mouse embryonic stem cells to cardiomyocytes. Cardiovasc. Ther. 2016, 34, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Baeyens, L.; Bouwens, L. Can beta-cells be derived from exocrine pancreas? Diabetes Obes. Metab. 2008, 10 (Suppl. 4), 170–178. [Google Scholar] [CrossRef]
- Koblas, T.; Leontovyč, I.; Zacharovová, K.; Berková, Z.; Kříž, J.; Girman, P.; Saudek, F. Activation of the Jak/Stat signalling pathway by leukaemia inhibitory factor stimulates trans-differentiation of human non-endocrine pancreatic cells into insulin-producing cells. Folia Biol. 2012, 58, 98–105. [Google Scholar]
- Valdez, I.A.; Dirice, E.; Gupta, M.K.; Shirakawa, J.; Teo, A.K.K.; Kulkarni, R.N. Proinflammatory Cytokines Induce Endocrine Differentiation in Pancreatic Ductal Cells via STAT3-Dependent NGN3 Activation. Cell Rep. 2016, 15, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Lemper, M.; Leuckx, G.; Heremans, Y.; German, M.S.; Heimberg, H.; Bouwens, L.; Baeyens, L. Reprogramming of human pancreatic exocrine cells to β-like cells. Cell Death Differ. 2015, 22, 1117–1130. [Google Scholar] [CrossRef] [PubMed]
- Makeeva, N.; Roomans, G.M.; Welsh, N. Role of TAB1 in nitric oxide-induced p38 activation in insulin-producing cells. Int. J. Biol. Sci. 2006, 3, 71–76. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Watanabe, H.; Saito, H.; Nishimura, H.; Ueda, J.; Evers, B.M. Activation of phosphatidylinositol-3 kinase regulates pancreatic duodenal homeobox-1 in duct cells during pancreatic regeneration. Pancreas 2008, 36, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, D.; Al-Amin, A.; Turpaev, K.; Li, T.; Idevall-Hagren, O.; Li, J.; Wuttke, A.; Fred, R.G.; Ravassard, P.; Scharfmann, R.; et al. Imatinib mesilate-induced phosphatidylinositol 3-kinase signalling and improved survival in insulin-producing cells: Role of Src homology 2-containing inositol 5’-phosphatase interaction with c-Abl. Diabetologia 2013, 56, 1327–1338. [Google Scholar] [CrossRef]
- Kemp, D.M.; Habener, J.F. Insulinotropic hormone glucagon-like peptide 1 (GLP-1) activation of insulin gene promoter inhibited by p38 mitogen-activated protein kinase. Endocrinology 2001, 142, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Choi, Y.-J.; Park, S.-Y.; Kim, J.-Y.; Won, K.-C.; Son, J.-K.; Kim, Y.-W. Hexane Extract of Orthosiphon stamineus Induces Insulin Expression and Prevents Glucotoxicity in INS-1 Cells. Diabetes Metab. J. 2015, 39, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Velayos, T.; Martínez, R.; Alonso, M.; Garcia-Etxebarria, K.; Aguayo, A.; Camarero, C.; Urrutia, I.; Martínez de LaPiscina, I.; Barrio, R.; Santin, I.; et al. An Activating Mutation in STAT3 Results in Neonatal Diabetes Through Reduced Insulin Synthesis. Diabetes 2017, 66, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- Wartchow, K.M.; Tramontina, A.C.; de Souza, D.F.; Biasibetti, R.; Bobermin, L.D.; Gonçalves, C.-A. Insulin Stimulates S100B Secretion and These Proteins Antagonistically Modulate Brain Glucose Metabolism. Neurochem. Res. 2016, 41, 1420–1429. [Google Scholar] [CrossRef]
- Chen, L.; Tredget, E.E.; Wu, P.Y.G.; Wu, Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS ONE 2008, 3, e1886. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Song, L.; Shen, K.; Wang, H.; Qian, M.; Niu, W.; Qin, X. Bone marrow mesenchymal stem cells promote the repair of islets from diabetic mice through paracrine actions. Mol. Cell. Endocrinol. 2014, 388, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Bourin, P.; Bunnell, B.A.; Casteilla, L.; Dominici, M.; Katz, A.J.; March, K.L.; Redl, H.; Rubin, J.P.; Yoshimura, K.; Gimble, J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013, 15, 641–648. [Google Scholar]
- Hidaka, H.; Endo, T.; Kawamoto, S.; Yamada, E.; Umekawa, H.; Tanabe, K.; Hara, K. Purification and characterization of adipose tissue S-100b protein. J. Biol. Chem. 1983, 258, 2705–2709. [Google Scholar]
- Michetti, F.; Dell’Anna, E.; Tiberio, G.; Cocchia, D. Immunochemical and immunocytochemical study of S-100 protein in rat adipocytes. Brain Res. 1983, 262, 352–356. [Google Scholar] [CrossRef]
- Gonçalves, C.A.; Leite, M.C.; Guerra, M.C. Adipocytes as an Important Source of Serum S100B and Possible Roles of This Protein in Adipose Tissue. Cardiovasc. Psychiatry Neurol. 2010, 2010, 790431. [Google Scholar] [CrossRef][Green Version]
- Bellaver, B.; Bobermin, L.D.; Souza, D.G.; Rodrigues, M.D.N.; de Assis, A.M.; Wajner, M.; Gonçalves, C.-A.; Souza, D.O.; Quincozes-Santos, A. Signaling mechanisms underlying the glioprotective effects of resveratrol against mitochondrial dysfunction. Biochim. Biophys. Acta 2016, 1862, 1827–1838. [Google Scholar] [CrossRef]
- Zanotto, C.; Simão, F.; Gasparin, M.S.; Biasibetti, R.; Tortorelli, L.S.; Nardin, P.; Gonçalves, C.-A. Exendin-4 Reverses Biochemical and Functional Alterations in the Blood-Brain and Blood-CSF Barriers in Diabetic Rats. Mol. Neurobiol. 2017, 54, 2154–2166. [Google Scholar] [CrossRef]
- Balboa, D.; Saarimäki-Vire, J.; Otonkoski, T. Concise Review: Human Pluripotent Stem Cells for the Modeling of Pancreatic β-Cell Pathology. Stem Cells 2019, 37, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Kellett, G.L.; Brot-Laroche, E.; Mace, O.J.; Leturque, A. Sugar absorption in the intestine: The role of GLUT2. Annu. Rev. Nutr. 2008, 28, 35–54. [Google Scholar] [CrossRef] [PubMed]
- Olson, A.L.; Pessin, J.E. Structure, function, and regulation of the mammalian facilitative glucose transporter gene family. Annu. Rev. Nutr. 1996, 16, 235–256. [Google Scholar] [CrossRef]
- Tang, D.-Q.; Cao, L.-Z.; Burkhardt, B.R.; Xia, C.-Q.; Litherland, S.A.; Atkinson, M.A.; Yang, L.-J. In vivo and in vitro characterization of insulin-producing cells obtained from murine bone marrow. Diabetes 2004, 53, 1721–1732. [Google Scholar] [CrossRef]
- Li, J.; Zhao, Z.; Liu, J.; Huang, N.; Long, D.; Wang, J.; Li, X.; Liu, Y. MEK/ERK and p38 MAPK regulate chondrogenesis of rat bone marrow mesenchymal stem cells through delicate interaction with TGF-beta1/Smads pathway. Cell Prolif. 2010, 43, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Dinić, S.; Grdović, N.; Uskoković, A.; Đorđević, M.; Mihailović, M.; Jovanović, J.A.; Poznanović, G.; Vidaković, M. CXCL12 protects pancreatic β-cells from oxidative stress by a Nrf2-induced increase in catalase expression and activity. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2016, 92, 436–454. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, S.; Zhou, Y.; Tan, J.; Che, H.; Ning, F.; Zhang, X.; Xun, W.; Huo, N.; Tang, L.; et al. Natural mineralized scaffolds promote the dentinogenic potential of dental pulp stem cells via the mitogen-activated protein kinase signaling pathway. Tissue Eng. Part A 2012, 18, 677–691. [Google Scholar] [CrossRef] [PubMed]
- Ba, P.; Duan, X.; Fu, G.; Lv, S.; Yang, P.; Sun, Q. Differential effects of p38 and Erk1/2 on the chondrogenic and osteogenic differentiation of dental pulp stem cells. Mol. Med. Rep. 2017. [Google Scholar] [CrossRef]
- Roussel, M.; Mathieu, J.; Dalle, S. Molecular mechanisms redirecting the GLP-1 receptor signalling profile in pancreatic β-cells during type 2 diabetes. Horm. Mol. Biol. Clin. Investig. 2016, 26, 87–95. [Google Scholar] [CrossRef]
- Yang, J.-L.; Chen, W.-Y.; Chen, Y.-P.; Kuo, C.-Y.; Chen, S.-D. Activation of GLP-1 Receptor Enhances Neuronal Base Excision Repair via PI3K-AKT-Induced Expression of Apurinic/Apyrimidinic Endonuclease 1. Theranostics 2016, 6, 2015–2027. [Google Scholar] [CrossRef] [PubMed]
- Zaragosi, L.-E.; Wdziekonski, B.; Villageois, P.; Keophiphath, M.; Maumus, M.; Tchkonia, T.; Bourlier, V.; Mohsen-Kanson, T.; Ladoux, A.; Elabd, C.; et al. Activin a plays a critical role in proliferation and differentiation of human adipose progenitors. Diabetes 2010, 59, 2513–2521. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Lu, H.; Wang, S.; Yin, W.; Liu, X.; Dong, L.; Chiu, R.; Shen, L.; Lu, W.-J.; Lan, F. Suppression of Nestin reveals a critical role for p38-EGFR pathway in neural progenitor cell proliferation. Oncotarget 2016, 7, 87052–87063. [Google Scholar] [CrossRef]
- Mao, G.-H.; Lu, P.; Wang, Y.-N.; Tian, C.-G.; Huang, X.-H.; Feng, Z.-G.; Zhang, J.-L.; Chang, H.-Y. Role of PI3K p110β in the differentiation of human embryonic stem cells into islet-like cells. Biochem. Biophys. Res. Commun. 2017, 488, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Kohn, A.D.; Summers, S.A.; Birnbaum, M.J.; Roth, R.A. Expression of a constitutively active Akt Ser/Thr kinase in 3T3-L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J. Biol. Chem. 1996, 271, 31372–31378. [Google Scholar] [CrossRef]
- Hori, Y.; Rulifson, I.C.; Tsai, B.C.; Heit, J.J.; Cahoy, J.D.; Kim, S.K. Growth inhibitors promote differentiation of insulin-producing tissue from embryonic stem cells. Proc. Natl. Acad. Sci. USA 2002, 99, 16105–16110. [Google Scholar] [CrossRef]
- Fan, N.; Sun, H.; Wang, Y.; Zhang, L.; Xia, Z.; Peng, L.; Hou, Y.; Shen, W.; Liu, R.; Peng, Y. Midkine, a Potential Link between Obesity and Insulin Resistance. PLoS ONE 2014, 9, e88299. [Google Scholar] [CrossRef] [PubMed]
- Galic, S.; Sachithanandan, N.; Kay, T.W.; Steinberg, G.R. Suppressor of cytokine signalling (SOCS) proteins as guardians of inflammatory responses critical for regulating insulin sensitivity. Biochem. J. 2014, 461, 177–188. [Google Scholar] [CrossRef]
- Caldarelli, I.; Speranza, M.C.; Bencivenga, D.; Tramontano, A.; Borgia, A.; Pirozzi, A.V.A.; Perrotta, S.; Oliva, A.; Della Ragione, F.; Borriello, A. Resveratrol mimics insulin activity in the adipogenic commitment of human bone marrow mesenchymal stromal cells. Int. J. Biochem. Cell Biol. 2015, 60, 60–72. [Google Scholar] [CrossRef]
- Gabr, M.M.; Zakaria, M.M.; Refaie, A.F.; Ismail, A.M.; Abou-El-Mahasen, M.A.; Ashamallah, S.A.; Khater, S.M.; El-Halawani, S.M.; Ibrahim, R.Y.; Uin, G.S.; et al. Insulin-producing cells from adult human bone marrow mesenchymal stem cells control streptozotocin-induced diabetes in nude mice. Cell Transplant. 2013, 22, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Toyoda, T.; Ito, R.; Funato, M.; Hosokawa, Y.; Matsui, S.; Sudo, T.; Nakamura, M.; Okada, C.; Zhuang, X.; et al. Identification of a small molecule that facilitates the differentiation of human iPSCs/ESCs and mouse embryonic pancreatic explants into pancreatic endocrine cells. Diabetologia 2017, 60, 1454–1466. [Google Scholar] [CrossRef] [PubMed]
- Gabr, M.M.; Zakaria, M.M.; Refaie, A.F.; Khater, S.M.; Ashamallah, S.A.; Ismail, A.M.; El-Badri, N.; Ghoneim, M.A. Generation of insulin-producing cells from human bone marrow-derived mesenchymal stem cells: Comparison of three differentiation protocols. BioMed Res. Int. 2014, 2014, 832736. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Meirelles, L.d.S.; Nardi, N.B. Murine marrow-derived mesenchymal stem cell: Isolation, in vitro expansion, and characterization. Br. J. Haematol. 2003, 123, 702–711. [Google Scholar] [CrossRef]
- Da Silva Meirelles, L.; Chagastelles, P.C.; Nardi, N.B. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J. Cell Sci. 2006, 119, 2204–2213. [Google Scholar] [CrossRef] [PubMed]
- Leite, M.C.; Galland, F.; Brolese, G.; Guerra, M.C.; Bortolotto, J.W.; Freitas, R.; de Almeida, L.M.V.; Gottfried, C.; Gonçalves, C.-A. A simple, sensitive and widely applicable ELISA for S100B: Methodological features of the measurement of this glial protein. J. Neurosci. Methods 2008, 169, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Timper, K.; Seboek, D.; Eberhardt, M.; Linscheid, P.; Christ-Crain, M.; Keller, U.; Müller, B.; Zulewski, H. Human adipose tissue-derived mesenchymal stem cells differentiate into insulin, somatostatin, and glucagon expressing cells. Biochem. Biophys. Res. Commun. 2006, 341, 1135–1140. [Google Scholar] [CrossRef]
- De Souza, D.F.; Wartchow, K.; Hansen, F.; Lunardi, P.; Guerra, M.C.; Nardin, P.; Gonçalves, C.-A. Interleukin-6-induced S100B secretion is inhibited by haloperidol and risperidone. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 43, 14–22. [Google Scholar] [CrossRef]
- Shiroi, A.; Yoshikawa, M.; Yokota, H.; Fukui, H.; Ishizaka, S.; Tatsumi, K.; Takahashi, Y. Identification of insulin-producing cells derived from embryonic stem cells by zinc-chelating dithizone. Stem Cells Dayt. Ohio 2002, 20, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Pellerin, L.; Magistretti, P.J. Glutamate uptake into astrocytes stimulates aerobic glycolysis: A mechanism coupling neuronal activity to glucose utilization. Proc. Natl. Acad. Sci. USA 1994, 91, 10625–10629. [Google Scholar] [CrossRef]
- Li, D.; Hao, J.; Yuan, Y.-H.; Yun, S.H.; Feng, J.-B.; Dai, L.-J.; Warnock, G.L. Pancreatic islet transplantation to the renal subcapsule in mice. Protoc. Exch. 2011. [Google Scholar] [CrossRef]
- Peterson, G.L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal. Biochem. 1977, 83, 346–356. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wartchow, K.M.; Rodrigues, L.; Suardi, L.Z.; Federhen, B.C.; Selistre, N.G.; Gonçalves, C.-A.; Sesterheim, P. Short-Term Protocols to Obtain Insulin-Producing Cells from Rat Adipose Tissue: Signaling Pathways and In Vivo Effect. Int. J. Mol. Sci. 2019, 20, 2458. https://doi.org/10.3390/ijms20102458
Wartchow KM, Rodrigues L, Suardi LZ, Federhen BC, Selistre NG, Gonçalves C-A, Sesterheim P. Short-Term Protocols to Obtain Insulin-Producing Cells from Rat Adipose Tissue: Signaling Pathways and In Vivo Effect. International Journal of Molecular Sciences. 2019; 20(10):2458. https://doi.org/10.3390/ijms20102458
Chicago/Turabian StyleWartchow, Krista Minéia, Letícia Rodrigues, Lucas Zingano Suardi, Barbara Carolina Federhen, Nicholas Guerini Selistre, Carlos-Alberto Gonçalves, and Patrícia Sesterheim. 2019. "Short-Term Protocols to Obtain Insulin-Producing Cells from Rat Adipose Tissue: Signaling Pathways and In Vivo Effect" International Journal of Molecular Sciences 20, no. 10: 2458. https://doi.org/10.3390/ijms20102458
APA StyleWartchow, K. M., Rodrigues, L., Suardi, L. Z., Federhen, B. C., Selistre, N. G., Gonçalves, C.-A., & Sesterheim, P. (2019). Short-Term Protocols to Obtain Insulin-Producing Cells from Rat Adipose Tissue: Signaling Pathways and In Vivo Effect. International Journal of Molecular Sciences, 20(10), 2458. https://doi.org/10.3390/ijms20102458