Towards Predictive Modeling of Sorghum Biomass Yields Using Fraction of Absorbed Photosynthetically Active Radiation Derived from Sentinel-2 Satellite Imagery and Supervised Machine Learning Techniques
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial Set-Up
2.2. Biomass Data Collection
2.3. Satellite Data Acquisition
2.4. Modeling Total Aboveground Biomass Yields
3. Results
3.1. fAPAR Index Pattern Across Sorghum Types
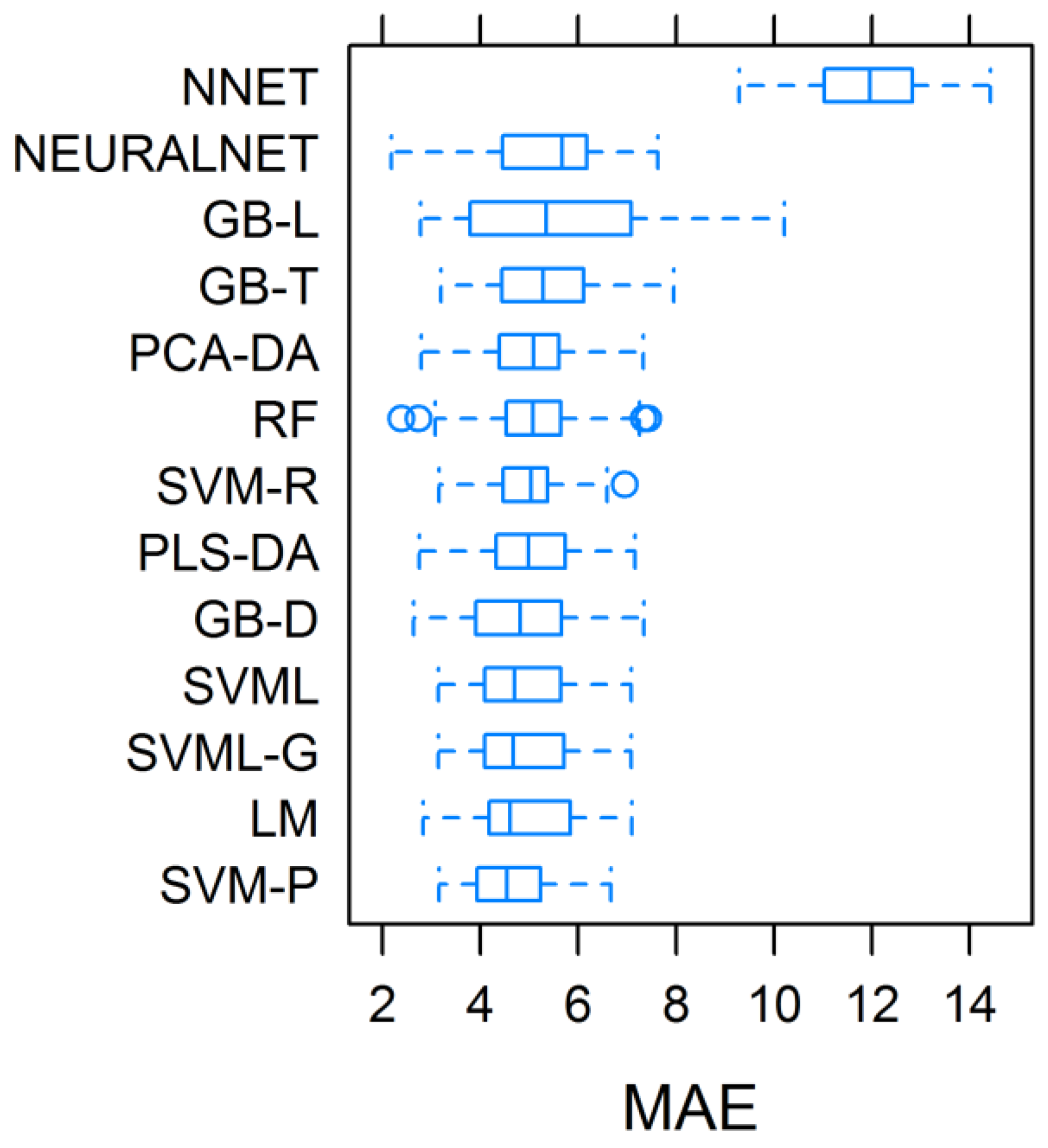
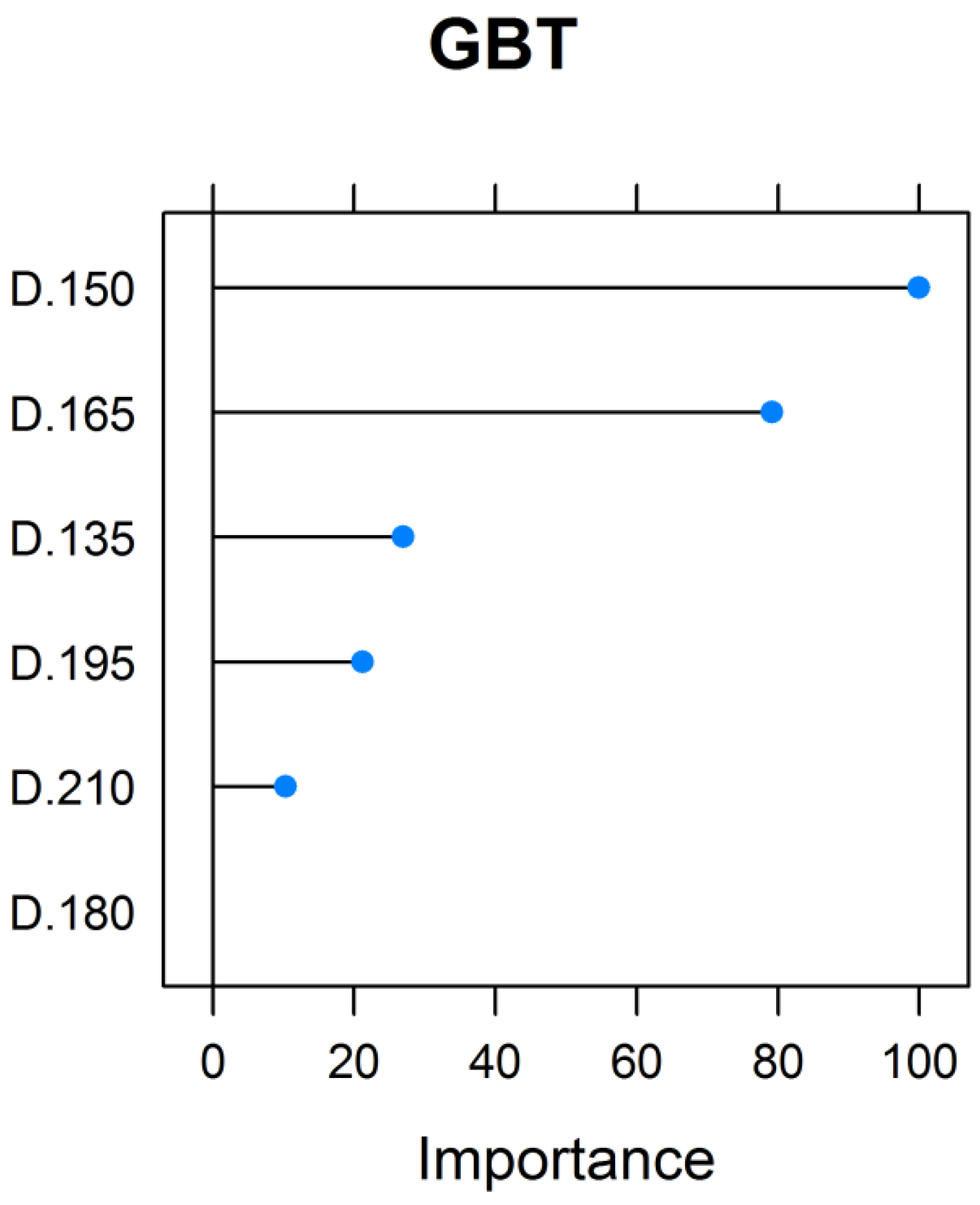
3.2. Assessment and Validation of the Predictive Models, and Importance of Regressors in Total Biomass Prediction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Habyarimana, E.; Lorenzoni, C.; Redaelli, R.; Alfieri, M.; Amaducci, S.; Cox, S. Towards a perennial biomass sorghum crop: A comparative investigation of biomass yields and overwintering of Sorghum bicolor x S. halepense lines relative to long term S. bicolor trials in northern Italy. Biomass Bioenergy 2018, 111, 187–195. [Google Scholar] [CrossRef]
- Damasceno, C.M.B.; Schaffert, R.E.; Duweikat, I. Mining Genetic Diversity of Sorghum as a Bioenergy Feedstock; Springer: New York, NY, USA, 2014; pp. 81–106. [Google Scholar]
- Hoffmann, L., Jr.; Rooney, W.L. Cytoplasm has no effect on the yield and quality of biomass sorghum hybrids. JSBS 2013, 3, 129–134. [Google Scholar] [CrossRef][Green Version]
- Prakasham, R.S.; Nagaiah, D.; Vinutha, K.S.; Uma, A.; Chiranjeevi, T.; Umakanth, A.V. Sorghum biomass: A novel renewable carbon source for industrial bioproducts. Biofuels 2014, 5, 159–174. [Google Scholar] [CrossRef]
- Habyarimana, E. Genomic prediction for yield improvement and safeguarding of genetic diversity in CIMMYT spring wheat (Triticum aestivum L.). Aust. J. Crop Sci. 2016, 10, 127–136. [Google Scholar]
- Rooney, W.L. Genetics and cytogenetics. In Sorghum: Origin, History, Technology, and Production; Smith, C.W., Frederiksen, R.A., Eds.; John Wiley & Sons: New York, NY, USA, 2000; pp. 261–307. [Google Scholar]
- El Bassam, N. Handbook of Bioenergy Crops: A Complete Reference to Species, Development and Applications; Earthscan Ltd.: London, UK, 2010; pp. 45–477. [Google Scholar]
- Stefaniak, T.R.; Dahlberg, J.A.; Bean, B.W.; Dighe, N.; Wolfrum, E.J.; Rooney, W.L. Variation in biomass composition components among forage, biomass, sorghum-sudangrass, and sweet sorghum types. Crop Sci. 2012, 52, 1949–1954. [Google Scholar] [CrossRef]
- Kussul, N.; Sokolov, B.V.; Zyelyk, Y.I.; Zelentsov, V.A.; Skakun, S.V.; Shelestov, A.Y. Disaster risk assessment based on heterogeneous geospatial information. J. Autom. Inform. Sci. 2010, 42, 32–45. [Google Scholar] [CrossRef]
- Kussul, N.; Shelestov, A.; Skakun, S. Flood Monitoring from SAR Data Use of Satellite and In-Situ Data to Improve Sustainability; Springer: Dordrecht, The Netherlands, 2011; pp. 19–29. [Google Scholar]
- Skakun, S.; Kussul, N.; Kussul, O.; Shelestov, A. Quantitative estimation of drought risk in Ukraine using satellite data. In Proceedings of the IEEE International Geoscience and Remote Sensing Symposium (IGARSS), Quebec City, QC, Canada, 13–18 July 2014; pp. 5091–5094. [Google Scholar]
- Skakun, S.; Kussul, N.; Shelestov, A.; Kussul, O. The use of satellite data for agriculture drought risk quantification in Ukraine. Geomat. Nat. Hazards Risk 2016, 7, 901–917. [Google Scholar] [CrossRef]
- Gallego, J.; Kravchenko, A.N.; Kussul, N.N.; Skakun, S.V.; Shelestov, A.Y.; Grypych, Y.A. Efficiency assessment of different approaches to crop classification based on satellite and ground observations. J. Autom. Inform. Sci. 2012, 44, 67–80. [Google Scholar] [CrossRef]
- Diouf, A.A.; Brandt, M.; Verger, A.; El Jarroudi, M.; Djaby, B.; Fensholt, R.; Ndione, J.A.; Tychon, B. Fodder Biomass Monitoring in Sahelian Rangelands Using Phenological Metrics from FAPAR time series. Remote Sens. 2015, 7, 9122–9148. [Google Scholar] [CrossRef]
- Duveiller, G.; López-Lozano, R.; Baruth, B. Enhanced Processing of 1-km Spatial Resolution fAPAR Time Series for Sugarcane Yield Forecasting and Monitoring. Remote Sens. 2013, 5, 1091–1116. [Google Scholar] [CrossRef]
- Johnson, D.M. A comprehensive assessment of the correlations between field crop yields and commonly used MODIS products. Int. J. Appl. Earth Obs. Geoinf. 2016, 52, 65–81. [Google Scholar] [CrossRef]
- Kogan, F.; Kussul, N.; Adamenko, T.; Skakun, S.; Kravchenko, O.; Kryvobok, O.; Shelestov, A.; Kolotii, A.; Kussul, O.; Lavrenyuk, A. Winter wheat yield forecasting in Ukraine based on Earth observation, meteorological data and biophysical models. Int. J. Appl. Earth Obs. Geoinf. 2013, 23, 192–203. [Google Scholar] [CrossRef]
- Kogan, F.; Kussul, N.; Adamenko, T.; Skakun, S.; Kravchenko, O.; Kryvobok, O.; Shelestov, A.; Kolotii, A.; Kussul, O.; Lavrenyuk, A. Winter wheat yield forecasting: A comparative analysis of results of regression and biophysical models. Int. J. Autom. Inform. Sci. 2013, 45, 68–81. [Google Scholar] [CrossRef]
- Kowalik, W.; Dabrowska-Zielinska, K.; Meroni, M.; Raczka, T.U.; de Wit, A. Yield estimation using SPOTVEGETATION products: A case study of wheat in European countries. Int. J. Autom. Inform. Sci. 2014, 32, 228–239. [Google Scholar]
- Kross, A.; McNairn, H.; Lapen, D.; Sunohara, M.; Champagne, C. Assessment of RapidEye vegetation indices for estimation of leaf areaindex and biomass in corn and soybean crops. Int. J. Appl. Earth Obs. Geoinf. 2015, 34, 235–248. [Google Scholar] [CrossRef]
- Camacho, F.; Cernicharo, J.; Lacaze, R.; Baret, F.; Weiss, M. GEOV1: LAI, FAPAR Essential Climate Variables and FCOVER global time series capitalizing over existing products. Part 2: Validation and intercomparison with reference products. Remote Sens. Environ. 2013, 137, 310–329. [Google Scholar] [CrossRef]
- Shelestov, A.; Kolotii, A.; Camacho, F.; Skakun, S.; Kussul, O.; Lavrenuik, M. Mapping of biophysical parameters based on high resolution EO imagery for JECAM test site in Ukraine. In Proceedings of the 2015 IEEE International Geoscience and Remote Sensing Symposium (IGARSS), Milan, Italy, 26–31 July 2015. [Google Scholar]
- Kussul, N.; Kolotii, A.; Skakun, S.; Shelestov, A.; Kussul, O.; Oliynuk, T. Efficiency estimation of different satellite data usage for winter wheat yield forecasting in Ukraine. In Proceedings of the 2014 IEEE International Geoscience and Remote Sensing Symposium (IGARSS), Quebec City, QC, Canada, 13–18 July 2014; pp. 5080–5082. [Google Scholar]
- López-Lozano, R.; Duveiller, G.; Seguini, L.; Meroni, M.; García-Condado, S.; Hooker, J.; Leo, O.; Baruth, B. Towards regional grain yield forecasting with 1km-resolution EO biophysical products: Strengths and limitations at pan-European level. Agric. For. Meteorol. 2015, 206, 12–32. [Google Scholar] [CrossRef]
- Baret, F.; Weiss, M.; Lacaze, R.; Camacho, F.; Makhmara, H.; Pacholcyzk, P.; Smets, B. Geov1: LAI and FAPAR essential climate variables and FCOVER global time series capitalizing over existing products. Part1: Principles of development and production. Remote Sens. Environ. 2013, 137, 299–309. [Google Scholar] [CrossRef]
- Fensholt, R.; Sandholt, I.; Rasmussen, M.S.; Stisen, S.; Diouf, A. Evaluation of satellite based primary production modelling in the semi-arid Sahel. Remote Sens. Environ. 2006, 105, 173–188. [Google Scholar] [CrossRef]
- Tucker, C.J.; Holben, B.N.; Elgin, J.H.; McMurtrey, J.E. Relationship of spectral data to grain yield variation. Photogramm. Eng. Remote Sens. 1980, 46, 657–666. [Google Scholar]
- Barnett, T.L.; Thompson, D.R. The use of large-area spectral data in wheatyield estimation. Remote Sens. Environ. 1982, 12, 509–518. [Google Scholar] [CrossRef]
- Hatfield, J.L. Remote sensing estimators of potential and actual crop yield. Remote Sens. Environ. 1996, 13, 301–311. [Google Scholar] [CrossRef]
- Panda, S.S.; Ames, D.P.; Panigrahi, S. Application of vegetation indices for agricultural crop yield prediction using neural network techniques. Remote Sens. 2010, 2, 673–696. [Google Scholar] [CrossRef]
- Shafian, S.; Rajan, N.; Schnell, R.; Bagavathiannan, M.; Valasek, J.; Shi, Y.; Olsenholler, J. Unmanned aerial systems-based remote sensing for monitoring sorghum growth and development. PLoS ONE 2018, 13, e0196605. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Everitt, J.H.; Bradford, J.M.; Escobar, D.E. Mapping grain sorghum growth and yield variations using airborne multispectral digital imagery. Trans. ASAE 2000, 43, 1927–1938. [Google Scholar] [CrossRef]
- Piper, J.K.; Kulakow, P.A. Seed yield and biomass allocation in Sorghum bicolor and F1 and backcross generations of S. bicolor x S. halepense hybrids. Can. J. Bot. 1994, 72, 468–474. [Google Scholar] [CrossRef]
- De Keukelaere, L.; Sterckx, S.; Adriaensen, S.; Knaeps, E.; Reusen, I.; Giardino, C.; Bresciani, M.; Hunter, P.; Neil, C.; Van der Zande, D.; et al. Atmospheric correction of Landsat-8/OLI and Sentinel-2/MSI data using iCOR algorithm: Validation for coastal and inland waters. Eur. J. Remote Sens. 2018, 51, 525–542. [Google Scholar] [CrossRef]
- Weiss, M.; Baret, F. ATBD S2ToolBox Level 2 Products: LAI, FAPAR, FCOVER (Version 1.1). 2016. Available online: http://step.esa.int/docs/extra/ATBD_S2ToolBox_L2B_V1.1.pdf (accessed on 7 November 2018).
- Jacquemoud, S.; Verhoef, W.; Baret, F.; Bacour, C.; Zarco-Tejada, P.J.; Asner, G.P.; François, C.; Ustin, S.L. PROSPECT + SAIL models: A review of use for vegetation characterization. Remote Sens. Environ. 2009, 113, S56–S66. [Google Scholar] [CrossRef]
- Sage, R.F. A portrait of the C4 photosynthetic family on the 50th anniversary of its discovery: Species number, evolutionary lineages, and Hall of Fame. J. Exp. Bot. 2016, 67, 4039–4056. [Google Scholar] [CrossRef]
- Eilers, P.H.C. A perfect smoother. Anal. Chem. 2003, 75, 3631–3636. [Google Scholar] [CrossRef]
- Atzberger, C.; Eilers, P.H.C. A smoothed 1-km resolution NDVI time series (1998–2008) for vegetation studies in South America. Int. J. Digit. Earth 2010, 4, 365–386. [Google Scholar] [CrossRef]
- Kuhn, M. Building predictive models in R using the caret Package. J. Stat. Softw. 2008, 28, 1–26. [Google Scholar] [CrossRef]
- Benor, D.; Baxter, M. Training and Visit Extension; The World Bank: Washington, DC, USA, 1984; pp. 21–212. [Google Scholar]
- Hoefsloot, P.; Ines, A.V.; van Dam, J.; Duveiller, G.; Kayitakire, F.; Hansen, J. Combining crop models and remote sensing for yield prediction: Concepts, applications and challenges for heterogeneous smallholder environments. In JRC Scientific and Policy Reports; Report of CCFAS-JRC Workshop at Joint Research Centre; Joint Research Centre of the European Commission: Ispra, VA, Italy, 2012; pp. 7–41. [Google Scholar]
- Meuwissen, T.H.E.; Hayes, B.J.; Goddard, M.E. Prediction of total genetic value using genome-wide dense marker maps. Genetics 2001, 157, 1819–1829. [Google Scholar] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Breiman, L.; Friedman, J.H.; Olshen, R.A.; Stone, C.J. Classification and Regression Trees; Wadsworth Inc.: Blelmont, CA, USA, 1984. [Google Scholar]
- Willmott, C.J.; Matsuura, K. Advantages of the mean absolute error (MAE) over the root mean square error (RMSE) in assessing average model performance. Clim. Res. 2005, 30, 79–82. [Google Scholar] [CrossRef]
- Duncan, D.B. Multiple range and multiple F tests. Biometrics 1955, 11, 1–42. [Google Scholar] [CrossRef]
- Spearman, C. The proof and measurement of association between two things. Am. J. Psychol. 1904, 15, 72–101. [Google Scholar] [CrossRef]
- Schellberg, J.; Hill, M.J.; Gerhards, R.; Rothmund, M.; Braun, M. Precision agriculture on grassland: Applications, perspectives and constraints. Eur. J. Agron. 2008, 29, 59–71. [Google Scholar] [CrossRef]
- Segarra, E. Precision Agriculture Initiative for Texas High Plains; Annual Comprehensive Report; Texas A&M University Research and Extension Center: Lubbock, TX, USA, 2002. [Google Scholar]
- Gao, F.; Anderson, M.; Daughtry, C.; Johnson, D. Assessing the Variability of Corn and Soybean Yields in Central Iowa Using High Spatiotemporal Resolution Multi-Satellite Imagery. Remote Sens. 2018, 10, 1489. [Google Scholar] [CrossRef]
- Habyarimana, E.; Lorenzoni, C.; Busconi, M. Search for new stay-green sources in Sorghum bicolor (L.) Moench. Maydica 2010, 55, 187–194. [Google Scholar]
- Habyarimana, E.; Laureti, D.; Di Fonzo, N.; Lorenzoni, C. Biomass production and drought resistance at the seedling stage and in field conditions in sorghum. Maydica 2002, 47, 303–309. [Google Scholar]
- Habyarimana, E.; Laureti, D.; De Ninno, M.; Lorenzoni, C. Performances of biomass sorghum [Sorghum bicolor (L.) Moench] under different water regimes in Mediterranean region. Ind. Crop Prod. 2004, 20, 23–28. [Google Scholar] [CrossRef]
- Habyarimana, E.; Bonardi, P.; Laureti, D.; Di Bari, V.; Cosentino, S.; Lorenzoni, C. Multilocational evaluation of biomass sorghum hybrids under two stand densities and variable water supply in Italy. Ind. Crop Prod. 2004, 20, 3–9. [Google Scholar] [CrossRef]
- Cox, T.S.; Van Tassel, D.L.; Cox, C.M.; DeHaan, L.R. Progress in breeding perennial grains. Crop. Pasture Sci. 2010, 61, 513–521. [Google Scholar] [CrossRef]
- Nabukalu, P.; Cox, T.S. Response to selection in the initial stages of a perennial sorghum breeding program. Euphytica 2016, 209, 103–111. [Google Scholar] [CrossRef]
- Battude, M.; Al Bitar, A.; Morin, D.; Cros, J.; Huc, M.; Sicre, C.M.; Le Dante, V.; Demarez, V. Estimating maize biomass and yield over large areas using high spatial and temporal resolution Sentinel-2 like remote sensing data. Remote Sens. Environ. 2016, 184, 668–681. [Google Scholar] [CrossRef]

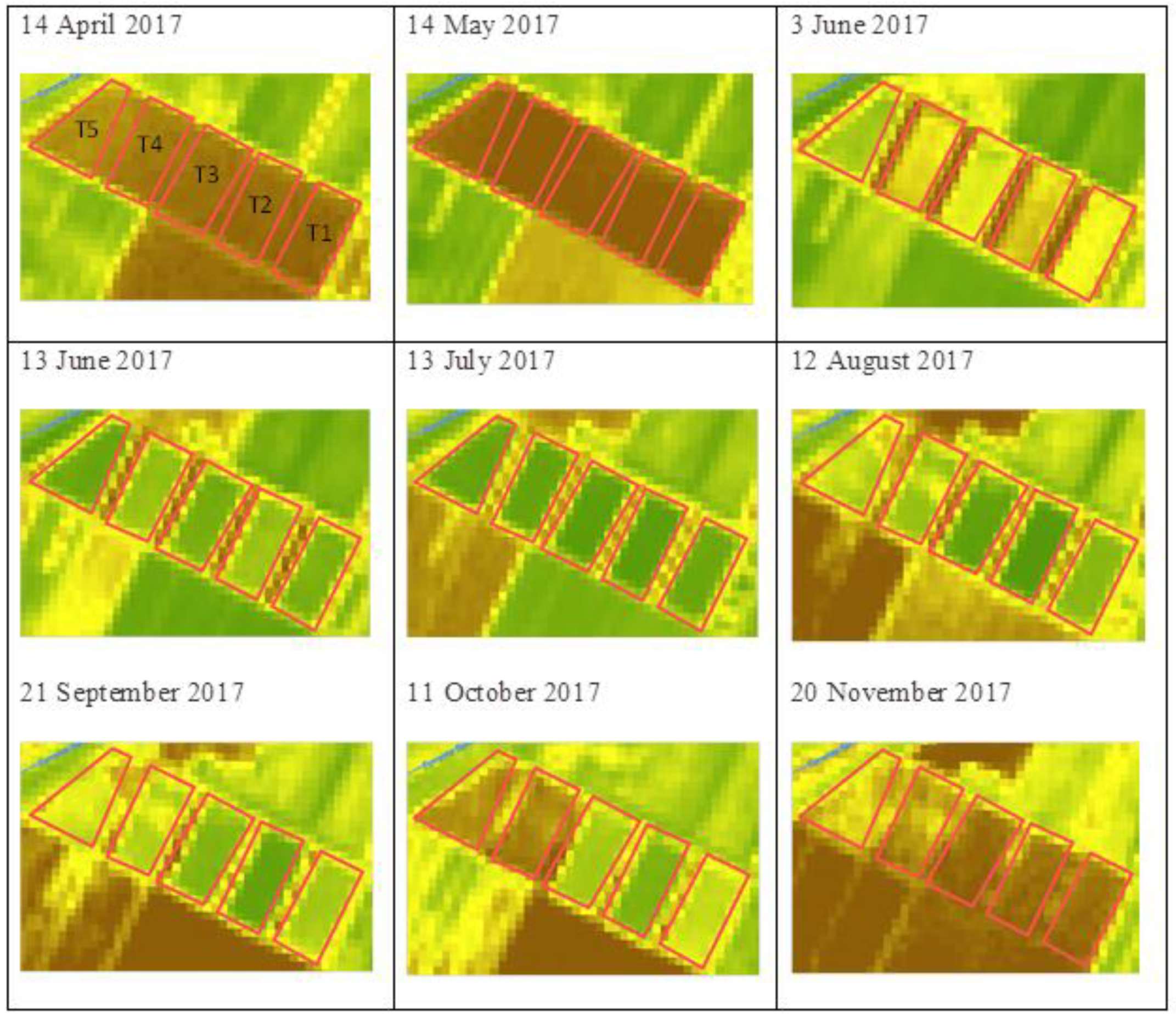

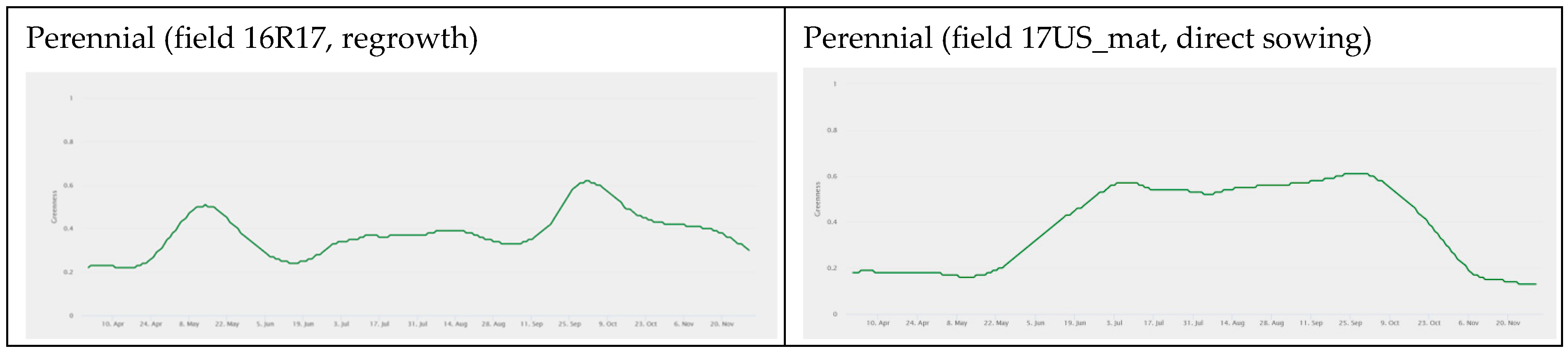
| Serial Number | Field/Pilot Name | Variety Name | Variety Type | Area (ha) | Dry Biomass Yield (t ha−1) | Cropping Season | Location Name |
|---|---|---|---|---|---|---|---|
| 1 | Botte 1 | Harmattan | Dual purpose | 9.00 | 14.13 | 2018 | Conselice |
| 2 | Saracca 5 | Harmattan | Dual purpose | 6.50 | 10.52 | 2018 | Conselice |
| 3 | V. serrata | Harmattan | Dual purpose | 44.87 | 9.69 | 2018 | Conselice |
| 4 | Magnana | P845F | Forage | 32.05 | 11.43 | 2018 | Conselice |
| 5 | Cà bianca | P845F | Forage | 3.72 | 11.11 | 2018 | Conselice |
| 6 | Gamberina 3 | Aralba | Dual purpose | 7.86 | 9.67 | 2018 | Conselice |
| 7 | Sagrate | Harmattan | Dual purpose | 50.00 | 8.90 | 2017 | Conselice |
| 8 | Prato_Mensa | Harmattan | Dual purpose | 3.29 | 19.10 | 2017 | Conselice |
| 9 | Comuna | P845F | Forage | 27.86 | 24.50 | 2017 | Conselice |
| 10 | Gamberina_1 | Aralba | Dual purpose | 7.60 | 12.80 | 2017 | Conselice |
| 11 | Botte | Harmattan | Dual purpose | 5.33 | 23.50 | 2017 | Conselice |
| 12 | Carafolo_G | Bulldozer | Biomass | 2.00 | 17.10 | 2017 | Nonantola |
| 13 | Cavriani_S | Merlin | Biomass | 2.00 | 19.50 | 2017 | Nonantola |
| 14 | Ferrari_R | Bulldozer | Biomass | 1.00 | 3.40 | 2017 | Nonantola |
| 15 | Mattioli_R | Bulldozer | Biomass | 1.00 | 4.90 | 2017 | Nonantola |
| 16 | Serafini_G | Bulldozer | Biomass | 2.00 | 15.30 | 2017 | Nonantola |
| 17 | Zavatti_E | Bulldozer | Biomass | 0.89 | 7.60 | 2017 | Mirandola |
| 18 | Grandi_Magonza | Bulldozer | Biomass | 0.80 | 12.80 | 2017 | Mirandola |
| 19 | Grandi_Ponte | Bulldozer | Biomass | 1.20 | 9.50 | 2017 | Mirandola |
| 20 | Zini_L | Palo Alto | Biomass | 2.50 | 8.00 | 2017 | Mirandola |
| 21 | Villa_verdetta | Bulldozer | Biomass | 1.00 | 6.25 | 2018 | Mirandola |
| 22 | Cama_grande | Bulldozer | Biomass | 4.40 | 14.94 | 2018 | Mirandola |
| 23 | Cama_piccolo | Bulldozer | Biomass | 4.00 | 15.35 | 2018 | Mirandola |
| 24 | Golinelli_Raimondo | Bulldozer | Biomass | 2.02 | 10.48 | 2018 | Mirandola |
| 25 | Barozzi_Lidia | Bulldozer | Biomass | 3.00 | 7.19 | 2018 | Mirandola |
| 26 | Molon_A | Palo Alto | Biomass | 5.00 | 8.30 | 2017 | Mirandola |
| 27 | T1_Anzola | Sole | Biomass | 0.74 | 13.00 | 2017 | Anzola |
| 28 | T2_Anzola | Trudan | Forage | 0.71 | 19.00 | 2017 | Anzola |
| 29 | T3_Anzola | Hannibal | Sweet | 0.71 | 17.00 | 2017 | Anzola |
| 30 | T4_Anzola | Harmattan | Dual purpose | 0.70 | 14.00 | 2017 | Anzola |
| 31 | T1_Anzola | Bulldozer | Biomass | 0.74 | 17.00 | 2018 | Anzola |
| 32 | T2_Anzola | Hannibal | Sweet | 0.71 | 19.00 | 2018 | Anzola |
| 33 | T3_Anzola | Tarzan | Biomass | 0.71 | 21.00 | 2018 | Anzola |
| 34 | T4_Anzola | Trudan | Forage | 0.70 | 13.00 | 2018 | Anzola |
| 35 | T5_Anzola | Harmattan | Dual purpose | 0.70 | 15.00 | 2018 | Anzola |
| 36 | 15R17 | Perennial | Biomass | 0.06 | 2.39 | 2017 | Anzola |
| 37 | 16R17 | Perennial | Biomass | 0.15 | 8.27 | 2017 | Anzola |
| 38 | 17IT_mat | Bicolor | Biomass | 0.17 | 18.30 | 2017 | Anzola |
| 39 | 17US_mat | Bicolor | Biomass | 0.15 | 21.00 | 2017 | Anzola |
| 40 | 16R18 | Perennial | Biomass | 0.15 | 14.35 | 2018 | Anzola |
| 41 | 15R18 | Perennial | Biomass | 0.06 | 10.73 | 2018 | Anzola |
| 42 | 17R18 | Perennial | Biomass | 0.15 | 17.47 | 2018 | Anzola |
| (1) Model | (2) Accuracy | (3) May_MAE.T t ha−1 | May_MAE.V | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| May | June | July | May–June | June–July | May–July | Mean | t ha−1 | % | ||
| PLS-DA | 0.77 | 0.49 | −0.02 | 0.69 | 0.32 | 0.56 | 0.47 ab | 5.01 bcd | 3.62 | 26.81 |
| PCA-DA | 0.76 | 0.49 | −0.13 | 0.67 | 0.29 | 0.52 | 0.43 ab | 5.00 bcd | 2.91 | 21.56 |
| RF | 0.82 | 0.39 | 0.64 | 0.68 | 0.53 | 0.74 | 0.63 a | 5.05 bcd | 2.27 | 16.81 |
| SVML | 0.80 | 0.49 | 0.58 | 0.67 | 0.59 | 0.70 | 0.64 a | 4.82 cd | 3.74 | 27.70 |
| SVML-G | 0.80 | 0.49 | 0.58 | 0.66 | 0.61 | 0.72 | 0.64 a | 4.84 cd | 3.74 | 27.70 |
| SVM-R | 0.88 | −0.36 | 0.51 | 0.02 | −0.15 | 0.08 | 0.16 b | 4.95 bcd | 1.87 | 13.85 |
| SVM-P | 0.81 | 0.49 | 0.09 | 0.63 | 0.42 | 0.53 | 0.50 a | 4.64 d | 6.22 | 46.07 |
| NNET | 0.78 | 0.56 | 0.16 | 0.75 | 0.38 | 0.70 | 0.56 a | 11.99 a | 12.50 | 92.59 |
| GBT | 0.78 | 0.56 | 0.69 | 0.58 | 0.81 | 0.57 | 0.66 a | 5.29 bc | 2.68 | 19.85 |
| GBD | 0.84 | 0.37 | −0.01 | 0.76 | 0.48 | 0.51 | 0.49 a | 4.80 d | 2.18 | 16.15 |
| GBL | 0.45 | 0.11 | 0.89 | 0.93 | 0.03 | 0.43 | 0.47 ab | 5.43 b | 3.40 | 25.19 |
| LM | 0.78 | 0.50 | 0.56 | 0.73 | 0.46 | 0.65 | 0.61 ab | 4.88 cd | 4.53 | 33.56 |
| NLNET | 0.79 | 0.09 | 0.33 | 0.79 | 0.01 | 0.79 | 0.47 ab | 5.36 b | 2.34 | 17.33 |
| MEAN | 0.77a | 0.36b | 0.37b | 0.66a | 0.37b | 0.58ab | 0.52 | 5.54 | 4.00 | 29.63 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habyarimana, E.; Piccard, I.; Catellani, M.; De Franceschi, P.; Dall’Agata, M. Towards Predictive Modeling of Sorghum Biomass Yields Using Fraction of Absorbed Photosynthetically Active Radiation Derived from Sentinel-2 Satellite Imagery and Supervised Machine Learning Techniques. Agronomy 2019, 9, 203. https://doi.org/10.3390/agronomy9040203
Habyarimana E, Piccard I, Catellani M, De Franceschi P, Dall’Agata M. Towards Predictive Modeling of Sorghum Biomass Yields Using Fraction of Absorbed Photosynthetically Active Radiation Derived from Sentinel-2 Satellite Imagery and Supervised Machine Learning Techniques. Agronomy. 2019; 9(4):203. https://doi.org/10.3390/agronomy9040203
Chicago/Turabian StyleHabyarimana, Ephrem, Isabelle Piccard, Marcello Catellani, Paolo De Franceschi, and Michela Dall’Agata. 2019. "Towards Predictive Modeling of Sorghum Biomass Yields Using Fraction of Absorbed Photosynthetically Active Radiation Derived from Sentinel-2 Satellite Imagery and Supervised Machine Learning Techniques" Agronomy 9, no. 4: 203. https://doi.org/10.3390/agronomy9040203
APA StyleHabyarimana, E., Piccard, I., Catellani, M., De Franceschi, P., & Dall’Agata, M. (2019). Towards Predictive Modeling of Sorghum Biomass Yields Using Fraction of Absorbed Photosynthetically Active Radiation Derived from Sentinel-2 Satellite Imagery and Supervised Machine Learning Techniques. Agronomy, 9(4), 203. https://doi.org/10.3390/agronomy9040203






